Abstract
Background:
The metabolome is a collection of exogenous chemicals and metabolites from cellular processes that may reflect the body’s response to environmental exposures. Studies of air pollution and metabolomics are limited.
Objectives:
To explore changes in the human metabolome before, during, and after the 2008 Beijing Olympics Games, when air pollution was high, low, and high, respectively.
Methods:
Serum samples were collected before, during, and after the Olympics from 26 participants in an existing panel study. Gas and ultra-high performance liquid chromatography/mass spectrometry were used in metabolomics analysis. Repeated measures ANOVA, network analysis, and enrichment analysis methods were employed to identify metabolites and classes associated with air pollution changes.
Results:
A total of 886 molecules were measured in our metabolomics analysis. Network partitioning identified four modules with 65 known metabolites that significantly changed across the three time points. All known molecules in the first module () were lipids (e.g., eicosapentaenoic acid, stearic acid). The second module consisted primarily of dipeptides (, e.g., isoleucylglycine) plus 8 metabolites from four other classes (e.g., hypoxanthine, 12-hydroxyeicosatetraenoic acid). Most of the metabolites in Modules 3 (19 of 23) and 4 (5 of 5) were unknown. Enrichment analysis of module-identified metabolites indicted significantly overrepresented pathways, including long- and medium-chain fatty acids, polyunsaturated fatty acids (n3 and n6), eicosanoids, lysolipid, dipeptides, fatty acid metabolism, and purine metabolism [(hypo) xanthine/inosine–containing pathways].
Conclusions:
We identified two major metabolic signatures: one consisting of lipids, and a second that included dipeptides, polyunsaturated fatty acids, taurine, and xanthine. Metabolites in both groups decreased during the 2008 Beijing Olympics, when air pollution was low, and increased after the Olympics, when air pollution returned to normal (high) levels. https://doi.org/10.1289/EHP3705
Introduction
The metabolome represents a collection of exogenous chemicals and metabolites from cellular processes that may provide a proximal indication of physiological responses to environmental exposures, disease processes, and drug therapies. Therefore, metabolomics may be a powerful tool to better understand the health effects of environmental stressors, including air pollution (Roessner and Bowne 2009).
Air pollution exposures have been associated with a wide array of health effects, including respiratory diseases, cardiovascular diseases, adverse pregnancy outcomes, and death (Brunekreef and Holgate 2002). However, the underlying mechanisms remain unclear. Over the past years, the use of metabolomics approaches to study environmental exposure-related biological mechanisms has increased. Previous studies using animal models have associated air pollution exposure with a small number of targeted metabolites including arachidonic acid (AA), prostaglandin (PGD2), and hydroxyoctadecadienoic acids (Brower et al. 2016; Cruickshank-Quinn et al. 2014; Joad et al. 1994; Li et al. 2015; Miller et al. 2015).
Metabolomics studies involving human subjects are limited. To our knowledge, only a few observational epidemiological studies have evaluated associations between environmental air pollution and metabolomics. In a study conducted in North Carolina, particulate matter with a diameter of or less () and ozone exposures were associated with changes in levels of targeted analytes along the creatine biosynthesis pathway and fatty acid oxidation metabolites (Breitner et al. 2016). Exposure to and was associated with differences in 8 metabolites (including and several amino acids) from different pathways among the 280 untargeted metabolites in the TwinsUK study (Menni et al. 2015). Nitrogen dioxide () exposure was associated with 10 metabolites in the lysophosphatidylcholines pathway among the 188 targeted serum metabolites (Ward-Caviness et al. 2016). Traffic-related air pollution has been associated with the elicitation of oxidative stress and inflammatory pathways (Liang et al. 2018), as well as the perturbation of linoleate metabolism pathway, which was further identified as a mediator in the association of air pollution with asthma and cardio-cerebrovascular diseases (Jeong et al. 2018). In addition, two experimental studies examined the impact of short-term air pollution exposure on metabolomics, including a study of 31 healthy volunteers exposed to ambient air pollution from different sources (e.g., subway, farm, traffic) for 5 h in which changes were reported in 89 of 3,873 metabolic features (Vlaanderen et al. 2017). A clinic-based crossover study of 24 participants reported increased levels of cortisol, corticosterone, and global lipid metabolism after 2 h of exposure to ozone (Miller et al. 2016).
The Beijing Olympics Air Pollution (BoaP) study is a panel study that was conducted during the Beijing Olympics in 2008 when temporary air pollution controls were implemented (Mu et al. 2014). The study enrolled 201 adults prior to Beijing’s air quality improvement initiative, when air pollution was high, and followed them during the Olympics, when air pollution was low, and after the Olympics, when air pollution returned to normal high levels. Biological specimens were collected and banked from each participant at baseline, during, and after the Olympics. For the present study, we performed an untargeted metabolomics analysis of biospecimens collected from a subset of BoaP participants to identify human serum metabolome changes before, during, and after the Olympics to better understand mechanisms underlying the health effects of air pollution exposures.
Methods
Study Design and Population
The present study is based on the BoaP study, which leveraged air pollution controls during the 2008 Beijing Olympic Games. The detailed design of the BoaP study can be found in our previous publications (Farhat et al. 2018; Mu et al. 2014). Briefly, the panel study recruited 201 participants residing in Beihang Community in Beijing, China, before the Olympics who were followed up during the Olympics (29 d after the opening of the Olympics) and again 74 d after the Olympics ended. Those with a prior medical history of cancer or serious immunological or chronic respiratory diseases were not included. Blood sample collection, in-person interviews, and physical examinations were conducted during each visit. Informed consent was obtained from each participant before their baseline visit. The study was approved by the institutional review boards of the University at Buffalo and the Peking University. A subset of participants from the parent study was selected for the metabolomics analysis.
Demographics and potential confounder measurement.
A total of 26 nonsmokers, 30–65 y of age, were randomly selected. All participants were Han Chinese. Weight and height were measured at baseline. Weekly vegetable and fruit consumption (grams/week) was calculated based on information of frequency and numbers of servings () per day during the past week collected at each visit. Approximately 12% of the data was missing for vegetable and fruit consumption. Missing data for each individual was imputed as the average of known values during other time points. An auxiliary variable for diet was defined as () for subsequent statistical modeling. Transportation data was collected at each time point by asking about the routine mode of transportation (i.e., bicycle, public transportation, or walking to work). Missing data for two individuals at a single time point were imputed as the transportation mode used during the other two time points, which was the same for both participants.
Air pollution assessment.
The method used to characterize aggregated air pollution has been previously described in detail (Farhat et al. 2018; Mu et al. 2014). Briefly, , , , , and total suspended particulates were measured using a particle mass monitor (Met One® 531 AEROCET Particulate Profiler; Met One Instruments, Inc.) twice per day during the study period along with temperature and relative humidity. The monitor was located in the center of the community in an open space close to the main road. Overall, the average levels of and were, respectively, and before the Olympics, and they decreased to () and () during the Olympics and returned to () and () after the Olympics. Therefore, we used three time points—before, during, and after the Olympics—to denote periods of high, low, and high air pollution exposures, respectively.
Metabolites measurement and processing.
The method used to collect blood samples has been previously described in detail (Farhat et al. 2018). Briefly, fasting blood samples were drawn at baseline and at the two follow-up visits. Collected biospecimens were immediately transferred to Peking University for processing. Serum and blood clots were separated by centrifugation, and aliquots were stored in cryogenic storage vials at . Samples were transferred on dry ice to the biorepository facility at the University at Buffalo.
Serum samples were sent on dry ice to Metabolon Inc. (in Durham, NC) for metabolomics analysis. Two platforms were used in the analysis: gas chromatography/mass spectrometry (GC/MS) and ultra-high performance liquid chromatography/tandem mass spectroscopy (UPLA-MS/MS). Untargeted metabolomic analysis was performed as described by Ohta et al. (2010) and Lawton et al. (2014). Briefly, of serum was subjected to a series of four liquid–liquid extractions, and the solvent extracts were pooled and then split into two equal aliquots, one for GC/MS and one for LC/MS. For LC/MS analysis, dry extracts were reconstituted in 10% methanol and 0.1% formic acid. GC/MS aliquots were derivatized using equal parts bistrimethylsilyl-trifluoroacetamide and solvent mixture acetonitrile: dichloromethane:cyclohexane (5:4:1) with 5% trimethylamine at 60˚C for 1 h. LC/MS was carried out using a Surveyor™ HPLC (Thermo Electron Corporation) with an electrospray ionization (Katajamaa and Oresic 2005) source coupled to an LTQ® MS (Thermo Electron Corporation). For GC/MS, the N,O-Bis(trimethylsilyl)trifluoroacetamide-derivatized samples were analyzed on a Thermo Finnigan™ Trace™ DSQ™ fast-scanning single-quadrupole MS operated at unit mass resolving power with a ( film phase consisting of 5% phenyldimethyl silicone) GC column. More than 3,500 commercially available purified standard compounds have been acquired and registered into the Laboratory Information Management System for distribution to both the LC/MS and GC/MS platforms for determination of their analytical characteristics. Briefly, metabolites are identified by comparison of retention time/index (IR), mass-to-charge ratio (m/z) of the ion, and MS fragmentation pattern to known metabolites. The peaks are matched to Metabolon’s library, then all peaks and matches are verified manually by a team of curators. Metabolites would be considered as Level 1 metabolites by the definitions of the Metabolites Standard Initiative. Metabolon maintains a library based on authenticated standards that contains the RI, m/z, and chromatographic data (including MS/MS spectral data) on all molecules present in the library. Furthermore, biochemical identifications are based on three criteria: the RI within a narrow RI window of the proposed identification, a nominal mass match to the library , and the MS/MS forward and reverse scores between the experimental data and authentic standards. Metabolites that lacked authenticated standards but had characteristics (RI, mass, and fragmentation) and correlated with known metabolites were considered as Level 2 if the metabolites were comparable to published spectra or as Level 3 if there were no published spectra. Level 3 metabolites are marked with single star to indicate a lack of 100% certainty in identity. The MS/MS scores are based on a comparison of the ions present in the experimental spectrum to the ions present in the library spectrum. Although there may be similarities between these molecules based on one of these factors, the use of all three data points can be utilized to distinguish and differentiate biochemicals.
Several quality control measures were used during the analysis:
Instrument variability was determined by calculating the median relative standard deviation (RSD) for the internal standards that were added to each sample prior to injection into the mass spectrometers.
Overall process variability was determined by calculating the median RSD for all endogenous metabolites present in 100% of the technical replicates of pooled client samples.
Each participant’s serum samples from all three time points were assayed in the same batch to avoid interassay variations.
Lab technicians at Metabolon were blinded to sample identity.
Recovery standards were added prior to the assay for quality control purposes.
The median RSD was 5% for internal variability and 11% for total process variability. Missing values were imputed using the minimum observed value for each metabolite following a log transformation.
Statistical Analysis
For each metabolite, scaling was done using the sample set median. Of the samples where a biochemical analyte was detected, the median value was set to 1.00, and all other values were scaled accordingly. After that step, any samples with missing values (i.e., a peak was not detected) were assigned the sample set minimum for that biochemical analyte. Subsequent statistics were performed after natural log transforming of the scaled, imputed data.
Statistical testing of individual metabolites.
The levels of each metabolite were compared among the three time points (before, during, and after the Olympics) using repeated measures Analysis of variance (ANOVA). Time point, sex, baseline body mass index (BMI), combined vegetable and fruit intake during the week before each visit (in grams), and mode of transportation to each study visit (categorized as bicycle, public transportation, or walking and modeled using indicator terms) were included as covariates. To take into account the multiple comparisons, q-value (Storey 2002; Storey and Tibshirani 2003) were used to estimate the false discovery rate, which is the proportion of false positives incurred when a particular test is classified as significant and is often used for high-dimensional testing problems. The q-values were calculated using the qvalue package in R (Dabney et al. 2010). Post hoc tests were used to contrast the levels of metabolites during versus before the Olympics and after versus during the Olympics.
Network analysis.
Network analysis was utilized to identify significant changes in metabolome classes. Specifically, we identified modules in which a group of metabolites were correlated with each other and associated with changes in air pollution levels in a concerted manner. These modules were further investigated for their functional roles in annotated class enrichment analysis.
As the first step, a correlation network was constructed with all metabolites. Each of the metabolites was represented as a node in the graph, and an edge (connecting line) was placed between two metabolites if the absolute value of their Spearman’s correlation coefficient exceeded a 0.7 threshold. Spearman’s correlation was utilized because it is rank based and therefore robust to nonlinear relationships and outliers. The threshold of 0.7 was carefully selected in order to a) minimize spurious correlations that may occur by chance because the probability of this occurring is close to zero and b) obtain a graph that is not too dense or too sparse, leading to too many or too few clusters, respectively.
The resulting network is partitioned into modules by greedy maximization of modularity (Clauset et al. 2004), using R package igraph (Csardi and Nepusz 2006). Modularity is a measure of the difference between the actual and the expected edge density, where the expected density assumes no module structure. Higher modularity indicates a stronger module structure. Only modules with sizes of at least five nodes were considered for further analysis.
In order to test for module significance and to control for confounding, we fit a series of two statistical models. First, we fit a linear regression for each metabolite in the module, with the independent variables of age, BMI, transportation, and vegetable and fruit consumptions. For each module, the residuals from these regression models were then used to fit the following three-way ANOVA to test the module significance:
where are the residuals from the th metabolite level for the th individual with sex at the th time point. The is the mean level of th metabolite at the th time point. The effects of th time point, th metabolite, and th sex are denoted as , , and , respectively. We calculated Benjamini and Hochberg–adjusted p-values to account for multiple comparisons (Benjamini and Hochberg 1995; Storey 2003). The modules with adjusted for the main effect of time were deemed significant. The marginal effects of sex, as well as interactions between sex and time, were also examined.
For the significant modules, pairwise comparisons of before versus during the Olympics, and during versus after the Olympics, were performed. Only modules with adjusted for both of the comparisons were retained as the final modules. All metabolites in the final modules were considered significantly related to air pollution as a group. To validate the concerted manner of metabolites in each module across the three time points, overall trends of each module were examined by taking the average of the normalized levels of all metabolites in the module.
As a second step, all of the identified metabolites from Modules 1 to 4 were further mapped to classes in the Metabolon database. Enrichment testing was performed to identify overrepresented classes in the significant modules by using Fisher’s exact test and adjusted using a Benjamini-Hochberg adjustment. Briefly, the contingency table for this test was constructed for each defined class within Metabolon’s database based on the metabolites within the class that are present in the significant modules and the “background” (e.g., “metabolite universe”), which consists of the 74 classes in the metabolon database itself (Huang et al. 2009). All statistical analysis was performed in R (R Development Core Team).
Results
Participants Characteristics
The study participants were 12 men and 14 women with an average age of 48.6 (SD 7.1) y and BMI of 23.6 (SD 3.2) at baseline. Shown in Table 1, medians of vegetable and fruit consumptions were similar across the three time periods, although the interquartile range was large for each period. Bicycling and walking were the two most common modes of routine transportation at each visits with few changes over the three periods.
Table 1.
Characteristics of the study population according to study visit, .
| Characteristic | Baseline (before Olympics) | During Olympics | After Olympics |
|---|---|---|---|
| Age [y ()] | NA | NA | |
| Female [n (%)] | 14 (53.8%) | NA | NA |
| BMI [ ()] | NA | NA | |
| Vegetable consumption {g/week [median (Q1, Q3)]} | 2,100.0 (1,837.5, 2,625.0) | 2,100.0 (1,750.0, 3,500.0) | 2,125.0 (1,862.5, 2,850.0) |
| Fruit consumption {g/week [median (Q1, Q3)]} | 425.0 (300.0, 450.0) | 450 (362.5, 1,162.5) | 425.0 (350.0, 737.5) |
| Transportation [ (%)] | |||
| Bicycle | 11 (45.8%) | 10 (38.5%) | 10 (38.5%) |
| Public transportation | 3 (12.5%) | 1 (3.9%) | 1 (3.9%) |
| Walking | 10 (41.7) | 15 (57.7%) | 15 (57.7%) |
Note: NA, not applicable.
Individual Metabolites Identified with Changes over the Study Period
A total of 886 metabolic signals were detected from the metabolomics analyses, among which 570 were known metabolites and 316 were of unknown identity. Based on individual ANOVA tests, 40 metabolites changed significantly ( prior to adjustment for multiple comparisons) over all three time periods or between at least one of the two pairs of time points (Table 2). These metabolites revealed two primary patterns of changes before, during, and after the Olympics: the first pattern (low–high–low) included 5 metabolites (e.g., N-acetylglutamate, p-toluic acid) that were lower before and after the Olympics than during the Olympics. The second pattern (high–low–high) included 28 metabolites (e.g., N-stearoyltaurine, 2-hydroxyglutarate) that were lower during the Olympics than before or after the Olympics. There are 7 metabolites that did not change with the above pattern, that is, 3 metabolites were lower during and after than before the Olympics (e.g., cortisone), and 4 metabolites were lower before than during and after the Olympics (e.g., oxalate). None of the comparisons reached significance after multiple testing adjustments. These metabolites belong to six super classes and 21 classes. Table S1 lists the numbers of metabolites in each class and the numbers of metabolites that changed significantly () across the three time points.
Table 2.
Forty metabolites with significant differences ( prior to adjustment for multiple comparisons) before, during, after the 2008 Beijing Olympics in 26 nonsmoking adults.
| Super class, class, and metabolite | Level of metabolitesa | Overall comparison | During vs. before the Olympics | After vs. during the Olympics | Missing (%)b | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | During | After | p-Value | Q-Value | Ratio | p-Value | Q-Value | Ratio | p-Value | Q-Value | ||
| Amino acid super class | ||||||||||||
| Glutamate metabolism class | ||||||||||||
| N-Acetylglutamate | 1.04 | 1.08 | 1.06 | 0.03* | 1.00 | 1.04* | 0.03 | 0.99 | 0.98 | 0.9 | 0.99 | 0 |
| Phenylalanine and tyrosine metabolism class | ||||||||||||
| p-Toluic acid | 0.95 | 1.07 | 0.87 | 0.08 | 1.00 | 1.13* | 0.02 | 0.99 | 0.81 | 0.14 | 0.99 | 0 |
| Carbohydrate super class | ||||||||||||
| Fructose, mannose, and galactose metabolism class | ||||||||||||
| Sorbitol | 1.20 | 1.07 | 2.02 | 0.10 | 1.00 | 0.89* | 0.05 | 0.99 | 1.90 | 0.82 | 0.99 | 0 |
| Pentose metabolism class | ||||||||||||
| Ribitol | 1.07 | 1.02 | 1.32 | 0.05 | 1.00 | 0.96 | 0.67 | 0.99 | 1.29 | 0.06 | 0.99 | 0 |
| Cofactors and vitamins super class | ||||||||||||
| Ascorbate and aldarate metabolism class | ||||||||||||
| Oxalate (ethanedioate) | 0.94 | 1.02 | 1.17 | 0.02* | 1.00 | 1.09 | 0.44 | 0.99 | 1.14* | 0.05 | 0.99 | 0 |
| Ascorbate (vitamin C) | 0.83 | 1.13 | 1.55 | 0.03* | 1.00 | 1.35 | 0.44 | 0.99 | 1.38 | 0.07 | 0.99 | 9.75 |
| Nicotinate and nicotinamide metabolism class | ||||||||||||
| Nicotinamide | 1.04 | 0.91 | 1.09 | 0.22 | 1.00 | 0.87* | 0.04 | 0.99 | 1.20 | 0.5 | 0.99 | 8.75 |
| Lipid super class | ||||||||||||
| Endocannabinoid subclass | ||||||||||||
| N-Stearoyltaurine | 1.79 | 0.98 | 1.52 | 0.05 | 1.00 | 0.55* | 0.02 | 0.99 | 1.55* | 0.03 | 0.99 | 5.50 |
| Fatty acid metabolism (acyl carnitine) class | ||||||||||||
| Hydroxybutyrylcarnitinec | 1.75 | 1.12 | 1.59 | 0.09 | 1.00 | 0.64* | 0.04 | 0.99 | 1.42 | 0.07 | 0.99 | 0 |
| Fatty acid, dicarboxylate class | ||||||||||||
| 2-Hydroxyglutarate | 1.10 | 0.92 | 1.14 | 0.02* | 1.00 | 0.83* | 0.02 | 0.99 | 1.24* | 0.01 | 0.92 | 0 |
| Sebacate (decanedioate) | 1.23 | 1.09 | 1.34 | 0.10 | 1.00 | 0.88 | 0.58 | 0.99 | 1.23* | 0.03 | 0.99 | 33.50 |
| Fatty acid, monohydroxy class | ||||||||||||
| 3-Hydroxydecanoate | 1.15 | 1.01 | 1.31 | 0.03* | 1.00 | 0.88* | 0.04 | 0.99 | 1.29* | 0.01 | 0.92 | 0 |
| 3-Hydroxyoctanoate | 1.24 | 1.10 | 1.52 | 0.06 | 1.00 | 0.89 | 0.29 | 0.99 | 1.38* | 0.02 | 0.95 | 0 |
| Glycerolipid metabolism class | ||||||||||||
| Glycerol 3-phosphate (G3P) | 1.03 | 1.41 | 1.02 | 0.06 | 1.00 | 1.37 | 0.21 | 0.99 | 0.72* | 0.03 | 0.99 | 0 |
| Long-chain fatty acid class | ||||||||||||
| Eicosenoate (20:1n9 or 11) | 1.18 | 1.12 | 1.46 | 0.02* | 1.00 | 0.95 | 0.69 | 0.99 | 1.30* | 0.01 | 0.92 | 0 |
| Pentadecanoate (15:0) | 1.03 | 0.89 | 1.09 | 0.03* | 1.00 | 0.86* | 0.02 | 0.99 | 1.22* | 0.04 | 0.99 | 0 |
| 10-Nonadecenoate (19:1n9) | 1.12 | 0.95 | 1.18 | 0.03* | 1.00 | 0.85 | 0.05 | 0.99 | 1.24* | 0.01 | 0.92 | 9.50 |
| Myristoleate (14:1n5) | 1.17 | 1.10 | 1.43 | 0.09 | 1.00 | 0.94 | 0.28 | 0.99 | 1.30* | 0.02 | 0.92 | 0 |
| Palmitoleate (16:1n7) | 1.08 | 1.00 | 1.25 | 0.10 | 1.00 | 0.92 | 0.30 | 0.99 | 1.25* | 0.02 | 0.95 | 0 |
| 10-Heptadecenoate (17:1n7) | 1.04 | 0.93 | 1.13 | 0.12 | 1.00 | 0.90 | 0.12 | 0.99 | 1.22* | 0.04 | 0.99 | 0 |
| Oleate (18:1n9) | 1.03 | 0.96 | 1.11 | 0.13 | 1.00 | 0.93 | 0.43 | 0.99 | 1.17* | 0.03 | 0.99 | 0 |
| Lysolipid class | ||||||||||||
| 1-Palmitoylglycerophosphoglycerolc | 1.55 | 1.22 | 1.31 | 0.14 | 1.00 | 0.78* | 0.04 | 0.99 | 1.08 | 0.42 | 0.99 | 0 |
| Medium-chain fatty acid class | ||||||||||||
| Caprylate (8:0) | 1.12 | 1.01 | 1.34 | * | 1.00 | 0.90 | 0.91 | 0.99 | 1.33* | 0.01 | 0.92 | 8.75 |
| Caprate (10:0) | 1.05 | 0.98 | 1.28 | 0.03* | 1.00 | 0.93 | 0.36 | 0.99 | 1.30* | 0.01 | 0.92 | 0 |
| Caproate (6:0) | 1.07 | 0.93 | 1.20 | 0.05 | 1.00 | 0.87 | 0.15 | 0.99 | 1.30* | 0.02 | 0.92 | 0 |
| 5-Dodecenoate (12:1n7) | 1.36 | 1.14 | 1.64 | 0.07 | 1.00 | 0.83 | 0.20 | 0.99 | 1.44* | 0.02 | 0.92 | 0 |
| Polyunsaturated fatty acid (n3 and n6) class | ||||||||||||
| Docosatrienoate (22:3n3) | 1.14 | 0.97 | 1.19 | 0.04* | 1.00 | 0.85 | 0.14 | 0.99 | 1.23* | 0.01 | 0.92 | 0 |
| Dihomo-linoleate (20:2n6) | 1.10 | 0.94 | 1.12 | 0.07 | 1.00 | 0.85 | 0.09 | 0.99 | 1.19* | 0.02 | 0.95 | 0 |
| Docosadienoate (22:2n6) | 1.08 | 0.98 | 1.19 | 0.07 | 1.00 | 0.91 | 0.36 | 0.99 | 1.22* | 0.03 | 0.99 | 5.50 |
| Arachidonate (20:4n6) | 1.08 | 0.99 | 1.04 | 0.15 | 1.00 | 0.91 | 0.57 | 0.99 | 1.06* | 0.04 | 0.99 | 20.50 |
| Mead acid (20:3n9) | 1.17 | 1.00 | 1.07 | 0.18 | 1.00 | 0.85 | 0.26 | 0.99 | 1.07* | 0.04 | 0.99 | 20.25 |
| Steroid Class | ||||||||||||
| Pregnanediol-3-glucuronide | 2.37 | 2.55 | 3.87 | 0.02* | 1.00 | 1.07 | 0.39 | 0.99 | 1.52* | 0.03 | 0.99 | 0 |
| Pregn steroid monosulfatec | 1.18 | 1.23 | 1.17 | 0.04* | 1.00 | 1.04 | 0.06 | 0.99 | 0.96 | 0.66 | 0.99 | 0 |
| Cortisone | 1.02 | 1.00 | 0.96 | 0.09 | 1.00 | 0.98* | 0.04 | 0.99 | 0.96 | 0.78 | 0.99 | 0 |
| Sterol class | ||||||||||||
| Campesterol | 0.89 | 0.81 | 1.04 | 0.10 | 1.00 | 0.92 | 0.99 | 1.00 | 1.27* | 0.03 | 0.99 | 1.75 |
| Peptide super class | ||||||||||||
| Dipeptide class | ||||||||||||
| Phenylalanyltryptophan | 0.97 | 1.03 | 1.14 | 0.14 | 1.00 | 1.06 | 0.22 | 0.99 | 1.11* | 0.04 | 0.99 | 0 |
| Threonylphenylalanine | 1.26 | 0.97 | 1.69 | 0.15 | 1.00 | 0.77 | 0.67 | 0.99 | 1.74* | 0.04 | 0.99 | 0 |
| γ-Glutamyl amino acid class | ||||||||||||
| γ-Glutamylthreoninec | 1.10 | 1.10 | 1.04 | 0.02* | 1.00 | 1.00 | 0.67 | 0.99 | 0.95* | 0.02 | 0.92 | 29.75 |
| Xenobiotics super class | ||||||||||||
| Chemical class | ||||||||||||
| Glycerol 2-phosphate | 1.06 | 1.37 | 1.03 | 0.08 | 1.00 | 1.30 | 0.54 | 0.99 | 0.75* | 0.05 | 0.99 | 0 |
| Food component/plant class | ||||||||||||
| Dihydroferulic acid | 1.33 | 1.11 | 1.05 | 0.17 | 1.00 | 0.83 | 0.27 | 0.99 | 0.95* | 0.04 | 0.99 | 0 |
Note: Statistical comparisons were adjusted for time-invariant variables (age, sex, BMI) and time-varying variables including diet and transportation mode. p-Value was calculated from ANOVA for overall comparison and from post hoc tests for during vs. before and after vs. during contrasts. Q-value was calculated as false discovery rate–adjusted p-value using Storey method. indicates increased, whereas indicates decreased levels of metabolites. *, .
Set median as 1 and scaled according to a per biochemical basis.
Averaged proportion of missing value from the three time points.
Indicates compounds with Level 3 identity confidence (lacked authenticated standards but had characteristics correlated with known metabolites).
Identified Metabolites Modules
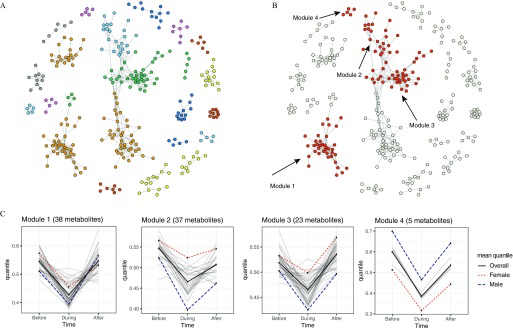
The module analysis generated 22 modules that showed significant differences across the three time points (adjusted ) using graph partitioning of the correlation matrix for all of the 876 compounds (Figure 1A). Three-way ANOVA analysis (accounting for interactions with time and sex) of the 22 modules identified four modules that were significantly different between both pairs of time points (before vs. during and during vs. after the Olympics) after multiple comparison adjustment (Figure 1B,C; see also Table S2). The mean normalized values of the individual metabolites in each module and the average values for all metabolites in each module (overall and according to sex) are depicted for each time point in Figure 1C. Metabolites in each of the four significant modules showed a similar pattern of changes, with lower levels during the Olympics than before or after the Olympics. Differences in average metabolite levels between men and women were significant for all modules after adjustment: Women had higher average levels of metabolites than men in Modules 1–3, but lower levels in Module 4 (Figure 1C). The adjusted p-value for the interaction between sex and time was significant for Modules 2 and 3 (), but not for Modules 1 and 4 (interaction p-values of 0.06 and 0.98, respectively), although the general pattern of changes according to time period (decreasing, then increasing) was consistent between men and women for all modules.
Figure 1.
(A) Twenty-two modules (shown in different colors) were identified by graph partition of 886 compounds with significant differences across the three time points (adjusted ). Each node represents a metabolite. An edge was placed between two metabolites if the absolute value of their Spearman’s correlation coefficient was . All modules were adjusted for age, sex, BMI, diet, and transportation mode. (B): Four modules including 69 metabolites that changed significantly across the three time points (before, during, and after the Olympics) are shown in red, whereas nodes in all other modules are shown in gray. (C) Average levels of individual metabolites (light gray lines) in each module during each of the three time points, and average levels for all metabolites combined, overall (solid line) and according to sex (dotted line for women, dashed line for men), at each time point.
There were 38, 37, 23, and 5 metabolites in the four significant modules that were significantly different for each pairwise comparison (before vs. during and during vs. after the Olympics) after multiple comparison adjustment. Most of the metabolic signals in Modules 1 (33/38) and 2 (32/37) were known metabolites, whereas most in Modules 3 (19/23) and 4 (5/5) were unidentified (see Table S3). Identified metabolites in Modules 1–3 were from a total of 6 super classes and 14 classes (Table 3). The 33 identified metabolites in Module 1 belonged to 8 classes within the lipid super class: long-chain fatty acids (LCFAs), polyunsaturated fatty acids [omega-3 and -6 (n3 and n6)] (PUFAs), medium-chain fatty acids (MCFAs), branched fatty acids, monohydroxy fatty acids, lysolipids, fatty acid metabolism, and glycerolipid metabolism. Module 2 included 32 identified metabolites, most of which belong to the peptide super class and dipeptide class. The remaining metabolites in Module 2 belonged to 4 super classes/classes including a) nucleotide super class, purine metabolism-(hypo)xanthine/inosine–containing class; b) lipid super class, eicosanoid class; c) amino acid super class, methionine–cysteine-S-adenosyl methione (SAM) and taurine metabolism class; and d) carbohydrate super class; glycolysis, gluconeogenesis, and pyruvate metabolism subclass. The four identified metabolites in Module 3 belonged to the lipid super class and the three 3 classes: monohydroxy fatty acids, fatty acid metabolism, and ketone bodies.
Table 3.
Sixty-nine known metabolites identified from module analyses with significant differences (Benjamini and Hochberg–adjusted ) before, during, and after the Beijing 2008 Olympics in 26 nonsmoking adults.
| Module | Super class | Class | Metabolites |
|---|---|---|---|
| Module 1 | Lipid super class | Long-chain fatty acid class | 10-Heptadecenoate (17:1n7) |
| Palmitoleate (16:1n7) | |||
| Margarate (17:0) | |||
| Palmitate (16:0) | |||
| Myristoleate (14:1n5) | |||
| Oleate (18:1n9) | |||
| 10-Nonadecenoate (19:1n9) | |||
| Myristate (14:0) | |||
| cis-Vaccenate (18:1n7) | |||
| Nonadecanoate (19:0) | |||
| Stearate (18:0) | |||
| Eicosenoate (20:1n9 or 11) | |||
| Polyunsaturated fatty acid (n3 and n6) class | Linolenate [ or ; (18:3n3 or 6)] | ||
| Docosapentaenoate (n3 DPA; 22:5n3) | |||
| Eicosapentaenoate (EPA; 20:5n3) | |||
| Stearidonate (18:4n3) | |||
| Dihomo-linoleate (20:2n6) | |||
| Linoleate (18:2n6) | |||
| Docosadienoate (22:2n6) | |||
| Docosatrienoate (22:3n3) | |||
| Dihomo-linolenate (20:3n3 or n6) | |||
| 5-Dodecenoate (12:1n7) | |||
| Medium-chain fatty acid class | Caprate (10:0) | ||
| Caprylate (8:0) | |||
| Laurate (12:0) | |||
| Fatty acid metabolism (acyl carnitine) class | cis-4-Decenoyl carnitine | ||
| Hexanoylcarnitine | |||
| Decanoylcarnitine | |||
| Octanoylcarnitine | |||
| Fatty acid, branched class | 17-Methylstearate | ||
| Fatty acid, monohydroxy class | 3-Hydroxydecanoate | ||
| Glycerolipid metabolism class | Glycerol | ||
| Lysolipid class | 1-Eicosapentaenoylglycerophosphocholine (20:5n3)a | ||
| Module 2 | Peptide | Dipeptide class | Isoleucylglycine |
| Prolylglycine | |||
| Valylglycine | |||
| Leucylglycine | |||
| Phenylalanylglycine | |||
| Isoleucylalanine | |||
| Prolylalanine | |||
| Phenylalanylalanine | |||
| Leucylphenylalanine | |||
| Prolylphenylalanine | |||
| Asparagylleucine | |||
| Valylleucine | |||
| Histidylleucine | |||
| Aspartylleucine | |||
| Threonylleucine | |||
| Leucylglutamate | |||
| Valylglutamate | |||
| Tyrosylglutamate | |||
| Valylglutamine | |||
| Valylaspartate | |||
| Isoleucylaspartate | |||
| Phenylalanylaspartate | |||
| Isoleucylvaline | |||
| Histidylvaline | |||
| Nucleotide | Purine metabolism, (hypo)xanthine/inosine–containing class | Hypoxanthine | |
| Xanthine | |||
| Xanthosine | |||
| Lipid | Eicosanoid class | 12-HETE | |
| 12-HEPE | |||
| Amino acid | Methionine, cysteine, SAM and taurine metabolism class | S-Adenosylhomocysteine (SAH) | |
| Taurine | |||
| Carbohydrate | Glycolysis, gluconeogenesis, and pyruvate metabolism class | Lactate | |
| Module 3 | Lipid | Fatty acid metabolism (acyl carnitine) | Acetylcarnitine |
| Hydroxybutyrylcarnitinea | |||
| Fatty acid, monohydroxy class | 3-Hydroxyoctanoate | ||
| Ketone bodies class | 3-Hydroxybutyrate (BHBA) |
Note: S-adenosyl methione.
Compounds with Level 3 identity confidence (lacked authenticated standards but had characteristics correlated with known metabolites).
Enrichment Analysis
Enrichment analysis of the 69 known metabolites in the significant modules identified seven overrepresented classes, which changed significantly across the three time points (Table 4). These overrepresented classes covered over half (33/53) of the metabolites in the five annotated lipid pathways; half (3/6) of the metabolites in the purine metabolism, (hypo)xanthine/inosine–containing pathway; and over a third (24/61) of the metabolites on the dipeptide pathway.
Table 4.
Enrichment analysis for significantly overrepresented classes of the 60 metabolites in the modules that showed significant changes before, during, and after the Olympics in 26 nonsmoking adults.
| Super pathways and pathways | p-Value | Adjusted p-Value | Metabolites (n) | Ratio (%) | |
|---|---|---|---|---|---|
| Module identified | In the pathway annotation | ||||
| Lipid super pathway | |||||
| Eicosanoid pathway | 0.01 | 0.03 | 2 | 2 | 100% |
| Long-chain fatty acid pathway | 12 | 17 | 71% | ||
| Polyunsaturated fatty acid (n3 and n6) pathway | 9 | 14 | 64% | ||
| Medium-chain fatty acid pathway | 0.01 | 0.02 | 4 | 8 | 50% |
| Fatty acid metabolism (acyl carnitine) pathway | 6 | 12 | 50% | ||
| Nucleotide super pathway | |||||
| Purine metabolism, (hypo)xanthine/inosine–containing pathway | 0.03 | 0.05 | 3 | 6 | 50% |
| Peptide super pathway | |||||
| Dipeptide pathway | 24 | 61 | 39% | ||
Note: Module analysis identified 69 metabolites that mapped to Metabolon classes, only classes with significant results (adjusted ) and more than one metabolite are shown. p-Values are calculated using Fisher’s exact test and adjusted with a Benjamini-Hochberg correction.
Discussion
The present study identified two major metabolic signatures in the pathways of lipid and peptide metabolism that decreased during the 2008 Beijing Olympics, when air pollution decreased relative to levels before and after the Olympics. Metabolites involved in these groups included LCFAs and PUFAs, eicosanoids and hydroxy fatty acid [e.g., 12-Hydroxyeicosatetraenoic acid (12-HETE), 12-Hydroxyeicosapentaenoate (12-HEPE)], lysolipids, and dipeptides. Metabolites in the purine metabolism (hypo)xanthine/inosine pathway (xanthine and hypoxanthine), and the taurine metabolism pathway [taurine and S-adenosylhomocysteine (SAH)] followed a pattern similar to that of the lipid and peptide metabolism around the Olympics. These pathways together might indicate a systemic reaction consistent with oxidative stress and inflammation before and after the Olympics when air pollution levels were high.
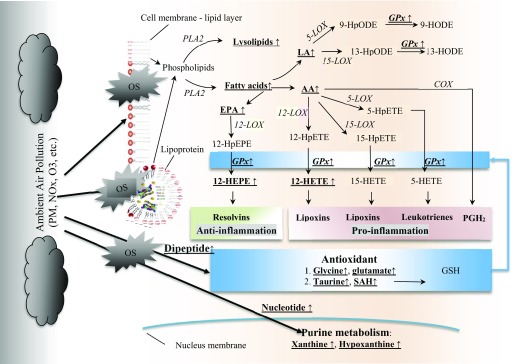
Exposure to ambient air pollution has been associated with oxidative stress that arises from the cellular response to reactive oxygen species (ROS) (Lodovici and Bigagli 2011). Free radicals and ROS injure the cells and induce damages to lipids, proteins, and nucleic acids. In the present study, we found the lipid metabolism pathway changed significantly across the three time points when air pollution changed drastically. Taken together with our previous reports on biomarkers of oxidative stress and antioxidant enzymes (Farhat et al. 2018) in the parent study population, these lipid metabolite changes might implicate a critical role of the lipid pathway in the oxidative process [e.g., eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), 12-HEPE], and the antioxidation/anti-inflammation process (e.g., taurine, glycine). A hypothesized mechanism is synthesized in Figure 2.
Figure 2.
The potential pathways of metabolomics changes before, during, after the Olympics. Bolded underlined metabolites changed significantly across the three time points with adjustment of age, sex, BMI, diet, and transportation mode. Note: AA, arachidonic acid; COX, cyclooxygenase; EPA, eicosapentaenoate; GPx, glutathione peroxidase; GSH, glutathione; HETE, Hydroxyeicosatetraenoic acid; HODE, Hydroxyoctadecadienoic acid; HpEPE, hydroperoxy-5Z,8Z,11Z,13E,17Z-eicosapentaenoic acid; HpETE, hydroperoxyeicosatetraenoic acid; HpODE, hydroperoxy-9Z,11E-octadecadienoic acid; LA, linoleic acid; LOX, lipoxygenase; NOx, Nitrogen oxides; O3, Ozone; OS, oxidative stress; , prostaglandins; PLA2, phospholipases A2; PM, particulate matter; SAH, S-adenosylhomocysteine.
First, we observed decreased levels of fatty acids during the Olympics than before or after the Olympics, particularly pathways of LCFAs and n3 (EPA and DHA) and n6 PUFAs (AA and linoleic acid [LA]). In response to free radicals and ROS, the cellular membrane phospholipid breaks down to free fatty acids (Valko et al. 2016). These broken-down free fatty acids include PUFAs (e.g., LA, EPA) because they were found at higher levels before and after than during the Olympics. The liberation of membrane fatty acids or lipoprotein fatty acids is consistent with an increased expression or activation of phospholipase A2 () (Brüske et al. 2011; Sevanian and Kim 1985; Tithof et al. 2002; van Kuijk et al. 1987). belongs to a group of enzymes that hydrolyze the sn-2 position of glycerophospholipids, thereby generating free fatty acids and lysolipids. Thus, the increased levels of free fatty acids may be indicative of cellular membrane or plasma lipoprotein phospholipid turnover during air pollution exposure.
Second, we observed that downstream metabolites of AA and EPA, that is, 12-HETE and 12-HEPE, respectively, significantly changed in a concerted manner similar to PUFAs. Free radical- and ROS-mediated lipid peroxidation of AA forms mixed hydroperoxy eicosatetraeneoic acids (HpETE) positional isomers (including 5-, 8-, 11-, 12-, or 15 HpETE). These hydroperoxy derivatives are rapidly reduced to their corresponding hydroxy derivatives by cellular and plasma glutathione peroxidase (GPx). Our metabolomics analysis indicated significant signals from the C12 positional isomers among the detected hydroxy fatty acids (i.e., 12-HETE, 12-HEPE) that might be indicative of 12-LOX activity. 12-LOX has catalytic activity against several substrates, including AA and EPA and lesser activity against LA (Ikei et al. 2012). Regulation of 12-LOX activity appears to be increased as a function of the availability of its PUFA substrates (Bidgood et al. 2000; Yeung and Holinstat 2011). 12-HETE is an important precursor of pro-inflammation mediators such as prostaglandins, leukotrienes, and thromboxane. In contrast, n3 PUFAs (mainly EPA and DPA) convert to 12-HEPE, which is an important precursor of anti-inflammatory mediators such as resolvins and the peroxisome proliferator-activated receptor, which attenuates the nuclear factor kappa-B (NF-κB) inflammatory pathway. Collectively, our data suggest that derivatives of lipid peroxidation may play a role in air pollution-induced inflammatory responses. Our results are in line with an experimental metabolomics study of mice exposed to ultrafine particulate matter that showed significantly increased intestine 12-HETE AA (Li et al. 2015). Given the key role of lipid peroxidation products in the development of atherosclerosis (Künzli et al. 2005), our results provide another line of evidence supporting a role of lipid metabolism, especially PUFA metabolism, in mechanisms linking air pollution and cardiovascular health (Brook et al. 2010). Previous studies have reported suggestive evidence that nutritional supplementation may attenuate air pollution-induced health effects (Hennig et al. 2007; Péter et al. 2015; Tong et al. 2012). Thus, further studies of the potential benefits of antioxidant vitamins, micronutrients, and n3 PUFA supplementation for reducing adverse health effects of ambient air pollution are warranted.
Dipeptide pathways, especially those involving glycine and glutamate metabolism, changed significantly across the three time points. We found significant changes in five metabolites belonging to the glycine-dipeptide class (e.g., isoleucylglycine, prolylglycine) and four metabolites belonging to the glutamate-dipeptide class (e.g., leucylglutamate, valylglutamate). Glycine and glutamate are both important mediators of the GSH metabolism pathways (Wu et al. 2004). Metabolites belonging to the glycine and glutamate metabolism classes decreased during, and then increased after, the Olympics. This is consistent with changes in GSH-dependent enzymes observed in the parent BoaP study (Farhat et al. 2018). In the parent study, GPx levels decreased by 12.0% during the Olympics and increased by 6.5% after the Olympics (Farhat et al. 2018). GPx is a key front-line defense phase II enzyme that breaks down hydrogen peroxide and lipid hydroperoxides into water and less toxic hydroxy lipids, respectively. GPx requires GSH as a cofactor for its activity and converts to GSH to glutathione disulfide (GSSG) as a byproduct. GSH also serves as the cofactor for glutathione-S-transferase, which detoxifies 4-hydroxyalkenal lipid peroxide breakdown products (Maulucci et al. 2016) and has direct antioxidant scavenging activity (Chaudière and Ferrari-Iliou 1999). Taken together, our findings further implicate a role of the GSH and the GSH-dependent family of enzymes in antioxidation processes related to air pollution exposure.
In addition to the lipid metabolism, we observed that xanthine and hypoxanthine significantly changed across the three time points. Xanthine and hypoxanthine are metabolites associated with the breakdown of purine nucleotides during the production of the endogenous antioxidant urate (Glantzounis et al. 2005). In animal studies, exposure was shown to significantly increase xanthine oxidase, coinciding with effects on oxidative stress, and endothelial NO synthase (eNOS) and inducible NO synthase (iNOS) mRNA expression levels (Dianat et al. 2016). Therefore, increased oxidative stress in air pollution exposure may threaten the integrity of the nucleus.
Our study also found changes in taurine, a product on the methionine–cysteine and taurine (MCT) metabolism pathway. Taurine is an important amino acid involved in various physiological processes such as bile acid conjugation, which produces taurine-conjugated bile acids and taurine chloramine (Huxtable 1992; Jacobsen and Smith 1968). These products are important mediators in anti-inflammatory effects, antioxidative effects, and lipid metabolism (Murakami 2015). An experimental study showed a higher level of taurine after air pollution exposures in rats that was consistent with a response to increased oxidative stress (Zhang et al. 2017). In a crossover study of healthy volunteers, taurine was increased in lung lavage fluid after exposure to pure biodiesel exhaust for 1 h (Surowiec et al. 2016). These findings suggest that air pollution exposure might induce oxidation first in the respiratory systems and then further spread into the circulation system. In the present study, SAH, another derivative on the MCT metabolism pathway, also changed significantly across the three time points in a manner similar to taurine. SAH is the immediate precursor of all homocysteine produced in the body, and there is growing evidence that SAH has an important role in different chronic diseases, including cardiovascular diseases and diabetes (Herrmann et al. 2005; Xiao et al. 2015).
Glycine metabolism decreased during the period of pollution control and then increased again after the removal of the controls. Glycine metabolism is a critical intermediate in the biosynthesis of neurotransmitters and their breakdown markers. The observed changes suggest that air pollution exposure might have the potential to affect neurophysiology (Hernandes and Troncone 2009). However, it must be noted that some neurotransmitters are also synthesized outside of the central nervous system where they serve signaling functions independent of neurotransmission.
Only a few epidemiological studies have investigated the effect of air pollution on human metabolomics. One untargeted metabolomics study analyzed 280 known metabolites in a subset of the TwinsUK cohort (Menni et al. 2015). The authors found was positively associated with lung function and inversely associated with air pollution exposure. α-Tocopherol is a biologically active form of vitamin E that plays an important role in antioxidation (Zingg et al. 2015). Consistently, another untargeted metabolomics study on serum and saliva samples from 54 healthy college students found associations of leukotriene and vitamin E metabolism pathways with air pollution exposures (Liang et al. 2018). In the present study, we found antioxidant reaction-related metabolites (e.g., isoleucylglycine on the glycine metabolism pathway, leucylglutamate on the glutamate metabolism pathway) also changed significantly across the three time points when air pollution changed drastically.
We found that lipid metabolism may be an important mechanistic pathway in air pollution exposure, which is consistent with other metabolomics studies. In one untargeted study, inoleate metabolism pathway was found as a mediator underlying the association of air pollution with asthma and cardio-cerebrovascular diseases (Jeong et al. 2018). In a targeted metabolomics analysis within the Cooperative Health Research in the Augsburg Region (KORA) cohort, two phospholipids that incorporated choline (i.e., lysophosphatidylcholines and phosphatidylcholines) were significantly associated with and ozone exposures (Ward-Caviness et al. 2016). In our study, we also found one similar phospholipid (i.e., 2-palmitoylglycerophosphocholine) decreased () during and then increased () after the Olympics. In a targeted metabolomics study within the CATHGEN cohort, short-term exposures to ambient and ozone were positively associated with LCFA (i.e., C16:1) (Breitner et al. 2016). Again, we found palmitoleate, which is also an LCFA with 16 carbons (C16:1n7), and 70.6% of other metabolites on the LCFA pathway significantly associated with air pollution level changes.
Our study has some strengths. First, the panel design took advantage of the air quality controls during the 2008 Olympics and thus mimicked a “natural experiment” manipulating air pollution levels. Although we cannot infer causal relations between air pollution and the metabolomic signatures, the metabolomic changes coincided with dramatic changes in air pollution. In addition, by evaluating repeat samples from the same individuals, we were able to minimize the influence of differences in individual characteristics such as genetic predisposition. We also included sex, age, BMI, diet, and mode of transportation as potential confounders in analyses. Second, we employed a graphic partition network analyses to identify modules of metabolites that were associated with air pollution level changes. This network analysis not only increased the power of our data analysis but also allowed us to identify classes in which metabolites were collectively associated with air pollution levels.
Our study also has certain limitations. First, although we had a total of 78 serum samples in this analysis, our sample size was relatively small. Therefore, many individual metabolites (Table 1) that were associated with air pollution level changes were not statistically significant after correcting for multiple comparisons. However, through network analyses, most of those identified metabolites were confirmed in the module analysis (Table 2) with significant changes across the three time points after correcting for multiple comparisons. False-positive results are common in -omics studies. However, given the fact that metabolites are systemically interrelated, traditional correction methods (e.g., Benjamin-Hochberg method) might be overly conservative for exploring novel pathways (Tzoulaki et al. 2014), and the issue needs to be further addressed statistically to balance multiple comparisons and the systemic complexity of metabolomics per se. The small sample size limited our ability to conduct sex-specific module analysis, although our data suggested a potential effect modification by sex. Future studies with sufficient sample size will be needed to examine the association of air pollution with metabolome by sex. Second, we also acknowledge that a potential bias may result from the limited annotation based on the extent of prior knowledge available. At present, there is a large portion of metabolites with unknown identities that may be functionally or mechanistically important. Third, we lack personal air pollution exposure assessments. Although overall air pollution changes were drastic across the three periods, participants may have had different individual exposures. In addition, the time intervals between each of the three time points were relatively short for both air pollution changes and metabolomic changes. Thus, the results may not be applicable to long-term exposure to air pollution or long-term changes in metabolomics. Given that our study was conducted in heavily air polluted areas, the findings may not be generalizable to populations in areas with low air pollution levels. Fourth, the metabolomic changes across the three time points may be explained by other factors that were not controlled in the analyses, such as changes in the epigenome, transcriptome, and other lifestyle factors.
Conclusions
Overall, our study identified two major metabolic signatures that showed significant changes before, during, and after the Beijing Olympics—when air pollution level changed drastically—including changes in lipid and peptide super classes and in polyunsaturated fatty acids, glycine-dipeptide, taurine, and xanthine.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health/National Institute of Environmental Health Sciences grants awarded to L. M. (grants R01ES018846 and R21ES026429) and National Cancer Institute grant awarded to H. Y. (grant P30CA016056). We acknowledge the technical support on the metabolomics assay from Metabolon, Inc.
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP3705).
These authors contributed equally to this work.
The authors declare they have no actual or potential competing financial conflicts.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57(1):289–300, 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- Bidgood MJ, Jamal OS, Cunningham AM, Brooks PM, Scott KF. 2000. Type IIA secretory phospholipase A2 up-regulates cyclooxygenase-2 and amplifies cytokine-mediated prostaglandin production in human rheumatoid synoviocytes. J Immunol 165(5):2790–2797, PMID: 10946311, 10.4049/jimmunol.165.5.2790. [DOI] [PubMed] [Google Scholar]
- Breitner S, Schneider A, Devlin RB, Ward-Caviness CK, Diaz-Sanchez D, Neas LM, et al. . 2016. Associations among plasma metabolite levels and short-term exposure to PM2.5 and ozone in a cardiac catheterization cohort. Environ Int 97:76–84, PMID: 27792908, 10.1016/j.envint.2016.10.012. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA III, Brook JR, Bhatnagar A, Diez-Roux AV, et al. . 2010. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121(21):2331–2378, PMID: 20458016, 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Brower JB, Doyle-Eisele M, Moeller B, Stirdivant S, McDonald JD, Campen MJ. 2016. Metabolomic changes in murine serum following inhalation exposure to gasoline and diesel engine emissions. Inhal Toxicol 28(5):241–250, PMID: 27017952, 10.3109/08958378.2016.1155003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunekreef B, Holgate ST. 2002. Air pollution and health. Lancet 360(9341):1233–1242, PMID: 12401268, 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- Brüske I, Hampel R, Baumgärtner Z, Rückerl R, Greven S, Koenig W, et al. . 2011. Ambient air pollution and lipoprotein-associated phospholipase A2 in survivors of myocardial infarction. Environ Health Perspect 119(7):921–926, PMID: 21356620, 10.1289/ehp.1002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudière J, Ferrari-Iliou R. 1999. Intracellular antioxidants: from chemical to biochemical mechanisms. Food Chem Toxicol 37(9–10):949–962, PMID: 10541450, 10.1016/S0278-6915(99)00090-3. [DOI] [PubMed] [Google Scholar]
- Clauset A, Newman ME, Moore C. 2004. Finding community structure in very large networks. Phys Rev E Stat Nonlin Soft Matter Phys 70(6 Pt 2):066111, PMID: 15697438, 10.1103/PhysRevE.70.066111. [DOI] [PubMed] [Google Scholar]
- Cruickshank-Quinn CI, Mahaffey S, Justice MJ, Hughes G, Armstrong M, Bowler RP, et al. . 2014. Transient and persistent metabolomic changes in plasma following chronic cigarette smoke exposure in a mouse model. PLoS One 9(7):e101855, PMID: 25007263, 10.1371/journal.pone.0101855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csardi G, Nepusz T. 2006. The igraph software package for complex network research. InterJournal, Complex Syst 1695:1–9.http://igraph.org. [Google Scholar]
- Dabney A, Storey JD, Warnes GR. 2010. qvalue: Q-value estimation for false discovery rate control. R package version 1.
- Dianat M, Radmanesh E, Badavi M, Mard SA, Goudarzi G. 2016. Disturbance effects of PM10 on iNOS and eNOS mRNA expression levels and antioxidant activity induced by ischemia–reperfusion injury in isolated rat heart: protective role of vanillic acid. Environ Sci Pollut Res Int 23(6):5154–5165, 10.1007/s11356-015-5759-x. [DOI] [PubMed] [Google Scholar]
- Farhat Z, Browne RW, Bonner MR, Tian L, Deng F, Swanson M, et al. . 2018. How do glutathione antioxidant enzymes and total antioxidant status respond to air pollution exposure? Environ Int 112:287–293, PMID: 29324239, 10.1016/j.envint.2017.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. 2005. Uric acid and oxidative stress. Curr Pharm Des 11(32):4145–4151, PMID: 16375736, 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- Hennig B, Ettinger AS, Jandacek RJ, Koo S, McClain C, Seifried H, et al. . 2007. Using nutrition for intervention and prevention against environmental chemical toxicity and associated diseases. Environ Health Perspect 115(4):493–495, PMID: 17450213, 10.1289/ehp.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandes MS, Troncone LR. 2009. Glycine as a neurotransmitter in the forebrain: a short review. J Neural Transm (Vienna) 116(12):1551–1560, PMID: 19826900, 10.1007/s00702-009-0326-6. [DOI] [PubMed] [Google Scholar]
- Herrmann W, Schorr H, Obeid R, Makowski J, Fowler B, Kuhlmann MK. 2005. Disturbed homocysteine and methionine cycle intermediates S-adenosylhomocysteine and S-adenosylmethionine are related to degree of renal insufficiency in type 2 diabetes. Clin Chem 51(5):891–897, PMID: 15774574, 10.1373/clinchem.2004.044453. [DOI] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA. 2009. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37(1):1–13, PMID: 19033363, 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable RJ. 1992. Physiological actions of taurine. Physiol Rev 72(1):101–163, PMID: 1731369, 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- Ikei KN, Yeung J, Apopa PL, Ceja J, Vesci J, Holman TR, et al. . 2012. Investigations of human platelet-type 12-lipoxygenase: role of lipoxygenase products in platelet activation. J Lipid Res 53(12):2546–2559, PMID: 22984144, 10.1194/jlr.M026385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JG, Smith LH. 1968. Biochemistry and physiology of taurine and taurine derivatives. Physiol Rev 48(2):424–511, PMID: 4297098, 10.1152/physrev.1968.48.2.424. [DOI] [PubMed] [Google Scholar]
- Jeong A, Fiorito G, Keski-Rahkonen P, Imboden M, Kiss A, Robinot N, et al. . 2018. Perturbation of metabolic pathways mediates the association of air pollutants with asthma and cardiovascular diseases. Environ Int 119:334–345, PMID: 29990954, 10.1016/j.envint.2018.06.025. [DOI] [PubMed] [Google Scholar]
- Joad JP, McDonald RJ, Giri SN, Bric JM. 1994. Ozone effects on mechanics and arachidonic acid metabolite concentrations in isolated rat lungs. Environ Res 66(2):186–197, PMID: 8055840, 10.1006/enrs.1994.1054. [DOI] [PubMed] [Google Scholar]
- Katajamaa M, Oresic M. 2005. Processing methods for differential analysis of LC/MS profile data. BMC Bioinformatics 6:179, PMID: 16026613, 10.1186/1471-2105-6-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzli N, Jerrett M, Mack WJ, Beckerman B, LaBree L, Gilliland F, et al. . 2005. Ambient air pollution and atherosclerosis in Los Angeles. Environ Health Perspect 113(2):201–206, PMID: 15687058, 10.1289/ehp.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton KA, Brown MV, Alexander D, Li Z, Wulff JE, Lawson R, et al. . 2014. Plasma metabolomic biomarker panel to distinguish patients with amyotrophic lateral sclerosis from disease mimics. Amyotroph Lateral Scler Frontotemporal Degener 15(5–6):362–370, PMID: 24984169, 10.3109/21678421.2014.908311. [DOI] [PubMed] [Google Scholar]
- Li R, Navab K, Hough G, Daher N, Zhang M, Mittelstein D, et al. . 2015. Effect of exposure to atmospheric ultrafine particles on production of free fatty acids and lipid metabolites in the mouse small intestine. Environ Health Perspect 123(1):34–41, PMID: 25170928, 10.1289/ehp.1307036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Moutinho JL, Golan R, Yu T, Ladva CN, Niedzwiecki M, et al. . 2018. Use of high-resolution metabolomics for the identification of metabolic signals associated with traffic-related air pollution. Environ Int 120:145–154, PMID: 30092452, 10.1016/j.envint.2018.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodovici M, Bigagli E. 2011. Oxidative stress and air pollution exposure. J Toxicol 2011:487074, PMID: 21860622, 10.1155/2011/487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulucci G, Daniel B, Cohen O, Avrahami Y, Sasson S. 2016. Hormetic and regulatory effects of lipid peroxidation mediators in pancreatic beta cells. Mol Aspects Med 49:49–77, PMID: 27012748, 10.1016/j.mam.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Menni C, Metrustry SJ, Mohney RP, Beevers S, Barratt B, Spector TD, et al. . 2015. Circulating levels of antioxidant vitamins correlate with better lung function and reduced exposure to ambient pollution. Am J Respir Crit Care Med 191(10):1203–1207, PMID: 25978575, 10.1164/rccm.201411-2059LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, Ghio AJ, Karoly ED, Bell LN, Snow SJ, Madden MC, et al. . 2016. Ozone exposure increases circulating stress hormones and lipid metabolites in humans. Am J Respir Crit Care Med 193(12):1382–1391, PMID: 26745856, 10.1164/rccm.201508-1599OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, Karoly ED, Jones JC, Ward WO, Vallanat BD, Andrews DL, et al. . 2015. Inhaled ozone (O3)-induces changes in serum metabolomic and liver transcriptomic profiles in rats. Toxicol Appl Pharmacol 286(2):65–79, PMID: 25838073, 10.1016/j.taap.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu L, Deng F, Tian L, Li Y, Swanson M, Ying J, et al. . 2014. Peak expiratory flow, breath rate and blood pressure in adults with changes in particulate matter air pollution during the Beijing Olympics: a panel study. Environ Res 133:4–11, PMID: 24906062, 10.1016/j.envres.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S. 2015. Role of taurine in the pathogenesis of obesity. Mol Nutr Food Res 59(7):1353–1363, PMID: 25787113, 10.1002/mnfr.201500067. [DOI] [PubMed] [Google Scholar]
- Ohta D, Kanaya S, Suzuki H. 2010. Application of Fourier-transform ion cyclotron resonance mass spectrometry to metabolic profiling and metabolite identification. Curr Opin Biotechnol 21(1):35–44, PMID: 20171870, 10.1016/j.copbio.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Péter S, Holguin F, Wood LG, Clougherty JE, Raederstorff D, Antal M, et al. . 2015. Nutritional solutions to reduce risks of negative health impacts of air pollution. Nutrients 7(12):10398–10416, PMID: 26690474, 10.3390/nu7125539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner U, Bowne J. 2009. What is metabolomics all about? Biotechniques 46(5):363–365, PMID: 19480633, 10.2144/000113133. [DOI] [PubMed] [Google Scholar]
- Sevanian A, Kim E. 1985. Phospholiphase A2 dependent release of fatty acids from peroxidized membranes. J Free Radic Biol Med 1(4):263–271, PMID: 3836246, 10.1016/0748-5514(85)90130-8. [DOI] [PubMed] [Google Scholar]
- Storey JD. 2002. A direct approach to false discovery rates. J R Stat Soc Series B Stat Methodol 64(3):479–498, 10.1111/1467-9868.00346. [DOI] [Google Scholar]
- Storey JD. 2003. The positive false discovery rate: a Bayesian interpretation and the q-value. Ann Stat 31(6):2013–2035, 10.1214/aos/1074290335. [DOI] [Google Scholar]
- Storey JD, Tibshirani R. 2003. Statistical significance for genomewide studies. Proc Natl Acad Sci USA 100(16):9440–9445, PMID: 12883005, 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surowiec I, Karimpour M, Gouveia-Figueira S, Wu J, Unosson J, Bosson JA, et al. . 2016. Multi-platform metabolomics assays for human lung lavage fluids in an air pollution exposure study. Anal Bioanal Chem 408(17):4751–4764, PMID: 27113461, 10.1007/s00216-016-9566-0. [DOI] [PubMed] [Google Scholar]
- Tithof PK, Elgayyar M, Cho Y, Guan W, Fisher AB, Peters-Golden M. 2002. Polycyclic aromatic hydrocarbons present in cigarette smoke cause endothelial cell apoptosis by a phospholipase A2-dependent mechanism. FASEB J 16(11):1463–1464, PMID: 12205049, 10.1096/fj.02-0092fje. [DOI] [PubMed] [Google Scholar]
- Tong H, Rappold AG, Diaz-Sanchez D, Steck SE, Berntsen J, Cascio WE, et al. . 2012. Omega-3 fatty acid supplementation appears to attenuate particulate air pollution–induced cardiac effects and lipid changes in healthy middle-aged adults. Environ Health Perspect 120(7):952–957, PMID: 22514211, 10.1289/ehp.1104472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzoulaki I, Ebbels TM, Valdes A, Elliott P, Ioannidis JP. 2014. Design and analysis of metabolomics studies in epidemiologic research: a primer on -omic technologies. Am J Epidemiol 180(2):129–139, PMID: 24966222, 10.1093/aje/kwu143. [DOI] [PubMed] [Google Scholar]
- Valko M, Jomova K, Rhodes CJ, Kuča K, Musílek K. 2016. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol 90(1):1–37, PMID: 26343967, 10.1007/s00204-015-1579-5. [DOI] [PubMed] [Google Scholar]
- van Kuijk FJGM, Sevanian A, Handelman GJ, Dratz EA. 1987. A new role for phospholipase-A2: protection of membranes from lipid peroxidation damage. Trends Biochem Sci 12:31–34, 10.1016/0968-0004(87)90014-4. [DOI] [Google Scholar]
- Vlaanderen JJ, Janssen NA, Hoek G, Keski-Rahkonen P, Barupal DK, Cassee FR, et al. . 2017. The impact of ambient air pollution on the human blood metabolome. Environ Res 156:341–348, PMID: 28391173, 10.1016/j.envres.2017.03.042. [DOI] [PubMed] [Google Scholar]
- Ward-Caviness CK, Breitner S, Wolf K, Cyrys J, Kastenmüller G, Wang-Sattler R, et al. . 2016. Short-term NO2 exposure is associated with long-chain fatty acids in prospective cohorts from Augsburg, Germany: results from an analysis of 138 metabolites and three exposures. Int J Epidemiol 45(5):1528–1538, PMID: 27892410, 10.1093/ije/dyw247. [DOI] [PubMed] [Google Scholar]
- Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. 2004. Glutathione metabolism and its implications for health. J Nutr 134(3):489–492, PMID: 14988435, 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Su X, Huang W, Zhang J, Peng C, Huang H, et al. . 2015. Role of S-adenosylhomocysteine in cardiovascular disease and its potential epigenetic mechanism. Int J Biochem Cell Biol 67:158–166, PMID: 26117455, 10.1016/j.biocel.2015.06.015. [DOI] [PubMed] [Google Scholar]
- Yeung J, Holinstat M. 2011. 12-Lipoxygenase: a potential target for novel anti-platelet therapeutics. Cardiovasc Hematol Agents Med Chem 9(3):154–164, PMID: 21838667, 10.2174/187152511797037619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hu H, Shi Y, Yang X, Cao L, Wu J, et al. . 2017. 1H NMR-based metabolomics study on repeat dose toxicity of fine particulate matter in rats after intratracheal instillation. Sci Total Environ 589:212–221, PMID: 28262365, 10.1016/j.scitotenv.2017.02.149. [DOI] [PubMed] [Google Scholar]
- Zingg JM, Azzi A, Meydani M. 2015. Induction of VEGF expression by alpha-tocopherol and alpha-tocopheryl phosphate via PI3Kγ/PKB and hTAP1/SEC14L2-mediated lipid exchange. J Cell Biochem 116(3):398–407, PMID: 25290554, 10.1002/jcb.24988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.