ABSTRACT
Clostridium difficile infection is the most prevalent health care-associated infection. Treatment relies on antimicrobial therapy with mounting evidence supporting fecal microbiota transplant (FMT) in refractory cases. Cohort studies have documented the safety of FMT in immunocompromised patients. However, the safety of FMT in patients with critically low (<500/μL) absolute neutrophil count is unknown. Currently, in severely immunocompromised bone marrow or solid organ transplant recipients, FMT is delayed until normalization of absolute neutrophil count. We present a patient with absolute neutropenia in whom sequential FMTs were safely and successfully administered, resulting in cure of fulminant C. difficile infection.
INTRODUCTION
Clostridium difficile infection (CDI) has become the most prevalent health care-associated infection with an attributable cost estimated at over $6 billion and mortality of 29,300 patients per year.1,2 Much of the costs, morbidity, and mortality are associated with recurrent and severe or fulminant CDI. Patients who are immunocompromised, particularly those after bone marrow transplant, are at increased risk of developing both recurrent and severe CDI often refractory to antimicrobial therapy.3 High-quality evidence supports the safety and efficacy of fecal microbiota transplant (FMT) in nonresponders and patients with severe or fulminant CDI.4–7 A protocol consisting of sequential FMTs administered in rapid cycles in combination with vancomycin was found to be efficacious at our institution for the treatment of refractory severe/fulminant CDI, and its success was echoed in a recent randomized clinical trial conducted in Italy.8,9
Recent reports suggest FMT is safe and effective in immunocompromised patients for the treatment of C. difficile infection (CDI).10–12 However, it is not recommended for patients with severe neutropenia.10 Ongoing clinical trials using FMT in bone marrow transplant recipients postpone FMT until the neutrophil count normalizes.
CASE REPORT
A 56-year-old man with a history of multiple myeloma status postbone marrow transplant with relapse undergoing salvage chemotherapy (day 4) presented with loose stools, fever of 101.6°F, and hypotension. Laboratory examination showed a white blood cell count of 1,600/μL (absolute neutrophil count [ANC] of 1,000), creatinine of 0.8 mg/dL, albumin of 2.2 g/L, and lactate of 4.4 mmol/L. Computed tomography imaging showed pancolitis, and he was diagnosed with severe-complicated CDI (Figure 1). Therapy was initiated with oral vancomycin (125 mg every 6 hours) and intravenous metronidazole (500 mg every 8 hours).
Figure 1.
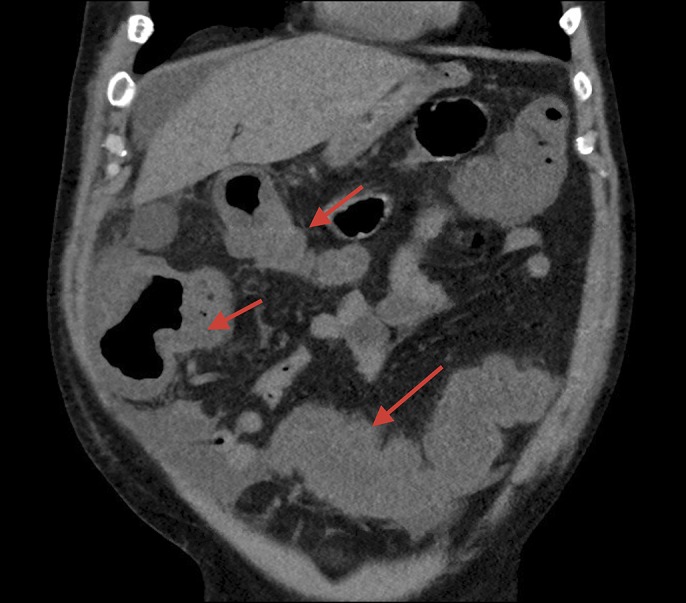
Computed tomography showing pancolitis.
The fever resolved, but the diarrhea continued along with the onset of severe abdominal pain on day 3, at which time a follow-up x-ray showed progressive colonic dilation consistent with toxic megacolon (Figure 2). Vancomycin dose was increased to 500 mg every 6 hours. Blood counts dropped with an ANC nadir of 10/μL. Given the lack of response to maximum antimicrobial therapy, the patient was evaluated by our surgery colleagues but deemed not a surgical candidate. After significant discussion of risks and benefits, a FMT via flexible sigmoidoscopy was pursued. Colon preparation was deferred given the patient's tenuous clinical status and lack of oral intake. The initial endoscopy showed dense and diffuse pseudomembranes, and FMT was performed with frozen/reconstituted stool obtained from the OpenBiome stool bank (Figure 3). The antimicrobial regimen was continued up to and after the procedure.
Figure 2.
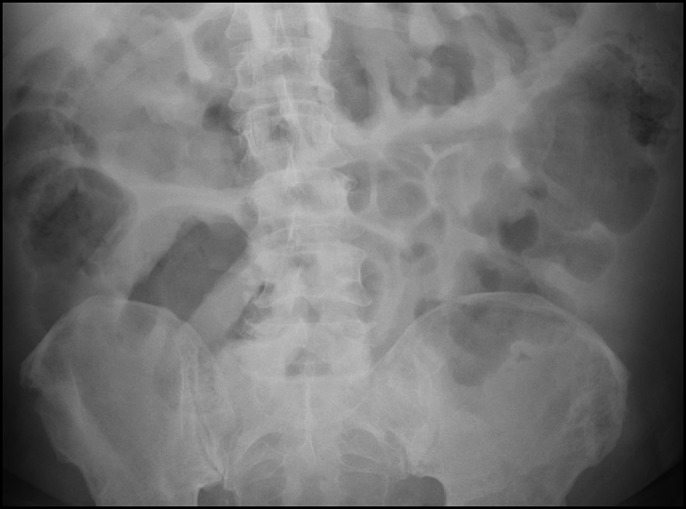
X-ray showing progressive colonic dilation consistent with toxic megacolon.
Figure 3.
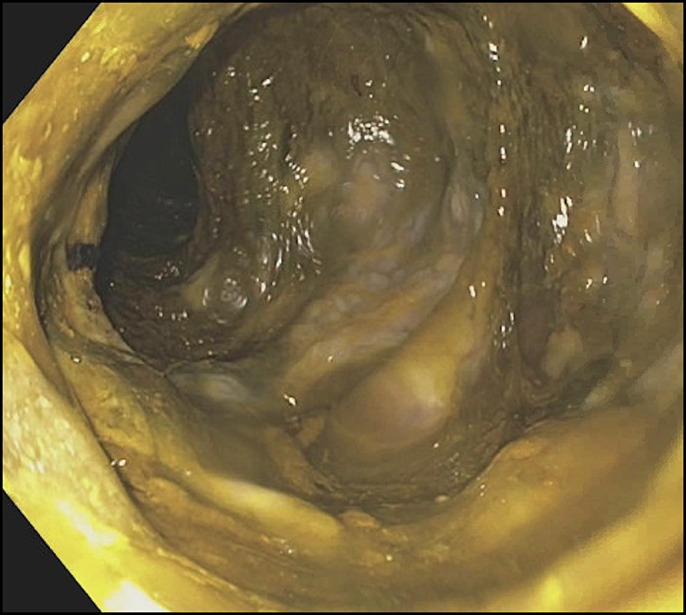
Initial endoscopy showing dense and diffuse pseudomembranes.
After the first FMT, the patient's symptoms including diarrhea, abdominal pain, and abdominal distention persisted, and he also developed hemodynamic instability. Endoscopic FMTs were repeated 4 times in the next 8 days. Subsequently, a nasoduodenal tube was placed, and FMT was repeated with 250 cc of frozen/reconstituted stool on 4 more occasions within 10 days. Initial decisions regarding recurrent FMTs every 1–3 days were based on persistent pseudomembranes along with the clinical status including diarrhea and abdominal distention. Subsequent recurrent FMTs administered via the nasoduodenal tube were based on the patient's clinical status (ie, abdominal pain and diarrhea). This is similar to our previously reported protocol for severe and severe-complicated CDI in which FMT was repeated at 5 days intervals.7 However, the protocol was adapted to this patient's specific clinical scenario in which FMTs were pursed more frequently and antibiotics were continued given profound immunosuppression. His clinical picture improved with decreased abdominal pain and diarrhea, and a diagnostic sigmoidoscopy showed only patchy distribution of pseudomembranes on day 22 (Figure 4). He was discharged on tapering doses of oral vancomycin.
Figure 4.
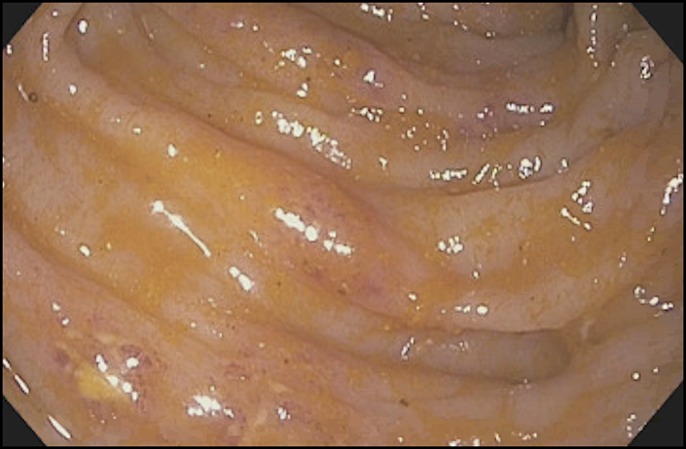
Sigmoidoscopy showing patchy distribution of pseudomembranes on day 22, consistent with improvement.
Immediately after completion of the vancomycin course at 6 weeks posthospital discharge, his profuse diarrhea and abdominal pain recurred and stool C. difficile was positive by polymerase chain reaction. The patient was readmitted, and colonoscopic FMT was performed at which time diffuse pseudomembranes were again noted. Fidaxomicin 200 mg twice daily was started, and repeat FMT was performed 3 days later. The diarrhea resolved, and he was discharged the next day with a plan for completing a course of fidaxomicin. In total, he received 11 FMTs, of which 7 were administered colonoscopically on days 2, 7, 8, 11, 12, 45, and 48 after the initial presentation and 4 via a nasojejunal tube on days 13, 14, 21, and 24. He did not experience recurrence of CDI during the 6 months of follow-up. Importantly, no procedure-related or infectious complications were noted.
DISCUSSION
Our case illustrates that FMT can be safely administered in a patient with critically low ANC. Our patient underwent a total of 11 FMTs, of which 2 were administered while the patient had an ANC <500/µL without any adverse events. It may not be necessary to delay FMT for safety reasons in critically ill patients until the neutrophil count recovery is achieved.
This case also points to the potential of FMT to cure severe or fulminant CDI in this high-risk patient population. Given the severely immunocompromised state, severe/fulminant CDI, and the need for continuation of non-CDI antibiotics for other infections, these patients will likely require several or serial FMTs with or without coadministration of anti-CDI antimicrobials for cure.
Evaluation by a multidisciplinary team including infectious disease specialists, gastroenterologists, and surgeons is key to determine the best management. A detailed discussion of risks/benefits, potential adverse events, and alternative therapies with the patient and family along with obtaining an informed consent is imperative. The high likelihood of needing multiple FMTs to achieve cure should be considered and planned for.
As illustrated in the presented case, FMT, a potentially life-saving therapy for severe and fulminant CDI, may be administered safely without delay in patients with critical neutropenia. Multiple FMTs will likely be needed for cure in this case scenario. Although this is a single case, it does provide anecdotal evidence regarding safety and efficacy in this population of patients who often have few options because they are not considered surgical candidates. We recommend that in severely neutropenic patients with severe or fulminant CDI refractory to conventional therapy, FMT should be considered without delay.
DISCLOSURES
Author contributions: E. Krajicek and M. Fischer wrote the manuscript. M. Bohm, S. Sagi, and M. Fischer edited the manuscript. E. Krajicek is the article guarantor.
Financial disclosure: None to report.
Previous presentation: This case report was presented as a poster at the 2018 ACG Annual Meeting; October 5–10, 2018; Philadelphia, Pennsylvania.
Informed consent was obtained for this case report from the deceased patient's next of kin.
REFERENCES
- 1.Lessa FC, Winston LG, McDonald LC. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(24):2369–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang S, Palazuelos-Munoz S, Balsells EM, Nair H, Chit A, Kyaw MH. Cost of hospital management of Clostridium difficile infection in United States-a meta-analysis and modelling study. BMC Infect Dis. 2016;16(1):447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamboj M, Son C,, Cantu S,, et al. Hospital-onset Clostridium difficile infection rates in persons with cancer or hematopoietic stem cell transplant: A C3IC network report. Infect Control Hosp Epidemiol. 2012;33(11):1162–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections . Am J Gastroenterol. 2013;108(4):478–98; quiz 499. [DOI] [PubMed] [Google Scholar]
- 5.McDonald LC, Gerding DN., Johnson S., et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):987–94. [DOI] [PubMed] [Google Scholar]
- 6.Quraishi MN, Widlak M, Bhala N, et al. Systematic review with meta-analysis: The efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther. 2017;46(5):479–93. [DOI] [PubMed] [Google Scholar]
- 7.Fischer M, Sipe B, Cheng YW, et al. Fecal microbiota transplant in severe and severe-complicated Clostridium difficile: A promising treatment approach. Gut Microbes. 2017;8(3):289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer M, Sipe BW, Rogers NA, et al. Faecal microbiota transplantation plus selected use of vancomycin for severe-complicated Clostridium difficile infection: Description of a protocol with high success rate. Aliment Pharmacol Ther. 2015;42(4):470–6. [DOI] [PubMed] [Google Scholar]
- 9.Ianiro G, Maida M, Burisch J, et al. Efficacy of different faecal microbiota transplantation protocols for Clostridium difficile infection: A systematic review and meta-analysis. United European Gastroenterol J. 2018;6(8):1232–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109(7):1065–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shogbesan O, Poudel DR, Victor S, et al. A systematic review of the efficacy and safety of fecal microbiota transplant for Clostridium difficile infection in immunocompromised patients. Can J Gastroenterol Hepatol. 2018;2018:1394379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng YW, Phelps E, Ganapini V, et al. Fecal microbiota transplantation for the treatment of recurrent and severe Clostridium difficile infection in solid organ transplant recipients: A multicenter experience. Am J Transplant. 2019;19(2):501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


