SUMMARY
Inflammasome activation and subsequent pyroptosis are critical defense mechanisms against microbes. However, overactivation of inflammasome leads to death of the host. While recent studies have uncovered the mechanism of pyroptosis following inflammasome activation, how pyroptotic cell death drives pathogenesis eventually leading to death of the host is unknown. Here we identified inflammasome activation as a trigger for blood clotting through pyroptosis. We have shown that canonical inflammasome activation by the conserved type III secretion system (T3SS) rod proteins from Gram-negative bacteria or noncanonical inflammasome activation by lipopolysaccharide (LPS) induced systemic blood clotting and massive thrombosis in tissues. Following inflammasome activation, pyroptotic macrophages released tissue factor (TF), an essential initiator of coagulation cascades. Genetic or pharmacological inhibition of TF abolished inflammasome-mediated blood clotting and protects against death. Our data reveal that blood clotting is the major cause of host death following inflammasome activation and demonstrate that inflammasome bridges inflammation with thrombosis.
Keywords: Inflammasome, Pyroptosis, Caspase, GSDMD, LPS, DIC, Coagulation, Tissue factor, Sepsis, T3SS, macrophage
Graphical Abstract

Overactivation of inflammasome leads to death of the host. Wu and colleagues demonstrate that activation of coagulation is responsible for inflammasome activation-induced death.
INTRODUCTION
Inflammasomes are intracellular sensory complexes that detect invading bacterial pathogens in the cytosol. Activation of the inflammatory caspases, including caspase-1 and caspase-11 (caspase-4 and −5 in human), by microbial products leads to interleukin (IL)-1β and IL-18 maturation and release and a lytic cell death termed pyroptosis (Fink and Cookson, 2006; Lamkanfi and Dixit, 2014; Schroder and Tschopp, 2010). Caspase-1 is activated by Gram-negative bacterial flagellin and type III secretion system (T3SS) apparatus proteins through NLRC4 inflammasome (Miao et al., 2010; Zhao et al., 2011). Caspase-11 is activated by cytoplasmic lipopolysaccharide (LPS) through noncanonical inflammasome pathway (Hagar et al., 2013; Kayagaki et al., 2013; Shi et al., 2014; Vanaja et al., 2016). Activated caspase-1 and −11 cleave gasdermin D (GSDMD), triggering the formation of pores in plasma membrane (Aglietti et al., 2016; Ding et al., 2016; Liu et al., 2016; Ruan et al., 2018; Sborgi et al., 2016). The pores formed by GSDMD have been shown to facilitate IL-1β release (Evavold et al., 2018; He et al., 2015; Monteleone et al., 2018). Formation of pores in cell membranes also drives pyroptosis, a form of osmotic cell lysis accompanied by ruptured cell membranes (He et al., 2015; Kayagaki et al., 2015; Shi et al., 2015). Inflammasome activation and subsequent pyroptosis support host defense against bacterial pathogens; however, their hyperactivity has devastating consequences, such as multiple organ dysfunction and lethality (Aziz et al., 2013; Zhao et al., 2016). T3SS rod proteins, flagellin, and LPS have been shown to trigger macrophage pyroptosis and death of the host (Kayagaki et al., 2013; Zhao et al., 2016). The underling mechanisms of host death following pyroptotic cell death of macrophages have not been defined.
Blood coagulation is essential for hemostasis to stop bleeding after injury. In contrast, disseminated intravascular coagulation (DIC) is a pathologic state of systemic activation of blood coagulation. DIC often accompanies sepsis and results in microvessel occlusion, organ dysfunction, and death (Gando et al., 2016; Levi and Cate, 1999). Mortality rate doubles in septic patients with DIC compared to patients without DIC (Fujishima et al., 2014; Gando et al., 2013; Rangel-Frausto et al., 1995; Venugopal, 2014). As the primary initiator of coagulation, tissue factor (TF) plays an essential role in initiating DIC in systemic inflammatory disorders including sepsis (Franco et al., 2000; Gando et al., 2016; Pawlinski et al., 2004; Pawlinski et al., 2010; Taylor et al., 1991). The mechanisms for blood coagulation induced by bacterial infection are often attributed to host inflammatory response to bacterial virulence factors (Gando et al., 2016), yet the molecular events linking bacterial infection to TF release that triggers coagulation cascade are unknown.
Here we show that inflammasome activation leads to TF release in the form of microvesicles (MV), which triggers systemic coagulation and lethality. TF release following inflammasome activation depends on pyroptosis. Deficiency of GSDMD, but not the receptor of IL-1β or IL-18, abolishes the activation of coagulation induced by E. coli T3SS rod protein EprJ. Pharmacological or genetical inhibition of TF prevented EprJ-induced DIC and lethality. Our findings identify a molecular mechanism of DIC in sepsis and reveal how inflammasome activation and pyroptosis lead to death of the host.
RESULTS
Inflammasome Activation by Bacterial Rod Protein EprJ Causes Systemic Coagulation
To identify the mechanism by which inflammasome activation leads to death of the host, we injected C57BL/6J mice with the E. coli T3SS inner rod protein EprJ. EprJ was fused to the cytosolic translocation domain of anthrax lethal factor (LFn) to enable efficient cytosolic delivery. LFn binds to anthrax protein protective agent (PA), which delivers the LFn-EprJ fusion protein into the cytoplasma through receptor-mediated endocytosis (Milne et al., 1995; Zhao et al., 2011). We found that purified EprJ (LFn-EprJ plus PA) induced robust caspase-1 activation, and pyroptosis (Figures S1A and S1B) in mouse primary bone marrow-derived macrophages (BMDMs). Intravenous injection of EprJ caused hemolysis in C57BL/6J mice (Figure S1C). Red blood cells did not rupture when incubated ex vivo with EprJ (Figure S1D), eliminating a direct effect of EprJ on red blood cells leading to hemolysis.
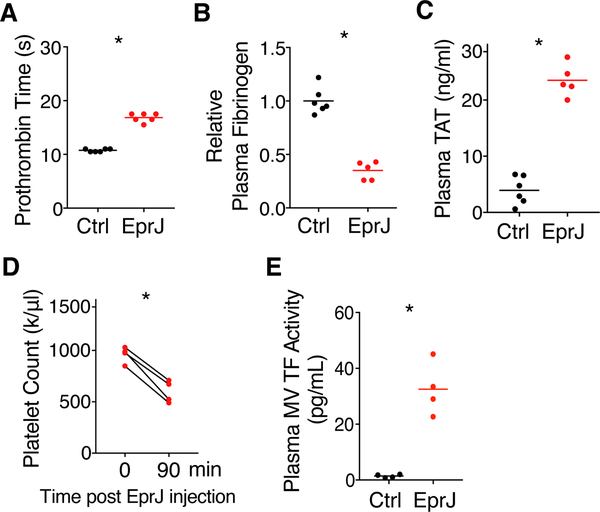
As hemolysis could be a consequence of DIC (Effenberger-Neidnicht and Hartmann, 2018), we investigated whether EprJ was capable of initiating blood coagulation. We first performed a series of assays commonly used for DIC diagnosis (Wada et al., 2014). Patients with DIC often have prolonged prothrombin time (PT) due to consumption of coagulation factors (Angus and van der Poll, 2013; Gando et al., 2016; Levi and Cate, 1999; Wada et al., 2014). Indeed, PT was prolonged significantly in C57BL/6J mice challenged with EprJ, as demonstrated by a standard PT assay (Figure 1A). PA alone had no effects (Figure S1E). During DIC, fibrinogen is cleaved into fibrin by thrombin (Wada et al., 2014), resulting in a decrease in plasma fibrinogen concentrations. As expected, plasma fibrinogen concentrations were reduced in C57BL/6J mice receiving EprJ (Figure 1B). EprJ elevated plasma thrombin-antithrombin (TAT) concentrations, indicating heightened conversion of prothrombin to thrombin (Figure 1C). EprJ also caused thrombocytopenia in C57BL/6J mice (Figure 1D), another clinical feature consistent with DIC. TF plays a key role in triggering blood clotting in sepsis (Bach et al., 1981; Levi et al., 1994; Morrissey et al., 1987; Pawlinski et al., 2004; Taylor et al., 1991), and TF activity in plasma microvesicles (MVs) was increased in mice challenged with EprJ (Figure 1E).
Figure 1. Administration of EprJ in vivo Induces Systemic Coagulation (A-E).
Mice (C57BL/6J) were injected intravenously with PBS (Ctrl) or EprJ (300 ng LFn-EprJ plus 3 μg PA per mouse). Blood were collected 90 minutes after PBS or EprJ injection. Prothrombin time (A), plasma fibrinogen concentrations (B), plasma TAT concentrations (C), total platelet count before and after EprJ injection (D), and TF activity in plasma microvesicles (MVs) (E) were measured. Solid circles represent individual mice, crossbars represent group mean. n = 4–6 for all experimental groups. One asterisk, P < 0.01 (Student’s t-test, unpaired for A-C, E, paired for D).
See also Figure S1
In DIC, systemic activation of the clotting cascade leads to fibrin deposition and formation of microthrombi throughout vasculature (Levi and Cate, 1999). Indeed, administration of EprJ in C57BL/6J mice induced thrombus formation, as evidenced by occlusion in cremaster vasculature, platelet accumulation, and fibrin deposition detected by intravital microscopy (Figure 2A and Video S1). The mice were injected with washed platelets isolated from the GFP-expressing mice C57BL/6-Tg(CAGEGFP)1Osb/J and Alexa 568-labled anti-fibrin antibody (59D8), which is a monoclonal antibody specifically recognizing mouse fibrin (Hui et al., 1983; Ivanciu et al., 2011; Neyman et al., 2008; Weiler-Guettler et al., 1998). Fibrin deposition was also detected in liver by immunostaining (Figure 2B). Immunoblot analysis revealed significant accumulation of fibrin in liver and spleen in mice challenged with EprJ (Figure 2C). Together, these data demonstrate that EprJ induces systemic coagulation in mice.
Figure 2. Caspase-1 is Required for EprJ-induced Blood Coagulation and Lethality (A).
C57BL/6J mice (WT) or Casp1/11 deficient mice were injected intravenously with washed platelets isolated from the C57BL/6-Tg(CAG-EGFP)1Osb/J mice (GFP) and an Alexa 568-labled (Red) anti-fibrin monoclonal antibody (59D8), followed by intravenous injection of EprJ. Thrombus formation in cremaster vasculatures was monitored using an intravital microscopy. Images were acquired at the same location, 15 min and 60 min after EprJ injection. Scale bar denotes 50 μm. Data are representative of 3 independent experiments (biological replicates). (B) C57BL/6J mice (WT) or Casp1/11 deficient mice were injected intravenously with EprJ. After 90 minutes, mice were euthanized and perfused with PBS then perfusion-fixed with 10% formalin under physiological pressure for 45 minutes. Liver sections were immunostained with the anti-fibrin monoclonal antibody (59D8). Wild type mice, but not Casp1/11−/− mice, showed fibrin deposition in liver (arrows). Scale bar denotes 50 μm. Data are representative of 3 independent experiments (biological replicates). (C) C57BL/6J mice (WT) or Casp1/11 deficient mice were injected intravenously with EprJ. After 90 minutes, mice were euthanized and tissues were isolated. Fibrin in the tissue lysates was detected by Immunoblot with the anti-fibrin monoclonal antibody (59D8). Data are representative of 3 independent experiments (biological replicates). (D-F) C57BL/6J mice (WT), Casp1/11 deficient mice, Casp11 deficient mice, and TLR4 deficient mice were injected intravenously with PBS or EprJ. Blood were collected 90 minutes after PBS or EprJ injection. Prothrombin time (D), plasma fibrinogen concentrations (E), and plasma TAT concentrations (F) were measured. Error bars denote SEM. n = 4–6 for all experimental groups. Two asterisks, P < 0.01 (two-way ANOVA with Holm-Sidak multiple comparisons). (G) C57BL/6J mice (WT) or Casp1/11 deficient mice were injected intravenously with a lethal dose of EprJ. Kaplan-Meier survival plots for mice challenged with EprJ. n = 12–15. Three asterisks, P < 0.01 versus WT [Log-rank (Mantel-Cox) test].
See also Figure S1
Coagulation Activation Induced by Rod Proteins Requires Caspase-1
Next, we examined the role of inflammasome in EprJ-induced coagulation with caspase-1 deficient mice. As widely used caspase-1 deficient mice lack both caspase-1 and caspase-11 (Kayagaki et al., 2011), they are referred to as Casp1/11−/− hereafter. Coagulation activation triggered by EprJ was diminished in Casp1/11−/− mice, as shown by loss of fibrin deposition in tissues (Figures 2A–2C and Video S1). EprJ-induced prolongation of PT (Figure 2D), reduction in plasma fibrinogen concentrations (Figure 2E), and increase in plasma TAT concentrations (Figure 2F) were diminished in Casp1/11−/− mice. Casp1/11−/− mice were protected from EprJ- induced lethality (Figure 2G). To clarify the role of caspase-1, we determined whether caspase- 11 was involved in EprJ-induced coagulation using Casp11−/− mice. Caspase-11 deficiency had no effect on PT, plasma fibrinogen concentrations or plasma TAT concentrations in mice challenged with EprJ (Figures 2D–2F). In addition, EprJ elicited coagulation in Tlr4−/− mice (Figures 2D–2F). These findings indicate that blood coagulation elicited by EprJ is dependent on caspase-1, the key protease of inflammasome activity. To verify that caspase 1 is responsible for EprJ-elicited coagulation, we used newly developed “clean” Casp1−/− mice (Kayagaki et al., 2015). As expected, EprJ-induced coagulation was abolished in Casp1−/− mice (Figures S1F and S1G).
Inflammasome Activation Drives Coagulation and Lethality through Pyroptosis
Inflammasome activation leads to IL-1β and IL-18 maturation and release (Lamkanfi and Dixit, 2014; Schroder and Tschopp, 2010) and rapid production of inflammatory lipid mediators (von Moltke et al., 2012). EprJ induced coagulation in Il1r−/−and Il18r−/− mice lacking the receptors of IL-1β and IL-18, respectively, to a similar extent as in wild type mice (Figures S2A and S2B). These data support that coagulation following inflammasome activation is independent of IL-1β and IL-18.
Eicosanoids synthesized by cyclooxygenases have been shown to be critical mediators of inflammation and vascular fluid loss following inflammasome activation by flagellin (von Moltke et al., 2012). To determine if cyclooxygenase (COX1- and COX2)-generated lipid mediators were involved in inflammasome-induced coagulation, mice were pretreated with the COX inhibitor aspirin prior to exposure to EprJ. Arachidonic acid-induced platelet aggregation requires COX-dependent TXA2 synthesis (Parise et al., 1984). As expected, arachidonic acid failed to elicit platelet aggregation in the mice pre-treated with aspirin (Figure S2C). However, aspirin had no effect on PT prolongation or elevated plasma TAT concentrations (Figures S2D and S2E), effectively ruling out a role for COX-mediated pathways in EprJ-induced coagulation.
Recent studies show that caspases-1 cleaves GSDMD and triggers pyroptosis—a form of programmed cell death with similar morphology to necrosis (Kayagaki et al., 2015; Shi et al., 2015). Consistent with these in vitro findings, EprJ injection caused peripheral monocyte depletion (Figure S3A) and macrophage depletion and cell death (Figure S3B and S3C). GSDMD deficiency protects against macrophage cell death and lethal endotoxemia (Kayagaki et al., 2015; Shi et al., 2015). We tested the hypothesis that GSDMD-dependent pyroptosis drives inflammasome-induced coagulation and lethality using Gsdmd−/− mice (Fujii et al., 2008). Indeed, fibrin deposition induced by EprJ was abolished by GSDMD deficiency (Figure 3A). EprJ- induced prolongation of PT (Figure 3B), reduction in plasma fibrinogen concentrations (Figure 3C), and increase in plasma TAT concentrations (Figure 3D) were also diminished in Gsdmd−/− mice. GSDMD deficiency also prevented systemic activation of coagulation induced by E.coli infection (Figures S4A and S4B). To determine whether inflammasome activation and subsequent pyroptosis are a common underlying mechanism for triggering coagulation, we examined the effects of rod proteins from other bacterial strains, such as Burkholderia BsaK and Salmonella PrgJ, on coagulation. Injection of BsaK and PrgJ proteins into C57BL/6 mice both elicited severe coagulation (Figures 3E and 3F), which was not observed in the Gsdmd−/− mice. Furthermore, GSDMD deficiency protected against EprJ- or BsaK-induced lethality (Figures 3G and 3H).
Figure 3. GSDMD-dependent Pyroptosis is Essential to Inflammasome-driven Coagulation and Lethality (A).
C57BL/6J (WT) or GSDMD deficient mice were injected intravenously with EprJ. After 90 minutes, mice were euthanized and tissues were isolated. Fibrin in the tissue lysates was detected by Immunoblot with the anti-fibrin monoclonal antibody (59D8). Data are representative of 3 independent experiments (biological replicates). (B-D) C57BL/6J mice (WT) or GSDMD deficient mice were injected intravenously with PBS (Ctrl) or EprJ. Blood were collected 90 minutes after PBS or EprJ injection. Prothrombin time (B), plasma fibrinogen concentrations (C), and plasma TAT concentrations (D) were measured. Error bars denote SEM. n = 4–6 for all experimental groups. Two asterisks, P < 0.01 (two-way ANOVA with Holm-Sidak multiple comparisons). (E and F) C57BL/6J mice (WT) or GSDMD deficient mice were injected intravenously with PBS (Ctrl) or BsaK or PrgJ. Blood were collected 90 minutes after the injection. Prothrombin time (E), plasma TAT concentrations (F) were measured. Error bars denote SEM. n = 5 for all experimental groups. Two asterisks, P < 0.01 (two-way ANOVA with Holm-Sidak multiple comparisons). (G and H) C57BL/6J mice (WT) or GSDMD deficient mice were injected intravenously with a lethal dose of EprJ or BsaK. Kaplan-Meier survival plots for mice challenged with EprJ. n = 8–12. Three asterisks, P < 0.01 versus WT [Log-rank (Mantel- Cox) test].
Monocytes and Macrophages are Major Contributors to Inflammasome Activation Induced Coagulation and Lethality
Monocytes and macrophages are essential in inflammasome detection of bacterial infection (Lamkanfi and Dixit, 2014; Latz et al., 2013; Schroder and Tschopp, 2010). To determine the contribution of monocytes and macrophages to EprJ-induced coagulation, we depleted these cells by administration of commonly used clodronate-containing liposomes. Consistent with our previous report (Xiang et al., 2013), clodronate administration reduced blood monocytes by 90% within 24 hours. Pre-depletion of monocytes and macrophages significantly attenuated EprJ- induced coagulation, as measured by PT, plasma fibrinogen and TAT concentrations (Figures 4A–4C), supporting a central role of monocytes and/or macrophages in EprJ-induced coagulation. Furthermore, their importance in inflammasome-induced lethality was established by the fact that depletion of monocytes and macrophages using clodronate-containing liposomes significantly improved survival in mice challenged with a lethal dose of EprJ (Figure 4D).
Figure 4. Macrophages Pyroptosis Plays a Critical Role in Inflammasome-induced Coagulation by Promoting the Releasing of TF-positive Microvesicles (A-C).
C57BL/6J mice were injected intravenously with control liposomes (Lipo) or clodronate- containing liposomes (Cldn) 24 hours prior to intravenous injection of PBS (Ctrl) or EprJ. Blood were collected 90 minutes after PBS or EprJ injection. Prothrombin time (A), plasma fibrinogen concentrations (B), and plasma TAT concentrations (C) were measured. Error bars denote SEM. n = 4–6 for all experimental groups. Two asterisks, P < 0.01 (two-way ANOVA with Holm- Sidak multiple comparisons). (D) C57BL/6J mice were injected intravenously with control liposomes (Lipo) or clodronate-containing liposomes (Cldn) 24 hours prior to intravenous injection of a lethal dose EprJ. Three asterisks, P < 0.01 [Log-rank (Mantel-Cox) test]. (E) BMDMs from C57BL/6J (WT), Casp1/11 deficient, Casp11 deficient mice, and TLR4 deficient mice were incubated with various T3SS rod proteins (100 ng/ml LFn-rod protein plus 1 μg/ml PA) for 45 minutes. TF and p20 caspase-1 in the supernatant were detected by fluorescent Immunoblot. Cell culture supernatants were precipitated to concentrate protein prior to Immunoblot. (F) BMDMs from C57BL/6J (WT) and TLR4 deficient mice were incubated with various T3SS rod proteins for 45 minutes. TF and p20 caspase-1 in the supernatant were detected by fluorescent Immunoblot. (G) BMDMs from C57BL/6J (WT) and GSDMD deficient mice were incubated with EprJ for 45 minutes. TF and p20 caspase-1 in the supernatant were detected by fluorescent Immunoblot. Glycine (+) designates addition of 5 mM glycine as osmoprotectant to prevent pyroptosis-associated membrane rupture. (H) THP-1 cells were incubated with T3SS needle protein EprI (1 ug/ml LFn-EprI plus 1μg/ml PA) for 45 minutes. Cell culture supernatants were precipitated to concentrate protein prior to Immunoblot analysis. Data are representative of 3 independent experiments for all the Immunoblot (biological replicates). (I) THP-1 were incubated with EprI for 45 minutes. Microvesicles (MVs) were isolated from supernatant and TF activity was measured. n = 4–6 for all experimental groups (biological replicates). One asterisk, P < 0.01 (Student’s t-test, unpaired). (J) Plasma MV TF activity. C57BL/6J (WT), Casp1/11 deficient, and GSDMD deficient mice were injected intravenously with EprJ. Blood were collected 90 minutes after EprJ injection. Microvesicles (MVs) were isolated from blood and TF activity was measured. Error bars denote SEM. n = 4–6 for all experimental groups. Four asterisks, P < 0.01 (one-way ANOVA with Holm-Sidak multiple comparisons).
See also Figure S5
Macrophage-derived TF is Required for Coagulation Activation Following Inflammasome Activation
Monocytes and macrophages are major sources of TF in vivo (Pawlinski et al., 2010). Hematopoietic cell-derived TF is a major contributor to the activation of coagulation in mouse models of endotoxemia and sepsis (Franco et al., 2000; Gando et al., 2016; Pawlinski et al., 2010). Macrophages can release TF-positive MVs upon caspase-1 activation by ATP in vitro (Rothmeier et al., 2015). As shown in Figure 4E, TF is abundantly expressed in BMDMs as detected by Immunoblot. TF is a transmembrane protein present in the cell membrane under resting condition. To determine whether TF is released following inflammasome activation, we incubated mouse BMDMs with EprJ and collected cell culture supernatants. EprJ challenged wild-type BMDMs exhibited caspase-1 activation, as revealed by the presence of p20 caspase- 1(Boucher et al.), which was accompanied by TF release into the cell culture supernatants (Figure 4E). EprJ-induced caspase-1 cleavage and TF release were not detected in the BMDMs from Casp1/11−/− mice. In contrast, caspase-11 or TLR4 deficiency had no effect on EprJ-induced caspase-1 cleavage and TF release (Figure 4E). These findings demonstrate that TF release from macrophages requires caspase-1 dependent inflammasome activity.
Rod proteins from other bacterial strains, such as Burkholderia BsaK, Salmonella PrgJ, and EPEC EscI, elicited TF release from BMDMs. Consistent with previous reports (Zhao et al., 2016; Zhao et al., 2011), all the rod proteins we examined led to caspase-1 activation (Figure S1A) and TF release from both wild type and TLR4 deficient BMDMs (Figure 4F). T3SS needle proteins can activate the inflammasome in human macrophages (Zhao et al., 2016). E. coli T3SS needle protein EprI elicited release of active TF from human monocyte-like THP-1 cells (Figures 4H and 4I), indicating inflammasome activation may be a conserved mechanism for the initiation of coagulation in both human and rodents.
We next investigated whether GSDMD-formed pores facilitate the release of TF from macrophages, because TF release elicited by EprJ was abolished in the Gsdmd−/− BMDMs (Figure 4G). To prevent pyroptosis-driven cell membrane rupture, we utilized glycine (5mM) as an osmoprotectant for BMDMs (Fink and Cookson, 2006). Glycine buffering prevented TF release from wild-type cells (Figure 4G), indicating that TF is not released through GSDMD-formed pores, rather rupture of the cell membrane is required for TF release.
Tissue Factor is Responsible for Inflammasome-induced Lethality
TF is released from cells in the form of MVs (Grover and Mackman, 2018). In contrast to elevated plasma MV TF activity in EprJ-challenged wild type mice, both Casp1/11−/− and Gsdmd−/− mice had significantly lower plasma MV TF activity when challenged with EprJ (Figure 4J), suggesting that GSDMD-dependent pyroptosis promotes the release of TF-positive MVs. Pre- depletion of monocytes and macrophages significantly decreased plasma MV TF activity (Figure S5A), demonstrating that MV TF released into plasma are mainly from pyroptotic monocytes and macrophages.
To establish the role of TF in pyroptosis-triggered coagulation, we utilized an inhibitory rat anti-mouse TF antibody, 1H1, to block TF activity (Kirchhofer et al., 2005). Mice administered with 1H1 but not a control IgG were protected from coagulation (Figures 5A and 5B). Mice injected with 1H1 also had improved survival (Figure 5C) after EprJ challenge in comparison to mice administered with a control IgG.
Figure 5. Tissue Factor Inhibition Protects Against Inflammasome-induced Coagulation and Lethality (A-C).
Pharmacological inhibition of TF. C57BL/6J mice were injected intravenously with a rat IgG or a rat anti-moue TF neutralizing antibody 1H1 (8 mg/kg). After 2 hours, the mice were injected intravenously with EprJ. Blood were collected 90 minutes after EprJ injection. Prothrombin time (A) and plasma TAT concentrations (B) were measured. Error bars denote SEM. n = 4–5 for all experimental groups. One asterisk, P < 0.01 (Student’s t-test, unpaired). (C) C57BL/6J mice were injected intravenously with a rat IgG or 1H1 (8 mg/kg). After 2 hours, the mice were injected intravenously with a lethal dose of EprJ. Three asterisks, P < 0.01 [Log-rank (Mantel-Cox) test]. (D-F) Inducible TF deficient mice was generated by crossing B6.Cg-Tg(UBC-cre/ERT2)1Ejb/J Cre transgenic mice with TF floxed mice. TF deficient mice or wild type littermates were injected intravenously with EprJ. Blood were collected 90 minutes after EprJ injection. Prothrombin time (D) and plasma TAT concentrations (E) were measured. Error bars denote SEM. n = 4–5 for all experimental groups. One asterisk, P < 0.01 (Student’s t-test, unpaired). (F) TF deficient mice or wild type littermates were injected with a lethal dose of EprJ. n = 8. Three asterisks, P < 0.01 [Log-rank (Mantel-Cox) test].
See also Figure S5
As a complementary approach, we generated a TF inducible deficient mouse model (because constitutive TF deficient mice are embryonic lethal). Deletion of TF was controlled by tamoxifen-inducible Cre recombinase expression under the control of the human ubiquitin C. Genetic deficiency of TF showed no effect on inflammasome activation and pyroptosis (Figure S5B and S5C), but blocked EprJ-induced coagulation and lethality (Figures 5D–5F). Thus, TF contributes to inflammasome activation-induced mortality, likely through inducing coagulation.
LPS Induces Coagulation through Noncanonical Inflammasome Pathway
Gram-negative bacteria activate inflammasomes through multiple mechanisms (Hagar et al., 2013; Kayagaki et al., 2013; Miao et al., 2010; Shi et al., 2014; Zanoni et al., 2016; Zhao et al., 2011). LPS is a known activator of inflammasome, although it acts in a noncanonical manner through caspase-11, instead of caspase-1 (Hagar et al., 2013; Kayagaki et al., 2013; Shi et al., 2014; Vanaja et al., 2016; Zanoni et al., 2016). Unlike caspase-1, activation of the caspase-11 pathway requires priming macrophages with toll-like receptor (TLR) ligands or interferons, which induces the expression of multiple components in the noncanonical inflammasome pathway, including caspase-11 (Broz et al., 2012; Case et al., 2013; Rathinam et al., 2012). We determined whether LPS could activate coagulation through the inflammasome activation. Intraperitoneal injection of LPS into wild-type mice primed with polyinosinic:polycytidylic acid [poly(I:C)], a TLR3 agonist, induced a systemic coagulopathy, as demonstrated by prolonged PT (Figures 6A), decreased plasma fibrinogen concentrations (Figure 6B), elevated plasma TAT concentrations (Figures 6C), and decreased total platelet counts (Figure 6D). Blood coagulation was not observed in Casp11−/− mice challenged with LPS (Figures 6A–6C), and thrombocytopenia was also attenuated (Figure 6D), suggesting that noncanonical inflammasome activation is required for LPS-elicited coagulation. In contrast, LPS-induced activation of coagulation was independent of TLR4 because Tlr4−/− mice responded to LPS to the same extent as wild-type mice in terms of coagulation (Figures 6A–6D). These data are consistent with previous findings that LPS-elicited inflammasome activation is TLR4 independent (Hagar et al., 2013; Kayagaki et al., 2013). Transfection of LPS into BMDMs, required to activate inflammasome (Figure S6A), promoted release of TF of BMDMs from wild-type mice and Tlr4−/− mice, but not from Casp11−/− mice (Figures 6E). Thus, LPS triggers blood coagulation through inflammasome-dependent TF release.
Figure 6. Noncanonical inflammasome activation by LPS triggers blood coagulation (A-D).
C57BL/6J mice (WT), Casp11 deficient, and TLR4 deficient mice were injected intraperitoneally with 4 mg/kg poly(I:C) for priming. After 8 hours, the mice were injected intraperitoneally with PBS (Ctrl) or 50 mg/kg LPS. Blood were collected 4 hours after injection of PBS or LPS injection. Prothrombin time (A), plasma fibrinogen concentrations (B), plasma TAT concentrations (C), and total platelet count before and after LPS injection (D) were measured. Error bars denote SEM. n = 4–6 for all experimental groups. Two asterisks, P < 0.01 (two-way ANOVA with Holm-Sidak multiple comparisons). (E) BMDMs from C57BL/6J (WT), Casp11 deficient mice, TLR4 deficient, and GSDMD deficient mice were incubated with poly(I:C) (1 μg/ml). After 5 hours, the cells were transfected with PBS (Ctrl) or LPS (2 μg/ml). Cell culture supernatants were precipitated to concentrate protein prior to Immunoblot. Data are representative of 3 independent experiments (biological replicates). (F-I) C57BL/6J mice (WT) or GSDMD deficient mice were injected intraperitoneally with 4 mg/kg poly(I:C) for priming. After 8 hours, the mice were injected intraperitoneally with PBS (Ctrl) or 50 mg/kg LPS. Blood were collected 4 hours after injection of PBS or LPS injection. Prothrombin time (F), plasma fibrinogen concentrations (G), plasma TAT concentrations (H), and total platelet count before and after LPS injection (I) were measured. Error bars denote SEM. n = 4–6 for all experimental groups. Two asterisks, P < 0.01 (two-way ANOVA with Holm-Sidak multiple comparisons). (J) C57BL/6J mice were injected intravenously with control liposomes (Lipo) or clodronate- containing liposomes (Cldn) 24 hours prior to intraperitoneal injection of 4 mg/kg poly(I:C). After 6 hours of poly(I:C) injection, the mice injected with a lethal dose of LPS. Three asterisks, P < 0.01 [Log-rank (Mantel-Cox) test]. (K) Model of coagulation triggered by canonical and noncanonical inflammasome activation.
See also Figure S6
Because LPS elicited coagulation through inflammasome activation, we hypothesiz that GSDMD-dependent pyroptosis also drives noncanonical LPS-induced coagulation and lethality using Gsdmd−/− mice. Indeed, LPS-induced prolongation of PT (Figure 6F), reduction in plasma fibrinogen concentrations (Figures 6G), and increase in plasma TAT concentrations (Figures 6H) were diminished in Gsdmd−/− mice. GSDMD deficiency showed modest but significant protection against LPS-induced thrombocytopenia (Figure I).
Two independent groups have reported that lethal sepsis in mice induced by LPS is attributed to caspase-11-dependent pyroptosis (Hagar et al., 2013; Kayagaki et al., 2011). However, it remains unclear which cell types drive lethality. Here, we extended their finding by demonstrating that depletion of monocytes and macrophages significantly decreased plasma MV TF activity (Figure S6B) and improved survival in mice administered a lethal dose of LPS (Figure 6J). Our observations are consistent with those in patients of septic shock where progression to DIC is associated with increased mortality (Fujishima et al., 2014; Gando et al., 2013; Rangel-Frausto et al., 1995; Venugopal, 2014).
DISCUSSION
Activation of inflammatory caspases is a central mechanism of innate immune response against bacterial infections. However, excessive activation of the inflammatory caspases leads to multiple organ damage and host lethality (Kayagaki et al.; Zhao et al.). Here, we provide a molecular mechanism by which inflammasome activation and pyroptosis cause death of the host. We demonstrate that TF released from pyroptotic macrophages initiates systemic coagulation and thrombosis in tissues, which plays a central role in inflammasome activation-induced lethality. Identifying DIC as a critical event following inflammasome and pyroptosis opens a direction in studying inflammasome and pyroptosis as current understanding of the inflammasome function is limited to inflammatory response.
Coagulation induced by EprJ was abolished by caspase 1 or GSDMD deficiency, suggesting that EprJ-induced coagulation activation depends on inflammasome activation and pyroptosis. Since pores formed by GSDMD facilitate the release of the IL-1β and IL-18 (Evavold et al.; He et al.; Monteleone et al.), GSDMD deficiency not only prevents pyroptosis but also diminishes IL-1β and IL-18 secretion. Thus, we utilized mice lacking IL-1β receptor or IL-18 receptor to investigate whether IL-1β and IL-18 contribute to coagulation following inflammasome activation. We found that deficiency of the receptors for IL-1β and IL-18 does not protect against EprJ-elicited coagulation. These data exclude the possibility that inflammasome activation-induced coagulation requires IL-1β and IL-18 dependent inflammation, thus establishing pyroptosis as a key event leading to coagulation upon inflammasome activation.
EprJ-induced coagulation is diminished in TF deficient mice and in mice administered a TF neutralizing antibody, demonstrating that TF is required for inflammasome-driven coagulation. These findings are consistent with previous studies that TF plays an essential role in sepsis-associated DIC (Franco et al., 2000; Gando et al., 2016; Pawlinski et al., 2004; Pawlinski et al., 2010; Taylor et al., 1991). Our data further show that EprJ-induced lethality was protected by the deficiency of TF or the TF neutralizing antibody, demonstrating that pyroptosis induced coagulation is a major cause of host death. Although TF has been recognized as a key initiator of DIC in sepsis, how TF is released to trigger coagulation is largely unknown. We show that microvesicles (MVs) isolated from the blood of EprJ-challenged mice have high TF activity, which was abolished by caspase-1 or GSDMD deficiency. Consistent with the in vivo data, EprJ treatment increased TF activity in the MVs from BMDMs isolated from wild-type, but not from Gsdmd−/− or Casp1/11−/− mice. Together, these data suggest that cell membrane fragments of pyroptotic macrophages form TF-positive MVs as a result of thermodynamics. This is consistent with previous findings that caspase-1 activation by ATP promotes TF-positive MV release in vitro (Rothmeier et al., 2015). Unlike IL-1β and IL-18, release of TF from macrophages is not through GSDMD-pores, but requires membrane rupture, suggesting distinct mechanisms are involved. This is not surprising because IL-1β and IL-18 are cytosolic proteins while TF is a transmembrane protein. Besides pyroptosis, necroptosis is another form of lytic programmed cell death (Wallach et al.). Although distinct signaling pathways induce pyroptosis and necroptosis, both forms of cell death lead to ruptured cell membrane, a requirement for TF-positive MV release. Thus, it is likely that necroptosis may also lead to TF release.
Sepsis is a complex process, involving severe inflammation, organ dysfunction, various types of cell death, etc. It is likely that different mechanisms contribute to the development of DIC during sepsis. In this regard, it has been shown that NETs, DNA, and histone play important roles in the development of DIC (Brinkmann and Zychlinsky, 2012; Fuchs et al., 2010; Liaw et al., 2016; McDonald et al., 2017). Inflammasome can be activated by both the pathogen- associated molecular patterns (PAMPs) and the damage-associated molecular patterns (DAMPs). E. coli induced coagulation activation was diminished in the GSDMD deficient mice, supporting that pyroptosis is at least one of the major mechanisms of DIC in bacterial infection.
While TF expression in monocytes is enhanced during sepsis or by LPS stimulation (Grover and Mackman, 2018), macrophages express high amount of TF, which is sufficient to trigger DIC. This conclusion is supported by our data that administration of EprJ or BsaK elicited DIC within 60 minutes and TF was detected by immunoblot in the cell lysates of macrophages. Consistent with these findings, administration of EprJ induced clearance of peripheral monocytes within 60 minutes. Clearance of peripheral monocytes was not due to migration of the cells to tissues, because the total monocytes and macrophages in tissues were also significantly reduced in tissues. Furthermore, we found that administration of EprJ induced macrophage death in tissues. Thus, administration of EprJ induced monocytes and macrophage depletion through pyroptosis, and TF released from pyroptotic cells triggered systemic coagulation. Although administration of clodronate into mice induced monocyte and macrophage depletion through apoptosis to a similar extent as injection of EprJ, clodronate failed to induce DIC and lethality in mice. In contrast, pre-depletion of monocytes and macrophages by clodronate protected against EprJ-elicited DIC and lethality. These data demonstrate that pyroptosis, but not apoptosis, is capable of TF release. Unlike apoptosis, cell death by pyroptosis results in plasma-membrane rupture and the release of cell contents into blood (Jorgensen and Miao, 2015; Wallach et al., 2016), which promotes inflammatory response. Our study identifieda function of pyroptosis in triggering coagulation activation through releasing TF.
Although TF is expressed in different cell types (Grover and Mackman, 2018), the major source of TF in blood following inflammasome activation appears to be monocytes and macrophages. These data are consistent with the previous findings that monocytes and macrophages derived TF plays an important role in sepsis-associated DIC, and also consistent with the findings that pyroptosis mainly occurs in monocytes and macrophages (Jorgensen and Miao, 2015; Wallach et al., 2016). Endothelial cells could undergo pyroptosis upon LPS stimulation (Cheng et al., 2017). However, they express minimal TF, thus unlikely to be a major source of TF in our experimental settings—coagulation was fully activated within 60 minutes after administration of EprJ, a time frame excluding enhanced TF expression in endothelial cells.
Our data establish inflammasome activation as an important link between inflammation and blood clotting. Specifically, inflammasome activation by bacterial products results in GSDMD-dependent macrophage pyroptosis, leading to the release of TF-positive MVs into the blood, which in turn triggers blood coagulation, resulting in organ damage and lethality. Our findings advance the understanding of the relationship between bacterial infections and coagulation as well as provide evidence that inflammasome may be a potential therapeutic target for sepsis. Our data also suggest that T3SS rod protein-induced coagulation is a robust systemic coagulation model that can be used for developing therapeutics against DIC. Whether similar mechanisms link inflammation to thrombosis in different settings remains to be determined.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Zhenyu Li (zhenyuli08@uky.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
Wild-type C57BL/6J, Casp1/11−/−, Casp1−/−, Casp11−/−, Tlr4−/−, Gsdmd−/−, B6.Cg-Tg(UBC-cre/ERT2)1Ejb/J Cre transgenic mice, and TF floxed mice were housed in the University of Kentucky Animal Care Facility, following institutional and National Institutes of Health guidelines after approval by the Institutional Animal Care and Use Committee. Il1r−/− mice and Il18r−/− mice were housed in the Animal Care Facility at National Institute of Biological Sciences, following the Ministry of Health national guidelines for housing and care of laboratory animals and performed in accordance with institutional regulations after review and approval by the Institutional Animal Care and Use Committee. Male mice at 8–12 weeks were used in all experiments.
In vivo Challenges
For EprJ challenge, purified PA and LFn-EprJ in PBS were administered via retro-orbital injection. For LPS challenge, mice were given poly(I:C) obtained from Invivogen at 4 mg/kg by intraperitoneal injection for priming. After 8 hours of priming, mice were injected intraperitoneally with LPS from E. coli O111:B4 (Sigma, Cat#L4130).
Intravital microscopy of cremaster vasculature
Mice were anaesthetized with Avertin and fixed on a custom built-stage to maintain a physiological temperature. Then the animals were injected (retro-orbital) with washed GFP- platelets (2.5 × 108) isolated from the C57BL/6-Tg(CAG-EGFP)1Osb/J mice and Alexa568-labeled fibrin antibody 59D8 (0.15 mg/kg) along with EprJ. Cremaster muscle was isolated immediately after injection. Images were acquired at the same locations 15 min and 60 min after injection on Zeiss LSM880 with 10X Zeiss W Plan-Apochromat under fast Airyscan mode (419×419 μm at 3.6 frames per second). Image and videos were processed in Imaris x64 9.2.0.
Pharmacological and Genetic TF inhibition
Pharmacological approach: Rat IgG (Sigma) or 1H1 anti-TF antibody (Genentech) at 8 mg/kg was given via retro-orbital injection 2 hours prior to PBS or EprJ injection. Genetic approach: B6.Cg-Tg(UBC-cre/ERT2)1Ejb/J Cre transgenic mice were crossed with TF floxed mice (F3fl/fl) to generate inducible TF deficient mice, F3fl/fl-ERT2+. CreERT2-recombinase expression for removal of TF was induced by intraperitoneal injections of tamoxifen (Sigma) at 100 mg/kg per day for 5 consecutive days at 4 to 5 weeks of age, and subsequent experiments were carried out at 5 weeks post-induction.
BMDM Cultures
BMDMs were isolated and seeded into 12-well cell culture plate at a density of 1 × 106 cells/well in 1 ml of RPMI-1640 medium containing 15% L929-cell conditioned medium (LCM). BMDMs were allowed to settle overnight and refreshed with 1 ml of Opti-MEM (Life Technologies, Cat#31985–070) before purified protein were added (EprJ at 100 ng/ml and PA at 1 μg/ml). For study of LPS, BMDMs were primed for 5 h with 1 μg/ml poly(I:C), then transfected with 2 μg/ml LPS using FuGENE HD (Promega, Cat#E2311). In brief, a mixture of 2 μl of LPS (1 mg/m) and 3 μl of FuGENE HD reagent was incubated for 5 minutes at room temperature then added to the 12-well plate.
THP-1 Culture
THP-1 cells were obtained from ATCC and cultured in RPMI1640 containing 10% FBS. Macrophage differentiation was induced by incubating THP-1 cells with PMA at 5 ng/ml for 48 hours.
Monocytes and Macrophages Depletion
Mice were administered clodronate liposome at 40 mg/kg or control liposome (Encapsula NanoSciences, Nashville, TN) via retro-orbital injection 24 hours before EprJ or LPS challenge.
METHOD DETAILS
LFn Fusion Protein Purification
Protein expression was induced in E. coli BL21 strains at 37 °C for 4 hours with 0.5 mM IPTG after OD600 reached 0.8–0.9. Bacteria were collected and lysed in 50 mM Tris-HCL and 300 mM NaCl. Proteins containing a His-tag were purified by affinity chromatography using HisPur Ni- NTA resin (Thermo scientific, Cat#88222). To minimize endotoxin contamination, 60% isopropanol was added to the wash buffer. Proteins were then eluted with 250 mM imidazole in 50 mM Tris-HCL and 300 mM NaCl, and subsequently dialyzed against PBS to remove imidazole. Protein concentrations were estimated against BSA standards on SDS-PAGE gels after Coomassie blue staining.
Prothrombin Time (PT)
Blood were collected from tribromoethanol (Avertin)-anaesthetized mice by cardiac puncture with a 23-gauge needle attached to a syringe pre-filled with 3.8% trisodium citrate as anticoagulant (final ratio at 1:10). Blood were centrifuged at 1,500 g for 15 minutes at 4° C to obtain plasma. Prothrombin time (PT) was determined with Thromboplastin-D (Pacific Hemostasis, Cat#100357/lot965299) in a manual setting according to manufacturer’s instruction, using CHRONO-LOG #367 plastic cuvette.
Plasma TAT Concentrations
Plasma TAT concentrations were determined using a mouse TAT ELISA kit (Abcam, Cat#ab137994) at 1:50 dilution according to manufacturer’s instruction. Plasma were collectedas mentioned above in PT.
Total Platelet Counts
Blood were collected via retro-orbital bleeding into tubes containing EDTA. Total platelet counts were acquired on HEMAVET 950 (Drew Scientific) or ProCyte DX Hematology Analyzer (IDEXX Laboratories, Inc.).
Tissue Preparation and Immunohistochemistry
Mice were perfused via both right and left ventricles with PBS and then perfusion-fixed with10% formalin under physiological pressure for 30–45 minutes. Tissues were collected and embedded in paraffin, then sectioned serially at 5 μm. Anti-fibrin antibody 59D8 (kindly provided by Dr. Hartmut Weiler at Medical College of Wisconsin and Dr. Rodney M. Camire at the University of Pennsylvania) at 4 μg/ml was used for staining fibrin deposition, with biotinylated goat anti-mouse IgG (Vector, Cat#BA-9200) at 1:200 dilution as secondary antibody for developing positive staining.
Glycine as osmoprotectant
Glycine (5 mM) was added to the cell culture media 30 min before EprJ stimulation to prevent pyroptosis-associated cell membrane rupture.
Fibrin extraction for Immunoblot
Frozen tissues were homogenized in 10 volumes (mg : μl) of T-PER tissue protein extraction reagent (Thermo#78510) containing cocktail inhibitor (Sigma#P8340) and PMSF. After centrifugation at 10,000 g for 10 min, supernatant was collected for β actin detection. Pellet was homogenized in 3 M urea and vortexed for 2 hrs at 37°C. After centrifugation at 14,000 g for 15 min, pellet was suspended in Immunoblot sample buffer (reducing) and vortexed at 65°C for 30 min and ready for fibrin detection.
Fluorescent Immunoblot
For detection of active caspase-1 and TF by Immunoblot, cells were washed with cold PBS and lysed with SDS sample buffer. Culture supernatants were precipitated with 1/10 volume of 2% sodium cholate and 1/10 volume of 100% trichloroacetic acid (TCA), and then dissolved in SDS sample buffer. Total protein from lysates and supernatants (equivalent to 5 ×104 cells) was analyzed by fluorescent Immunoblot for multiplex detection. TF was detected using anti-tissue factor (Abcam, Cat#ab151748, rabbit monoclonal) at 1:1000 dilution. Both pro-caspase-1 and p20 caspase-1 were determined using anti-caspase-1(p20) (Adipogen, Cat#AG-20B-0042-C100 for mouse BMDMs, AG-20B-0048-C100 for THP-1 cells) at 1:1000 dilution. IL-1β (p17) was detected using anti-IL-1β (GeneTex Cat#GTX74034). Tissue factor and caspase-1 were visualized on the same blot in the 800 nm (IRDye 800CW) and 700 nm (IRDye 680RD) channel, respectively, with LI-COR Odyssey Classic Imager. Plasma fibrinogen (equivalent to 0.03 μl of plasma) was detected using anti-fibrinogen (Dako Cat#A0080, rabbit polyclonal) at 1:3000 dilution, and imaged in the 800 nm (IRDye 800CW) channel. Tissue fibrin was detected using anti-fibrin (59D8) at 1 μg/ml and imaged in the 700 nm (IRDye 680RD) channel.
Isolation of MVs from mouse plasma
Plasma was collected as mentioned above in PT. Then 50 μl of mouse plasma was diluted with 1 ml of HBSA (137 mM NaCl, 5.38 mM KCl, 5.55 mM glucose, 10 mM HEPES, 0.1% bovine serum albumin, pH 7.5). MVs were pelleted at 20,000 g for 20 min at 4 °C, washed once with 1 ml of HBSA and re-suspended in 100 μl HBSA.
Isolation of MVs from cell culture supernatant
Cell debris were removed from cell culture supernatant by centrifugation at 2600 x g for 5 min. MVs were then pelleted at 20,000 g for 20 min at 4 °C, resuspended in PBS.
MV TF activity assay
Samples (50 μl each) were incubated with the 1H1 anti-TF antibody at 100 μg/ml (Genentech) for murine samples, HTF-1 anti-TF antibody at 8 μg/ml (BD Bioscience) for human samples or respective IgG controls for 15 min at room temperature. Next, 50 μl of HBSA containing 10 nM mouse FVIIa (Enzyme Research Laboratories, South Bend, IN), 300 nM human FX (Enzyme Research Laboratories) and 10 mM CaCl2 were added to the sample and incubated for 2 hours at 37 °C in a 96-well plate. FXa generation was stopped by the addition of 25 μl of 25 mM EDTA buffer. Finally, 25 μl of the chromogenic substrate Pefachrome FXa 8595 (4 mM, Pentapharm, Switzerland) was added and the mixture incubated at 37 °C for 15 min. Absorbance at 405 nm was measured using a Spectramax microplate reader (Molecular Devices, San Jose, CA). The procoagulant activity (PCA) of the sample was calculated by reference to a standard curve generated using recombinant human relipidated TF (0– 55 pg/ml, Siemens, Munich, Germany). The TF-dependent PCA generation (pg/ml) was determined by subtracting the amount of PCA generated in the presence of blocking antibodies from the amount of total PCA generated in the presence of the IgG controls.
Cell Viability
BMDMs cell viability was determined using CellTiter-Glo Luminescent Cell Viability Assay (Promega, Cat#G7571). Briefly, 100 μl of assay buffer was added to each well containing 100 μl cell culture (1 ×105 cells) in a 96-well plate. After incubation for 5–10 minutes, luminescence was recorded as an indicator of ATP concentrations in metabolically active cells.
Cytotoxicity
BMDMs cell cytotoxicity, as LDH concentration in cell culture supernatant, was determined using CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, Cat#G1780) according to manufacturer’s instruction.
Flow cytometry
Anti-CD115-Alexa 488, clone AFS98 (eBioscience, Cat#53–1152-81); anti-CD45-APC-Cy7, clone 30-F11 (BD Biosciences, Cat#557659); and anti-F4/80-APC, clone C1:A3–1, (Biorad antibody, Cat#MCA497APC) were used for flow cytometric analysis in the study. Monocytes were identified as CD45+CD115− in blood and CD45+CD115+F4/80− in lung and spleen. Macrophages were identified as CD45+F4/80+. Data were acquired on an LSRII (BD Biosciences) and analyzed with FlowJo v10.07. To obtain single cell suspension, tissues were digested with a cocktail of 1 mg/ml collagenase A (Sigma, Cat#0103586001) and 100 ug/ml DNase I (Sigma, Cat#1010415900s1) in PBS at 37°C for 30 minutes.
QUANTIFICATION AND STATISTICAL ANALYSIS
Data are represented as mean ± SEM. Student’s t-test (two-sided) was used to compare two- group data with normal distribution and equivalent variance; for multiple-group with two independent factors, two-way ANOVA with Holm-Sidak multiple comparisons was used for normally distributed variables. P < 0.05 was considered statistically significant. All statistical analyses were conducted on biological replicates in GraphPad Prism 7.
DATA AND SOFTWARE AVAILABILITY
N/A
Supplementary Material
Video S1. Intravital imaging of DIC in cremaster muscle, Related to Figure 2. Platelets were GFP-positive (isolated from C57BL/6-Tg(CAG-EGFP)1Osb/J mice) and fibrin were detected with Alexa568-labeled fibrin antibody 59D8. Time series images were acquired at the same location of cremaster vasculature, 15 min and 60 min after EprJ injection, on Zeiss LSM880 with 10X Zeiss W Plan-Apochromat under fast Airyscan mode (419×419 μm at 3.6 frames per second). Image and videos were processed in Imaris x64 9.2.0.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| 1H1 anti-TF antibody | Genentech | Kirchhofer et al., 2005 |
| HTF-1 anti-TF antibody | BD Biosciences | Cat #550252 RRID:AB_393557 |
| Anti-TF antibody (for Western) | Abcam | Cat #ab151748 |
| Anti-caspase-1(p20) (mouse) | Adipogen | Cat #AG-20B-0042-C100 RRID:AB_2755041 |
| Anti-caspase-1(p20) (human) | Adipogen | Cat #AG-20B-0048-C100 RRID:AB_2490257 |
| Anti-fibrin antibody 59D8 | Medical College of Wisconsin, University of Pennsylvania | Hui et al., 1983 |
| Anti-IL1β | GeneTex | Cat #GTX74034 |
| Anti-fibrinogen | Dako | Cat #A0080 |
| Anti-β-Actin | Biorad antibodies | Cat #MCA5775GA |
| Anti-CD115-Alexa 488, clone AFS98 | eBioscience | Cat #53–1152-81 |
| Anti-CD45-APC-Cy7, clone 30-F11 | BD Biosciences | Cat #557659 |
| anti-F4/80-APC, clone C1:A3–1 | Biorad antibody | Cat #MCA497APC |
| Biotinylated goat anti-mouse IgG | Vector | Cat #BA-9200 |
| IRDye® 680RD Goat anti-Mouse IgG (H + L) | LI-COR | Cat #925–68070 |
| IRDye® 800CW Goat anti-Rabbit IgG (H + L) | LI-COR | Cat #925–32211 |
| Bacterial and Virus Strains | ||
| E.coli | ATCC | Cat #49106 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| PA | Z.L. laboratory | Zhao et al., 2011 |
| LFn-EprJ | Z.L. laboratory | This paper |
| LPS (E. coli O111:B4) | Sigma | Cat #L4130 |
| Poly(I:C) | Invivogen | Cat #tlrl-picw |
| Tamoxifen | Sigma | T5648 |
| FuGENE HD | Promega | Cat #E2311 |
| Opti-MEM | Life Technologies | Cat #31985–070 |
| Clodronate liposome kit | Encapsula NanoSciences | Cat # CLD-8909 |
| HisPur Ni-NTA resin | ThermoFisher | Cat #88222 |
| T-PER tissue protein extraction | ThermoFisher | Cat #78510 |
| Cocktail inhibitor | Sigma | Cat #P8340 |
| Alexa Fluor™ 568 Antibody Labeling Kit | ThermoFisher | Cat # A20184 |
| Critical Commercial Assays | ||
| Thromboplastin-D | Pacific Hemostasis | Cat #100357 |
| TAT ELISA kit | Abcam | Cat #ab137994 |
| CellTiter-Glo Luminescent Cell Viability Assay | Promega | Cat #G7571 |
| CytoTox 96 Non-Radioactive Cytotoxicity Assay | Promega | Cat #G1780 |
| Experimental Models: Cell Lines | ||
| Mouse Primary Bone Marrow Derived Macrophages | Z.L. laboratory | This paper |
| THP-1 | ATCC | Cat #TIB-202 |
| Experimental Models: Organisms/Strains | ||
| C57BL/6J | The Jackson Laboratory | JAX:000664 |
| Casp1/11−/− | The Jackson Laboratory | JAX:016621 |
| Casp1−/− | Genentech Inc | Kayagaki et al., 2015 |
| Casp11−/− | The Jackson Laboratory | JAX:024698 |
| Tlr4−/− | The Jackson Laboratory | JAX:007227 |
| Gsdmd−/− | National Institute of Genetics, Japan | Fujii et al., 2008 |
| Il1r−/− | The Jackson Laboratory | JAX:003245 |
| Il18r−/− | The Jackson Laboratory | JAX:004131 |
| B6.Cg-Tg(UBC-cre/ERT2)1Ejb/J | The Jackson Laboratory | JAX: 007001 |
| C57BL/6-Tg(CAG-EGFP)1Osb/J | The Jackson Laboratory | JAX:003291 |
| F3fl/fl | Mackman Laboratory | RRID:IMSR_JAX:028721 |
| Software and Algorithms | ||
| FlowJo v10.07 (mac) | FLOWJO | |
| GraphPad Prism 7 (mac) | GraphPad | |
HIGHLIGHTS.
Canonical or noncanonical inflammasome activation leads to blood clotting
Inflammasome activation induces blood clotting through pyroptosis
Tissue factor released from pyroptotic macrophages drives blood blotting
Interfering tissue factor prevents pyroptosis-induced lethality
ACKNOWLEDGMENTS
C.W. is supported by AHA Great Rivers Affiliate Postdoctoral Fellowship 16POST31140008 and is an K99 awardee (K99HL145117; NHLBI). J.S. is supported by National Natural Science Foundation of China 81372391. X.L. is supported by NIH R01 GM121796. Y.W. is supported by AHA Great Rivers Affiliate Grant-in-Aid 17GRNT33410327, NSF CHE-1709381, and NIH/NIAID R56 AI137020 and R21 AI142063, and NIH/NHLBI R01 HL142640. Z.L. is supported by NIH/NHLBI R01 HL123927 and R01 HL142640. Dr. Wendy Katz provided help with Tissue paraffin embedding and sectioning and was supported by NIH/NIGMS Institutional Development Award P20GM103527. Dr. Thomas Wilkop at UK Light Microscopy Core provided help with intravital microscopy. Dr. Wei Li at Marshall University provided help with isolation of mouse cremaster muscle. Dr. Hartmut Weiler at Medical College of Wisconsin and Dr. Rodney M. Camire at the University of Pennsylvania providesed fibrin antibodies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
D.K. is an employee of Genentech Inc. The other authors declare no competing interests.
REFERENCES
- Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, Ciferri C, Dixit VM, and Dueber EC (2016). GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci U S A 113, 7858–7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus DC, and van der Poll T (2013). Severe sepsis and septic shock. N Engl J Med 369, 840–851. [DOI] [PubMed] [Google Scholar]
- Aziz M, Jacob A, Yang WL, Matsuda A, and Wang P (2013). Current trends in inflammatory and immunomodulatory mediators in sepsis. J Leukoc Biol 93, 329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach R, Nemerson Y, and Konigsber W (1981). Purification and characterization of bovine tissue factor. The Journal of Biological Chemistry 256, 8324–8331. [PubMed] [Google Scholar]
- Boucher D, Monteleone M, Coll RC, Chen KW, Ross CM, Teo JL, Gomez GA, Holley CL, Bierschenk D, Stacey KJ, et al. (2018). Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. J Exp Med 215, 827–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, and Zychlinsky A (2012). Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol 198, 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, and Monack DM (2012). Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490, 288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case CL, Kohler LJ, Lima JB, Strowig T, de Zoete MR, Flavell RA, Zamboni DS, and Roy CR (2013). Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proc Natl Acad Sci U S A 110, 1851–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KT, Xiong S, Ye Z, Hong Z, Di A, Tsang KM, Gao X, An S, Mittal M, Vogel SM, et al. (2017). Caspase-11-mediated endothelial pyroptosis underlies endotoxemia-induced lung injury. J Clin Invest 127, 4124–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, and Shao F (2016). Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116. [DOI] [PubMed] [Google Scholar]
- Effenberger-Neidnicht K, and Hartmann M (2018). Mechanisms of Hemolysis During Sepsis. Inflammation 41, 1569–1581. [DOI] [PubMed] [Google Scholar]
- Evavold CL, Ruan J, Tan Y, Xia S, Wu H, and Kagan JC (2018). The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity 48, 35–44 e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink SL, and Cookson BT (2006). Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol 8, 1812–1825. [DOI] [PubMed] [Google Scholar]
- Franco RF, de Jonge E, Dekkers PEP, Timmerman JJ, Spek CA, van Deventer SJH, van Deursen P, van Kerkhoff L, van Gemen B, ten Cate H, et al. (2000). The in vivo kinetics of tissue factor messenger RNAexpression during human endotoxemia: relationship with activation of coagulation. Blood 96, 554–559. [PubMed] [Google Scholar]
- Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr., Wrobleski SK, Wakefield TW, Hartwig JH, and Wagner DD (2010). Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A 107, 15880–15885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Tamura M, Tanaka S, Kato Y, Yamamoto H, Mizushina Y, and Shiroishi T (2008). Gasdermin D (Gsdmd) is dispensable for mouse intestinal epithelium development. Genesis 46, 418–423. [DOI] [PubMed] [Google Scholar]
- Fujishima S, Gando S, Saitoh D, Mayumi T, Kushimoto S, Shiraishi S, Ogura H, Takuma K, Kotani J, Ikeda H, et al. (2014). A multicenter, prospective evaluation of quality of care and mortality in Japan based on the Surviving Sepsis Campaign guidelines. J Infect Chemother 20, 115–120. [DOI] [PubMed] [Google Scholar]
- Gando S, Levi M, and Toh CH (2016). Disseminated intravascular coagulation. Nat Rev Dis Primers 2, 16037. [DOI] [PubMed] [Google Scholar]
- Gando S, Saitoh D, Ogura H, Fujishima S, Mayumi T, Araki T, Ikeda H, Kotani J, Kushimoto S, Miki Y, et al. (2013). A multicenter, prospective validation study of the Japanese Association for Acute Medicine disseminated intravascular coagulation scoring system in patients with severe sepsis. Crit Care 17, R111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover SP, and Mackman N (2018). Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler Thromb Vasc Biol 38, 709–725. [DOI] [PubMed] [Google Scholar]
- Hagar JA, Powell DA, Aachoui Y, Ernst RK, and Miao EA (2013). Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, and Han J (2015). Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res 25, 1285–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui KY, Haber E, and Matsueda GR (1983). Monoclonal antibodies to a synthetic fibrin-like peptide bind to human fibrin but not fibrinogen. Science 222, 1129–1132. [DOI] [PubMed] [Google Scholar]
- Ivanciu L, Toso R, Margaritis P, Pavani G, Kim H, Schlachterman A, Liu JH, Clerin V, Pittman DD, Rose-Miranda R, et al. (2011). A zymogen-like factor Xa variant corrects the coagulation defect in hemophilia. Nat Biotechnol 29, 1028–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen I, and Miao EA (2015). Pyroptotic cell death defends against intracellular pathogens. Immunol Rev 265, 130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, et al. (2015). Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al. (2011). Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, et al. (2013). Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249. [DOI] [PubMed] [Google Scholar]
- Kirchhofer D, Moran P, Bullens S, Peale F, and Bunting S (2005). A monoclonal antibody that inhibits mouse tissue factor function. J Thromb Haemost 3, 1098–1099. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, and Dixit VM (2014). Mechanisms and functions of inflammasomes. Cell 157, 1013–1022. [DOI] [PubMed] [Google Scholar]
- Latz E, Xiao TS, and Stutz A (2013). Activation and regulation of the inflammasomes. Nat Rev Immunol 13, 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi M, and Cate HT (1999). Disseminated intravascular coagulation. N Engl J Med 341, 586–592. [DOI] [PubMed] [Google Scholar]
- Levi M, ten Cate H, Bauer KA, van der Poll T, Edgington TS, Buller HR, van Deventer SJ, Hack CE, ten Cate JW, and Rosenberg RD (1994). Inhibition of endotoxin-induced activation of coagulation and fibrinolysis by pentoxifylline or by a monoclonal anti-tissue factor antibody in chimpanzees. J Clin Invest 93, 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw PC, Ito T, Iba T, Thachil J, and Zeerleder S (2016). DAMP and DIC: The role of extracellular DNA and DNA-binding proteins in the pathogenesis of DIC. Blood Rev 30, 257–261. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, and Lieberman J (2016). Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B, Davis RP, Kim SJ, Tse M, Esmon CT, Kolaczkowska E, and Jenne CN (2017). Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood 129, 1357–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE, Leaf IA, and Aderem A (2010). Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci U S A 107, 3076–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne JC, Blanke SR, Hanna PC, and R.J. C (1995). Protective antigen-binding domain of anthrax lethal factor mediates translocation of a heterologous protein fused to its amino- or carboxy-terminus. Molecular Microbiology 15, 661–666. [DOI] [PubMed] [Google Scholar]
- Monteleone M, Stanley AC, Chen KW, Brown DL, Bezbradica JS, von Pein JB, Holley CL, Boucher D, Shakespear MR, Kapetanovic R, et al. (2018). Interleukin-1beta Maturation Triggers Its Relocation to the Plasma Membrane for Gasdermin-D-Dependent and - Independent Secretion. Cell Rep 24, 1425–1433. [DOI] [PubMed] [Google Scholar]
- Morrissey JH, Fakhrai H, and Edgington TS (1987). Molecular Cloning of the cDNA for Tissue Factor, the Cellular Receptor for the Initiation of the Coagulation Protease Cascade. Cell 50, 129–135. [DOI] [PubMed] [Google Scholar]
- Neyman M, Gewirtz J, and Poncz M (2008). Analysis of the spatial and temporal characteristics of platelet-delivered factor VIII-based clots. Blood 112, 1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parise LV, Venton DL, and Le Breton GC (1984). Arachidonic acid-induced platelet aggregation is mediated by a thromboxane A2/prostaglandin H2 receptor interaction. J Pharmacol Exp Ther 228, 240–244. [PubMed] [Google Scholar]
- Pawlinski R, Pedersen B, Erlich J, and Mackman N (2004). Role of tissue factor in haemostasis, thrombosis, angiogenesis and inflammation: lessons from low tissue factor mice. Thromb Haemost 92, 444–450. [DOI] [PubMed] [Google Scholar]
- Pawlinski R, Wang JG, Owens AP 3rd, Williams J, Antoniak S, Tencati M, Luther T, Rowley JW, Low EN, Weyrich AS, et al. (2010). Hematopoietic and nonhematopoietic cell tissue factor activates the coagulation cascade in endotoxemic mice. Blood 116, 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, and Wenzel RP (1995). The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA 273, 117–123. [PubMed] [Google Scholar]
- Rathinam VA, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM, and Fitzgerald KA (2012). TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150, 606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothmeier AS, Marchese P, Petrich BG, Furlan-Freguia C, Ginsberg MH, Ruggeri ZM, and Ruf W (2015). Caspase-1-mediated pathway promotes generation of thromboinflammatory microparticles. J Clin Invest 125, 1471–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan J, Xia S, Liu X, Lieberman J, and Wu H (2018). Cryo-EM structure of the gasdermin A3 membrane pore. Nature 557, 62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sborgi L, Ruhl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, Farady CJ, Muller DJ, Broz P, and Hiller S (2016). GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J 35, 1766–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, and Tschopp J (2010). The inflammasomes. Cell 140, 821–832. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, and Shao F (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, and Shao F (2014). Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192. [DOI] [PubMed] [Google Scholar]
- Taylor FB Jr., Chang A, Ruf W, Morrissey JH, Hinshaw L, Catlett R, Blick K, and Edgington TS (1991). Lethal E. coli septic shock is prevented by blocking tissue factor with monoclonal antibody. Circ Shock 33, 127–134. [PubMed] [Google Scholar]
- Vanaja SK, Russo AJ, Behl B, Banerjee I, Yankova M, Deshmukh SD, and Rathinam VA (2016). Bacterial Outer Membrane Vesicles Mediate Cytosolic Localization of LPS and Caspase-11 Activation. Cell 165, 1106–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal A (2014). Disseminated intravascular coagulation. Indian J Anaesth 58, 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Moltke J, Trinidad NJ, Moayeri M, Kintzer AF, Wang SB, van Rooijen N, Brown CR, Krantz BA, Leppla SH, Gronert K, et al. (2012). Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature 490, 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Matsumoto T, and Yamashita Y (2014). Diagnosis and treatment of disseminated intravascular coagulation (DIC) according to four DIC guidelines. Journal of Intensive Care 2, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D, Kang TB, Dillon CP, and Green DR (2016). Programmed necrosis in inflammation: Toward identification of the effector molecules. Science 352, aaf2154. [DOI] [PubMed] [Google Scholar]
- Weiler-Guettler H, Christie PD, Beeler DL, Healy AM, Hancock WW, Rayburn H, Edelberg JM, and Rosenberg RD (1998). A targeted point mutation in thrombomodulin generates viable mice with a prethrombotic state. J Clin Invest 101, 1983–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang B, Zhang G, Guo L, Li XA, Morris AJ, Daugherty A, Whiteheart SW, Smyth SS, and Li Z (2013). Platelets protect from septic shock by inhibiting macrophage-dependent inflammation via the cyclooxygenase 1 signalling pathway. Nat Commun 4, 2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Tang Y, Di Gioia M, Broggi A, Ruan J, Shi J, Donado CA, Shao F, Wu H, Springstead JR, et al. (2016). An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science 352, 1232–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Shi J, Shi X, Wang Y, Wang F, and Shao F (2016). Genetic functions of the NAIP family of inflammasome receptors for bacterial ligands in mice. J Exp Med 213, 647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, and Shao F (2011). The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477, 596–600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Intravital imaging of DIC in cremaster muscle, Related to Figure 2. Platelets were GFP-positive (isolated from C57BL/6-Tg(CAG-EGFP)1Osb/J mice) and fibrin were detected with Alexa568-labeled fibrin antibody 59D8. Time series images were acquired at the same location of cremaster vasculature, 15 min and 60 min after EprJ injection, on Zeiss LSM880 with 10X Zeiss W Plan-Apochromat under fast Airyscan mode (419×419 μm at 3.6 frames per second). Image and videos were processed in Imaris x64 9.2.0.
Data Availability Statement
N/A