Objectives
IPX203 is an investigational oral extended-release capsule formulation of carbidopa and levodopa. The pharmacodynamics and efficacy of IPX203 were compared with immediate-release carbidopa-levodopa (IR CD-LD) in this open-label, rater-blinded, multicenter, crossover study in patients with advanced Parkinson disease (PD).
Methods
Twenty-eight patients were randomized to 2 weeks of treatment with IR CD-LD followed by IPX203 or IPX203 followed by IR CD-LD. Pharmacokinetic and motor assessments were conducted on days 1 and 15 of each treatment period. Efficacy was assessed using a 3-day PD diary. Pharmacodynamics were assessed by rater-blinded Movement Disorder Society—Unified Parkinson's Disease Rating Scale Part III and Investigator Assessment of Subject's Motor State.
Results
After a single dose, levodopa concentrations were sustained above 50% of peak concentration for 4.6 hours with IPX203 versus 1.5 hours with IR CD-LD (P < 0.0001). Based on the PD diary, patients experienced significantly less Off time with IPX203 as a percentage of waking hours than IR CD-LD (mean 19.3% vs 33.5%, respectively; P < 0.0001), translating into 2.3 hours less Off time than IR CD-LD with most of this improvement (1.9 hours) being Good On time. There was no significant difference in the amount of On time with troublesome dyskinesia between treatments. Pharmacodynamic assessments demonstrated similar outcomes in favor of IPX203 on day 1 and a significant predose benefit on motor examination after multiple dosing.
Conclusions
IPX203 demonstrated a sustained effect to reduce Off time and improve Good On time in patients with PD and motor fluctuations. Both treatments were well tolerated.
Key Words: IPX203, extended-release carbidopa-levodopa, motor effects, Parkinson disease, pharmacokinetics
Levodopa (LD) in combination with an aromatic amino acid decarboxylase inhibitor, such as carbidopa (CD), is a highly effective symptomatic treatment of Parkinson disease (PD). As PD progresses, nigrostriatal dopaminergic transmission deteriorates and the brain becomes dependent on exogenous LD. Patients with advanced PD typically require frequent administration of immediate-release (IR) CD-LD formulations.1 Short-acting CD-LD formulations are associated with the expected pharmacodynamic consequences of their pharmacokinetic profiles. Many PD patients (50% after 5 years of treatment2) using short-acting dopaminergic therapies eventually develop motor complications, including slowness in turning On, end-of-dose wearing-off, unpredictable On-Off motor fluctuations or On-Off phenomena, and peak-dose dyskinesias. These symptoms are believed to correlate with widely fluctuating plasma LD levels and are managed by optimizing dosing intervals, use of extended-release (ER) CD-LD formulations, and use of combination regimens that include CD-LD, longer acting dopamine agonists, enzyme inhibitors (catechol-O-methyltransferase [COMT] and monoamine oxidase [MAO]), and amantadine. Eventually, some patients require direct continuous duodenal infusion and deep brain stimulation. Although the mechanisms underlying motor complications involving dyskinesias and On-Off fluctuations in PD are not fully understood, the pulsatile stimulation of dopamine receptors by fluctuating LD concentrations from frequent oral administrations of short-acting CD-LD formulations together with the progressive loss of nigrostriatal neurons are thought to contribute to this occurance.3 Chronic intermittent pulsatile stimulation of dopamine receptors contributes to the development of dyskinesia in PD animal models when compared with animals treated with continuous infusion.4–6 Continuous administration or long-acting dopaminergic agents are associated with a decreased risk of motor complications in patients with PD compared with short-acting agents or standard CD-LD formulations.3
IPX203 (carbidopa and levodopa extended-release capsules; ER CD-LD) is a multiparticulate oral capsule formulation of CD-LD being developed in dose-proportional strengths. The CD:LD ratio in IPX203 is 1:4, identical to marketed formulations of CD-LD. IPX203 comprises different types of beads in a specific ratio designed to provide the desired LD plasma profile of a rapid initial rise in plasma LD followed by prolonged, steady concentrations that extend beyond currently available products.
Background information on IPX203 and the single-dose pharmacokinetics and pharmacodynamics of IPX203 have been reported7; results in patients with advanced PD demonstrated that IPX203 had a longer pharmacodynamic effect compared with immediate-release carbidopa-levodopa (IR CD-LD) and with extended-release CD-LD capsules (Rytary; Impax Laboratories, LLC, Bridgewater, NJ) as measured by the Investigator Assessment of Subject's Motor State (IASMS).
This present study compared the single- and multiple-dose pharmacokinetics, pharmacodynamics, efficacy, and safety of individually adjusted therapeutic doses of IPX203 with IR CD-LD in patients with advanced PD.
MATERIALS AND METHODS
This open-label, rater-blinded, multicenter, randomized crossover study was conducted at 11 US sites comparing IPX203 and IR CD-LD in 28 patients with advanced PD who were experiencing motor fluctuations. The protocol was approved by the institutional review boards of the participating institutions, patients provided written informed consent, and the study was conducted in accordance with ethical principles that are consistent with good clinical practice (ClinicalTrials.gov identifier, NCT03007888).
Study Design
Patients were randomized in a 1:1 manner to IR CD-LD followed by IPX203 or IPX203 followed by IR CD-LD with a 1-week washout between treatment periods. Patients took their prestudy PD medication regimen during the washout period. Each treatment period was 15 days. During the first 9 days of each treatment period, investigators could adjust the dose regimens of IPX203 and IR CD-LD for optimal therapeutic effect. Dosing regimens on days 10 to 15 of each treatment period were to remain stable with no further dose adjustments.
On days 1 and 15 of each treatment period, patients arrived at the clinic having fasted for at least 8 hours. On day 1 of each treatment period, patients were instructed to withhold study drug after 10 pm the previous evening; after dosing with study drug, patients could receive rescue treatment if they experienced an Off state lasting 3 or more consecutive hours postdose or at the investigator's discretion. On day 15 of each treatment period, patients were instructed to withhold study drug for at least 5 hours before arriving at the clinic and were dosed with study drug during the clinic visit according to the stable regimen noted in their 3-day dosing diary.
Eligible patients were diagnosed with idiopathic PD8 at age 40 years and older and on stable regimens of CD-LD but still experiencing motor complications and awakening on most mornings in an Off state as confirmed by a 3-day PD diary9 with an average of at least 2 hours per day of Off time during waking hours and at least 1 hour Off time each day. At entry, the Movement Disorder Society—Unified Parkinson's Disease Rating Scale Part III (MDS-UPDRS-III) total score in the Off state was at least 20 units and at least 25% or 10 units greater than in the On state. The prestudy IR CD-LD regimen included a morning dose between 100 to 250 mg of LD, a total daily dose of at least 400 mg of LD, with a maximum of 1600 mg of LD, and a dosing frequency of at least 4 times daily. Stable doses of dopamine agonists (except apomorphine), selective MAO-type B inhibitors, anticholinergic PD medication, and amantadine were permitted. Use of extended-release CD-LD products was permitted except as the first morning dose or after 10 pm the evening before clinic visits.
Key exclusion criteria included malabsorption, hepatic or renal insufficiency, history of psychosis, or treatment with dopamine antagonist. Other CD-LD formulations, additional CD or benserazide, COMT and nonselective MAO inhibitors, and apomorphine were not permitted during the study.
Pharmacodynamic and Pharmacokinetic Evaluations
Pharmacodynamics were assessed by qualified treatment-blinded clinicians using the IASMS and the MDS-UPDRS-III scores. Clinicians conducting pharmacodynamic assessments were blinded to treatment assignment and were not involved in patient dosing or in adjusting the treatment regimen. The IASMS is similar to the patient Hauser Diary9; in-clinic assessments were made by the blinded rater predose and then every half-hour postdose during the in-clinic observation period. The MDS-UPDRS-III scores were obtained predose and hourly over each study period. Pharmacokinetic samples were obtained for up to 8 hours postdose on day 1 (single dose) and for up to 10 hours on day 15 (multiple dose) of each treatment period.
Patients completed their PD and dosing diaries for 3 days before day 1 of their first treatment period and for 3 days before each of the day 15 visits. Pharmacokinetic parameters were estimated, including relative bioavailability, time to threshold concentrations of LD, peak-trough fluctuation, and drug accumulation.
Study Endpoints
The primary efficacy endpoint was the average percent Off time during waking hours based on patient PD diaries collected at the end of each treatment period. Secondary efficacy endpoints based on the 3-day PD diary included the average total times for each motor state (Off, Good On [defined as the sum of On without dyskinesia plus On with nontroublesome dyskinesia], On with no dyskinesia, On with troublesome dyskinesia, and On with nontroublesome dyskinesia), the average number of clinical (motor) fluctuations, the average duration of Good On state and average duration of any On states, and the proportion of patients with an improvement from baseline of at least 0.5, 1, 1.5, 2, 3, and 4 hours in the total Off time.
Between-treatment hourly differences in the MDS-UPDRS-III scores were assessed based on the change from day 1 predose values and the area under the curve (AUC) of these changes. The average duration of effect was estimated beginning at the time point at which an improvement of at least 4 points, 7 points, and 13 points in the MDS-UPDRS-III score10 from predose value on day 1 is first observed and continuing until the time point at which the improvement was no longer observed. Bradykinesia was assessed by the average of change in sum of questions 4 to 8 and question 14 MDS-UPDRS-III score from day 1 predose value at each time point.
Treatment effects were also compared using the IASMS in the clinic on days 1 and 15. The average total Off time, total Good On time, and total On time with troublesome dyskinesia were captured by treatment for each observation period.
Safety assessments included spontaneous reporting of adverse events (AEs), vital signs, clinical laboratory tests, 12-lead electrocardiogram, and physical examinations.
Statistical Analyses
Assuming patients would have an average of 16 hours of awake time during the day, 26 patients were required for this 2-way crossover study to detect a difference of 1.75 hours between treatments in Off time, with the standard deviation of the treatment differences of 3 hours, and 80% power.
The primary efficacy parameter, difference in average percent Off time between treatments, was analyzed using a mixed-model analysis of variance. The model included fixed-effect factors for treatment, period, sequence, and subject-within-sequence random effect.
All continuous secondary efficacy endpoints were evaluated similar to the primary efficacy parameter. Categorical efficacy endpoints were analyzed using Fisher exact test. Secondary endpoints were tested at 5% significance level without multiplicity correction.
Time to event data were analyzed using a Cox model, adjusting for treatment, period, and sequence.
For day 1, MDS-UPDRS assessments after rescue were imputed as baseline observation carried forward and motor state assessments were imputed as Off. For day 15, postredose data were actual observed values.
Study Medication
IPX203 is an investigational product manufactured by Impax Laboratories, LLC (Bridgewater, NJ). Two IPX203 dose strengths were used in this study: 45–180 mg of CD-LD and 67.5–270 mg of CD-LD. The comparator was Sinemet 25–100 mg of CD-LD (Merck & Company, Whitehouse Station, NJ). During the IR CD-LD treatment period, the initial regimen of IR CD-LD was the same as the subject's stable prestudy regimen. Any bedtime doses of CR CD-LD, either alone or in combination with IR CD-LD, were discontinued and substituted with a 1:1 milligram-equivalent dose of IR CD-LD. Based on the results from a single-dose study in patients with advanced PD,7 a dose conversion algorithm for the first morning dose of IPX203 was provided that converted a 100 mg dose of IR LD to 360 mg IPX203 LD. For subsequent afternoon and evening doses of IR CD-LD, the most frequent dose was determined and 100 mg of IR LD was converted to 270 mg of IPX203 LD. The recommended dosing frequency for IPX203 was approximately every 7 to 8 hours. Investigators were allowed to titrate the dosing regimen of each treatment during the first 9 days of each period.
RESULTS
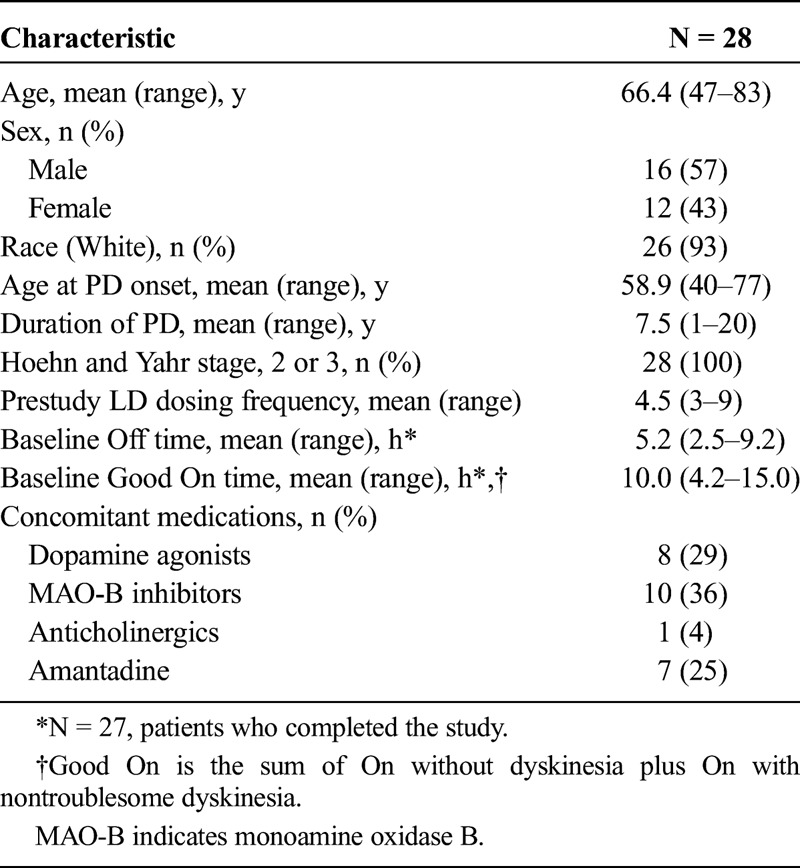
Of the 39 patients who were screened, 28 were randomized and 27 (96.4%) received both treatments and completed the study. One patient was withdrawn from the study because of an AE while receiving IPX203 and did not receive IR CD-LD. All subsequent study dosing, pharmacokinetics, and efficacy/pharmacodynamic results refer to these 27 study completers. Baseline demographics and clinical characteristics of the patients are provided in Table 1.
TABLE 1.
Baseline Characteristics and Demographics
Study Medication Dosing
At study entry, the mean total daily LD dose was 737 mg with the mean first morning dose and most frequent afternoon/evening LD doses being 159 mg and 148 mg, respectively. Based on the 3-day dosing diaries completed at the end of each treatment period, mean total daily doses of LD from IR CD-LD and IPX203 were 713 and 1453 mg, respectively, and the mean dosing frequency was 4.7 for IR CD-LD and 3.1 for IPX203 (P < 0.0001). Mean morning LD doses on day 1 for IR CD-LD and IPX203 were 159 mg and 573 mg, respectively. The median time to complete dose titrations was 5 and 0 days for IPX203 and IR CD-LD, respectively. All but 1 subject completed dose titration within the 9-day window. The median number of dose titrations for IPX203 and IR CD-LD was 2.0 versus 0, respectively, with a range of 0 to 6 steps for both treatments. On day 15 during the clinic visit, the mean first morning LD dose for IR CD-LD and IPX203 was 159 mg and 560 mg, and the mean dosing frequency was 3.1 for IR CD-LD and 2.0 for IPX203 (P < 0.0001). Time to rescue after a single dose on day 1 was in favor of IPX203 (hazard ratio, 0.267; P = 0.0002).
Pharmacokinetics
A summary of LD pharmacokinetics is presented in Table 2. Levodopa plasma concentrations increased rapidly after both treatments, and the initial increase in LD concentration was similar for both treatments. Average time to reach 430 ng/mL was 0.3 hours for both treatments (P = 0.7243). After a single dose, LD plasma concentrations were sustained above 50% of Cmax for 4.6 hours with IPX203 compared with 1.5 hours with IR CD-LD (P < 0.0001). After multiple dosing, peak LD concentrations were similar for both treatments, and the accumulation ratio for LD was 1.0 for IPX203 and 1.1 for IR CD-LD (P = 0.1422). IPX203 resulted in a lower fluctuation index for plasma LD (1.7 vs 2.7; P < 0.0001). Levodopa plasma concentration immediately before administration of the first IPX203 dose on day 15 was significantly higher compared with IR CD-LD (361 ng/mL vs 71 ng/mL; P < 0.0001). Levodopa bioavailability of IPX203 relative to IR CD-LD based on the arithmetic mean AUC∞ ratios was 89%. Figure 1 presents the time course of the percentage deviation in plasma LD concentration from each patient's average LD concentration on each treatment. This method of presentation accommodates the individualized dosing regimen of both treatments that each patient took in the study. A similar approach has been used previously to compare the performance of an LD prodrug.11
TABLE 2.
Summary of LD Pharmacokinetics After IPX203 and IR CD-LD (N = 27)

FIGURE 1.
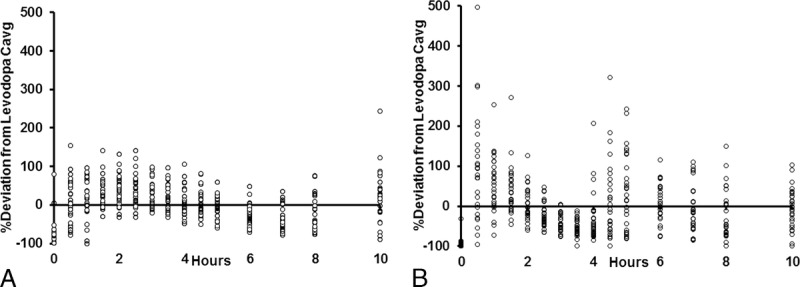
Time course of percentage deviation from each patient's average LD concentration (Cavg) after (A) IPX203 and (B) IR CD-LD (N = 27).
Single-Dose Pharmacodynamics (Day 1)
Movement Disorder Society—Unified Parkinson's Disease Rating Scale Part III
On day 1, motor function improved rapidly after dosing and to a similar extent for the first 2 hours with both treatments, as noted for the MDS-UPDRS-III (Fig. 2). The improvements in motor scores were significantly better after IPX203 compared with IR CD-LD at every time point from 3 to 8 hours (all P < 0.025). Similarly, IPX203 was associated with an average improvement of 19.3 units over the 8-hour observation compared with an 8.4-unit improvement for IR CD-LD (P < 0.0001). There was no carryover effect in the day 1 predose value (P = 0.3109) or the day 1 postdose average (P = 0.368), indicating that the 7-day washout period was sufficient.
FIGURE 2.
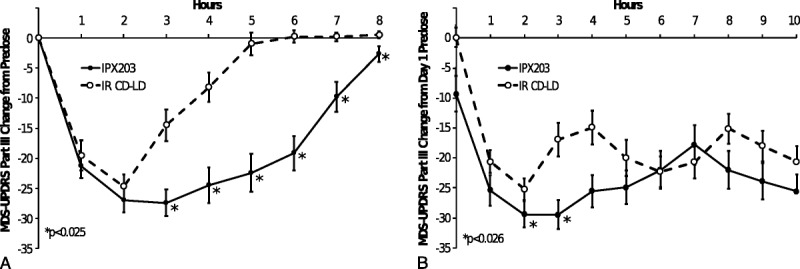
Comparative MDS-UPDRS-III motor scores on day 1 and day 15.
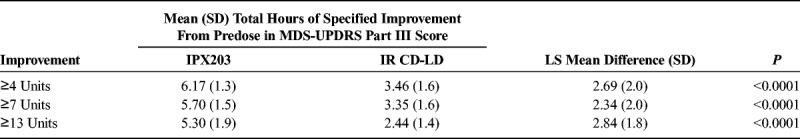
Table 3 summarizes the duration of pharmacodynamic effect based on the prespecified 4-, 7-, and 13-point improvement thresholds in the MDS-UPDRS-III. IPX203 consistently demonstrated a significantly longer duration of effect (2.3–2.8 hours) compared with IR CD-LD regardless of the threshold used. On day 1, beginning with hour 3 and continuing through hour 7, significant reductions in total bradykinesia score were seen after treatment with IPX203; a nonsignificant improvement was observed at hour 8 (least squares [LS] means difference, −1.5 [SD, 5.4]; P = 0.0613). Over the entire 8-hour observation period, the mean postdose average (SD) change in total bradykinesia score for IPX203 versus IR CD-LD was −7.79 (5.1) versus −3.75 (3.2) (LS means difference, −3.93 (3.7); P < 0.0001). The 8-hour postdose average for each bradykinesia individual question score was also significantly improved with IPX203.
TABLE 3.
Mean (SD) Total Hours of 4-, 7-, or 13-Point Improvement in MDS-UPDRS Part III Scores on Day 1 (N = 27)
Investigator Assessment of Subject's Motor State
IPX203 treatment resulted in significantly less Off time and significantly more Good On time than IR CD-LD (LS means differences, −2.57 hours and 2.57 hours, respectively, P < 0.0001 for each comparison) (Table 4). Mean total On times with troublesome dyskinesia were similar during both treatments. There was no carryover effect for any of the measures of the IASMS (P ≥ 0.3489), indicating that the 7-day washout period was sufficient.
TABLE 4.
Investigator Assessment of Subject's Motor State: Mean Total Times on Day 1 (N = 27)
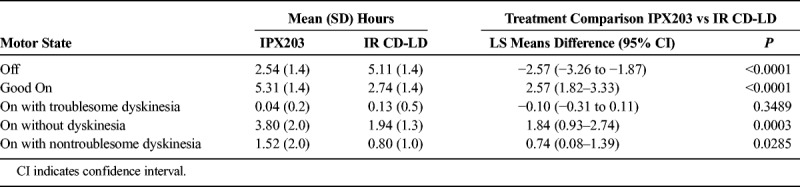
Figure 3 presents the proportion of patients achieving various durations of Good On time. After treatment with IR CD-LD, most patients achieved less than 3 hours of Good On time. In contrast, after IPX203 treatment, almost 50% of patients achieved at least 6 hours of Good On time.
FIGURE 3.
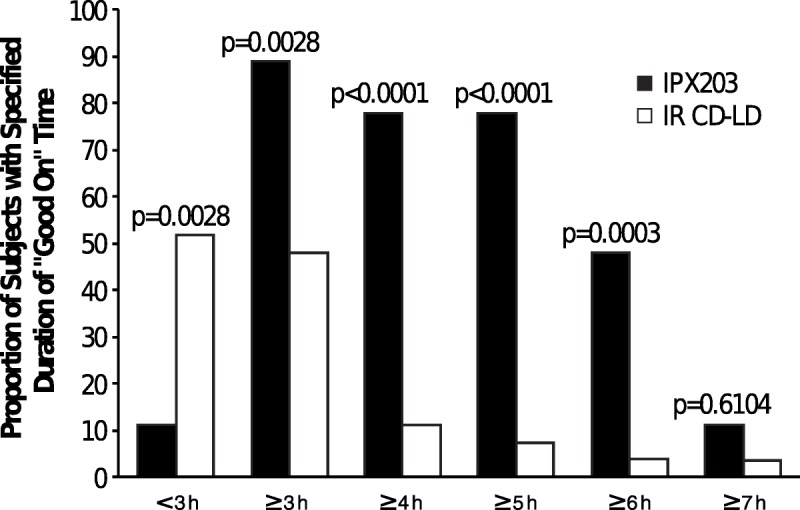
Proportions of patients with specified duration of Good On by investigator assessment on day 1. The calculated state of Good On is the sum of On without dyskinesia plus On with nontroublesome dyskinesia.
Multiple-Dose Pharmacodynamics (Day 15)
Movement Disorder Society—Unified Parkinson's Disease Rating Scale Part III
MDS-UPDRS-III (motor) predose score on day 15 of IPX203 treatment was significantly lower compared with the day 15 predose score during IR CD-LD treatment, consistent with the significantly higher LD plasma concentrations at predose with IPX203 (Fig. 4, Table 2). The change in MDS-UPDRS-III (motor) score from day 1 to day 15 was −9.3 for IPX203 versus 0.1 for IR CD-LD (LS means difference, −9.0; P = 0.0087). When predose mean MDS-UPDRS Parts I to IV scores were compared by treatment on day 15, scores were significantly lower during IPX203 treatment for only individual Part IV (in addition to Part III). Parts I and II improvements did not reach significance.
FIGURE 4.
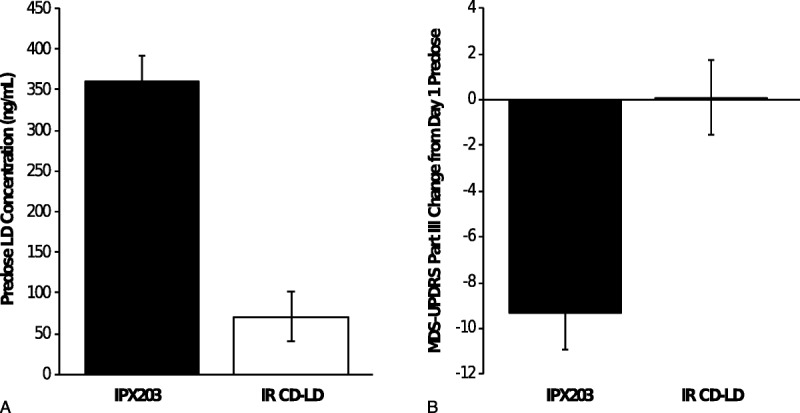
Day 15 predose pharmacokinetics and pharmacodynamics. A, Predose LD plasma concentrations with IPX203 and IR CD-LD (P < 0.0001). B, The MDS-UPDRS-III change from day 1 predose (P = 0.0087).
After multiple dosing, the initial rates of improvement in motor symptoms were similar between the 2 treatments (Fig. 2). However, improvements in MDS-UPDRS-III scores lasted longer after IPX203 than IR CD-LD, which resulted in patients requiring less frequent dosing of IPX203 than IR CD-LD. Similarly, the change-from-day-1-predose scores across 10 hours postdose also demonstrated a significant advantage for IPX203 over IR CD-LD (LS means difference of −24.6 for IPX203 versus −19.4 for IR CD-LD, P = 0.0226). No significant between-treatment differences were noted in the day 15 bradykinesia change from baseline.
Investigator Assessment of Subject Motor State
Total Off time was approximately 1.1 hours less, and total Good On time was approximately 1.4 hours greater during IPX203 treatment than during IR CD-LD treatment (P ≤ 0.0008).
Efficacy Based on Patient PD Diary
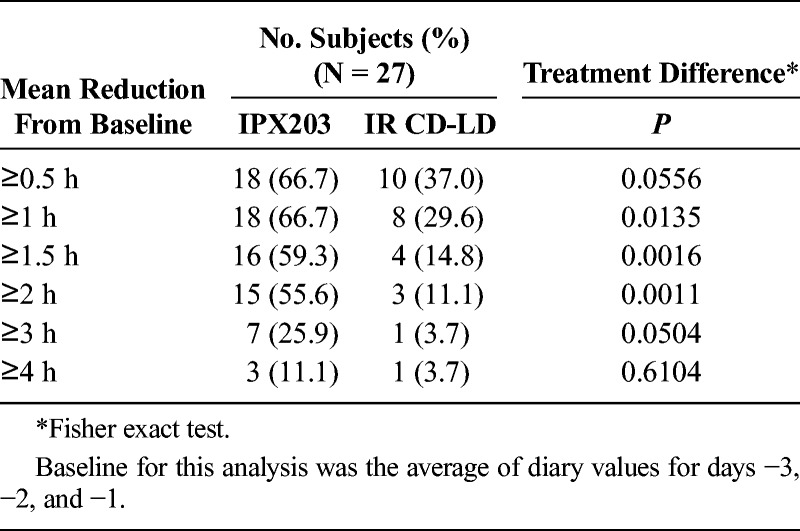
Table 5 presents a summary of the 3-day PD Diary. IPX203 treatment provided a 2.3-hour advantage in mean total Off time during waking hours compared with IR CD-LD (P < 0.0001), and Good On time was improved by 1.9 hours compared with IR CD-LD (P = 0.0001). There was no significant difference in the On time with troublesome dyskinesia between the 2 treatments (P = 0.148). Treatment with IPX203 was also associated with significantly longer Good On episodes than IR CD-LD (LS means, 6.26 vs 3.38 hours; LS mean difference, 2.89 hours; P < 0.0001) and significantly longer On episodes (LS means, 7.56 vs 3.77 hours; LS mean difference, 3.79 hours; P < 0.0001). Treatment with IPX203 was associated with significantly fewer motor fluctuations per day than IR CD-LD (LS means, 3.6 vs 6.1; LS mean difference, −2.6; P < 0.0001). Treatment with IPX203 resulted in a significantly higher proportion of patients with an average reduction in Off time of at least 1.0, 1.5, and 2.0 hours when compared with IR CD-LD (Table 6). Analysis indicated that there was no carryover effect (P > 0.399).
TABLE 5.
Summary of Patient PD Diary Data on Day 15 (N = 27)
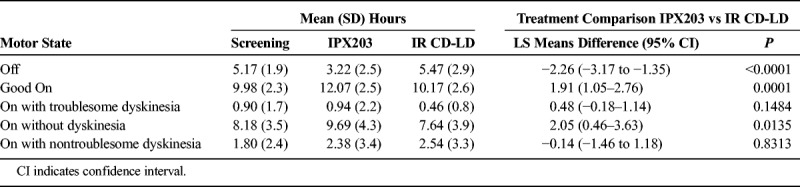
TABLE 6.
Proportion of Subjects With a Specified Reduction From Baseline in Off Time in Study IPX203-B16-01 (PD Diary Completers)
Safety
Eleven patients (39.3%) reported at least 1 treatment-emergent adverse event (TEAE). A higher percentage of patients reported TEAE(s) while taking IPX203 (35.7%) than while taking IR CD-LD (7.4%). The most common TEAEs were dyskinesia (n = 5), dizziness (n = 2), and nausea (n = 2), all occurring during the IPX203 treatment period. All TEAEs were considered mild or moderate. Six patients reported TEAEs during IPX203 treatment that were considered dopaminergic; most of these events resolved with a dose reduction.
One patient (3.6%) experienced a treatment-emergent serious adverse event of mild increased hypertension during treatment with IPX203, which was assessed as not related to treatment. Another patient experienced 3 serious adverse events (moderate dehydration, severe diarrhea, and moderate atrial fibrillation) during the IR CD-LD washout period between the 2 treatments. One patient (3.6%) was withdrawn from the study during treatment with IPX203 owing to intermittent moderate orthostatic hypertension.
The MDS-UPDRS Part IV captures details related to motor complications (dyskinesias, motor fluctuations, and painful Off state dystonia). Analysis of MDS-UPDRS Part IV questions did not demonstrate between-treatment differences for the dyskinesia questions or for complexity (predictability) of motor fluctuations. Significant benefits of IPX203 versus IR CD-LD were noted on time spent in Off state (LS means difference, −0.5 [0.51]; P < 0.0001) and on functional impact of fluctuations (LS means difference, −0.5 [0.99]; P = 0.011). A nonsignificant benefit of IPX203 versus IR CD-LD was suggested on painful Off-state dystonia.
DISCUSSION
Various approaches to extend the duration of effect of LD by prolonging plasma concentrations have been explored including metabolism inhibitors (such as COMT inhibitors), prodrugs, gastroretentive formulations, and duodenal infusion systems. Gastroretentive dosage forms require concomitant administration of food to retain the dosage form in the stomach. This may not be practical for PD patients. Continuous intraduodenal infusion systems, reserved for the most severe patients, require invasive surgery, have the risk of infections, the inconvenience of carrying and refilling the pump, and issues related to device malfunction. A convenient and improved oral ER LD product that can rapidly achieve and sustain constant LD plasma concentrations with every 8 hours dosing would be a useful addition to the current treatment options for PD patients.12
The clinical effects of IPX203 versus IR CD-LD were consistent with the pharmacokinetics. Subjects converted to IPX203 within 4.5 days using a total of 2.0 conversion steps. The PD diary data demonstrated that patients experienced significantly less Off time as a percentage of their waking hours (mean 19.3% for IPX203 vs 33.5% with IR CD-LD) and had a 2.3-hour greater reduction in Off time and a 1.9-hour increase in Good On time with IPX203 versus IR CD-LD, despite less frequent dosing. There were significantly fewer motor fluctuations per day and significantly longer average durations of Good On episodes with IPX203 compared with IR CD-LD. There was no significant difference in the duration of On time with troublesome dyskinesia between the 2 treatments. During IPX203 treatment, patients were 73.3% less likely to receive rescue medication (day 1) than during IR CD-LD treatment. Day 1 IPX203 pharmacodynamic improvements were consistent with the PD diary: the IASMS demonstrated improvements in Off time and in Good On times of almost 2.6 hours, consistent with the improvements in duration of effect of 2.3 to 2.8 hours using the MDS-UPDRS-III 4-, 7-, and 13-point improvement thresholds. Hourly assessments demonstrated significant improvements in MDS-UPDRS-III mean scores for patients receiving IPX203 than IR CD-LD from 3 to 8 hours postdose. On day 15, when patients had not had a study treatment or any CD-LD treatment for at least 5 hours, mean predose MDS-UPDRS-III scores were significantly lower in IPX203-treated patients, consistent with the significantly higher LD plasma concentrations at predose with IPX203 and suggesting that the clinical effects of IPX203 may carry over to the next morning. The literature suggests that an improvement of at least 4 units on MDS-UPDRS-III represents the minimal change that is clinically meaningful to the patient.13 The magnitude of this sustained effect, that is, a 9-unit predose advantage in the MDS-UPDRS-III in favor of IPX203, is well above the minimal clinically important difference noted in the literature for advanced PD patients.14 Some asymmetry in reported dopaminergic AEs was observed during dose conversion, but after dose stabilization, neither the Patient PD diary nor the MDS-UPDRS-IV suggested that IPX203 increased dyskinesia.
This multiple-dose crossover study in patients with PD had some methodological limitations. The study was in a relatively small number of patients who were not blinded to treatment. Investigators could adjust the dosing regimen during the first 9 days of each treatment period to optimize dosing, and patients were on a stable dose for only 6 days. This may be too short to characterize the long-acting effects of LD. Notwithstanding these limitations, the findings support the safety and efficacy of IPX203 at the recommended doses given approximately every 7 to 8 hours. A larger study is planned to confirm the safety and effectiveness of IPX203 in patients with advanced PD.
In summary, IPX203 provides a sustained and consistent effect on multiple measures including MDS-UPDRS-III scores, the IASMS, and patients' 3-day PD diaries. The reduced peak-to-trough fluctuations of LD achieved with IPX203 resulted in a consolidation of On periods in contrast to the frequent cycles of On and “Off’ periods with IR CD-LD therapy.
ACKNOWLEDGMENT
The authors acknowledge study investigators Jason Aldred, MD; Victor Biton, MD; Daniel Burdick, MD; John Campbell, MD; Rohit Dhall, MD, MSPH; and Aaron Ellenbogen, DO, MPH; Ramon Gil, MD; Steven Gunzler, MD; Robert Hauser, MD, MBA, FAAN; Stuart Isaacson, MD; and Mark Stacy, MD, for their contributions to the conduct of this study; Karen Getz of Impax Laboratories, LLC, for medical writing support in drafting the article; and Sejal Vora, PharmD, of Impax Laboratories, LLC, for editorial support.
Footnotes
Conflicts of Interest and Source of Funding: N.B.M., A.M., P.D., R.R., and S.G. were employees of Impax Laboratories, LLC, during study execution and article development. The study was funded by Impax Laboratories, LLC.
REFERENCES
- 1.Verhagen Metman L, Stover N, Chen C, et al. Gastroretentive carbidopa/levodopa, DM-1992, for the treatment of advanced Parkinson's disease. Mov Disord 2015;30:1222–1228. [DOI] [PubMed] [Google Scholar]
- 2.Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 2001;16:448–445. [DOI] [PubMed] [Google Scholar]
- 3.Olanow CW, Obeso JA, Stocchi F. Continuous dopamine-receptor treatment of Parkinson's disease: scientific rationale and clinical implications. Lancet Neurol 2006;5:677–687. [DOI] [PubMed] [Google Scholar]
- 4.Juncos JL, Engber TM, Raisman R, et al. Continuous and intermittent levodopa differentially affect basal ganglia function. Ann Neurol 1989;25:473–478. [DOI] [PubMed] [Google Scholar]
- 5.Engber TM, Susel Z, Juncos JL, et al. Continuous and intermittent levodopa differentially affect rotation induced by D-1 and D-2 dopamine agonists. Eur J Pharmacol 1989;168:291–298. [DOI] [PubMed] [Google Scholar]
- 6.Blanchet PJ, Calon F, Martel JC, et al. Continuous administration decreases and pulsatile administration increases behavioral sensitivity to a novel dopamine D2 agonist (U-91356A) in MPTP-exposed monkeys. J Pharmacol Exp Ther 1995;272:854–859. [PubMed] [Google Scholar]
- 7.Modi NB, Mittur A, Rubens R, et al. Single-dose pharmacokinetics and pharmacodynamics of IPX203 in patients with advanced Parkinson's disease: a comparison with immediate-release carbidopa-levodopa and with extended-release carbidopa-levodopa capsules. Clin Neuropharmacol 2019;42:4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauser RA, Deckers F, Lehert P. Parkinson's disease home diary: further validation and implications for clinical trials. Mov Disord 2004;19:1409–1413. [DOI] [PubMed] [Google Scholar]
- 10.Hauser RA, Gordon MF, Mizuno Y, et al. Minimal clinically important difference in Parkinson's disease as assessed in pivotal trials of pramipexole extended release. Parkinsons Dis 2014;2014:467131; Article ID 467131, 8 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeWitt PA, Huff FJ, Hauser RA, et al. Double-blind study of the actively transported levodopa prodrug XP21279 in Parkinson's disease. Mov Disord 2014;29:75–82. [DOI] [PubMed] [Google Scholar]
- 12.Stocchi F, Vacca L, Ruggieri S, et al. Intermittent vs continuous levodopa administration in patients with advanced Parkinson disease: a clinical and pharmacokinetic study. Arch Neurol 2005;62:905–910. [DOI] [PubMed] [Google Scholar]
- 13.Horváth K, Aschermann Z, Ács P, et al. Minimal clinically important difference on the Motor Examination part of MDS-UPDRS. Parkinsonism Relat Disord 2015;21:1421–1426. [DOI] [PubMed] [Google Scholar]
- 14.Makkos A, Kovács M, Aschermann Z, et al. Are the MDS-UPDRS-based composite scores clinically applicable? Mov Disord 2018;33:835–839. [DOI] [PubMed] [Google Scholar]


