Abstract
Magnetic resonance imaging (MRI) is an appealing technology for fetal cardiovascular assessment. It can be used to visualize fetal cardiac and vascular anatomy, to quantify fetal blood flow, and to quantify fetal blood oxygen saturation and hematocrit. However, there are practical limitations to the use of conventional MRI for fetal cardiovascular assessment, including the small size and high heart rate of the human fetus, the lack of conventional cardiac gating methods to synchronize data acquisition, and the potential corruption of MRI data due to maternal respiration and unpredictable fetal movements. In this review, we discuss recent technical advances in accelerated imaging, image reconstruction, cardiac gating, and motion compensation that have enabled dynamic MRI of the fetal heart.
Keywords: accelerated imaging, congenital heart disease, fetal heart, magnetic resonance imaging, motion correction
Imaging plays a vital role in the diagnosis and treatment planning for fetal cardiac abnormalities discovered in utero. Ultrasound is the primary modality for evaluating the fetus due to its spatial and temporal resolutions, widespread availability, and ease-of-use. Nevertheless, there is a growing interest in the use of magnetic resonance imaging (MRI) as an adjunct diagnostic tool for the fetal heart, brain, lungs, liver, and other organs when ultrasound is limited by maternal obesity, oligohydramnios, multiple gestations, fetal diaphragmatic hernia, or fetal bone in late gestation.1 Several studies have investigated the safety of fetal MRI with respect to peripheral nerve stimulation, acoustic noise, and energy deposition.2 Overall, it has been concluded that MRI is a safe modality for the fetus when operated within standard limits (normal mode) and scan durations.2 Moreover, studies have not demonstrate any adverse outcomes in children previously exposed to MRI during fetal development.3
In the context of fetal cardiac imaging, several studies have focused on the development of methods for evaluating fetal cardiac anatomy, function, blood flow, and oximetry.
The purpose of this review is to describe technical developments that have led to the current state-of-the-art MRI methods for assessing the structure and function of the fetal heart. We outline the major challenges facing fetal cardiac MRI and describe advancements ranging from static 2-dimensional (2D) images using readily available sequences to time-resolved volumetric images of the fetal heart using advanced acquisition and reconstruction methods. We conclude with a discussion of the clinical impact of these techniques, although still investigational, and comparisons to the current gold standard of ultrasound.
CHALLENGES OF FETAL CARDIAC MRI
Several practical challenges exist when imaging the fetal heart with MRI. The size of major fetal vessels at full gestation is in the range of 5 to 10 mm in diameter, the width of the fetal ventricles is in the range of 10 to 30 mm,4 the duration of the fetal cardiac cycle is between 330 and 540 ms with a systolic period between 20 and 50 ms,5–7 and a relatively large field-of-view (260 to 480 mm) is required to avoid wrap-around artifact from maternal anatomy. Consequently, achieving clinically useful spatial and temporal resolution while maintaining a reasonable scan time is difficult with the conventional sequences available on most scanners. Managing the length of fetal MRI acquisitions is particularly important given the periodic motions (fetal respiratory movements, fetal cardiac motion, maternal respiration) and stochastic motions (gross fetal movement) that can degrade image quality.
In pediatric and adult patients, a combination of cardiac gating and breath-holding or respiratory navigation is used to maintain diagnostically useful image quality. Unfortunately, a fetal electrocardiogram (ECG) signal is not readily available in the MRI environment and maternal breath-holds are brief and ideally avoided to optimize maternal comfort. As a result, standard methods for dynamic imaging of the heart are not directly translatable to fetal subjects. Considering these limitations, early feasibility of fetal cardiac MRI was demonstrated in animals,8–12 while human studies focused on fast 2D acquisitions as described in the following section.
STATIC IMAGING
Several groups have reported the use of single-shot balanced steady-state free precession (bSSFP) and fast spin echo sequences (HASTE, SS-FSE) to evaluate fetal cardiovascular anatomy and identify abnormalities.13–20 These sequences are attractive because they provide relatively high resolution (∼1.0 to 1.5 mm in-plane) static 2D images in short acquisition times (≳500 ms), allowing for multislice (ie, multiplanar) protocols. Although the temporal resolution of these early methods was too low to resolve fetal cardiac motion, resulting in anatomical blur of dynamic structures, static MRI remains useful for identifying gross anatomy and unusual extracardiac abnormalities that may affect the diagnosis of cardiovascular malformations, such as persistent left superior vena cava or inferior vena caval interruption.21 These strengths become more apparent in late gestation when greater fetal size facilitates static MRI, and when echocardiography is more challenging. For reference, Fig. 1 shows example SSFP and SS-FSE images of the fetal heart highlighting both bright-blood and black-blood strategies, respectively, which can be used to assess the fetal cardiac anatomy. In addition to the structural information provided by these sequences, the repeated acquisition of bSSFP images in the same anatomical location over time has been used to provide a dynamic “real-time” assessment of the fetal heart including measurements of cardiac function.13,15,17,22–24 However, the spatiotemporal resolution of such real-time methods has historically been inadequate to resolve valvular functionality and to visualize the end-systolic phases of the fetal cardiac cycle, leading groups to focus instead on methods for CINE imaging.
FIGURE 1.

Static balanced steady-state free precession (bSSFP) bright-blood image (left) and single-shot fast spin-echo (ss-FSE) black-blood image (right) in a 38-week fetus with a right ventricular mass (arrowed). Reprinted with permission from Prenat Diagn 2016; 36:916–925 used under CC BY. Caption adapted from the original. Right (R) and left (L) heart sides are labelled.
CINE IMAGING
Time-resolved (CINE) bSSFP acquisitions are an integral facet of postnatal cardiac MRI examinations providing assessment of both cardiac structure and function. Using readily available sequences, high spatial and high temporal resolution images (∼1 × 1 mm2, <50 ms) may be acquired by synchronizing the sequence to the patient's heart rate using an ECG signal (cardiac gating). Unfortunately, a fetal ECG signal is not available in the MRI environment precluding conventional CINE imaging of the fetal heart. Nevertheless, much work has been done to develop fetal CINE imaging using novel post-processing methods and external hardware devices for retrospective fetal cardiac gating.
FETAL CARDIAC GATING
Metric Optimized Gating
Metric optimized gating (MOG) extracts the fetal heart rate by iteratively reconstructing CINE images using a parameterized model of the cardiac cycle and then outputs the optimum image quality according to the minimized image entropy through time,25 space,26,27 or both.28 While this method was first developed for time-resolved phase-contrast measurements of fetal blood flow,25 it has since been applied to both Cartesian and radial bSSFP acquisitions and was the first method to demonstrate dynamic CINE MRI of the human fetal heart.26 By independently parameterizing each fetal cardiac trigger, this model accounts for beat-to-beat fluctuations in fetal heart rate for subsequent CINE reconstruction. For Cartesian data without acceleration, the computational time needed for MOG is minor (eg, <5 minutes per slice using conventional desktop computing); however, for acquisitions with more complex reconstructions (ie, compressed sensing), the increased computational demand has led groups to explore alternative fetal gating methods based on intermediate real-time reconstructions, including an alternative formulation of MOG as described by the next section.
Self-gating
Self-gating refers to strategies that extract periodic gating signals from the MRI data itself. These signals can then be used to retrospectively sort the data into high-quality gated images.29 Implementations of self-gating have used various k-space trajectories for data acquisition, including Cartesian, radial, and spiral trajectories, and also various metrics to detect the cardiac or respiratory cycles, including signal intensity modulation, center-of-mass tracking, and image correlation.30 In the context of fetal MRI, self-gating has been used to extract the heart-rate from radial data using a principal component-based filtering of the repeatedly sampled k-space center.31 Alternatively, self-gating signals have been extracted from intermediate real-time reconstructions of radial data by measuring the correlation between frames32 or applying MOG to the real-time images, with compressed sensing reconstruction of each real-time image series requiring approximately 120 minutes per slice using conventional desktop computing.33 Potential challenges of non-Cartesian sampling include nonuniform clustering of data after temporal sorting, k-space trajectory errors, and off-resonance artifact (these latter issues being fairly benign for in utero radial imaging at 1.5T).34,35 In addition, fetal heart rates have been estimated directly from the temporal frequency spectrum of Cartesian real-time images, that is, the temporal Fourier transform of the image series, as the periodicity of the beating fetal heart appears as a local maximum in the spectrum within the range of expected heart rates.36
Hardware
Dramatic advances in fetal cardiovascular MRI have been demonstrated using the tailored acquisition and reconstruction methods described above. These methods, however, have limitations including relatively long reconstruction times that currently prevent evaluation of images during the MRI examination.
To address these limitations, researchers are developing novel external hardware to monitor the fetal cardiac cycle during MRI data acquisition. The most mature device for this purpose uses an MR-compatible Doppler Ultrasound Gating (DUS) probe placed over the maternal abdomen.37,38 This nonimaging device monitors motions associated with the fetal cardiac cycle, such as blood flow and cardiac contraction, based on a Doppler waveform. Triggers derived from this waveform are supplied to the MRI scanner to synchronize data acquisition. A practical benefit of this approach is the ability to use established cardiovascular protocols for fetal imaging, which provide immediate in-line image reconstructions to support clinical work-flow.39 Such devices may also facilitate studies that require prospective triggering, for example, in applications such as triggered T1 or T2 mapping.40 Drawbacks to this approach, as with any external device, include patient preparation time and equipment procurement. Furthermore, monitoring the fetal position to ensure it lays within the detection range will likely require pairing such devices with appropriate acquisition schemes, resulting in more elaborate and slower reconstructions.
ACCELERATED CINE IMAGING
With the advent of methods for fetal cardiac gating, CINE imaging of the fetal heart is possible. However, the increased scan time of CINE acquisitions relative to their static 2D counterparts, and the various sources of motion that can occur during the scan, necessitates methods for accelerating fetal acquisitions. Conventional parallel imaging methods are available on most scanners and have been used for fetal cardiac imaging, albeit with modest acceleration factors due to the often poor coil coverage of the fetus within the large maternal abdomen.26 Conversely, multiple studies have investigated the use of compressed sensing to achieve highly accelerated fetal acquisitions using either Cartesian27,41 or radial sequences.31–33 Both compressed sensing and k-t SENSE36 have been used to reconstruct real-time images with greater spatial and temporal resolution than those described in the static imaging section; however, these were primarily used for motion estimation and correction, before CINE reconstruction. Table 1 summarizes different implementations of fetal CINE MRI from the literature, recognizing that exact comparisons between methods are difficult because actual spatial and temporal resolutions depend on factors such as regularization, motion correction, volumetric reconstruction approach, and gating accuracy, while some publications may also quote effective (interpolated) resolutions.
TABLE 1.
Comparison of CINE MRI Methods Applied to the Human Fetal Heart
| Reference | GA Range, wks | Field, T | Trajectory | Spatial Res, mm | Temporal Res, ms | Gating Method | Motion Correction | Outlier Rejection |
| Roy et al26 | [35–37] (N = 2) | 1.5 | Cartesian | 1.5 × 1.5 × 4.0 | 50 | MOG | None | None |
| Roy et al33 | [34–38] (N = 7) | 1.5 | Radial | 1.0 × 1.0 × 4.0 | 12–15 | MOG | 2D rigid (k-space) | Semi-automatic |
| Haris et al31 | [29–37] (N = 5) | 1.5 | Radial | 1.4 × 1.4 × 4.0 | 56 | SG k-t | None | None |
| Kording et al37 | [30–37] (N = 15) | 1.5 and 3.0 | Cartesian | 1.0 × 1.0 × 5.0 | 30 | DUS | None | None |
| Chaptinel et al32 | [26–32] (N = 6) | 1.5 | Radial | 1.0 × 1.0 × 4.0 | 25 | SG x-t | None | None |
| van Amerom et al36 | [25–35] (N = 30) | 1.5 | Cartesian | 2.0 × 2.0 × 2.0 | 72-83 | SG x-f | 2D rigid (image-space) | Automatic |
| van Amerom et al42 | [25–33] (N = 20) | 1.5 | Cartesian | 2.0 × 2.0 × 2.0 | 64-76 | SG x-f (2D) SG x-t (3D) | 3D rigid (image space) | Automatic |
Note: All studies performed using a balance steady-state free precession (bSSFP) sequence.
DUS indicates Doppler ultrasound gating; GA, gestation age; MOG, metric optimized gating; SG, self-gating based on k-space versus time (k-t), images versus time (x-t), or image temporal frequency (x-f).
MOTION COMPENSATION
To prevent artifact from maternal respiration, studies have acquired 2D fetal MRI data under maternal breathhold, but this can be challenging for pregnant subjects and limits the number of slices that can be acquired consecutively.31,32 Alternatively, free-breathing methods have been developed using motion correction to suppress artifact from both maternal respiration and gross fetal movement, thereby enabling multislice acquisitions covering the whole fetal heart in multiple orientations.33,42
Such motion compensation is achieved using real-time 2D imaging to estimate motion parameters that inform higher resolution CINE reconstructions from the same data. Examples of real-time fetal imaging, with increasing levels of motion, are shown in Fig. 2. From such real-time image series, in-plane motion (Dx,y in Fig. 2) may be tracked and corrected. Conversely, through-plane motion results in mixing of anatomical information from adjacent structures, producing image degradation if combined blindly. It is thus important to identify periods of through-plane motion and discard data acquired during those periods. As shown in Fig. 2I, real-time images can also be used for manual or semi-automatic detection and rejection of data acquired during periods of through-plane motion associated with fetal motion or maternal respiration.32,33Figure 3 provides an example of multislice CINE imaging of the fetal heart using such a motion-robust acquisition and reconstruction.
FIGURE 2.
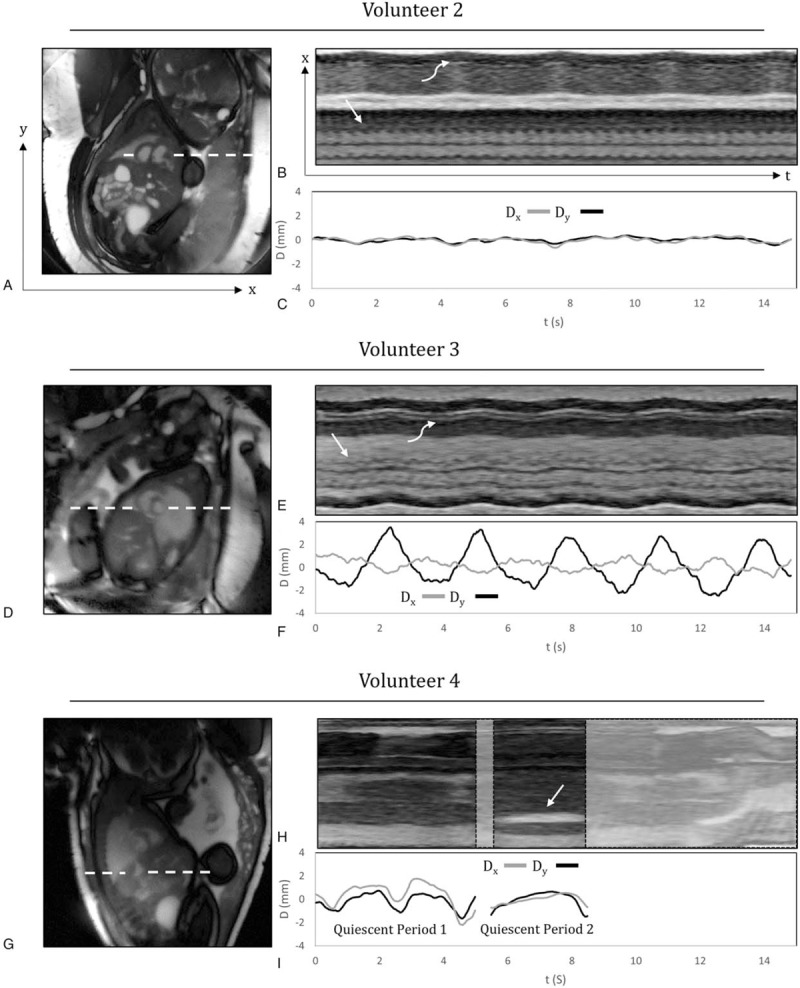
Real-time reconstructions of the fetal heart for 3 volunteers with increasing levels of motion. Time-averaged images of the heart and surrounding anatomy (A, D, G), M-mode displays of signal along the white dashed lines (B, E, H), and plots of displacement (Dx,y) versus time (C, F, I), calculated from the real-time reconstructions over a region of interest containing the heart. Dx and Dy represent the horizontal and vertical components of displacement, respectively. For volunteers 2 and 3, the M-modes show fetal cardiac motion (straight arrow) and maternal respiratory motion (curved arrow). For volunteer 4, the M-mode display shows the fetal stomach (straight arrow), which is used to identify through-plane motion. Reprinted with permission from J Cardiovasc Magn Reson 2017; 19:29 used under CC BY. Caption adapted from the original.
FIGURE 3.

Motion-corrected CINE short axis images of the fetal heart using the radial acquisition and reconstruction framework described in J Cardiovasc Magn Reson 2017; 19:29 by Roy et al.33
More recently, fully automated methods have been implemented to identify periods of motion during fetal cardiac MRI,36,42 leveraging statistical outlier-rejection methods previously established for fetal brain volumetric reconstruction.43,44 Outlier rejection reduces the impact of inconsistent data during reconstruction, resulting in reduced artifact and sharper images. This automated approach estimates the probability of each voxel and real-time image frame being classified as either an inlier or an outlier, and then the probabilities are used to downweight or completely reject outlier voxels and frames from the final CINE reconstruction. As mentioned, these approaches have previously enabled volumetric reconstruction of the fetal brain from multiple 2D static images, and recently, these approaches have been adapted to dynamic, volumetric MRI of the fetal heart.
VOLUMETRIC RECONSTRUCTION
The majority of fetal cardiac MRI is done using fast 2D acquisitions to minimize the impact of fetal and maternal motion. However, the underlying fetal anatomy is 3-dimensional and would be best represented as volumetric data to aid interpretation and assessment. Unfortunately, 3D MR data sets require several seconds to acquire and, consequently, are likely to be corrupted by motion and cardiac pulsation. An inventive solution to this problem is to use a multiplanar acquisition and combine the 2D images using volumetric reconstruction methods.
Volumetric reconstruction of 2D fetal MRI was first demonstrated for fetal brain imaging43–46 and subsequently applied to the fetal body47 as well as the placenta.48 These techniques use scattered data interpolation to achieve volumetric reconstructions from multiplanar 2D SS-FSE MR images. Initial implementations interleaved interpolation with motion correction to generate 3D data, using either cubic B-spline45 or Gaussian kernel-based46 interpolation. Subsequently, an error minimization (super-resolution) approach was introduced to reduce the blurring effect from thick-slice MR images when using Gaussian kernel-based interpolation43 and outlier rejection was employed to reduce the impact of voxels corrupted by motion and images misaligned to the volume,43,44 removing the need for manual exclusion of inconsistent data. Signal intensity-matching was also added to increase data consistency leading to improved volumetric reconstructions.44,49
Methods for volumetric reconstruction of the fetal heart have now been developed for both static (3D) visualization of the fetal heart and extracardiac vasculature50 as well as CINE (4D) whole-heart visualization.42 Volumetric reconstruction from 2D MRI has several characteristics that are advantageous for fetal cardiac imaging. First, the acquisition of multiplanar 2D MRI for volumetric reconstruction is robust to motion and does not require highly specific scan plane prescriptions to capture the desired anatomical features, which can be a challenge in 2D single-slice MRI. Second, both in-plane and through-plane motion can be corrected. Lastly, the reconstructed data can provide full coverage of the entire fetal heart, allowing for comprehensive assessment of fetal cardiovascular anatomy.
Figure 4 shows an example of the motion-corrected 3D volumetric reconstruction framework, originally used for fetal brain MRI,44 adapted to T2-weighted SS-FSE MR images of the fetal heart. Blood flowing through the cardiovascular system has a hypointense signal in the SS-FSE images and the extracardiac vasculature is well defined. Such 3D reconstructions allow for detailed depiction of cardiovascular anatomy, particularly the aortic arch and pulmonary vessels, with improved diagnostic quality compared with uncorrected 2D SS-FSE.50 However, due to the signal characteristics of SS-FSE and the long acquisition time relative to the cardiac cycle (∼500 to 1000 ms), the heart and the roots of the great vessels appear as a nearly homogeneous signal region with little definition of intracardiac features in both the acquired 2D images and, consequently, the static 3D reconstruction.
FIGURE 4.
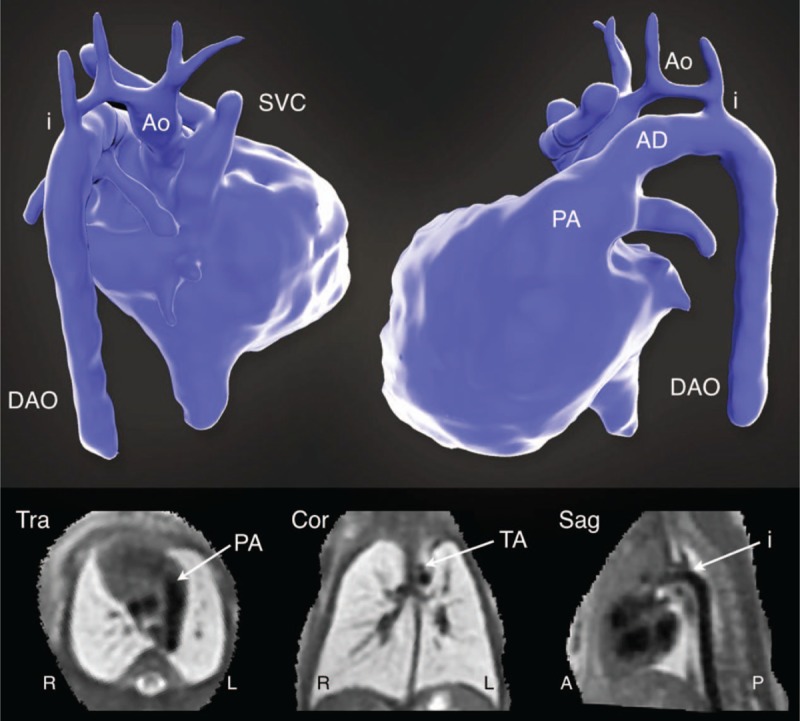
Volumetric reconstruction of T2-weighted multiplanar single shot fast spin echo (ss-FSE) MRI in a 33-week gestational age fetus with coarctation of the aorta. A volume rendering of the blood pool is shown in posterior projection (top left) and left lateral projection (top right). The bottom panel shows planes from the volumetric reconstructed data in transverse (Tra), coronal (Cor), and sagittal (Sag) orientations. The aorta (Ao), arterial duct (AD), descending aorta (DAo), aortic isthmus (i), pulmonary artery (PA), transverse arch (TA), and superior vena cava (SVC) are labeled. Reprinted with permission from Lancet 2019; 393:1619–1627 used under CC BY.
Figure 5 shows an example 4D reconstruction of a healthy fetal heart, acquired using real-time bSSFP instead of SS-FSE, showing the great vessels as well as intracardiac anatomy. These data could be resliced in any 2D plane or cardiac phase, allowing for optimal anatomical views and facilitating understanding of the spatial relationships of cardiovascular structures. This 4D reconstruction framework42 combines the motion-tolerant, image-domain 2D fetal cardiac CINE technique36 with a 3D volumetric reconstruction technique,44 and adds a temporal component to the volumetric reconstruction to generate dynamic volumes.
FIGURE 5.
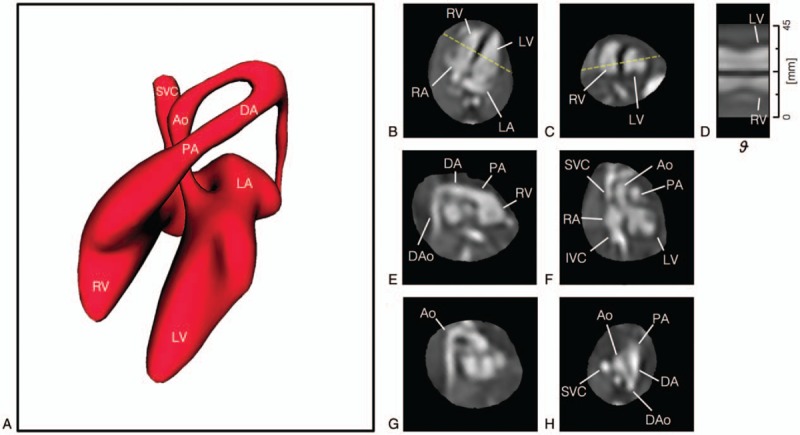
4D CINE volumetric reconstruction of the heart of a healthy 28-week gestational age fetus from multi-planar real-time balanced steady-state free precession (bSSFP) MRI. (A) Volume rendering of blood pool in diastole showing arrangement and connections of chambers and vessels, for reference. The reconstructed 4D data are shown resliced in (B) 4-chamber, (C) mid-short axis, (E) right ventricular outflow tract, (F) left ventricular outflow tract, (G) aortic arch, and (H) 3-vessel views. (D) A line profile at the intersection of the 4-chamber and mid-short axis views (dashed yellow lines) shows the contraction and dilation of the ventricles with cardiac phase (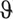 ). Ventriculoarterial connections can be seen in outflow tract views with the pulmonary artery (PA) from the right ventricle (RV) in (E) and the aorta (Ao) arising from the left ventricle (LV) in (F). Systemic venous connections of the superior (SVC) and inferior (IVC) vena cava with the right atrium (RA) can also be seen in (F). The ductal arch (DA) can be seen in both (E) and (H), connecting the PA to the descending aorta (DAo), while the Ao arch can be seen in (G) and (H). All boxes bounding the resliced views measure 65 × 65 mm. The fetal heart is shown in radiological orientation, that is, image axes up and right relative to the page correspond to left, anterior, and/or superior anatomical directions. Views are shown using spatial B-spline interpolation to avoid voxel distortion. Reprinted with permission from Magn Reson Med 2019; 82:1055–1072 used under CC BY.
). Ventriculoarterial connections can be seen in outflow tract views with the pulmonary artery (PA) from the right ventricle (RV) in (E) and the aorta (Ao) arising from the left ventricle (LV) in (F). Systemic venous connections of the superior (SVC) and inferior (IVC) vena cava with the right atrium (RA) can also be seen in (F). The ductal arch (DA) can be seen in both (E) and (H), connecting the PA to the descending aorta (DAo), while the Ao arch can be seen in (G) and (H). All boxes bounding the resliced views measure 65 × 65 mm. The fetal heart is shown in radiological orientation, that is, image axes up and right relative to the page correspond to left, anterior, and/or superior anatomical directions. Views are shown using spatial B-spline interpolation to avoid voxel distortion. Reprinted with permission from Magn Reson Med 2019; 82:1055–1072 used under CC BY.
CLINICAL UTILITY AND COMPARISON TO ULTRASOUND
The technical advancements in fetal cardiac MRI described in this review provide new opportunities for evaluating high-risk pregnancies. Recent studies have explored the clinical utility of these methods for detecting and characterizing fetal cardiac abnormalities, and compared their performance against ultrasound, the primary fetal imaging modality.32,42,50,51 For example, a recent MRI study of fetal CHD investigated motion-corrected volumetric reconstructions (see Fig. 4) of fetal cardiovascular anatomy and found good spatial agreement between these static reconstructions and ultrasound (intraclass correlation coefficient 0.78).50 Moreover, volumetric visualizations were of significantly higher diagnostic quality than the individual 2D MRI data used to generate the volumes. Dynamic fetal cardiac MRI studies have shown similarly good agreement32,39,42,51 (Fig. 6) and have proved useful for evaluating specific abnormalities as evidenced by case studies of a cardiac mass42,52 and an unbalanced common atrioventricular canal.53 In an evaluation of the utility of 4D volumetric reconstruction,42 readers had high confidence in their comprehensive assessment of fetal cardiovascular anatomy using reconstructed 4D cine MRI (Fig. 5) and there was good agreement between ventricular dimensions at systole and diastole on 2D M-mode ultrasound and 4D MRI.
FIGURE 6.

A 36-week fetus with a septal defect and diverticulum. Fetal cardiac MRI (left) and echocardiography (right) images demonstrate a diverticulum (Div.) attached to the right ventricle (RV) in long-axis (A) and short-axis views (B), and an interruption of the myocardium indicates a ventricular septal defect (VSD) in a short-axis view (C). Reprinted with permission from Circ Cardiovasc Imaging 2018; 11:e007745 used under CC BY.
In parallel with these MRI studies of fetal cardiovascular anatomy, groups have also demonstrated and validated MRI techniques for quantifying fetal blood flow,28,54,55 oxygenation,56–58 and hematocrit59 noninvasively. These techniques have subsequently been combined to study the impact of pathology on fetal development including the effect of CHD60 and intrauterine growth restriction61 on fetal brain development. Specific CHD cohorts that have been investigated using these techniques include fetal left-sided heart disease62 and transposition of the great arteries.63,64
CONCLUSION
Over the last 15 years, fetal cardiac MRI has transformed from static, single-shot imaging into multi-dimensional, motion-tolerant, and high-resolution dynamic imaging of the fetal heart and surrounding vasculature. This information, combined with MRI measurements of fetal physiology, promises to improve our understanding of fetal development and the impact of disease. As such, these methods are a promising adjunct to fetal echocardiography, but challenges remain—especially when imaging small and mobile fetuses at earlier gestation, which require even higher spatial and temporal resolutions with improved motion compensation. Nevertheless, the field of fetal cardiovascular MRI is advancing quickly, and it will be exciting to witness its capabilities grow over the next 15 years.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Gholipour A, Estroff JA, Barnewolt CE, et al. Fetal MRI: a technical update with educational aspirations. Concepts Magn Reson Part A Bridg Educ Res 2014; 43:237–266.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gowland P. Safety of Fetal MRI Scanning. 2010; Berlin Heidelberg: Springer, 49–54. [Google Scholar]
- 3.Ray JG, Vermeulen MJ, Bharatha A, et al. Association Between MRI Exposure During Pregnancy and Fetal and Childhood Outcomes. 2016; Vancouver, BC, Canada: JAMA Fraser Institute, 316:952. [DOI] [PubMed] [Google Scholar]
- 4.Firpo C, Hoffman JIE, Silverman NH. Evaluation of fetal heart dimensions from 12 weeks to term. Am J Cardiol 2001; 87:594–600.. [DOI] [PubMed] [Google Scholar]
- 5.National Institute of Child Health and Human Development Research Planning Workshop Electronic fetal heart rate monitoring: research guidelines for interpretation. Am J Obstet Gynecol 1997; 177:1385–1390.. [PubMed] [Google Scholar]
- 6.Wheeler T, Murrills A. Patterns of fetal heart rate during normal pregnancy. Br J Obstet Gynaecol 1978; 85:18–27.. [DOI] [PubMed] [Google Scholar]
- 7.Tsyvian P, Malkin K, Wladimiroff JW. Assessment of fetal left cardiac isovolumic relaxation time in appropriate and small-for-gestational-age fetuses. Ultrasound Med Biol 1995; 21:739–743.. [DOI] [PubMed] [Google Scholar]
- 8.Wedegärtner U, Kooijman H, Yamamura J, et al. In vivo MRI measurement of fetal blood oxygen saturation in cardiac ventricles of fetal sheep: a feasibility study. Magn Reson Med 2010; 64:32–41.. [DOI] [PubMed] [Google Scholar]
- 9.Yamamura J, Schnackenburg B, Kooijmann H, et al. Magnetic resonance angiography of fetal vessels: feasibility study in the sheep fetus. Jpn J Radiol 2010; 28:720–726.. [DOI] [PubMed] [Google Scholar]
- 10.Yamamura J, Schnackenburg B, Kooijmann H, et al. High resolution MR imaging of the fetal heart with cardiac triggering: a feasibility study in the sheep fetus. Eur Radiol 2009; 19:2383–2390.. [DOI] [PubMed] [Google Scholar]
- 11.Yamamura J, Frisch M, Ecker H, et al. Self-gating MR imaging of the fetal heart: comparison with real cardiac triggering. Eur Radiol 2011; 21:142–149.. [DOI] [PubMed] [Google Scholar]
- 12.Schrauben EM, Saini BS, Darby JRT, et al. Fetal hemodynamics and cardiac streaming assessed by 4D flow cardiovascular magnetic resonance in fetal sheep. J Cardiovasc Magn Reson 2019; 21:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorincour G, Bourlière-Najean B, Bonello B, et al. Feasibility of fetal cardiac magnetic resonance imaging: preliminary experience. Ultrasound Obstet Gynecol 2007; 29:105–108.. [DOI] [PubMed] [Google Scholar]
- 14.Saleem SN. Feasibility of MRI of the fetal heart with balanced steady-state free precession sequence along fetal body and cardiac planes. Am J Roentgenol 2008; 191:1208–1215.. [DOI] [PubMed] [Google Scholar]
- 15.Manganaro L, Savelli S, Di Maurizio M, et al. Potential role of fetal cardiac evaluation with magnetic resonance imaging: preliminary experience. Prenat Diagn 2008; 28:148–156.. [DOI] [PubMed] [Google Scholar]
- 16.Votino C, Jani J, Damry N, et al. Magnetic resonance imaging in the normal fetal heart and in congenital heart disease. Ultrasound Obstet Gynecol 2012; 39:322–329.. [DOI] [PubMed] [Google Scholar]
- 17.Dong S-Z, Zhu M, Li F. Preliminary experience with cardiovascular magnetic resonance in evaluation of fetal cardiovascular anomalies. J Cardiovasc Magn Reson 2013; 15:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaur L, Talemal L, Bulas D, et al. Utility of fetal magnetic resonance imaging in assessing the fetus with cardiac malposition. Prenat Diagn 2016; 36:752–759.. [DOI] [PubMed] [Google Scholar]
- 19.Dong S-Z, Zhu M. MR imaging of fetal cardiac malposition and congenital cardiovascular anomalies on the four-chamber view. Springerplus 2016; 5:1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lloyd DFA, van Amerom JFP, Pushparajah K, et al. An exploration of the potential utility of fetal cardiovascular MRI as an adjunct to fetal echocardiography. Prenat Diagn 2016; 36:916–925.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong S-Z, Zhu M. Magnetic resonance imaging of fetal persistent left superior vena cava. Sci Rep 2017; 7:4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manganaro L, Savelli S, Di Maurizio M, et al. Assessment of congenital heart disease (CHD): is there a role for fetal magnetic resonance imaging (MRI)? Eur J Radiol 2009; 72:172–180.. [DOI] [PubMed] [Google Scholar]
- 23.Fogel MA, Wilson RD, Flake A, et al. Preliminary investigations into a new method of functional assessment of the fetal heart using a novel application of “real-time” cardiac magnetic resonance imaging. Fetal Diagn Ther 2005; 20:475–480.. [DOI] [PubMed] [Google Scholar]
- 24.Tsuritani M, Morita Y, Miyoshi T, et al. Fetal cardiac functional assessment by fetal heart magnetic resonance imaging. J Comput Assist Tomogr 2019; 43:104–108.. [DOI] [PubMed] [Google Scholar]
- 25.Jansz MS, Seed M, van Amerom JFP, et al. Metric optimized gating for fetal cardiac MRI. Magn Reson Med 2010; 64:1304–1314.. [DOI] [PubMed] [Google Scholar]
- 26.Roy CW, Seed M, van Amerom JFP, et al. Dynamic imaging of the fetal heart using metric optimized gating. Magn Reson Med 2013; 70:1598–1607.. [DOI] [PubMed] [Google Scholar]
- 27.Roy CW, Seed M, Macgowan CK. Accelerated MRI of the fetal heart using compressed sensing and metric optimized gating. Magn Reson Med 2017; 77:2125–2135.. [DOI] [PubMed] [Google Scholar]
- 28.Goolaub DS, Roy CW, Schrauben E, et al. Multidimensional fetal flow imaging with cardiovascular magnetic resonance: a feasibility study. J Cardiovasc Magn Reson 2018; 20:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spraggins TA. Wireless retrospective gating: application to cine cardiac imaging. Magn Reson Imaging 1990; 8:675–681.. [DOI] [PubMed] [Google Scholar]
- 30.Kellman P, Chefd’hotel C, Lorenz CH, et al. High spatial and temporal resolution cardiac cine MRI from retrospective reconstruction of data acquired in real time using motion correction and resorting. Magn Reson Med 2009; 62:1557–1564.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haris K, Hedström E, Bidhult S, et al. Self-gated fetal cardiac MRI with tiny golden angle iGRASP: a feasibility study. J Magn Reson Imaging 2017; 46:207–217.. [DOI] [PubMed] [Google Scholar]
- 32.Chaptinel J, Yerly J, Mivelaz Y, et al. Fetal cardiac cine magnetic resonance imaging in utero. Sci Rep 2017; 7:15540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy CW, Seed M, Kingdom JC, et al. Motion compensated cine CMR of the fetal heart using radial undersampling and compressed sensing. J Cardiovasc Magn Reson 2017; 19:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell-Washburn AE, Xue H, Lederman RJ, et al. Real-time distortion correction of spiral and echo planar images using the gradient system impulse response function. Magn Reson Med 2016; 75:2278–2285.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen W, Meyer CH. Semiautomatic off-resonance correction in spiral imaging. Magn Reson Med 2008; 59:1212–1219.. [DOI] [PubMed] [Google Scholar]
- 36.van Amerom JFP, Lloyd DFA, Price AN, et al. Fetal cardiac cine imaging using highly accelerated dynamic MRI with retrospective motion correction and outlier rejection. Magn Reson Med 2018; 79:327–338.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kording F, Schoennagel BP, de Sousa MT, et al. Evaluation of a portable doppler ultrasound gating device for fetal cardiac MR imaging: initial results at 1.5T and 3T. Magn Reson Med Sci 2018; 17:308–317.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kording F, Yamamura J, de Sousa MT, et al. Dynamic fetal cardiovascular magnetic resonance imaging using Doppler ultrasound gating. J Cardiovasc Magn Reson 2018; 20:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tavares de Sousa M, Hecher K, Yamamura J, et al. Dynamic fetal cardiac magnetic resonance four chamber view imaging using Doppler ultrasound gating in the normal fetal heart and in congenital heart disease: comparison to fetal echocardiography. Ultrasound Obstet Gynecol 2019; 53:669–675.. [DOI] [PubMed] [Google Scholar]
- 40.Messroghli DR, Moon JC, Ferreira VM, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2∗ and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imagi. J Cardiovasc Magn Reson 2017; 19:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roy CW, Seed M, Macgowan C. Accelerated phase contrast measurements of fetal blood flow using compressed sensing. J Cardiovasc Magn Reson 2016; 18:30.27209219 [Google Scholar]
- 42.van Amerom JFP, Lloyd DF, Deprez M, et al. Fetal whole-heart 4D imaging using motion-corrected multi-planar real-time MRI. Magn Reson Med 2019; 82:1055-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gholipour A, Estroff JA, Warfield SK. Robust super-resolution volume reconstruction from slice acquisitions: application to fetal brain MRI. IEEE Trans Med Imaging 2010; 29:1739–1758.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuklisova-Murgasova M, Quaghebeur G, Rutherford MA, et al. Reconstruction of fetal brain MRI with intensity matching and complete outlier removal. Med Image Anal 2012; 16:1550–1564.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang S, Xue H, Glover A, et al. MRI of moving subjects using multislice snapshot images with volume reconstruction (SVR): application to fetal, neonatal, and adult brain studies. IEEE Trans Med Imaging 2007; 26:967–980.. [DOI] [PubMed] [Google Scholar]
- 46.Rousseau F, Glenn OA, Iordanova B, et al. Registration-based approach for reconstruction of high-resolution in utero fetal MR brain images. Acad Radiol 2006; 13:1072–1081.. [DOI] [PubMed] [Google Scholar]
- 47.Kainz B, Alansary A, Malamateniou C, et al. Flexible reconstruction and correction of unpredictable motion from stacks of 2D images. Lect Notes Comput Sci 2015; 9350:555–562.. [Google Scholar]
- 48.Miao H, Mistelbauer G, Karimov A, et al. Placenta maps: in utero placental health assessment of the human fetus. IEEE Trans Vis Comput Graph 2017; 23:1612–1623.. [DOI] [PubMed] [Google Scholar]
- 49.Kim K, Habas PA, Rajagopalan V, et al. Bias field inconsistency correction of motion-scattered multislice mri for improved 3d image reconstruction. IEEE Trans Med Imaging 2011; 30:1704–1712.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lloyd DFA, Pushparajah K, Simpson JM, et al. Three-dimensional visualisation of the fetal heart using prenatal MRI with motion-corrected slice-volume registration: a prospective, single-centre cohort study. Lancet 2019; 393:1619–1627.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roy CW, Marini D, Lloyd DFA, et al. Preliminary experience using motion compensated CINE magnetic resonance imaging to visualise fetal congenital heart disease. Circ Cardiovasc Imaging 2018; 11:e007745. [DOI] [PubMed] [Google Scholar]
- 52.Roy CW, Macgowan CK. Dynamic MRI of a large fetal cardiac mass. Radiology 2019; 290:288. [DOI] [PubMed] [Google Scholar]
- 53.Bhat M, Haris K, Bidhult S, et al. Fetal iGRASP cine CMR assisting in prenatal diagnosis of complicated cardiac malformation with impact on delivery planning. Clin Physiol Funct Imaging 2019; 39:231–235.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bidhult S, Töger J, Heiberg E, et al. Independent validation of metric optimized gating for fetal cardiovascular phase-contrast flow imaging. Magn Reson Med 2019; 81:495–503.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schoennagel BP, Yamamura J, Kording F, et al. Fetal dynamic phase-contrast MR angiography using ultrasound gating and comparison with Doppler ultrasound measurements. Eur Radiol 2019; 29:4169–4176.. [DOI] [PubMed] [Google Scholar]
- 56.Portnoy S, Osmond M, Zhu MY, et al. Relaxation properties of human umbilical cord blood at 1.5 Tesla. Magn Reson Med 2017; 77:1678–1690.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodríguez-Soto AE, Langham MC, Abdulmalik O, et al. MRI quantification of human fetal O2 delivery rate in the second and third trimesters of pregnancy. Magn Reson Med 2018; 80:1148–1157.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Portnoy S, Milligan N, Seed M, et al. Human umbilical cord blood relaxation times and susceptibility at 3 T. Magn Reson Med 2018; 79:3194–3206.. [DOI] [PubMed] [Google Scholar]
- 59.Portnoy S, Seed M, Sled JG, et al. Non-invasive evaluation of blood oxygen saturation and hematocrit from T 1 and T 2 relaxation times: in-vitro validation in fetal blood. Magn Reson Med 2017; 78:2352–2359.. [DOI] [PubMed] [Google Scholar]
- 60.Sun L, Macgowan CK, Sled JG, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation 2015; 131:1313–1323.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu MY, Milligan N, Keating S, et al. The hemodynamics of late-onset intrauterine growth restriction by MRI. Am J Obstet Gynecol 2016; 214:367.e1–367.e17.. [DOI] [PubMed] [Google Scholar]
- 62.Al Nafisi B, van Amerom JFP, Forsey J, et al. Fetal circulation in left-sided congenital heart disease measured by cardiovascular magnetic resonance: a case-control study. J Cardiovasc Magn Reson 2013; 15:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Porayette P, van Amerom JFP, Yoo S-J, et al. MRI shows limited mixing between systemic and pulmonary circulations in foetal transposition of the great arteries: a potential cause of in utero pulmonary vascular disease. Cardiol Young 2015; 25:737–744.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jørgensen DES, Tabor A, Rode L, et al. Longitudinal brain and body growth in fetuses with and without transposition of the great arteries. Circulation 2018; 138:1368–1370.. [DOI] [PubMed] [Google Scholar]


