Abstract
Introduction:
Currently, hot melt extrusion (HME) is a promising technology in the pharmaceutical industry, as evidenced by its application to manufacture various FDA-approved commercial products in the market. HME is extensively researched for enhancing the solubility and bioavailability of poorly water-soluble drugs, taste masking, and modifying release in drug delivery systems. Additionally, its other novel opportunities or pharmaceutical applications, and capability for continuous manufacturing are being investigated. This efficient, industrially scalable, solvent-free, continuous process can be easily automated and coupled with other novel platforms for continuous manufacturing of pharmaceutical products.
Areas covered:
This review focuses on updates on solubility enhancement of poorly water- soluble drugs and process analytical tools such as UV/visible spectrophotometry; near-infrared spectroscopy; Raman spectroscopy; and rheometry for continuous manufacturing, with a special emphasis on fused deposition modeling 3D printing.
Expert opinion:
The strengths, weakness, opportunities, threats, (SWOT) and availability of commercial products confirmed wide HME applicability in pharmaceutical research. Increased interest in continuous manufacturing processes makes HME a promising strategy for this application. However, there is a need for extensive research using process analytical tools to establish HME as a dependable continuous manufacturing process.
Keywords: 3D Printing, Continuous Manufacturing, Hot Melt Extrusion, Process Analytical Tools, Solubility enhancement, SWOT analysis
1. Introduction
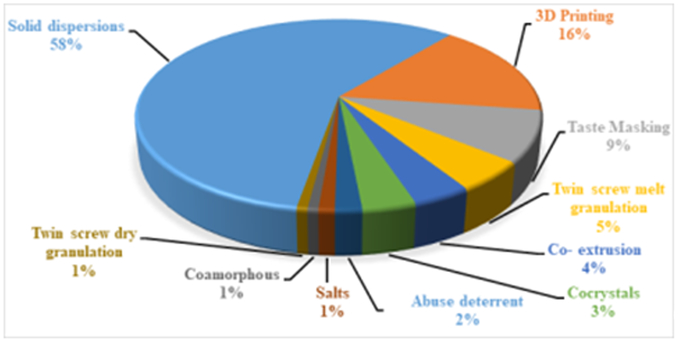
Over the past three decades, hot melt extrusion (HME) has emerged as an essential processing technology in the pharmaceutical industry, and it is steadily rising owing to its utilization to manufacture novel pharmaceutical products [1, 2, 3, 4]. Most pharmaceutical industries are moving towards HME technology mainly to improve the dissolution profile of poorly water-soluble drugs to enhance bioavailability and achieve therapeutic plasma drug concentration [5, 6, 7]. Lately, HME is being explored for various applications, and it is found successful in the development of various drug delivery strategies. Literature reports showed the use of HME in the development of pharmaceutical cocrystals [8], co-extrusion for producing fixed-dose combinations [9], chronotherapeutic systems [10], self-emulsifying drug delivery systems [11], twin-screw granulation [12], semisolid dosage forms [13], abuse-deterrent formulations [14], novel three-dimensional (3D) printing filaments [15], and other miscellaneous applications [2, 3, 4, 16]. Figure 1 illustrates the volume of research publications on HME for preparing different drug delivery sytems during the last five years. This suggests that the use of HME is predominantly devoted to solid dispersions. Remarkably, in a relatively short period of time, the use of 3D printing has exploded for the development of dosage forms.
Figure 1.
Piechart illustrating the percentage of research publications on hot melt extrusion for preparation of different drug delivery systems during last five years (Scopus and Pubmed)
In a previous review [4], the authors focused on the advantages of HME applications, such as solubility enhancement, taste masking, and targeted and shaped drug delivery systems, with special emphasis on innovations in HME. As an update to this earlier review, the current review focuses on the innovative opportunities and continuous manufacturing (CM) of HME in pharmaceutical drug delivery.
The quality by design (QbD) principle is used in drug product development and manufacturing to improve process capability, reduce product variability, enhance product quality, and enable CM. This QbD principle involves the use of experiment design, data and risk analysis, and process analytical tools to collect real-time data to produce quality product [17, 18, 19]. This principle initiated the pharmaceutical industry to shift into CM. Thus, in this new era of pharmaceutical product development, there is an increased interest in the use of CM to produce pharmaceutical products [20, 21, 22, 23, 24]. Application of CM and the required process controls for traditional manufacturing processes are challenging tasks for the pharmaceutical industry; therefore, single batch operations are used to manufacture most pharmaceutical products. However, advanced manufacturing processes, such as HME and 3D printing, promise to offer an advanced manufacturing approach to develop a CM process [25]. The structure of this review is categorized into Part I and Part II. Within this Part I the authors emphasize strengths, weakness, opportunities, and threats (SWOT) analysis of HME, process analytical tools, and CM. In addition, an update on solubility enhancement is presented, as well as HME based fused deposition modeling (FDM) 3D printing with case studies are detailed.
1.1. SWOT analysis of HME
SWOT analysis of HME was presented in the literature from the same group in “Melt extrusion with poorly-soluble drugs: An integrated review” [3]. In the current review, strengths of HME and its application on CM are discussed, along with a special emphasis on 3D printing using HME technology.
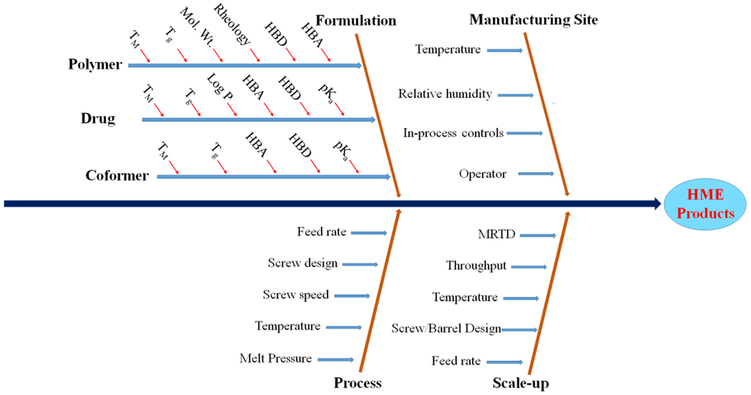
In general, the advantages of HME technology are the absence of solvents, which prevents risk of chemical degradation (in case of aqueous solvents) and residual organic solvents limits [26]; ease of scalability and CM by adapting process analytical tools [27, 28]; few processing steps, which makes it cost- and time-effective [29, 30]; improved solubility and bioavailability of poorly water-soluble drugs [26, 31, 32]; modification of drug release to delayed or sustained release [33, 34]; and development of various pharmaceutical drug delivery systems [35, 36, 37, 38, 39, 40]. Figure 2 represents the critical parameters involved in the development of various dosage forms by HME. Likewise, its disadvantages are high processing temperatures and high energy input, which may affect the stability of processing materials [41, 42]. Feedstock materials with suitable properties for HME, such as flow characteristics, and use of additional additives may overcome this issue to a certain extent [28, 43]. Alternative technologies or threats for HME technology include spray-drying and KinetiSol® technology. However, these technologies may be helpful at times when HME is not appropriate (as in thermolabile drugs) to enhance the solubility of poorly water-soluble drugs. Finally, considering the overall applicability of HME techniques (CM, 3D printing, potential alternative to dry granulation, cocrystals, fixed-dose combination products), it is a versatile platform technology in the pharmaceutical industry.
Figure 2.
Fishbone diagram representing the critical parameters involved in development of various dosage forms by hot melt extrusion (HME). TM - melting point, Tg - glass transition, HBA - hydrogen bond acceptor, HBD - hydrogen bond donor, MRTD – Mean residence time distribution.
2. Continuous manufacturing and process analytical tools
In recent years, there is an increased interest in the development of CM of pharmaceutical products. The use of process analytical tools for real-time monitoring is a suitable option for designing CM processes. The concept of Process Analytical Technologies (PAT) was introduced by the United States Food and Drug Administration (FDA) in 2004 for a greater understanding of fundamental manufacturing processes to ensure end-product quality [44, 45]. The main objective was to implement suitable tools for in-line or on-line monitoring and data analysis through a scientific approach, such as the QbD approach and chemometrics [46].
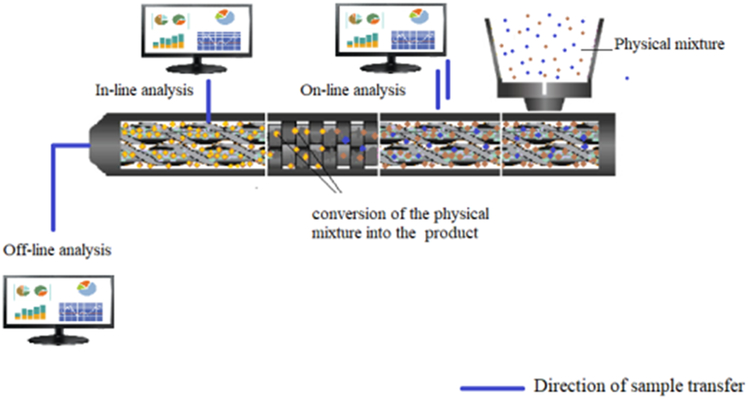
In the pharmaceutical industry, for CM, HME can be supported with different PAT tools, such as UV/visible (UV/VIS) spectrophotometry, near-infrared spectroscopy (NIR), Raman spectroscopy, and rheometry. These tools enable real-time analysis in CM and immediate process control to correct deviations. The schematic representation of in-line, on-line and off-line analysis in hot melt extrusion is shown in Figure 3. In summary, PAT tools may potentially improve safety, reduce batch losses, and meet applicable specifications [28].
Figure 3.
Schematic representation of in-line, on-line and off-line analysis within a hot melt extruder
2.1. UV/Visible spectrophotometry
In-line UV/VIS spectrophotometry has been used for many years in inline-colorimetric monitoring of polymer extrusion [47] and in the food industry [48]. In UV/VIS spectrophotometry, only the color coordinates obtained from the spectra can be used for process control. However, UV/VIS in-line spectrophotometry in extrusion, in addition to the color, provides information on particle size, solid state, and concentration [28].
Wesholowski et al. [49] used in-line UV/VIS spectrophotometry as a PAT tool for manufacturing solid dispersions of carbamazepine and theophylline with copovidone. The method was proved to be accurate and repeatable for the formulations tested. The range of linearity differed with different formulations because the range of linearity for carbamazepine formulations was 5–30%, whereas that for theophylline formulations was 2.5–10%. Evaluation of recorded data was appropriate because univariant data analysis was found to be adequate for the evaluated range of experimental parameters.
Becker et al. [50] used inline-visible spectrophotometry to determine particle size distribution density of polypropylene/clay composites. Particle distribution density was obtained from transmission spectra and using numerical linear equation systems. From the results, it is evident that distribution density depends on parameters such as dosage and screw speed.
2.2. Near-infrared spectroscopy
NIR is a nondestructive monitoring tool to measure the effect of critical process parameters (temperature, feed rate, and screw speed) on product quality and process efficiency. Due to this feasibility to make adjustments to the process, correction can be performed in real-time when deviations in the process are noticed during the HME process [51]. In the HME process, a transmission NIR probe can be used for in-line monitoring of extrudates, with the probe inserted in the die. Detection of physicochemical transformations can produce vital information about product quality and performance [52].
Islam et al. [51] used two NIR probes as in-line monitoring tools for extrusion of poorly water-soluble indomethacin (IND) with Soluplus® and Kollidon® VA 64. Two probes were used to increase the rationale for studying the transitions during the process. The first reflectance probe near the feeding zone collects the spectra of a crystalline drug and the transmission probe at the die collects the spectra of a molten drug. The NIR data provided valuable information on the relation between torque or die pressure and screw speed, as well as transparency of extrudates and peaks, revealing amorphous conversion of IND and drug-polymer interaction. Differential scanning calorimetry (DSC) and X-ray diffraction (XRD) data were in accordance with the results provided by NIR spectra. Thus, NIR data provide valuable inputs regarding the optimization of process and formulation parameters.
Krier et al. [53] used NIR as an in-line technique in a process involving celecoxib and ethyl vinyl acetate 2803G to monitor co-extrusion of implants. Spectral intensity of the active pharmaceutical ingredient (API) data was found to be directly proportional to drug concentration and can be correlated to increasing polymer concentration. These changes in drug concentration according to the experimental conditions reflect the ability of NIR to analyze the process in real-time. NIR assay values were in close agreement with those of off-line high performance liquid chromatography (HPLC) methods. This NIR technique employed was found to be very fast (5 sec) and sensitive compared to the reference methods used (4 min). Experimental data infer that the employed NIR tool monitors the critical quality attributes (CQAs) of the process and allows modification of the critical parameters of the process (such as drug content and extruder diameter).
2.3. Raman spectroscopy
Raman spectroscopy is a possible analytical technique for in-line quantification of API. This technique is a molecular vibrational spectroscopic method that is nondestructive and fast [44]. Additionally, it is a useful PAT tool requiring no sample pretreatment and containing multivariate data as quantitative and qualitative physicochemical information. Compared with NIR spectroscopy, owing to its much lower sensitivity to water interference, Raman spectroscopy is also suitable as a monitoring tool for wet granulation process. There are few applications of Raman spectroscopy in determining the endpoint of a blending process. De Beer et al. [54] used Raman spectroscopy to determine end point in a high shear mixing process. Hence, the collected Raman spectra were matched with reference spectra using SIMCA models. Nagy et al. [55] employed Raman spectroscopy successfully in a continuous blending and tableting process. They used it for the first time for quantitative analysis in a continuous real-time monitoring and to evaluate drug content in the blending step performed by a twin-screw blender.
Recently, Ibrahim et al. [56] employed terahertz Raman (THz Raman) imaging to characterize the solid-state characteristics of acetaminophen (APAP)/ hydroxyl propyl methyl cellulose (HPMC) solid dispersions manufactured via HME. The peak from the crystalline lattice vibrations provides clear discrimination between the crystalline and amorphous portions of APAP in the extrudates. Extrudates manufactured at different screw speeds and temperatures were evaluated and the results showed that both higher thermal energy and screw speed successfully assisted the amorphous conversion of APAP. High-spatial-resolution Raman images confirmed the amorphous nature of APAP dispersed in HPMC matrix. The study proves the suitability of THz Raman imaging for identifying the solid state of API in solid dispersions. This technique also provides visual distribution patterns of formulation ingredients in solid dispersions.
Harting et al. [57] evaluated Raman spectroscopy as a PAT tool for quantifying ibuprofen and diclofenac sodium in a twin-screw wet granulation process. A Raman probe was installed in front of the barrel and spectra were collected upon continuous flow of granules. The first step was to develop a partial least square (PLS) calibration model and the second step was to develop an in-line Raman spectroscopy method for both APIs through a split-feeding method. When the in-line method was applied for the process with both APIs, the detectable saturation concentration range of API was found to be different for both APIs. The in-line analysis was further checked by an off-line UV analysis, which proved that the twin-screw granulation process was suitable for monitoring drug content uniformity in the granules.
Netchacovitch et al. [58] used in-line Raman spectroscopy for real-time monitoring to determine drug content. Two approaches, univariate and multivariate analyses, were employed to determine content uniformity of itraconazole in dosage forms. Extrusion was performed at 155°C with a screw speed of 100 rpm and drug contents ranging from 70–110%. In-line Raman measurements were performed, and drug concentrations of extrudates were analyzed by a valid off-line confocal Raman microscopic reference method. Drug content values, as determined by in-line and off-line analyses, were closer in multivariate analysis than in a univariate method, proving the accuracy of multivariate method. This study provided valuable information on successful application of in-line Raman spectroscopy for regulating content uniformity in dosage forms.
2.4. Rheometry
In the HME process, primary process variables, such as pressure and temperature, can be freely monitored to test process stability (such as process and product homogeneity). In CM, in-line measurement of rheological characteristics plays an important role in real-time monitoring of torque, influence of drug load, and effect of formulation ingredients on the process [59].
Real-time evaluation of rheology in the extrusion process can be achieved by determining the pressure drop inside an extruder die connected to the instrument. In-line process measurement is an evaluation made inside the main process stream, rather than in the sample obtained from the main flow (on-line). In-line rheological characterization enhances process control and understanding. Recently, Kelly et al. [60] used in-process rheometry as a PAT tool for HME. The API (a developmental API), was dispersed in polyvinylpyrrolidone-vinylacetate (PVP-VA) copolymer (Kollidon® VA 64). The ranges of % drug load and temperature studied were 0–40 and 120–140°C, respectively. A 16-mm extruder was fitted with a rheological slit die with a temperature control adaptor and slit-die block. A linear fitting was performed between the change of pressure along the slit length and predicted pressure at the die exit. The results reflected a trend of miscibility between the API and polymer, as well as a plasticizing effect of the API on the polymer. The viscosity of pure polymer was reduced by approximately 30 folds at 40% drug load and by 20 folds with increment of the process temperature from 120°C to 160°C. The observed relation between shear rates and pressure drop due to shear thinning provided a valuable real-time data about process control.
Monteyne et al. [61] employed vibrational spectroscopy to study the relation between continuous twin-screw melt granulation (TSMG) and rheology. There is a possible relationship between chemical changes or interactions and physical properties such as the micro-structure of granules. Modified physical properties may reflect the modification of chemical properties, indicating the changes brought by the applied process. Therefore, an in-line Raman and in situ Fourier-transform infrared spectroscopy (FTIR) were applied to study rheological changes during the melt granulation process based on analysis of physicochemical properties. This study aimed to detect molecular level changes in a caffeine anhydrous/Soluplus® combination in the TSMG process, with reference to changes in rheometric analysis. With the applied pressure and force in the twin-screw granulation process, the materials, while being converted into the granule form, showed changes in rheological properties, which were further reflected in their chemical properties, as showed by in situ FTIR. Changes in the chemical properties of the formulation, such as polymorphic conversion at specific process settings applied, including temperature and shear, showed a correlation between the data measured by plate-plate rheology and FTIR data.
This in-line rheometric analysis can be a valuable tool for HME and CM. The data on viscosity and degradation provided by this technique could play a vital role in setting process parameters and thus improving product quality.
3. Solubility enhancement by HME
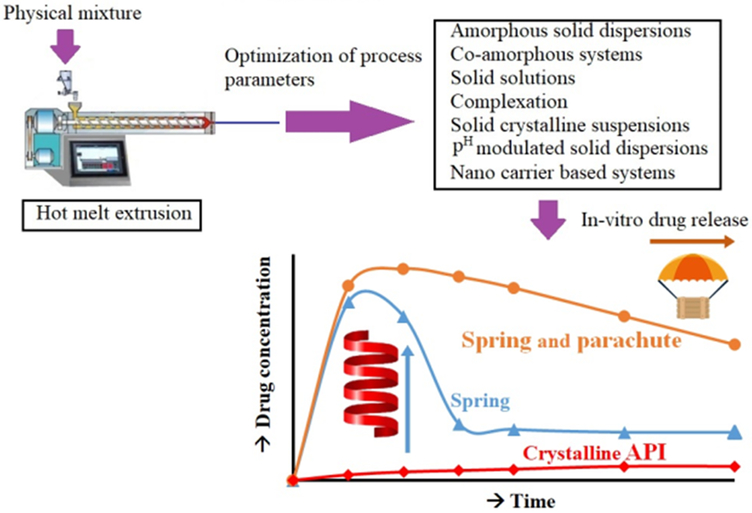
The use of HME for improving the solubility and dissolution rate of poorly water-soluble drugs through dispersion or distribution in suitable carriers is well-established, as reflected by its use in the manufacturing of commercial products on the market (Table 1) [62, 63, 64]. The different mechanisms used to improve drug solubility are improving wettability, modifying physicochemical properties of API, preventing recrystallization, and stabilizing amorphous formulations[65]. The melt-mixing process during HME converts crystalline APIs to the amorphous form by dispersing it in a carrier and prevents drug recrystallization through formation of non-covalent bond between the drug and the carrier chain or through the stearic hindrance phenomenon [2]. Because the drug is dissolved in the hydrophilic carrier, its dissolution in gastrointestinal fluids is enhanced compared to that of the native crystalline form of APIs. This improvement is caused by Gibb’s free energy and absence of lattice energy barrier. The hydrophilic nature of the carriers aids in improving drug dissolution in aqueous fluids, whereas the nature and concentration of the carrier plays a vital role [66]. These solid dispersions may tend to recrystallize based on polymer characteristics and processing conditions employed [65]. Figure 4 shows spring-parachute effect and the various HME strategies studied for enhancing the solubility for poorly water-soluble drugs.
Table 1.
Examples of marketed products for solubility enhancement via HME
| Trade name | Company | Nature of product |
API | Polymer system | Therapeutic treatment |
|---|---|---|---|---|---|
| Mavyret® | AbbVie | Amorphous Dispersion | Glecaprevir/Pibrentasvir | Copovidone/Vitamin E Polyethylene glycol succinate | Hepatitis C |
| Venclexta® | AbbVie | Amorphous Dispersion | Venetoclax | Copovidone | Chronic lymphocytic leukemia |
| Technivie® | AbbVie | Amorphous Dispersion | Ombitasvir, paritaprevir, and ritonavir | Copovidone/Vitamin E polyethylene glycol succinate | Hepatitis C |
| Viekira pak® | AbbVie | Amorphous Dispersion | Ombitasvir, paritaprevir, ritonavir and dasabuvir | Copovidone | Hepatitis C |
| Belsomra® | Merck | Amorphous Dispersion | Suvorexant | Copovidone | Insomnia |
| Onmel ® | Merz | Amorphous Dispersion | Itraconazole | HPMC | Onychomycosis |
| Noxafil® | Merck | Amorphous dispersion | Posaconazole | HPMCAS/PVA | Antifungal |
| Fenoglide® | Life cycle Pharma | Solid dispersion | Fenofibrate | PEG 6000 | Dyslipidaemia |
| Cesamet ® | Meda Pharmaceuticals | Solid dispersion | Nabilone | PVP | Antiemetic |
| Rezulin ® | Parke-Davis | Amorphous dispersion | Troglitazone | PVP | Diabetes |
| Gris-PEG® | Penidol Ph. | Crystalline dispersion | Griseofulvin | PEG | Onychomycosis |
| Norvir® | Abbott | Amorphous Dispersion | Ritonavir | PEG-glyceride | HIV |
| Kaletra ® | Abbott | Amorphous Dispersion | Lopinavir/Ritonavir | PVP/PVA | HIV |
Figure 4.
Representation of HME technology studied for enhancement of solubility for poorly soluble drugs
Various reports on solubility enhancement through HME were discussed in recent reviews [3, 52, 63], including solid dispersions of tadalafil [67], co-amorphous system of indomethacin/arginine [68], solid crystalline suspensions of efavirenz [69], solid solutions of artesunate [70], and ternary inclusion complexes of itraconazole/cyclodextrin [71]. In this section of this review, we present new reports on HME applications for drug solubility enhancement for the readers’ interest.
Recently, Kelleher et al. [72] investigated the effect of process parameters for developing solubility-enhanced, fixed-dose combination of ramipril (melting point (M.P) 117°C) and hydrochlorothiazide (M.P 272°C) by spray-drying and HME techniques. The hydrophilic carriers Kollidon® VA 64 and Soluplus® were employed, and the effect of the plasticizer polyethylene glycol (PEG) 3350 on the extrusion process was evaluated. Considering ramipril degradation, the maximum temperature used for extrusion was 140°C, which was above the glass transition temperature (Tg) of the two APIs and Kollidon® VA 64 or Soluplus®. The formulations with Kollidon® VA 64 showed a significant improvement in drug dissolution, whereas Soluplus®-based formulations showed relatively low improvement in drug release. This might be attributed to the chemical composition of Soluplus®, which rearranges itself into multi-chain micelle assembly at increased concentrations [73].
McFall et al. [74] reported pH-modulated solid dispersions of poorly water-soluble aripiprazole (137–142°C). The formulations were prepared at 20–40% of drug load and 60–80% of hydrophilic polymer (Kollidon® 12PF) and at 0–10% concentration of succinic acid. The process was carried out at 120°C, which was the minimum possible temperature for extrusion. Further, the extrudates were milled, blended with excipients, and compressed into tablets. There was significant improvement in dissolution rate because of the micro-environment provided by succinic acid, which helps in the dissolution of weakly basic drugs such as aripiprazole. In vivo studies showed the same trend to that in in vitro release studies. The applicability of HME with pH modifiers was revealed as a suitable approach for APIs with pH-dependent solubility.
Ong et al. [75] used a co-processing/formulation approach to improve the solubility of poorly water-soluble drugs. Their study utilized surface-modified dendrimer nanoparticles (phytoglucan whose surface was modified by octinyl succinate) as solubility enhancers that modify micro-environment by forming locked aliphatic chains and aiding the formation of amorphous solid dispersions. Drugs with different hydrophobicities, including ibuprofen, phenytoin, griseofulvin, and loratadine, were selected for the study. Based on the crystal properties (log X ideal) and hydrophobicity (-log γ) calculated in the preliminary studies, the order of hydrophobicity was found to be phenytoin > griseofulvin> ibuprofen > loratadine. Results of XRD studies revealed that the crystallinity values of the formulations after extrusion were in accordance with the original crystallinity of the respective APIs. Results of drug release studies revealed that dissolution rate was markedly improved for all APIs owing to the wetting effect of the carrier, except for phenytoin. The reason might be mismatched polarity between phenytoin and the C8 chains of the carrier, as well as the inability of the atmosphere of the carrier molecule to accommodate a drug with relatively low hydrophobicity, such as phenytoin.
Huang et al. [76] prepared an amorphous solid dispersion (ASD) of gliclazide (M.P 166°C) and investigated the effect of processing conditions on the formation of amorphous compounds and degradation potential of the process while the drug converts to the highly energetic amorphous form. During preformulation studies, Affinisol™ HPMC 100LV was found to be more miscible with the API than Soluplus®, and a drug load of 10% was found feasible for extrusion. This study confirmed that processing conditions such as shear and processing temperature affect drug degradation, as well as drug conversion to the amorphous form. The minimum energy (mechanical and thermal) required to attain complete, amorphous, single-phase solid dispersions without degrading the product is an essential factor in developing ASDs for drugs with high M.Ps.
Hanada et al. [77] investigated the effect of process parameters on the ternary ASD of IND/mesoporous silica/HPMC. The results showed that the trials with 2 and 3 kneading zones exhibited a dark yellow/brown color due to the high shear caused by the process, whereas the trials with 0 and 1 kneading zone produced products not completely converted to the amorphous form. As shown through dissolution studies, the ASDs obtained by using 0 and 1 kneading zones showed rapid dissolution for 2 h, followed by precipitation. However, the ASDs prepared by 2 and 3 kneading zones showed rapid dissolution and demonstrated a parachute effect with high drug concentration for up to 24 h. These findings indicate the relationship between specific mechanical energy of the process and product performance. Results of NMR studies also showed that higher specific mechanical energy results in higher drug/polymer miscibility, a greater parachute effect, and reduced recrystallization upon exposure to higher temperature/humidity conditions. These studies further confirm the use of HME for drug solubility enhancement.
4. HME and 3D Printing
Recently, 3D printing has emerged as a novel technology for pharmaceutical and biomedical applications. It is a process where materials are deposited layer-by-layer using a computer design to produce a desired 3D product. The first 3D-printed pharmaceutical product, Spritam® (levetiracetam; Aprecia Pharmaceuticals), is a rapidly disintegrating tablet used to treat partial onset seizures, and its approval by the FDA in 2015 initiated an increase in interest in the development of 3D-printed pharmaceutical products [78, 79]. In recent years, various researchers have proposed and developed 3D-printed drug delivery systems of various drugs. The various 3D printing techniques reported in literature are fused deposition modeling (FDM), binder deposition, material jetting, inkjet printing, photopolymerization, powder bed fusion, and pen-based 3D printing [56, 80, 81, 82, 83]. For pharmaceutical applications, among the various 3D printing technologies available, FDM is a prominent technology that can be coupled with HME to provide patient-centric dosage forms. In this part of the review, we highlight applications of extrusion-based FDM 3D printing technology for the development of personalized drug delivery systems.
4.1. HME coupled with FDM 3D printing
FDM is a typical extrusion process in which a thermoplastic polymer filament is extruded through a nozzle, where it is heated and then deposited layer-by-layer as designed [84]. This FDM technology requires filaments for printing pharmaceutical products. The polymer filaments used in FDM should have suitable thermal and mechanical properties because these filaments will experience high temperature and compressive forces during the printing process, which may hinder the printing of thermolabile drugs. However, most of the currently available filaments on the market do not have appropriate characteristics to be used directly for FDM 3D printing. The pharmaceutical-grade polymers that can be useful for FDM 3D printing are polyvinyl alcohol, polyvinyl pyrrolidine, and polylactic acid [15]. This limited availability of polymers restricts the use of FDM 3D printing in the development of pharmaceutical products. However, HME is an established technology for extrusion of polymers to produce filaments of desired thickness based on die diameter. Consequently, there is an increased interest in the development of filaments of various polymers through HME followed by FDM 3D printing.
4.1.1. Applications of HME coupled with FDM 3D printing
Tan et al. [85] comprehensively discussed pharmaceutical applications of HME coupled with FDM 3D printing for personalized drug delivery. This combination of HME and FDM 3D printing is utilized and reported in the development of various 3D-printed tablets with different drug-release characteristics. Likewise, controlled-release tablets of acetaminophen with HPMC E5 [86, 87], budesonide with polyvinyl alcohol [88], and glipizide with polyvinyl alcohol [89]; modified-release tablets using hydroxypropyl methylcellulose acetyl succinate [90]; sustained-release tablets of theophylline and metformin hydrochloride using thermoplastic polyurethanes [91]; immediate-release, 3D-printed tablets of pantoprazole sodium with five different pharmaceutical-grade polymers (polyvinylpyrrolidone K12, PEG 6000, 20000, Kollidon® VA 64, and poloxamer 407) [92]; and intragastric floating system of domperidone [93] are discussed elsewhere in the literature. The following are several literature reports that showed extensive research on HME-based FDM 3D printing for the development of various types of drug delivery systems.
Solanki et al. [94] screened polymers, studied drug polymer miscibility and printability, as well as formulated 3D-printed, rapid-release tablets of haloperidol by FDM. The various polymers studied were Kollidon® VA 64, Kollicoat® IR, Affinsiol™15 cP, and hypromellose acetate succinate (HPMCAS) either alone or as binary blends (Kollidon® VA 64 and Affinisol™ 15 cP, 1:1; Kollidon® VA64 and HPMCAS, 1:1). Finally, HME-extruded filaments of the Kollidon® VA 64-Affinisol™ 15 cP mixture were identified as a suitable polymer system that is flexible and possesses the desired mechanical 3D printing properties and rapid drug-release characteristics. All of the polymers or blends of polymers evaluated were commonly employed pharmaceutical-grade materials, which confirms the feasibility of FDM 3D printing technology for the development of various pharmaceutical dosage forms.
Recently, Okwuosa et al. [95] for the first time developed a method to produce liquid capsules with the potential of drug dose and release modifications. This study coordinated the use of HME to produce filaments with the use of modified dual FDM 3D printing and liquid dispensing to manufacture personalized dosage forms on demand in a fully automated fashion. The polymethacrylate polymers Eudragit® EPO (immediate release) and Eudragit® RL (controlled release) were extruded to produce filaments by HME, and the shells for optimizing drug release using FDM 3D printing were simultaneously filled using a computer-controlled liquid dispenser loaded with the model drug suspension (dipyridamole) or solution (theophylline). The 1.6 mm thickness and concentric architecture of the shells allowed successful containment of liquid core while maintaining the release properties of the 3D-printed liquid capsule. The modification of shell thickness modified the release characteristics without changing the formulation. This approach also overcomes the compatibility of formulation ingredients to the high temperature of FDM 3D printing. Thus, owing to its low cost and versatility, this approach can be adapted to a wide spectrum of small- or large-molecule liquid formulations. Kollamaram et al. [96] reported low temperature FDM 3D printing at 70–90 °C using ramipril and 4-aminosalicylic acid as thermolabile drugs as well as Kollidon® VA 64 and Kollidon® 12PF as potential polymers. Therefore, this study showed that selection and use of appropriate excipients can overcome a major limitation of drug degradation due to thermal heating by FDM, thus making this technology suitable for drugs with low melting points.
This HME coupled FDM 3D printing is combined with nanotechnology to produce novel dosage forms. Beck et al. developed 3D-printed tablets loaded with polymeric nanocapsules, combining HME and FDM 3D printing with nanotechnology. Initially, 3D-printed tablets were produced using FDM 3D printing of poly(ε-caprolactone) and Eudragit® RL100 filaments with or without a channeling agent (mannitol). These tablets were soaked in deflazacort-loaded nanocapsule dispersions for adsorption to obtain drug nanocapsule-loaded 3D-printed tablets. The drug release characteristics were dependent on the type of polymers in the tablets with or without a channeling agent (mannitol). This study again revealed the adaptability of HME coupled with FDM 3D printing and approaches to produce personalized novel drug delivery systems [97]. Scoutaris et al. developed 3D-printed Starmix®-loaded dosage forms for pediatric applications by using HME and FDM. They prepared filaments of IND, HPMCAS, and PEG, which were fed into a 3D printer to print ring-, heart-, bottle-, bear-, and lion-shaped tablets. The products were characterized using DSC, XRPD, FTIR, and Raman spectroscopy, and the results demonstrated a molecular dispersion of IND in the printed tablets, with excellent content uniformity and taste-masking efficiency, as well as immediate drug-release characteristics [98]. The use of PEG in the formulation in appropriate ratios provided the plasticizing effect of the filaments, which can be used for FDM printing. FDM of HME filaments was also utilized for developing orodispersible films. Jamroz et al. [99] reported 3D-printed orodispersible films of aripiprazole using polyvinyl alcohol. These formulations suggest the feasibility of HME coupled with FDM 3D printing as an option for the development of pediatric dosage forms.
Palekar et al. [100] developed a pediatric formulation (minicaplets) of baclofen by a QbD approach using HME-based FDM 3D printing. In this study, PVA with sorbitol as polymer was used to prepare baclofen-loaded filaments that were printed into minicaplets in different sizes, with four different infill patterns and three different infill percentages. Drug release was marginally affected by infill percentage but significantly affected by caplet dimension. This study yet again proved that low-cost FDM 3D printing can be a promising alternative for the development of customized pediatric formulations.
Arafat et al. [101] developed a 3D-printed oral dosage form of tailored, on-demand, anticoagulant (warfarin) dosing as a strategy to improve anticoagulation therapy. For effective anticoagulation treatment, the target International Normalized Ratio of the patients needs to be monitored and maintained in the normal range. To achieve this, the dose of an anticoagulant drug needs to be altered accordingly before being administered to patients. In this study, sodium warfarin-loaded filaments were produced by HME, and the filaments were further fabricated via FDM 3D printing to obtain capsular-ovoid-shaped immediate-release dosage forms with varying doses. In vivo studies of the obtained dosage forms in Sprague-Dawley rats showed proportionate results compared to the drug solution. These studies further revealed the applicability of HME coupled with 3D printing in the development of personalized drug delivery.
Furthermore, HME coupled with FDM 3D printing has been utilized for the development of drug-loaded implants. Kempin et al. produced quinine-loaded filaments using various polymers, such as Eudragit® RS, polycaprolactone, poly(L-lactide), and ethyl cellulose. These filaments were used in FDM printing to produce drug-loaded, hollow, cylinder-shaped implants. Drug release from these implants varied over 51 to 100 days, suggesting that this technology can be useful to develop implants in any desired shape for a desired purpose over a period [102].
Genina et al. [103] utilized ethylene vinyl acetate (EVA) to produce IND-loaded EVA filaments using HME. The drug-loaded EVA filaments were employed in FDM 3D printing to produce custom-made, T-shaped intrauterine devices and subcutaneous rods. Similarly, Fu et al. developed personalized progesterone-loaded vaginal rings using FDM 3D printing. The progesterone-loaded filaments were fed into FDM 3D printers to print vaginal rings in different shapes, namely “O”, “Y”, or “M”. These vaginal rings showed long-term sustained-release of progesterone for more than a week [104]. This HME-based FDM 3D printing was also utilized as a platform for miscellaneous applications, such as development of polypills (multiple drugs in one dosage form) as bilayer dosage forms [105], duocaplets, multilayer tablets [106], and two compartment capsule dosage forms [107]. This two-compartment capsule form can be used for pulsatile release of one drug or two compartments filled with two different drugs as polypill. The printing of these two compartments can be tailored with different polymer materials for free solubility or gastric resistant.
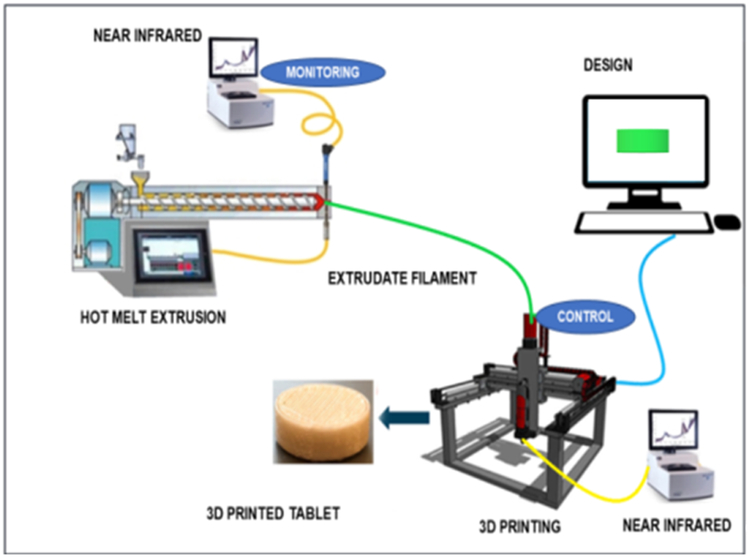
The abovementioned studies established HME coupled with FDM 3D printing as a versatile approach for the development of various drug delivery systems. Thus, the combined advantages of HME and FDM 3D printing prompted the exploration of HME coupled with FDM 3D printing as a CM process for the development of personalized drug delivery systems. In the new era of pharmaceutical product development, there is an increased interest in the use of CM to manufacture pharmaceutical products. These two advanced manufacturing processes, HME and FDM 3D printing, offer an advanced manufacturing approach to achieve CM. This continuous HME coupled with FDM 3D printing, along with PAT, will be a novel application for the CM and designing of optimized personalized drug delivery products in the future (Figure 5). The availability of Fourier-transform NIR spectroscopy and Raman spectroscopy as PAT in conjunction with multivariate analysis techniques and PLS provides real-time monitoring of the process and product [19]. The combination of these two technologies as a CM process offers advantages of producing efficient and cost-effective pharmaceutical products.
Figure 5.
Schematic illustration of hot melt extrusion coupled with FDM 3D printing for continuous manufacturing.
5. Conclusion
HME has become an essential technology in the pharmaceutical industry because of its numerous advantages and applicability for CM. This solvent-free, environmentally friendly technology is utilized in the development of various pharmaceutical drug delivery systems. Further, this technology is being explored as a continuous process in various novel strategies, specially fused deposition modelling 3D printing. As discussed in Part I of this review, process analytical tools may be successfully applied along with appropriate Design of Experiments for this technology to understand in-line processing and establish CM processes. Nevertheless, the major challenges encountered such as thermal and chemical degradation can be overcome by investigating and optimizing the various physicochemical properties, such as miscibility and interaction of drug polymer systems, rheological properties, physical states, and ultimate stability of hot melt-extruded products.
6. Expert opinion
Increased patents and publications on HME, availability of commercial products, and SWOT analysis are indicative of the extensive applicability of HME in the pharmaceutical industry as a platform technology. HME is employed for development of various pharmaceutical drug delivery systems, such as granules, tablets, oral disintegrating formulations, films, and implants. However, increased interest in the use of CM processes has marked HME as an effective strategy, which is supported by different PAT tools, such as NIR spectroscopy, Raman spectroscopy, and rheometry. PAT facilitates real-time analysis in CM and it potentially improves safety, reduces batch losses, and meets the specifications to produce products with critical attributes of quality medicines. However, extensive research is imperative to establish HME as a viable CM process.
HME for improvement of solubility and dissolution rate of poorly water-soluble drugs with suitable polymers is very well-established, and this trend is evidenced by the availability of commercial products on the market. Recently, approval of the first 3D-printed product by USFDA resulted in increased interest in the development of 3D-printed pharmaceutical products for personalized drug delivery. Especially, the suitability of FDM with HME processes for various 3D-printed dosage forms is evidenced by the literature. FDM and HME technologies possess a unique opportunity to be coupled for CM of patient-centric dosage forms, including pediatric and geriatric products. As presented in Figure 1, the use of HME is predominantly devoted to solid dispersions. Remarkedly, however, in a relatively short period of time, 3D printing has exploded for the development of dosage forms, including patient-centric medicines. Thus, it seems evident that HME coupled with 3D printing provides a paradigm shift in pharmaceutical manufacturing.
The limitations of HME, namely potential high processing temperatures and high energy input, may affect the stability of hot melt-extruded products. Studies on the physicochemical properties, nature of the extrudate products, and use of appropriate additives may overcome these issues to a certain extent. Effective strategies and experimental designs are also necessary to manage product quality matters. An alternative to the HME process may be KinetiSol® technology; however, its shortcomings, such as scalability, need to be further investigated.
In summary, the application of HME technology in the pharmaceutical industry has expanded its previous focus on the development of pharmaceutical products with improved therapeutic efficacy. The United States FDA has been supportive of this versatile technology. However, industries are need of regulatory guidance to ensure adequate quality control and continuous manufacturing of HME products.
Article highlights.
This review emphasizes a SWOT analysis and provides updates on solubility enhancement and hot-melt extrusion (HME) based 3D printing in formulation of different dosages
HME processing is supported with different PAT tools and can be established as a continuous manufacturing process
Melt extrusion is a solvent free, cost-effective technology for various conventional and novel formulations
HME is an adaptable technology, which can be coupled with Fused Deposition Modelling (FDM) 3D printing for various dosage forms
The high processing temperatures and high energy input in HME need to be assessed for HME manufacturing.
Acknowledgments
Funding
This paper was supported by Grant Number P20GM104932 from the National Institute of General Medical Sciences and the Biopharmaceutics-Clinical and Translational Core E of the COBRE, a component of the National Institutes of Health.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
The articles on HME and its applications have been highlighted as either “important” (*) or “extremely important” (**) for benefit of readers.
- 1.Censi R, Gigliobianco M, Casadidio C, et al. Hot Melt Extrusion: Highlighting Physicochemical Factors to Be Investigated While Designing and Optimizing a Hot Melt Extrusion Process. Pharmaceutics. 2018;10(3):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crowley MM, Zhang F, Repka MA, et al. Pharmaceutical applications of hot-melt extrusion: part I. Drug development and industrial pharmacy. 2007;33(9):909–926. [DOI] [PubMed] [Google Scholar]
- 3.Repka MA, Bandari S, Kallakunta VR, et al. Melt extrusion with poorly soluble drugs–An integrated review. International journal of pharmaceutics. 2018;535(1–2):68–85.**A detailed review on solubility, scale up and characterization of HME dosage forms.
- 4.Tiwari RV, Patil H, Repka MA. Contribution of hot-melt extrusion technology to advance drug delivery in the 21st century. Expert opinion on drug delivery. 2016;13(3):451–464.**An Interesting review on applications of HME in the field of pharmaceutics.
- 5.Fule R, Meer T, Amin P, et al. Preparation and characterisation of lornoxicam solid dispersion systems using hot melt extrusion technique. Journal of Pharmaceutical Investigation. 2014;44(1):41–59. [Google Scholar]
- 6.Kate L, Gokarna V, Borhade V, et al. Bioavailability enhancement of atovaquone using hot melt extrusion technology. European Journal of Pharmaceutical Sciences. 2016;86:103–114. [DOI] [PubMed] [Google Scholar]
- 7.Maddineni S, Battu SK, Morott J, et al. Influence of process and formulation parameters on dissolution and stability characteristics of Kollidon® VA 64 Hot-melt extrudates. AAPS PharmSciTech. 2015;16(2):444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douroumis D, Ross SA, Nokhodchi A. Advanced methodologies for cocrystal synthesis. Advanced drug delivery reviews. 2017;117:178–195. [DOI] [PubMed] [Google Scholar]
- 9.Vynckier AK, Voorspoels J, Remon JP, et al. Co‐extrusion as a processing technique to manufacture a dual sustained release fixed‐dose combination product. Journal of Pharmacy and Pharmacology. 2016;68(5):721–727. [DOI] [PubMed] [Google Scholar]
- 10.Dumpa NR, Sarabu S, Bandari S, et al. Chronotherapeutic Drug Delivery of Ketoprofen and Ibuprofen for Improved Treatment of Early Morning Stiffness in Arthritis Using Hot-Melt Extrusion Technology. AAPS PharmSciTech. 2018;19(6):2700–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva LAD, Almeida SL, Alonso EC, et al. Preparation of a solid self-microemulsifying drug delivery system by hot-melt extrusion. International journal of pharmaceutics. 2018;541(1–2):1–10. [DOI] [PubMed] [Google Scholar]
- 12.Kallakunta VR, Tiwari R, Sarabu S, et al. Effect of formulation and process variables on lipid based sustained release tablets via continuous twin screw granulation: A comparative study. European Journal of Pharmaceutical Sciences. 2018;121:126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhagurkar AM, Angamuthu M, Patil H, et al. Development of an ointment formulation using hot-melt extrusion technology. AAPS PharmSciTech. 2016;17(1):158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wening K, Schwier S, Hans-J Stahlberg M, et al. Application of hot-melt extrusion technology in immediate-release abuse-deterrent formulations. Journal of opioid management. 2017;13(6):473–484. [DOI] [PubMed] [Google Scholar]
- 15.Melocchi A, Parietti F, Maroni A, et al. Hot-melt extruded filaments based on pharmaceutical grade polymers for 3D printing by fused deposition modeling. International journal of pharmaceutics. 2016;509(1–2):255–263. [DOI] [PubMed] [Google Scholar]
- 16.Nasr M, Karandikar H, Abdel-Aziz RT, et al. Novel nicotinamide skin-adhesive hot melt extrudates for treatment of acne. Expert opinion on drug delivery. 2018;15(12):1165–1173. [DOI] [PubMed] [Google Scholar]
- 17.Chablani L, Taylor MK, Mehrotra A, et al. Inline real-time near-infrared granule moisture measurements of a continuous granulation–drying–milling process. Aaps PharmSciTech. 2011;12(4):1050–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markl D, Wahl PR, Menezes JC, et al. Supervisory control system for monitoring a pharmaceutical hot melt extrusion process. AAPS PharmSciTech. 2013;14(3):1034–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vo AQ, He H, Zhang J, et al. Application of FT-NIR Analysis for In-line and Real-Time Monitoring of Pharmaceutical Hot Melt Extrusion: a Technical Note. Aaps Pharmscitech. 2018;19(8):3425–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abboud L, Hensley S. Factory Shift: New Prescription For Drug Makers: Update the Plants; After Years of Neglect, Industry Focuses on Manufacturing; FDA Acts as a Catalyst; The Three-Story Blender. The Wall Street Journal. 2003:1-1. [Google Scholar]
- 21.Byrn S, Futran M, Thomas H, et al. Achieving continuous manufacturing for final dosage formation: challenges and how to meet them. May 20–21, 2014 continuous manufacturing symposium. Journal of pharmaceutical sciences. 2015;104(3):792–802. [DOI] [PubMed] [Google Scholar]
- 22.DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. Journal of health economics. 2003;22(2):151–185. [DOI] [PubMed] [Google Scholar]
- 23.Islam MT, Maniruzzaman M, Halsey SA, et al. Development of sustained-release formulations processed by hot-melt extrusion by using a quality-by-design approach. Drug delivery and translational research. 2014;4(4):377–387. [DOI] [PubMed] [Google Scholar]
- 24.Kossik J Think small: Pharmaceutical facility could boost capacity and slash costs by trading in certain batch operations for continuous versions. Pharmamag. com. Pharmamag com, article ID/DDAS-SEX B. 2002;52. [Google Scholar]
- 25.Lovett David, Tahir F. Hot melt extrusion technology for continuous manufacturing. European Pharmaceutical Review 2017. (6). [Google Scholar]
- 26.Hengsawas Surasarang S, Keen JM, Huang S, et al. Hot melt extrusion versus spray drying: hot melt extrusion degrades albendazole. Drug development and industrial pharmacy. 2017;43(5):797–811. [DOI] [PubMed] [Google Scholar]
- 27.Food, Administration D. Guidance for industry, PAT-A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance. http://www.fda.gov/cder/guidance/published.html. 2004.
- 28.Hitzer P, Bäuerle T, Drieschner T, et al. Process analytical techniques for hot-melt extrusion and their application to amorphous solid dispersions. Analytical and bioanalytical chemistry. 2017;409(18):4321–4333. [DOI] [PubMed] [Google Scholar]
- 29.Griff AL. Plastics extrusion technology. Krieger Pub Co; 1976. [Google Scholar]
- 30.Sauceau M, Fages J, Common A, et al. New challenges in polymer foaming: A review of extrusion processes assisted by supercritical carbon dioxide. Progress in Polymer Science. 2011;36(6):749–766. [Google Scholar]
- 31.Jaiswar DR, Jha D, Amin PD. Preparation and characterizations of stable amorphous solid solution of azithromycin by hot melt extrusion. Journal of Pharmaceutical Investigation. 2016;46(7):655–668. [Google Scholar]
- 32.Lee J-Y, Kang W-S, Piao J, et al. Soluplus®/TPGS-based solid dispersions prepared by hot-melt extrusion equipped with twin-screw systems for enhancing oral bioavailability of valsartan. Drug design, development and therapy. 2015;9:2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Jaeghere W, De Beer T, Van Bocxlaer J, et al. Hot-melt extrusion of polyvinyl alcohol for oral immediate release applications. International journal of pharmaceutics. 2015;492(1–2):1–9. [DOI] [PubMed] [Google Scholar]
- 34.Vo AQ, Feng X, Morott JT, et al. A novel floating controlled release drug delivery system prepared by hot-melt extrusion. European Journal of Pharmaceutics and Biopharmaceutics. 2016;98:108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cossé A, König C, Lamprecht A, et al. Hot melt extrusion for sustained protein release: matrix erosion and in vitro release of PLGA-based implants. AAPS PharmSciTech. 2017;18(1):15–26. [DOI] [PubMed] [Google Scholar]
- 36.Esra’aAlbarahmieh, Qi S, Craig DQ. Hot melt extruded transdermal films based on amorphous solid dispersions in Eudragit RS PO: The inclusion of hydrophilic additives to develop moisture-activated release systems. International journal of pharmaceutics. 2016;514(1):270–281. [DOI] [PubMed] [Google Scholar]
- 37.Melocchi A, Loreti G, Del Curto MD, et al. Evaluation of Hot‐Melt Extrusion and Injection Molding for Continuous Manufacturing of Immediate‐Release Tablets. Journal of pharmaceutical sciences. 2015;104(6):1971–1980. [DOI] [PubMed] [Google Scholar]
- 38.Pimparade MB, Vo A, Maurya AS, et al. Development and evaluation of an oral fast disintegrating anti-allergic film using hot-melt extrusion technology. European Journal of Pharmaceutics and Biopharmaceutics. 2017;119:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salmoria G, Sibilia F, Henschel V, et al. Structure and properties of polycaprolactone/ibuprofen rods prepared by melt extrusion for implantable drug delivery. Polymer Bulletin. 2017;74(12):4973–4987. [Google Scholar]
- 40.Vo AQ, Feng X, Pimparade M, et al. Dual-mechanism gastroretentive drug delivery system loaded with an amorphous solid dispersion prepared by hot-melt extrusion. European Journal of Pharmaceutical Sciences. 2017;102:71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta SS, Meena A, Parikh T, et al. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion-I: Polyvinylpyrrolidone and related polymers. Journal of Excipients and Food Chemicals. 2016;5(1):1001. [Google Scholar]
- 42.Li Y, Pang H, Guo Z, et al. Interactions between drugs and polymers influencing hot melt extrusion. Journal of Pharmacy and Pharmacology. 2014;66(2):148–166. [DOI] [PubMed] [Google Scholar]
- 43.Di Martino P, Magnoni F, Vargas Peregrina D, et al. Formation, physicochemical characterization, and thermodynamic stability of the amorphous state of drugs and excipients. Current pharmaceutical design. 2016;22(32):4959–4974. [DOI] [PubMed] [Google Scholar]
- 44.De Beer T, Burggraeve A, Fonteyne M, et al. Near infrared and Raman spectroscopy for the in-process monitoring of pharmaceutical production processes. International journal of pharmaceutics. 2011;417(1–2):32–47. [DOI] [PubMed] [Google Scholar]
- 45.Saerens L, Vervaet C, Remon JP, et al. Process monitoring and visualization solutions for hot‐melt extrusion: a review. Journal of Pharmacy and Pharmacology. 2014;66(2):180–203. [DOI] [PubMed] [Google Scholar]
- 46.Food, Administration D. Guidance for industry PAT—a framework for innovative pharmaceutical development, manufacturing, and quality assurance, September 2004. (http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070305.pdf). Accessed; 2011.
- 47.Gilmor C, Balke S, Calidonio F, et al. In‐line color monitoring of polymers during extrusion using a charge coupled device spectrometer: Color changeovers and residence time distributions. Polymer Engineering & Science. 2003;43(2):356–368. [Google Scholar]
- 48.Valadez-Blanco R, Virdi A, Balke S, et al. In-line colour monitoring during food extrusion: Sensitivity and correlation with product colour. Food research international. 2007;40(9):1129–1139. [Google Scholar]
- 49.Wesholowski J, Prill S, Berghaus A, et al. Inline UV/Vis spectroscopy as PAT tool for hot-melt extrusion. Drug delivery and translational research. 2018:8 (6):1595–1603. [DOI] [PubMed] [Google Scholar]
- 50.Becker W, Guschin V, Mikonsaari I, et al. Turbidimetric method for the determination of particle sizes in polypropylene/clay-composites during extrusion. Analytical and bioanalytical chemistry. 2017;409(3):741–751. [DOI] [PubMed] [Google Scholar]
- 51.Islam MT, Scoutaris N, Maniruzzaman M, et al. Implementation of transmission NIR as a PAT tool for monitoring drug transformation during HME processing. European Journal of Pharmaceutics and Biopharmaceutics. 2015;96:106–116. [DOI] [PubMed] [Google Scholar]
- 52.Maniruzzaman M, Rana M, Boateng J, et al. Dissolution enhancement of poorly water-soluble APIs processed by hot-melt extrusion using hydrophilic polymers. Drug development and industrial pharmacy. 2013;39(2):218–227. [DOI] [PubMed] [Google Scholar]
- 53.Krier F, Mantanus J, Sacré P-Y, et al. PAT tools for the control of co-extrusion implants manufacturing process. International journal of pharmaceutics. 2013;458(1):15–24. [DOI] [PubMed] [Google Scholar]
- 54.De Beer T, Bodson C, Dejaegher B, et al. Raman spectroscopy as a process analytical technology (PAT) tool for the in-line monitoring and understanding of a powder blending process. Journal of pharmaceutical and biomedical analysis. 2008;48(3):772–779. [DOI] [PubMed] [Google Scholar]
- 55.Nagy B, Farkas A, Gyürkés M, et al. In-line Raman spectroscopic monitoring and feedback control of a continuous twin-screw pharmaceutical powder blending and tableting process. International journal of pharmaceutics. 2017;530(1–2):21–29. [DOI] [PubMed] [Google Scholar]
- 56.Ibrahim M, Zhang J, Repka M, et al. Characterization of the Solid Physical State of API and Its Distribution in Pharmaceutical Hot Melt Extrudates Using Terahertz Raman Imaging. AAPS PharmSciTech. 2019;20(2):62. [DOI] [PubMed] [Google Scholar]
- 57.Harting J, Kleinebudde P. Development of an in-line Raman spectroscopic method for continuous API quantification during twin-screw wet granulation. European Journal of Pharmaceutics and Biopharmaceutics. 2018;125:169–181. [DOI] [PubMed] [Google Scholar]
- 58.Netchacovitch L, Thiry J, De Bleye C, et al. Global approach for the validation of an in-line Raman spectroscopic method to determine the API content in real-time during a hot-melt extrusion process. Talanta. 2017;171:45–52. [DOI] [PubMed] [Google Scholar]
- 59.Cogswell FN. Polymer melt rheology: a guide for industrial practice. Cambridge, England: Woodhead Publishing Limited; 1981. [Google Scholar]
- 60.Kelly AL, Gough T, Isreb M, et al. In-process rheometry as a PAT tool for hot melt extrusion. Drug development and industrial pharmacy. 2018;44(4):670–676. [DOI] [PubMed] [Google Scholar]
- 61.Monteyne T, Heeze L, Oldörp K, et al. Vibrational spectroscopy to support the link between rheology and continuous twin-screw melt granulation on molecular level: a case study. European Journal of Pharmaceutics and Biopharmaceutics. 2016;103:127–135. [DOI] [PubMed] [Google Scholar]
- 62.Jermain SV, Brough C, Williams III RO. Amorphous solid dispersions and nanocrystal technologies for poorly water-soluble drug delivery–An update. International journal of pharmaceutics. 2018;535(1–2):379–392. [DOI] [PubMed] [Google Scholar]
- 63.Maniruzzaman M, Nokhodchi A. Continuous manufacturing via hot-melt extrusion and scale up: regulatory matters. Drug discovery today. 2017;22(2):340–351. [DOI] [PubMed] [Google Scholar]
- 64.Stanković M, Frijlink HW, Hinrichs WL. Polymeric formulations for drug release prepared by hot melt extrusion: application and characterization. Drug Discovery Today. 2015;20(7):812–823.*An interesting article on polymers for hot melt extrusion
- 65.Fousteris E, Tarantili PA, Karavas E, et al. Poly (vinyl pyrrolidone)–poloxamer-188 solid dispersions prepared by hot melt extrusion. Journal of thermal analysis and calorimetry. 2013;113(3):1037–1047. [Google Scholar]
- 66.Terife G, Wang P, Faridi N, et al. Hot melt mixing and foaming of soluplus® and indomethacin. Polymer Engineering & Science. 2012;52(8):1629–1639. [Google Scholar]
- 67.Krupa A, Cantin O, Strach B, et al. In vitro and in vivo behavior of ground tadalafil hot-melt extrudates: how the carrier material can effectively assure rapid or controlled drug release. International journal of pharmaceutics. 2017;528(1–2):498–510. [DOI] [PubMed] [Google Scholar]
- 68.Lenz E, Löbmann K, Rades T, et al. Hot melt extrusion and spray drying of co-amorphous indomethacin-arginine with polymers. Journal of pharmaceutical sciences. 2017;106(1):302–312. [DOI] [PubMed] [Google Scholar]
- 69.Pawar JN, Fule RA, Maniruzzaman M, et al. Solid crystal suspension of Efavirenz using hot melt extrusion: Exploring the role of crystalline polyols in improving solubility and dissolution rate. Materials Science and Engineering: C. 2017;78:1023–1034. [DOI] [PubMed] [Google Scholar]
- 70.Fule R, Paithankar V, Amin P. Hot melt extrusion based solid solution approach: exploring polymer comparison, physicochemical characterization and in-vivo evaluation. International journal of pharmaceutics. 2016;499(1–2):280–294. [DOI] [PubMed] [Google Scholar]
- 71.Thiry J, Krier F, Ratwatte S, et al. Hot-melt extrusion as a continuous manufacturing process to form ternary cyclodextrin inclusion complexes. European Journal of Pharmaceutical Sciences. 2017;96:590–597. [DOI] [PubMed] [Google Scholar]
- 72.Kelleher J, Gilvary G, Madi A, et al. A comparative study between hot-melt extrusion and spray-drying for the manufacture of anti-hypertension compatible monolithic fixed-dose combination products. International journal of pharmaceutics. 2018;545(1–2):183–196. [DOI] [PubMed] [Google Scholar]
- 73.Cavallari C, Fini A, Ternullo S, et al. Release problems for nifedipine in the presence of Soluplus. Journal of Pharmacy and Pharmaceutics. 2016;3(2):70–82. [Google Scholar]
- 74.McFall H, Sarabu S, Shankar V, et al. Formulation of aripiprazole-loaded pH-modulated solid dispersions via hot-melt extrusion technology: In vitro and in vivo studies. International journal of pharmaceutics. 2019;554:302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ong HJ, Pinal R. Drug Solubilization by Means of a Surface-Modified Edible Biopolymer Enabled by Hot Melt Extrusion. Journal of pharmaceutical sciences. 2018;107(1):402–411. [DOI] [PubMed] [Google Scholar]
- 76.Huang S, O'Donnell KP, de Vaux SMD, et al. Processing thermally labile drugs by hot-melt extrusion: The lesson with gliclazide. European Journal of Pharmaceutics and Biopharmaceutics. 2017;119:56–67.*This is an important article on HME processing
- 77.Hanada M, Jermain SV, Lu X, et al. Predicting physical stability of ternary amorphous solid dispersions using specific mechanical energy in a hot melt extrusion process. International journal of pharmaceutics. 2018;548(1):571–585.*It is an interesting study on stability issues in melt extrusion
- 78.Goole J, Amighi K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. International journal of pharmaceutics. 2016;499(1–2):376–394. [DOI] [PubMed] [Google Scholar]
- 79.Ursan ID, Chiu L, Pierce A. Three-dimensional drug printing: a structured review. Journal of the American Pharmacists Association. 2013;53(2):136–144. [DOI] [PubMed] [Google Scholar]
- 80.Daly R, Harrington TS, Martin GD, et al. Inkjet printing for pharmaceutics–a review of research and manufacturing. International journal of pharmaceutics. 2015;494(2):554–567. [DOI] [PubMed] [Google Scholar]
- 81.Khaled SA, Burley JC, Alexander MR, et al. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. International journal of pharmaceutics. 2014;461(1–2):105–111. [DOI] [PubMed] [Google Scholar]
- 82.Norman J, Madurawe RD, Moore CM, et al. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Advanced drug delivery reviews. 2017;108:39–50. [DOI] [PubMed] [Google Scholar]
- 83.Skowyra J, Pietrzak K, Alhnan MA. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. European Journal of Pharmaceutical Sciences. 2015;68:11–17. [DOI] [PubMed] [Google Scholar]
- 84.Zhang J, Vo AQ, Feng X, et al. Pharmaceutical additive manufacturing: a novel tool for complex and personalized drug delivery systems. AAPS PharmSciTech. 2018:1–15.** A detailed review Pharmaceutical additive manufacturing
- 85.Tan D, Maniruzzaman M, Nokhodchi A. Advanced pharmaceutical applications of Hot-Melt Extrusion coupled with Fused Deposition Modelling (FDM) 3D printing for personalised drug delivery. Pharmaceutics. 2018;10(4):203.* An interesting review on Hot-Melt Extrusion coupled with Fused Deposition Modelling (FDM) 3D printing
- 86.Zhang J, Feng X, Patil H, et al. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int J Pharm. 2017. March 15;519(1–2):186–197. [DOI] [PubMed] [Google Scholar]
- 87.Zhang J, Yang W, Vo AQ, et al. Hydroxypropyl methylcellulose-based controlled release dosage by melt extrusion and 3D printing: Structure and drug release correlation. Carbohydrate polymers. 2017. December 1;177:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goyanes A, Chang H, Sedough D, et al. Fabrication of controlled-release budesonide tablets via desktop (FDM) 3D printing. International journal of pharmaceutics. 2015;496(2):414–420. [DOI] [PubMed] [Google Scholar]
- 89.Li Q, Wen H, Jia D, et al. Preparation and investigation of controlled-release glipizide novel oral device with three-dimensional printing. International journal of pharmaceutics. 2017;525(1):5–11. [DOI] [PubMed] [Google Scholar]
- 90.Goyanes A, Fina F, Martorana A, et al. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. International journal of pharmaceutics. 2017;527(1–2):21–30. [DOI] [PubMed] [Google Scholar]
- 91.Verstraete G, Samaro A, Grymonpré W, et al. 3D printing of high drug loaded dosage forms using thermoplastic polyurethanes. International journal of pharmaceutics. 2018;536(1):318–325. [DOI] [PubMed] [Google Scholar]
- 92.Kempin W, Domsta V, Grathoff G, et al. Immediate Release 3D-Printed Tablets Produced Via Fused Deposition Modeling of a Thermo-Sensitive Drug. Pharmaceutical research. 2018;35(6):124. [DOI] [PubMed] [Google Scholar]
- 93.Chai X, Chai H, Wang X, et al. Fused deposition modeling (FDM) 3D printed tablets for intragastric floating delivery of domperidone. Scientific reports. 2017;7(1):2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Solanki NG, Tahsin M, Shah AV, et al. Formulation of 3D printed tablet for rapid drug release by fused deposition modeling: screening polymers for drug release, drug-polymer miscibility and printability. Journal of pharmaceutical sciences. 2018;107(1):390–401. [DOI] [PubMed] [Google Scholar]
- 95.Okwuosa TC, Soares C, Gollwitzer V, et al. On demand manufacturing of patient-specific liquid capsules via co-ordinated 3D printing and liquid dispensing. European Journal of Pharmaceutical Sciences. 2018;118:134–143. [DOI] [PubMed] [Google Scholar]
- 96.Kollamaram G, Croker DM, Walker GM, et al. Low temperature fused deposition modeling (FDM) 3D printing of thermolabile drugs. International journal of pharmaceutics. 2018;545(1–2):144–152. [DOI] [PubMed] [Google Scholar]
- 97.Beck R, Chaves P, Goyanes A, et al. 3D printed tablets loaded with polymeric nanocapsules: an innovative approach to produce customized drug delivery systems. International journal of pharmaceutics. 2017;528(1–2):268–279. [DOI] [PubMed] [Google Scholar]
- 98.Scoutaris N, Ross SA, Douroumis D. 3D printed “Starmix” drug loaded dosage forms for paediatric applications. Pharmaceutical research. 2018;35(2):34. [DOI] [PubMed] [Google Scholar]
- 99.Jamróz W, Kurek M, Łyszczarz E, et al. 3D printed orodispersible films with Aripiprazole. International journal of pharmaceutics. 2017;533(2):413–420. [DOI] [PubMed] [Google Scholar]
- 100.Palekar S, Nukala PK, Mishra SM, et al. Application of 3D printing technology and quality by design approach for development of age-appropriate pediatric formulation of baclofen. International journal of pharmaceutics. 2019;556:106–116. [DOI] [PubMed] [Google Scholar]
- 101.Arafat B, Qinna N, Cieszynska M, et al. Tailored on demand anti-coagulant dosing: An in vitro and in vivo evaluation of 3D printed purpose-designed oral dosage forms. European Journal of Pharmaceutics and Biopharmaceutics. 2018;128:282–289. [DOI] [PubMed] [Google Scholar]
- 102.Kempin W, Franz C, Koster LC, et al. Assessment of different polymers and drug loads for fused deposition modeling of drug loaded implants. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2017. June;115:84–93. [DOI] [PubMed] [Google Scholar]
- 103.Genina N, Hollander J, Jukarainen H, et al. Ethylene vinyl acetate (EVA) as a new drug carrier for 3D printed medical drug delivery devices. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2016. July 30;90:53–63. [DOI] [PubMed] [Google Scholar]
- 104.Fu J, Yu X, Jin Y. 3D printing of vaginal rings with personalized shapes for controlled release of progesterone. International journal of pharmaceutics. 2018;539(1–2):75–82. [DOI] [PubMed] [Google Scholar]
- 105.Gioumouxouzis CI, Baklavaridis A, Katsamenis OL, et al. A 3D printed bilayer oral solid dosage form combining metformin for prolonged and glimepiride for immediate drug delivery. European Journal of Pharmaceutical Sciences. 2018;120:40–52. [DOI] [PubMed] [Google Scholar]
- 106.Goyanes A, Wang J, Buanz A, et al. 3D Printing of Medicines: Engineering Novel Oral Devices with Unique Design and Drug Release Characteristics. Molecular pharmaceutics. 2015. November 2;12(11):4077–84. [DOI] [PubMed] [Google Scholar]
- 107.Maroni A, Melocchi A, Parietti F, et al. 3D printed multi-compartment capsular devices for two-pulse oral drug delivery. Journal of controlled release : official journal of the Controlled Release Society. 2017. December 28;268:10–18. [DOI] [PubMed] [Google Scholar]