Background and Purpose:
Exercise programs for people with dementia need to be optimized. We therefore evaluated the applicability of a high-intensity functional exercise program among people with dementia in nursing homes with regard to attendance, achieved exercise intensity, adverse events, a focus on dementia type, and whether symptoms of dementia or other medical conditions common in this population were associated with program applicability.
Methods:
The Umeå Dementia and Exercise study, a cluster-randomized controlled trial set in 16 nursing homes in Umeå, Sweden. Ninety-three people with dementia (mean [SD] Mini-Mental State Examination score of 15.4 [3.4]) were randomized to the exercise intervention. Thirty-four participants had Alzheimer's disease (AD) and 59 non-Alzheimer's dementia (non-AD). High-Intensity Functional Exercise (HIFE) program was conducted in groups of 3 to 8 participants. Two physiotherapists led 5 sessions (45 minutes each) per fortnight for 4 months (total 40 sessions).
Results:
Median attendance rate was 82.5%. Lower limb strength exercises were performed at high or medium intensity at a median interquartile range of 94.7% (77.8%-100%) of attended sessions. Participants with non-AD performed more sessions with high intensity in strength exercises than participants with AD (median interquartile range, 53.8% [25.7%-80%] vs 34.9% [2.02%-62.9%]; P = .035). Balance exercises were performed at high intensity at a median interquartile range of 75% (33.3%-88.6%). Adverse events (all minor and temporary, mostly musculoskeletal) occurred during the exercise sessions in 16% of attended sessions. Low motivation was the most common barrier for attendance. Buildup period, low motivation, and pain were common barriers for achieving high intensity in balance and strength exercises, and fear was a barrier in balance exercises. Of medical conditions, only behavioral and psychological symptoms of dementia, including apathy, were negatively associated with applicability.
Conclusion:
A group-based, supervised, and individualized high-intensity functional exercise program seems to be applicable with regard to attendance, achieved intensity, and adverse events during the exercise sessions, in people with mild to moderate dementia in nursing homes. Effective strategies to enhance motivation to participate in exercise, as well as prevention and treatment of pain and behavioral and psychological symptoms of dementia, are important when promoting exercise participation in this population.
Keywords: dementia, exercise, long-term care, mobility limitation, rehabilitation
INTRODUCTION
Dementia is common, progressive, and the leading cause of dependency in activities of daily living (ADLs) in older adults, leading many to reside in nursing homes.1 Gradually, it reduces cognitive function and impairs walking and balance,2 increasing the risks of falls, fractures,3 physical inactivity,4 and dependency in ADLs.5 Physical exercise may postpone or prevent these consequences in people with dementia,6–10 and thus may be an important way to maintain independence and quality of life.
To improve functional ability in older adults with mobility problems, resistance11,12 and balance13 training is recommended. To obtain optimal effects, exercise should be task specific14,15 and performed with high intensity,11,15 that is, performed near the individual's maximum capacity and with sufficient frequency and duration.12,16 However, these recommendations may be challenging for some people with dementia due to complicating symptoms of the disease.6 Cognitive deficits (eg, impaired memory, impaired executive function, aphasia, and apraxia), and behavioral and psychological symptoms of dementia (BPSD) may impede participation in high-intensity exercise programs, especially with increasing dementia severity. Furthermore, common comorbidities and medical conditions, such as pain, vision or hearing impairment,1,17 and an unstable health status,18,19 may impact attendance, reduce the ability to achieve high exercise intensity, and increase the risk of adverse events. In addition, symptom patterns and comorbidities differ among dementia types,1,20 which could affect exercise program applicability.
Studies exploring applicability of exercise programs have been requested with the aim of optimizing exercise programs for people with dementia.6,9 Furthermore, it is also important to evaluate whether different dementia types, dementia symptoms, and other medical conditions are associated with applicability of exercise programs. The Umeå Dementia and Exercise (UMDEX) study evaluated the effects of a high-intensity functional exercise program in people with dementia living in nursing homes. The exercise program affected ADLs and balance positively, but only in participants with other types of dementia than Alzheimer's disease (non-AD).21 The primary aim of the current study was to evaluate the applicability of this high-intensity functional exercise program among people with dementia in nursing homes with regard to attendance, achieved exercise intensity, and adverse event occurrence, with a focus on dementia type. The secondary aim was to evaluate whether symptoms of dementia or other medical conditions common in this population were associated with program applicability.
METHODS
This study was part of the UMDEX study, a cluster-randomized controlled trial conducted in 16 nursing homes in Umeå, Sweden, including general and dementia units all with private rooms and staff on hand, as well as units with private apartments with access to on-site nursing and care. A group of 864 residents were screened by physiotherapists (PTs) and physicians. The UMDEX study is described in detail elsewhere.21 Those randomized to participate in the exercise intervention were included in this study. The study protocol (ISRCTN31767087) is published on the ISRCTN registry. Ethical approval was obtained from the Regional Ethical Review Board in Umeå in August 2011 (2011-205-31M).
Participants
Participants had dementia according to the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision) (DSM-IV-TR),22 aged 65 years and older, had Mini-Mental State Examination (MMSE) scores of 10 or more,23 had dependence in personal ADLs according to the Katz Index (score > A) administered in an interview by health care staff,24 had the ability to rise from a chair with an armrest with assistance from 1 person or less, had the ability to hear and understand spoken Swedish, and had their physicians' approval. All participants provided informed oral consent, affirmed by next of kin.
Exercise Intervention
The intervention was based on the High-Intensity Functional Exercise (HIFE) program developed by research team members. The HIFE program, designed to improve lower limb strength, balance, and mobility, is available as a booklet25 and on a Web page.26 Exercise sessions were conducted at participating nursing homes in small groups (N = 3-8), each supervised by 2 PTs. The sessions were mediated by a total of 7 PTs (clinical experience range, 1-11 years), who had experience working with geriatric patients diagnosed with dementia. Before intervention, all of the administering PTs participated in a 1-day education session held by the developers (H.L., N.L., and E.R.) for training to deliver the exercise program, during which they were also taught about the signs and symptoms of heart disease by a geriatric medicine and internal medicine specialist (Y.G.) on the research team. Five sessions (∼45 minutes each) were held per fortnight at set times for 4 months (total, 40 sessions). Before starting each exercise session, the PTs or nursing home staff gave verbal reminders or aided transfer to the exercise sessions, and also when needed, motivated the participants to join the sessions. When possible, supervised individual sessions were provided for participants unable to attend group sessions. Physiotherapists were encouraged to obtain updates on participants' health status before sessions and could contact physicians or nurses when necessary. Based on the patients health status, PTs were able to adjust attendance arrangements (fewer exercises, individual sessions, or nonattendance) and modify exercise intensity.
Each group session started with group warm-up exercises for all participants. Participants were then supervised individually to safely promote the highest possible exercise intensity. Participants took turns exercising and resting during each session. The high-intensity functional exercise program comprises 39 exercises performed in functional weight-bearing positions, similar to those used in everyday situations (eg, rising from a chair, trunk rotation, walking, climbing stairs).25,27 The exercises are distributed over 5 categories: A, static and dynamic balance exercises in combination with lower limb strength exercises; B, dynamic balance exercises in walking; C, static and dynamic balance exercises in standing; D, lower limb strength exercises with continuous balance support; and E, walking with continuous balance support. Exercises were planned depending on each individual's degree of functional deficit and according to a hierarchical model based on level of support required while walking a short distance (5-10 m). We recommended performance of at least 2 lower limb strength exercises and 2 balance exercises in 2 sets each session. High intensity was the aim, with adaptation through progressive adjustment of load (performance adjustment or weighted waist belt [maximum 12 kg] use) and support (eg, by narrowing base of support or surface alteration), while considering participants' symptoms and changes in health and functional status. Physiotherapists defined the intensity of each strength exercise set relative to the repetition maximum (RM), that is, “the maximum number of times a load can be lifted before fatigue using good form and technique,”28 (high, 8-12 RM; medium, 13-15 RM; low, >15 RM). Balance exercise intensity, estimated by the supervising PT through observation, was defined according to the level of postural stability challenge exhibited: high, fully challenged (ie, balance exercises performed near limits of maintaining an upright position); medium, not fully challenged or fully challenged in a minority of exercises; low, not challenged. Participants wore belts with handles so that PTs could provide support when needed, thereby preventing falls. Participants conducted moderate-intensity strength exercises for the first 2 weeks (buildup period).
Outcome Variables
Attendance, intensity of lower limb strength and balance exercises, and adverse events were the outcome variables. At the end of each exercise session, activity leaders completed a structured protocol for each participant, including exercise intensity,25 reasons for not achieving high intensity, effective workout time without rest, reasons for nonattendance, and adverse event occurrence, that is, development or worsening of discomfort during the exercise session (observed by a leader or expressed by a participant spontaneously or upon questioning). Leaders assessed whether moderate- to high-intensity strength exercises primarily strained either peripheral (muscular) or central (cardiorespiratory) systems. They assessed by observation or upon questioning whether the reason for stopping exercise primarily was muscle fatigue in the lower limb or shortness of breath in chest. Two geriatric medicine specialists (including Y.G.) and 1 PT (E.R. or H.L.) assessed adverse event severity in consensus as (1) minor or temporary (incurred or worsened by exercise), (2) serious symptoms (potential risk of severe injury or life threatening), (3) manifest injury or disease, and (4) death. During adverse event assessment, they had access to medical records to follow up the course and severity of the symptoms. Two geriatric medicine specialists assessed whether deaths occurring during the intervention period were related to the exercise.
Baseline Assessment
Physiotherapists and physicians performed baseline assessments before randomization, evaluating functional balance capacity (Berg Balance Scale),29 usual gait speed over 4 m with a walking aid, pain while walking (self-reported pain directly after gait speed test), depressive symptoms (15-item Geriatric Depression Scale administered by interviewing the participant),30 global cognitive function (MMSE),23 BPSD (Neuropsychiatric Inventory) (proxy reported by nursing home staff),31 and nutritional status (Mini Nutritional Assessment).32 Diagnoses were based on information gathered from assessments, medical records, and medication prescriptions. Physicians specialized in geriatric medicine diagnosed dementia type according to the DSM-IV-TR.22
Data Analysis
Individual attendance rate was calculated as the number of sessions attended divided by the total number of sessions offered (n = 40), regardless of study completion. Intensity and adverse event rates were calculated as the number of sessions attended, with specific intensity and with adverse event occurrence, respectively, divided by the number of attended sessions. Baseline characteristics of participants with AD and non-AD were compared using Student t test or the χ2 test. Attendance, high intensity, and adverse event rates and effective workout time were compared using the Mann-Whitney U test (due to skewed distribution). Spearman rank correlations between outcome variables and medical conditions (Table 1) were examined. Analyses were performed using SPSS software (version 23; IBM Corporation, Armonk, New York), with a 2-tailed significance level of P < .05.
Table 1. Correlations Between Outcome Variables and Medical Conditionsa.
| Variable | Attendance Rate | High-Intensity Strength Rate | High-Intensity Balance Rate | Adverse Event Rate |
|---|---|---|---|---|
| MMSE | –0.016 | 0.182 | 0.153 | 0.027 |
| GDS-15 | 0.009 | 0.014 | 0.163 | 0.057 |
| NPI, total | –0.217b | –0.150 | –0.295c | 0.063 |
| NPI, apathy | –0.216b | 0.067 | 0.055 | –0.082 |
| BBS | 0.055 | –0.001 | 0.093 | –0.085 |
| MNA | –0.026 | 0.028 | –0.048 | –0.061 |
| Pain while walking | 0.016 | –0.119 | 0.013 | 0.215b |
| Analgesic use | –0.064 | –0.183 | –0.041 | 0.238b |
| Neuroleptic use | 0.185b | 0.107 | 0.185 | 0.036 |
| Heart failure | –0.025 | 0.175 | 0.040 | –0.006 |
| Angina pectoris | –0.107 | 0.223b | 0.034 | 0.049 |
| Chronic lung disease | –0.193 | 0.043 | –0.094 | 0.091 |
| Vision impairment | 0.166 | –0.070 | –0.178 | 0.053 |
| Hearing impairment | 0.077 | 0.074 | –0.164 | 0.026 |
| Sex (female) | 0.043 | –0.097 | –0.016 | 0.148 |
Abbreviations: BBS, Berg Balance Scale; GDS-15, 15-item Geriatric Depression Scale; MMSE, Mini-Mental State Examination; MNA, Mini Nutritional Assessment; NPI, Neuropsychiatric Inventory.
aSpearman rank correlations used in analyses.
bP < .05.
cP < .01.
RESULTS
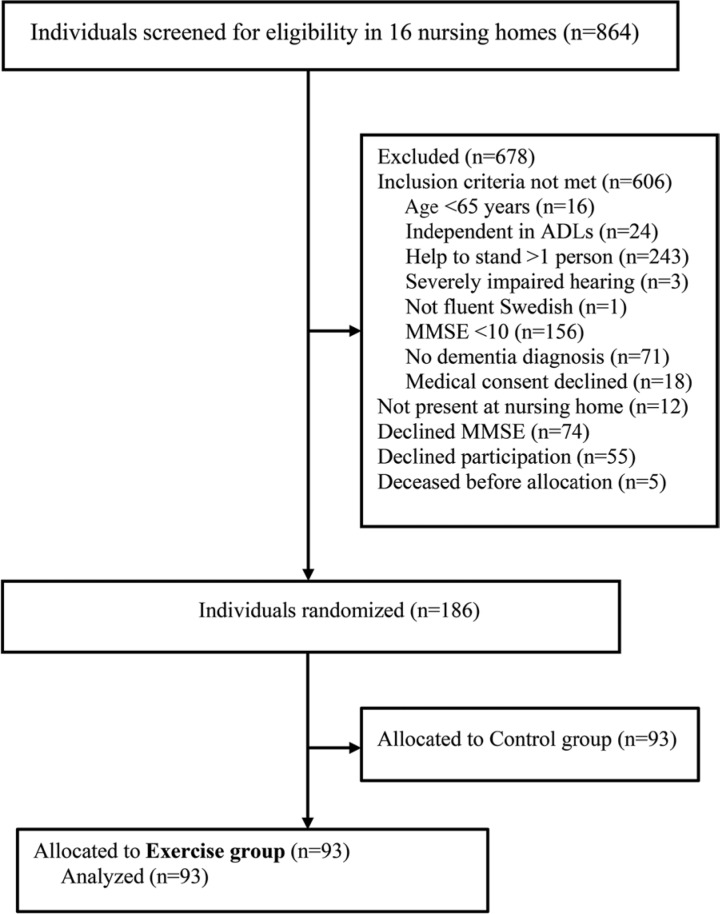
Ninety-three participants (70 women, 23 men) were included in the study (Figure 1). Thirty-four (36.6%) participants had AD and 59 (63.4%) had non-AD, including vascular, mixed Alzheimer's and vascular, Lewy body, frontotemporal, and Parkinson's dementia (Table 2). Fifty-three (57.0%) participants had depressive disorders, 48 (51.6%) had delirium in the past week, and 21 (22.6%) had apathy symptoms according to the Neuropsychiatric Inventory. Relative to those with AD, participants with non-AD had better global cognitive function (MMSE score of 16.0 vs 14.4), a greater occurrence of previous stroke (50.8% vs 8.8%), more mobility devise use (93.2% vs 61.8%), more prescribed medications (9.1 vs 7.3), and worse balance (Berg Balance Scale score of 25.9 vs 33.2) (Table 2).
Figure 1.
Flowchart of the study. ADLs indicates activities of daily living; MMSE, Mini-Mental State Examination.
Table 2. Baseline Characteristics of Participantsa.
| Characteristic | Total (n = 93) | AD (n = 34) | Non-AD (n = 59) |
|---|---|---|---|
| Age, y | 84.4 (6.2) | 84.9 (6.6) | 84.1 (6.0) |
| Sex, female | 70 (75.3) | 27 (79.4) | 43 (72.9) |
| Dementia type | |||
| Alzheimer's | 34 (36.6) | ||
| Vascular | 36 (38.7) | ||
| Mixed AD/vascular | 8 (8.6) | ||
| Other | 15 (16.1) | ||
| Diagnoses and medical conditions | |||
| Depressive disorders | 53 (57.0) | 18 (52.9) | 35 (59.3) |
| Delirium, previous weekb | 48 (51.6) | 15 (44.1) | 33 (55.9) |
| Previous stroke | 33 (35.5) | 3 (8.8) | 30 (50.8)c |
| Heart failure | 24 (25.8) | 6 (17.6) | 18 (30.5) |
| Angina pectoris | 21 (22.6) | 5 (14.7) | 16 (27.1) |
| Previous hip fracture | 28 (30.1) | 7 (20.6) | 21 (35.6) |
| Diabetes mellitus | 18 (19.4) | 6 (17.6) | 12 (20.3) |
| Rheumatic disease | 14 (15.1) | 4 (11.8) | 10 (16.9) |
| Chronic lung disease | 20 (21.5) | 6 (17.6) | 14 (23.7) |
| Osteoarthritis | 35 (37.6) | 12 (35.3) | 23 (39.0) |
| Hearing impairmentd | 20 (21.5) | 9 (26.5) | 11 (18.6) |
| Vision impairment | 10 (10.8) | 6 (17.6) | 4 (6.8) |
| Pain while walking, n = 90 | 15 (16.1) | 7 (20.6) | 8 (13.6) |
| Prescription medications | |||
| Analgesics | 55 (59.1) | 19 (55.9) | 36 (61.0) |
| Antidepressants | 58 (62.4) | 22 (64.7) | 36 (61.0) |
| Benzodiazepine | 19 (20.4) | 8 (23.5) | 11 (18.6) |
| Diuretics | 41 (44.1) | 13 (38.2) | 28 (47.5) |
| Cholinesterase inhibitor | 25 (26.9) | 13 (38.2) | 12 (20.3) |
| Neuroleptics | 11 (11.8) | 5 (14.7) | 6 (10.2) |
| Number of medications | 8.4 (4.0) | 7.3 (3.6) | 9.1 (4.1)e |
| Assessments | |||
| Barthel ADL Index (0-20)f | 10.7 (4.5) | 11.0 (4.5) | 10.6 (4.5) |
| MMSE (range: 0-30)f | 15.4 (3.4) | 14.4 (3.0) | 16.0 (3.6)e |
| Berg Balance Scale (range: 0-56)f | 28.6 (14.3) | 33.2 (12.9) | 25.9 (14.5)e |
| Gait speed: 4 m, m/s, n = 88 | 0.45 (0.2) | 0.5 (0.2) | 0.45 (0.2) |
| NPI (range: 0-144)g | 15.2 (15.8) | 15.7 (12.9) | 15.0 (17.4) |
| NPI apathy (range: 0-12)g | 0.97 (2.2) | 0.47 (1.4) | 1.3 (2.5) |
| GDS-15 (range: 0-15)g, n = 92 | 4.0 (3.4) | 3.2 (3.0) | 4.4 (3.5) |
| MNA (range: 0-30)f | 21.3 (2.8) | 21.1 (2.9) | 21.3 (2.7) |
| Use of mobility device | 76 (81.7) | 21 (61.8) | 55 (93.2)c |
| Self-reported health, good | 60 (62.5) | 21 (61.8) | 39 (66.1) |
Abbreviations: ADL, activities of daily living; GDS-15, 15-item Geriatric Depression Scale; MMSE, Mini-Mental State Examination; MNA, Mini Nutritional Assessment; NPI, Neuropsychiatric Inventory.
aValues are expressed as mean (SD) or as n (%). Numbers reported after covariates indicate numbers of measures available when values are missing.
bReported by staff based on the confusion subscales of the Organic Brain Syndrome Scale.
cP < .01.
dUnable to hear conversation from 1-m distance with or without hearing aid.
eP < .05.
fHigher scores indicate better status.
gLower scores indicate better status.
Attendance
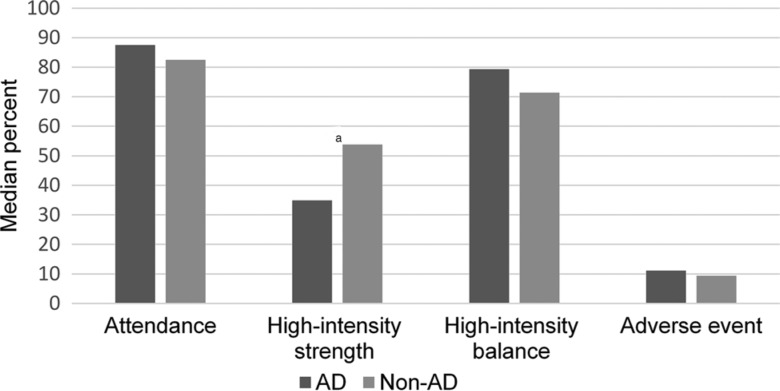
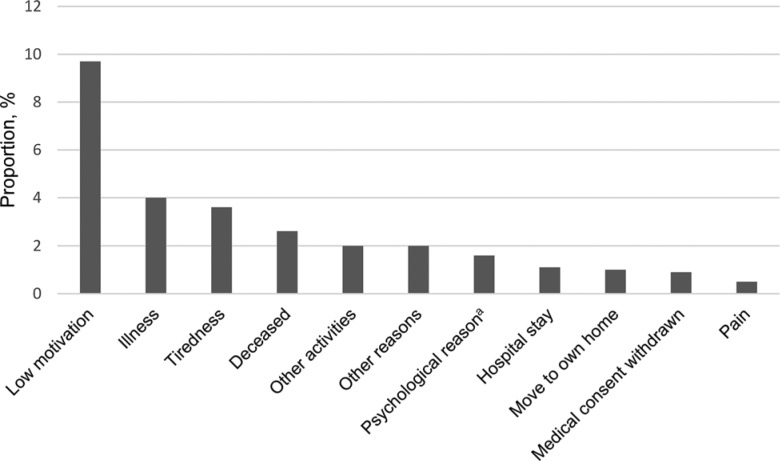
The overall attendance rate was in mean (SD): 73.4% (28.5) (2729/3720 sessions, including 247 [9.1%] individual sessions). The median (interquartile range) individual attendance rate and effective workout time per session were 82.5% (70.0%-92.5%) and 17.0 (15.0-19.5) minutes, respectively, with no significant differences between the AD and non-AD groups (P = .308), (P = .64), respectively (Figure 2). The most common reasons for session nonattendance were lack of motivation (9.7% of all sessions), illness (4.0%), tiredness (3.6%), and deceased status (2.6%; Figure 3).
Figure 2.
Attendance, high-intensity strength, high-intensity balance, and adverse event rates in participants with AD and non-AD dementia. aP < .05, AD versus non-AD. AD indicates Alzheimer's disease.
Figure 3.
Reasons for not attending exercise sessions, as percentage of total sessions. aIncludes anxiety, restlessness, and delirium.
Exercise Intensity
In total, lower limb strength exercises were performed at high intensity in 49.4% of the attended sessions, moderate intensity in 39.7%, and low intensity in 10.8% (1349, 1084, and 296 out of 2729 sessions, respectively). Corresponding figures for balance intensity were 67.6%, 26.5%, and 5.9% (1845, 722, and 162 sessions, respectively). The median (interquartile range) individual lower limb strength exercises rate for high intensity was 47.2% (12.5%-77.8%) of attended sessions, and the rate for high or medium intensity at 94.7% (77.8%-100%). Balance exercises were performed at high intensity at 75% (33.3%-88.6%) of attended sessions and at high or medium intensity at 100% (91.2%-100%). High-intensity strength and balance exercises were performed in the same session at 28.6% (9.1%-40.5%). A significant difference was observed between the AD and non-AD groups in the high-intensity strength rate (34.9% [2.0%-62.9%] vs 53.8% [25.7%-80.0%] P = .035) but not in the high-intensity balance rate (P = .771) (Figure 2).
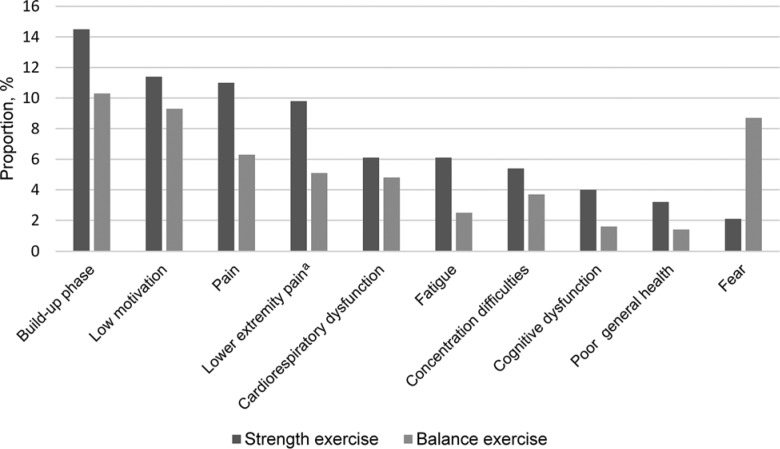
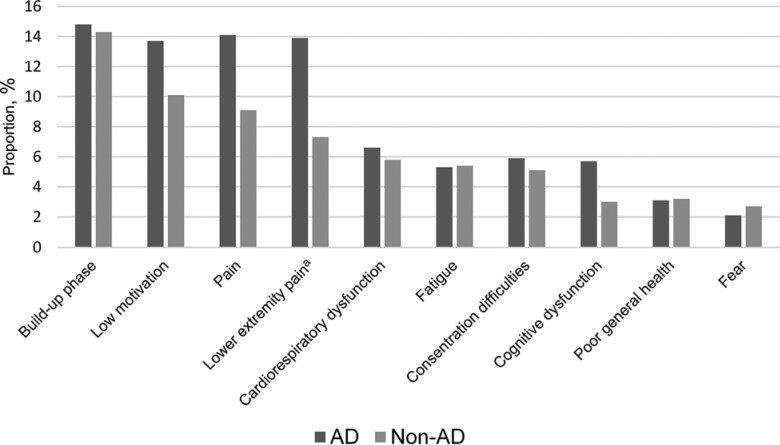
Strength exercises resulted in primary straining of peripheral (muscular) and central (cardiorespiratory) systems in 82.9% and 17.1% of attended sessions, respectively. The most common reasons for not achieving high-intensity performance in lower limb strength exercises were buildup training in the beginning of the exercise period or after a period of absence due to, for example, illness (14.5% of attended sessions), low motivation (11.4%), and pain (11.0%; 9.8% in the lower extremities; Figure 4). Corresponding figures divided in participants with AD and non-AD, respectively, are shown in Figure 5. The most common reasons for not achieving high intensity in balance exercises were buildup training (10.3% of attended sessions), low motivation (9.3%), fear (8.7%), and pain (6.1%; Figure 4).
Figure 4.
Reasons for not achieving high intensity, as percentage of all attended sessions. More than 1 reason per participant could be recorded. aIncluded in the pain category.
Figure 5.
Reasons for not achieving high intensity in strength exercises in participants with AD and non-AD dementia, as percentage of all attended sessions. More than 1 reason per participant could be recorded. aIncluded in the pain category. AD indicates Alzheimer's disease.
Adverse Events
In total, adverse events were recorded in 446/2729 (16.3%) attended sessions among 72 (77.4%) participants. All adverse events (N = 455) were minor or temporary. The median (interquartile range) individual adverse event rate was 10.0% (2.6%-27.3%) of attended sessions, with no significant difference between participants with AD and non-AD (P = .821) (Figure 2). Adverse events were musculoskeletal (pain, soreness; 64.0%), general/unspecified (eg, fatigue, headache, stomach pain, nausea; 13.6%), psychological (eg, anxiety, restlessness, anger; 13.3%), dizziness (5.2%), and cardiorespiratory (eg, breathlessness, chest discomfort; 3.9%).
Among the deaths that occurred during the intervention period, there was 1 case in which an indirect association with exercise could not be excluded with complete certainty. The individual fell ill 1 day after participating in an exercise session and later died from causes attributed to circulatory failure and general atherosclerosis.21 The participant suffered from multimorbidity, including poststroke, heart failure, atrial fibrillation, and general atherosclerosis. During the last 3 exercise sessions, the participant experienced some reduced endurance but without any discomfort. The participant's responsible physician at the nursing home was consulted after the 2nd of these 3 sessions and approved further exercise, which was adjusted according to the program instructions.
Correlation Between Outcome Variables and Medical Conditions
The occurrence of more BPSD symptoms, according to the Neuropsychiatric Inventory, was correlated with lower attendance (P = .037) and lower balance intensity (P = .005), and apathy was correlated with lower attendance (P = .037; Table 1). Neuroleptic use was correlated with higher attendance (P = .012). Angina pectoris diagnosis was correlated with higher strength intensity (P = .034). Use of analgesics (P = .023) and pain while walking (P = .041) were correlated with the occurrence of more adverse events.
DISCUSSION
This study showed that in a group-based, supervised, and individualized high-intensity functional exercise program, among older people with mild to moderate dementia living in nursing homes, a majority of the participants had high attendance rate and could exercise with moderate to high lower limb strength intensity and high balance intensity. The exercise program led to only minor and temporary (mostly musculoskeletal) adverse events during the exercise sessions. Participants with non-AD performed more sessions with a high intensity in strength exercises than participants with AD. The most common barriers for not attending exercise sessions were low motivation, illness, and tiredness. While fear was a common reason for not achieving high intensity in balance exercises, buildup period, low motivation, and pain were all common barriers in both balance and strength exercises. Of the medical conditions and symptoms of dementia analyzed in our study, only BPSD, including apathy, was negatively associated with the applicability of the program.
The attendance rate in this study was similar to those reported in other evaluations of high-intensity functional exercises among people with dementia living in nursing homes9,33 and only slightly lower than that reported in community-dwelling sedentary older adults participating in various exercise regimes,34 despite participants' significant cognitive and mobility impairment and the high prevalence of medical conditions.35,36 Exercise studies reporting higher attendance in people with dementia living in nursing homes included participants with greater mobility37–40 and cognitive capacity,40 only completing participants,37–39 and exercises of seemingly lower intensity.37,40 The high intensity of the present intervention may have negatively impacted motivation to attend, as participants with cognitive impairment have been found to prefer simple, light, and safe exercises.41 However, UMDEX participants reported that the exercise was challenging, but achievable, gave them moments of pleasure, and improved their mental and physical strength.42 In addition, no serious adverse event occurred during exercise, which may have increased motivation to attend. Other factors contributing to attendance in this study were the possibility of individual sessions and help with transfer to sessions, as shown previously.43 The latter factor may have contributed to the similarity of attendance rates of participants with AD and non-AD, despite differences in cognitive and balance capacity.35,36 Although the median attendance rate was 82.5%, only half of participants fulfilled the recommendation to exercise at least 2 times per week.12,44 To increase this proportion, the provision of additional weekly exercise sessions and individualization of session times according to participants' daily routines and needs may be necessary.
Although many participants did not reach high intensity, especially in strength exercises, participants achieved moderate to high intensity in strength and balance exercises in almost all attended sessions, in accordance with exercise recommendations12,44 and findings of similar studies in this population.27,33 Factors potentially contributing to this result are that the program's functional exercises are easy to follow for people with cognitive impairment and were adapted individually. As lower limb strength is important for functional performance, the greater intensity with which participants with non-AD were able to perform strength exercises may have contributed to the superior effects on ADLs and balance compared with those with AD, as shown previously.21 Despite comorbidity, including cardiorespiratory diseases, strength exercises strained mostly peripheral lower extremity muscles in this study. This straining is a positive result because exercising to muscle fatigue is a prerequisite to increasing muscle strength.
The incidence of recorded adverse events during exercise sessions in the study was higher than previously reported incidences in people with dementia.27,45 This difference could be explained by a relatively high prevalence of medical conditions such as osteoarthritis, stroke, depression, and delirium in our cohort. The participants were also more dependent in ADLs and more cognitively impaired than prior study groups, indicating that our population had progressed further in the course of their diseases, making them more sensitive to internal and external disturbances.18 The high prevalence of osteoarthritis (37.6%) in our cohort may explain, at least in part, why the majority of adverse events were musculoskeletal (pain and soreness; 64.0%). Muscle-strengthening physical activity to reduce pain and improve function is recommended for people with osteoarthritis, with no contraindication for minor or temporary pain during exercise.46–48 Although all discomforts experienced by the participants during the exercise sessions were minor or temporary, there was 1 death for which an indirect association with exercise could not be excluded with complete certainty. The participant, who had multimorbidity, fell ill a day after an exercise intervention. The patient experienced no discomfort during the preceding exercise session, which suggests that the death was probably unrelated to the exercise intervention. This inference is supported by other studies of exercise in people with dementia in nursing homes in which no exercise-related deaths were reported.33,40,49,50 Factors important for the safe achievement of moderate to high intensity were participants' use of waist belts with handles and PTs' ability to modify exercise according to participants' health status. This was based on PTs' close attention to participants' nonverbal communication51 and PTs' ability to obtain updates on participants' health status by communication with nursing home staff and to contact physicians or nurses when needed.
Low motivation, illness, and tiredness were the most common barriers to session attendance, as in other studies of people with dementia living in nursing homes.38,49 Despite individual exercise adaptation and the leader's effort to get to know participants and interpret their needs,51 low motivation was a common barrier to exercise attendance and intensity. Future studies should explore reasons for low motivation in this population, in which apathy is common, to develop more effective strategies, including ways to deliver exercise programs, to enhance the applicability of high-intensity functional exercise programs. Pain was a common barrier to the achievement of high-intensity performance, especially in strength exercises and among participants with AD. Pain while walking and analgesic use were also associated with adverse events. Thus, pain reduction and exercise individualization are needed in this population. Fear was a common barrier to the achievement of high intensity in balance exercises, perhaps due to difficulties in understanding the purpose of the exercises because of cognitive impairment and/or fear of falling when challenging postural stability; addressing these issues might be important.
Behavioral and psychological symptoms of dementia, including apathy, but not depression, functional balance capacity, or cognitive level, affected program applicability negatively in this study. Similarly, depression and dementia severity did not affect attendance,33 and cognitive function did not affect the applicability of high-intensity exercise,9 in previous studies. Unexpectedly, neuroleptic use, which was present in approximately one-tenth of the participants, had a positive association with attendance. This result might be due to a positive medication effect (eg, on BPSD) or random significance. This might also be the reasons for the positive association between angina pectoris and high intensity in balance exercises. In summary, these results support the provision of high-intensity exercise functional programs to individuals with dementia living in nursing homes.
Some limitations of this study should be considered. Although the inclusion criteria were broad, the exclusion of people with MMSE scores of less than 10 and need of assistance from 2 or more to rise from a chair with an armrest diminishes the generalizability of the findings. Strength, in the present study, is the attempt to estimate the exercise intensity in an adequate manner, that is, quantify the challenge of a task to the capability of that individual.52 A limitation is that the reliability of the rating scales was not tested, which makes the assessment more uncertain. However, the standardized rating system was predefined and all PTs were familiar with its application. There was no monitoring of new adverse events between exercise sessions, which might have led to missing potential adverse events. However, when assessing the course and severity of adverse events present during exercise sessions, it was possible to follow up them between sessions by reviewing medical records. In addition, it is possible that participants experienced delayed-onset muscle soreness or other pain between sessions and which is not known to the research team. However, pain was not a common reason for not attending exercise session (0.5% of total sessions, shown in Figure 3). Furthermore, the non-AD group was heterogeneous, as it included participants with several dementia subtypes. Further subdivision was not possible due to power issues in this analysis. Future studies of high-intensity functional exercise program applicability in participants with various dementia subtypes, as well as studies recording adverse events between sessions are needed.
CONCLUSION
A group-based, supervised, and individualized high-intensity functional exercise program seems to be applicable with regard to attendance, achieved intensity, and adverse events during the exercise sessions, in people with mild to moderate dementia in nursing homes. People with non-AD might be able to exercise with higher intensity in strength exercises than people with AD. To fulfill the recommendations to exercise at least twice per week for a majority of people in this population, the provision of additional weekly exercise sessions and individualization of exercise time may be needed. Since low motivation was a common barrier for attendance as well as for achieving high intensity, effective strategies to enhance motivation to participate in exercise are of importance. Furthermore, it is also important to prevent and treat pain and BPSD when promoting an high-intensity functional exercise program in this population. Although medical conditions are common in people with dementia in nursing homes, the results from our study support the inclusion of this population in high-intensity functional exercise programs.
ACKNOWLEDGMENTS
The authors express their sincere gratitude to all who contributed to data collection and implementation of the UMDEX study, as well as the social authorities of the municipality of Umeå, care staff, and participants.
Footnotes
This work was supported by the Swedish Research Council (grant numbers K2009-69P-21298-01-4, K2009-69X-21299-01-1, K2009-69P-21298-04-4, and K2014-99X-22610-01-6); Forte—Swedish Research Council for Health, Working Life and Welfare (formerly FAS—Swedish Council for Working Life and Social Research); the Vårdal Foundation; the Swedish Dementia Association; the Promobilia Foundation; the Swedish Society of Medicine; the Swedish Alzheimer Foundation; the County Council of Västerbotten, the Umeå University Foundation for Medical Research; the Ragnhild and Einar Lundström's Memorial Foundation; and the Erik and Anne-Marie Detlof's Foundation.
H.L. and N.L. developed and have received royalties on the weighted belt used in the exercise program. All other authors declare no conflicts of interest.
Richard Bohannon was the Decision Editor.
REFERENCES
- 1.World Health Organization. Dementia: a public health priority. http://www.who.int/mental_health/publications/dementia_report_2012/en/. Published 2012. Accessed December 14, 2014.
- 2.Allali G, Annweiler C, Blumen HM, et al. Gait phenotype from mild cognitive impairment to moderate dementia: results from the GOOD initiative. Eur J Neurol. 2016;23(3):527–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherrington C, Michaleff ZA, Fairhall N, et al. Exercise to prevent falls in older adults: an updated systematic review and meta-analysis. Br J Sports Med. 2017;51(24):1750–1758. [DOI] [PubMed] [Google Scholar]
- 4.Morie M, Reid KF, Miciek R, et al. Habitual physical activity levels are associated with performance in measures of physical function and mobility in older men. J Am Geriatr Soc. 2010;58(9):1727–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wennie Huang WN, Perera S, VanSwearingen J, Studenski S. Performance measures predict onset of activity of daily living difficulty in community-dwelling older adults. J Am Geriatr Soc. 2010;58(5):844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forbes D, Thiessen EJ, Blake CM, Forbes SC, Forbes S. Exercise programs for people with dementia. Cochrane Database Syst Rev. 2013;(12):CD006489. [DOI] [PubMed] [Google Scholar]
- 7.Rao AK, Chou A, Bursley B, Smulofsky J, Jezequel J. Systematic review of the effects of exercise on activities of daily living in people with Alzheimer's disease. Am J Occup Ther. 2014;68(1):50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitkälä K, Savikko N, Poysti M, Strandberg T, Laakkonen ML. Efficacy of physical exercise intervention on mobility and physical functioning in older people with dementia: a systematic review. Exp Gerontol. 2013;48(1):85–93. [DOI] [PubMed] [Google Scholar]
- 9.Littbrand H, Stenvall M, Rosendahl E. Applicability and effects of physical exercise on physical and cognitive functions and activities of daily living among people with dementia: a systematic review. Am J Phys Med Rehabil. 2011;90(6):495–518. [DOI] [PubMed] [Google Scholar]
- 10.Burton E, Cavalheri V, Adams R, et al. Effectiveness of exercise programs to reduce falls in older people with dementia living in the community: a systematic review and meta-analysis. Clin Interv Aging. 2015;10:421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009(3):CD002759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American College of Sports Medicine, Chodzko-Zajko WJ, Proctor DN, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–1530. [DOI] [PubMed] [Google Scholar]
- 13.Howe TE, Rochester L, Neil F, Skelton DA, Ballinger C. Exercise for improving balance in older people. Cochrane Database Syst Rev. 2011(11):CD004963. [DOI] [PubMed] [Google Scholar]
- 14.de Vreede PL, Samson MM, van Meeteren NL, Duursma SA, Verhaar HJ. Functional-task exercise versus resistance strength exercise to improve daily function in older women: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(1):2–10. [DOI] [PubMed] [Google Scholar]
- 15.American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687–708. [DOI] [PubMed] [Google Scholar]
- 16.Lesinski M, Hortobagyi T, Muehlbauer T, Gollhofer A, Granacher U. Effects of balance training on balance performance in healthy older adults: a systematic review and meta-analysis. Sports Med. 2015;45(12):1721–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox C, Smith T, Maidment I, et al. The importance of detecting and managing comorbidities in people with dementia? Age Ageing. 2014;43(6):741–743. [DOI] [PubMed] [Google Scholar]
- 18.Kovach CR, Logan BR, Simpson MR, Reynolds S. Factors associated with time to identify physical problems of nursing home residents with dementia. Am J Alzheimers Dis Other Demen. 2010;25(4):317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strandberg TE, Pitkälä KH. Frailty in elderly people. Lancet. 2007;369(9570):1328–1329. [DOI] [PubMed] [Google Scholar]
- 20.Allan LM, Ballard CG, Burn DJ, Kenny RA. Prevalence and severity of gait disorders in Alzheimer's and non-Alzheimer's dementias. J Am Geriatr Soc. 2005;53(10):1681–1687. [DOI] [PubMed] [Google Scholar]
- 21.Toots A, Littbrand H, Lindelöf N, et al. Effects of a high-intensity functional exercise program on dependence in activities of daily living and balance in older adults with dementia. J Am Geriatr Soc. 2016;64(1):55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 24.Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721–727. [DOI] [PubMed] [Google Scholar]
- 25.Littbrand H, Lindelöf N, Rosendahl E. The HIFE Program: The High-Intensity Functional Exercise Program, 2nd ed. Umeå, Sweden: Department of Community Medicine and Rehabilitation, Geriatric Medicine, Umeå University; 2014. [Google Scholar]
- 26.Littbrand H, Lindelöf N, Rosendahl E. The High Intensity Functional Exercise (HIFE) Program. Umeå, Sweden: Department of Community Medicine and Rehabilitation, Umeå University; 2018. https://www.hifeprogram.se/en. Accessed February 1, 2018 [Google Scholar]
- 27.Littbrand H, Rosendahl E, Lindelöf N, Lundin-Olsson L, Gustafson Y, Nyberg L. A high-intensity functional weight-bearing exercise program for older people dependent in activities of daily living and living in residential care facilities: evaluation of the applicability with focus on cognitive function. Phys Ther. 2006;86(4):489–498. [PubMed] [Google Scholar]
- 28.Pollock ML, Gaesser GA, Butcher JD, et al. American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30(6):975–991. [DOI] [PubMed] [Google Scholar]
- 29.Muir SW, Berg K, Chesworth B, Speechley M. Use of the Berg Balance Scale for predicting multiple falls in community-dwelling elderly people: a prospective study. Phys Ther. 2008;88(4):449–459. [DOI] [PubMed] [Google Scholar]
- 30.Sheikh LI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986(5):165–172. [Google Scholar]
- 31.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. [DOI] [PubMed] [Google Scholar]
- 32.Guigoz Y, Vellas B, Garry PJ. Mini nutritional assessment: a practical assessment tool for grading nutritional state of elderly patients. Facts Res Gerontol. 1994; 4 (supp. 2):15–59. [Google Scholar]
- 33.Telenius EW, Engedal K, Bergland A. Effect of a high-intensity exercise program on physical function and mental health in nursing home residents with dementia: an assessor blinded randomized controlled trial. PLoS One. 2015;10(5):e0126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong SY, Hughes S, Prohaska T. Factors affecting exercise attendance and completion in sedentary older adults: a meta-analytic approach. J Phys Act Health. 2008;5(3):385–397. [DOI] [PubMed] [Google Scholar]
- 35.van Alphen HJ, Hortobagyi T, van Heuvelen MJ. Barriers, motivators, and facilitators of physical activity in dementia patients: a systematic review. Arch Gerontol Geriatr. 2016;66:109–118. [DOI] [PubMed] [Google Scholar]
- 36.Stubbs B, Eggermont L, Soundy A, Probst M, Vandenbulcke M, Vancampfort D. What are the factors associated with physical activity (PA) participation in community dwelling adults with dementia? A systematic review of PA correlates. Arch Gerontol Geriatr. 2014;59(2):195–203. [DOI] [PubMed] [Google Scholar]
- 37.Kemoun G, Thibaud M, Roumagne N, et al. Effects of a physical training programme on cognitive function and walking efficiency in elderly persons with dementia. Dement Geriatr Cogn Disord. 2010;29(2):109–114. [DOI] [PubMed] [Google Scholar]
- 38.Bossers WJ, van der Woude LH, Boersma F, Hortobagyi T, Scherder EJ, van Heuvelen MJ. A 9-week aerobic and strength training program improves cognitive and motor function in patients with dementia: a randomized, controlled trial. Am J Geriatr Psychiatry. 2015;23(11):1106–1116. [DOI] [PubMed] [Google Scholar]
- 39.Venturelli M, Scarsini R, Schena F. Six-month walking program changes cognitive and ADL performance in patients with Alzheimer. Am J Alzheimers Dis Other Demen. 2011;26(5):381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santana-Sosa E, Barriopedro MI, Lopez-Mojares LM, Perez M, Lucia A. Exercise training is beneficial for Alzheimer's patients. Int J Sports Med. 2008;29(10):845–850. [DOI] [PubMed] [Google Scholar]
- 41.Chong TW, Doyle CJ, Cyarto EV, et al. Physical activity program preferences and perspectives of older adults with and without cognitive impairment. Asia Pac Psychiatry. 2014;6(2):179–190. [DOI] [PubMed] [Google Scholar]
- 42.Lindelöf N, Lundin-Olsson L, Skelton DA, Lundman B, Rosendahl E. Experiences of older people with dementia participating in a high-intensity functional exercise program in nursing homes: “While it's tough, it's useful.” PLoS One. 2017;12(11):e0188225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tak SH, Kedia S, Tongumpun TM, Hong SH. Activity engagement: perspectives from nursing home residents with dementia. Educ Gerontol. 2015;41(3):182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Souto Barreto P, Morley JE, Chodzko-Zajko W, et al. Recommendations on physical activity and exercise for older adults living in long-term care facilities: a taskforce report. J Am Med Dir Assoc. 2016;17(5):381–392. [DOI] [PubMed] [Google Scholar]
- 45.Dawson N, Judge KS, Gerhart H. Improved functional performance in individuals with dementia after a moderate-intensity home-based exercise program: a randomized controlled trial. J Geriatr Phys Ther. 2019;42(1):18–27. [DOI] [PubMed] [Google Scholar]
- 46.Ageberg E, Link A, Roos EM. Feasibility of neuromuscular training in patients with severe hip or knee OA: the individualized goal-based NEMEX-TJR training program. BMC Musculoskelet Disord. 2010;11:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med. 2015;49(24):1554–1557. [DOI] [PubMed] [Google Scholar]
- 48.Fransen M, McConnell S, Hernandez-Molina G, Reichenbach S. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014(4):CD007912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rolland Y, Pillard F, Klapouszczak A, et al. Exercise program for nursing home residents with Alzheimer's disease: a 1-year randomized, controlled trial. J Am Geriatr Soc. 2007;55(2):158–165. [DOI] [PubMed] [Google Scholar]
- 50.de Souto Barreto P, Cesari M, Denormandie P, Armaingaud D, Vellas B, Rolland Y. Exercise or social intervention for nursing home residents with dementia: a pilot randomized, controlled trial. J Am Geriatr Soc. 2017;65(9):E123–E129. [DOI] [PubMed] [Google Scholar]
- 51.Fjellman-Wiklund A, Nordin E, Skelton DA, Lundin-Olsson L. Reach the person behind the dementia—physical therapists' reflections and strategies when composing physical training. PLoS One. 2016;11(12):e0166686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farlie MK, Robins L, Keating JL, Molloy E, Haines TP. Intensity of challenge to the balance system is not reported in the prescription of balance exercises in randomised trials: a systematic review. J Physiother. 2013;59(4):227–235. [DOI] [PubMed] [Google Scholar]