Right after giving birth to her daughter in 1962, Marcy Hartman—a newspaper reporter at the time—watched a nurse remove a syringe full of blood from the placenta. “It was a small contribution I made to the future,” remembers Hartman, now 75.
Between 1959 and 1967, Hartman and more than 15,000 other pregnant women in the San Francisco Bay Area enrolled in a long-term cohort called the Child Health and Development Studies (CHDS).1 The cohort was intended to help scientists better understand what makes pregnancies, and children, healthy. Researchers are now using the biological specimens and other data provided decades ago by Hartman and the other cohort members to tackle one of the most vexing scientific questions about the role of the environment in health—whether an individual’s exposures can impact the health of his or her descendants.
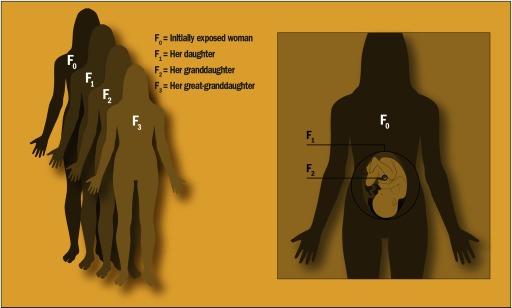
Unlike males, who make sperm throughout life, females are thought to be born with all the eggs they will ever produce. That means that each prenatal exposure has the potential to directly impact three generations, says epidemiologist Barbara Cohn, principal investigator of the CHDS. They include the mother (known as the generation), the fetus ( generation), and, if the fetus is a girl, all her immature egg cells—any of which may one day become the generation.
When a pregnant woman is exposed to an environmental agent, the exposure can affect not only herself () and her unborn child (), but also the germ cells developing within the fetus, if that fetus is a girl (). The woman’s great-grandchild () is the first generation not directly affected by the original exposure. Image: EHP.
In the past decade, studies in laboratory animals have pointed to the possibility that exposures to potentially toxic environmental chemicals and other stressors in utero may affect normal development of not just the growing fetus, but multiple generations to come.2 In one mouse study, for instance, the descendants of mice treated during pregnancy with di-(2-ethylhexyl) phthalate had lower baseline and stress-induced plasma levels of the stress hormone corticosterone than untreated mice.3 Another study showed differences in the social interactions of mice of the and generations of dams fed bisphenol A compared with those of unexposed dams.4
Unsurprisingly, evidence from human studies has proved elusive.5 People, after all, reproduce far more slowly than mice, and keeping tabs on thousands of humans for decades is no small feat. There are very few cohorts in the world that are able to generate the kind of long-term, multigenerational data that would be needed to address questions about the potential cross-generational effects of human exposures. Yet the few that do exist are beginning to yield clues about how a person’s exposures and experiences could influence the neurodevelopment and behavior of future generations.6,7
Findings in Humans Emerge
In 2017, researchers studying a large group of parents and children in the United Kingdom reported associations between smoking by grandmothers during pregnancy and autism symptoms and diagnoses in grandchildren.6 Study leader Jean Golding, an epidemiologist at the University of Bristol, had founded the Avon Longitudinal Study of Parents and Children (ALSPAC) in 1991 to answer questions about child development.8
Golding did not design the 14,000-family cohort around autism risk, specifically. “At the time, the incidence of autism was thought to be lower than 1 in 1,000 people,” remembers Golding. She did not think that 14,000 children would be a large enough number to address questions about such a rare disorder. In fact, she expected fewer than 10 cases of autism to be counted among the cohort children.
But Golding became interested in risk factors for autism after studies documented a fivefold increase over previous years in the incidence of autism in kids born in the United Kingdom between 1988 and 1995.9 Over the years, the ALSPAC researchers identified more than 200 study children with possible or diagnosed autism.
Golding and colleagues had reason to think there could be a link between children’s neurodevelopment and their grandparents’ smoking habits. Previously they had found preliminary evidence suggesting that among boys whose mothers smoked during pregnancy, average head circumference was smaller for boys whose fathers, too, were exposed prenatally, compared with boys whose fathers were not exposed prenatally. (No such evidence was found for girls, however, and average head circumferences of boys or girls exposed to maternal smoking in utero did not differ depending on whether their mothers were also exposed prenatally.)10
Grandparents were not included in the ALSPAC cohort, so researchers asked the study parents whether their own mothers had smoked during pregnancy. Doctors, for the most part, did not discourage smoking during pregnancy in the 1950s and 1960s, when many of the parents were born.
The researchers found that after adjusting for known autism risk factors, including mother’s education and socioeconomic status, children whose maternal grandmothers smoked were more likely to be diagnosed with autism than those whose maternal grandmothers did not smoke.6 However, this association was only apparent if the child was also exposed to maternal smoking in utero.
Then in 2018, an analysis of data from the Nurses’ Health Study II (NHS-II), which had followed a large group of U.S. women since the late 1980s, also found evidence of a multigenerational exposure–outcome association. Researchers reported that children whose grandmothers used diethylstilbestrol (DES) during pregnancy were more likely to be diagnosed with attention-deficit/hyperactivity disorder (ADHD).7 Specifically, 7.7% of mothers who reported being exposed to DES in utero also reported ADHD in their children versus 5.2% of mothers who did not report in utero DES exposure.
DES was prescribed from 1938 to 197119 to prevent miscarriage and other pregnancy complications. Doctors stopped prescribing it to pregnant women after it was shown to increase cancer risk in the daughters exposed prenatally.20 Use of DES during pregnancy has also been associated with an increase in hypospadias (a penile birth defect) in grandsons11 and higher odds of irregular menstrual periods in granddaughters.12 Image: CC BY-NC-SA 2.0 via https://www.flickr.com/photos/diethylstilbestrol/24559986905.
A few previous human studies had suggested that multigenerational health effects could be possible with endocrine-disrupting chemicals such as DES.11,12 The study of the NHS-II cohort, however, is the first to report an association between a known endocrine disrupter and potential effects on neurodevelopment, specifically, across three generations, according to lead study author Marianthi-Anna Kioumourtzoglou, an epidemiologist at Columbia University.
The research is intriguing, because if replicated, such findings could lead to a paradigm shift in how we think about the etiology of mental disorders,5 says Joel Nigg, a neuropsychiatrist at Oregon Health & Science University. Such findings also could help to explain why some complex disorders, such as ADHD, run in families to what Nigg says is a greater extent than can be currently explained by known gene effects alone.
Yet it is far from clear, at this point, how such multigenerational changes might actually occur. How is it possible for people’s environmental exposures to trigger changes in the brains and bodies of their grandchildren?
Some researchers suspect that epigenetic inheritance may play a role. Others raise the possibility of a direct hit to either the female or male germ line.2 All agree there are a lot of puzzle pieces still missing.
Elucidating the Role of the Epigenome
The concept that an organism’s genetic sequence determines its biology—that genes are the primary regulator for processes like disease and even evolution—has been dominant in science and medicine since the early 1900s. Now it is becoming increasingly clear that many diseases do not have a single genetic cause, but are instead due to a combination of genetic factors modulated by interactions with the organism’s environment.
But how one’s environment influences behaviors and neurodevelopment remains largely a mystery.5 The new science of environmental epigenetics offers some ideas. “What we are now seeing is that epigenetics is probably equally important [to genetics] in every biological system,” says Michael Skinner, an epigeneticist at Washington State University.
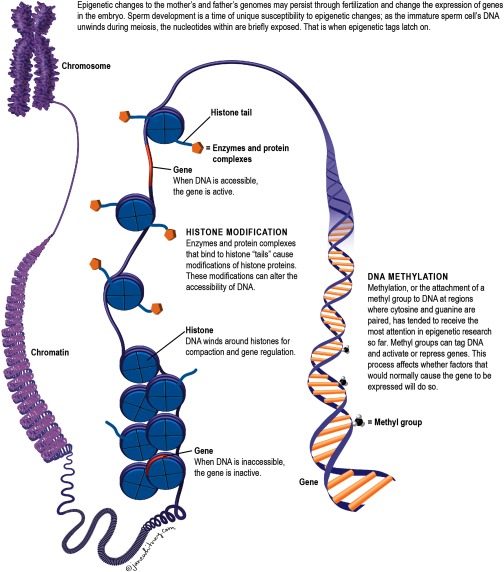
The epigenome contains all the chemical modifications that turn certain genes “on” in some parts of the body during specific periods of development and “off” in other parts during other periods. Every cell in the body has the same genetic blueprint, but not every cell in the body performs the same function. Chemical modifications to DNA determine how different cells read the same blueprint. It is why brain cells perform different functions than skin cells, even though they have the same DNA.
Image: EHP.
For many years, scientists thought that all these modifications, or epigenetic “marks,” were reset with each subsequent generation.2 Early on in the formation of a new embryo, the epigenetic marks from the parent are erased. This normal biological process ensures the pluripotency of embryonic stem cells—their unique potential to grow into any cell in the body.
But sometimes the process does not work that way. Scientists now know that some of these epigenetic marks are not erased or reset. Instead, they get imprinted on the genome and passed on. It is not yet clear how and why this happens normally.2 However, Skinner and colleagues have shown in animal models that exposures to environmental toxicants may lead to new regions of the genome where epigenetic marks are not erased.13
“Essentially, you are taking the normal sites and creating a shift,” says Skinner. If these shifts occur at just the right time during development, they may become permanently programmed and passed on, he explains.
That’s the idea behind epigenetic inheritance. Animal studies suggest that environmental exposures can cause changes in an organism’s epigenome, and these changes can be transmitted from one generation to the next long after the initial exposure.14
Skinner and colleagues were among the first to demonstrate this phenomenon. In 2005, they reported that reproductive abnormalities, such as decreased sperm motility, carried out to the fourth generation after dosing pregnant mice with the fungicide vinclozolin.15 Skinner hypothesized that these fourth-generation offspring had inherited the molecular effects of a previous generation’s exposure without ever being exposed to the fungicide themselves. With subsequent animal studies, he reported evidence that several other environmental toxicants may increase the susceptibility of future generations to reproductive and kidney problems, obesity, and cancer.16,17
“Toxicological studies—rodent studies—are extremely helpful, but I think to have more confidence we need both toxicological and human studies,” says Columbia’s Kioumourtzoglou. “At the end of the day, rodents are not humans.” Yet epidemiological studies on their own are not ideal, because they are limited in what they can prove or show. “If we have corroboration from both, the evidence becomes much more powerful,” says Kioumourtzoglou.
It is impossible to prove whether epigenetic inheritance is responsible for the associations of tobacco smoking and DES exposures with autism and ADHD symptoms in grandchildren observed in the ALSPAC and NHS-II cohorts. Despite the tantalizing clues they offer, the two studies have a lot of limitations. Both, for instance, relied on self-reports from one generation’s recall of a previous generation’s exposures and health habits.6,7
The researchers cannot rule out the possibility that the associations they saw were due to other exposures, information errors, or chance. These studies also cannot account for the influence of other environmental factors that may have been shared across generations. And it is difficult—if not impossible—to directly assess epigenetic status in the living human brain. Epigenetic markings in DNA are tissue specific, and there is no good way, at present, to obtain and analyze brain tissue from healthy people.5
Skinner points out another potential caveat if umbilical cord blood samples from past human cohorts were to be used in epigenetic studies of human generations. Studies with cord blood or blood require purification of cell types for optimal epigenetic analysis, he says. But it can be difficult to purify frozen cell populations.
Multigenerational studies are challenging because investigators cannot rule out the possibility that the associations they observe are due to other exposures in the initial generation, exposures shared across generations, or chance. Image: © iStockphoto/kali9.
That said, from a public health perspective, epigenetics offers the promise not only for a better understanding of how the environment contributes to disease risk, but potentially an inkling of how to intervene, according to Brandon Pearson, a neuroscientist at Columbia University who studies epigenetics and environmental health. Currently, however, the idea of epigenetic interventions for complex disorders remains a stretch. “We do not yet understand what makes the epigenetic risk for something like ADHD,” says Pearson. “There’s unlikely any single epigenetic mark or profiles in genetic locations that could lead to ADHD or other neuropsychiatric disorder.”
A New Research Effort
It is impossible to say how many of the chemicals to which people are exposed today may have neurodevelopmental and endocrine effects. “You have a rolling set of environmental circumstances and new exposures created in every generation,” says Cohn. How these layers of exposures may compound or interact with each other—and with our genes—remains largely unknown. These associations are extremely difficult to quantify. “There are no human studies that are perfect,” says Cohn. She knows that hers will not be, either.
In a second-floor walkup perched over a French deli in the heart of Berkeley, California’s “Gourmet Ghetto,” Cohn and her colleagues at the CHDS are culling data from more than 20,000 births between the mid-1980s and 2013. These births represent a portion of the cohort’s third generation—the grandchildren of the expectant mothers and fathers, including Marcy Hartman and her husband, who volunteered for the study more than five decades ago.
Cohn and colleagues have linked the birth records of the study grandchildren to an existing database of autism cases maintained by the California Department of Health. The researchers are now working to identify and analyze any links between grandparents’ exposures and behaviors—things like smoking, alcohol, and medication use—and autism diagnoses in the third generation.18
Whereas the ALSPAC and NHS-II studies relied on the second generation to report their parents’ exposures, CHDS researchers now have direct evidence. They have data from medical records for pharmaceutical exposures such as DES. Both mothers and fathers provided blood and urine shortly before and after their children were born.
Those samples were prepared and have been stored in a biorepository for the last several decades. Now Cohn and colleagues can use the urine and blood to measure how much of certain chemicals the grandparent generation would have had in their bodies at the time. They can confirm, for instance, whether and how much a mother may have smoked during pregnancy by measuring urine levels of cotinine—a metabolite of nicotine. Having these measurements, according to Cohn, will help to increase confidence that any associations they find are real.
Another unique thing about the CHDS dataset is that the researchers have information on environmental exposures in both mothers and fathers of the first generation. Down the paternal line, three generations is far enough to rule out the possibility of direct exposure, notes Cohn.
Each generation has its own set of potential environmental circumstances and exposures. Some of these exposures remain relevant to the day-to-day lives of subsequent generations. But even for those that become obsolete, potential health effects may persist. Image: CC BY 2.0 via https://www.flickr.com/photos/simpleinsomnia/25128717624/.
And initial counts of the California autism database show just over 100 cohort children with autism. That’s a large enough number, say the researchers, to find major associations, but may not allow them to pick up on minor factors that would lead to weaker associations.
Each new study, though imperfect, adds a little bit more knowledge. New methods, such Mendelian randomization—a technique that may strengthen causal inferences from epidemiological data in some contexts—are providing researchers with more powerful tools to assess potential effects of environmental pollutants. Nigg and colleagues recently used this approach to further support evidence that lead exposure may contribute to ADHD.5
As the scientific landscape expands and researchers are able to address increasingly complex questions about interactions between health and environment, the small contributions made years ago by Hartman and other members of prospective longitudinal cohorts offer a wealth of data that will keep on giving for years to come.
Biography
Lindsey Konkel is a New Jersey–based journalist who reports on science, health, and the environment. In 2018, she traveled to the San Francisco Bay Area and interviewed CHDS cohort members and researchers with support from a Lizzie Grossman Freelance Grant for Environmental Health Reporting awarded through the Society for Environmental Journalism’s Fund for Environmental Journalism.
References
- 1.Child Health and Development Studies. 2019. About Us http://www.chdstudies.org/about_us/index.php [accessed 25 June 2019].
- 2.Nilsson EE, Sadler-Riggleman I, Skinner MK. 2018. Environmentally induced epigenetic transgenerational inheritance of disease. Environ Epigenet 4(2):dvy016, PMID: 30038800, 10.1093/eep/dvy016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quinnies KM, Doyle TJ, Kim KH, Rissman EF. 2015. Transgenerational effects of di-(2-ethylhexyl) phthalate (DEHP) on stress hormones and behavior. Endocrinology 156(9):3077–3083, PMID: 26168342, 10.1210/EN.2015-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolstenholme JT, Edwards M, Shetty SRJ, Gatewood JD, Taylor JA, Rissman EF, et al. 2012. Gestational exposure to bisphenol a produces transgenerational changes in behaviors and gene expression. Endocrinology 153(8):3828–3838, PMID: 22707478, 10.1210/en.2012-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nigg JT. 2018. Toward an emerging paradigm for understanding attention-deficit/hyperactivity disorder and other neurodevelopmental, mental, and behavioral disorders: environmental risks and epigenetic associations. JAMA Pediatr 172(7):619–621, PMID: 29799950, 10.1001/jamapediatrics.2018.0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golding J, Ellis G, Gregory S, Birmingham K, Iles-Caven Y, Rai D, et al. 2017. Grand-maternal smoking in pregnancy and grandchild’s autism traits and diagnosed autism. Sci Rep 7:46179, PMID: 28448061, 10.1038/srep46179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kioumourtzoglou MA, Coull BA, O'Reilly ÉJ, Ascherio A, Weisskopf MG. 2018. Association of exposure to diethylstilbestrol during pregnancy with multigenerational neurodevelopmental deficits. JAMA Pediatr 172(7):670–677, PMID: 29799929, 10.1001/jamapediatrics.2018.0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, et al. 2013. Cohort profile: the ‘children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 42(1):111–127, PMID: 22507743, 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagberg KW, Jick H. 2010. Autism in the UK for birth cohorts 1988–2001. Epidemiology 21(3):426–427, PMID: 20386177, 10.1097/EDE.0b013e3181d61dd9. [DOI] [PubMed] [Google Scholar]
- 10.Pembrey M, Northstone K, Gregory S, Miller LL, Golding J. 2014. Is the growth of the child of a smoking mother influenced by the father’s prenatal exposure to tobacco? A hypothesis generating longitudinal study. BMJ Open 4(7):e005030, PMID: 25015471, 10.1136/bmjopen-2014-005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalfa N, Paris F, Soyer-Gobillard MO, Daures JP, Sultan C. 2011. Prevalence of hypospadias in grandsons of women exposed to diethylstilbestrol during pregnancy: a multigenerational national cohort study. Fertil Steril 95(8):2574–2577, PMID: 21458804, 10.1016/j.fertnstert.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 12.Titus-Ernstoff L, Troisi R, Hatch EE, Wise LA, Palmer J, Hyer M, et al. 2006. Menstrual and reproductive characteristics of women whose mothers were exposed in utero to diethylstilbestrol (DES). Int J Epidemiol 35(4):862–868, PMID: 16723367, 10.1093/ije/dyl106. [DOI] [PubMed] [Google Scholar]
- 13.Skinner MK, Guerrero-Bosagna C, Haque M, Nilsson E, Bhandari R, McCarrey JR. 2013. Environmentally induced transgenerational epigenetic reprogramming of the primordial germ cells and the subsequent germ line. PLoS One 8(7):e66318, PMID: 23869203, 10.1371/journal.pone.0066318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Otterdijk SD, Michels KB. 2016. Transgenerational epigenetic inheritance in mammals: how good is the evidence? FASEB J 30(7):2457–2465, PMID: 27037350, 10.1096/fj.201500083. [DOI] [PubMed] [Google Scholar]
- 15.Anway MD, Cupp AS, Uzumcu M, Skinner MK. 2005. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308(5727):1466–1469, PMID: 15933200, 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manikkam M, Guerrero-Bosagna C, Tracey R, Haque MM, Skinner MK. 2012. Transgenerational actions of environmental compounds on reproductive disease and identification of epigenetic biomarkers of ancestral exposures. PLoS One 7(2):e31901, PMID: 22389676, 10.1371/journal.pone.0031901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McBirney M, King SE, Pappalardo M, Houser E, Unkefer M, Nilsson E, et al. 2017. Atrazine induced epigenetic transgenerational inheritance of disease, lean phenotype and sperm epimutation pathology biomarkers. PLoS One 12(9):e0184306, PMID: 28931070, 10.1371/journal.pone.0184306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Defense Technical Information Center. 2018. Grandparental Exposures and Risk of Autism in the Third Generation. Fort Detrick, MD: Defense Technical Information Center. [Google Scholar]
- 19.Wingard DL, Cohn BA, Helmrich SP, Edelstein SL. 1996. DES awareness and exposure: the 1994 California Behavioral Risk Factor Survey. Am J Prev Med 12(5):437–441, PMID: 8909659, 10.1016/S0749-3797(18)30305-2. [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute. 2011. Diethylstilbestrol (DES) and Cancer. https://www.cancer.gov/about-cancer/causes-prevention/risk/hormones/des-fact-sheet [accessed 25 June 2019].