Abstract
Elevated blood pressure (BP), or “hypertension,” has been one of the main exclusion criteria for living kidney donation, as it is a risk factor for renal and cardiovascular disease. The effect of elevated BP in living kidney donors is not well studied or understood. The most current living kidney donation guidelines state that donors with a BP >140/90 mm Hg with 1–2 antihypertensive medications or evidence of end-organ damage should be excluded from living kidney donation. Yet, the definitions of “hypertension” have changed with the release of the American Heart Association (AHA)/American College of Cardiology (ACC) clinical practice guidelines suggesting that 120–129 mm Hg is elevated BP and Stage 1 hypertension is 130 mm Hg. However, the kidney function (in terms of estimated GFR) of “hypertensive” living kidney donors does not fare significantly worse postdonation compared with that of “normotensive” donors. In addition, even though living kidney donation itself is not considered to be a risk factor for developing hypertension, there exist certain risk factors (African American or Hispanic descent, obesity, age) that may increase the risk of living kidney donors developing elevated BP postdonation. The choice of BP targets and medications needs to be carefully individualized. In general, a BP <130/80 mm Hg is needed, along with lifestyle modifications.
The waiting list for a kidney transplant continues to expand on an annual basis. However, the demand for kidney donations has far outstripped the supply of deceased donor allografts. One of the most effective solutions to this issue has been transplanting kidneys from living donors.1 Studies have shown that recipients who received allografts from living donors survive longer and perform significantly better than those who received grafts from deceased donors.2,3 In addition, live kidney donations can be used as preemptive (before dialysis) or early interventional (for those who are already on dialysis) treatment.4
Despite all the benefits and promise of living kidney donation, there has been an overall decline in number of living kidney donations since 2004, while the waiting list for kidney donors increases every year.5,6 This decline can largely be attributed to the medical unsuitability of potential donors, who often present with a multitude of health concerns, such as hypertension, obesity, diabetes, and impaired kidney function.6 Elevated blood pressure (BP) is one of the leading exclusion criteria for living kidney donation, as it increases the risk for detrimental outcomes for both kidney transplant recipients and living kidney donors alike. Despite hypertension affecting both kidney recipients and donors, the impact of hypertension on the latter has not been as well studied and understood. The definitions of “hypertension” are variable depending on the guidelines. The definitions have also evolved over time. If one were to define “hypertension” as that level of BP in which the benefits of treatment outweigh the risks of inaction, then one could more appropriately individualize the care plan. For kidney donors, this is the clinical conundrum: how best to assess this balance between BP and long-term outcomes.
In this review, we will examine the current living kidney donation guidelines with respect to “hypertension,” the impact of living kidney donation on “hypertensive” donors, the risk of and factors contributing to the development of “hypertension” postdonation in normotensive donors, and medical and lifestyle management of BP in living kidney donors. Understanding “hypertension” in living kidney donors will improve not only patient education during the donor recruitment process but also patient care postdonation. With this knowledge, it may be possible to delineate more specific and standardized guidelines for living kidney donation, which may very well be what we need to narrow the gap between the supply and demand for living kidney transplants.
Current Living Kidney Donation Guidelines
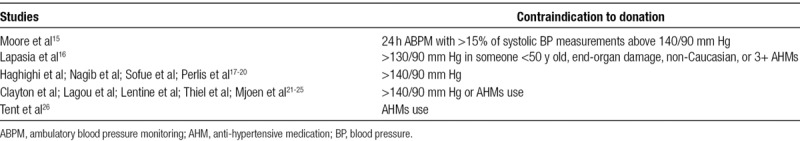
Elevated BP has long been considered as one of the main contraindications to live kidney donation, but the risks of donation in mild, well-controlled “hypertension” are not well understood. This has led to the creation of several different guidelines that try to delineate the exact benchmark of “hypertension” (Table 1). In addition, despite the release of such consensus guidelines, most transplant centers adhere to their own standards when evaluating for hypertension in potential live kidney donors (Table 2).
TABLE 1.
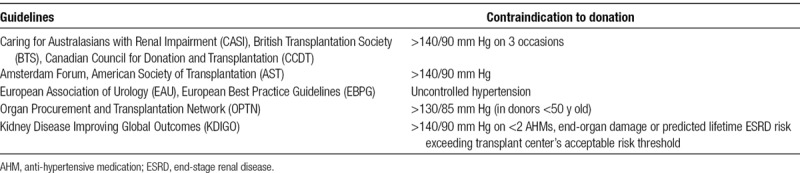
TABLE 2.
With the recent release of the 2017 High Blood Pressure Clinical Practice Guidelines by ACC/AHA, the definitions of hypertension have been revised.7 Elevated BP is 120–129 mm Hg systolic and Stage 1 hypertension is 130–139 mm Hg or diastolic 80–89 mm Hg. The implications of these new guidelines on other published guidelines regarding kidney donation and hypertension remain to be seen. Traditionally, most guidelines have raised concerns about donors with systolic BP >140 mm Hg, although some have also raised questions about even donations in the patients with systolic blood pressure >130 mm Hg, especially in younger donors. Whether kidney donors need somewhat different BP treatment goals is also an intriguing question, especially in light of these newer guidelines. The SPRINT (Systolic Blood Pressure Intervention Trial) clearly demonstrated that a lower BP goal (<120/80 mm Hg) was associated with a reduction in cardiovascular events in a population with a mean age of 68 years, no evidence of diabetes, and an estimated glomerular filtration rate (GFR) of approximately 72 mL/min/1.73 m2.8 One could argue that the SPRINT study does not provide evidence that lower systolic pressure goals are important in delaying the progression of kidney disease. Yet, the consistency of benefit for reducing cardiovascular events was substantial regardless of the GFR of the participants. Of note, the mean GFR in the SPRINT study participants of ~70 mL per minute is not substantially different than what one observes in people who have donated a kidney.8 Thus, there may be reason to believe that kidney donors might need lower BP targets, especially if they are younger, overweight, or have African ancestry. The issue of age, obesity, and African heritage will be discussed in greater detail in the next section.
Evaluation and Assessment of BP Predonation
In general, screening for hypertension in potential donors should include BP measurement on 2 separate occasions by clinical staff who are trained to measure BP accurately, with equipment that has been calibrated. If BP is determined to be high, or high normal, especially if there is variability, or the patient is younger, overweight, or has African heritage, then BP should be evaluated with ambulatory BP monitoring (ABPM) or repeated with standardized BP measurements9-11 (Figure 1). It should be noted that misclassification of BP is common, especially if there is variability. The differences between office, home, and ABPM readings can be substantial, and there are phenotypes such as white coat hypertension and masked hypertension which may have prognostic significance. The variability and phenotype differences have not been prospectively studied in kidney donors. A recent study by Armanyous et al12 using daytime ABPM as a gold standard found a 16% prevalence of hypertension in kidney donors using Joint National Commission-713 definitions and 34% using ACC/AHA7 guidelines. They also noted that office and automated office BP readings demonstrated a high level of specificity but low sensitivity, and a substantial percentage of patients had masked hypertension, defined with ABPM.
FIGURE 1.
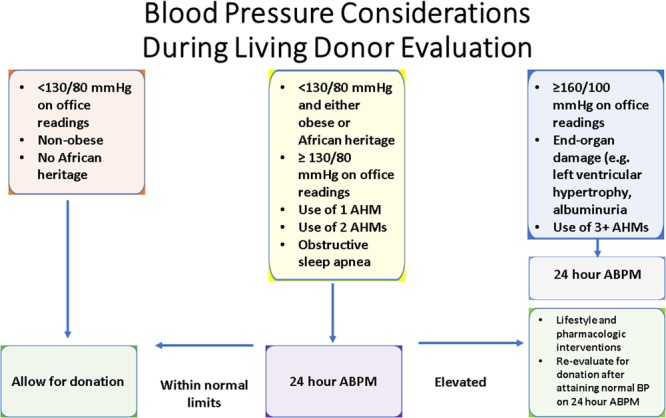
Blood pressure considerations during living donor evaluation. ABPM, ambulatory blood pressure monitoring; AHM, anti-hypertensive medication; BP, blood pressure.
In the past, a BP reading >140/90 mm Hg and/or use of antihypertensive medications (AHMs) was considered as contraindications to donation. However, patients with easily controlled hypertension with 1 or 2 agents and no evidence of target organ damage may be accepted as low-risk kidney donors on a case-by-case basis.2 Additional examinations and imaging studies may be considered in those with borderline hypertension or abnormalities to assess their qualification to donate. From a short-term standpoint, Lentine et al concluded that predonation hypertension was not associated with an increased risk of any perioperative complications, such as gastrointestinal, bleeding, respiratory, and surgical injuries.14
The KDIGO working group report of 201711 suggested that potential donors with hypertension should be individualized in relation to the transplant program’s acceptable risk profile threshold and that they should be counseled that donation may accelerate the rise in BP and increase the need for more antihypertensive therapy. We agree with these statements and would add that this is also a concern in younger donors, especially if they are overweight or have African ancestry, or both.
Preexisting Hypertension in Kidney Donor Candidates
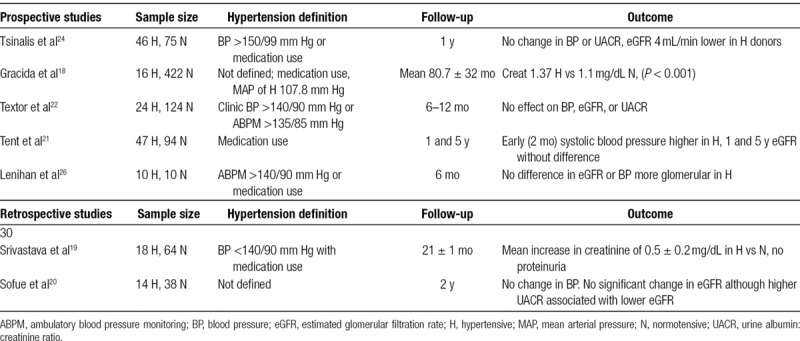
Despite hypertension being a known risk factor for renal and cardiovascular disease,15-17 more and more transplant centers are relaxing their selection criteria to include donors with well-regulated hypertension in response to decreasing supply of donor organs and increasing demand for kidney transplants. Observational studies of hypertensive donors have produced conflicting results (Table 3). Some studies have found that donors with predonation hypertension have a decline in kidney function (evidenced by increasing serum creatinine and decreased estimated GFR),18,19 while others have maintained that there is no significant difference in kidney function between normotensive and hypertensive donors.20-24 The discrepancy between these studies could be explained by the variance in follow-up length, study population, hypertension definitions, and sample size. Other existing retrospective studies have also been limited by short follow-up times, unrepresentative patient populations, and low rates of follow-up.11 When looking at the relationship between predonation BP and end-stage renal disease (ESRD), Kiberd noted that a 5-mm Hg increase in systolic BP had a very small effect on ESRD risk compared with other factors, such as increased proteinuria or reduced GFR.25 Lenihan et al26 discovered that although there was marked glomerulopenia in hypertensive donors, there was a nonsignificant difference in GFR, hyperfiltration capacity, or compensatory renocortical hypertrophy. A recent observational study of 24 533 older donors (≥50 y of age), including 2265 with predonation hypertension, demonstrated that older donors with hypertension had a higher risk of ESRD but not mortality for 15 years postdonation.27 Nevertheless, most retrospective studies concur that there is no significant effect on BP or change in AHM use in hypertensive donors postdonation.
TABLE 3.
Published clinical data comparing influence of blood pressure on renal outcomes in living kidney donors
In summary, donors with well-controlled BP (BP <140/90 controlled with 1–2 antihypertensive drugs and no evidence of target organ damage) seem to be at minimal risk of developing worsening kidney function or hypertension, strengthening the support for their inclusion into, and consequent expansion of, the living kidney donor pool. Yet, the available studies are of short duration, and there are phenotypes, such as obese, or people with African heritage who may be at more risk, and genotypes such as APOL1 which may confer more risk over time. For example, as 25-year-old overweight male homozygous for APOL1 risk variants may not be an ideal candidate despite a BP of 115/70 mm Hg. However, proper counseling on the aforementioned risks (increased BP and potential end-organ damage), albeit small, should be conducted.11
Development of Hypertension in Kidney Donors Postdonation
Even though kidney donation often leads to physiological alterations (kidney hyperfiltration, upregulation of renin-angiotensin-aldosterone system, and changes in vascular tone)28,29 that may elevate BP, it is not considered to be a risk factor in developing hypertension postdonation.
An important prospective study by Kasiske et al followed living kidney donors over a 3-year period.30 They observed systolic and diastolic BP increased slightly and significantly over time in both donors and controls, but there were no significant differences between the 2 groups; in addition, after 3 years, the 24-hour ABPM of both groups was not statistically significant either.
There are also reports that suggest that there is an increased risk of developing hypertension postdonation, but these studies often fail to compare their study population with age-matched controls. One by Kim et al31 looked at the long-term risk of hypertension in living kidney donors and found that there was a statistically significant increase in BP postdonation, with 21% of the donors developing hypertension that required AHM use. However, the increase in BP was from 113/75 to 116/77 mm Hg, values that are well below the normal benchmarks for hypertension; in addition, the total sample size of donors whose hypertension histories could be obtained was 43, with only 11% of the total sample size having a follow-up of >1 year. Most importantly, there was no comparison of these statistics with that of the normal population, which, if it had been done, would have shown the lack of any statistical or significant difference.
The trial of preventing hypertension study which was conducted in patients with normal kidney function, demonstrated that 2 of 3 patients with untreated prehypertension developed stage 1 hypertension over a course of 4 years, while treatment with candesartan decreased this incidence of “hypertension.”32 This signifies that not only are there inherent factors that contribute to the development of elevated BP aside from living kidney donation but also that there are ways to modify and control the upward trajectory of BP.
Thiel et al33 found that the risk of developing hypertension, defined as >140/90 mm Hg, increased 3.64-fold 1-year postdonation. However, the mean predonation systolic and diastolic BP values of the normotensive donors who were hypertensive after 1 year postdonation were significantly higher than those donors who were normotensive after 1 year postdonation. This suggests that these patients may have been prehypertensive or more susceptible to developing hypertension. In addition, in the subset of the donor cohort who remained normotensive after 1 year and 5 years, there was only a modest and nonsignificant increase in risk of developing hypertension. Thus, this study suggests that kidney donation itself does not significantly increase the risk of developing hypertension, but there are factors that lead to a progressive rise in BP, and if your predonation systolic BP is 135 mm Hg, you are more likely to reach 140 mm Hg than if your BP was 125 mm Hg over the course of several years.
However, it is important to keep in mind that the above studies were conducted with positively selected and homogenous patient populations who satisfied highly stringent health requirements to become living kidney donors. Many of these studies failed to include more “marginal” donors who, at baseline, are at higher risk of developing hypertension, such as those of African American and Hispanic descent, the obese, or the elderly.34
There have been a few studies that have looked at these at-risk groups to see how they fare postdonation. Hypertension (defined as >140/90 mm Hg) was identified in 41% of African American donors in a study with an average follow-up length of 7 years, compared with 30% of Caucasians.35 African American and Hispanic donors are also at about a 50% increased relative risk of developing hypertension postdonation.36,37 One retrospective study demonstrated a 2.4-fold increased risk of hypertension postdonation in African Americans compared with a matched group of African American nondonors from the Coronary Artery Risk Development in Young Adults study.13 African American donors had a 37% higher relative likelihood of any antihypertensive medication use after donation compared with Caucasian living donors.38 One study attributes these findings to health disparities, as they found that African American patients with hypertension were less likely to have their BP controlled despite equal access to care.39
However, African Americans have higher rates of incident ESRD than European Americans. MYH9 and APOL1 loci on Chromosome 22 have been linked with nondiabetic kidney disease in several recent studies.40-42 Two alleles of the APOL1 gene, G1, and G2, are associated with higher risk of focal glomerulosclerosis and hypertension-attributed kidney disease.41 While the risk allele frequency in African Americans for G1 or G2 is ~30%, it is much lower in Hispanics with African admixture and extremely low in European Americans. These variants may explain 70% of the excess focal segmental glomerulosclerosis, HIV-associated nephropathy, and hypertensive kidney disease in African Americans. Screening for APOL1 susceptibility variants in kidney donors before surgery may have an important impact on outcomes for kidney transplant recipients. Several small studies suggest the presence of donor variants is associated with adverse outcomes for the recipients of kidneys APOL1 susceptibility.43,44 Donors with 2 APOL1 variants convey higher risk of allograft failure to recipients.43,44 Those who receive kidneys with 1 APOL1 variant have outcomes similar to that of patients who receive a kidney from a donor without these variants, in small studies. The number of observations published, however, also remains relatively small. Screening of African American kidney donors for APOL1 alleles may exclude many potential donors, which may further reduce the availability of transplants in a population already less likely to undergo transplantation. The National Institute of Diabetes Digestive and Kidney Diseases-funded APOL1 Long-Term Kidney Transplantation Outcomes Research Network will examine the implications of one or more variants on clinical outcomes for both donors and recipients. However, the number of transplant donors needed to detect statistically significant differences in their outcomes is likely to be considerable.
Current consensus guidelines have contraindicated donors with a BMI >30–35.2 Lentine et al14 confirmed that obese donors were more likely to have predonation hypertension and each increase in year of donor age was associated with a 5%–7% higher likelihood of hypertension.14 Another study demonstrated that the frequency of hypertension increased by 10% for each 1-unit increase in BMI, with higher BMI being associated with higher rates of hypertension 5 years postnephrectomy.39 After controlling for multiple risk factors, including age, gender, and ethnicity, on average, obese donors (BMI >30) had higher mean systolic and diastolic BPs than nonobese donors, leading to a significant increase in ESRD risk.45 Also, since BMI does not seem to change significantly after donation, BMI status at time of evaluation determines one’s postdonation prognosis.46 Finally, studies have also shown that hip-to-waist ratio may be a more sensitive prognostic for determining health outcomes than BMI; however, there is a lack of data with regards to this up and coming measurement of obesity that warrants further study and validation.47 Even though current consensus guidelines have taken some of these risk factors into account, again, more studies need to be conducted to determine the specific BMI with which the risk outweighs the benefits of live kidney donation.
In summary, it is important to keep these risk factors (African American and Hispanic descent, BMI, and age) in mind when evaluating potential living kidney donors, as they seem to pose a higher risk of developing hypertension and other long-term medical complications as a result of donation. As discussed below, donors with risk factors for hypertension may require earlier and more intensive strategies to control BP.
Management of BP and Hypertension Postdonation in Living Kidney Donors
Early after donation, it is suggested to allow BP (<160/90 mm Hg) to be elevated to allow for optimal kidney perfusion, which can be maintained with selective, short-acting hypertensive agents (such as calcium channel blocker or clonidine).48 Long-term control of BP in kidney donors depends on the individual patient, and thus it is difficult to recommend a general medication regimen.49 Specific pharmacotherapy must be individualized according to comorbidities, drug-drug interactions, drug side effects, and kidney function. Townsend et al23 recommend that angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and diuretics should be avoided perioperatively due to adaptive hyperfiltration and expected shifts in volume.
Along with a personalized medication regimen, living kidney donors also need to make positive lifestyle changes, including weight control, modest dietary sodium reduction and regular exercise, smoking avoidance, and modest alcohol intake.11,49 They should also receive proper education on taking home BP for self-BP monitoring and management.
The KDIGO Clinical Practice Guidelines suggest that proper BP measurements should be performed annually as part of postdonation follow-up care.11 Hypertensive living kidney donors (whether the hypertension developed pre or postdonation) should be followed more frequently than the average, normotensive donor and to have regular BP, laboratory, and urinary albumin:creatinine ratio tests conducted. They should also have their other cardiovascular risk factors well controlled.11,23 However, these recommendations are somewhat unrealistic, given frequent lack of follow-up of living kidney donors. Ideally, there should be a systematic approach. Moreover, higher risk patients may also require lower BP targets for treatment.
Our Suggestions for Living Kidney Donor Evaluation With Respect to BP
Based on the current literature, we suggest the following protocol in evaluating potential living kidney donors on the basis of their BPs (Figure 1).
Office readings should be carried out as recommended in most guidelines: patient will rest 5 minutes before readings are conducted. BP should be measured with a properly sized BP cuff. The final BP will be averaged over 3 readings conducted with a minimum of a 1-minute rest in between each reading. If there are additional concerns about a potential donor’s BP or younger age, overweight, or African heritage, then ABPM should be considered and measurements take place every 15–30 minutes during the daytime and 30–60 minutes at night for 24 hours with measurements.
Based on the most recent ACC/AHA guidelines, if the patient has an average office BP reading <130/80 mm Hg over 3 measurements, then they should be considered for donation with appropriate individualization and counseling. If the patient has an average office BP reading ≥160/100 mm Hg, evidence of end-organ damage (such as left ventricular hypertrophy, albuminuria), or is taking >2 antihypertensive medication, then they should be excluded from kidney donation.
In the absence of any available clinical data, we raise concern about patients with an average office BP reading ≥130/80 mm Hg but <<160/100 mm Hg, and is taking 2 or fewer antihypertensive medication, who has obstructive sleep apnea or African heritage, or has a waist circumference of ≥94 cm for men and ≥80 cm for women as obesity has been shown to be a risk factor for ESRD.45,47 They should have an ABPM and preferably try to lose some weight. If the patient’s 24 hour ABPM BP reading is <125/75 mm Hg, they should be considered for donation. If the patient’s 24 hour ABPM reading is ≥125/75 mm Hg, they should be given pharmacologic and lifestyle interventions to control their BP and weight; when their BP becomes <125/75 mm Hg on a subsequent 24 hour ABPM reading or over multiple office measurements, they could then be reevaluated for living kidney donation.
With respect to BP assessment postdonation, we suggest maintaining a clinic BP ≤130/80 mm Hg, which is in line with current guidelines, not only for living kidney donors but for the general population. Whether patients who are obese or possibly carry high risk APOL1 variants require lower BP targets is an intriguing question.
There are a few caveats to our suggestions. Our proposal does not take into account “masked hypertension,” defined as normal BP in the office with elevated BP in nonoffice settings, as the prevalence of masked hypertension in the healthy population has not been clearly elucidated. In addition, there is currently not enough data to support the use of ABPM for all potential donors, as we have to be mindful of the cost, availability, and expertise needed to perform ABPM correctly. However, 1 recent study by Banegas et al9 demonstrated that ABPM more strongly predicted all-cause and cardiovascular mortality than clinic systolic BP measurements, which could lend more credence to the validity of ABPM. Nevertheless, more studies should be conducted to verify and gauge the role of ABPM. We recommend that ideally all potential donors should monitor their BP with home BP readings.
We acknowledge the fact that there are no evidence-based guidelines currently available for identifying levels of BP which would pose risk from kidney donation, especially if they are obese, African heritage, or have an APOL1 high risk variant. Nor is there any data on an optimal BP target or treatment plan. There is a need for a well-designed, randomized clinical trial to examine prospectively the influence of predonation elevated BP, treated and untreated, on kidney outcomes. Short of this, we are basing our suggestions on the currently available data, our understanding that these as they apply to the living kidney donors, and our practice. Personalized and individualized care should be provided as needed.
CONCLUSIONS
“Hypertension” criteria have changed substantially in the last few years. The goal of being more inclusive for kidney donors with elevated BP may be considered given the opportunity to control BP in an effective manner. No longer should they be considered “marginal” donors. Currently, some of the guidelines recommend that a 24-hour ABPM BP reading >140/90 mm Hg or use of AHMs be a contraindication to donation. However, when hypertensive donors are allowed to donate, they do not seem to be at a significantly increased risk of developing renal or cardiovascular health issues. Another concern for living kidney donors is the possibility of developing hypertension after kidney donation, especially if they are younger, overweight, or of African heritage. Studies have shown that in healthy, well-selected donors with no known risk factors, living kidney donation does not significantly predispose them to developing hypertension postdonation; however, certain risk factors, such as being African American or Hispanic, obese, or older, are associated with higher likelihoods of developing hypertension postdonation. Personalized pharmacotherapy along with beneficial lifestyle changes and more follow-up appointments should be utilized to manage BP and hypertension after kidney donation. Lastly, we have put forth suggestions for evaluating BP in potential living kidney donors and maintaining BP control postdonation.
Footnotes
Published online 19 September, 2019.
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. Furthermore, each author certifies that this material or similar material has not been and will not be submitted to or published in any other publication before its appearance in Transplantation. A.R. and M.R.W. performed the conception and design of study, acquisition of data, analysis and/or interpretation of data, and drafting the manusript. A.R., S.Y., F.A., L.S., K.S., M.K., N.N., J.S.B., and M.R.W revised the manuscript critically for important intellectual content and approved of the version of the manuscript to be published.
The authors declare no funding or conflicts of interest.
REFERENCES
- 1.O’Connor KJ, Delmonico FL. Increasing the supply of kidneys for transplantation. Semin Dial 200518460–462 [DOI] [PubMed] [Google Scholar]
- 2.Delmonico F; Council of the Transplantation Society A report of the Amsterdam Forum on the Care of the Live Kidney Donor: data and medical guidelines. Transplantation 2005796 SupplS53–S66 [PubMed] [Google Scholar]
- 3.Ogden DA. Consequences of renal donation in man. Am J Kidney Dis 19832501–511 [DOI] [PubMed] [Google Scholar]
- 4.Meier-Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation 2002741377–1381 [DOI] [PubMed] [Google Scholar]
- 5.Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2013 Annual Data Report: kidney. Am J Transplant 201515Suppl 21–34 [DOI] [PubMed] [Google Scholar]
- 6.Rodrigue JR, Schold JD, Mandelbrot DA. The decline in living kidney donation in the United States: random variation or cause for concern? Transplantation 201396767–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/apha/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol 201871e127–e248 [DOI] [PubMed] [Google Scholar]
- 8.Wright JT, Jr, Williamson JD, et al. ; SPRINT Research Group A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 20153732103–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banegas JR, Ruilope LM, de la Sierra A, et al. Relationship between clinic and ambulatory blood-pressure measurements and mortality. N Engl J Med 20183781509–1520 [DOI] [PubMed] [Google Scholar]
- 10.Ramesh Prasad GV. Ambulatory blood pressure monitoring in solid organ transplantation. Clin Transplant 201226185–191 [DOI] [PubMed] [Google Scholar]
- 11.Lentine KL, Kasiske BL, Levey AS, et al. KDIGO clinical practice guideline on the evaluation and care of living kidney donors. Transplantation 2017101Suppl 1S1–S109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armanyous S, Ohashi Y, Lioudis M, et al. Diagnostic performance of blood pressure measurement modalities in living kidney donor candidates. Clin J Am Soc Nephrol 201914738–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doshi MD, Goggins MO, Li L, et al. Medical outcomes in African American live kidney donors: a matched cohort study. Am J Transplant 201313111–118 [DOI] [PubMed] [Google Scholar]
- 14.Lentine KL, Lam NN, Axelrod D, et al. Perioperative complications after living kidney donation: a national study. Am J Transplant 2016161848–1857 [DOI] [PubMed] [Google Scholar]
- 15.Clayton PA, Saunders JR, McDonald SP, et al. Risk-factor profile of living kidney donors: the Australia and New Zealand Dialysis and Transplant Living Kidney Donor Registry 2004-2012. Transplantation 20161001278–1283 [DOI] [PubMed] [Google Scholar]
- 16.Feldstein C, Julius S. Establishing targets for hypertension control in patients with comorbidities. Curr Hypertens Rep 201012465–473 [DOI] [PubMed] [Google Scholar]
- 17.Grams ME, Sang Y, Levey AS, et al. ; Chronic Kidney Disease Prognosis Consortium Kidney-failure risk projection for the living kidney-donor candidate. N Engl J Med 2016374411–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gracida C, Espinoza R, Cedillo U, et al. Kidney transplantation with living donors: nine years of follow-up of 628 living donors. Transplant Proc 200335946–947 [DOI] [PubMed] [Google Scholar]
- 19.Srivastava A, Sinha T, Varma PP, et al. Experience with marginal living related kidney donors: are they becoming routine or are there still any doubts? Urology 200566971–975 [DOI] [PubMed] [Google Scholar]
- 20.Sofue T, Inui M, Hara T, et al. Short-term prognosis of living-donor kidney transplantation from hypertensive donors with high-normal albuminuria. Transplantation 201497104–110 [DOI] [PubMed] [Google Scholar]
- 21.Tent H, Sanders JS, Rook M, et al. Effects of preexistent hypertension on blood pressure and residual renal function after donor nephrectomy. Transplantation 201293412–417 [DOI] [PubMed] [Google Scholar]
- 22.Textor SC, Taler SJ, Driscoll N, et al. Blood pressure and renal function after kidney donation from hypertensive living donors. Transplantation 200478276–282 [DOI] [PubMed] [Google Scholar]
- 23.Townsend RR, Reese PP, Lim MA. Should living kidney donors with hypertension be considered for organ donation? Curr Opin Nephrol Hypertens 201524594–601 [DOI] [PubMed] [Google Scholar]
- 24.Tsinalis D, Binet I, Steiger J. Can ‘borderline’ living kidney donors (BLKD) be used safely for transplantation? Kidney Blood Press 199922388–389 [Google Scholar]
- 25.Kiberd BA. Estimating the long term impact of kidney donation on life expectancy and end stage renal disease. Transplant Res. 2013;2:2. doi: 10.1186/2047-1440-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenihan CR, Busque S, Derby G, et al. The association of predonation hypertension with glomerular function and number in older living kidney donors. J Am Soc Nephrol 2015261261–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al Ammary F, Luo X, Muzaale AD, et al. Risk of ESKD in older live kidney donors with hypertension. Clin J Am Soc Nephrol 2019141048–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boudville N, Prasad GV, Knoll G, et al. ; Donor Nephrectomy Outcomes Research (DONOR) Network Meta-analysis: risk for hypertension in living kidney donors. Ann Intern Med 2006145185–196 [DOI] [PubMed] [Google Scholar]
- 29.Garg AX, Prasad GV, Thiessen-Philbrook HR, et al. ; Donor Nephrectomy Outcomes Research (DONOR) Network Cardiovascular disease and hypertension risk in living kidney donors: an analysis of health administrative data in Ontario, Canada. Transplantation 200886399–406 [DOI] [PubMed] [Google Scholar]
- 30.Kasiske BL, Anderson-Haag T, Israni AK, et al. A prospective controlled study of living kidney donors: three-year follow-up. Am J Kidney Dis 201566114–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SH, Hwang HS, Yoon HE, et al. Long-term risk of hypertension and chronic kidney disease in living kidney donors. Transplant Proc 201244632–634 [DOI] [PubMed] [Google Scholar]
- 32.Julius S, Nesbitt SD, Egan BM, et al. ; Trial of Preventing Hypertension (TROPHY) Study Investigators Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med 20063541685–1697 [DOI] [PubMed] [Google Scholar]
- 33.Thiel GT, Nolte C, Tsinalis D, et al. Investigating kidney donation as a risk factor for hypertension and microalbuminuria: findings from the Swiss prospective follow-up of living kidney donors. BMJ Open. 2016;6:e010869. doi: 10.1136/bmjopen-2015-010869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferdinand KC, Nasser SA. Understanding the importance of race/ethnicity in the care of the hypertensive patient. Curr Hypertens Rep. 2015;17:15. doi: 10.1007/s11906-014-0526-9. [DOI] [PubMed] [Google Scholar]
- 35.Nogueira JM, Weir MR, Jacobs S, et al. A study of renal outcomes in African American living kidney donors. Transplantation 2009881371–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lentine KL, Patel A. Risks and outcomes of living donation. Adv Chronic Kidney Dis 201219220–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mueller TF, Luyckx VA. The natural history of residual renal function in transplant donors. J Am Soc Nephrol 2012231462–1466 [DOI] [PubMed] [Google Scholar]
- 38.Lentine KL, Schnitzler MA, Garg AX, et al. Understanding antihypertensive medication use after living kidney donation through linked national registry and pharmacy claims data. Am J Nephrol 201440174–183 [DOI] [PubMed] [Google Scholar]
- 39.Downie DL, Schmid D, Plescia MG, et al. Racial disparities in blood pressure control and treatment differences in a Medicaid population, North Carolina, 2005-2006. Prev Chronic Dis. 2011;8:A55. [PMC free article] [PubMed] [Google Scholar]
- 40.Bostrom MA, Freedman BI. The spectrum of MYH9-associated nephropathy. Clin J Am Soc Nephrol 201051107–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freedman BI, Murea M. Target organ damage in African American hypertension: role of APOL1. Curr Hypertens Rep 20121421–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kao WH, Klag MJ, Meoni LA, et al. ; Family Investigation of Nephropathy and Diabetes Research Group MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet 2008401185–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freedman BI, Julian BA, Pastan SO, et al. Apolipoprotein L1 gene variants in deceased organ donors are associated with renal allograft failure. Am J Transplant 2015151615–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freedman BI, Pastan SO, Israni AK, et al. APOL1 genotype and kidney transplantation outcomes from deceased African American donors. Transplantation 2016100194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Locke JE, Reed RD, Massie A, et al. Obesity increases the risk of end-stage renal disease among living kidney donors. Kidney Int 201791699–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yazawa M, Kido R, Shibagaki Y, et al. Kidney function, albuminuria and cardiovascular risk factors in post-operative living kidney donors: a single-center, cross-sectional study. Clin Exp Nephrol 20111514–521 [DOI] [PubMed] [Google Scholar]
- 47.Noble RE. Waist-to-hip ratio versus BMI as predictors of cardiac risk in obese adult women. West J Med 2001174240–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olyaei AJ, deMattos AM, Bennett WM. A practical guide to the management of hypertension in renal transplant recipients. Drugs 1999581011–1027 [DOI] [PubMed] [Google Scholar]
- 49.Rossi AP, Vella JP. Hypertension, living kidney donors, and transplantation: where are we today? Adv Chronic Kidney Dis 201522154–164 [DOI] [PubMed] [Google Scholar]


