Abstract
This review assesses the abuse potential of medically-administered psilocybin, following the structure of the 8 factors of the US Controlled Substances Act (CSA). Research suggests the potential safety and efficacy of psilocybin in treating cancer-related psychiatric distress and substance use disorders, setting the occasion for this review. A more extensive assessment of abuse potential according to an 8 factor analysis would eventually be required to guide appropriate schedule placement.
Psilocybin, like other 5-HT2A agonist classic psychedelics, has limited reinforcing effects, supporting marginal, transient non-human self-administration. Nonetheless, mushrooms with variable psilocybin content are used illicitly, with a few lifetime use occasions being normative among users. Potential harms include dangerous behavior in unprepared, unsupervised users, and exacerbation of mental illness in those with or predisposed to psychotic disorders. However, scope of use and associated harms are low compared to prototypical abused drugs, and the medical model addresses these concerns with dose control, patient screening, preparation and follow-up, and session supervision in a medical facility.
Conclusions:
(1) psilocybin has an abuse potential appropriate for CSA scheduling if approved as medicine; (2) psilocybin can provide therapeutic benefits that may support the development of an approvable new drug application (NDA) but further studies are required which this review describes; (3) adverse effects of medical psilocybin are manageable when administered according to risk management approaches; and (4) although further study is required, this review suggests that placement in Schedule IV may be appropriate if a psilocybin-containing medicine is approved.
Keywords: psilocybin, abuse potential, Controlled Substances Act, depression, anxiety, addiction
1. Introduction
Psilocybin (4-phosphoryloxy-N,N-dimethyltryptamine) is under development for the treatment of depression and anxiety for patients with life-threatening cancer diagnoses (Griffiths et al., 2016; Grob et al., 2011; Ross et al., 2016). Although at a more preliminary research state, promising open label results have also been reported for treatment-resistant major depression (Carhart-Harris et al., 2016a; Rucker et al., 2017) and addiction to tobacco (Johnson et al., 2014) and alcohol (Bogenschutz et al., 2015). Such treatments would be in the form of a clinically tested drug product that would provide psilocybin doses demonstrated to be safe and effective in a formulation that assures precision in dosing, which is rarely the case for illicitly consumed mushrooms (Bigwood and Beug, 1982), and in a clinical framework that would minimize the possibility of misuse or diversion. These drug formulation and intervention parameters would be addressed in an agreed upon risk management plan and would also likely be addressed in a legally binding Risk Evaluation and Mitigation Strategies (REMS) plan (U.S. Food and Drug Administration, 2015). The REMS would be based on the studies and approaches used to ensure safe and effective use and could include: a) limitations on the dose and the number of doses that could be administered to a given patient, b) administration of the drug in clinic settings with psychological support of specially trained staff, c) a variety of restrictions on distribution, access and storage, and d) a post-marketing surveillance plan to provide the FDA with timely and comprehensive communication of unintended consequences (Blanchette et al., 2015; Brandenburg et al., 2017; Dart, 2009; Dasgupta and Schnoll, 2009; U.S. Food and Drug Administration, 2015; Wu and Juhaeri, 2016).
The benefits of psilocybin in the treatment of depression, anxiety and other disorders were first suggested in the 1960s when psilocybin was marketed in many countries, including the United States (US) under the trade name Indocybin® by the Swiss pharmaceutical company, Sandoz. Indocybin® provided a shorter acting alternative to lysergic acid diethylamide (LSD) which has a similar primary pharmacological mechanism of action, now known to be agonist or partial agonist effects at the 5-HT2A receptor (Nichols, 2016). While Indocybin® was used safely as an adjunct to psychotherapy, eventually the societal backlash in the US and other countries in the 1960s (Matsushima et al., 2009) led to a ban on marketing and possession of “hallucinogenic” drugs in the US in 1965, and led Sandoz to discontinue manufacturing and marketing of Indocybin® in 1966 (Belouin and Henningfield, 2018; Bonson, 2018; Novak, 1997). The 1970 placement of psilocybin, LSD, and other “hallucinogens” in Schedule I of the CSA did not reflect an absence of therapeutic benefit, although the scientific evidence at the time was mixed. This mixed evidence included strong (at least for the time) pharmacological studies as discussed later in this review, along with clinical studies suggesting potential safety and efficacy that were nonetheless considered by leading researchers during the 1960s to be limited and not sufficient to support efficacy and safety claims for LSD or other hallucinogens. This situation is discussed by Bonson (2018) in her review of human LSD research and regulation, and would appear to generally apply to psilocybin, which was being administered by some of the same research programs that administered LSD. These limitations in the evidence base and the rising tide of sensational media accounts of adverse consequences of classic psychedelic use, discussed later, fueled the perception by many public and political leaders that psilocybin posed serious risks to patients and the public that did not outweigh its benefits (Belouin and Henningfield, 2018; Hofmann, 1980; Nutt et al., 2013). Therefore, having not been formally approved by the FDA for therapeutic use, psilocybin was placed in Schedule I of the CSA in 1970 and remains in Schedule I.1
As discussed in section 1.1, removal from Schedule I can only occur if a medicinal product containing a Schedule I substance is approved for therapeutic use as a drug by the FDA. Then, whether it will be scheduled, and, if so, into what schedule it will be placed, will be subject to the FDA’s abuse potential assessment that will include an analysis of the 8 factors of the CSA (Drug Enforcement Administration, 2017a; U.S. Food and Drug Administration, 2017a). As discussed by Calderon, Hunt and Klein in this journal issue, schedule placement is a process that considers “potential for abuse, medical use, and physical or psychological dependence liability,” among other lines of evidence (Calderon et al., 2017). For example, approval of the Schedule I compounds dextrorphan and difenoxin (with atropine) resulted in dextrophan becoming unscheduled, and difenoxin (with atropine) being placed into either Schedule IV or V, depending on dose. Similarly, the previously Schedule I compound piperazine was descheduled. Approval of an oral form of dronabinol (marinol) was initially placed in Schedule II and, in 1999, rescheduled to Schedule III, leaving cannabis and forms of dronabinol that were not approved drug products in Schedule I. As noted by Calderon et al., approved drugs with hallucinogenic effect vary widely in the scheduling from the Schedule I status of most hallucinogenic drugs without approved medical use, to Schedule II phencyclidine, Schedule III ketamine, and Schedule IV lorcaserin, and the not scheduled 2,5-dimethoxy-4-iodoamphatamine, also known as DOI (Calderon et al., 2017).
Thus, if an NDA for a psilocybin product is submitted to the FDA and approved, then the CSA would require its rescheduling, and schedule placement would be determined by evaluation of its overall abuse potential (Drug Enforcement Administration, 2017a; Henningfield et al., 2017; U.S. Food and Drug Administration, 2017a). In fact, as discussed in Belouin and Henningfield (2018) (in this journal issue), there is increasing evidence supporting the eventual development and submission of an NDA for a psilocybin-containing product. Emerging science suggesting benefits of a psilocybin product warrant an official breakthrough designation by the FDA to address the large number of cancer sufferers whose depression and anxiety are not responsive to conventional therapies (Belouin and Henningfield, 2018; Griffiths and Johnson, 2015; Ross et al., 2016). In addition, advances in risk management and monitoring, which were absent in the earlier heyday of psychedelic research, necessitate that we revisit the potential for approving a classic psychedelic (i.e., psilocybin) as a medicine because risk management, particularly in the legally binding approach of REMS, is intended to provide conditions for distribution, use, oversite and other factors to ensure safe use (McCormick et al., 2009; U.S. Food and Drug Administration, 2015).
Clinically, chemically, and pharmacologically, psilocybin has similarities with several substances that were generally termed “hallucinogens” in the 1950s and have been termed “psychedelics” since the 1960s. Although both of these terms are sometimes used to refer to compounds with other primary mechanisms of action (e.g., ketamine; salvinorin A, methylenedioxymethamphetamine or MDMA), 5-HT2A receptor agonist compounds, including psilocybin, LSD, mescaline, and dimethyltryptamine (DMT), are specifically referred to as “classic psychedelics” or “classic hallucinogens.” Although there are similarities in the effects, patterns of use and past clinical applications of LSD, psilocybin, and other classic psychedelics, the present evaluation is focused on a drug product in which the active ingredient is psilocybin. Moreover, approval would include not only the compound, but also its labeling and restrictions on manufacturing, marketing and use. These additional domains are critical to the benefit to risk evaluations which are foundational for drug evaluation and approval (U.S. Food and Drug Administration, 2017c).
Research and licit clinical use of LSD and psilocybin greatly slowed in the 1960s as amendments in 1962 and 1965 to the 1938 US Food Drug and Cosmetic Act imposed severe restrictions on distribution, possession, use, and research (Barrigar, 1964; Bonson, 2018; Grabowski, 1976; Grinspoon and Bakalar, 1979). As discussed elsewhere in this journal issue and in other publications (Nutt, 2015; Nutt et al., 2013; Scientific American Editors, 2014; Sinha, 2001; Spillane, 2004; Woodworth, 2011), legal restrictions have greatly constrained research; however, research did not altogether cease, and began to accelerate by the late 1980s in preclinical laboratories, and in clinical settings by the late 1990s. This resurgence has been fueled in part by renewed appreciation of the potential importance of these substances in advancing the science of the brain and behavior and for their potential significance in the treatment of disease. Moreover, since the 1970s extensive national drug use and effects surveillance systems have been developed in the US, which show that the prevalence of abuse and serious adverse events associated with psilocybin and other classic psychedelics are relatively low compared to other major classes of abused drugs (Johnson, Hendricks, Barrett, Griffiths, submitted). In addition to the more recent clinical research, the reassuring results from these epidemiological data also increase interest in the evaluation of psilocybin as a potential therapeutic medicine (Roseman et al., 2017; Rucker et al., 2017). Because the FDA approved therapeutic medicines cannot be listed in Schedule I of the CSA, consideration of changes in scheduling recommendations becomes an important part of the clinical development of psilocybin. As discussed in this review the evidence continues to support the conclusion that if a psilocybin drug product was approved by the FDA, CSA scheduling would remain appropriate. Considerable additional study will be required for the development of an FDA-acceptable NDA, including the abuse potential assessment section of the NDA according to the FDA’s abuse potential assessment guidance (U.S. Food and Drug Administration, 2017a). Thus, it is premature to come to a definitive conclusion about which schedule would be most appropriate. This review is intended to stimulate further research and thinking in this area through its evaluation of key abuse potential-related science presently available and considered through the approach of the CSA 8-factor analysis which is the key approach of the CSA for developing scheduling recommendations. The review includes a preliminary scheduling conclusion based on the research considered and the opinions of these authors, along with key gaps in the research that will also likely be of importance to the FDA.
1.1. Abuse potential and drug scheduling in the context of the CSA
The scheduling process for new drugs officially commences upon approval of the product by the Controlled Substances Staff (CSS) of the FDA, who provide an 8-factor analysis based, in part, on the sponsor’s submission of an NDA that includes the sponsor’s abuse potential assessment that has been prepared according to the recommendations in the FDA’s guidance for sponsors: Assessment of the Abuse Potential of Drugs (U.S. Food and Drug Administration, 2017a). The FDA obtains review and input from the National Institute on Drug Abuse (NIDA). Then, the Assistant Secretary of the US Department of Health and Human Services transmits her/his recommendation to the Drug Enforcement Administration (DEA) within the Department of Justice (DOJ). Since the spring of 2016, the schedule recommendation by the Department of Health and Human Services must be accepted and finalized by the DOJ/DEA within 90 days unless there is a compelling basis for placement in a different schedule (U.S. Congress, 2015). Finalization of the scheduling action will follow the standard federal rulemaking process (U.S. Food and Drug Administration, 2015; U.S. Office of the Federal Register, 2011).
The scientific assessment of the abuse potential (also commonly referred to as “abuse liability” and “addiction potential”) is based on the scientific evaluation of substances going back to the early twentieth century search for less abusable analgesics (Jasinski et al., 1984). By the 1960s such evaluations included stimulants, sedatives, and psychedelics. This science and its methods of assessment, along with other considerations including population level public health impact, were brought together in the 1970 CSA in the form of 8 specific factors for the assessment of what was then termed “abuse potential.” That term recognized that problematic use of substances could occur in people who were not physiologically dependent or addicted, and by drugs (e.g., cocaine, cannabis, LSD and psilocybin) for which it was unclear (at the time) if they posed a physiological dependence risk.
Analysis of all 8 factors is required to guide the FDA and DEA recommendations for CSA scheduling of approved medicines (Drug Enforcement Administration, 2017a; U.S. Food and Drug Administration, 2017a). Consistent with the observations that abuse potential varies widely across substances, approved medicines can vary from control in Schedule II to Schedule V (i.e., C-II to C-V), in which C-II is for those of greatest concern (e.g., cocaine, morphine, and phencyclidine), C-V is for those of sufficient concern to warrant control but for which abuse potential appears lowest among controlled substances (e.g., low dose codeine in combination with acetaminophen, lacosamide, and pregabalin). Of intermediate concern for control is Schedule IV, which includes diazepam, mazindol and tramadol, and Schedule III, which includes dronabinol, ketamine, and nalorphine.
1.1.1. FDA is the sponsors’ focal point for the NDA including its abuse potential assessment
The FDA is the focal point for abuse potential assessment, and works with the sponsor to determine the range of studies needed to enable its review of the NDA in order to determine approvability, the scheduling recommendation, and all aspects of labeling (some of which are based on the abuse potential assessment and scheduling). The NDA’s abuse potential assessment submission required by FDA is comprised of 5 modules that include the sponsor’s scheduling proposal and rationale in Module 1, and a summary and thorough discussion of all abuse related nonclinical and clinical data in Module 2. Modules 3, 4 and 5 include complete study protocols and data addressing chemistry, in vitro and nonhuman pharmacology, and clinical studies including the integrated summary of safety (ISS), respectively. The sponsor need not submit an 8-factor analysis but sponsors often include one in their module 1 rationale.
The present 8-factor analysis benefits from the fact that psilocybin is not a new chemical entity devoid of real world (i.e., “community”) data. Rather we have been able to draw from more than a half century of research and various types of therapeutic use, as well surveillance epidemiology. However, it suffers from the fact that most of the research has not been conducted as part of a cohesive sponsored drug development program that had FDA input throughout much of development. Thus, in this review we attempt to note particular strengths and weaknesses in studies and gaps in the study portfolio that will likely need to be addressed before filing an NDA.
2. Evaluation of the abuse potential of psilocybin according to the 8 factors of the CSA
The following 8-factor evaluation of psilocybin may be considered a substantially abbreviated effort compared to the 100–200 page Module 1 and Module 2 abuse potential assessment submitted as part of a potential new drug application, though substantially more detailed than the summary 8-factor analysis that might be prepared by the FDA and published by DEA in the US Federal Register in support of their scheduling recommendations (Drug Enforcement Administration, 2002, 2013, 2014, 2017b).
2.1. Factor 1. Actual or relative potential for abuse
Although the 1970 placement of psilocybin in Schedule I impeded research, more than a half century of research, clinical experience, and surveillance provide a substantial basis for evaluating the abuse potential of psilocybin according to Factor 1 and the seven additional factors. This experience has shown that psilocybin does have a potential for abuse, with preclinical and clinical studies providing information about this potential for abuse relative to other substances, scheduled and nonscheduled.
2.1.1. Preclinical studies
Psilocybin has been evaluated in a variety of preclinical models of physical dependence and abuse potential, yielding qualitatively generally similar findings with LSD. These similarities included increased pulse, respiratory rate, and pupil diameter but no physical dependence or withdrawal (Martin, 1973). Preclinical models of abuse potential suggest weak reinforcing effects and weak stimulus generalization to substances of high abuse potential (Baker, 2017; de Veen et al., 2017; Fantegrossi et al., 2008). For example, Fantegrossi, Woods and Winger (Fantegrossi et al., 2004) evaluated the classic psychedelic compounds N,N-dimethyltryptamine (DMT), mescaline, and psilocybin in rhesus monkeys with histories of self-administering 3,4-methylenedioxymethamphetamine (MDMA), a compound which is not a classic psychedelic but which produces some overlapping subjective effects in humans (Studerus et al., 2010). As shown in Figure 1 generated reliable self-administration, none of the classic psychedelics generated reliable self-administration though during occasional sessions, animals self-administered all available doses and appeared intoxicated post-session. The study authors concluded “… the present data provide further evidence that several classic psychedelic drugs from two distinct structural classes do not reliably maintain contingent responding in rhesus monkeys. This pattern of sporadic self-administration may indicate that these compounds have weak reinforcing effects, or, alternatively, mixed reinforcing and aversive effects.”
Figure 1:
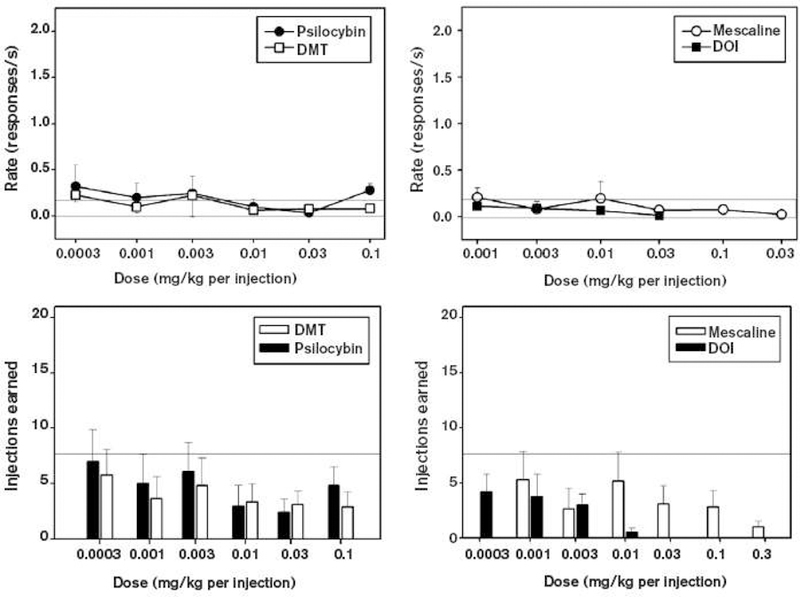
The two upper panels show mean response rates (±SEM) during self-administration of classic psychedelic compounds by rhesus monkeys making lever presses under an FR-30 schedule of reinforcement. Left panel shows psilocybin and DMT; right panel shows mescaline and 4-iodo-2,5-dimethoxyphenylisopropylamine (DOI). The two bottom panels show the corresponding mean number of injections earned (±SEM) during these self-administration sessions. For all panels, the light horizontal lines show the range for saline response rates (upper panels) and saline injections earned (bottom panels; with the bottom of the range at 0). For all panels, n=4. Figure from Fantegrossi et al, 2004, Figure 1)
The apparent weak reinforcing effects of psilocybin and other classic psychedelics may account for why there have been relatively few nonhuman studies examining reinforcement models. In contrast, many more nonhuman research studies with classic psychedelics have used drug discrimination models. Discriminative stimulus effects refer to the ability of a drug, upon administration, to serve as a cue that can predict environmental contingencies, e.g., which of two levers will result in the delivery of a reward if pressed. Discriminative stimulus effects can therefore be thought of as the ability of the drug to be recognizable to the organism (and therefore serve as a cue). Discriminative stimulus effects are different from reinforcing effects, and have different biological bases (Johnson and Ettinger, 2000). Discriminative stimulus effects may be relevant to drug reinforcement when a test drug reliably substitutes in discrimination testing for a drug with well-established reinforcing effects, e.g., when a drug reliably substitutes for amphetamine. In such cases it is likely (although not certain) that the test drug will also be shown to be reinforcing when directly tested with self-administration procedures. Discrimination studies have strongly contributed to our understanding of psilocybin and other classic psychedelics. For example, Harris and Balster compared psilocybin to amphetamine in a rodent model for assessing behavioral and discriminative effects (Harris and Balster, 1971). They found that psilocybin served as a discriminative stimulus but that these stimulus-control effects were weak compared to amphetamine. Schechter and Rosecrans (Schechter and Rosecrans, 1972) employed a T-maze discrimination procedure and found psilocybin and mescaline, but not amphetamine, reliably substituted for LSD in rats trained to discriminate LSD from saline. Similarly, another study found the psilocybin failed to substitute for amphetamine in rats trained to discriminate amphetamine from saline (Kuhn et al., 1974). In another study rats trained with psilocybin generalized fully to psilocin (the active metabolite of psilocybin) and to LSD but not to mescaline, which is considered a classic psychedelic of the phenethylamine-based structural class rather than the tryptamine-based structural class of which psilocybin is a member (Cunningham and Appel, 1987; Koerner and Appel, 1982). Another study, however found that psilocybin fully substituted for mescaline in rats trained to discriminate mescaline from saline (Appel and Callahan, 1989). A study in pigeons found psilocybin to fully substitute for LSD in LSD trained subjects (Jarbe, 1980).
Winter, Rice, Amorosis and Rabina (Winter et al., 2007) evaluated psilocybin and other classic psychedelics following treatment with several antagonists for specific serotonin receptor subtypes. They concluded: “the present data indicate that the stimulus properties of psilocybin in the rat are broadly compatible with those of other ergoline, indoleamine, and phenethylamine classic psychedelics. However, significant differences are apparent as well” and “psilocybin induces a compound stimulus in which activity at the 5-HT2A receptor plays a prominent but incomplete role” and “the full generalization of psilocybin to LSD and to DOM is completely blocked by the selective 5-HT2A receptor antagonist, M100907, but stimulus control by psilocybin is only partially antagonized by M100907” (Halberstadt and Geyer, 2011; Winter et al., 2007).
These studies confirm that psilocybin produces discriminative effects that do not generalize to amphetamine, and psilocybin does not substitute in amphetamine trained animals. Moreover, psilocybin discriminative effects are likely mediated by psilocin, the active metabolite produced in vivo by dephosphorylation of psilocybin (Passie et al., 2002). In addition, findings demonstrate that psilocybin produces weak and transient reinforcing effects that are consistent with community level observations (also see Factor 4) suggesting that the vast majority of people who have used psilocybin do not develop compulsive patterns of use. Instead, more typically individuals report only a few uses of psilocybin, consistent with a substance of low overall abuse potential. The findings also suggest a need for additional studies to better understand the mechanisms of action of psilocybin and other psychedelic substances and how these may contribute to their apparent low overall abuse potential (Baker, 2017; Hayes and Greenshaw, 2011).
2.1.2. Human abuse potential assessment.
Psilocybin has not been examined in an abuse potential study that would meet the criteria recommended by the FDA in its 2017 Guidance: Assessment of the Abuse Potential of Drugs; however, many clinical laboratory studies have been conducted since the mid-1950s in which key measures of abuse potential have been assessed. This work began at the US Public Health Service Addiction Research Center (ARC) of the National Institute of Mental Health, during the time that the methods of human abuse potential were being developed. Studies with psilocybin and LSD contributed to the development of abuse potential assessment methods, in part because it was quickly recognized that they differed in several key respects from opioids, sedatives, and stimulants which were then emerging as prototypic substances of abuse. In contrast to these drugs, any abuse potential-related effects associated with LSD, psilocybin, and related substances appeared to be unreliable and limited to specific conditions such as time of assessment, dose, and individual, social and experiential factors. In further contrast, the predominant and most reliable effects seemed to be effects thought to limit use and abuse (e.g., fear, anxiety, dysphoria, and physical discomfort including gastrointestinal upset). Thus, a leading addiction scientist and director of the ARC, Dr. William Martin, stated the following in a 1973 review of preclinical studies of psychedelic drugs: “The abuse of LSD-like hallucinogens came as somewhat of a surprise to many of the early experimenters with these drugs” (page 149)(Martin, 1973). Nonetheless, while he did acknowledge that certain doses of LSD could produce pleasure in some volunteers (Belleville et al., 1956), Martin’s 1973 review indicated that most of the preclinical and clinical findings of the 1950s and 1960s were not indicative of a prototypic drug of abuse.
Psilocybin studies at the ARC commenced a few years following studies of LSD, with the first human reports published in 1959 by Isbell (Isbell, 1959a, b). The initial studies occurred early in the development of human abuse potential assessment research when human volunteers with histories of substance abuse were evaluated for potential euphoriant effects, which were considered predictive of abuse potential (Isbell, 1956). These studies contributed to the development of human abuse potential assessment as measures evolved to characterize not only the euphoriant effects that characterized opioids and stimulants, but also the dysphoric effects that distinguished classic psychedelics such as LSD and psilocybin. At the same time theories of addiction and addiction liability assessment were evolving from the focus on physical dependence and withdrawal that had dominated the prior few decades of opioid-focused studies to a greater focus on the acute subjective and behavioral effects of drugs that contributed to their self-administration and abuse, regardless of whether physical dependence and withdrawal were evident (Isbell, 1956; Wikler, 1961).
During the 1950s and 1960s, the ARC demonstrated that among the strongest predictors of abuse potential was the reliable and dose-related production of euphoriant effects as measured by self-reported, and observer-evaluated effects including liking of the drug, apparent pleasure, confidence, and sense of well-being (Isbell, 1956). These findings led to development of systematic approaches to the assessment of drug liking, drug type identification, and frequent physiological correlates including pupil diameter and withdrawal symptoms (Fraser et al., 1961; Jasinski and Henningfield, 1989; Jasinski et al., 1984). The methods developed have continued to be refined over the past half century and remain the foundation for human abuse potential assessment studies (Carter and Griffiths, 2009; Griffiths et al., 2003; U.S. Food and Drug Administration, 2017a).
In the early 1960s, an important addition to the study of human abuse potential was the development of the ARC Inventory (ARCI), a participant-completed questionnaire. Studies of LSD and psilocybin contributed to the development of this questionnaire and a broader understanding of abuse (Haertzen and Hickey, 1987; Haertzen et al., 1963; Hill et al., 1963). Table 1 provides more background on the ARCI and its importance in characterizing the abuse potential of LSD and psilocybin. The full ARCI contained more than 500 items, however, 49 items or fewer were found to provide valid and reliable characterization of abuse-related qualitative effects of several categories of drugs with various subscales emerging from studies of drug administration in human volunteers. The most prominent predictor of abuse potential was the Morphine Benzedrine Group (MBG) scale that came to be accepted as an important measure of euphoria. In contrast, a scale that was derived from LSD studies, the LSD scale, came to be known as the dysphoria and psychotomimetic scale, which captured fear and anxiety and seemed to predict low abuse potential. LSD and psilocybin most reliably elevated scores on the LSD scale, but frequently also, at a certain dose and in some individuals, elevated scores on the MBG scale, but generally at a lesser magnitude than opioids and stimulants (Haertzen and Hickey, 1987; Jasinski and Henningfield, 1989; Jasinski et al., 1984).
Table 1.
The Addiction Research Center Inventory
| Through the 1950s the term for assessing potential addictive and abuse-related drug effects was “addiction liability” assessment and the major focus of assessment was on the development of tolerance and the emergence of withdrawal signs and symptoms upon discontinuation of drug administration (Himmelsbach and Andrews, 1943). In the late 1950s Isbell, Frazier and colleagues at the ARC came to conclude that the mood and behavior altering effects of drugs contributed to and were predictive of the risk of abuse and addiction and that these could be evaluated by psychometric instruments. The simplest and most commonly relied upon measure in human abuse potential studies to support new drug applications to the FDA is the drug liking scale that was originally a five-point scale in which subjects rated their liking of the drug from 0 (not at all ) to 4 (an awful lot). This scale development benefitted from the then recent observations of Beecher (Beecher, 1952, 1957) who demonstrated that such scales could be used to reliably assess pain and analgesia (Beecher, 1952, 1957; Lasagna et al., 1955). Such positive mood alterations could be produced by drugs of abuse that were not then known to produce physical dependence and withdrawal, and by single doses of opioids in former opioid users (referred to as “post-addicts”) who were no longer physically dependent (Jasinski, 1977; Jasinski and Henningfield, 1989; Jasinski et al., 1984; U.S. Food and Drug Administration, 2017a). |
| As predominant theories of addiction at the time included the potential importance of personality disorders, a psychologist who was expert in the Minnesota Multiphasic Personality Inventory and testing, Charles Haertzen, was hired in 1959, to take the lead in developing a comprehensive instrument to better characterize and differentiate the several categories of substances that were abused as well as the personality characteristics of those who used them. The resulting Addiction Research Center Inventory (ARCI) contained more than 500 true and false items, but shorter versions containing 40 or 49 items were most commonly used in human abuse potential studies. The ARCI scale that provided the most robust indicator of high abuse potential was the Morphine Benzedrine Group (MBG) scale, commonly referred to as the “euphoria” scale because it was empirically derived based on the response of volunteers to the prototypic euphoriants morphine and Benzedrine® (hence, the MBG scale) which produced robustly elevated mood and feeling states. In contrast, a scale based on responses to LSD (LSD scale) was distinguished by a cluster of items, that included unpleasant, dysphoric, or psychotomimetic responses to LSD (hence the LSD scale) that were associated with a lower propensity to compulsively or frequently self-administer the substance; it was often referred to as the “dysphoria” scale (Haertzen and Hill, 1963; Jasinski et al., 1984). It also included scales based on clusters of items that were associated with amphetamine administration (the A scale) and one that reflected the somewhat overlapping and sedating effects of pentobarbital, chlorpromazine, and atropine group of drugs (the PCAG scale). Most drugs of high abuse potential produced elevations in the scores on the MBG scale as well as on the specific scale that reflected their pharmacological class. Thus, alcohol, barbiturates, opioids, and stimulants could all increase MBG scale robustly as well as the scale that was specific to their class. Chlorpromazine and atropine, by contrast, which were rarely abused, did not reliably elevate MBG scale scores but might elevate LSD scale scores. LSD elevated LSD scale scores and sometimes elevated MBG scale scores and liking scores, reflecting their overall low abuse potential and diverse effects that can range from fear and anxiety to pleasure, depending much on dose, time since drug, experience, and other factors (Griffiths et al., 2008). |
| Examples of a few of the items that distinguished drugs likely to elevate scores on the MBG scale as compared to items characterizing the LSD scale are the following: “I would be happy all the time if I felt as I do now” - scored positively on the MBG scale and negatively on the LSD scale; “I am in the mood to talk about the feeling I have” and “I feel more clear-headed than dreamy” - were both score positively on the MBG scale and were not included on the LSD scale. The LSD scale also contained numerous items reflective of mixed mood effects, e.g., “I feel anxious and upset” and “I have a weird feeling” – both scored positively; negatively scored items included “I feel very patient”, and “My movements are free, relaxed and pleasurable”; and, items reflective of introspection and negative feelings included “I have a negative disturbance in my stomach”, “Some parts of my body are tingling”, and “It seems I’m spending longer than I should on each of these questions” (Haertzen and Hickey, 1987; Jasinski and Henningfield, 1989). |
| Over more than 50 years of research, it became clear that drugs with the highest overall abuse potential were those that produced robust increases in scores on drug liking scale and the MBG scale, and low effects on the LSD scale (Griffiths et al., 1980; Griffiths and Balster, 1979; Haertzen and Hickey, 1987; Jasinski and Henningfield, 1989; Jasinski et al., 1984). Liking scales have since evolved into the more commonly used 100-point (or 100mm) visual line analog scales and the ARCI often replaced with scales to assess positive (pleasant) and negative (unpleasant) effects as described in early 2000 expert reviews and advised by FDA in its abuse potential assessment guidance (Carter and Griffiths, 2009; Griffiths et al., 2003; U.S. Food and Drug Administration, 2017a). |
| The ARCI helped elucidate a major difference in nature and magnitude of the abuse potential that is associated with psychedelics, as compared to substances that carry a high risk of compulsive patterns of repetitive use and abuse including amphetamine, cocaine, the cigarette form of nicotine delivery, prototypic opioids, and sedatives, as compared to substances with substantially lower potential for compulsive use and abuse, such as LSD and psilocybin (see also Table 1). |
A seminal study that was that published by Isbell in 1959 found that psilocybin produced qualitatively similar effects to LSD with spontaneously reported onset of subjective effects at about 10-15 mins following oral ingestion (Isbell, 1959a). In contrast to the initial euphoric effects that characterized opioids, stimulants, sedatives, and cannabis, Isbell found that the initial effects of psilocybin were more likely to include anxiety along with altered sensations. These effects were often followed within the next 15 min by increasingly strong anxiety, and fear, visual distortions and difficulty thinking, though some subjects experienced elation and expressed “continuous gales of laughter” (page 32). He concluded that LSD was approximately 100-150 times as potent as psilocybin on subjective effects and physiologic measures including increased pupil diameter, heart and respiratory rate, and reduced threshold of the patellar reflex, with similar time course of onset but shorter duration of effects by psilocybin compared to LSD. Additional ARC studies are described in factor 2 as they pertain to understanding the mechanisms of action of psilocybin.
2.1.3. Clinical trials relevant to abuse potential assessment since 2000.
Since 2000 there have been several clinical trials that have included measures related to the assessment of abuse potential. For example, one study (Griffiths et al., 2011) showed that all four oral doses of psilocybin examined (~0.071, ~0.143, ~0.286, and ~0.429 mg/kg) produced statistically significant increases over placebo for both the A (amphetamine) scale and LSD scales of the ARCI. The MGB scale did not significantly differ between placebo and psilocybin at any dose. Another study (Bogenschutz et al., 2015) included a short form of the ARCI. Unfortunately, the open label study was neither placebo controlled, nor did it include a positive control for comparison. Such conditions are especially important for drugs that produced mixed and weak signs of abuse potential. Nonetheless, their findings were typical of those previously observed for psilocybin and LSD. The authors observed weak elevations of both the MBG and LSD scales following oral administration of 0.3 and 0.4 mg/kg psilocybin, in volunteers with histories of alcohol dependence. Whereas these effects do not indicate substantial abuse potential, they cannot be used to rule out significant potential for abuse because in the absence of comparators, the weak MBG effect might be related to the population and other design aspects of the study. This study, like others discussed in Factor 6 (Griffiths et al., 2016; Ross et al., 2016) also documented reports of acute elevations in fear and anxiety in some patients that are predictive of low abuse potential as well as a subsequently emerging sense of contentment that is not associated with a strong motivation to use repeatedly and chronically. It is also important to note that these recent studies have gone to further lengths to maximize the pleasantness of the physical environment and establish interpersonal rapport between participants and staff (Johnson et al., 2008) compared to the older ARC studies. Therefore, MBG scores in these recent studies might overestimate the drug euphoria that would be experienced in a less than optimal environment. As in Factor 6, the mixed acute subjective effects of psilocybin included fear, anxiety, pleasure, happiness and contentment, and thus are consistent with those of the early 1960s from the ARC, however, these studies were not designed as human abuse potential studies and the putative abuse potential related effects must be interpreted cautiously. In particular, the participants in the recent cancer trials (Griffiths et al., 2016; Ross et al., 2016) were patients with severe anxiety and or depression whose therapeutic improvements in mood were long-lasting and not necessarily reflective of abuse potential.
2.2. Factor 2. Scientific evidence of its pharmacological effect
It has been estimated that there were more than one thousand scientific and clinical studies of classic psychedelics including LSD and psilocybin published through the 1960s (Drug Enforcement Administration, 1995; Grinspoon, 1981; Grinspoon and Bakalar, 1979; Johnson and Griffiths, 2017), and several thousand more published since the 1960s (Sellers et al., 2017).
Initial conclusions drawn by ARC researchers have been replicated by others as discussed in various reviews (Johnson et al., 2008; Nichols et al., 2017). In brief, in addition to physiological and behavioral effects discussed in Factor 1, it was demonstrated that repeated dosing produces diminished effects (tolerance) and that cross-tolerance occurs between psilocybin and LSD (Abramson et al., 1960; Isbell et al., 1961), but not to tetrahydrocannabinol (THC) indicating different mechanisms of action (Isbell and Jasinski, 1969). Effects of psilocybin are qualitatively similar to those produced by mescaline, however, mescaline is less potent but longer acting (Wolbach et al., 1962). The effects of psilocin are the same as those by psilocybin except that it is more potent and shorter acting than psilocybin (Isbell et al., 1961). It is now understood that psilocybin is a pro-drug, converted by dephosphorylation to the pharmacologically active psilocin (Nichols et al., 2017; Passie et al., 2002). Strong early support for this contention was provided by data showing that although psilocin is slightly more potent than psilocybin, the ratio difference in potency between the two compounds (in both humans and nonhumans) is nearly identical to the ratio of their respective molecular weights (i.e., they are equipotent on a molecular basis) (Koerner and Appel, 1982; Wolbach et al., 1962). Isbell and Logan (1957) demonstrated that chlorpromazine administration reduced and could partially reverse the effects of LSD. Nonetheless, the pharmacology and mechanisms of action of psilocybin and LSD are similar in many respects, although psilocybin is shorter acting and at least 100 times less potent than LSD (Isbell, 1959a; Sellers et al., 2017). Research has also shown the 5-HT2A antagonist ketanserin to block most of the effects of psilocybin (Kometer et al., 2012; Kometer et al., 2013; Quednow et al., 2012; Vollenweider et al., 1998), although ketanserin does not block certain psilocybin effects including the slowing of binocular rivalry, reductions in arousal/vigilance (Carter et al., 2007), and attentional impairment (Carter et al., 2005).
More than 100 species of mushrooms, in the genus Psilocybe, contain psilocybin (Johnson and Griffiths, 2017; Stamets, 1996). Its agonist activity at the 5-hydroxytryptamine (HT)2A receptor appears to account partially for its behavioral effects, however, the mechanisms of action of its full range of effects have not been fully elucidated (Nichols, 2016; Winter et al., 2007). Psilocybin is a substituted indolealkylamine and with diverse serotonergically mediated effects and little affinity for dopamine D2 receptors (Halberstadt and Geyer, 2011; Passie et al., 2002). It is among the structural class of classic psychedelics based on the tryptamine structure, including an indole ring (Passie et al., 2002). Albert Hofmann, the discoverer of LSD and chemist at the Swiss Sandoz Pharmaceutical Company, isolated psilocybin from Central American mushrooms (Psilocybe mexicana) in 1957, and synthesized the substance in 1958 (Passie et al., 2002). Its binding to and agonist effects at 5-HT2A serotonin receptors are associated with dilation of the pupils (mydriasis), reduced threshold for knee reflex, and commonly increased heartrate and blood pressure, and feelings of nausea (Isbell, 1959a, b). Its effects on mood and feeling can include visual and auditory hallucinations and distortion of visual and auditory stimuli, altered temporal sense, and alteration of body image. Its effects have the potential to mimic psychotic states which contributed to its designation, along with LSD, as a psychotomimetic. The effects that contribute to introspection and often increased receptivity to advice and psychotherapy contributed to its use in psychotherapy, as well as to investigations by psychologists and psychiatrists in efforts to better understand the moods and states of their patients (Hofmann, 1980; Matsushima et al., 2009; Passie et al., 2002).
Studies of LSD began in the 1940s with many of the same laboratories, including Sandoz, investigating the generally similar-acting psilocybin in the 1950s and 1960s. However, as discussed above in Factor 1, caution must be made in generalizing findings, including mechanisms of action, from LSD to psilocybin and vice versa. The resurgence of research beginning slowly in the 1970s and accelerating in particular since the 1990s has been rapidly increasing the understanding of the effects and mechanisms of action of psilocybin, including its general safety and the conditions of safe use (Griffiths et al., 2008; Nichols et al., 2017).
2.2.1. Tolerance and physical dependence
Tolerance refers to decreased response with repeated administration of a drug. Tolerance to the psychological and physiological effects of psilocybin is strong. Moreover, there is cross-tolerance between psilocybin and LSD. However, physical dependence and withdrawal, which refer to adverse effects upon discontinuing repeated use of a drug, have not been documented (Abramson et al., 1956; Abramson and Rolo, 1965; Balestrieri, 1967; Isbell, 1959a; Isbell et al., 1961; Passie et al., 2002; Wolbach et al., 1962). It is plausible that the FDA would recommend that sponsors collect a more rigorous evaluation of physical dependence and withdrawal in animals consistent with its 2017 abuse potential guidance, perhaps as part of a safety evaluation of high dosages. However, it is also plausible that the FDA might not require such additional studies given that there is little evidence that psilocybin produces physical dependence and withdrawal, and the treatment protocols under investigation would not involve repeated daily dosing.
2.2.2. Toxicity
Unlike prototypic opioids and sedatives of abuse, psilocybin carries a low risk of overdose toxicity by respiratory depression or cardiovascular events or other causes of death associated with substances of abuse. The LD50 of intravenous psilocybin has been determined to be above 250 mg/kg (with 200 mg/kg killing no animals, and 250 mg/kg killing a small portion of animals (Cerletti, 1958). Its lethal dose in humans has been theoretically estimated at approximately 1000 times an effective dose (Gable, 2004), which is an amount that is likely not possible for an individual to consume when in the form of psilocybin-containing mushrooms. The authors are aware of only one documented case of acute overdose poisoning death likely caused by psilocybin (Lim et al., 2012). Specifically, a 24-year old female, who had received a heart transplant 10 years prior due to end-stage rheumatic heart disease, experienced cardiac arrest 2–3 hr after consuming psilocybin-containing mushrooms, and subsequently died. Toxicology revealed only psilocin (active metabolite of psilocybin) and THC. Thus, the only known acute fatal overdose from psilocybin appears to be in a medically compromised individual. Given psilocybin’s moderate pressor effects, individuals with such serious cardiac vulnerability would be excluded from recently approved psilocybin trials and should be excluded from any potential non-research future approved clinical use.
One study examined isolated nonhuman animal organs and found no significant effect in the rat uterus or the guinea pig duodenum or seminal vesicle (Cerletti, 1958). Administering relatively large doses to waking nonhuman animals of a variety of species led to acute autonomic effects including mydriasis, piloerection, hyperglycemia, hypertonia, and pulse and breathing irregularities (Cerletti, 1958), with similar effects later observed in Rhesus macaques (Horibe, 1974; Passie et al., 2002). A micronucleus study in mice found no evidence that psilocybin administration resulted in chromosome breaking (Van Went, 1978).
Hollister reported that human administration of psilocybin resulted in decreased urinary excretion of inorganic phosphorus and reduced circulating eosinophil levels, as well as pupillary dilation and increased deep tendon reflexes (Hollister, 1961). In addition, Hollister (1961) reported on a single participant who was administered psilocybin on a daily basis for 22 days, with doses ranging from 1.5 to 27 mg per day. Before and during that course of administration, no chronic changes were observed for any metric assessed: total leukocyte count, absolute eosinophil count, hemoglobin, curea nitrogen, creatinine, glucose, serum proteins, cholinesterage activity, serum glutamic-oxaloacetic transaminase titer, cholesterol and EEG tracing. Gouzoulis-Mayfrank et al. found that human psilocybin administration resulted in no change in cortisol, prolactin, or growth hormone (Gouzoulis-Mayfrank et al., 1999). Johnson et al. found that in a within-subject, double-blind, placebo-controlled study, oral psilocybin (0, ~0.071, ~0.143, ~0.286, and ~0.429 mg/kg) caused headaches which were dose-dependent in terms of incidence, duration, and severity (Johnson et al., 2012). Headaches had delayed onset relative to subjective drug effects, were transient, and ceased within 24 hr of psilocybin administration. Although mechanisms response for these delayed onset headaches are not known, one possible mechanism is nitric oxide release.
2.2.3. Pharmacodynamics
The acute effects of psilocybin have been studied in animals and humans over a broad range of doses over several decades (Isbell et al., 1961; Johnson et al., 2008; Nichols et al., 2017; Wolbach et al., 1962). Like other classic psychedelics, the acute psychological effects following psilocybin administration are varied and often intense, although strongly dose-dependent and dependent on the interpersonal and physical environment (Griffiths et al., 2011; Hasler et al., 2004; Johnson et al., 2008). These psychological effects often include perceptual changes that are primarily visual but can also include synesthesia across sense modalities, emotional changes in which both positive and negative emotions can be far more intense than normal, cognitive changes that can include alterations in time perception, and an introspective focus on personal history, life relationships and circumstances, and changes in sense of self (Johnson et al., 2008). In a retrospective analysis of 409 psilocybin administrations to 261 healthy participants by a single research group, a few interpersonal factors among many were found to influence psilocybin response (Studerus et al., 2012). Specifically, high trait absorption scores, being in an emotionally excitable and active state before administration, and having fewer recent psychological problems all predicted pleasant and mystical-type effects, while high trait emotional excitability, younger age, and a PET imaging setting, all predicted unpleasant or anxious effects (note that pleasant and unpleasant effects within the same session are not mutually exclusive).
The early studies by Isbell and colleagues documented the time courses of onset of autonomic and psychological effects, generally beginning within 30 min of oral ingestion, peaking within 1–2 h, and subsiding over the next few hours, with a duration of action shorter than those produced by LSD and mescaline (Wolbach et al., 1962). Since 2000, several studies have been conducted in which the pharmacodynamics have been evaluated over multiple measures and doses. Hasler et al. investigated the acute psychological and physiological effects of oral psilocybin in a double-blind, placebo-controlled study in healthy volunteers at dose of 0, 0.045, 0.115, 0.215, and 0.315 mg/kg administered in a cross-over design at intervals of at least two weeks (Hasler et al., 2004). Measures included cardiovascular variables, plasma concentrations of a several hormones, and several measures of mood, subjective response and behavioral performance. Blood samples were collected pre-dosing and at 105 and 300 min post-administration. Blood pressure was measured 30 min pre-dosing and at 5, 30, 60, 90, 120, 165, and 210 min post-administration. Electrocardiograms (EKG) were continuously monitored for 24 hr. The main findings were orderly dose- and time-dependent effects that were significantly altered at many measures and timepoints. Subjective effects began to onset about 20–40 min post-administration, peaking at about 60–90 min and diminishing over the next 60–90 min. One subject became markedly anxious at the 0.315 mg/kg dose and his anxiety gradually subsided to complete resolution within 6 hr after drug administration. No significant changes were observed in EKG or body temperature, but prolactin, thyroid-stimulating hormone, adrenocorticotropic hormone, and cortisol were increased by at least the 0.315 mg/kg dose. Another dose effect study of psilocybin ranging into higher doses examined 0, ~0.071, ~0.143, ~0.286, and ~0.429 mg/kg using a placebo-controlled, double-blind, crossover design (Griffiths et al., 2011). Sessions were 1 month apart, and a 14-month follow-up was conducted. Acute psychological effects largely replicated those shown in the earlier study, with time course data showing orderly dose- and time-related effects. In addition, this study found that 39% of participants reported extreme anxiety/fear for at least one of the two highest doses. End of session data showed psilocybin caused significant dose-related increases in mystical experience using the Mystical Experience Questionnaire. Moreover, a month after sessions, the experiences associated with the two highest doses were rated as having substantial personal and spiritual significance. Participants attributed improvements in attitudes, mood, and behavior to the two highest doses. At the 14-month follow-up, such ratings were largely unchanged from ratings made a month after each session. Improvements in attitudes, mood, and behavior were also observed in dose-blinded community members who had regular contact with participants.
More recently, two clinical trials discussed below in Factor 6 (Griffiths et al., 2016; Ross et al., 2016) also documented the time course of several physiological, mood and behavioral variables. However, persisting for far longer than these acute effects were the therapeutic effects. Specifically, both studies showed that psilocybin caused significantly and clinically significant reductions in symptoms of depression and anxiety lasting at last 6 months after psilocybin administration. Griffiths et al. studied patients with clinical anxiety and depression related to their life-threatening cancer diagnoses (Griffiths et al., 2016). Informed by data from previous psilocybin dose effects studies (Griffiths et al., 2011; Hasler et al., 2004) they compared a moderately high dose (~0.314 or ~0.429 mg/kg) to a dose sufficiently low that it was expected to be devoid of therapeutic effects (~0.014 or ~0.043 mg/kg), using a randomized, double-blind, cross-over counterbalanced design. The two doses were administered 5 weeks apart, and participants returned for 6-month follow-up. Measures of mood, attitudes, and behaviors were self-reported by participants and rated by staff and community observers throughout the study. On drug administration days, research staff were present with the patients continually during the approximately 7–8 hr long experimental session that included a battery of physiological, subjective and behavioral measures 10 min before capsule administration, repeated 30, 60, 90, 120, 180, 340, 300, and 360 min after oral capsule administration. As shown in Figure 2, there were significant dose and time-related effects on most measures including non-clinically severe increases in heart rate and blood pressure, and observer-rated anxiety, nausea, joy/intense happiness, peace/harmony, psychological discomfort and physical discomfort, but no serious adverse events attributed to psilocybin. Ross et al. used a largely similar design with a moderately high dose of psilocybin (0.3 mg/kg) being administered in one session, and a comparison compound administered in another session, with the exception that the comparison compound was niacin rather than a very low dose of psilocybin (Ross et al., 2016). Largely similar acute effects were reported, and no serious adverse effects were attributed to psilocybin.
Figure 2:
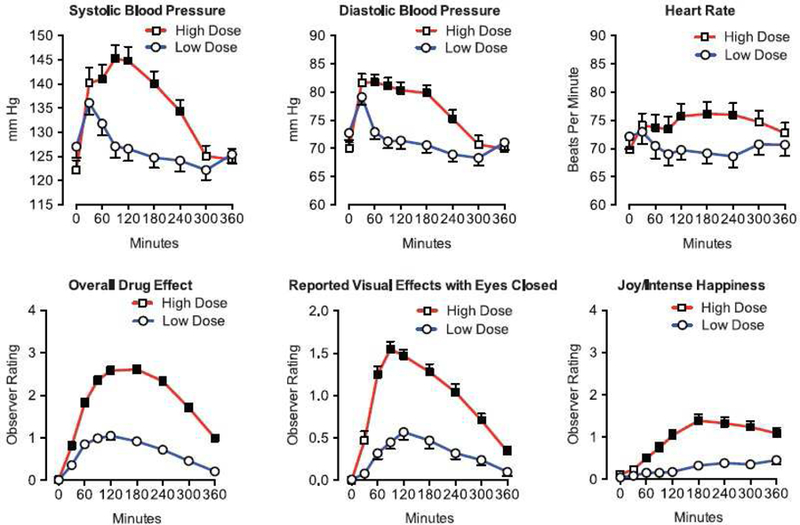
Cardiovascular and observer-rated effects of oral psilocybin in cancer patients (n=50). Each panel shows the mean (±SEM) within-subject time-course effect of a moderately-high (~0.314 or ~0.429 mg/kg) versus low, placebo-like (~0.014 or ~0.043 mg/kg) dose of psilocybin. For observer ratings, the Y-axis spans the range of possible scores. Filled squares indicate that planned comparisons showed the high dose condition significantly differed from the low dose condition at that time-point (p<0.05). Figure from Griffiths et al, 2016, Figure 2)
2.3. Factor 3. Current scientific knowledge regarding drug
Psilocybin is a phosphate derivative of N,N-dimethyltryptamine that is typically is observed in concentrations ranging from 0.1 to 1.5% at least ten species of the Psilocybe genus of mushrooms, and in some species of other genera (Stamets, 1996). Virtually all illicit use is in the form of mushrooms, including dried and fresh mushrooms. They are often eaten whole, with or without food, but can also be heated in water to produce an active aqueous extraction (a “tea”), or powdered and consumed in capsules (if dried) (Stamets, 1996). Cultivated psilocybin-containing mushrooms have been shown to vary in psilocybin content by a factor of 4, while “street samples” of psilocybin-containing mushrooms have been shown to vary in psilocybin content by an astonishing factor of 10 (Bigwood and Beug, 1982). These wild variations in psilocybin content, combined with the variations in methods for consumption described above, suggest that dosing is not well controlled in typical illicit use. This contrasts with approved studies that administer known doses of psilocybin. There have been occasional reports of intravenous injection psilocybin in research (Carhart-Harris et al., 2016b; Petri et al., 2014; Schartner et al., 2017; Waugh, 2016) although we are aware of no reports of illicit use of psilocybin by injection.
There has been considerable progress elucidating the effects and mechanisms of action of psilocybin in animal and human studies. It is well-established that psilocybin, like other classic psychedelics, has agonist or partial agonist activity at 5-HT2A receptors (Nichols, 2016). Carbon 14-label psilocybin studies revealed that approximately 50% of orally ingested psilocybin is absorbed and rapidly systemically distributed. The isotope is distributed almost uniformly throughout the whole body. Studies of metabolites by Holzman and Hasler (Hasler, 1997; Holzmann, 1995) reported by Passie et al. (Passie et al., 2002), found four metabolites: d 4-hydroxy-N,N-dimethyltrypt-amine (Psilocin); d 4-hydroxyindole-3-yl-acetaldehyde (4H1A); d 4-hydroxyindole-3-yl-acetic-acid (41-IIAA); and d 4-hydroxytryptophol (41-IT), with a first hepatic bypass effect leading to extensive conversion to psilocin within 30 min. This corresponds to the beginning of physiological and psychological effects in the time course described below. Passie et al. (2002) reported that psilocin levels peak at about 50 min post oral administration and then slowly decline over the next 5 hr, again roughly corresponding to physiological and psychological effects, for a half-life estimated at 163 ± 64 min orally (Passie et al., 2002; Sellers et al., 2017).
Considerable progress has been made in recent years to understand the mechanisms of psilocybin’s therapeutic effects. Resting state function magnetic resonance imaging shows that psilocybin administration acutely alters brain network activity. This includes decreased connectivity within the default mode network, which is a system of brain regions that supports internal focus (Carhart-Harris et al., 2012; Johnson and Griffiths, 2017). However, there is no well-documented theory about how such acute effects, lasting only hours, lead to therapeutic benefits lasting months and possibly a year or more. It has been suggested that the acute destabilization of brain networks by psilocybin (which may stem from receptor level effects via amplification of neuronal avalanches) may provide the opportunity to alter brain network activity in a persisting fashion (Johnson and Griffiths, 2017; Nichols et al., 2017). Such a mechanism has been suggested as consistent with the evident importance of the appropriate context and importance of psychotherapy in the therapeutic benefits of both psilocybin and LSD (Hofmann, 1980; Johnson et al., 2008; Johnson and Griffiths, 2017). That is, the acute effects of psilocybin in altering brain network dynamics may set the occasion for such networks to re-establish themselves in altered ways after the conclusion of acute effects; the overall context and the non-drug therapeutic aspects of the intervention may play a role in shaping such re-established networks.
As reviewed by Nichols et al. (2017), it is now known that serotonergic-acting psychedelics, including psilocybin, have anti-inflammatory effects and may have efficacy in treating some inflammatory diseases. They observed that inflammation of the brain “has been linked to several psychiatric disorders including depression, addiction, and neurodegenerative disorders such as Parkinson’s and Alzheimer’s disease.” Insofar as elevated serotonin levels are associated with inflammation it is plausible that psilocybin has anti-inflammatory effects in the brain, possibly involving serotonergic systems that contribute to its therapeutic effects (Nichols et al., 2017).
2.4. Factor 4. History and current pattern of abuse
Table 2 provides a summary overview of psilocybin and psilocybin-containing mushrooms in cultures dating back at least 7 millennia. From the perspective of understanding the abuse potential of psilocybin it is important to note that the history of psilocybin use has primarily involved naturally occurring psilocybin containing mushrooms. Use of these mushrooms by non-indigenous individuals in the US and elsewhere began soon after Wasson’s discovery of mushroom ceremonies in the late 1950s (Stevens, 1987). An exception was the brief distribution of a pure psilocybin containing drug product branded as Indocybin® as an adjuvant to psychotherapy or a tools in experimental psychiatry, free of charge for a few years in the early 1960s by the Swiss Sandoz pharmaceutical company (Lee and Shlain, 1992; Passie et al., 2002). In those days this general approach was permitted for drugs that were not approved for therapeutic use (Bonson, 2018). Nonetheless, research on psychedelic substances began to slow in 1962/1963 when US scientists were required to seek federal approval for evaluations of psilocybin or LSD (Stevens, 1987).
Table 2.
History of psilocybin use and in culture
| 7000 BCE-5000 BCE – Mushroom cave paintings from Tassilli, modern-day Algeria (Samorini, 1992) |
| 4000 BCE – Possible evidence of psilocybin-containing mushroom use in cave paintings in modern-day Spain (Akers et al., 2011) |
| 4000 BCE-900 CE – Mushroom stones and other artifacts from cultures throughout the Americas, including Mayan (de Borhegyi, 1961; Lowy, 1971; Schultes, 1969; Schultes et al., 2001; Truttman, 2012) |
| 1600 – Spanish colonizers documented religious mushroom use by indigenous people in Mexico, considered it devil worship, and persecuted its use. Sacramental use was driven underground for the next 400 years (Schultes, 1969; Schultes et al., 2001). |
| 1957 – Spanish conqueror accounts of mushroom use had come to be considered myth (Schultes, 1969). Then, following earlier suggestive evidence by R. Schultes (Schultes, 1939, 1940), R.G. Wasson became the first non-indigenous individual to participate in and document sacramental psilocybin-containing mushroom use by indigenous people (Mazatec society in Mexico) since European colonization (Wasson, 1959; Wasson and Wasson, 1957) |
| 1958–1959 – A. Hofmann, using mushrooms provided by R.G. Wasson, isolated psilocybin and psilocin, then developed synthesis of each (Hofmann, 1958; Hofmann et al., 1958; Hofmann et al., 1959) |
| 1959 – Clinical research was begun; initial research did not appreciate the powerful influences of set and setting, resulting in erratic outcomes (Delay et al., 1959) |
| 1960s – Societal, legal, and political backlash emerged against the psychoactive drug excesses of the 1960s, along with the associated “counter-culture”, the promotion of psychedelics as a panacea for achieving personal enlightenment and a utopian transformation of society, as opposed to use primarily as potential medicines in people with illness |
| Early 1960s – Indocybin marketing for research by Sandoz requiring therapeutic interventions, ending in 1966 |
| 1970 – US Controlled Substances Act listed psilocybin in Schedule I, along with LSD, heroin and other substances of serious societal and public health concern, thus prohibiting therapeutic use, and imposing extensive barriers to possession and research |
| 1971–1990s – Human psilocybin research was largely dormant until the late 1990s when a few laboratories in Europe renewed interest (Spitzer et al., 1996; Vollenweider et al., 1997). Human psilocybin research then began in the U.S. at the University of New Mexico (Bogenschutz et al., 2015; Strassman, 2001) [initiated but unpublished psilocybin results], Johns Hopkins University (Griffiths et al., 2006), the University of Arizona (Moreno et al., 2006), the University of California, Los Angeles (Grob et al., 2011), and New York University (Ross et al., 2016). |
2.4.1. United States national surveys
Various national agencies monitor a broad range of substance use related behaviors, effects, concomitants and treatment seeking. Together, these characterize the prevalence and trends and effects related to various substances geographically and demographically. A brief summary of the major surveillance systems follows.
Treatment Episode Datasets (TEDS)
TEDS is an annual record of U.S. substance abuse treatment admissions. The methods of the survey and data collection are described elsewhere (Substance Abuse and Mental Health Services Administration, 2017a). An estimate of treatment for psilocybin use disorder specifically cannot be assessed because it has not emerged as a sufficiently large cause of substance use disorders to warrant its own category, thus, the TEDS assesses a composite category termed “hallucinogens,” which includes LSD, DMT, “STP” (2,5-dimethoxy-4-methylamphetamine or DOM), mescaline, peyote, psilocybin, and other (unnamed) “hallucinogens”. Common substances sometimes considered to be “hallucinogens” but which are included in other TEDS categories (rather than the “hallucinogen” category) are MDMA and phencyclidine (PCP). As shown in Table 3 for all years from 2005 to 2015, “hallucinogens” were consistently reported as the primary substance of abuse in 0.1% of all admissions aged 12+ years. In 2015 those who reported “hallucinogens” as their primary substance of abuse at admission were 74.9% male and – on average – 28 years of age, and 43.6% had not used “hallucinogens” in the past month (only 25.9% had used daily in the past month). To provide some perspective we include TEDS data for opiates, cocaine and alcohol. Together these data show that among substances of abuse, treatment seeking for the entire category of “hallucinogens” constitutes a very small fraction of reports to TEDS with no evidence of increasing trends over the last decade of reports
Table 3.
Treatment Episode Datasets (TEDS): Rate of Various Drugs as the Primary Substance of Abuse Among Persons 12 Years and Older, 2005–2015
| Primary Substance | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 1,896,299 | 1,962,664 | 1,969,862 | 2,074,974 | 2,055,914 | 1,932,524 | 1,936,278 | 1,834,591 | 1,762,015 | 1,639,125 | 1,537,025 |
| Hallucinogens | |||||||||||
| n | 2,045 | 1,644 | 1,651 | 1,917 | 1,880 | 1,791 | 1,998 | 2,155 | 2,177 | 1,899 | 1,917 |
| % | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% |
| Opiates | |||||||||||
| n | 332,401 | 353,899 | 364,614 | 411,301 | 439,826 | 443,405 | 486,729 | 488,038 | 507,989 | 501,680 | 526,686 |
| % | 17.5% | 18.0% | 18.5% | 19.8% | 21.4% | 22.9% | 25.1% | 26.6% | 28.8% | 30.6% | 34.3% |
| Cocaine | |||||||||||
| n | 268,402 | 277,852 | 259,973 | 239,342 | 193,419 | 158,780 | 152,349 | 126,371 | 106,594 | 88,623 | 74,710 |
| % | 14.2% | 14.2% | 13.2% | 11.5% | 9.4% | 8.2% | 7.9% | 6.9% | 6.0% | 5.4% | 4.9% |
| Alcohol* | |||||||||||
| n | 746,544 | 781,349 | 804,581 | 860,742 | 856,180 | 782,764 | 759,017 | 709,891 | 654,808 | 591,404 | 521,089 |
| % | 39.4% | 39.8% | 40.8% | 41.5% | 41.6% | 40.5% | 39.2% | 38.7% | 37.2% | 36.1% | 33.9% |
Alcohol only or with a secondary drug
Drug Abuse Warning Network (DAWN)
The DAWN, which monitored U.S. drug-related visits to emergency departments, was discontinued after 2011. The methods and its scope of data collection are described elsewhere (Substance Abuse and Mental Health Services Administration, 2013). As shown in Table 4, from 2004 to 2011, the data suggest an increasing trend in psilocybin-related emergency department (ED) visits. However, the signal is so small, compared to “pain relievers,” cocaine, and alcohol that an increase from 0.2 to 0.4 of all ED visits must be interpreted with caution. In terms of rates, psilocybin-related ED visits increased from 1.0 per 100,000 population in 2004 to 1.9 per 100,000 population in 2011.
Table 4.
Drug Abuse Warning Network (DAWN): Total ED Visits (Any Type) for Various Drugs, 2004–2011
| Drugs | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 |
|---|---|---|---|---|---|---|---|---|
| Total ED visits | 2,537,722 | 3,009,025 | 3,441,855 | 3,998,228 | 4,383,494 | 4,595,261 | 4,916,328 | 5,067,374 |
| Psilocybin | ||||||||
| number of ED visits | 2,947 | 2,937 | 3,557 | 4,006 | 5,422 | 4,087 | 4,539 | 6,048 |
| % of all ED visits | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% |
| Rate per 100,000 population | 1.0 | 1.0 | 1.2 | 1.3 | 1.8 | 1.3 | 1.5 | 1.9 |
| Opiates/opioids | ||||||||
| number of ED visits | 299,498 | 388,873 | 452,929 | 542,699 | 668,803 | 769,330 | 851,453 | 855,348 |
| % of all ED visits | 11.8% | 12.9% | 13.2% | 13.6% | 15.3% | 16.7% | 17.3% | 16.9% |
| Rate per 100,000 population | 102.3 | 131.6 | 151.8 | 180.2 | 219.9 | 250.8 | 275.3 | 274.5 |
| Cocaine | ||||||||
| number of ED visits | 475,425 | 483,865 | 548,608 | 553,535 | 482,188 | 422,902 | 488,101 | 505,224 |
| % of all ED visits | 18.7% | 16.1% | 15.9% | 13.8% | 11.0% | 9.2% | 9.9% | 10.0% |
| Rate per 100,000 population | 162.4 | 163.7 | 183.9 | 183.8 | 158.6 | 137.9 | 157.8 | 162.1 |
| Alcohol | ||||||||
| number of ED visits | 674,914 | 527,198 | 577,525 | 634,663 | 656,911 | 658,263 | 687,574 | 724,306 |
| % of all ED visits | 26.6% | 17.5% | 16.8% | 15.9% | 15.0% | 14.3% | 14.0% | 14.3% |
| Rate per 100,000 population | 230.5 | 178.4 | 193.6 | 210.7 | 216.0 | 214.6 | 222.3 | 232.5 |
National Survey on Drug Use and Health (NSDUH)
The NSDUH is an annual survey of substance use and mental health issues in US civilians ≥ age 12. Methods for some NSDUH items changed in 2015, necessitating trend breaks in some cases. However, items related to “hallucinogens” were not modified. As shown in Table 5, between 2009 and 2015, lifetime use of psilocybin was consistently reported by about 8.5% of NSDUH respondents aged 12 and older, with a low of 8.1% (in both 2011 and 2012) and a high of 8.7% (in 2013). The reported lifetime use rate in 2015 was 8.5%. The methods of the survey, including specific questions are described in detail elsewhere (Substance Abuse and Mental Health Services Administration, 2017b).
Table 5.
National Survey on Drug Use and Health (NSDUH): Lifetime Use of Various Drugs Among Persons Aged 12 and Older, 2009–2015
| 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | |
|---|---|---|---|---|---|---|---|
| Psilocybin | |||||||
| % lifetime | 8.4% | 8.3% | 8.1% | 8.1% | 8.7% | 8.5% | 8.5% |
| % past year | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Pain Relievers | |||||||
| % lifetime | 14.0% | 13.8% | 13.3% | 14.2% | 13.5% | 13.6% | 10.3%* |
| % past year | 4.9% | 4.8% | 4.3% | 4.8% | 4.2% | 3.9% | 4.7%* |
| Cocaine | |||||||
| % lifetime | 14.6% | 14.7% | 14.3% | 14.5% | 14.3% | 14.8% | 14.5% |
| % past year | 1.9% | 1.8% | 1.5% | 1.8% | 1.6% | 1.7% | 1.8% |
| Alcohol | |||||||
| % lifetime | 82.8% | 82.5% | 82.2% | 82.3% | 81.5% | 82.1% | 81.0% |
| % past year | 66.8% | 66.4% | 66.2% | 66.7% | 66.3% | 66.6% | 65.7% |
NSDUH metric was “non-medical use” from 2009–2014, but changed to “misuse” in 2015. Additionally, the focus of the survey shifted from lifetime to past-year (for most drugs) in 2015. SAMHSA has suggested that these methods changes may cause trend breaks for some drugs, including pain relievers. Thus, caution needs to be applied when comparing 2015 estimates to those from 2009–2014.
N/A = not assessed
Monitoring the future (MTF)
The MTF is a survey of substance use and attitudes of U.S. secondary school students, college students, and young adults. It does not ask its participants about prevalence of psilocybin use; however, the survey does ask about “hallucinogens”, which is broken down into LSD and “hallucinogens” other than LSD. The two substances most commonly identified in the class “hallucinogens” other than LSD, has been psilocybin or “shrooms.” From 2006 to 2011, lifetime prevalence of high schoolers using hallucinogens other than LSD (of which psilocybin/shrooms comprise the largest proportion), stayed relatively stable around 5.0%, but from 2011 to 2016, lifetime prevalence has decreased from 4.9% to 3.0%. Past year use among high schoolers mirrored this trend, staying relatively stable from 2006–2011 (around 3.0–3.3%) and declining from 3.1% in 2011 to 1.8% in 2016. Among college students, lifetime prevalence of use of “hallucinogens” other than LSD has steadily declined in the past 10 years from 10.1% in 2006 to 6.6% in 2016. Among college students, past year prevalence for “hallucinogens” other than LSD has also steadily declined from 5.4% in 2006 to 3.0% in 2016. Among young adults aged 19–28, lifetime prevalence for “hallucinogens” other than LSD declined from 14.9% in 2006 to 10.6% in 2016. Among young adults aged 19–28, past year prevalence for “hallucinogens” other than LSD has declined from 3.8% in 2006 to 3.0 in 2016.
National Forensic Laboratory Information System (NFLIS)
The NFLIS system of the DEA is based on results from drug chemistry analyses conducted by state, local and federal forensic laboratories, from drug seizures by law enforcement. It is not a measure of human use, abuse, overdose or effects but rather is intended to provide information about what substances are being found in drug seizures (also known as “busts” or “raids”) across the country (Drug Enforcement Administration Diversion Control Division, 2016). As shown in Table 6, the estimated number of total drug reports for psilocin/psilocybin has slightly declined from a high of 0.30% of total drug reports in 2010 to staying relatively stable from 2013 to 2015 (0.27% of all drug reports in 2013 and 0.26% of all drug reports in 2014 and 2015), however these rates are so small in comparison to other substances that interpretation must be made with caution.
Table 6.
National Forensic Laboratory Information System (NFLIS): Estimated percentage of total drug reports submitted to laboratories for various drugs, 2010–2015
| Drug | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|---|---|
| Psilocin/Psilocybin | 0.30% | 0.31% | 0.31% | 0.27% | 0.26% | 0.26% |
| Cocaine | 21.44% | 20.10% | 16.54% | 15.63% | 14.10% | 13.95% |
| Heroin | 6.44% | 7.21% | 8.11% | 9.85% | 10.83% | 12.12% |
| Oxycodone | 3.56% | 3.61% | 3.40% | 2.96% | 2.85% | 2.70% |
| Hydrocodone | 2.81% | 2.82% | 2.66% | 2.41% | 2.19% | 1.76% |
| Buprenorphine | 0.61% | 0.66% | 0.73% | 0.78% | 1.01% | 1.16% |
| MDMA | 1.48% | 0.78% | 0.37% | 0.31% | 0.32% | 5,188 |
American Association of Poison Control Centers’ (AAPCC) National Poison Data System (NPDS)
As shown in Table 7, from 2007 to 2015, there were 5559 case mentions of psilocybin and psilocin reported to the National Poison Data System (NPDS). A mention indicates that the substance was associated with, but not necessarily the cause of, a reported suspected poisoning. Of these 5559 mentions, there was one death, in 2012. Whether this death was the result of psilocybin use or other concomitant drug use is unknown. Case reports mentioning psilocybin and psilocin have decreased from 773 reports in 2007 to 473 in 2015.
Table 7.
American Association of Poison Control Centers’ (AAPCC) National Poison Data System (NPDS), 2007–2015
| Drug | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mushrooms: Hallucinogenics (Psilocybin and Psilocin) | ||||||||||
| # of Case Mentions | 773 | 758 | 727 | 643 | 633 | 593 | 476 | 484 | 473 | |
| # of Single Exposures | 609 | 574 | 565 | 478 | 462 | 409 | 342 | 335 | 311 | |
| Unintentional | 83 | 82 | 59 | 74 | 40 | 44 | 50 | 49 | 32 | |
| Intentional | 511 | 479 | 495 | 394 | 408 | 350 | 285 | 266 | 266 | |
| No Outcome | 40 | 37 | 33 | 23 | 27 | 24 | 38 | 23 | 18 | |
| Minor Outcome | 112 | 92 | 111 | 92 | 104 | 69 | 64 | 83 | 75 | |
| Moderate Outcome | 257 | 248 | 243 | 193 | 187 | 180 | 142 | 142 | 137 | |
| Major Outcome | 9 | 9 | 11 | 6 | 4 | 8 | 5 | 7 | 5 | |
| Death | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| Cocaine | ||||||||||
| # of Case Mentions | 7634 | 6351 | 5293 | 5130 | 5485 | 4850 | 4749 | 4289 | 4738 | |
| # of Single Exposures | 2748 | 2075 | 1707 | 1582 | 1597 | 1345 | 1265 | 1171 | 1160 | |
| Unintentional | 281 | 261 | 184 | 162 | 168 | 140 | 133 | 105 | 127 | |
| Intentional | 2323 | 1695 | 1448 | 1329 | 1327 | 1133 | 1041 | 974 | 933 | |
| No Outcome | 488 | 419 | 349 | 234 | 231 | 191 | 197 | 175 | 195 | |
| Minor Outcome | 301 | 281 | 264 | 248 | 245 | 219 | 213 | 195 | 189 | |
| Moderate Outcome | 649 | 474 | 431 | 426 | 435 | 372 | 313 | 343 | 320 | |
| Major Outcome | 140 | 121 | 88 | 90 | 101 | 70 | 77 | 60 | 65 | |
| Death | 20 | 18 | 6 | 10 | 34 | 28 | 20 | 9 | 7 | |
| Codeine | ||||||||||
| # of Case Mentions | 974 | 965 | 2056 | 1993 | 2054 | 1953 | 1935 | 1709 | 1824 | |
| # of Single Exposures | 629 | 616 | 1550 | 1501 | 1542 | 1467 | 1395 | 1254 | 1327 | |
| Unintentional | 499 | 449 | 1307 | 1270 | 1280 | 1215 | 1164 | 1049 | 1073 | |
| Intentional | 90 | 109 | 163 | 152 | 186 | 163 | 160 | 133 | 185 | |
| No Outcome | 158 | 123 | 413 | 409 | 403 | 389 | 364 | 345 | 332 | |
| Minor Outcome | 84 | 71 | 176 | 155 | 192 | 177 | 166 | 148 | 182 | |
| Moderate Outcome | 13 | 17 | 27 | 31 | 26 | 33 | 28 | 29 | 30 | |
| Major Outcome | 1 | 1 | 5 | 1 | 3 | 1 | 2 | 5 | 3 | |
| Hydrocodone Alone or in Combinationa, b | ||||||||||
| # of Case Mentions | - | - | - | 316 | 1986 | 1989 | 1943 | 1956 | 1853 | |
| # of Single Exposures | - | - | - | 193 | 1089 | 1065 | 974 | 989 | 862 | |
| Unintentional | - | - | - | 116 | 675 | 698 | 604 | 646 | 538 | |
| Intentional | - | - | - | 59 | 297 | 247 | 271 | 243 | 234 | |
| No Outcome | - | - | - | 26 | 190 | 203 | 157 | 188 | 163 | |
| Minor Outcome | - | - | - | 47 | 246 | 215 | 188 | 211 | 161 | |
| Moderate Outcome | - | - | - | 14 | 67 | 51 | 63 | 47 | 46 | |
| Major Outcome | - | - | - | 0 | 10 | 3 | 2 | 2 | 2 | |
| Death | - | - | - | 0 | 1 | 0 | 0 | 4 | 1 | |
| Oxycodone Alone or in Combinationc | ||||||||||
| # of Case Mentions | 6515 | 7692 | 8065 | 9157 | 8963 | 8460 | 7742 | 7740 | 8170 | |
| # of Single Exposures | 3340 | 3741 | 3803 | 4278 | 3973 | 3644 | 3363 | 3300 | 3506 | |
| Unintentional | 1667 | 1980 | 1945 | 2102 | 1886 | 1820 | 1806 | 1763 | 1912 | |
| Intentional | 1271 | 1415 | 1463 | 1746 | 1700 | 1449 | 1231 | 1286 | 1319 | |
| No Outcome | 488 | 700 | 621 | 700 | 659 | 657 | 656 | 649 | 745 | |
| Minor Outcome | 560 | 615 | 714 | 804 | 758 | 673 | 655 | 782 | 775 | |
| Moderate Outcome | 260 | 289 | 368 | 478 | 469 | 409 | 387 | 397 | 431 | |
| Major Outcome | 78 | 85 | 91 | 112 | 108 | 105 | 90 | 81 | 109 | |
| Death | 9 | 11 | 8 | 12 | 37 | 26 | 20 | 15 | 13 | |
| Alcohol (Ethanol Beverages) | ||||||||||
| # of Case Mentions | 47202 | 50919 | 51909 | 51549 | 53021 | 54445 | 50763 | 49305 | 51811 | |
| # of Single Exposures | 8668 | 8560 | 9937 | 9307 | 9166 | 9753 | 7954 | 6026 | 6761 | |
| Unintentional | 2428 | 2496 | 2640 | 2381 | 2371 | 2363 | 2218 | 2076 | 2190 | |
| Intentional | 5668 | 5512 | 6729 | 6223 | 6169 | 6738 | 5099 | 3340 | 3947 | |
| No Outcome | 1010 | 1153 | 1124 | 894 | 880 | 694 | 706 | 662 | 704 | |
| Minor Outcome | 1280 | 1174 | 1570 | 1498 | 1446 | 1567 | 1220 | 984 | 1237 | |
| Moderate Outcome | 915 | 935 | 1074 | 1099 | 1062 | 1221 | 1162 | 1021 | 1127 | |
| Major Outcome | 185 | 185 | 202 | 225 | 208 | 220 | 234 | 219 | 260 | |
| Death | 5 | 20 | 8 | 21 | 71 | 111 | 79 | 15 | 20 |
Excluding Combination Products with Acetaminophen, Acetylsalicylic Acid or Ibuprofen
NFLIS started reporting Hydrocodone alone or in combination in 2010
Excluding Combination Products with Acetaminophen or Acetylsalicylic Acid
Sources: (Bronstein et al., 2011; Bronstein et al., 2009, 2010; Bronstein et al., 2008; Bronstein et al., 2012; Mowry et al., 2015;Mowry et al., 2016; Mowry et al., 2013; Mowry et al., 2014)
2.4.2. A Note on “Microdosing”
Psychedelic “microdosing,” which involves use of very low, sub-perceptual, doses of psychedelics, has recently received attention in popular press articles and books (Fadiman, 2011; Koebler, 2015; Malone, 2016; Waldman, 2017). Although popular attention to microdosing is relatively new, Albert Hofmann discussed the medical potential of using very low doses of LSD for antidepressant effects (Horowitz, 1976) as early as 1976. Six percent of individual responding to a drug-related survey indicated having microdosed with LSD at least once in their lifetime (Global Drug Survey, 2017). However, nothing is currently known about the population-level prevalence of psychedelic microdosing, nor about microdosing of psilocybin mushrooms among psychedelic users. Given the substantial variability in psilocybin-content in mushrooms (Bigwood and Beug, 1982), one risk of microdosing with mushrooms is accidentally consuming a higher psilocybin dose than intended, resulting in strong and possibly overwhelming psychological effects in a dangerous or otherwise problematic environment, for example, while driving or working.
2.5. Factor 5. The scope, duration, and significance of abuse
There is an extensive history that provides important insights concerning patterns of psilocybin, LSD and other classic psychedelic use, abuse, and place in culture in the US and globally. Unlike, LSD, psilocybin is not a new molecular entity but rather is a naturally occurring substance that has been used ritualistically for at least hundreds and likely thousands years in Central and South America and possibly Africa and Europe (Akers et al., 2011; de Borhegyi, 1961; Lowy, 1971; Samorini, 1992; Schultes, 1969; Schultes et al., 2001; Truttman, 2012), with an apparently revered place in many cultures through history (Schultes et al., 2001). By way of contrast, alcohol, cocaine, opioids, and tobacco also have histories of use dating thousands of years, but these substances were recognized as addicting and harmful to the lives of many users for centuries (Corti, 1931; Crocq, 2007; Lewin, 1998; Rush, 1808; Terry and Pellens, 1970). As discussed in the foregoing citations, many users of these classic substances of abuse developed patterns of daily use that interfered with social and occupational functioning and caused harm to users. Moreover, with these drugs abstinence often came with great difficulty and was sometimes associated with sickness. Such sickness was eventually recognized as part of a withdrawal syndrome that contributed to the persistence of chronic daily use (Koob and Le Moal, 2006; O’Brien, 2011).
In contrast, whereas many experts (Gable, 1993, 2004) and expert organizations including NIDA and the DEA recognize psilocybin as a drug of abuse, they universally differentiate it from drugs that cause dependence/addiction and carry a high risk of overdose and harm. For example, NIDA Drug Facts website describes LSD and psilocybin type classic psychedelics as not addicting in contrast to NMDA antagonist phencyclidine (PCP) which may be considered an addicting “hallucinogen,” broadly speaking. See Table 1.
The characterization of psilocybin as a substance with high abuse potential is based largely on social lore, sensationalized media coverage, and misinformation and misunderstanding about the actual risk of dependence and harms during the 1960s. This coincided with nonmedical use of classic psychedelics, primarily LSD, by the public in the 1960s (British Psychological Society, 2014; Costandi, 2014; Hofmann, 1980; Penner, 2015; Pollan, 2015). There is no question that such use involved motivation for intoxicating effects, and frequently involved co-administration of other substances. Furthermore, even though medical use by experienced practitioners had shown these drugs to be remarkably safe, use in the population for nonmedical reasons, often in high doses, in combination with other drugs, and in unsafe environments, led to highly sensationalized adverse consequences that contributed to the characterization of these substances as dangerous and highly abusable and ultimately in their placement in Schedule I of the CSA when it was codified in 1970. See further discussion in Belouin and Henningfield in this journal issue and Hofmann, 1980.
Scientific and medical studies, and US national surveillance systems yield a different characterization of psilocybin use, abuse, and risks than the 1960s media accounts as summarized in this factor and other factors. The scientific evidence confirms that there has been abuse and supports regulation as a controlled substance, however, that actual risk of dependence and harm associated with psilocybin has been estimated to be among the lowest of all major substances of abuse and dependence over the past several decades by several expert analyses, and lines of evidence evaluated in this factor and other factors of the CSA. For example, in a comparative overview of the dependence potential and acute toxicity of psychoactive substances, Gable concluded that psilocybin carried a lower risk of dependence than caffeine and among the lowest risks of death of all major substance abuse categories including cannabis (Gable, 1993). In a subsequent analysis using different methods Gable again found that psilocybin was amongst the least physiologically toxic drugs (Gable, 2004).
Similarly, Nutt, King, Saulsbury and Blakemore developed an instrument to assess drug harms and misuse that considered “physical” and “social” harm and dependence risk, and had a group of UK drug experts rank a large group of licit and illicit drugs (Nutt et al., 2007). Heroin, cocaine, sedatives and alcohol were ranked highest in overall harm. Although psilocybin was not specifically evaluated, the related drug LSD was ranked among the drugs with the lowest harm. This general approach was extended to use a more advanced decision-making approach, and included 16 specific criteria for evaluation by experts in the United Kingdom (Nutt et al., 2010). Alcohol was ranked most harmful with an overall harm score of 72 out of a possible 80, followed by heroin (overall harm score of 55 out of 80) and crack cocaine (overall harm score of 54 out of 80); the lowest overall score, as show in Figure 3, was assigned to “mushrooms, with an overall harm score of 6 out of 80.
Figure 3:
Normalized ratings of harm potential of psilocybin (“mushrooms”) relative to other drugs as rated by experts in the United Kingdom using on a multidimensional scale. Drugs are ranked by overall harm from left (most harmful) to right (least harmful), with harm to users (blue) and harm to others (red) shown separately. Abbreviations: CW=cumulative weight, GHB=gamma-hydroxybutyric acid. (Figure from Nutt et al., 2010, Figure 2)
A large survey of 1501 UK drug users (Morgan et al., 2010) assessed ratings of harms for the drugs previously examined by the UK drug experts in Nutt et al. (Nutt et al., 2007). Although psilocybin was not assessed, LSD was ranked relatively low in harm among other drugs (Morgan et al., 2010). In a similar study (Carhart-Harris and Nutt, 2013), experienced drug users rated harms to “self” and to “others.” The ratings by substance users and experts were overall similar, placing LSD among the lowest in harm to self and others with psilocybin-containing mushrooms receiving the lowest ratings (Carhart-Harris and Nutt, 2013). A study utilizing Dutch experts, using a framework based on that developed by Nutt and colleagues (Nutt et al., 2007), similarly concluded psilocybin-containing mushrooms to be the least harmful of all licit and illicit drugs examined, both to the individual and to the population (van Amsterdam et al., 2010). In turn, similar findings were obtained by 40 European Union addiction experts who scored 20 drugs on 16 factors related to harm (van Amsterdam et al., 2015). As shown in Figure 4, harm ratings at the population and individual level were among the lowest for “magic mushrooms” among all substances that were evaluated.
Figure 4:
Normalized ratings of harm potential of psilocybin (“magic mushrooms”) relative to other drugs as rated by experts in the European Union using a multidimensional scale. Drugs are ranked by overall harm from left (most harmful) to right (least harmful), with harm to users (shaded texture) and harm to others (solid texture) shown separately. (Figure 2 from van Amsterdam et al., 2015)
Lending confidence to these various assessments of drug harm rankings is the remarkable correspondence among them. Specifically, using the drugs in common between studies, the correlation between Nutt et al. (2007) expert rankings and the Nutt et al., (2010) expert rankings were strong (Pearson’s r = 0.70) despite methodological differences (Nutt et al., 2010). The van Amsterdam et al., (2010) Dutch expert rankings and Nutt et al., (2010) UK expert rankings were also strongly correlated (Pearson’s r: individual harm: 0.80, population harm: 0.84). The correlation between the UK drug user rankings in the Morgan et al. (2010) study and the UK expert rankings in Nutt et al. (2007) were strong (Pearson’s r = 0.90) (Morgan et al., 2010). The correlation between the UK drug user rankings in the Carhart-Harris et al. (2013) study were strongly correlated with both of UK expert rankings (Nutt et al., 2010: User harms Spearman’s rho = 0.90, harm to others Spearman’s rho = 0.76) and the Dutch expert rankings (van Amsterdam et al., 2010) (Individual level: Spearman’s rho = 0.93; Population level: Spearman’s rho = 0.94) (Carhart-Harris and Nutt, 2013). The rankings of European Union addiction experts showed remarkably high correlations to UK experts (Nutt et al., 2010; van Amsterdam et al., 2015) (Overall harm: Pearson’s r = 0.99). Collectively, these studies suggest strong international, cross-laboratory consensus, across academics, clinicians, and drug users themselves, regarding the relatively low harm potential of psilocybin compared to other drugs of abuse.
An evaluation of the harm-potential of psilocybin-containing mushrooms use, sanctioned by the Minister of Health of the Netherlands, “concluded that the physical and psychological dependence potential of magic mushrooms was low, that acute toxicity was moderate, chronic toxicity low and public health and criminal aspects negligible” (van Amsterdam et al., 2011). Further, the evaluation concluded that while “the use of magic mushrooms is relatively safe as only few and relatively mild adverse effects have been reported,” the most harmful instances of use tended to involve the combination of other drugs including alcohol with mushrooms, and suboptimal settings such as the absence of a sober companion.
An important evaluation of the comparative epidemiology of dependence across a broad range of substances, including “psychedelics” was performed by Anthony, Warner and Kessler using data from the National Comorbidity Survey (Anthony et al., 1994). With respect to the rank ordering of the risk of transition from “drug use” to “dependence” they concluded as follows: “For both men and women, and for all but the oldest age group of drug users, tobacco and heroin were top ranked; psychedelic drugs (defined in report as “e.g., LSD, peyote, mescaline” which presumably would have included psilocybin) and inhalants were at the bottom.” (Anthony et al., 1994). The inhalant results are unfortunately difficult to interpret because “inhalant” included compounds that widely varied in mechanism of action and related harms, from volatile solvents such as gasoline to nitrous oxide.
2.6. Factor 6. Risk to public health
Risks to public health can be estimated by a variety of approaches that help capture consequences of use among users and to nonusers. Carbonaro et al. (2016) reported on an online survey of psilocybin users about their single most psychologically difficult or challenging experience after consuming mushrooms. Eleven percent reported putting her/himself or others at risk of physical harm. Greater estimated dose, duration and difficulty of the experience, and lack of physical comfort and social support, were all related to increased risk. Approximately three percent reported behaving in a physically aggressive or violent manner, and the approximately three percent reported receiving medical help. Including only individuals whose reference psilocybin exposure occurred more than a year before survey completion, approximately eight percent reported seeking treatment for persisting psychological symptoms. Three of the respondents reported their psilocybin use to be followed by the onset of enduring psychotic symptoms. Three respondents reported attempting suicide.
As discussed in Factor 2, the risk of overdose poisoning by psilocybin is low due to its low physiological toxicity. In addition, it is possible that the often undesirable effects of high doses of psilocybin (Griffiths et al., 2011; Johnson et al., 2012), combined with large variability in the psilocybin-content of mushrooms (Bigwood and Beug, 1982) may lead many users to be cautious about dosing. On the other hand, its well documented sensory altering and impairing effects suggest a potential concern for the safety of users and others. By way of contrast, more than 10,000 or almost one third of all driving-related deaths in 2015 involved alcohol (Centers for Disease Control and Prevention, 2017), in addition to more than 2000 alcohol overdose poising deaths (Centers for Disease Control and Prevention, 2015), and nearly 80,000 alcohol related liver disease deaths (National Institute on Alcohol Abuse and Alcoholism, 2017). Recent trends suggest that an increasing fraction of highway motor vehicle accidents involve substances other than alcohol, including prescription drugs and possibly cannabis. The exception to this trend appears to be the category of “hallucinogens” (Rudisill et al., 2014). A plausible explanation is that the acute effects of classic psychedelics are so disrupting that persons under the influence are less likely to drive than those who are under the influence of intoxicating, sedating, and inhibition releasing substances that are more commonly associated with traffic accidents and fatalities. Another plausible contribution is the fact that psilocybin is typically used far less frequently than these other drugs which more readily lead to daily use and use disorders; therefore, there are fewer instances of drug intoxication involving driving and therefore fewer driving-related deaths.
Nonetheless, concerns about the safety of users and others have been voiced since early research with psilocybin and other psychedelics. Therefore, the relative rarity of apparent cases of classic psychedelic involved deaths does not mean that this should be of no concern (de Veen et al., 2017; Hofmann, 1980). Thus, despite an apparently low risk of addiction and physiological toxicity, there is concern about abuse because of potential adverse effects, including panic reactions, possible precipitation of enduring psychiatric conditions (i.e., psychotic disorders), and long-lasting visual perceptual disturbances. Importantly, these risks can be minimized by control of dose, setting, patient selection and other factors (Carhart-Harris and Nutt, 2013; de Veen et al., 2017; Johnson et al., 2008). What is reassuring, and at odds with one of the conditions for CSA Schedule I control (“There is a lack of accepted safety for use of the drug or other substance under medical supervision.”) is that decades of experience and recent clinical research demonstrate that psilocybin can be used safely under medical supervision and the conditions of safe use are increasingly well-defined (Griffiths et al., 2016; Johnson et al., 2008; Ross et al., 2016).
It is likely that in the approval of psilocybin for therapeutic application, the FDA would not simply assume low risk, but rather would require that such serious but mitigatable concerns warrant a REMS to contribute to safe use and minimize unintended negative effects (U.S. Food and Drug Administration, 2015). Approval of drugs with REMS anticipates the likelihood that emerging clinical experience, further research, and the relatively high level of oversight and data collection provided by the REMS can support expansion of the conditions and indications for use and result in modifications of the REMS itself, as was the case for sodium oxybate (Xyrem®), the medication whose active pharmaceutical ingredient is the controversial substance commonly known as GHB (Carter et al., 2009; Carter et al., 2006; Johnson and Griffiths, 2013; McCormick et al., 2009; The Medical Letter, 2006; Wang et al., 2009). Data important in understanding the safety, mechanisms of action, and potential future indications for psilocybin-assisted treatment have included the treatment of substance use disorders (Bogenschutz et al., 2015; Garcia-Romeu et al., 2014; Johnson et al., 2014; Johnson et al., 2017; Johnson and Griffiths, 2017; Johnson et al., 2012; Nichols et al., 2017; Sessa and Johnson, 2015; Tupper et al., 2015), obsessive-compulsive disorder (Schindler et al., 2015), and cluster headaches (Matsushima et al., 2009; Sewell et al., 2006).
Ideally REMS are designed with knowledge gained from clinical trials to provide a basis for a plan that will contribute to beneficial effects and mitigate the risk of undesired effects. In this case there is knowledge that goes back to the 1950s efforts of Sandoz to ensure safe use by health care providers and the 21st century clinical trials have carefully designed and documented their programs to minimize unintended consequences. Furthermore, history and clinical research indicate that adverse events are not random but are related to controllable factors that can be addressed in labeling and by the requirement of elements to assure safe use (ETASU) of REMS that would likely be required by the FDA given (a) the 1960s history that did include problems, and (b) the apparent ability to minimize problems by following protocols employed in clinical research. In fact, information that would contribute to the development of a REMS is already emerging from recent clinical safety and efficacy trials.
2.6.1. Potential public health benefits
Risk to public health and overall public health impact must include consideration of benefits in order to provide a balance risk to benefit analysis. This concept has received increasing attention from the FDA in recent years. For example, in the 2012 Food and Drug Administration Safety and Innovation Act (FDASIA) Section X, is entitled “Enhancing Benefit-Risk Assessment in Regulatory Decision-Making.” This section required the FDA to “develop a five-year plan to further develop and implement a structured benefit/risk assessment in the new drug approval process” and “An evaluation plan to ascertain the impact of the benefit-risk framework in the human drug review process. The evaluation will consider the utility of the framework in facilitating decision-making and review team discussions across disciplines, risk management plan decision-making, training of new review staff, and communicating regulatory decisions. In particular, the evaluation will consider the degree to which the framework supports or facilitates balanced consideration of benefits and risks, a more consistent and systematic approach to discussion and decision-making, and communication of benefits and risks.” (U.S. Food and Drug Administration, 2012). The plan included holding two public workshops addressing benefit-risk considerations in drug regulation, one of which was held September 18, 2017 (U.S. Food and Drug Administration, 2017b).
The importance of public health benefits in drug scheduling decision-making is not new but its prominence seems to be increasing and in fact, the standard for evaluation of new tobacco products and for potential approval of some harm reduction tobacco products as “Modified Risk Tobacco Products” invokes a public health standard and not an efficacy standard by the 2009 Family Smoking Prevention and Tobacco Control Act (U.S. Congress, 2009). Nicotine is a drug that meets criteria for placement in Schedule III of the CSA (if marketed as a drug but not in the form of tobacco products which are exempted from CSA scheduling along with alcoholic beverages by the CSA) but the potential public health benefits of nicotine were prominent in the decision by the FDA not to recommend scheduling upon approval of nicotine gum in 1985, and in 1996 not to recommend scheduling of a nasal nicotine product that clearly met criteria for such control (Henningfield et al., 2016; U.S. Food and Drug Administration, 1996). Similarly, public health considerations were prominent in the FDA’s resistance to reschedule low dose hydrocodone plus acetaminophen products from Schedule III to Schedule II (Anson, 2014; Coleman, 2015).
In this context, it is important to recognize the potential public health benefits of psilocybin and to avoid unduly restrictive scheduling that would pose an unnecessary barrier to potential life-saving and public health enhancing access. For example, placement in Schedule II is intended to pose high barriers to patient prescribing by health care providers and access by patients, and this was a consideration in advocacy by the FDA, pain patient advocacy organizations, and many people with pain in sustaining the low dose acetaminophen combination form of hydrocodone in Schedule III as discussed above (Coleman, 2015).
As discussed in the summaries of analyses of Factors 4 and 5 in this article and earlier in this section, the overall risks to public health posed by illicit psilocybin are low compared to most scheduled drugs and certainly lower than most Schedule II and III drugs. Clinical studies of psilocybin suggest that the public health risk of an approved medicine would be lower still due to the restrictions on its access imposed by distribution only through pharmacies and potentially at least initially limited to a single central pharmacy provider if that was recommended as part of its REMS program (Griffiths and Johnson, 2015).
The potential medical and public health benefits of medicinal psilocybin were demonstrated by research up until the 1960s, and with some resurgence beginning in the 1990s. The clinical development program for psilocybin as a potential medicine as for the treatment of depression and anxiety and to improve quality of life in patients with life-threatening cancer diagnoses (Griffiths et al., 2016; Grob et al., 2011; Ross et al., 2016), provides more recent data, from studies that are intended to meet FDA standards for Phase 1 and 2 studies to support an eventual new drug application. In summary, Grob et al. assessed the effects of one-time psilocybin (14mg/70kg doses) using a double-blind, placebo-controlled design, with administration in a therapeutic setting in patients with life-threatening illnesses including cancer (Grob et al., 2011). There were reductions in measures of trait anxiety and depressed mood that persisted through the 6-month follow-up observation. There were no serious adverse events. Carhart-Harris et al. conducted an open label study of 10 and 25 mg doses of psilocybin administered 7 days apart in a supportive setting in patients with treatment-resistant depression. This demonstrated strong reductions in measures of depression at 1 week and 3 months by the 16-item Quick Inventory of Depressive Symptoms, with no serious adverse events (Carhart-Harris et al., 2016b).
The most rigorous study of psilocybin for treatment of depressed mood and anxiety in severely distressed cancer patients was by Griffiths et al. (Griffiths et al., 2016), as described under Factor 2. Acute effects during the sessions were described (see Figure 2). As shown in Figure 5, the therapeutic benefits of the high dose of psilocybin (~0.314 or ~0.429 mg/kg) were profound and persistent as reported by both patients and observers. The overall rates of clinician-rated therapeutic effects at 6 months were 78% for depression and 83% for anxiety. Ross et al. conducted a study that was generally similar to that by Griffiths et al., with the most important difference being the use of small doses of niacin as an active placebo instead of low doses of psilocybin (Griffiths et al., 2016; Ross et al., 2016). Ross et al. also found robust acute and sustained antidepressant effects by psilocybin. Ross et al. and Griffiths et al. have assisted a nonprofit program that has been coordinated by the Heffter Research Institute (Heffter Research Institute, 2017) and Usona Institute (Usona Institute, 2017) which are working together to sponsor the development of psilocybin for approval as a medicine by the FDA. These studies include measures of mood enhancement in patient populations that are not discussed in Factor 1 (regarding euphoriant effects) l because the relevance of persisting mood improvement in depressed and anxious patients to abuse potential is not clear (Griffiths et al., 2016).
Figure 5:
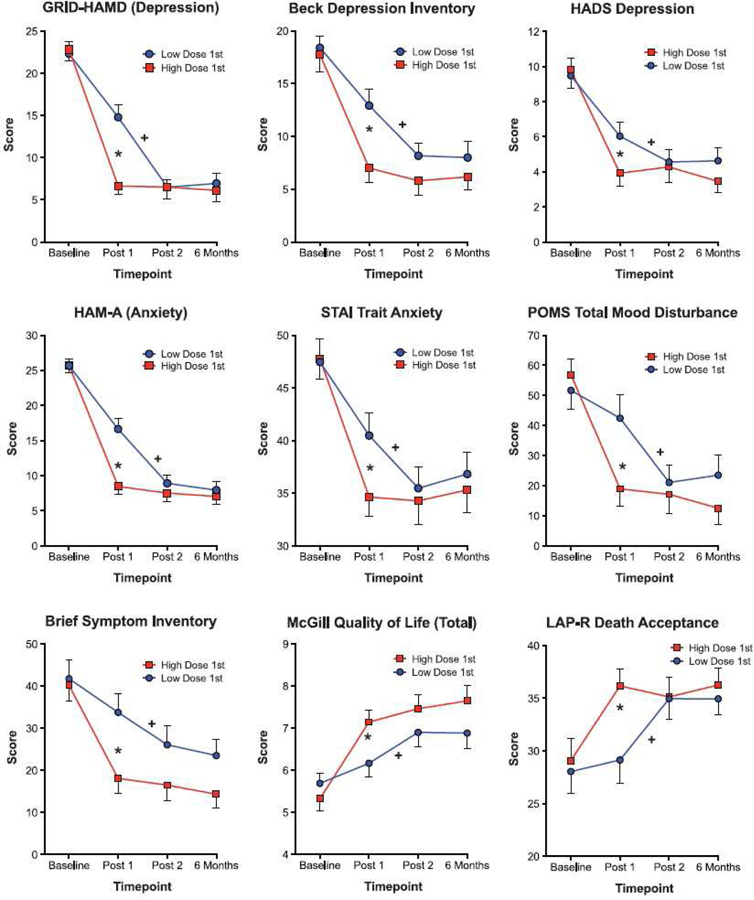
Persisting effects of psilocybin on depression- and anxiety-related outcome measures Outcomes were measured at baseline (pre-psilocybin), post session 1 (5 weeks after the first psilocybin session), post session 2 (5 weeks after the second psilocybin session), and the 6-month follow-up (n = 25, 25, 24, and 22 at baseline, post session 1, post session 2, and 6 months, respectively). Each panel shows the mean (±SEM) scores for two groups: The “Low Dose 1st” group received a low, placebo-like (~0.014 or ~0.043 mg/kg) dose of psilocybin in Session 1, and a moderately-high (~0.314 or ~0.429 mg/kg) dose of psilocybin in Session 2; the “High Dose 1st” group received the doses in the opposite order. Stars show a significant difference between the two groups at post session 1 by planned comparison (p<0.05). Crosses show a significant difference between the post session 1 and post session 2 times in the Low-Dose-1st group by planned comparison (p<0.05). (Figure from Griffiths et al., 2016, Figure 3)
Non-therapeutic laboratory studies of psilocybin in healthy volunteers also suggest positive persisting effects of psilocybin. Two studies administering doses of up to ~0.429 mg/kg to healthy volunteers showed increased participant-ratings of well-being or life satisfaction (Griffiths et al., 2008; Griffiths et al., 2011) 14 months after psilocybin administration. Data pooled across these studies showed an increases in personality over a year after psilocybin administration (MacLean et al., 2011). A recent, large laboratory study examining the interactive effects of psilocybin and spiritual practices (including meditation) in 75 healthy volunteers showed high-dose psilocybin (~0.286 and ~0.429 mg/kg in two separate sessions) to cause significant increases in ratings of interpersonal closeness, gratitude, and life meaning/purpose 6 months after psilocybin administration, suggesting persisting improvements prosocial traits and psychological functioning (Griffiths et al., in press).
Larger, population- and cohort-based studies are consistent with findings from these experimental investigations. For example, Hendricks et al. tested the relationships of classic psychedelic use and psilocybin use per se with psychological distress and suicidality among over 190,000 adult respondents pooled from years 2008 through 2012 of the NSDUH (Hendricks et al., 2015a; Hendricks et al., 2015b). They found that lifetime classic psychedelic use was associated with a reduced odds of past month psychological distress (aOR = 0.81), past year suicidal thinking (aOR = .86), past year suicidal planning (aOR = 0.71), and past year suicidal attempt (aOR = 0.64), with these results extending to psilocybin per se. Lifetime illicit use of other drugs was, by and large, associated with an increased odds of these outcomes. Building on these findings, Argento et al. (2017) found that psychedelic drug use, broadly defined (i.e., not restricted only to 5HT2A agonists but also including MDMA) prospectively predicted a reduced likelihood of suicide ideation or attempts among 290 marginalized Canadian women (aHR = 0.40). Moreover, consistent with pilot studies of psilocybin-assisted psychotherapy for drug dependence (Bogenschutz et al., 2015; Johnson et al., 2014), Pisano et al. found that lifetime classic psychedelic use was associated with a reduced risk of past year opioid dependence (weighted risk ratio = 0.73) and past year opioid abuse (weighted risk ratio = 0.60) among over 44,000 illicit opioid users who completed the NSDUH in years 2008 through 2013 (Pisano et al., 2017). Finally, a growing literature suggests protective effects for individuals in the criminal justice system, who suffer from numerous comorbid psychopathologies including depression, anxiety, and drug dependence that exacerbate criminal behavior. Hendricks et al. found that naturalistic “hallucinogen” use predicted a reduced likelihood of recidivism among over 25,000 individuals under community corrections supervision with a history of substance involvement (aOR = 0.60) (Hendricks et al., 2014) and Walsh et al. found that naturalistic “hallucinogen” use predicted reduced arrest for intimate partner violence among 302 jail inmates (aOR = 0.62) (Walsh et al., 2016). Of course, as “hallucinogens” are a broader class of substance that includes classic psychedelics such as psilocybin in addition to other substances, these studies were not able to test the unique relationships of classic psychedelics or psilocybin in particular with criminal behavior. Toward that end, Hendricks et al. (2018) evaluated the associations of classic psychedelic use, and psilocybin use per se, with criminal behavior among over 480,000 adult respondents pooled from years 2002 through 2014 of the NSDUH. They found that lifetime classic psychedelic use was associated with a reduced odds of past year larceny/theft (aOR = 0.73), past year assault (aOR = 0.88), past year arrest for a property crime (aOR = 0.78) and past year arrest for a violent crime (aOR = 0.82). Results also were consistent with a protective effect of lifetime psilocybin use for past year antisocial behavior. Lifetime illicit use of other drugs was largely associated with an increased odds of these outcomes.
To be clear, it is not a conclusion of this review that psilocybin or other psychedelics should currently be recommended as a general or blanket approach for the prevention of suicide or other behaviors and conditions discussed in this section. Nor is it proposed that approval of psilocybin for depression and anxiety disorders related to advanced cancer diagnosis will translate to reduced suicide or other problems at the population level in the near term, if ever. In part this is because self-selection and other factors may contribute to the population level effect. Furthermore, psilocybin and related substances can produce adverse effects that were documented by Hoffman in the 1940s and since, and the risks of such adverse events can be minimized by appropriate protocols, conditions for use, dosing and other factors. However, in the evaluation of the potential public health effects, the data suggest that psilocybin is overall more likely to contribute to public health improvement than to adversely affect public health. Taken together, the evidence suggest that, at least with respect to certain mental disorders, psilocybin appears to offer potential benefits to patients and little risk to public health (Belouin and Henningfield, 2018).
2.7. Factor 7. Psychic or physiological dependence liability
No apparent physiological dependence as evidenced by withdrawal symptoms has been documented in humans (clinical observations) or animals (laboratory studies), although tolerance has been observed (Abramson et al., 1960; Appel and Freedman, 1968; Isbell et al., 1961). For example, no withdrawal was reported following chronic psilocybin use in humans in ARC studies including a study by Isbell et al. (1961) of 19 participants that included up to 12 days of psilocybin (ascending up to 0.15 mg/kg or 0.21 mg/kg) followed by up to 13 days monitoring after termination of administration. With the exception of MDMA, which is distinct from classic psychedelics both in effects and primary pharmacological mechanism of action, the Fifth edition of the Diagnostic and Statistical Manual of the American Psychiatric Association (DSM 5) does not include a diagnosis of Withdrawal for “hallucinogens” (American Psychiatric Association, 2013). As concluded by O’Brien (2011), “Frequent, repeated use of psychedelic drugs is unusual, and thus tolerance is not commonly seen. Tolerance does develop to the behavioral effects of LSD after three or four daily doses, but no withdrawal syndrome has been observed” (O’Brien, 2011). The Isbell et al., (1961) study discussed above observed tolerance (decreased drug effect after chronic treatment) to all measured effects of psilocybin, some of which met statistical significance. Hollister reported on a single participant who was administered psilocybin on a daily basis for 22 days, with doses ranging from 1.5 to 27 mg per day, and noted strong tolerance, with minimal apparent effects, to 15 mg on day 22 (Hollister, 1961). After several weeks of abstinence the same 15 mg dose resulted in a robust and typical response, demonstrating a recovery from tolerance. Cross-tolerance occurs between psilocybin and LSD (Abramson et al., 1960; Appel and Freedman, 1968; Isbell et al., 1961).
2.8. Factor 8. Immediate precursor of substance controlled
Psilocybin is a prodrug to the active entity, psilocin, both of which are currently placed in Schedule I of the CSA.
3. Discussion
3.1. Summary and recommendation for CSA scheduling
All 8 factors and other lines of evidence taken together indicate the profile of a substance that is characterized by some level of abuse potential and potential risks. However, the findings do not support placement more restrictively than Schedule IV. The current placement in Schedule I is presently necessitated by the absence of FDA approval for a psilocybin containing medicine and Schedule I is the only Schedule into which substances of abuse can be placed that do not have an approved medical indication. However, it is the opinion of the authors of this review that the original placement of psilocybin was the result of a substantial overestimation of the risk of harm and abuse potential. The CSA stipulates that Schedule I is for substances with a high potential for abuse, lack of therapeutic approval, and that cannot be used safely in medicine. History of use and available scientific data show that the first criterion is questionable, and the third criterion is likely not true. The second of these criteria can only be negated by FDA approval of a psilocybin-containing products, but at this point the data suggest that the potential therapeutic benefits of psilocybin-assisted therapy are real, and of potential medical and public health significance.
Schedule placement is guided by an analysis of the 8 factors of the CSA that will be drafted by the FDA with input from NIDA. The 8-factor analysis contained in this review should be considered an abbreviated assessment of abuse potential as compared to what would be required by the FDA to accompany the submission of an NDA for approval of a psilocybin containing drug product. Furthermore, considerable additional study will yet be required to support the submission of a complete and reviewable NDA and its abuse potential assessment. This will include at least one major phase 3 clinical efficacy and safety trial that includes assessments relevant to abuse potential, additional Phase 1 and/or 2 clinical studies, and possibly some animal testing (Calderon et al., 2017; Heal et al., 2018; Sellers et al., 2017). Thus data yet to be collected will influence the final scheduling proposal that will be made by the sponsor and, in turn by the FDA, NIDA, and DEA. Nonetheless, considerable data from animal self-administration and discrimination studies, and human abuse potential studies since the 1960s provide a substantial basis for the present preliminary evaluation. In contrast to Schedule III drugs and even to many drugs placed in Schedule IV, the reinforcing effects in preclinical studies are marginal. There is no clear evidence of physical dependence and withdrawal in preclinical or clinical studies, or among those who chronically used illicit products. Euphoriant effects can occur under limited circumstances but appear attenuated by dysphoric effects. The doses that pose a risk of acute poisoning death (“overdose”) appear to be approximately 1000 times the likely highest clinical dose to be marketed, psychological dependence resulting in daily use appears rare, and all major drug surveillance systems reviewed in Factors 4, 5, and 6 of this analysis indicate rates of abuse, emergency department reports, and treatment seeking in youth and adults that are substantially lower than are evident for many Schedule IV drugs. It is possible, of course that subsequent study with larger populations and different designs in animals and humans, would yield different outcomes, but this review suggests that psilocybin would be appropriately placed in Schedule IV of the CSA if the FDA approves a psilocybin NDA.
The authors of this review recognize that opinions in the general population may differ substantially as it is clear that there remains a legacy of fear regarding psychedelics since the 1960s. The role of the 8-factor analysis of the CSA is to bring science to bear to support the foundation for scheduling, implications for other aspects of scheduling which are based on much of the same data. In particular, this means the labeling that will be specific to the label section, Drug Abuse and Dependence (section 9 of the drug labeling), and warnings including the possible requirement of a Boxed Warning (U.S. Food and Drug Administration, 2017d). As with all approved drug products, determination of safe and effective by FDA does not mean without risk, and the conclusion that the science does not support scheduling more restrictive than IV does not mean no abuse or dependence risk.
3.2. Implications for research and policy
This analysis has implications for future research with psilocybin and for the possible development of related drugs. Perhaps most challenging and important is research to better understand the mechanisms of action of psilocybin and related drugs that can produce profound and very long lasting positive changes in mood and well-being in people who were resistant to standard care and approved medicines. Given the extent to which undertreated and treatment resistant mental and behavioral disorders, including mood, anxiety, and substance use disorders, remain serious problems at the personal and societal levels in the US and globally (Belouin and Henningfield, 2018), it could be concluded that the need for such research is urgent.
The dearth of therapeutic and mechanistic studies of psilocybin and other classic psychedelics over the past half-century does not stem from a lack of interest among psychologists, psychiatrists, pharmacologists and neuroscientists. Research has been and continues to be limited by the provisions of the CSA and the lack of prioritization of such research by potential federal funding agencies. As discussed elsewhere, the barriers to research imposed by Schedule I regulation are formidable and although they do not outright ban such research, the consequence has been that this area of science and potential clinical application has been greatly under-researched (Belouin and Henningfield, 2018; Nutt, 2015; Nutt et al., 2013; Scientific American Editors, 2014; Sinha, 2001; Spillane, 2004; Woodworth, 2011). Several of the key clinical studies have been primarily supported by private foundations rather than federal institutions such as NIH (Bogenschutz et al., 2015; Griffiths et al., 2016; Johnson et al., 2014; Ross et al., 2016).
The science of drug abuse potential assessment has evolved considerably in recent decades and this is evident in the FDA’s 2017 guidance document, “Assessment of Abuse Potential of Drugs,” that summarizes research strategies, and methods and discusses how these can be brought to bear to provide the regulatory science foundation for drug scheduling decisions. The application of this scientific approach to further evaluate the abuse potential of psilocybin provides an example of how this area of regulatory science has the potential to facilitate innovative therapeutic breakthroughs by replacing fear and misinformation with scientifically based conclusions and facts.
Supplementary Material
Highlights.
Psilocybin mushrooms have been used for millennia for spiritual and medical purposes
Animal and human studies indicate low abuse and no physical dependence potential
Major national surveys indicate low rates of abuse, treatment-seeking and harm
Psilocybin may provide therapeutic benefits supporting its development as a new drug
Analysis supports the scheduling of psilocybin no more restrictively than Schedule IV
Acknowledgements
The authors are grateful for the care and efforts of Yolanda Green, and Evan Schnoll for referencing and formatting of the manuscript, Daniel Wang and Mark Sembrower for assistance with the surveillance data presented in factors 4, 5, and 6, and to Reginald Fant for advice on the abuse potential assessment, and Nora Belblidia for proofreading assistance.
Funding:
Drs. Johnson, Griffiths and Hendricks have received research grants from the Heffter Research Institute, and support from Usona for travel related to research with psilocybin but not directly related to this manuscript. Dr. Griffiths acknowledges partial support in writing this paper by NIH grant RO1DA03889. Dr. Henningfield’s efforts in the writing of this review were supported by Pinney Associates without input from any commercial sources. Neither Heffter, Usona, or any other organization or commercial interest have had input into the writing of this review or its conclusions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Roland Griffiths is on the Board of Directors of the Heffter Research Institute which supports psilocybin research and the potential development and submission of an NDA to the United States Food and Drug Administration (US FDA).
Through, Pinney Associates, Jack Henningfield has consulted and/or are presently consulting to the Heffter Research Institute and to the Usona Institute which are supporting the development of psilocybin as a new medication to be submitted for approval by the U.S. FDA, as well as to other sponsors of central nervous system acting products concerning their abuse potential, appropriate regulation, and medicinal application.
Schedule I of the CSA is reserved for substances determined by DEA to “have a high potential for abuse, no currently accepted medical use in treatment in the United States, and a lack of accepted safety for use under medical supervision.” This includes substances that were determined to warrant placement in Schedule I when the CSA was enacted into law in 1970, and substances that have not been approved by FDA for medical use but were placed in Schedule I based on DEA’s 8-factor analysis, or temporarily placed (also commonly termed “emergency scheduled”) in Schedule I if DEA determines such placement “is necessary to avoid an imminent hazard to the public safety.” For such scheduling the DEA is required to consider only factors 4, 5 and 6 of the CSA, namely, the substance’s history and current pattern of abuse; the scope, duration and significance of abuse; and what, if any, risk there is to the public health, respectively (Calderon et al., 2017; Drug Enforcement Administration, 2017a; Henningfield et al., 2017; Pinney Associates, 2016; U.S. Food and Drug Administration, 2017a).
References
- Abramson HA, Jarvik ME, Gorin MH, Hirsch MW, 1956. Lysergic Acid Diethylamide (LSD-25): XVII. Tolerance Development and its Relationship to a Theory of Psychosis. The Journal of Psychology 41, 81–105. [Google Scholar]
- Abramson HA, Rolo A, 1965. Lysergic acid diethylamide (LSD-25). 38. Comparison with action of methysergide and psilocybin on test subjects. J Asthma Res 3, 81–96. [DOI] [PubMed] [Google Scholar]
- Abramson HA, Rolo A, Stache J, 1960. Lysergic acid diethylamide (LSD-25) antagonists: chlorpromazine. J Neuropsychiatr 1, 307–310. [PubMed] [Google Scholar]
- Akers BP, Ruiz JF, Piper A, Ruck CAP, 2011. A Prehistoric Mural in Spain Depicting Neurotropic Psilocybe Mushrooms?1. Economic Botany 65, 121–128. [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and statistical manual of mental disorders : DSM-5 [Google Scholar]
- Anson P, 2014. Pain Experts Predict Problems with Hydrocode Rescheduling http://nationalpainreport.com/pain-experts-predict-problems-with-hydrocodone-rescheduling-8824655.html (Accessed; August 17)
- Anthony JC, Warner LA, Kessler RC, 1994. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Experimental and Clinical Psychopharmacology 2, 244–268. [Google Scholar]
- Appel JB, Callahan PM, 1989. Involvement of 5-HT receptor subtypes in the discriminative stimulus properties of mescaline. European Journal of Pharmacology 159, 41–46. [DOI] [PubMed] [Google Scholar]
- Appel JB, Freedman DX, 1968. Tolerance and cross-tolerance among psychotomimetic drugs. Psychopharmacologia 13, 267–274. [DOI] [PubMed] [Google Scholar]
- Baker LE, 2017. Hallucinogens in Drug Discrimination. Curr Top Behav Neurosci [DOI] [PubMed] [Google Scholar]
- Balestrieri C, 1967. On the action mechanisms of LSD 25. In: Abramson HA, (Ed), The use of LSD in psychotherapy and alcoholism Bobbs Merrill, Indianapolis, New York, Kansas City, pp. 653–660. [Google Scholar]
- Barrigar RH, 1964. The Regulation of Psychedelic Drugs. The Psychedelic Review 1, 394–441. [Google Scholar]
- Belleville RE, Fraser HF, Isbell H, Logan CR, Wikler A, 1956. Studies on lysergic acid diethylamide (LSD-25). I. Effects in former morphine addicts and development of tolerance during chronic intoxication. AMA Arch Neurol Psychiatry 76, 468–478. [PubMed] [Google Scholar]
- Belouin SJ, Henningfield JE, 2018. Psychedelics: Where we are now, why we got here, what we must do. Neuropharmacology [DOI] [PubMed] [Google Scholar]
- Bigwood J, Beug MW, 1982. Variation of psilocybin and psilocin levels with repeated flushes (harvests) of mature sporocarps of Psilocybe cubensis (Earle) Singer. J Ethnopharmacol 5, 287–291. [DOI] [PubMed] [Google Scholar]
- Blanchette CM, Nunes AP, Lin ND, Mortimer KM, Noone J, Tangirala K, Johnston S, Gutierrez B, 2015. Adherence to risk evaluation and mitigation strategies (REMS) requirements for monthly testing of liver function. Drugs Context 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa PC, Strassman RJ, 2015. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol 29, 289–299. [DOI] [PubMed] [Google Scholar]
- Bonson KR, 2018. Regulation of human research with LSD in the United States (1949–1987). Psychopharmacology (Berl) 235, 591–604. [DOI] [PubMed] [Google Scholar]
- Brandenburg NA, Bwire R, Freeman J, Houn F, Sheehan P, Zeldis JB, 2017. Effectiveness of Risk Evaluation and Mitigation Strategies (REMS) for Lenalidomide and Thalidomide: Patient Comprehension and Knowledge Retention. Drug Saf 40, 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Psychological Society, 2014. Hallucinogens: heaven and hell [special issue] [Google Scholar]
- Calderon SN, Hunt J, Klein M, 2017. A regulatory perspective on the evaluation of hallucinogen drugs for human use. Neuropharmacology [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Bolstridge M, Rucker J, Day CM, Erritzoe D, Kaelen M, Bloomfield M, Rickard JA, Forbes B, Feilding A, Taylor D, Pilling S, Curran VH, Nutt DJ, 2016a. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry 3, 619–627. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Erritzoe D, Williams T, Stone JM, Reed LJ, Colasanti A, Tyacke RJ, Leech R, Malizia AL, Murphy K, Hobden P, Evans J, Feilding A, Wise RG, Nutt DJ, 2012. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci U S A 109, 2138–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Muthukumaraswamy S, Roseman L, Kaelen M, Droog W, Murphy K, Tagliazucchi E, Schenberg EE, Nest T, Orban C, Leech R, Williams LT, Williams TM, Bolstridge M, Sessa B, McGonigle J, Sereno MI, Nichols D, Hellyer PJ, Hobden P, Evans J, Singh KD, Wise RG, Curran HV, Feilding A, Nutt DJ, 2016b. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proceedings of the National Academy of Sciences 113, 4853–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Nutt DJ, 2013. Experienced drug users assess the relative harms and benefits of drugs: a web-based survey. J Psychoactive Drugs 45, 322–328. [DOI] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR, 2009. Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug Alcohol Depend 105 Suppl 1, S14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Pardi D, Gorsline J, Griffiths RR, 2009. Illicit gamma-hydroxybutyrate (GHB) and pharmaceutical sodium oxybate (Xyrem): differences in characteristics and misuse. Drug Alcohol Depend 104, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Richards BD, Mintzer MZ, Griffiths RR, 2006. Relative abuse liability of GHB in humans: A comparison of psychomotor, subjective, and cognitive effects of supratherapeutic doses of triazolam, pentobarbital, and GHB. Neuropsychopharmacology 31, 2537–2551. [DOI] [PubMed] [Google Scholar]
- Carter OL, Burr DC, Pettigrew JD, Wallis GM, Hasler F, Vollenweider FX, 2005. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J Cogn Neurosci 17, 1497–1508. [DOI] [PubMed] [Google Scholar]
- Carter OL, Hasler F, Pettigrew JD, Wallis GM, Liu GB, Vollenweider FX, 2007. Psilocybin links binocular rivalry switch rate to attention and subjective arousal levels in humans. Psychopharmacology (Berl) 195, 415–424. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2015. Alcohol Poisoning Deaths. Vital Signs https://www.cdc.gov/vitalsigns/alcohol-poisoning-deaths/index.html (Accessed; October 18) [Google Scholar]
- Centers for Disease Control and Prevention, 2017. Impaired Driving: Get the Facts. Motor Vehicle Safety https://www.cdc.gov/motorvehiclesafety/impaired_driving/impaired-drv_factsheet.html (Accessed; October 18) [Google Scholar]
- Cerletti A, 1958. Etude pharmacologique de la psilocybine. In: Heim R, Wasson RG, (Eds), Les champignons hallucinogenes du Mexique Museum d’Historie Naturelle, Paris, pp. 268–271. [Google Scholar]
- Coleman JJ, 2015. Rescheduling Hydrocodone Combination Products: Addressing the Abuse of America’s Favorite Opioid. American Society of Addiction Medicine Magazine https://www.asam.org/resources/publications/magazine/read/article/2015/04/10/rescheduling-hydrocodone-combination-products-addressing-the-abuse-of-america-s-favorite-opioid [Google Scholar]
- Corti EC, 1931. A History of Smoking George G. Harrap & Co. Ltd., London [Google Scholar]
- Costandi M, 2014. A brief history of psychedelic psychiatry. The Guardian https://www.theguardian.com/science/neurophilosophy/2014/sep/02/psychedelic-psychiatry (Accessed; August 16) [Google Scholar]
- Crocq M-A, 2007. Historical and cultural aspects of man’s relationship with addictive drugs. Dialogues in Clinical Neuroscience 9, 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Appel JB, 1987. Neuropharmacological reassessment of the discriminative stimulus properties of d-lysergic acid diethylamide (LSD). Psychopharmacology (Berl) 91, 67–73. [DOI] [PubMed] [Google Scholar]
- Dart RC, 2009. Monitoring risk: post marketing surveillance and signal detection. Drug Alcohol Depend 105 Suppl 1, S26–32. [DOI] [PubMed] [Google Scholar]
- Dasgupta N, Schnoll SH, 2009. Signal detection in post-marketing surveillance for controlled substances. Drug Alcohol Depend 105 Suppl 1, S33–41. [DOI] [PubMed] [Google Scholar]
- de Borhegyi SF, 1961. Miniature Mushroom Stones from Guatemala. American Antiquity 26, 498–504. [Google Scholar]
- de Veen BT, Schellekens AF, Verheij MM, Homberg JR, 2017. Psilocybin for treating substance use disorders? Expert Rev Neurother 17, 203–212. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration, 1995. LSD in the United States In: Department of Justice, (Ed). http://www.druglibrary.org/schaffer/dea/pubs/lsd/intro.htm [Google Scholar]
- Drug Enforcement Administration, 2002. Schedule of Controlled Substances: Proposed Rule: Rescheduling of Buprenorphine From Schedule V to Schedule III. In: Justice, U. S. D. o, (Ed). Federal Register, pp. 13114–13116. https://www.federalregister.gov/documents/2002/03/21/02-6767/schedule-of-controlled-substances-proposed-rule-rescheduling-of-buprenorphine-from-schedule-v-to [Google Scholar]
- Drug Enforcement Administration Diversion Control Division, 2011. National Forensic Laboratory Information System (NFLIS) 2010 Annual Report. Springfield, VA: https://www.nflis.deadiversion.usdoj.gov/DesktopModules/ReportDownloads/Reports/NFLIS2010AR.pdf (Accessed; July 25, 2017). [Google Scholar]
- Drug Enforcement Administration Diversion Control Division, 2012. National Forensic Laboratory Information System (NFLIS) 2011 Annual Report. Springfield, VA: https://www.nflis.deadiversion.usdoj.gov/DesktopModules/ReportDownloads/Reports/NFLIS2011AR.pdf (Accessed; July 25, 2017). [Google Scholar]
- Drug Enforcement Administration Diversion Control Division, 2013. National Forensic Laboratory Information System (NFLIS) 2012 Annual Report. Springfield, VA: https://www.nflis.deadiversion.usdoj.gov/DesktopModules/ReportDownloads/Reports/NFLIS2012AR.pdf (Accessed; July 25, 2017). [Google Scholar]
- Drug Enforcement Administration Diversion Control Division, 2014. National Forensic Laboratory Information System (NFLIS) 2013 Annual Report. Springfield, VA: https://www.nflis.deadiversion.usdoj.gov/DesktopModules/ReportDownloads/Reports/NFLIS2013AR.pdf (Accessed; July 25, 2015). [Google Scholar]
- Drug Enforcement Administration Diversion Control Division, 2015. National Forensic Laboratory Information System (NFLIS) 2014 Annual Report. Springfield, VA: https://www.nflis.deadiversion.usdoj.gov/DesktopModules/ReportDownloads/Reports/NFLIS2014AR.pdf (Accessed; July 25, 2017). [Google Scholar]
- Drug Enforcement Administration, 2013. Schedules of Controlled Substances: Placement of Perampanel into Schedule III. In: Justice, U. S. D. o., (Ed). Federal Register, pp. 62500–62506. https://www.federalregister.gov/documents/2013/10/22/2013-24600/schedules-of-controlled-substances-placement-of-perampanel-into-schedule-iii [PubMed] [Google Scholar]
- Drug Enforcement Administration, 2014. Schedules of Controlled Substances: Removal of Naloxegol From Control. In: Justice, U. S. D. o., (Ed). Federal Register, pp. 64349–64353. [PubMed] [Google Scholar]
- Drug Enforcement Administration, 2017a. Controlled Substances Act https://www.dea.gov/druginfo/csa.shtml (Accessed; August 28)
- Drug Enforcement Administration, 2017b. Schedules of Controlled Substances: Placement of FDA-Approved Products of Oral Solutions Containing Dronabinol [(−)-delta-9-trans-tetrahydrocannabinol (delta-9-THC)] in Schedule II. In: Justice, U. S. D. o., (Ed). Federal Register, pp. 14815–14820. https://www.federalregister.gov/documents/2017/03/23/2017-05809/schedules-of-controlled-substances-placement-of-fda-approved-products-of-oral-solutions-containing [PubMed] [Google Scholar]
- Drug Enforcement Administration Diversion Control Division, 2016. National Forensic Laboratory Information System (NFLIS) 2015 Annual Report Springfield, VA: https://www.nflis.deadiversion.usdoj.gov/DesktopModules/ReportDownloads/Reports/NFLIS2015AR.pdf (Accessed; July 25, 2017) [Google Scholar]
- Fadiman J, 2011. The Psychedelic Explorer’s Guide : Safe, therapeutic, and sacred journeys Park Street Press, Rochester, Vt: xiv, 336 p. [Google Scholar]
- Fantegrossi WE, Murnane KS, Reissig CJ, 2008. The behavioral pharmacology of hallucinogens. Biochem Pharmacol 75, 17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Woods JH, Winger G, 2004. Transient reinforcing effects of phenylisopropylamine and indolealkylamine hallucinogens in rhesus monkeys. Behav Pharmacol 15, 149–157. [DOI] [PubMed] [Google Scholar]
- Fraser HF, Van Horn GD, Martin WR, Wolbach AB, Isbell H, 1961. Methods for evaluating addiction liability. (A) “Attitude” of opiate addicts toward opiate-like drugs. (B) a short-term “direct” addiction test. J Pharmacol Exp Ther 133, 371–387. [PubMed] [Google Scholar]
- Gable RS, 1993. Toward a comparative overview of dependence potential and acute toxicity of psychoactive substances used nonmedically. Am J Drug Alcohol Abuse 19, 263–281. [DOI] [PubMed] [Google Scholar]
- Gable RS, 2004. Comparison of acute lethal toxicity of commonly abused psychoactive substances. Addiction 99, 686–696. [DOI] [PubMed] [Google Scholar]
- Garcia-Romeu A, Griffiths RR, Johnson MW, 2014. Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Curr Drug Abuse Rev 7, 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Drug Survey, 2017. Global Drug Survey https://www.globaldrugsurvey.com/ (Accessed; February 2)
- Gouzoulis-Mayfrank E, Thelen B, Habermeyer E, Kunert HJ, Kovar KA, Lindenblatt H, Hermle L, Spitzer M, Sass H, 1999. Psychopathological, neuroendocrine and autonomic effects of 3,4-methylenedioxyethylamphetamine (MDE), psilocybin and d-methamphetamine in healthy volunteers. Results of an experimental double-blind placebo-controlled study. Psychopharmacology (Berl) 142, 41–50. [DOI] [PubMed] [Google Scholar]
- Grabowski HG, 1976. Drug regulation and innovation : empirical evidence and policy options American Enterprise Institute for Public Policy Research, Washington: 82 p. [Google Scholar]
- Griffiths R, Johnson M, 2015. The Case for Rescheduling Psilocybin as a Treatment Medication: Regulatory Rationale, Abuse Liability, Safety, and Treatment Efficacy. Conference on Problems of Drug Dependence, Phoenix, Arizona. [Google Scholar]
- Griffiths R, Richards W, Johnson M, McCann U, Jesse R, 2008. Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J Psychopharmacol 22, 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Ator NA, 2003. Principles of initial experimental drug abuse liability assessment in humans. Drug Alcohol Depend 70, S41–54. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Carducci MA, Umbricht A, Richards WA, Richards BD, Cosimano MP, Klinedinst MA, 2016. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharmacol 30, 1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, Richards BD, Jesse R, MacLean KA, Barrett FS, Cosimano MP and Klinedinst MA, in press. Psilocybin-occasioned mystical-type experience in combination with meditation and other spiritual practices produces enduring positive changes in psychological functioning and in trait measures of prosocial attitudes and behaviors. Journal of Psychopharmacology, p.0269881117731279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, Richards BD, McCann U, Jesse R, 2011. Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects. Psychopharmacology (Berl) 218, 649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinspoon L, 1981. LSD Reconsidered. The Sciences 21, 20–23. [Google Scholar]
- Grinspoon L, Bakalar JB, 1979. Psychedelic drugs reconsidered Basic Books, New York: xiv, 343 p. [Google Scholar]
- Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstadt AL, Greer GR, 2011. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry 68, 71–78. [DOI] [PubMed] [Google Scholar]
- Haertzen CA, Hickey JE, 1987. Addiction Research Center Inventory (ARCI): Measurement of Euphoria and Other Drug Effects. In: Bozarth MA, (Ed), Methods of Assessing the Reinforcing Properties of Abused Drugs Springer-Verlag, New York, pp. 489–524. [Google Scholar]
- Haertzen CA, Hill HE, Belleville RE, 1963. Development of the Addiction Research Center Inventory (Arci): Selection of Items That Are Sensitive to the Effects of Various Drugs. Psychopharmacologia 4, 155–166. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA, 2011. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology 61, 364–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RT, Balster RL, 1971. An Analysis of the Function of Drugs in the Stimulus Control of Operant Behavior. In: Thompson T, Pickens R, (Eds), Stimulus Properties of Drugs. Appleton-Century-Crofts Educational Division/Meredith Coporation, New York, pp. 111–132. [Google Scholar]
- Hasler F, 1997. Untersuchungen zur Humanpharmakokinetik von Psilocybin [Google Scholar]
- Hasler F, Grimberg U, Benz MA, Huber T, Vollenweider FX, 2004. Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology (Berl) 172, 145–156. [DOI] [PubMed] [Google Scholar]
- Hayes DJ, Greenshaw AJ, 2011. 5-HT receptors and reward-related behaviour: a review. Neurosci Biobehav Rev 35, 1419–1449. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Gosden J, Smith SL, 2018. Evaluating the abuse potential of psychedelic drugs as part of the safety pharmacology assessment for medical use in humans. Neuropharmacology [DOI] [PubMed] [Google Scholar]
- Heffter Research Institute, 2017. Heffter Research Institute http://heffter.org/ (Accessed; August 22)
- Hendricks PS, Clark CB, Johnson MW, Fontaine KR, Cropsey KL, 2014. Hallucinogen use predicts reduced recidivism among substance-involved offenders under community corrections supervision. J Psychopharmacol 28, 62–66. [DOI] [PubMed] [Google Scholar]
- Hendricks PS, Johnson MW, Griffiths RR, 2015a. Psilocybin, psychological distress, and suicidality. J Psychopharmacol 29, 1041–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks PS, Thorne CB, Clark CB, Coombs DW, Johnson MW, 2015b. Classic psychedelic use is associated with reduced psychological distress and suicidality in the United States adult population. J Psychopharmacol 29, 280–288. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Fant RV, Wang DW, 2017. The abuse potential of kratom according the 8 factors of the controlled substances act: implications for regulation and research. Psychopharmacology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Smith TT, Kleykamp BA, Fant RV, Donny EC, 2016. Nicotine self-administration research: the legacy of Steven R. Goldberg and implications for regulation, health policy, and research. Psychopharmacology (Berl) 233, 3829–3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill HE, Haertzen CA, Wolbach AB Jr., Miner EJ, 1963. The Addiction Research Center Inventory: Standardization of Scales Which Evaluate Subjective Effects of Morphine, Amphetamine, Pentobarbital, Alcohol, Lsd-25, Pyrahexyl and Chlorpromazine. Psychopharmacologia 4, 167–183. [DOI] [PubMed] [Google Scholar]
- Hofmann A, 1980. LSD, my problem child McGraw-Hill, New York: xiii, 209 p. [Google Scholar]
- Hollister LE, 1961. Clinical, biochemical and psychologic effects of psilocybin. Arch Int Pharmacodyn Ther 130, 42–52. [PubMed] [Google Scholar]
- Holzmann PP, 1995. Bestimmung Von Psilocybin-Metaboliten Im Humanplasma Und Urin University of Tuebingen, Tuebingen, Germany. [Google Scholar]
- Horibe M, 1974. The effects of psilocybin on EEG and behaviour in monkeys. Act Nerv Super (Praha) 16, 40–42. [PubMed] [Google Scholar]
- Horowitz M, 1976. Interview with Albert Hofmann. High Times [Google Scholar]
- Isbell H, 1956. Trends in research on opiate addiction. Trans Stud Coll Physicians Phila 24, 1–10. [PubMed] [Google Scholar]
- Isbell H, 1959a. Comparison of the reactions induced by psilocybin and LSD-25 in man. Psychopharmacologia 1, 29–38. [DOI] [PubMed] [Google Scholar]
- Isbell H, 1959b. Effects of various drugs on the LSD reaction. In: Kline NS, (Ed), Psychopharmacology Frontiers Little, Brown and Co., Boston, pp. 362–364. [Google Scholar]
- Isbell H, Jasinski DR, 1969. A comparison of LSD-25 with (−)-delta-9-trans-tetrahydrocannabinol (THC) and attempted cross tolerance between LSD and THC. Psychopharmacologia 14, 115–123. [DOI] [PubMed] [Google Scholar]
- Isbell H, Wolbach AB, Wikler A, Miner EJ, 1961. Cross tolerance between LSD and psilocybin. Psychopharmacologia 2, 147–159. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, 1980. LDS-25 as a discriminative stimulus for response selection by pigeons. Pharmacol Biochem Behav 13, 549–554. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Henningfield JE, 1989. Human abuse liability assessment by measurement of subjective and phsyiological effects. In: Fischman MW, Mello NK, (Eds), Testing for abuse liability of drugs in humans U.S. Government Printing Office, Washington, D.C., pp. 73–100. [PubMed] [Google Scholar]
- Jasinski DR, Johnson RE, Henningfield JE, 1984. Abuse liability assessment in human subjects. Trends in Pharmacological Sciences 5, 196–200. [Google Scholar]
- Johnson M, Ettinger RH, 2000. Active cocaine immunization attenuates the discriminative properties of cocaine. Experimental and Clinical Psychopharmacology 8, 163–167. [DOI] [PubMed] [Google Scholar]
- Johnson M, Richards W, Griffiths R, 2008. Human hallucinogen research: guidelines for safety. J Psychopharmacol 22, 603–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Garcia-Romeu A, Cosimano MP, Griffiths RR, 2014. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol 28, 983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Garcia-Romeu A, Griffiths RR, 2017. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse 43, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Griffiths RR, 2013. Comparative abuse liability of GHB and ethanol in humans. Exp Clin Psychopharmacol 21, 112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Griffiths RR, 2017. Potential Therapeutic Effects of Psilocybin. Neurotherapeutics 14, 734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Sewell RA, Griffiths RR, 2012. Psilocybin dose-dependently causes delayed, transient headaches in healthy volunteers. Drug Alcohol Depend 123, 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebler J, 2015. A Brief History of Microdosing. Motheboard https://motherboard.vice.com/en_us/article/gv5p5y/a-brief-history-of-microdosing (Accessed; February 2) [Google Scholar]
- Koerner J, Appel JB, 1982. Psilocybin as a discriminative stimulus: lack of specificity in an animal behavior model for ‘hallucinogens’. Psychopharmacology (Berl) 76, 130–135. [DOI] [PubMed] [Google Scholar]
- Kometer M, Schmidt A, Bachmann R, Studerus E, Seifritz E, Vollenweider FX, 2012. Psilocybin biases facial recognition, goal-directed behavior, and mood state toward positive relative to negative emotions through different serotonergic subreceptors. Biol Psychiatry 72, 898–906. [DOI] [PubMed] [Google Scholar]
- Kometer M, Schmidt A, Jancke L, Vollenweider FX, 2013. Activation of serotonin 2A receptors underlies the psilocybin-induced effects on alpha oscillations, N170 visual-evoked potentials, and visual hallucinations. J Neurosci 33, 10544–10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M, 2006. Neurobiology of addiction Elsevier/Academic Press, Amsterdam ; Boston: viii, 490 p. [Google Scholar]
- Kuhn D, Appel JB, Greenberg I, 1974. An analysis of some discriminative properties of d-amphetamine. Psychopharmacologia 39, 57–66. [DOI] [PubMed] [Google Scholar]
- Lee MA, Shlain B, 1992. Acid dreams : the complete social history of LSD : the CIA, the sixties, and beyond Grove Weidenfeld, New York: xxv, 345 p., 312 p. of plates [Google Scholar]
- Lewin L, 1998. Phantastica : a classic survey on the use and abuse of mind-altering plants Park Street Press, Rochester, Vt: xvi, 288 p. [Google Scholar]
- Lim TH, Wasywich CA, Ruygrok PN, 2012. A fatal case of ‘magic mushroom’ ingestion in a heart transplant recipient. Intern Med J 42, 1268–1269. [DOI] [PubMed] [Google Scholar]
- Lowy B, 1971. New records of mushroom stones from Guatemala. Mycologia 63, 983–993. [PubMed] [Google Scholar]
- MacLean KA, Johnson MW and Griffiths RR, 2011. Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. Journal of Psychopharmacology, 25(11), pp.1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone N, 2016. Why Power Women Are Micro-Dosing LSD at Work. Marie Claire http://www.marieclaire.com/culture/news/a23669/power-women-microdosing-lsd/ (Accessed; February 2) [Google Scholar]
- Martin WR, 1973. Assessment of the abuse potentiality of amphetamines and LSD-like hallucinogens in man and its relationship to basic animal assessment programs. Psychic [Google Scholar]
- Matsushima Y, Eguchi F, Kikukawa T, Matsuda T, 2009. Historical overview of psychoactive mushrooms. Inflammation and Regeneration 29, 47–58. [Google Scholar]
- McCormick CG, Henningfield JE, Haddox JD, Varughese S, Lindholm A, Rosen S, Wissel J, Waxman D, Carter LP, Seeger V, Johnson RE, 2009. Case histories in pharmaceutical risk management. Drug Alcohol Depend 105 Suppl 1, S42–55. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Muetzelfeldt L, Muetzelfeldt M, Nutt DJ, Curran HV, 2010. Harms associated with psychoactive substances: findings of the UK National Drug Survey. J Psychopharmacol 24, 147–153. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism, 2017. Alcohol Facts and Statistics. Alcohol & Your Health https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/alcohol-facts-and-statistics (Accessed; October 18)
- National Institute on Drug Abuse, 2016. Understanding Drug Use and Addiction. DrugFacts https://www.drugabuse.gov/publications/drugfacts/understanding-drug-use-addiction (Accessed; August 16)
- Nichols DE, 2016. Psychedelics. Pharmacol Rev 68, 264–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE, Johnson MW, Nichols CD, 2017. Psychedelics as Medicines: An Emerging New Paradigm. Clin Pharmacol Ther 101, 209–219. [DOI] [PubMed] [Google Scholar]
- Novak SJ, 1997. LSD before Leary. Sidney Cohen’s critique of 1950s psychedelic drug research. Isis 88, 87–110. [DOI] [PubMed] [Google Scholar]
- Nutt D, 2015. Illegal drugs laws: clearing a 50-year-old obstacle to research. PLoS Biol 13, e1002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D, King LA, Saulsbury W, Blakemore C, 2007. Development of a rational scale to assess the harm of drugs of potential misuse. Lancet 369, 1047–1053. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, King LA, Nichols DE, 2013. Effects of Schedule I drug laws on neuroscience research and treatment innovation. Nat Rev Neurosci 14, 577–585. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, King LA, Phillips LD, Independent Scientific Committee on, D., 2010. Drug harms in the UK: a multicriteria decision analysis. Lancet 376, 1558–1565. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, 2011. Drug Addiction. In: Brunton LL, (Ed), Goodman & Gilman’s The Pharmacological Basis of Therapeutics McGrawHill Medical, New York, pp. 649–668. [Google Scholar]
- Passie T, Seifert J, Schneider U, Emrich HM, 2002. The pharmacology of psilocybin. Addict Biol 7, 357–364. [DOI] [PubMed] [Google Scholar]
- Penner J, 2015. The Rise and Fall of LSD Los Angeles Review of Books; https://lareviewofbooks.org/article/the-rise-and-fall-of-lsd/#! [Google Scholar]
- Petri G, Expert P, Turkheimer F, Carhart-Harris R, Nutt D, Hellyer PJ, Vaccarino F, 2014. Homological scaffolds of brain functional networks. Journal of The Royal Society Interface 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney Associates, 2016. Assessment of Kratom under the CSA Eight Factors and Scheduling Recommendation http://www.pinneyassociates.com/wp-content/uploads/2017/07/Kratom-8-Factor-by-PinneyAssoc-11.28.16.pdf
- Pisano VD, Putnam NP, Kramer HM, Franciotti KJ, Halpern JH, Holden SC, 2017. The association of psychedelic use and opioid use disorders among illicit users in the United States. J Psychopharmacol 31, 606–613. [DOI] [PubMed] [Google Scholar]
- Pollan M, 2015. The Trip Treatment The New Yorker; http://www.newyorker.com/magazine/2015/02/09/trip-treatment [Google Scholar]
- Quednow BB, Kometer M, Geyer MA, Vollenweider FX, 2012. Psilocybin-induced deficits in automatic and controlled inhibition are attenuated by ketanserin in healthy human volunteers. Neuropsychopharmacology 37, 630–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman L, Demetriou L, Wall MB, Nutt DJ, Carhart-Harris RL, 2017. Increased amygdala responses to emotional faces after psilocybin for treatment-resistant depression. Neuropharmacology 10.1016/j.neuropharm.2017.12.041 pii: S0028-3908(17)30639-1 [DOI] [PubMed] [Google Scholar]
- Ross S, Bossis A, Guss J, Agin-Liebes G, Malone T, Cohen B, Mennenga SE, Belser A, Kalliontzi K, Babb J, Su Z, Corby P, Schmidt BL, 2016. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol 30, 1165–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker JJH, Iliff J, Nutt DJ, 2017. Psychiatry & the psychedelic drugs. Past, present & future. Neuropharmacology [DOI] [PubMed] [Google Scholar]
- Rudisill TM, Zhao S, Abate MA, Coben JH, Zhu M, 2014. Trends in drug use among drivers killed in U.S. traffic crashes, 1999–2010. Accid Anal Prev 70, 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush B, 1808. An inquiry into the effects of ardent spirits upon the human body and mind : with an account of the means of preventing, and of the remedies for curing them Printed for Thomas Dobson ... Archibald Bartram, printer, Philadelphia: 50 p. [Google Scholar]
- Samorini G, 1992. The oldest representations of hallucinogenic mushrooms in the world (Sahara Desert, 9000–7000 B.P.). Integration 2, 69–78. [Google Scholar]
- Schartner MM, Carhart-Harris RL, Barrett AB, Seth AK, Muthukumaraswamy SD, 2017. Increased spontaneous MEG signal diversity for psychoactive doses of ketamine, LSD and psilocybin. Sci Rep 7, 46421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter MD, Rosecrans JA, 1972. Lysergic acid diethylamide (LSD) as a discriminative cue: drugs with similar stimulus properties. Psychopharmacologia 26, 313–316. [DOI] [PubMed] [Google Scholar]
- Schindler EA, Gottschalk CH, Weil MJ, Shapiro RE, Wright DA, Sewell RA, 2015. Indoleamine Hallucinogens in Cluster Headache: Results of the Clusterbusters Medication Use Survey. J Psychoactive Drugs 47, 372–381. [DOI] [PubMed] [Google Scholar]
- Schultes RE, 1969. Hallucinogens of plant origin. Science 163, 245–254. [DOI] [PubMed] [Google Scholar]
- Schultes RE, Hofmann A, Rätsch C, 2001. Plants of the gods : their sacred, healing, and hallucinogenic powers Healing Arts Press, Rochester, Vt: 208 p. [Google Scholar]
- Scientific American Editors, 2014. End the Ban on Psychoactive Drug Research. Scientific Americans https://www.scientificamerican.com/article/end-the-ban-on-psychoactive-drug-research/ [Google Scholar]
- Sellers EM, Romach MK, Leiderman DB, 2017. Studies with psychedelic drugs in human volunteers. Neuropharmacology [DOI] [PubMed] [Google Scholar]
- Sessa B, Johnson MW, 2015. Can psychedelic compounds play a part in drug dependence therapy? Br J Psychiatry 206, 1–3. [DOI] [PubMed] [Google Scholar]
- Sewell RA, Halpern JH, Pope HG Jr., 2006. Response of cluster headache to psilocybin and LSD. Neurology 66, 1920–1922. [DOI] [PubMed] [Google Scholar]
- Sinha J, 2001. The History and Development of the Leading International Drug Control Conventions. In: Division L. a. G., (Ed). Library of Parliament, Canada. [Google Scholar]
- Spillane JF, 2004. Debating the Controlled Substances Act. Drug Alcohol Depend 76, 17–29. [DOI] [PubMed] [Google Scholar]
- Stamets P, 1996. Psilocybin mushrooms of the world : an identification guide Ten Speed Press, Berkeley, Calif: ix, 245 p. [Google Scholar]
- Stevens J, 1987. Storming heaven : LSD and the American dream Atlantic Monthly Press, New York: xvii, 396 p. [Google Scholar]
- Studerus E, Gamma A, Kometer M, Vollenweider FX, 2012. Prediction of psilocybin response in healthy volunteers. PLoS One 7, e30800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studerus E, Gamma A, Vollenweider FX, 2010. Psychometric evaluation of the altered states of consciousness rating scale (OAV). PLoS One 5, e12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2013. Dug Abuse Warning Network, 2011: Selected Tables of National Estimates of Drug-Related Emergency Department Visits Center for Behavioral Health Statistics and Quality, Rockville, MD. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2017a. Client Level Data/TEDS https://www.samhsa.gov/data/client-level-data-teds/reports?tab=18 (Accessed; July 25)
- Substance Abuse and Mental Health Services Administration, 2017b. Population Data/NSDUH https://www.samhsa.gov/data/population-data-nsduh/reports (Accessed; July 25)
- Terry CE, Pellens M, 1970. The Opium Problem Patterson Smith, Montclair, New Jersey [Google Scholar]
- The Medical Letter, 2006. A New Indication for Gamma Hydroxybutyrate (Xyrem) for Narcolepsy. Med Lett Drugs Ther 48, 11–12. [PubMed] [Google Scholar]
- Truttman P, 2012. The Forgotten Mushrooms of Ancient Peru Global Mountain Action, Fungi and Mountains Publication Series; 1, 33. [Google Scholar]
- Tupper KW, Wood E, Yensen R, Johnson MW, 2015. Psychedelic medicine: a re-emerging therapeutic paradigm. CMAJ 187, 1054–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Congress, 2009. Family Smoking Prevention and Tobacco Control Act U.S. Government Printing Office, Washington, D.C. [Google Scholar]
- U.S. Congress, 2015. Improving Regulatory Transparency for New Medical Therapies Act U.S. Government Printing Office, Washington, D.C. [Google Scholar]
- U.S. Food and Drug Administration, 1996. Regulations Restricting the Sale and Distribution of Cigarettes and Smokeless Tobacco to Protect Children and Adolescents. In: U.S. Department of Health and Human Services, (Ed). Federal Register, pp. 44396–44618. https://www.gpo.gov/fdsys/pkg/FR-1996-08-28/pdf/X96-10828.pdf [PubMed] [Google Scholar]
- U.S. Food and Drug Administration, 2012. PDUFA Reauthorization Performance Goals and Procedures Fiscal Years 2013 Through 2017 In: U.S. Department of Health and Human Services, (Ed). https://www.fda.gov/downloads/ForIndustry/UserFees/PrescriptionDrugUserFee/UCM270412.pdf [Google Scholar]
- U.S. Food and Drug Administration, 2015. Risk Evaluation and Mitigation Strategies: Modifications and Revisions: Guidance for Industry In: U.S. Department of Health and Human Services, (Ed), Silver Spring, MD: https://www.fda.gov/downloads/Drugs/.../Guidances/UCM184128.pdf [Google Scholar]
- U.S. Food and Drug Administration, 2017a. Assessment of Abuse Potential of Drugs: Guidance for Industry In: U.S. Department of Health and Human Services, (Ed). https://www.fda.gov/downloads/drugs/guidances/ucm198650.pdf [Google Scholar]
- U.S. Food and Drug Administration, 2017b. Benefit-Risk Assessments in Drug Regulatory Decision-Making; Public Meeting; Request for Comments. Federal Register, pp. 37230–37232. https://www.federalregister.gov/documents/2017/08/09/2017-16720/benefit-risk-assessments-in-drug-regulatory-decision-making-public-meeting-request-for-comments [Google Scholar]
- U.S. Food and Drug Administration, 2017c. Development & Approval Process (Drugs) https://www.fda.gov/drugs/developmentApprovalProcess/default.htm (Accessed; OCtober 18)
- U.S. Food and Drug Administration, 2017d. Labeling. 21 https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=201.56
- U.S. Office of the Federal Register, 2011. A Guide to the Rulemaking Process. Federal Register https://www.federalregister.gov/uploads/2011/01/the_rulemaking_process.pdf [Google Scholar]
- Usona Institute, 2017. Usona Institute http://www.usonainstitute.org/ (Accessed; August 22)
- van Amsterdam J, Nutt D, Phillips L, van den Brink W, 2015. European rating of drug harms. J Psychopharmacol 29, 655–660. [DOI] [PubMed] [Google Scholar]
- van Amsterdam J, Opperhuizen A, Koeter M, van den Brink W, 2010. Ranking the harm of alcohol, tobacco and illicit drugs for the individual and the population. Eur Addict Res 16, 202–207. [DOI] [PubMed] [Google Scholar]
- van Amsterdam J, Opperhuizen A, van den Brink W, 2011. Harm potential of magic mushroom use: a review. Regul Toxicol Pharmacol 59, 423–429. [DOI] [PubMed] [Google Scholar]
- Van Went GF, 1978. Mutagenicity testing of 3 hallucinogens: LSD, psilocybin and delta 9-THC, using the micronucleus test. Experientia 34, 324–325. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D, 1998. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9, 3897–3902. [DOI] [PubMed] [Google Scholar]
- Waldman A, 2017. A really good day : how microdosing made a mega difference in my mood, my marriage, and my life Alfred A. Knopf, New York: xxii, 229 pages [Google Scholar]
- Walsh Z, Hendricks PS, Smith S, Kosson DS, Thiessen MS, Lucas P, Swogger MT, 2016. Hallucinogen use and intimate partner violence: Prospective evidence consistent with protective effects among men with histories of problematic substance use. J Psychopharmacol 30, 601–607. [DOI] [PubMed] [Google Scholar]
- Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL, 2009. Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem): abuse, misuse, dependence, and diversion. J Clin Sleep Med 5, 365–371. [PMC free article] [PubMed] [Google Scholar]
- Waugh R, 2016. Professor was injected with magic mushrooms to have ‘near death experience’. Metro http://metro.co.uk/2016/07/19/professor-was-injected-with-magic-mushrooms-to-have-near-death-experience-6016804/ (Accessed; August 17)
- Wikler A, 1961. On the nature of addiction and habituation. British Journal of Addiction 57, 73–80. [Google Scholar]
- Winter JC, Rice KC, Amorosi DJ, Rabin RA, 2007. Psilocybin-induced stimulus control in the rat. Pharmacol Biochem Behav 87, 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbach AB Jr., Miner EJ, Isbell H, 1962. Comparison of psilocin with psilocybin, mescaline and LSD-25. Psychopharmacologia 3, 219–223. [DOI] [PubMed] [Google Scholar]
- Woodworth TW, 2011. How Will DEA Affect Your Clinical Study? Journal of Clinical Research Best Practices 7. [Google Scholar]
- World Health Organization, 1993. ICD-10 Classification of Mental and Behavioural Disorders (The): Diagnostic Criteria for Research World Health Organization [Google Scholar]
- Wu J, Juhaeri J, 2016. The US Food and Drug Administration’s Risk Evaluation and Mitigation Strategy (REMS) Program - Current Status and Future Direction. Clin Ther 38, 2526–2532. [DOI] [PubMed] [Google Scholar]
- Strassman R, 2001. DMT : The Spirit Molecule : A Doctor's Revolutionary Research Into the Biology of Near-Death And Mystical Experiences. Park Street Press, Rochester, Vt xviii, 358 p. [Google Scholar]
- Mowry JB, Spyker DA, Cantilena LR Jr., Bailey JE, Ford M, 2013. 2012 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 30th annual report. Clin. Toxicol (Phila) 51, 949–1229. [DOI] [PubMed] [Google Scholar]
- Mowry JB, Spyker DA, Cantilena LR Jr., McMillan N, Ford M, 2014. 2013 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin. Toxicol. (Phila) 52, 1032–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowry JB, Spyker DA, Brooks DE, McMillan N, Schauben JL, 2015. 2014 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 32nd Annual Report. Clin. Toxicol. (Phila) 53, 962–1147. [DOI] [PubMed] [Google Scholar]
- Mowry JB, Spyker DA, Brooks DE, Zimmerman A, Schauben JL, 2016. 2015 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 33rd Annual Report. Clin. Toxicol. (Phila) 54, 924–1109. [DOI] [PubMed] [Google Scholar]
- Moreno FA, Wiegand CB, Taitano EK, Delgado PL, 2006. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J. Clin. Psychiatr 67, 1735–1740. [DOI] [PubMed] [Google Scholar]
- Himmelsbach CK, Andrews HL, 1943. Studies on modification of the morphine abstinence syndrome by drugs. J. Pharmacol. Exp. Therapeut 77, 17–23. [Google Scholar]
- Isbell H, Logan CR, 1957. Studies on the diethylamide of lysergic acid (LSD-25). II. Effects of chlorpromazine, azacyclonol, and reserpine on the intensity of the LSD-reaction. AMA Arch. Neurol. Psychiatr 77 (4), 350–358. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, Angst J, 1997. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology 16, 357–372. [DOI] [PubMed] [Google Scholar]
- Wasson RG, 1959. The hallucinogenic mushrooms of Mexico: an adventure in enthomycological exploration. Trans. N. Y. Acad. Sci 21, 325. [Google Scholar]
- Wasson VP, Wasson RG, 1957. Mushrooms, Russia, and History. Pantheon Books, New York, 432 p. [Google Scholar]
- Spitzer M, Thimm M, Hermle L, Holzmann P, Kovar KA, Heimann H, Gouzoulis-Mayfrank E, Kischka U, Schneider F, 1996. Increased activation of indirect semantic associations under psilocybin. Biol. Psychiatr 39, 1055–1057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


