ABSTRACT
Through the roles of vitamin B1 and B12 in neuroprotection and in improving cerebral palsy symptoms have been previously noticed, the action mechanism is still unclear. This study aims to investigate the protective effect of vitamin B1 and B12 on neuron injury in cerebral palsy and to clarify the mechanism of vitamin B1 and B12 inhibiting neurons apoptosis, and to focus on the role of lncRNA MALAT1 in this process. In order to investigate the effect of vitamin B1 and B12 on neurons injury in vivo and on neuron apoptosis in vitro, we, respectively, introduced vitamin B1 and B12 into cerebral palsy rat and in apoptosis-induced N2A neurons by Oxygen Glucose Deprivation/reoxygenation (OGD/R). Our results demonstrated that vitamin B1 and B12 treatment improved the motor and memory functions and ameliorated the neurons injury in cerebral palsy rats. OGD/R treatment repressed the expression of MALAT1 and BDNF and the phosphorylation of PI3K and Akt, and enhanced the miR-1 expression, which were all reversed by vitamin B1 and B12 treatment in N2A neurons. Vitamin B1 and B12 inhibited miR-1 expression through MALAT1, promoted BDNF expression and activated PI3K/Akt signaling through the MALAT1/miR-1 axis. Vitamin B1 and B12 suppressed neuron apoptosis by up-regulating BDNF via MALAT1/miR-1 pathway. MALAT1 interference abolished the neuroprotective effect of vitamin B1 and B12 in cerebral palsy rats. Collectively, vitamin B1 and B12 up-regulates BDNF and its downstream PI3K/Akt signaling through MALAT1/miR-1 axis, thus suppressing neuron apoptosis and mitigating nerve injury in cerebral palsy rats.
KEYWORDS: Cerebral palsy, vitamin B1 and B12, MALAT1, miR-1, BDNF
Introduction
Cerebral palsy (CP) refers to the non-progressive brain injury occurs before birth and within one month after birth, featuring with central dyskinesia and abnormal posture, as well as dysfunction in intelligence and verbal behavior [1]. Cerebral palsy is a complicate multifactorial disorder, and the hypoxia-ischemia brain damage is the primary cause in clinical [2]. The main mechanism of nerve injury in cerebral ischemia is neurons apoptosis [3], which is the key point for the treatment of cerebral palsy. Hence, factors that influence neuron apoptosis have been widely explored. In addition to neuronal apoptosis in cerebral ischemia, an increase of neuron-specific enolase (NSE) in the blood is also noted, which is a sensitive marker of neurons injury [4].
Hydro-acupuncture (HA) is a method of drug injection at the acupuncture point to cure a certain disease, serving as a significant therapy in traditional Chinese medicine [5]. Use of HA makes the target delivery of drugs a reality, which greatly helps to improve the curative effect of drugs. As Ma et al. reported, substantial improvement in limb dysmyotonia has been observed in children with spastic cerebral palsy after vitamin B1 and B12 injection by HA [6]. Vitamin B1 and B12 have neurotropic effects, playing a crucial role in neurotransmission and neurite outgrowth [7]. But the underlying mechanism of vitamin B1 and B12 relieving cerebral palsy still remains uncertain yet.
Brain-derived neurotrophic factor (BDNF) is a kind of neurotrophins that has critical roles in the development of central nervous system (CNS) and neurons survival, differentiation, and growth [8,9]; and up-regulation of BDNF significantly restrained apoptosis of hippocampal neurons [10]. It has been reported that BDNF expression in peripheral blood mononuclear cells from cerebral palsy children was significantly higher than that in the cord blood or that from healthy adults [11]. In neonatal rats, acupuncture up-regulated BDNF expression in hippocampus and reduced apoptosis following hypoxia-ischemia [12]. Furthermore, BDNF mediated the activation of PI3K/AKT signaling pathway [13], which generally functions to suppressing neurons apoptosis and exhibits a protective role in ischemia and reperfusion [14,15].
Many regulators of BDNF have been identified in biological processes, among them are the microRNAs (miRNAs) that repress BDNF expression via targeting its 3ʹ UTR, such as miR-206 and miR-15a [16,17]. In Schwann cells and peripheral nervous system, BDNF has been identified as a target of miR-1 [18,19]. With the positive effect of miR-1 in hypoxia-induced neuronal cell apoptosis validated [20], we believed that miR-1 may play a part in the cerebral palsy pathogenesis. It made more sense when the interaction between miR-1 and long non-coding RNA (lncRNA) MALAT1 revealed [21,22], indicating a competitive endogenous RNA (ceRNA) regulating network between them. In addition, MALAT1 devoted itself to playing the anti-apoptotic and anti-inflammatory roles to reduce ischemic cerebral vascular and parenchymal damages in ischemic stroke [23]. Above all, we inferred that vitamin B1 and B12 introduced by HA may be involved in affecting neurons apoptosis in cerebral palsy by regulating BDNF and its downstream PI3K/Akt through MALAT1/miR-1 axis.
Materials and methods
Cerebral palsy model in rats
All animal experimental procedures were approved by the Laboratory Animals Ethics Committee at the Fifth Affiliated Hospital of Zhengzhou University and were performed in accordance with local guidelines for the Care and Use of Laboratory Animals. Male SD postnatal day 7 rats were used to establish the cerebral palsy model via hypoxia-ischemia induction [24]. Before surgery, rats were randomly assigned into three groups: sham-operated group (Sham), cerebral palsy group (CP), and cerebral palsy plus vitamin B1 and B12 injection with HA (CP+HA-VitB). After induction of anesthesia with an intraperitoneal injection of 10% chloral hydrate, an incision was made at the middle of the neck of rats to isolate the left common carotid artery, which was ligated and coagulated using a surgical silk thread. After the wound was sutured, all the rats were immediately placed into an incubator at 37℃ with 8% oxygen and 92% nitrogen, for 2 h. Only anesthesia and vessels exposure was carried out on the rats in sham group, without common carotid artery ligation and coagulation.
Hydro-acupuncture treatment
For rats in the CP+HA-VitB group, HA was applied at the “Bai hui (GV20)” and Si shencong (EX-HN-1) acupoints [12]. Vitamin B1 (300 mg) and B12 (1 mg) were dissolved in normal saline and injected for 0.1 ml in one acupoint one time per day, and lasted for 5d in each course; and they were subjected for four courses, with a 2d interval between courses.
Rota-rod test
28d after surgery, rota-rod test was conducted to assess the motor function of rats [25]. Briefly, rats were positioned in a rota-rod with a diameter rod of 60 mm, a length of 75 mm, and a rotating speeding of 25rpm. The time spent on the rota-rod was recorded and considered as the latency to fall. Each rat was tested for 5 times with a 2-min interval between two trials, and the duration was limited within 3 min.
Step-down avoidance task
Step-down avoidance task was carried out to estimate the memory function [25]. Rats were placed on a platform (7 × 25 cm) with a height of 2.5 cm and allowed to have a rest on the platform for 1 min with spaced 1 cm apart, the platform faced a 42 × 25 cm grid of parallel 0.1 cm caliber stainless steel bars. In the training session, rats received a 0.5 mA scramble foot shock for 2 sec immediately upon stepping off the platform. 48 h after the training session, retention time was evaluated. The latency time of the step-down avoidance task referred to the interval of rats stepping down and placing all four paws in the grid.
Enzyme-linked immunosorbent assay (ELISA)
The venous blood of rats in each group was obtained and the serum NSE concentration was determined by ELISA using NSE ELISA kits (R&D, USA) according to the manufacturer’s instructions. The absorbance at 450 nm was measured on a microplate reader. Each blood sample was tested in duplicate.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from hippocampus tissue using the RNeasy Plus Mini Kit (Qiagen, USA) according to the supplier’s manual. After concentration detection with the NanoVue plus (GE Healthcare, USA), 2μg of total RNA was taken for cDNA synthesis with a SuperScript Reverse Transcription Kit (Invitrogen, USA). SYBR Green Master Mix (Applied Biosystems, USA) and an ABI PRISM7900 Sequence Detection System (Applied Biosystems, USA) were used to complete the qRT-PCR. Sangon Biotech (Shanghai, China) undertook the design and synthesis of the Oligonucleotide primers. The relative expression was calculated with 2−∆∆Ct method. The sequence of all primers used in qRT-PCR was listed. MALAT1 (F 5ʹ-ACGCAGGTGTGGCTTTCCAT-3ʹ, R 5ʹ-GTCCTCCCTCACCACAATGG-3ʹ); miR-1 (F 5ʹ -TGGAATGTAAAGAAGT-3ʹ, R 5ʹ -ATCCAGTGCAGGGTCCGAGG-3ʹ); U6 (F 5ʹ-GCGCTCTCTACTGGCATCACATA-3ʹ, R 5ʹ-AATTTACGCTACGCTGTCTGCG-3ʹ); BDNF (F 5ʹ-GTAGTTTTTGTAGGATGAGGAAGTG-3ʹ, R 5ʹ-TATAAATTAACAACCCCAATACACA-3ʹ); β-actin (F 5ʹ-CCTCTATGCCAACACAGT-3ʹ, R 5ʹ-AGCCACCAATCCACACAG-3ʹ). The amplification efficiency of lncRNA MALAT1 primer is 1.01.
Western blot
Hippocampus tissues were incubated with Radio-Immunoprecipitation Assay (RIPA) buffer (Beyotime, Shanghai, China) for 30 min and centrifuged at 14,000 rpm for 15 min at 4°C. Then, the extract was run on 10% SDS polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto polyvinylidene fluoride (PVDF) membranes and blocked in 5% skim milk in tris-buffered saline tween (TBST) for 2 h at room temperature. Blots were probed with BDNF polyclonal antibody (1:250; Santa Cruz Biotechnology), and then incubated with horseradish-peroxidase-coupled goat anti-rabbit antibodies (1:200; Abcam) at 37°C for 2 h and visualized on an ECL Plus Western Blotting Substrate (Thermo Scientific, Shanghai, China). In addition, β-action (Sigma-Aldrich) served as an internal control.
Cell culture and oxygen glucose deprivation/reoxygenation (OGD/R)
Mouse N2A neuroblastoma cells were grown to 50% confluence in DMEM (Gibco, USA) containing 10% fetal bovine serum (FBS) in a 37 ◦C anaerobic chamber (Thermo Electron LED GmbH, Langenselbold, Germany) under normoxic conditions (5% CO2, 95% O2). Prior to OGD treatment, vitamin B1 and B12 (300:1) solution at different concentrations were added into the medium. To mimic the ischemic-like condition in vitro, cells were exposed to OGD treatment, in which the culture medium was replaced by glucose-free DMEM, and cells were maintained in the hypoxic chamber (5% CO2, 1% O2, 94% N2) for 3 h. After OGD exposure, N2A cells were returned to glucose-containing DMEM under normoxic conditions for 24 h reoxygenation.
Neurons apoptosis assessed by TUNEL
After OGD treatment, cell apoptosis was evaluated by TUNEL staining using In Situ Cell Death Detection Kit (Roche) according to the manufacturer’s protocol. In brief, after dewaxing, hydration, the slices were washed three times in 0.1 mol/L PBS and treated with proteinase K working solution at 37°C for 15 min. The slices were washed in PBS and incubated with TUNEL reaction mixture in a dark humidified chamber at 37°C for 1 h and then incubated with Converter-POD solution in a humidified chamber for 30 min. The color was developed using DAB and counterstained with hematoxylin. The photomicrographs were observed with a light microscope (Olympus, Japan).
Down-regulation of MALAT1 in cerebral palsy rats
Rats were randomly allocated into several groups (n = 8 in each group): Sham, CP, CP+HA-VitB, CP+HA+si-control, and CP+HA+si-MALAT1. 72 h before surgery, rats in the last two groups were injected with si-control or si-MALAT1 at the cortex for 3 times, followed by vitamin B1 and B12 or normal saline introduction through HA. 28d after surgery, the motor and memory functions of rats were evaluated by Rota-rod test and Step-down avoidance task, respectively. Hippocampus tissues of the rats were collected for MALAT1, miR-1, and BDNF expression detection.
Statistical analysis
Data were expressed as the mean±standard deviation (S.D.). Comparisons between two groups were assessed by two-sided Student’s t-test using the SPSS 22.0 (IBM, Armonk, NY, USA), besides, P < 0.05 was considered statistically significant.
Results
Vitamin B1 and B12 introduced by HA ameliorated nerve injury in cerebral palsy rats
To make it clear the impact of HA-mediated vitamin B1 and B12 on nerve injury in cerebral palsy, cerebral palsy rat model was constructed. Rats were allocated into three groups (n = 8): Sham, CP, and CP+HA-VitB (B1 and B12); 28d following model construct, the motor and memory functions of rats were evaluated with the latency to fall and step-down latency time-detected. Compared with the sham group, the motor and memory functions in CP group were notably lower and the NSE level was clearly increased, but they were reversed by vitamin B1 and B12 injection through HA (Figure 1(a)). Then, the expression of MALAT1, miR-1, and BDNF in the hippocampus was determined. As shown in Figure 1(b), the expression levels of lncRNAs including N1LR, NEAT1, C2dat1, and MALAT1 were significantly reduced in CP group, while only MALAT1 expression was restored after vitamin B1 and B12 injection through HA. However, miR-1 expression was markedly increased in the CP group when compared with the sham group, which was then inverted by vitamin B1 and B12 injection through HA (Figure 1(c)). The expression of BDNF mRNA and protein was just the opposite with that of miR-1 in these groups (Figure 1(c)).
Figure 1.
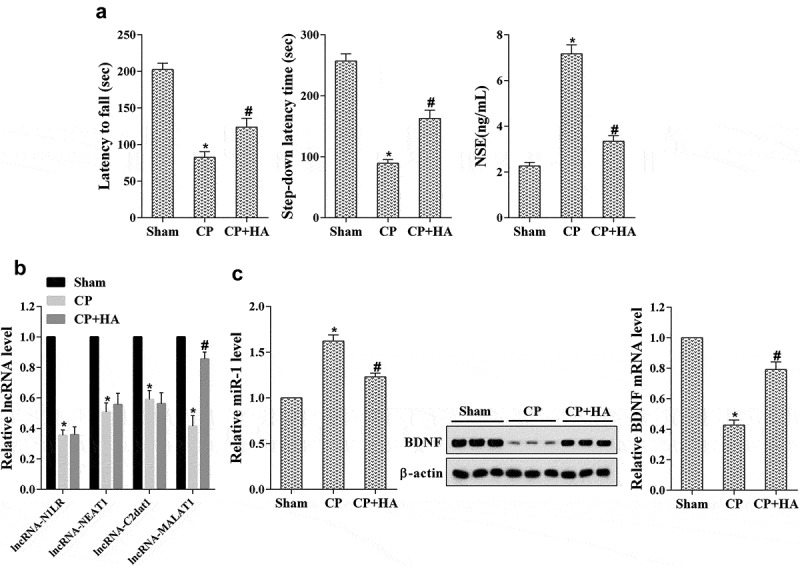
Vitamin B1 and B12 introduced by HA ameliorated nerve injury in cerebral palsy rats. Rats were allocated into three groups (n = 6): Sham, CP, CP+HA. (a) The motor and memory functions of rats were evaluated by Rota-rod test and Step-down avoidance task, respectively; the NSE level was detected by ELISA. (b) The expression of lncRNAs including MALAT1 in hippocampus tissues was quantified by qRT-PCR. (c) The expression level of miR-1, BDNF mRNA and protein in hippocampus tissues was analyzed by qRT-PCR and western blot, respectively. *P < 0.05 compared with sham; #P < 0.05 compared with CP.
The expression of MALAT1 in OGD/R induced N2A neurons was increased by vitamin B1 and B12
In mouse N2A neuroblastoma cells, OGD/R and VitB treatment were introduced and cells were divided into six groups: control, OGD/R, OGD/R + 1 μM VitB, OGD/R + 2.5 μM VitB, OGD/R + 5 μM VitB, and OGD/R + 7.5 μM VitB. The MALAT1 expression was down-regulated up OGD/R treatment, while VitB elevated its level with a dose-dependent method (Figure 2(a)). On the contrary, the miR-1 level was promoted with OGD/R treatment but suppressed by VitB with a concentration-dependent manner (Figure 2(b)). The expression of BDNF mRNA and protein was remarkably inhibited by OGD/R treatment, but presented a dose-dependent increase after treatment with different VitB concentrations (Figure 2(c)). It could be noted that the effect of VitB at a concentration of 5μM caused a significant influence on the expression of MALAT1, miR-1, and BDNF, suggesting that 5μM of VitB was suitable for N2A neurons treatment.
Figure 2.
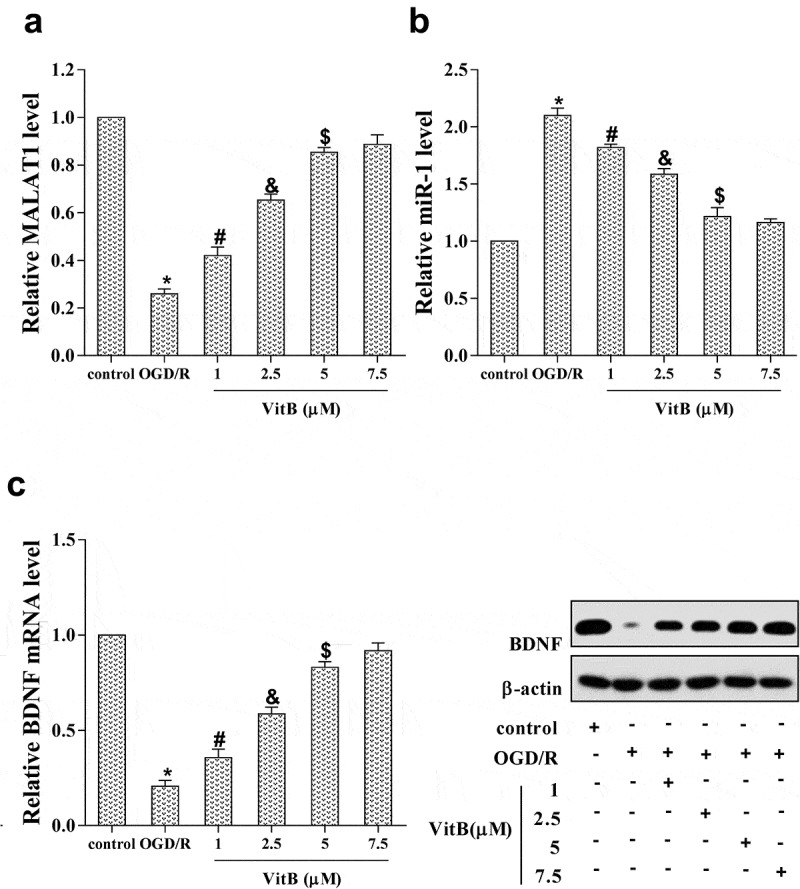
The expression of MALAT1 in OGD/R induced N2A neurons was increased by vitamin B1 and B12. To evaluate the effect of vitB on MALAT1 expression and which levels of vitB were suitable in N2A cells, OGD/R and VitB at different concentrations were introduced to allocate into six groups: control, OGD/R, OGD/R + 1 μM VitB, OGD/R + 2.5 μM VitB, OGD/R + 5 μM VitB, and OGD/R + 7.5 μM VitB. (a) The expression of MALAT1 in N2A cells was detected by qRT-PCR. (b, c) The expression level of miR-1, BDNF mRNA and protein in N2A cells was determined by qRT-PCR and western blot, respectively. *P < 0.05 compared with control; #P < 0.05 compared with OGD/R; &P < 0.05 compared with 1μM; $P < 0.05 compared with 2.5μM.
Vitamin B1 and B12 reduced miR-1 expression in OGD/R induced N2A neurons through up-regulating MALAT1
With the role of vitamin B1 and B12 in promoting MALAT1 expression revealed, their influence on miRNAs level in neurons was then explored. N2A cells were assigned into three groups: control, OGD/R, OGD/R+ VitB (5μM), and the levels of miRNAs that involved in nerve injury were determined, including miR-140, miR-141, miR-142, miR-150, miR-155, and miR-1. Figure 3(a) demonstrates that the expression of these miRNAs was notably higher in the OGD/R group than that in the control group, but only miR-1 was rescued by VitB treatment. N2A cells were then divided into five groups via different treatments: control, OGD/R, OGD/R+ VitB (5μM), OGD/R+ VitB+si-control, and OGD/R+ VitB+si-MALAT1, for the miR-1 expression detected. It was illustrated that increase of miR-1 induced by OGD/R was reduced by VitB treatment, which was further inverted by si-MALAT1 transfection (Figure 3(b)). These findings confirmed a ceRNA regulating the relationship between MALAT1 and miR-1 in N2A cells, and proved that vitamin B1 and B12 decreased miR-1 expression in OGD/R induced N2A neurons through up-regulating MALAT1.
Figure 3.
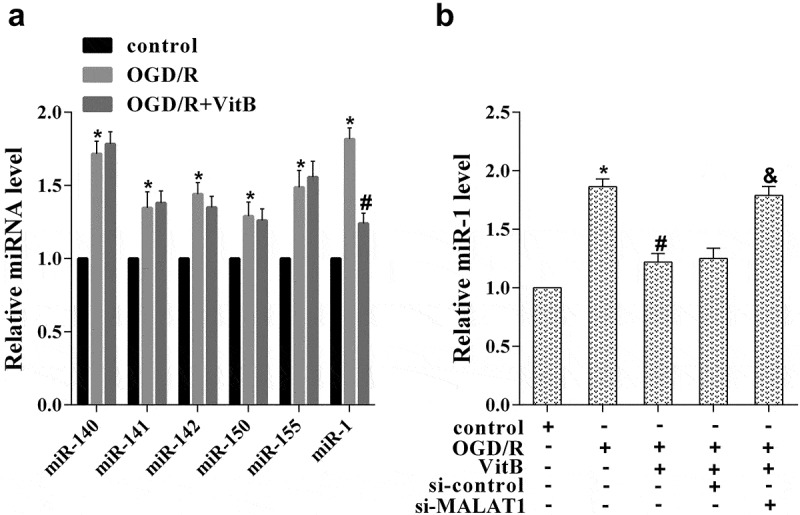
Vitamin B1 and B12 reduced miR-1 expression in OGD/R induced N2A neurons through up-regulating MALAT1. (a) To evaluate which miRNA was affected by vitB, N2A cells were assigned into three groups: control, OGD/R, OGD/R+ VitB (5μM). The level of miR-140, miR-141, miR-142, miR-150, miR-155, and miR-1 in N2A cells was evaluated using qRT-PCR. (b) N2A cells were then divided into five groups via different treatments: control, OGD/R, OGD/R+ VitB (5μM), OGD/R+ VitB+si-control, and OGD/R+ VitB+si-MALAT1. The expression of miR-1 in N2A cells of different groups was examined by qRT-PCR. *P < 0.05 compared with control; #P < 0.05 compared with OGD/R; &P < 0.05 compared with the OGD/R+ VitB+si-control group.
Vitamin B1 and B12 affected BDNF and PI3K/Akt pathway through MALAT1/miR-1 axis
To further clarify the downstream of MALAT1 regulated by vitamin B1 and B12, N2A cells were allocated into seven groups: control, OGD/R, OGD/R+VitB (5μM), OGD/R+VitB+si-control, OGD/R+VitB+si-MALAT1 (20μM), OGD/R+VitB+si-MALAT1+NC, and OGD/R+VitB+si-MALAT1+miR-1 inhibitor. Compared with the control group, the miR-1 expression was promoted in OGD/R group, but it was repressed after VitB treatment, while it was elevated with si-MALAT1 transfected and finally reversed by miR-1 inhibitor (Figure 4(a)). Oppositely, BDNF protein level was lowered upon OGD/R treatment, accompanied with PI3K and Akt phosphorylation inhibited; with BDNF protein increased by VitB, the PI3K and Akt phosphorylation was enhanced (Figure 4(b)). Nonetheless, transfection with si-MALAT1 led to decrease of BDNF protein and low PI3K and Akt phosphorylation, which were rescued by miR-1 inhibitor transfection (Figure 4(b)). From the above, it could be seen that vitamin B1 and B12 affected BDNF and PI3K/Akt pathway through MALAT1/miR-1 axis.
Figure 4.
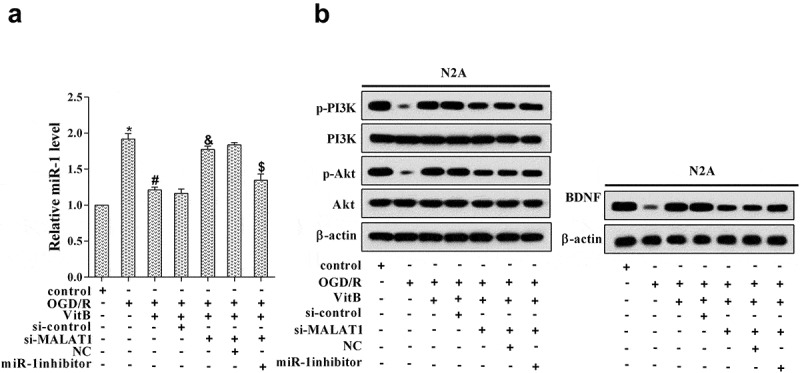
Vitamin B1 and B12 affected BDNF and PI3K/Akt pathway through MALAT1/miR-1 axis. N2A cells were allocated into seven groups: control, OGD/R, OGD/R+ VitB (5μM), OGD/R+ VitB+si-control, OGD/R+ VitB+si-MALAT1 (20μM), OGD/R+ VitB+si-MALAT1+ NC, and OGD/R+ VitB+si-MALAT1+ miR-1 inhibitor. (a) The expression of miR-1 in N2A cells of different groups was assessed with qRT-PCR. (b) The protein level of p-PI3K, PI3K, p-Akt, Akt, and BDNF in N2A cells was determined using western blot. *P < 0.05 compared with control; #P < 0.05 compared with OGD/R; &P < 0.05 compared with the OGD/R+ VitB+si-control group; $P < 0.05 compared with the OGD/R+ VitB+si-MALAT1+ NC group.
Vitamin B1 and B12 repressed neuron apoptosis via the MALAT1/miR-1/BDNF/PI3K/Akt pathway
Next, we investigated the action mechanism of vitamin B1 and B12 on affecting neuron apoptosis. N2A cells were grouped according to different treatments: control, OGD/R, OGD/R+VitB (5μM), OGD/R+VitB+si-control, OGD/R+VitB+si-MALAT1, OGD/R+VitB+si-MALAT1+NC, OGD/R+VitB+si-MALAT1+miR-1 inhibitor, and OGD/R+VitB+si-MALAT1+miR-1 inhibitor+LY294002 (inhibitor of PI3K/Akt pathway). The results illuminated that OGD/R induced neuron apoptosis when compared with the control group, and VitB restrained cell apoptosis caused by OGD/R, which was subsequently altered by miR-1 inhibitor but ultimately promoted with the PI3K/Akt inhibitor introduced (Figure 5(a)). The variation trend of cleaved-caspase-3 in these groups was completely consistent with that of neuron apoptosis level (Figure 5(b)). Here, we illustrated that vitamin B1 and B12 suppressed neuron apoptosis via the MALAT1/miR-1/BDNF/PI3K/Akt pathway.
Figure 5.
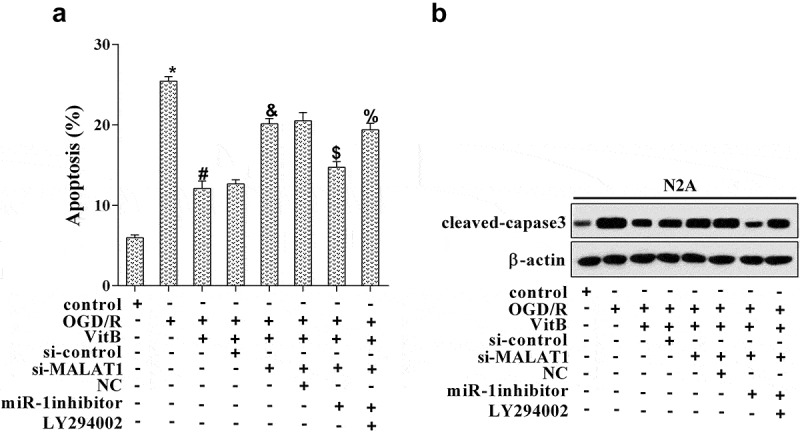
Vitamin B1 and B12 participated in neuron apoptosis via the MALAT1/miR-1/BDNF/PI3K/Akt pathway. N2A cells were grouped according to different treatments: control, OGD/R, OGD/R+ VitB (5μM), OGD/R+ VitB+si-control, OGD/R+ VitB+si-MALAT1, OGD/R+ VitB+si-MALAT1+ NC, OGD/R+ VitB+si-MALAT1+ miR-1 inhibitor, and OGD/R+ VitB+si-MALAT1+ miR-1 inhibitor+LY294002 (inhibitor of PI3K/Akt pathway). (a) Neuron apoptosis was estimated using TUNEL staining. (b) The level of cleaved-capase3 was analyzed by western blot. *P < 0.05 compared with control; #P < 0.05 compared with OGD/R; &P < 0.05 compared with the OGD/R+ VitB+si-control group; $P < 0.05 compared with the OGD/R+ VitB+si-MALAT1+ NC group; %P < 0.05 compared to the OGD/R+ VitB+si-MALAT1+ miR-1 inhibitor group.
Down-regulation of MALAT1 abolished the neuroprotective effect of HA-introduced vitamin B1 and B12 in cerebral palsy rats
To validate the role of MALAT1 in cerebral palsy treatment mediated by HA-introduced vitamin B1 and B12, rats were randomly divided into five groups (n = 8 in each group): Sham, CP, CP+HA-VitB, CP+HA+si-control, and CP+HA+si-MALAT1. Before surgery, rats in the last two groups were injected with si-control or si-MALAT1, followed by vitamin B1 and B12 or normal saline introduction through HA. The results demonstrated that rats in CP group were dull in motor and memory, and the NSE level was augmented, which were improved after vitamin B1 and B12 introduction by HA (Figure 6(a)). However, the relief of nerve injury in rats by HA treatment was then abolished by si-MALAT1 transfection (Figure 6(a)). In addition, the expression of MALAT1 and BDNF protein in the hippocampus of rats in different groups was examined. Figure 6(b) reveals that the MALAT1 and BDNF protein level were reduced in the CP group, but restored by HA-mediated vitamin B1 and B12 introduction, which were further inverted by si-MALAT1 transfection. These results elucidated that MALAT1 played an essential role in mitigating nerve injury by HA-introduced vitamin B1 and B12 in cerebral palsy.
Figure 6.
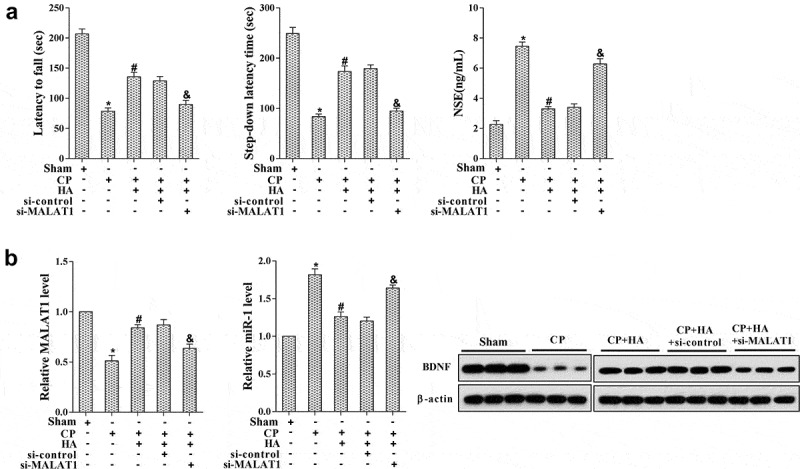
Down-regulation of MALAT1 abolished the neuroprotective effect of HA-introduced vitamin B1 and B12 in cerebral palsy rats. Rats were randomly allocated into several groups (n = 6 in each group): Sham, CP, CP+HA-VitB, CP+HA+si-control, and CP+HA+si-MALAT1. (a) The motor and memory functions of rats were evaluated by Rota-rod test and Step-down avoidance task, respectively; the NSE level was detected by ELISA. (b) The expression of MALAT1, miR-1, and BDNF mRNA in hippocampus tissues was quantified with qRT-PCR; the level of BDNF protein in hippocampus tissues was analyzed by western blot. *P < 0.05 compared with sham; #P < 0.05 compared with CP.
Discussion
In this study, we explored the molecular mechanism that vitamin B1 and B12 injected by HA ameliorates nerve injury in cerebral palsy, revealing the crucial role of lncRNA MALAT1 in this event. With a series of experiments in vitro and in vivo performed, a conclusion can be drawn that vitamin B1 and B12 introduced by HA contributes to mitigating neurons apoptosis and nerve injury in cerebral palsy via the MALAT1/miR-1/BDNF signaling and its downstream PI3K/Akt pathway. Herein, not only the practicability of HA was underlined in cerebral palsy, but also the neurotropic effect of vitamin B1 and B12 and the neuroprotective role of MALAT1 were highlighted, providing novel insight and effective targets for cerebral palsy treatment and symptom improvement.
It is well known that vitamin B is a class of significant nutrient that highly benefit neurological system, and vitamin B complex in low-doses promote the development, maturation, and enlargement of human embryonic brain cells [26]. Actually, vitamin B complex deficiency has been noted in children with cerebral palsy [27], and the effects of vitamin B1 and B12 on facilitating the regeneration of the peripheral have been previously confirmed [28]. Regretfully, seldom progress has been made in cerebral palsy treatment with vitamin B. By accelerating blood circulation and exciting CNS, acupuncture is an essential part of the traditional Chinese medicine and has been widely used in neurological system disorders, including cerebral palsy [29]. Above all, with the merits of acupuncture and drug integrated into HA, a huge improvement in the nerve injury, motor and memory functions was achieved, manifesting the satisfactory outcome of a combination of vitamin B1 and B12 and HA in cerebral palsy. Our study demonstrated that vitamin B1 and B12 introduced by hydro-acupuncture may become a promising therapeutic strategy in treating cerebral palsy.
CeRNAs affect the transcription of other RNAs through competitive binding with miRNAs, to participate in biological process and pathologic mechanism [30]. LncRNA MALAT1 is one of the ceRNAs that generally regulates cell proliferation, migration, and invasion to promote angiogenesis and tumor progression [31,32]. In N2A cells, MALAT1 contributed to neurite outgrowth through activation of ERK/MAPK signaling pathway [33], and it also devoted itself to modulating synaptogenesis by interacting with the genes involved in synapse formation and/or maintenance [34]. The competitive binding relationship between MALAT1 and miR-1 has been previously identified in breast cancer cells [22], and in this study, we clarified the ceRNA regulating the relationship between MALAT1 and miR-1 in N2A cells, reflecting the neuroprotective role of MALAT1 via inhibiting neuron apoptosis.
Our study illustrated the inhibitory effect of BDNF on neuron apoptosis, as well its gene expression controlled by miR-1. By targeting the heat-shock protein 70, miR-1 has been reported to mediate hypoxia-induced apoptotic insults to neuronal cells [20]; and miR-1 was implicated in neural stem cell differentiation via repressing the expression of Hes1 [35]. BDNF is a typical neurotrophic factor that influences neuronal activity, function, and survival [36], and is involved in diverse neurological disorders, such as attention-deficit hyperactivity disorder [37], Alzheimer’s Disease [38], and Huntington’s disease [39]. These reports validated together that miR-1 and BDNF may become effective targets in the treatment of other neurological disorders except for cerebral palsy. In addition, the key role of PI3K/Akt pathway in neuron apoptosis was also emphasized in our study, in accordance with its function in mediating the neuroprotective effects of electro-acupuncture treatment for ischemic stroke [40].
In conclusion, vitamin B1 and B12 introduced by HA up-regulates BDNF and its downstream PI3K/Akt through the MALAT1/miR-1 axis, which contributes to mitigating neurons apoptosis and nerve injury in cerebral palsy. The current study highlighted the enormous potential of vitamin B1 and B12 introduced by hydro-acupuncture in ameliorating neuron injury in cerebral palsy. Even much more studies are needed in choosing the optimum concerning dosage and acupuncture point, this therapy may bring about a breakthrough in clinical for treatment of cerebral palsy and other neurological system diseases.
Funding Statement
This study was supported by the grants from the Key Scientific Research Project of Colleges and Universities in Henan province [Grant No. 14A360016], Zhengzhou Collaborative Innovation Major Project [Grant No. 18XTZX12003], Special Research Project of Traditional Chinese Medicine in Henan Province [Grant No. 2018ZY3013], Henan medical science and technology project [Grant No. 2018020223], Key Technological Research Projects of Henan Science and Technology Department [Grant No. 192102310366], Henan Science and Technology Development Plan Project [Grant No. 172102310170], Key Scientific Research Projects Plan of Henan Higher Education Institutions [Grant No. 17A320007], Key Scientific Research Projects Plan of Henan Higher Education Institutions [Grant No. 16B320026], Key Scientific Research Projects Plan of Henan Higher Education Institutions [Grant No. 14A360016], Research Project on Teaching Reform of Vocational Education in Henan Province [Grant No. ZJC18051].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Mathewson MA, Lieber RL.. Pathophysiology of muscle contractures in cerebral palsy. Phys Med Rehabil Clin N Am. 2015;26(1):57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rumajogee P, Bregman T, Miller SP, et al. Rodent hypoxia-ischemia models for cerebral palsy research: a systematic review. Front Neurol. 2016;7:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ding ZM, Wu B, Zhang WQ, et al. Neuroprotective effects of ischemic preconditioning and postconditioning on global brain ischemia in rats through the same effect on inhibition of apoptosis. Int J Mol Sci. 2012;13(5):6089–6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gelderblom M, Daehn T, Schattling B, et al. Plasma levels of neuron specific enolase quantify the extent of neuronal injury in murine models of ischemic stroke and multiple sclerosis. Neurobiol Dis. 2013;59:177–182. [DOI] [PubMed] [Google Scholar]
- [5].Wang JL, Li RL, Jia CS. Establishment of data warehouse of needling and moxibustion literature based on data mining. Zhen Ci Yan Jiu. 2012;37(1):67–71. [PubMed] [Google Scholar]
- [6].Ma Y, Zhai H. Curative effect observation of the effect of hydro-acupuncture after muscle function localization on cerebral palsy with lower limb dysmyotonia. Mod J Integr Traditional Chin West Med. 2009;18(1):1–2. [Google Scholar]
- [7].Fujii A, Matsumoto H, Yamamoto H. Effect of vitamin B complex on neurotransmission and neurite outgrowth. Gen Pharmacol. 1996;27(6):995–1000. [DOI] [PubMed] [Google Scholar]
- [8].Cardenas-Aguayo Mdel C, Kazim SF, Grundke-Iqbal I, et al. Neurogenic and neurotrophic effects of BDNF peptides in mouse hippocampal primary neuronal cell cultures. PLoS One. 2013;8(1):e53596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang L, Meece K, Williams DJ, et al. Differentiation of hypothalamic-like neurons from human pluripotent stem cells. J Clin Invest. 2015;125(2):796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Huang X, Mao YS, Li C, et al. Venlafaxine inhibits apoptosis of hippocampal neurons by up-regulating brain-derived neurotrophic factor in a rat depression model. Pharmazie. 2014;69(12):909–916. [PubMed] [Google Scholar]
- [11].Koh H, Hwang K, Lim HY, et al. Mononuclear cells from the cord blood and granulocytecolony stimulating factor-mobilized peripheral blood: is there a potential for treatment of cerebral palsy? Neural Regen Res. 2015;10(12):2018–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang Y, Lan R, Wang J, et al. Acupuncture reduced apoptosis and up-regulated BDNF and GDNF expression in hippocampus following hypoxia-ischemia in neonatal rats. J Ethnopharmacol. 2015;172:124–132. [DOI] [PubMed] [Google Scholar]
- [13].Song D, Diao J, Yang Y, et al. MicroRNA382 inhibits cell proliferation and invasion of retinoblastoma by targeting BDNFmediated PI3K/AKT signalling pathway. Mol Med Rep. 2017;16(5):6428–6436. [DOI] [PubMed] [Google Scholar]
- [14].Vazquez de la Torre A, Junyent F, Folch J, et al. PI3 k/akt inhibition induces apoptosis through p38 activation in neurons. Pharmacol Res. 2013;70(1):116–125. [DOI] [PubMed] [Google Scholar]
- [15].Mullonkal CJ, Toledo-Pereyra LH. Akt in ischemia and reperfusion. J Invest Surg. 2007;20(3):195–203. [DOI] [PubMed] [Google Scholar]
- [16].Wang Z, Zhang C, Huang J, et al. MiRNA-206 and BDNF genes interacted in bipolar I disorder. J Affect Disord. 2014;162:116–119. [DOI] [PubMed] [Google Scholar]
- [17].Gao Y, Su J, Guo W, et al. Inhibition of miR-15a promotes BDNF expression and rescues dendritic maturation deficits in MeCP2-Deficient neurons. Stem Cells. 2015;33(5):1618–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Neumann E, Hermanns H, Barthel F, et al. Expression changes of microRNA-1 and its targets Connexin 43 and brain-derived neurotrophic factor in the peripheral nervous system of chronic neuropathic rats. Mol Pain. 2015;11:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yi S, Yuan Y, Chen Q, et al. Regulation of Schwann cell proliferation and migration by miR-1 targeting brain-derived neurotrophic factor after peripheral nerve injury. Sci Rep. 2016;6:29121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chang CY, Lui TN, Lin JW, et al. Roles of microRNA-1 in hypoxia-induced apoptotic insults to neuronal cells. Arch Toxicol. 2016;90(1):191–202. [DOI] [PubMed] [Google Scholar]
- [21].Jin C, Yan B, Lu Q, et al. The role of MALAT1/miR-1/slug axis on radioresistance in nasopharyngeal carcinoma. Tumour Biol. 2016;37(3):4025–4033. [DOI] [PubMed] [Google Scholar]
- [22].Chou J, Wang B, Zheng T, et al. MALAT1 induced migration and invasion of human breast cancer cells by competitively binding miR-1 with cdc42. Biochem Biophys Res Commun. 2016;472(1):262–269. [DOI] [PubMed] [Google Scholar]
- [23].Zhang X, Tang X, Liu K, et al. Long noncoding RNA Malat1 regulates cerebrovascular pathologies in ischemic stroke. J Neurosci. 2017;37(7):1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang H, Gao J, Wang M, et al. Effects of scalp electroacupuncture on the PI3K/Akt signalling pathway and apoptosis of hippocampal neurons in a rat model of cerebral palsy. Acupunct Med. 2018;36(2):96–102. [DOI] [PubMed] [Google Scholar]
- [25].Jung SY, Kim DY. Treadmill exercise improves motor and memory functions in cerebral palsy rats through activation of PI3K-Akt pathway. J Exerc Rehabil. 2017;13(2):136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Danielyan KE, Abramyan RA, Galoyan AA, et al. Vitamin B-complex initiates growth and development of human embryonic brain cells in vitro. Bull Exp Biol Med. 2011;151(5):579–583. [DOI] [PubMed] [Google Scholar]
- [27].Hariprasad PG, Elizabeth KE, Valamparampil MJ, et al. Multiple nutritional deficiencies in cerebral palsy compounding physical and functional impairments. Indian J Palliat Care. 2017;23(4):387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hasegawa K, Homma S, Kanda K. Effects of vitamin B1, B6 and B12 complex on the regeneration of the peripheral nerve and muscle receptors. Nihon Yakurigaku Zasshi. 1973;69(3):483–497. [DOI] [PubMed] [Google Scholar]
- [29].Yang C, Hao Z, Zhang LL, et al. Efficacy and safety of acupuncture in children: an overview of systematic reviews. Pediatr Res. 2015;78(2):112–119. [DOI] [PubMed] [Google Scholar]
- [30].Xu J, Li Y, Lu J, et al. The mRNA related ceRNA-ceRNA landscape and significance across 20 major cancer types. Nucleic Acids Res. 2015;43(17):8169–8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sun JY, Zhao ZW, Li WM, et al. Knockdown of MALAT1 expression inhibits HUVEC proliferation by upregulation of miR-320a and downregulation of FOXM1 expression. Oncotarget. 2017;8(37):61499–61509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xia C, Liang S, He Z, et al. Metformin, a first-line drug for type 2 diabetes mellitus, disrupts the MALAT1/miR-142-3p sponge to decrease invasion and migration in cervical cancer cells. Eur J Pharmacol. 2018;830:59–67. [DOI] [PubMed] [Google Scholar]
- [33].Chen L, Feng P, Zhu X, et al. Long non-coding RNA Malat1 promotes neurite outgrowth through activation of ERK/MAPK signalling pathway in N2a cells. J Cell Mol Med. 2016;20(11):2102–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bernard D, Prasanth KV, Tripathi V, et al. A long nuclear-retained non-coding RNA regulates synaptogenesis by modulating gene expression. Embo J. 2010;29(18):3082–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zheng J, Yi D, Liu Y, et al. Long nonding RNA UCA1 regulates neural stem cell differentiation by controlling miR-1/Hes1 expression. Am J Transl Res. 2017;9(8):3696–3704. [PMC free article] [PubMed] [Google Scholar]
- [36].Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10(3):209–219. [DOI] [PubMed] [Google Scholar]
- [37].Liu DY, Shen XM, Yuan FF, et al. The physiology of BDNF and its relationship with ADHD. Mol Neurobiol. 2015;52(3):1467–1476. [DOI] [PubMed] [Google Scholar]
- [38].Song JH, Yu JT, Tan L. Brain-derived neurotrophic factor in Alzheimer’s disease: risk, mechanisms, and therapy. Mol Neurobiol. 2015;52(3):1477–1493. [DOI] [PubMed] [Google Scholar]
- [39].Zuccato C, Cattaneo E. Huntington’s disease. Handb Exp Pharmacol. 2014;220:357–409. [DOI] [PubMed] [Google Scholar]
- [40].Xue X, You Y, Tao J, et al. Electro-acupuncture at points of Zusanli and Quchi exerts anti-apoptotic effect through the modulation of PI3K/Akt signaling pathway. Neurosci Lett. 2014;558:14–19. [DOI] [PubMed] [Google Scholar]


