Background.
Ischemia-reperfusion injury (IRI) after lung transplantation triggers a cascade of inflammatory changes that can contribute to acute allograft injury. This influences both the short- and long-term survival of the lung allograft. Alpha-1 antitrypsin (AAT) is a protease inhibitor with known anti-inflammatory and immune-regulatory properties that mitigate tissue damage. This study explores the protective effects of AAT in the setting of IRI utilizing a rat lung transplant model.
Methods.
Orthotopic left single lung transplantation was performed from Lewis to Sprague-Dawley rats; recipients did not receive systemic immunosuppression. Before transplantation, the donor lungs were primed with either albumin (control) or AAT. Starting the day of transplantation, recipient rats also received either albumin (control) or AAT with subsequent doses administered over the next 7 days. On the eighth postoperative day, lung allografts were recovered and analyzed.
Results.
Degree of inflammatory infiltrate, as quantified by the allograft weight (g)/body weight (kg) ratio, was significantly reduced in the AAT-treated group compared with controls (3.5 vs 7.7, respectively, P < 0.05). Treatment with AAT also significantly decreased allograft necrosis in treated animals, as measured by a semiquantitative score that ranged from 0 to 4 (1.25 vs 4, P < 0.05). In addition, lymphocytes isolated from recipients treatment group showed significant proliferative inhibition via a mixed lymphocyte response assay in response to donor antigens.
Conclusions.
AAT attenuates acute allograft injury and necrosis in a rat model of lung transplantation, suggesting that AAT may play a role in reducing IRI-induced inflammation.
Lung transplantation is an important, and often the only, therapeutic option for patients with end-stage lung disease. Despite the optimization of surgical techniques, postoperative care, and advances in immunosuppression, long-term allograft outcomes have not significantly improved over the past 10 years.1 Ischemia-reperfusion injury (IRI), a process that occurs at the time of organ implantation, is marked by endothelial and epithelial injury and results in noncardiogenic pulmonary edema and development of a complex inflammatory milieu. Tissue injury triggers the expression of damage-associated molecular patterns that subsequently activate and recruit cells from both the innate and adaptive immune systems. These cells can further promote allograft injury.2 Ultimately, this inflammatory cascade contributes to the development of primary graft dysfunction, a major cause of early mortality after lung transplantation and known risk factor for the development of long-term chronic allograft lung dysfunction.2-5
Alpha-1 antitrypsin (AAT) is a 52-kDa glycoprotein that is primarily produced in the liver and is the most prevalent serine protease inhibitor present in human plasma. In addition to its predominant role in protease-antiprotease homeostasis, AAT is a potent anti-inflammatory, antiapoptotic, and immune-regulatory protein for both the innate and adaptive immune systems.6 Prior work has demonstrated the role of AAT in several disease models and suggested a potential role in several transplant models.5,7-10 Götzfried et al11 recently demonstrated in a preclinical model that perfusion and storage of lung allografts with a preservation solution containing AAT significantly reduced the inflammatory response after reperfusion. Gao et al12 used a rat pulmonary artery ischemia-reperfusion model to demonstrate that AAT infusion significantly improved lung oxygenation and mechanics with a corresponding reduction in pulmonary edema. A subsequent follow-up study by Iskender et al13 used a pig transplant model to show that pretransplantation infusion of AAT improved pulmonary compliance and reduced lung permeability during the 4-hour postoperative reperfusion period. AAT also reduced the number of inflammatory mediators detectable in plasma and inhibited neutrophil infiltration into the lung.12,13
Taken together, these studies demonstrate that preservation of donor lung allografts in AAT-containing solution, or treatment of the recipient with AAT infusion before lung transplantation, reduces the inflammation triggered by IRI. However, these observations are limited due to the short postoperative observation periods of the respective studies. Therefore, it is unclear if this effect is sustained. This is of particular importance because severe IRI, present at >48 hours after transplantation (rather than early posttransplant allograft changes), is associated with chronic lung allograft dysfunction and all-cause mortality.14-16 This study aimed to test the hypothesis that AAT treatment of both the donor lung and recipient protects the lung allograft from delayed IRI and attenuates allograft necrosis.
MATERIALS AND METHODS
Rats
Pathogen-free male Lewis and Sprague-Dawley (SD) rats were purchased from Charles River Laboratories (Boston, MA). All rats were maintained under pathogen-free conditions throughout the experiments and cared for according to methods approved by the American Association for the Accreditation of Laboratory Animal Care. All live animal experiments and procedures were performed with the approval of the Institutional Animal Care and Use Committee at the Malcom Randall VA Medical Center in Gainesville, FL.
Determination of Human AAT Kinetics
SD male rats were injected with a single intraperitoneal dose of human AAT (200 mg/kg) (Prolastin C, Grifols Therapeutic Inc., Research Triangle Park, NC). Blood (0.2 mL) was serially collected from each recipient at 1, 3, 6, 12, 24, 48, and 72 hours post-AAT injection. Samples were centrifuged (800g × 15 min) and the plasma stored at −80°C until analysis. AAT levels were determined using a house-made human AAT-specific enzyme-linked immunosorbent assay.17
Orthotopic Left Lung Transplant
Orthotopic left single lung transplant (LTx) was performed between Lewis (donor) and SD (recipient) rats, as previously described.18,19 Briefly, donor rats underwent surgical tracheostomy and were placed on mechanical ventilation (with a rate of 80 breaths/min, fraction of inspired oxygen 100%, and positive end-expiratory pressure 3 cm H2O). General anesthesia was maintained with inhaled isoflurane. The main pulmonary artery was isolated, and the lungs were flushed with 20 mL of cold (4°C) Perfadex (Vitrolife, Uppsala, Sweden) solution or cold Perfadex that contained human AAT (100 µM). After perfusion was complete, the lungs were inflated to peak vital capacity, and the heart-lungs were excised en bloc. Following excision, cuffs were attached to the pulmonary artery, pulmonary vein, and left mainstem bronchus. The lungs were subsequently stored for 4 hours at 4°C in either Perfadex solution or Perfadex that contained human AAT (100 µM). Following cold storage, the left lung was orthotopically transplanted into recipient rats. Recipients in the experimental group were injected intraperitoneal with 200 mg/kg of human AAT at 2 hours before transplantation. Subsequent doses were administered on days 2, 4, and 6 posttransplant (4 doses total). Recipients in the control group received injections of normal saline at the indicated time points. The rats were euthanized on day 8 posttransplantation, and the right native lung and left lung allograft were recovered at this time.
Assessment of Lung Allograft Injury and Necrosis
The left lung allograft was recovered from recipient animals on postoperative day 8. Upon recovery, the allograft was weighed to calculate the wet allograft weight (GW)/body weight (BW) ratio. Both the allograft and native lung were divided into 3 sections (upper, middle, and lower). The upper, middle, and lower sections of the allograft lung, as well as the middle sections of the native lung, were fixed in 10% formalin, embedded in paraffin, cut into 4-μm sections, and stained with hematoxylin and eosin. The hematoxylin and eosin–stained lung sections were examined independently by 2 pathologists who were blinded to the treatment groups. A semiquantitative scoring method was utilized to assess the degree of necrosis. This score uses a 5-point scale based on the percent necrosis present in each section (0 [0%], 1 [1%–25%], 2 [26%–50%], 3 [51%–75%], and 4 [76%–100%]), as previously described.12,20,21 In addition, the nonnecrotic areas of the lungs were assessed for acute cellular rejection per standardized international grading criteria.22
One-way Mixed Lymphocyte Reaction Assay
A one-way mixed lymphocyte reaction (MLR) was performed utilizing recipient T-cells obtained at the time of allograft recovery, as previously described.23 Briefly, donor (Lewis) spleen or lung cells were incubated with mitomycin C, washed, and used as stimulator cells. Splenocytes from the recipient were enriched for T-cells by nylon wool purification and used as responder cells. We cocultured 1 × 105 responder cells with 5 × 105 cells/well of stimulator cells for 5 days in a round-bottom 96-well plate in RPMI-1640 culture medium supplemented with 10% fetal calf serum, 100 U/mL penicillin, and 100 mg/mL streptomycin. 3H-thymidine was added for the final 16 hours (1 µCi/well). The cells were harvested onto fiberglass filters, and incorporated 3H-thymidine was measured using a scintillation counter.
Statistical Analysis
Experimental results are expressed as mean ± SEM. Statistical differences between groups were determined using an unpaired 2-tailed Student’s t-test. A P value of <0.05 was considered statistically significant.
RESULTS
Kinetics of Human AAT in Rats
To determine the time-dependent circulating levels of human AAT and baseline kinetics in the plasma obtained from rats, a single dose of 200 mg/kg was injected into nontransplanted SD rats, after which serial blood samples were obtained. AAT levels peaked at 206 ± 9 mg/dL at 6 hours postinjection. The half-life of human AAT in rat plasma was approximately 24 hours, and levels were lowest by 72 hours postinjection (Figure 1).
FIGURE 1.
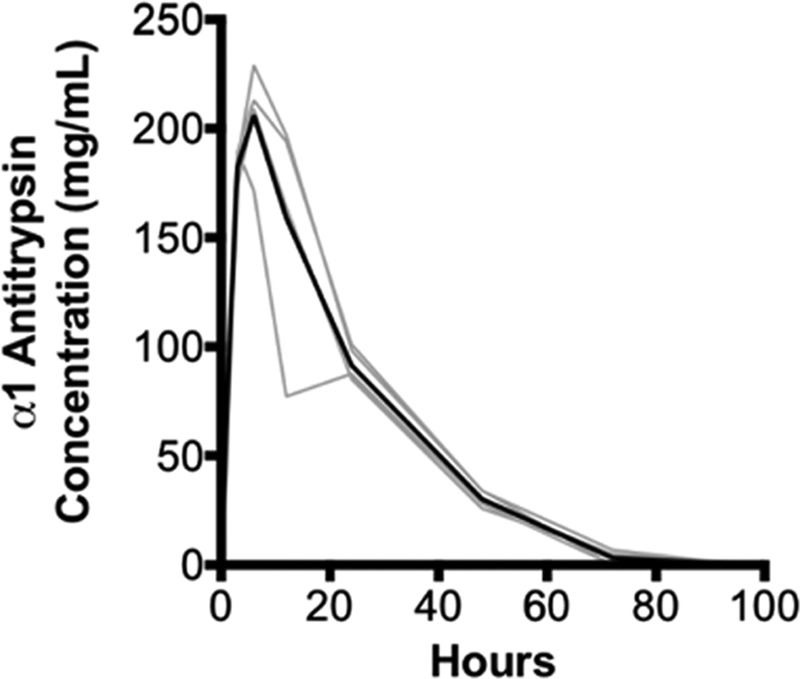
Kinetics of human AAT in rats. Nontransplanted Sprague-Dawley rats (n = 5) were injected with a single intraperitoneal dose of 200 mg/kg human AAT (Prolastin C). Blood samples were then serially collected at 1, 3, 6, 12, 24, 48, 72, and 96 h postinjection. Levels of AAT were determined using ELISA; data from individual rats are expressed in gray with the mean values delineated in black. AAT, alpha-1 antitrypsin; ELISA, enzyme-linked immunosorbent assay.
Effects of Treatment With AAT on the IRI After Transplantation
To investigate the potential therapeutic benefit of AAT on posttransplantation IRI, orthotopic left single LTxs were performed between Lewis (donor) and SD (recipient) rats. In the treatment group, both donor allografts and recipient animals were treated with AAT. Based on the results from the kinetic study performed above, recipient rats were injected 2 hours before transplantation and on days 2, 4, and 6 posttransplantation (4 total doses) (Figure 2). We previously demonstrated evidence of acute lung injury and necrosis continuing up through 5 days posttransplantation in this donor-recipient combination.18,19 However, to remove the confounding factor of postsurgical inflammation, as well as to more fully assess the effects of AAT given our limited sample size, the analysis was conducted on day 8 posttransplantation. In the control group, the left lung allograft was notably enlarged with evidence of hemorrhagic and consolidative changes on gross examination in comparison to the right native lung (Figure 3A, panel a). In contrast, the left lung allograft and right native lung appeared similar to each other in the AAT treatment group (Figure 3A, panel b). The GW-to-BW ratio was significantly lower in the AAT-treated allograft, compared with untreated allograft (3.5 vs 7.7, respectively, P < 0.05; Figure 3B).
FIGURE 2.
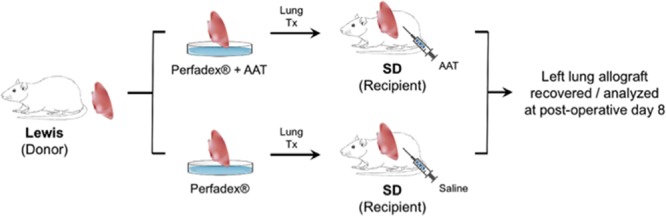
Orthotopic left lung transplant was performed using Lewis (donor) and Sprague-Dawley (SD) (recipient) rats. The donor lung was primed with Perfadex (±100 µM AAT) after procurement and preserved at 4°C for 4 h before transplantation. Recipient rats in the treatment group received one dose (200 mg/kg) human AAT 2 h before transplantation and on days 2, 4, and 6 posttransplant. Recipient rats in the control group received saline at these time points. All recipients were euthanized and lung allografts were recovered on day 8 posttransplantation. AAT, alpha-1 antitrypsin; Tx, transplant.
FIGURE 3.
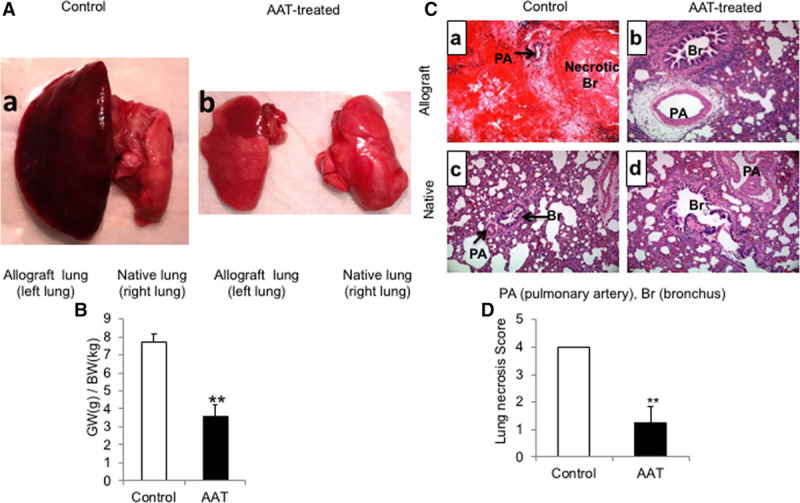
Treatment with AAT attenuated lung allograft injury and necrosis. To investigate the potential therapeutic benefit of AAT on posttransplantation IRI, the orthotopic left single lung transplant was performed between Lewis (donor) and SD (recipient) rats. A, Representative gross images of lungs in the control rats (panel a) and treatment group (panel b). B, Treatment with AAT significantly reduced the GW:BW ratio in the treatment group compared with the control group. Data represent the mean plus SEM; **P < 0.01, (n = 6 rats in control group and 5 rats in the AAT treatment group). C, Histologic examination (H&E stained, ×200 magnification) showed an extensive necrosis of lung allografts in the control group (panel a) in comparison to lung allografts in the treatment group (panel b) and native lungs (panels c and d) on day 8 posttransplantation. D, Semiquantitative lung necrosis scoring was performed using a 5-point scale according to the percent involvement of necrosis in each section. The mean percent necrosis score was significantly less in the AAT treatment group in comparison to the control group. Data represent the mean plus SEM; n = 6 in control group and n = 5 in the AAT-treated group. AAT, alpha-1 antitrypsin; GW/BW, allograft weight/body weight; H&E, hematoxylin and eosin; IRI, ischemia-reperfusion injury; SD, Sprague-Dawley; SEM, standard error of the mean.
Histologic examination of control allografts showed diffuse hemorrhagic necrosis involving 75%–90% of the lung allograft area (Figure 3C). A semiquantitative scoring method12,20,21 was used to assess the extent of posttransplantation IRI-induced necrosis. The mean percent necrosis score was significantly less in the AAT treatment group in comparison to the control group (1.25 vs 4, P < 0.05; Figure 3D). Due to the extensive necrosis in the lung allografts of the control group, grading for acute cellular rejection (based on established International Society for Heart and Lung Transplantation guidelines)22 was not possible. Nonetheless, diffuse interstitial and perivascular infiltrates were observed in areas of less severe necrosis that were suggestive of severe acute cellular rejection. It should be noted that the nonnecrotic lungs in the AAT treatment group also showed interstitial and perivascular lymphocytic infiltrates, consistent with moderate-to-severe acute cellular rejection (Figure 3C).
One-way MLR Experiment
AAT modulates the proliferation and function of T-cells by modifying monocyte-lymphocyte interaction24 and altering the cytokine milieu.25,26 To investigate the effects of AAT treatment on the ability of recipient lymphocytes to proliferate after exposure to donor antigen(s), a one-way MLR was performed23 using the donor (Lewis) rat spleen or lung cells as stimulator cells. Lymphocytes isolated from recipients (SD) in both the control and AAT-treated groups were used as responder cells (Figure 4). Results demonstrated that lymphocyte proliferation of cells from recipients treated with AAT was significantly inhibited in comparison to lymphocytes obtained from control animals. This level of proliferation was not significantly different from that observed with the use of responder lymphocytes from naïve (nontransplanted) SD rats. This result occurred irrespective of the use of either Lewis spleen (Figure 4A) or lung cells (Figure 4B) as stimulator cells. However, it should be noted that the use of Lewis spleen cells as the stimulator cell (vs Lewis lung cells) led to more proliferation, suggesting this cell type is more potent for inducing T cell responses. Overall, these results suggest that administration of AAT to presensitized recipients attenuates lymphocyte proliferation to a level comparable to that observed when no prior exposure to donor antigen has occurred.
FIGURE 4.
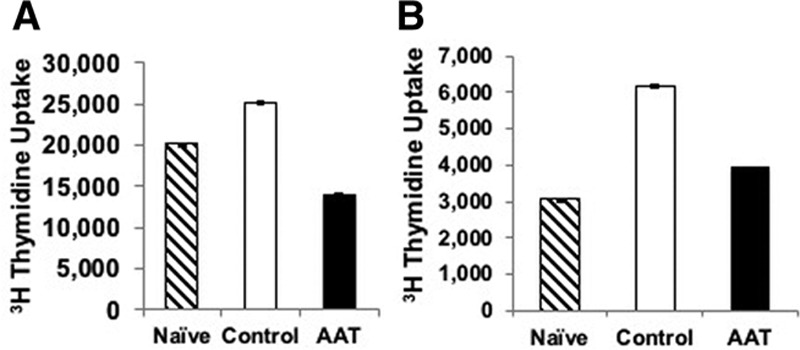
AAT treatment attenuates the recipients’ spleen T-cell proliferation in vitro. A one-way mixed lymphocyte reaction (MLR) was performed with the donor (Lewis) rat spleen (A) or lung (B) cells as stimulator cells. Lymphocytes isolated from recipients (SD) in both the control and AAT-treated groups were used as responder cells. Lymphocyte proliferation of cells from recipients treated with AAT was significantly inhibited in comparison to lymphocytes obtained from control animals. This level of proliferation was not significantly different from that observed with the use of responder lymphocytes from naïve (nontransplanted) SD rats. This result was irrespective of the use of either Lewis spleen (A) or the lung (B) as the stimulator cell. Data represent mean + SEM, n = 3 for each group; **P < 0.01 vs control. AAT, alpha-1 antitrypsin; SD, Sprague-Dawley; SEM, standard error of the mean.
DISCUSSION
AAT, a serine protease inhibitor, plays a major role in protease-antiprotease homeostasis by protecting the lung from damage that can occur due to unopposed activation of neutrophil elastases and other proteinases.6 In addition to its anti-protease activity, AAT also has numerous anti-inflammatory and tissue-protective effects. AAT modulates the activation and maturation of antigen-presenting cells,26-28 improves mitochondrial membrane stability, and inhibits caspases. In combination, these actions prevent cell apoptosis and enhance cell survival during ischemia.26,29-31 AAT downregulates proinflammatory cytokines (IL-6, IL-8, IL-1b, and TNF-α) and promotes anti-inflammatory mediators (IL-10, IL-1βRα, and TGF-β).24,26,28 Given these properties, this study set out to determine whether conditioning of the lung allograft, and subsequent treatment of the recipient with AAT, reduced IRI in the specific setting of a fully allogeneically mismatched LTx, and without systemic immunosuppression.
Our results demonstrated that priming the donor lung with AAT, in addition to posttransplantation treatment of the recipient with AAT, reduced histologic evidence of IRI-associated acute lung injury and necrosis. IRI, a process initiated at the time of organ implantation, is marked by an endothelial and epithelial injury resulting in noncardiogenic pulmonary edema. Treatment with AAT reduces lung GW and severity of lung allograft necrosis on histologic evaluation. Prior studies evaluated the effect of pretransplantation infusion of AAT on IRI using a rat pulmonary artery ischemia-reperfusion model12 and pig model of lung transplantation13 within few hours postreperfusion. Our study extends the model of allograft dysfunction to 8 days postreperfusion. This is particularly important because, in clinical practice, severe IRI beyond the first 48 hours after LTx, is strongly correlated with poor outcomes.14-16 Therefore, the clinical relevance of assessing allograft changes during the early posttransplant period, without further assessment of the allograft at later time points, is unclear.
The donor and recipient rats used within these experiments were allogenic mismatches, and the recipients in our study did not receive systemic immunosuppression. Thus, we also assessed the presence and severity of acute cellular rejection. Recipients in both the control and AAT treatment group demonstrated histologic findings consistent with moderate-to-severe acute cellular rejection (when identified). This suggests that AAT administration did not prevent acute cellular rejection, even though in vitro assays showed reduced recipient T-cell proliferation in treated, versus control, animals. Thus, while our study supports the tissue-protective properties of AAT in the setting of IRI-induced lung allograft necrosis, the tempo and severity of acute cellular rejection appear unchanged. This suggests that just reducing T-cell proliferation, in response to donor antigen, is insufficient for preventing acute allograft rejection; therefore, other immune mechanisms are likely involved.
Result interpretation should consider that recipients received human, not rat, AAT. Human AAT only has a 70% sequence homology with its rat counterpart,32 and prior studies have shown that it is biologically active in rodents and large animals.12,13,26,27 However, it still remains unclear if there is an appreciable change in functionality due to this interspecies difference. Last, the administered dose in these experiments was chosen based on a previous study13 and the kinetic data generated herein. However, it is not known if there is a “target” serum level of AAT that achieves certain immunomodulatory and/or immunosuppressive effects. In other words, questions remain as to whether a higher AAT serum level would have produced a greater effect on our measured outcomes.
This pilot study, although novel, has several notable limitations. Although our data demonstrate the tissue-protective effects of AAT in the setting of IRI, the mechanism by which these effects occur has not been elucidated. Our focus and primary outcome measure were related to “late” IRI-related histological changes; therefore, data from the immediate posttransplant period (0–72 h), which is the main focus of clinical interest, were not obtained. Systemic immunosuppression was also not administered to recipient animals, and it is unclear if combining AAT with these medications would alter its effect(s). Last, as both the donor lungs and recipient were treated with AAT, it is unclear if the observed protective effects were related to donor lung priming, extended treatment of the recipient, or both. Future studies will include allograft assessment at earlier time points to further assess the evolution of acute allograft injury and necrosis; in addition, we plan to obtain blood and bronchoalveolar lavage samples at the time of allograft recovery to further characterize the immune cell and cytokine profiles present in the recipients.
In conclusion, AAT appears to protect against IRI. To our knowledge, the combination of donor lung AAT priming with subsequent posttransplant administration of AAT to the recipient is a novel approach that has not been described in previous preclinical animal models. Although the underlying mechanism(s) by which this occurs is unclear, our data argue for a conceivable therapeutic role for AAT in this setting and the potential to affect allograft outcome.
ACKNOWLEDGMENTS
The authors would like to thank Lin Ai, Carmen M. Swaisgood, and Humberto Herrera for assistance during surgery and other technical expertise.
Footnotes
Published online 29 May, 2019.
A.M.E. and H.H. contributed equally to this work. A.M.E. analyzed the data, wrote the manuscript, and contributed to the preparation of the figures. H.H. performed surgical operations and MRL experiments. H.H. and L.L. interpreted the pathology slides for scoring lung necrosis and acute cellular rejection. M.L.B. designed this study, supervised the experiments, and supervised interpretation of the data.
The authors declare no conflicts of interest.
This study was supported by grants from the Gatorade Trust at the University of Florida, VA Medical Research, and Grifols Therapeutics Inc. (Research Triangle Park, NC). Alpha-1 antitrypsin for in vivo use was generously provided by Grifols Therapeutics Inc.
REFERENCES
- 1.Yusen RD, Edwards LB, Dipchand AI, et al. ; International Society for Heart and Lung Transplantation. The registry of the International Society for Heart and Lung Transplantation: thirty-third adult lung and heart-lung transplant report-2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant. 2016;35:1170–1184.. [DOI] [PubMed] [Google Scholar]
- 2.Gelman AE, Fisher AJ, Huang HJ, et al. Report of the ISHLT working group on primary lung graft dysfunction part III: mechanisms: a 2016 consensus group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36:1114–1120.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christie JD, Carby M, Bag R, et al. ; ISHLT Working Group on Primary Lung Graft Dysfunction. Report of the ISHLT Working Group on primary lung graft dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24:1454–1459.. [DOI] [PubMed] [Google Scholar]
- 4.Burton CM, Iversen M, Carlsen J, et al. Acute cellular rejection is a risk factor for bronchiolitis obliterans syndrome independent of post-transplant baseline FEV1. J Heart Lung Transplant. 2009;28:888–893.. [DOI] [PubMed] [Google Scholar]
- 5.Sharples LD, McNeil K, Stewart S, et al. Risk factors for bronchiolitis obliterans: a systematic review of recent publications. J Heart Lung Transplant. 2002;21:271–281.. [DOI] [PubMed] [Google Scholar]
- 6.Cosio MG, Bazzan E, Rigobello C, et al. Alpha-1 antitrypsin deficiency: beyond the protease/antiprotease paradigm. Ann Am Thorac Soc. 2016;13Suppl 4S305–S310.. [DOI] [PubMed] [Google Scholar]
- 7.Breit SN, Wakefield D, Robinson JP, et al. The role of alpha 1-antitrypsin deficiency in the pathogenesis of immune disorders. Clin Immunol Immunopathol. 1985;35:363–380.. [DOI] [PubMed] [Google Scholar]
- 8.Zhang B, Lu Y, Campbell-Thompson M, et al. Alpha1-antitrypsin protects beta-cells from apoptosis. Diabetes. 2007;56:1316–1323.. [DOI] [PubMed] [Google Scholar]
- 9.Petrache I, Fijalkowska I, Medler TR, et al. Alpha-1 antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am J Pathol. 2006;169:1155–1166.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daemen MA, Heemskerk VH, van’t Veer C, et al. Functional protection by acute phase proteins alpha(1)-acid glycoprotein and alpha(1)-antitrypsin against ischemia/reperfusion injury by preventing apoptosis and inflammation. Circulation. 2000;102:1420–1426.. [DOI] [PubMed] [Google Scholar]
- 11.Götzfried J, Smirnova NF, Morrone C, et al. Preservation with α1-antitrypsin improves primary graft function of murine lung transplants. J Heart Lung Transplant. 2018;37:1021–1028.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao W, Zhao J, Kim H, et al. Α1-antitrypsin inhibits ischemia reperfusion-induced lung injury by reducing inflammatory response and cell death. J Heart Lung Transplant. 2014;33:309–315.. [DOI] [PubMed] [Google Scholar]
- 13.Iskender I, Sakamoto J, Nakajima D, et al. Human α1-antitrypsin improves early post-transplant lung function: pre-clinical studies in a pig lung transplant model. J Heart Lung Transplant. 2016;35:913–921.. [DOI] [PubMed] [Google Scholar]
- 14.Prekker ME, Nath DS, Walker AR, et al. Validation of the proposed International Society for Heart and Lung Transplantation grading system for primary graft dysfunction after lung transplantation. J Heart Lung Transplant. 2006;25:371–378.. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki Y, Cantu E, Christie JD. Primary graft dysfunction. Semin Respir Crit Care Med. 2013;34:305–319.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diamond JM, Lee JC, Kawut SM, et al. ; Lung Transplant Outcomes Group. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2013;187:527–534.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye GJ, Oshins RA, Rouhani FN, et al. Development, validation and use of ELISA for antibodies to human alpha-1 antitrypsin. J Immunol Methods. 2013;388:18–24.. [DOI] [PubMed] [Google Scholar]
- 18.Hu H, Zhu X, Joshi S, et al. Thioredoxin priming prolongs lung allograft survival by promoting immune tolerance. PLOS One. 2015;10:e0124705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu H, Lu L, Mu W, et al. Priming donor lungs with thioredoxin-1 attenuates acute allograft injury in a rat model of lung transplantation. J Heart Lung Transplant. 2008;27:1142–1149.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han B, Haitsma JJ, Zhang Y, et al. Long pentraxin PTX3 deficiency worsens LPS-induced acute lung injury. Intensive Care Med. 2011;37:334–342.. [DOI] [PubMed] [Google Scholar]
- 21.Oishi H, Okada Y, Kikuchi T, et al. Transbronchial human interleukin-10 gene transfer reduces acute inflammation associated with allograft rejection and intragraft interleukin-2 and tumor necrosis factor-alpha gene expression in a rat model of lung transplantation. J Heart Lung Transplant. 2010;29:360–367.. [DOI] [PubMed] [Google Scholar]
- 22.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242.. [DOI] [PubMed] [Google Scholar]
- 23.Beyer M, Bartz H, Hörner K, et al. Sustained increases in numbers of pulmonary dendritic cells after respiratory syncytial virus infection. J Allergy Clin Immunol. 2004;113:127–133.. [DOI] [PubMed] [Google Scholar]
- 24.Bata J, Revillard JP. Interaction between alpha 1 antitrypsin and lymphocyte surface proteases: immunoregulatory effects. Agents Actions. 1981;11:614–616.. [DOI] [PubMed] [Google Scholar]
- 25.Bergin DA, Hurley K, McElvaney NG, et al. Alpha-1 antitrypsin: a potent anti-inflammatory and potential novel therapeutic agent. Arch Immunol Ther Exp (Warsz). 2012;60:81–97.. [DOI] [PubMed] [Google Scholar]
- 26.Marcondes AM, Karoopongse E, Lesnikova M, et al. Α-1-antitrypsin (AAT)-modified donor cells suppress GVHD but enhance the GVL effect: a role for mitochondrial bioenergetics. Blood. 2014;124:2881–2891.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis EC, Mizrahi M, Toledano M, et al. Alpha1-antitrypsin monotherapy induces immune tolerance during islet allograft transplantation in mice. Proc Natl Acad Sci U S A. 2008;105:16236–16241.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elshikha AS, Lu Y, Chen MJ, et al. Alpha 1 antitrypsin inhibits dendritic cell activation and attenuates nephritis in a mouse model of lupus. PLOS One. 2016;11:e0156583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou X, Shapiro L, Fellingham G, et al. HIV replication in CD4+ T lymphocytes in the presence and absence of follicular dendritic cells: inhibition of replication mediated by α-1-antitrypsin through altered iκbα ubiquitination. J Immunol. 2011;186:3148–3155.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shahaf G, Moser H, Ozeri E, et al. Α-1-antitrypsin gene delivery reduces inflammation, increases T-regulatory cell population size and prevents islet allograft rejection. Mol Med. 2011;17:1000–1011.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berger M, Liu M, Uknis ME, et al. Alpha-1-antitrypsin in cell and organ transplantation. Am J Transplant. 2018;18:1589–1595.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chao S, Chai KX, Chao L, et al. Molecular cloning and primary structure of rat alpha 1-antitrypsin. Biochemistry. 1990;29:323–329.. [DOI] [PubMed] [Google Scholar]


