Abstract
Both auditory evoked responses and metabolites measured by magnetic resonance spectroscopy (MRS) are altered in schizophrenia and other psychotic disorders, but the relationship between electrophysiological and metabolic changes are not well characterized. We examined the relation of MRS metabolites to cognitive and electrophysiological measures in individuals during the early phase of psychosis (EPP) and in healthy control subjects. The mismatch negativity (MMN) of the auditory event-related potential to duration deviant tones and the auditory steady response (ASSR) to 40 Hz stimulation were assessed. MRS was used to quantify glutamate+glutamine (Glx), N-Acetylasparate (NAA), creatine (Cre), myo-inositol (Ins) and choline (Cho) at a voxel placed medially in the frontal cortex. MMN amplitude and ASSR power did not differ between groups. The MRS metabolites Glx, Cre and Cho were elevated in the psychosis group. Partial least squares analysis in the patient group indicated that elevated levels of MRS metabolites were associated with reduced MMN amplitude and increased 40 Hz ASSR power. There were no correlations between the neurobiological measures and clinical measures. These data suggest that elevated neurometabolites early in psychosis are accompanied by altered auditory neurotransmission, possibly indicative of a neuroinflammatory or excitotoxic disturbance which disrupts a wide range of metabolic processes in the cortex.
Keywords: Mismatch negativity, auditory steady state response, magnetic resonance spectroscopy, psychosis, schizophrenia, event-related potentials
1. Introduction
1.1. Auditory evoked responses in schizophrenia
Individuals with schizophrenia (SZ) exhibit impaired electrophysiological responses to auditory stimuli, as robustly observed in mismatch negativity (MMN) amplitude and auditory steady state response (ASSR) power to 40 Hz stimulation. The MMN is elicited in response to deviant auditory stimuli interspersed among a series of more frequent standard stimuli. The MMN peaks at 150-250 ms post stimulus onset and is thought to index echoic memory processes, sound-discrimination accuracy and predictive coding (Kujala et al., 2007; Naatanen and Kahkonen, 2009; Todd et al., 2012; Wacongne et al., 2012; Winkler et al., 1996). Early conceptualizations of the MMN attribute change detection in sensory memory to temporal generators and attention shift to frontal generators (Deouell, 2007). A role of frontal cortex in the generation of the MMN has been supported by evidence from functional magnetic resonance imaging (fMRI), positron-emission tomography (PET), optical imaging, EEG source imaging, and lesion studies (Kim et al., 2017; Molnar et al., 1995; Randau et al., 2019; Rissling et al.,2014; Tse et al., 2006). MMN amplitude is usually reduced in patients with chronic schizophrenia, with a larger effect size for duration deviant compared to frequency deviant stimuli (Naatanen and Kahkonen, 2009; Umbricht and Krljes, 2005). MMN amplitude reduction has been less consistently found in first episode schizophrenia or psychosis, and there is some evidence that the MMN deficit may increase over the course of the illness (Erickson et al., 2016; Salisbury et al., 2017; Salisbury et al., 2002).
The ASSR is elicited by periodic auditory stimuli which rapidly entrain the electroencephalogram (EEG) to the frequency and phase of the stimulus with a maximal response at stimulus rates of about 40 Hz in humans. Like the MMN, the scalp recorded ASSR appears to be generated by auditory cortex with contributions from other regions, including the prefrontal cortex (Reyes et al., 2005; Reyes et al., 2004). The ASSR to 40 Hz stimulation is usually reduced in power or phase synchronization in patients with schizophrenia compared to healthy adults. Deficits have also been observed in first-episode and high-risk patients (Tada et al., 2016), and first-degree relatives (Hong et al., 2004; Rass et al., 2012). While both the MMN and ASSR implicate disturbances of auditory and frontal cortical circuits, the neural basis for these deficits are not well characterized. Both MMN and ASSR are sensitive to N-methyl-D-aspartate receptor (NMDAR) antagonists, consistent with models of NMDAR dysfunction in schizophrenia (Javitt et al., 2012; Kocsis et al., 2013; McCarley et al., 1999; Thune et al., 2016).
1.2. MRS metabolites in schizophrenia
Since scalp recorded electrophysiological responses are primarily generated by post-synaptic graded potentials, they would likely be sensitive to alterations in inter- and extracellular metabolites which reflect neural integrity and signaling. In vivo proton magnetic resonance spectroscopy (MRS) can quantify regional metabolites in the brain in persons with psychiatric disorders to better understand their underlying neurobiological mechanisms. MRS allows for the examination of neurochemical correlates of relevant ERPs and their relation to neurobiological models proposed in the etiology of SZ, such as NMDA receptor hypofunction, abnormal glutamatergic and dopaminergic transmission and neuroinflammation (Port and Agarwal, 2011). The majority of MRS studies have focused on chronic SZ, with more recent studies also examining individuals at clinical high risk for psychosis or first episode patients. A variety of metabolites within relevant brain regions and circuits have been assessed using MRS to examine the neurobiological mechanisms underlying SZ, with studies examining glutamatergic metabolites being the most prevalent (Merritt et al., 2016; Poels et al., 2014). MRS glutamate metabolites include glutamine (Gln), glutamate (Glu) and their sum, Glx. Glu is an amino acid and neurotransmitter that is synthesized from Gln within glutamatergic neurons, then synaptically released during neurotransmission. Glial cells recycle Glu from the extracellular space and convert it into Gln (Bak et al., 2006; Niciu et al., 2012).
The glutamatergic system has been implicated in the pathophysiology of SZ by pharmacological, animal model, post-mortem and imaging investigations, possibly secondary to NMDA receptor dysfunction (Hu et al., 2015; Javitt, 2010; Stone et al., 2007; Veerman et al., 2014). A meta-analysis examining studies of the glutamatergic metabolites concluded that persons with schizophrenia, or at high risk for the disorder, had elevated concentrations of Glu and Glx in the basal ganglia, Glx in the medial temporal lobe, and Gln in the thalamus compared to control subjects (Merritt et al., 2016). However, secondary analyses also showed differences across phases of the illness. Individuals at high-risk had higher Glx concentrations in the medial frontal cortex, individuals within their first episode showed increased Glx in the basal ganglia and individuals with chronic schizophrenia had elevated Glx levels within frontal white matter and the medial temporal lobe. Several subsequent MRS studies have evaluated Glu levels in the anterior cingulate cortex in first-episode of psychosis, but results have been inconsistent, reporting elevated (Kim et al., 2018), reduced (Reid et al., 2019), and intact levels of Glu (Egerton et al., 2018) compared to control subjects.
1.3. Relationship between ERPs and MRS metabolites in EPP
Evidence for a relationship between the MMN and ASSR with metabolites in psychosis remains sparse, particularly in the early stages of the illness. To our knowledge, there are no studies of the 40 Hz ASSR examining the relationship between glutamatergic metabolites and EPP. For MMN, studies have associated increased thalamic Glx with smaller (less negative) frontal MMN amplitude in individuals at high-risk for psychosis (Stone et al., 2010), and a trend for increased ACC Glu and smaller frontal MMN amplitude in EPP (Kaur et al., 2019). In contrast, smaller MMN amplitude has been associated with lower frontal and anterior cingulate cortex Glu levels in individuals with chronic schizophrenia (Rowland et al., 2016). Consequently, there is a pressing need to characterize the metabolic correlates of MMN amplitude and the 40 Hz ASSR in persons with EPP. Such relationships could shed light on the pathophysiological abnormalities associated with psychosis, such as NMDAR hypofunction and altered glutamatergic neurotransmission.
1.3. Aims of current study
The primary aim of the current study was to examine correlations between MMN amplitude and 40 Hz ASSR power with concentrations of frontal cortex Glx in persons with EPP. Secondarily, glutamatergic levels and MMN amplitude were compared between subjects with EPP and healthy adults. We predicted that compared to control subjects, a) MMN amplitude and ASSR power would be reduced in EPP compared to control subjects, b) Glx would be increased in EPP and c) increased Glx would be associated with smaller MMN amplitude and reduced ASSR power in the EPP group. We also examined associations between the electrophysiological measures and other metabolites that have been investigated in schizophrenia and related disorders (Brugger et al., 2011; Kraguljac et al., 2012; Wijtenburg et al., 2015), including N-acetyl aspartate (NAA), myo-inositol (Ins), creatine (Cre), and choline (Cho; composed of phosphocholine and glycerophosphocholine). Finally, exploratory analyses evaluated the relationship of both biological measures with clinical features and cognitive function.
2. Methods
2.1. Participants
Thirty-four participants with EPP (EPP) were recruited from the Prevention and Recovery Center for Early Psychosis (PARC), which is part of Indiana University School of Medicine (IUSM) and the Eskenazi Health System. Subjects had a DSM-IV diagnosis of schizophrenia, schizoaffective, schizophreniform or psychosis not otherwise specified, as determined by the Structured Clinical Interview for DSM-IV-TR (SCID-I/P Patient Edition) (First et al., 2002) and corroborated by family informants and medical records. Subjects were between 16 and 35 years of age and within five years of the first onset of a non-affective, non-substance use-induced psychosis. First onset was operationally defined as first emergence of psychotic symptoms coupled with evidence of seeking treatment. Cumulative antipsychotic drug dosages prior to study enrollment and during the trial were quantified as chlorpromazine equivalent doses (Woods, 2003). Exclusionary criteria included IQ less than 70, current substance use disorders, pregnancy, neurologic illness or other serious medical disorders or inability to provide informed consent. All 34 subjects received MRS assessment and 31 subjects received both MRS and electrophysiological assessment.
Nineteen control participants were recruited using advertisements in local community newspapers and flyers. The EPP and control groups did not differ in sex distribution (Pearson Chi-Square (1) = 0.034, p = 0.85). All control participants were interviewed using the SCID-NP (non-patient edition (First, 2002)) to exclude individuals with psychiatric diagnoses. Exclusionary criteria were the same as for the early psychosis group. All 19 subjects received MRS assessment and 16 subjects received both MRS and electrophysiological assessment. The research was approved by the IU Institutional Review Board and informed consent was obtained from all participants.
2.2. Electrophysiological assessment
Participants were seated comfortably in a dark, electrically isolated enclosure for electrophysiological assessment. For both paradigms, the electroencephalogram was continuously recorded (band pass 0.1–200 Hz, sampling rate 1000 Hz) and digitized (Neuroscan SynAmps) from the scalp, using a 28-channel electrode cap (10–20 system; Falk-Minow Services, Munich, Germany) and additional electrodes to obtain vertical and horizontal electrooculograms. Recordings were referenced to the nose. Electrode impedances were maintained at <10 kOhm. All auditory stimuli were presented through Etymotic insert earphones.
ASSRs.
During the evaluation, participants kept their eyes open while listening to trains of clicks. The individual stimuli were 1 ms duration clicks (80 dB SPL), presented in 500 ms duration 40 Hz click trains. Eighty trains were presented with a 700 ms inter-train interval. Fordata processing, EEG was digitally filtered with a bandpass of .02 to 100 Hz, and corrected for ocular artifacts using the Gratton et al. algorithm (Gratton et al, 1983). The EEG data was segmented into 500 ms epochs concurrent with the click train stimulus. Epochs with voltage exceeding ±100 μV at any site were automatically excluded from analyses. A fast Fourier Transform (FFT) was applied to the averaged ASSR to generate power spectra. The 40 Hz signal power (μV2) was measured at the FZ electrode site.
Duration Mismatch Negativity.
Participants sat with eyes open while gazing at a fixation cross and listening to a series of tones. The paradigm was composed of 765 auditory tones with a duration of 100 ms (“frequent” tones; probability=0.90) and 85 tones with a duration of 50 ms (“deviant” tones; probability=0.10). Tones were presented at 800 Hz, 70 dB SPL, and had a rise/fall of 10 ms. The inter-stimulus interval (ISI) was 500 ms. EEG data was stored offline for subsequent analysis. Using BrainVision Analyzer software (Brain Products, Munich, Germany), data were filtered using high pass (0.01Hz) and low pass (30Hz) filters. After VEOG correction, data were segmented into individual epochs with 100 ms baseline and 400 ms poststimulus duration. After baseline correction, individual epochs were rejected if they exceeded ±100 μV. Trials within the frequent and deviant conditions were averaged for each subject. Difference waveforms were computed by subtracting the averaged deviant tone waveform from the averaged frequent tone waveform. MMN amplitude was measured as the most negative voltage between 140 and 280 ms in the difference waveform at FZ for each participant, which is typically the location of the largest MMN deflection in nose-referenced EEG recordings (Javitt et al, 2000; Umbricht et al, 2006),
2.3. MRS Assessment
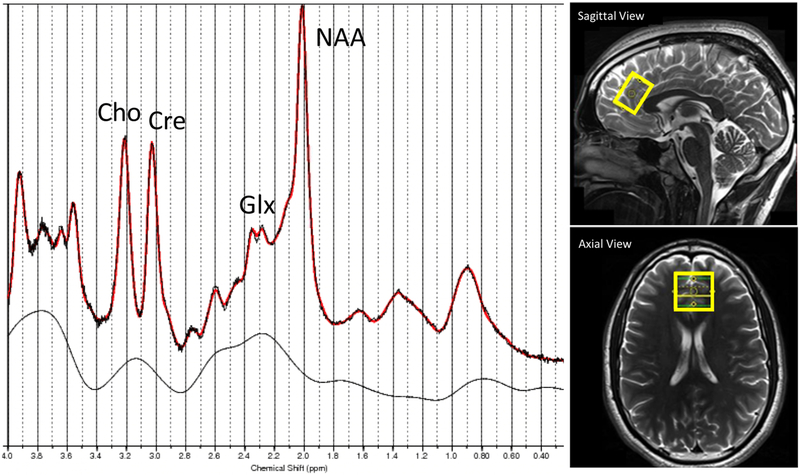
All MRI/MRS acquisitions were performed on a Siemens 3T TIM Trio whole-body scanner with a 32-channel head coil. T1-weighted MPRAGE images (resolution 1×1×1 mm3) were acquired for anatomical information. A single voxel PRESS sequence was used with the following parameters: echo time (TE) = 30ms, repetition time (TR) = 1500 ms, 128 averages, resulting in a total acquisition time of 4 min. In addition, for every scan a water-unsuppressed scan with 24 averages was acquired to serve as water reference for quantification purposes. The MRS volume of interest (VOI) was placed medially in the frontal cortex (size = 2×2×2 cm3). VOI placement and a representative spectrum of the frontal cortex are shown in Fig 1. All spectroscopy data were quantified using LCModel V6.2-0R and scaled to the internal water signal. MPRAGE images were segmented into gray matter, white matter and cerebral spinal fluid with SPM12. The percentage of each type of tissue within the MRS VOI was calculated using in-house Matlab code. The metabolites of interest were Glx, NAA, Cre, Ins and Cho. Metabolite concentrations were expressed in institutional units and corrected for the percentage of cerebrospinal fluid (CSF) within the VOI (Chowdhury et al, 2015).
Fig. 1.
Location of MRS voxel of interest (VOI) in medial frontal lobe and representative spectrum. VOI placement in sagittal and axial views, together with a representative MR spectrum (red: LCModel fit, dashed: raw data, solid black line: fitted baseline) from a EPP subject, showing the peaks of Cho, Cre, Glx and NAA.
2.4. Clinical and cognitive assessments
Symptoms were assessed by the Positive and Negative Syndrome Scale (PANSS (Kay et al, 1987)) with total score and three sub-scale scores defined by Marder et al.(Marder et al, 1997) assessing positive, negative and disorganized thought symptoms. The Brief Assessment of Cognition in Schizophrenia (BACS; (Keefe et al, 2004; Keefe et al, 2008)) was used to evaluate cognitive function. The BACS assesses four domains of cognition, including verbal memory, working memory, processing speed, and reasoning/problem solving. All BACS scores were corrected for norms based upon age and gender of participants. The BACS composite score was used for analysis. One patient did not complete the BACS.
2.5. Statistical analysis
T-tests were used to test for group differences on the metabolite and electrophysiological measures. Partial least squares analysis (PLS) was used to test for an overall relationship between the six MRS measures and the two electrophysiological measures (MMN, ASSR power) in the EPP group and the entire sample. PLS computes a singular value decomposition of a cross-correlation matrix between two sets or blocks of measures, which produces a series of latent variables (singular vectors) (Bookstein et al, 1996; O’Donnell et al, 1999). Salience or weight measures for each variable indicate the contribution of a variable to a given latent variable. In order to test the significance of the association between the two blocks of measures, the first pair of latent variables was compared with the distribution of covariances arising from random permutations of the data set. Data were permuted 10,000 times to estimate the likelihood that the obtained covariance of a latent pair is due to chance alone. The calculated p value indicates whether or not the relationship between the two variable blocks is significant.
Exploratory analyses tested for possible relationships between the MRS metabolites and electrophysiological measures and clinical features in the EPP group using Spearman correlation coefficients. Features included the PANSS negative, positive and disorganized factor scores, illness duration, age, CPZ dosage and the composite score of the BACS. A criterion of p < .05 (two-tailed) was used for significance testing across tests.
3. Results
3.1. Participant characteristics
Demographic, clinical, and cognitive characteristics are displayed in Table 1 for the patient and control groups. The BACS composite score was about two standard deviations below the control group mean (p < .001), indicative of marked cognitive impairment.
Table 1.
Participant Characteristics
| Gender and Diagnoses | ||
|---|---|---|
| EPP | Control | |
| Sample size | 34 | 19 |
| Female/Male | 7/24 | 14/5 |
| Diagnosis, n (%) | ||
| Psychosis disorder NOS | 4 (13%) | - |
| Schizoaffective | 6 (19%) | - |
| Schizophrenia | 17 (55%) | - |
| Schizophreniform | 4 (13%) | - |
| M (SD) | M (SD) | |
| EPP | Control | |
| Age, years | 22.0 (4.3) | 22.9 (3.6) |
| BACS composite score | 29.9 (14.2) | 48.8 (11.7)** |
| Illness Duration, years | 2.01 (1.33) | - |
| CPZ Lifetime Exposure, grams | 139.6 (173.0) | - |
| PANSS total scoreb | 53.7 (13.6) | - |
| PANSS cognitive/disorganized factor | 12.6 (3.7) | - |
| PANSS negative symptom factor | 12.7 (5.6) | - |
| PANSS positive symptom factor | 16.9 (6.5) | - |
Note. EPP = Early Phase Psychosis, CPZ = chlorpromazine, PANSS =the Positive and Negative Syndrome Scale, BACS = Brief Assessment of Cognition in Schizophrenia.
p < .001.
3.2. Group differences for MRS metabolites, MMN amplitude and ASSR power
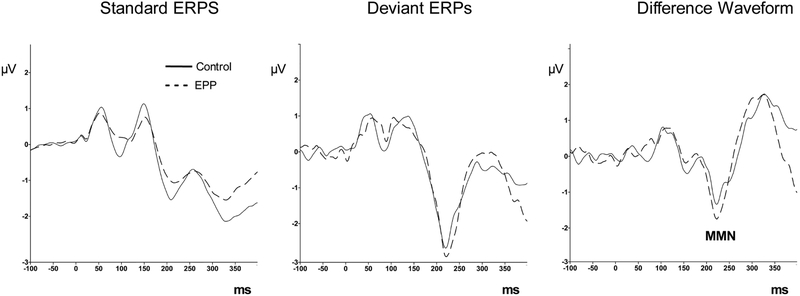
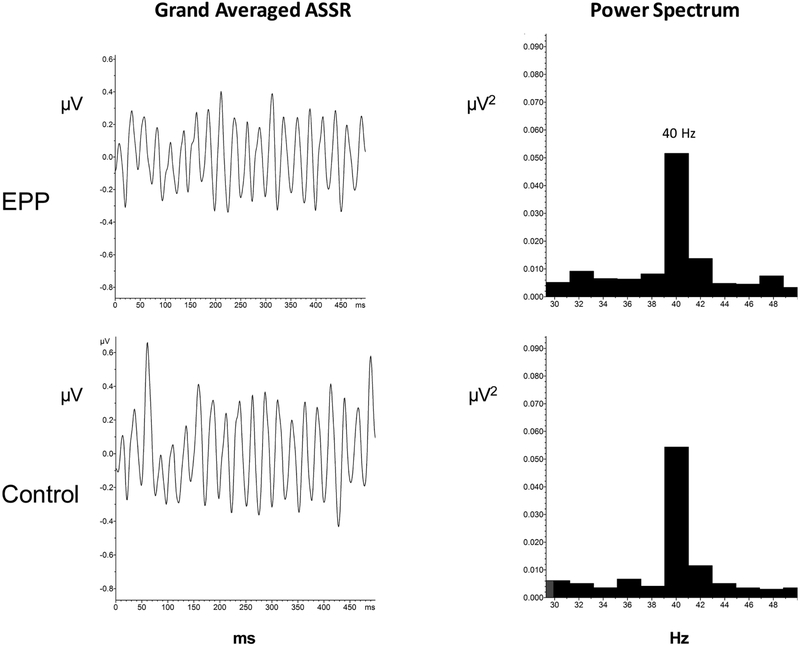
MRS and electrophysiological measures are shown in Table 2. T-tests between groups for each metabolite showed increased levels (p < .05) of Glx , Cre and Cho in EPP compared to the control group. Figure 2 shows the grand average event-related potentials elicited from each group in the MMN paradigm. MMN amplitude did not differ between groups. Figure 3 shows the averaged time domain and frequency domain 40 Hz ASSR response in both groups. ASSR power at 40 Hz did not differ between groups.
Table 2.
MRS Metabolite, MMN Amplitude and 40 Hz ASSR Power
| M (SD) EPP |
M (SD) Control |
Test statistic | 95% CI | Effect size (d) |
|
|---|---|---|---|---|---|
| Metabolite concentration (i.u.) | |||||
| Glx | 4.93 (1.44) | 4.07 (1.18) | t [51] = 2.24* | [1.64, 0.09] | 0.62 |
| NAA | 4.23 (1.05) | 3.71 (0.82) | t [51] = 1.85 | [1.08, −0.04] | 0.52 |
| Cho | 0.96 (0.23) | 0.73 (0.18) | t [51] = 3.80** | [0.35, 0.11] | 0.97 |
| Cre | 3.34 (0.86) | 2.75 (0.77) | t [51] = 2.50* | [1.07, 0.12] | 0.68 |
| Ins | 2.76 (0.88) | 2.40 (0.66) | t [51] = 1.59 | [0.83, −0.10] | 0.45 |
| Electrophysiological measure | |||||
| MMN (μV) | −2.71 (2.76) | −2.27 (2.40) | t [45] = 0.54 | [1.20, −2.07] | −0.17 |
| 40 Hz Power (μV2) | 0.05 (0.05) | 0.05 (0.04) | t [45] = 0.28 | [0.02, −0.03] | −0.09 |
Note. Group differences were tested using t-tests. EPP = Early Phase Psychosis, Glx = Glutamate+Glutamine, NAA = N-Acetylasparate, Cho = Choline, Cre = creatine, Ins = Myoinositol, MMN = mismatch negativity.
p < 0.05,
p < 0.001
Fig. 2.
Event-related potential grand average responses to deviant and standard tones in the EPP (dashed line) and Control group (solid line). Deviant - Standard grand average difference waveforms showing the MMN deflection (third column).
Fig. 3.
ASSR grand average responses to 500 Hz click trains in the EPP and Control groups (left column). Grand Average power spectra showing 40 Hz power in the two groups (right column).
3.3. Partial Least Squares Analysis of MRS metabolites and electrophysiological measures in the EPP group.
PLS analysis revealed an overall relationship between the MRS metabolites and the electrophysiological measures in the EPP group (Table 3). This relationship was captured in the salience or weights of the first latent factor of the PLS analysis, which accounted for 98.5% of the variance contributed to the overall sum of the summed squared cross-block correlations in the analysis. Permutation tests revealed a significant relationship between the two blocks of measures (p = 0.01). The magnitude of the salience value indicates estimates the contribution of that measure to the latent variable. For MMN amplitude, the positive salience value indicated that MMN amplitude became smaller (i.e. less negative) as MRS metabolite levels increased. For the 40 Hz ASSR, the positive salience value indicated that power increased as metabolite levels increased. The larger salience value for MMN amplitude (0.88) indicated that it had a stronger relationship to the set of MRS metabolite values than 40 Hz power (0.47).
Table 3.
Partial Least Squares Analysis of Association Between MRS Metabolites and Electrophysiological Responses in the EPP Group
| Latent variable | 1 | 2 |
|---|---|---|
| Singular Values | 1.027 | 0.128 |
| Percent variance | 98.5 | 1.5 |
| MRS Metabolite Level | ||
| Glx | 0.445 | 0.574 |
| NAA | 0.458 | −0.770 |
| Cho | 0.501 | 0.256 |
| Cre | 0.480 | 0.010 |
| Ins | 0.333 | −0.108 |
| Electrophysiological Measure | ||
| 40 Hz ASSR power | 0.473 | 0.881 |
| Mismatch Negativity Amplitude | 0.881 | −0.473 |
A second PLS using the same variables was computed using the entire sample of EPP and control subjects (Table 4). The overall relationships were comparable to the PLS on the EPP sample. The relationship between the MRS and electrophysiological measures was significant (p = 0.01), with 96% of the covariance loading on the first latent factor. As MRS metabolite levels increased, MMN decreased in amplitude and ASSR increased in power.
Table 4.
Partial Least Squares Analysis of Association Between MRS Metabolites and Electrophysiological Responses in Pooled EPP and Control Subjects
| Latent variable | 1 | 2 |
|---|---|---|
| Singular Values | 0.828 | 0.170 |
| Percent variance | 96.0 | 4.0 |
| MRS Metabolite Level | ||
| Glx | 0.316 | 0.645 |
| NAA | 0.492 | −0.681 |
| Cho | 0.449 | 0.266 |
| Cre | 0.538 | −0.121 |
| Ins | 0.408 | 0.187 |
| Electrophysiological Measure | ||
| 40 Hz ASSR power | 0.287 | 0.958 |
| Mismatch Negativity Amplitude | 0.958 | −0.287 |
3.3. Correlations between neurobiological variables and clinical features
MRS metabolites, MMN amplitude and ASSR power did not show significant correlations with PANSS symptom severity, illness duration, CPZ dosage, age or the BACS composite score.
4. Discussion
Consistent with hypotheses, the EPP group showed higher Glx levels compared to the control group, as well as elevations of Cre and Cho. In contrast, and inconsistent with hypotheses, there were no differences between groups for MMN amplitude or 40 Hz ASSR power. Partial least squares analysis group showed that increased MRS metabolite levels were associated with smaller (less negative) MMN amplitude within the EPP group and in the combined sample. There was a weaker association between increased metabolite levels and increased ASSR 40 Hz power. Additional analyses indicated that neither the MRS metabolites nor electrophysiological measures were correlated with symptom severity, age, CPZ lifetime dosage or cognitive function in the EPP group. These data can be interpreted in terms of the specific implications of abnormal glutamatergic signaling, or of the consequence of pathophysiological processes which could broadly interfere with metabolic functioning.
While MMN has been a robust finding in patients with chronic schizophrenia, MMN amplitude reduction has been less consistently found in first episode psychosis. The current findings in EPP are congruent with several other studies showing an unaffected MMN in first episode psychosis (Salisbury et al., 2007; Salisbury et al., 2018; Salisbury et al., 2017; Salisbury et al., 2002; Umbricht et al., 2006). Forty Hz ASSR power was also unaffected in the present EPP sample. This differs from Tada et al. (2016), who found reduced a 40 HZ ASSR deficit in persons with first episode schizophrenia. The findings suggest that these two electrophysiological measures may be less affected in a diagnostically heterogeneous EPP sample compared to persons with chronic SZ.
The MRS metabolites Glx, Cre and Cho were elevated in the FEP group. Increased levels of Glx and Glu are common in first-episode patients (Kahn and Sommer, 2014). Prior MRS studies suggest that the course of schizophrenia may be characterized by an initial increase in prefrontal Glx during the prodromal and early phase, followed by a decrease with age and illness progression (Abbott and Bustillo, 2006; Kahn and Sommer, 2014; Liemburg et al., 2016). Results from a longitudinal study suggest that increased Glu levels in the associative-striatum are associated with conversion to psychosis (de la Fuente-Sandoval et al., 2013). Creatine is thought to reflect energy metabolism, while choline reflects membrane integrity. Elevated Cre has been reported in the frontal lobe of children with schizophrenia (O'Neill et al., 2004), while reduced frontal Cre has been reported in first-episode patients relative to healthy controls (Ohrmann et al., 2007). Increases in Ins and Cho have been found in the right associative striatum, along with increased Ins in the bilateral medial temporal lobes in previous MRS studies of first-episode psychosis (de la Fuente-Sandoval et al., 2013; Plitman et al., 2016b; Wood et al., 2008). Contrary to the current findings, several previous studies assessing NAA concentrations in EPP have found reduced NAA (Brugger et al., 2011; Liemburg et al., 2016; Schwerk et al., 2014).
Increases in Glx in the EPP group and entire sample were associated with lower MMN amplitude and greater ASSR power. The relationship of Glx to MMN and ASSR in the present study may indicate that the pathophysiological processes involved in the onset of psychosis may be associated with disrupted glutamatergic neurotransmission, which contributes to variation in electrophysiological responses. Consistent with this model, acute administration of NMDA receptor antagonists in rodents can produce increased 40 Hz ASSR power or phase locking (Leishman et al., 2015; Sullivan et al., 2015) particularly at low levels of receptor occupancy (Sivarao et al., 2016). Importantly, glutamatergic disturbances may affect other metabolites. For example, elevated Cre has been attributed to hypermetabolism induced by aberrant glutamatergic signaling (Olney and Farber, 1995; Smesny et al., 2015; Tibbo et al., 2013) and higher levels of glutamatergic neurotransmission may produce excitotoxic damage to neurons (Plitman et al., 2014).
The present findings suggest that individuals with EPP may display a different relationship between neurochemical metabolites and event-related potentials than individuals with chronic illness. Increases of glutamate metabolites have been associated with reduced MMN amplitude in clinical high-risk subjects (Stone et al., 2010) and in EPP (Kaur et al., 2019). In contrast, smaller MMN amplitude has been associated with lower Glu levels in individuals with chronic schizophrenia (Rowland et al., 2016). In addition to a relationship between higher levels of Glu and larger MMN, a higher ratio of glutamine to glutamate has been associated with smaller (less negative) MMN in chronic SZ (Rowland et al., 2016), suggesting that altered glutamatergic signaling impacts MMN generation. These findings support the possibility that glutamatergic transmission or metabolism changes during the transition from early psychosis to more chronic illness (Kahn and Sommer, 2014).
The elevation of multiple metabolites observed in the present data may reflect a pathophysiological mechanism that interferes with a broad range of metabolic processes, such as neuroinflammation. Converging evidence has implicated neuroinflammatory responses to the risk for and expression of psychosis (Radhakrishnan et al., 2017). The present findings are similar to the results observed in the associative striatum of sixty antipsychotic naïve patients during their first episode of psychosis. In these patients, Glu, Cho and Ins were significantly increased in the patient group, while Glx, NAA and Cre were elevated but did not reach significance (Plitman et al., 2016a). Plitman et al. hypothesized that elevated levels of Cho and Ins may reflect neuroinflammatory disruption of astrocyte function, which in turn disturbs the conversion of glutamate to glutamine within astrocytes. Notably, this research group had previously found higher levels of NAA, Glu, Ins and Cho in an anti-psychotic naïve first episode group in the associative striatum or cerebellum (de la Fuente-Sandoval et al., 2013).
There are several limitations affecting interpretation of the present findings. All of the patients were medicated, which could affect electrophysiological responses and MRS metabolites (de la Fuente-Sandoval et al., 2013). The MRS voxel was placed in a different brain region than the temporal lobe generators of the MMN and ASSRs. MRS levels may be differentially affected in regions of the brain which were not assessed in the present study. The psychosis group was diagnostically heterogeneous, unlike many MRS studies which only include persons with schizophrenia or schizoaffective disorder. Mood disturbances were not assessed with rating scales. The number of control subjects was appreciably smaller than the number of EPP subjects.
Despite these limitations, these findings suggest that joint use of human metabolite and electrophysiological measures could help better characterize psychosis pathophysiology. When coupled with a longitudinal design, this multimethod approach could be highly informative in characterizing neurophysiological and neurochemical changes that occur with the emergence and varied outcomes of psychotic disorders. Finally, these non-invasive methods are well suited for measurement of the neurobiological effects of therapeutic interventions.
Highlights.
Electrophysiological responses and brain metabolites are affected in psychosis.
The relationship between these biomarkers in the early phase of psychosis was examined.
The brain metabolites Glx, creatine and choline were elevated in patients.
Elevated metabolites were associated with decreased mismatch and increased gamma responses.
Metabolic changes may contribute to neurophysiological alterations in early psychosis.
Acknowledgments
We wish to express appreciation to the participants in this study. We gratefully acknowledge support for this project from the following agencies and foundations: The Stanley Medical Research Institute (AB), Sandra Eskenazi Center for Brain Care Innovation (AB), National Institute of Mental Health R01 MH074983 (WPH) and R21 MH091774 (BFO), the Brain and Behavior Research Foundation NARSAD Young Investigator Award (ARB), the Dr. Victor Milstein Clinical and Research Foundation Fund for Psychiatry IUSM (AB) and Larue Carter Hospital (AB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data from this study is available at Bartolomeo et al, 2019.
REFERENCES
- Abbott C, Bustillo J, 2006. What have we learned from proton magnetic resonance spectroscopy about schizophrenia? A critical update. Curr Opin Psychiatry 19, 135–139. [DOI] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Waagepetersen HS, 2006. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. Journal of neurochemistry 98, 641–653. [DOI] [PubMed] [Google Scholar]
- [dataset] Bartolomeo Lisa., Wright Andrew., Ma Ruoyun., Hummer Tom., Visco Andrew., Mehdiyoun Nicole., Bolbecker-Hosking Amanda., Hetrick William P., O'Donnell Brian F., Dydak Ulrike., Breier Alan., 2019. Relationship of Auditory Electrophysiological Responses to Magnetic Resonance Spectroscopy Metabolites in Early Phase Psychosis. Mendeley Data, V1 DOI: 10.17632/nrcg43224r.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein FL, Sampson PD, Streissguth AP, Barr HM, 1996. Exploiting redundant measurement of dose and developmental outcome: New methods from the behavioral teratology of alcohol. Developmental Psychology 32, 404–415. [Google Scholar]
- Brugger S, Davis JM, Leucht S, Stone JM, 2011. Proton magnetic resonance spectroscopy and illness stage in schizophrenia--a systematic review and meta-analysis. Biol Psychiatry 69, 495–503. [DOI] [PubMed] [Google Scholar]
- Chowdhury FA, O'Gorman RL, Nashef L, Elwes RD, Edden RA, Murdoch JB, Barker GJ, Richardson MP, 2015. Investigation of glutamine and GABA levels in patients with idiopathic generalized epilepsy using MEGAPRESS. Journal of magnetic resonance imaging : JMRI 41, 694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, Stephano S, Favila R, Diaz-Galvis L, Alvarado-Alanis P, Ramirez-Bermudez J, Graff-Guerrero A, 2013. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiatry 70, 1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deouell LY, 2007. The Frontal Generator of the Mismatch Negativity Revisited. Journal of Psychophysiology 21, 188–203. [Google Scholar]
- Egerton A, Broberg BV, Van Haren N, Merritt K, Barker GJ, Lythgoe DJ, Perez-Iglesias R, Baandrup L, During SW, Sendt KV, Stone JM, Rostrup E, Sommer IE, Glenthoj B, Kahn RS, Dazzan P, McGuire P, 2018. Response to initial antipsychotic treatment in first episode psychosis is related to anterior cingulate glutamate levels: a multicentre (1)H-MRS study (OPTiMiSE). Molecular psychiatry 23, 2145–2155. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Ruffle A, Gold JM, 2016. A Meta-Analysis of Mismatch Negativity in Schizophrenia: From Clinical Risk to Disease Specificity and Progression. Biological psychiatry 79, 980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP. Biometrics Research, New York State Psychiatric Institute, New York. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB, 2002. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition SCID-I/P. [Google Scholar]
- Gratton G, Coles MG, Donchin E, 1983. A new method for off-line removal of ocular artifact. Electroencephalography and clinical neurophysiology 55, 468–484. [DOI] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, McMahon R, Adami H, Francis G, Elliott A, Buchanan RW, Thaker GK, 2004. Evoked gamma band synchronization and the liability for schizophrenia. Schizophr Res 70, 293–302. [DOI] [PubMed] [Google Scholar]
- Hu W, MacDonald ML, Elswick DE, Sweet RA, 2015. The glutamate hypothesis of schizophrenia: evidence from human brain tissue studies. Annals of the New York Academy of Sciences 1338, 38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, 2010. Glutamatergic theories of schizophrenia. The Israel journal of psychiatry and related sciences 47, 4–16. [PubMed] [Google Scholar]
- Javitt DC, Shelley A, Ritter W, 2000. Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clin Neurophysiol 111, 1733–1737. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR, Heresco-Levy U, Umbricht D, 2012. Has an angel shown the way? Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Schizophr Bull 38, 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn RS, Sommer IE, 2014. The neurobiology and treatment of first-episode schizophrenia. Molecular Psychiatry 20, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M, Chitty KM, Lagopoulos J, Hickie IB, Duffy SL, Hermens DF, 2019. Elucidating the glutamatergic processes underlying mismatch negativity deficits in early stage bipolar disorder and schizophrenia: A combined (1)H-MRS and EEG study. Journal of psychiatric research 113, 83–89. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA, 1987. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L, 2004. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res 68, 283–297. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Harvey PD, Goldberg TE, Gold JM, Walker TM, Kennel C, Hawkins K, 2008. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS). Schizophr Res 102, 108–115. [DOI] [PubMed] [Google Scholar]
- Kim M, Cho KI, Yoon YB, Lee TY, Kwon JS, 2017. Aberrant temporal behavior of mismatch negativity generators in schizophrenia patients and subjects at clinical high risk for psychosis. Clin Neurophysiol 128, 331–339. [DOI] [PubMed] [Google Scholar]
- Kim SY, Kaufman MJ, Cohen BM, Jensen JE, Coyle JT, Du F, Ongur D, 2018. In Vivo Brain Glycine and Glutamate Concentrations in Patients With First-Episode Psychosis Measured by Echo Time-Averaged Proton Magnetic Resonance Spectroscopy at 4T. Biol Psychiatry 83, 484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis B, Brown RE, McCarley RW, Hajos M, 2013. Impact of ketamine on neuronal network dynamics: translational modeling of schizophrenia-relevant deficits. CNS neuroscience & therapeutics 19, 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac NV, Reid M, White D, Jones R, den Hollander J, Lowman D, Lahti AC, 2012. Neurometabolites in schizophrenia and bipolar disorder - a systematic review and meta-analysis. Psychiatry Res 203, 111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujala T, Tervaniemi M, Schroger E, 2007. The mismatch negativity in cognitive and clinical neuroscience: theoretical and methodological considerations. Biol Psychol 74, 1–19. [DOI] [PubMed] [Google Scholar]
- Leishman E, O'Donnell BF, Millward JB, Vohs JL, Rass O, Krishnan GP, Bolbecker AR, Morzorati SL, 2015. Phencyclidine Disrupts the Auditory Steady State Response in Rats. PLoS One 10, e0134979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liemburg E, Sibeijn-Kuiper A, Bais L, Pijnenborg G, Knegtering H, van der Velde J, Opmeer E, de Vos A, Dlabac-De Lange J, Wunderink L, Aleman A, 2016. Prefrontal NAA and Glx Levels in Different Stages of Psychotic Disorders: a 3T (1)H-MRS Study. Sci Rep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder SR, Davis JM, Chouinard G, 1997. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry 58, 538–546. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Niznikiewicz MA, Salisbury DF, Nestor PG, O'Donnell BF, Hirayasu Y, Grunze H, Greene RW, Shenton ME, 1999. Cognitive dysfunction in schizophrenia: unifying basic research and clinical aspects. European archives of psychiatry and clinical neuroscience 249 Suppl 4, 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt K, Egerton A, Kempton MJ, Taylor MJ, McGuire PK, 2016. Nature of Glutamate Alterations in Schizophrenia: A Meta-analysis of Proton Magnetic Resonance Spectroscopy Studies. JAMA Psychiatry 73, 665–674. [DOI] [PubMed] [Google Scholar]
- Molnar M, Skinner JE, Csepe V, Winkler I, Karmos G, 1995. Correlation dimension changes accompanying the occurrence of the mismatch negativity and the P3 event-related potential component. Electroencephalography and clinical neurophysiology 95, 118–126. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Kahkonen S, 2009. Central auditory dysfunction in schizophrenia as revealed by the mismatch negativity (MMN) and its magnetic equivalent MMNm: a review. Int J Neuropsychopharmacol 12, 125–135. [DOI] [PubMed] [Google Scholar]
- Niciu MJ, Kelmendi B, Sanacora G, 2012. Overview of glutamatergic neurotransmission in the nervous system. Pharmacology, biochemistry, and behavior 100, 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill J, Levitt J, Caplan R, Asarnow R, McCracken JT, Toga AW, Alger JR, 2004. 1H MRSI evidence of metabolic abnormalities in childhood-onset schizophrenia. Neuroimage 21, 1781–1789. [DOI] [PubMed] [Google Scholar]
- O’Donnell BF, McCarley RW, Potts GF, Salisbury DF, Nestor PG, Hirayasu Y, Niznikiewicz MA, Barnard J, Shen ZJ, Weinstein DM, Bookstein FL, Shenton ME, 1999. Identification of neural circuits underlying P300 abnormalities in schizophrenia. Psychophysiology 36, 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohrmann P, Siegmund A, Suslow T, Pedersen A, Spitzberg K, Kersting A, Rothermundt M, Arolt V, Heindel W, Pfleiderer B, 2007. Cognitive impairment and in vivo metabolites in first-episode neuroleptic-naive and chronic medicated schizophrenic patients: a proton magnetic resonance spectroscopy study. J Psychiatr Res 41, 625–634. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB, 1995. Glutamate receptor dysfunction and schizophrenia. Archives of general psychiatry 52, 998–1007. [DOI] [PubMed] [Google Scholar]
- Plitman E, de la Fuente-Sandoval C, Reyes-Madrigal F, Chavez S, Gomez-Cruz G, Leon-Ortiz P, Graff-Guerrero A, 2016a. Elevated Myo-Inositol, Choline, and Glutamate Levels in the Associative Striatum of Antipsychotic-naive Patients With First-Episode Psychosis: A Proton Magnetic Resonance Spectroscopy Study With Implications for Glial Dysfunction. Schizophr Bull 42, 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plitman E, Nakajima S, de la Fuente-Sandoval C, Gerretsen P, Chakravarty MM, Kobylianskii J, Chung JK, Caravaggio F, Iwata Y, Remington G, Graff-Guerrero A, 2014. Glutamate-mediated excitotoxicity in schizophrenia: a review. Eur Neuropsychopharmacol 24, 1591–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plitman E, Patel R, Chung JK, Pipitone J, Chavez S, Reyes-Madrigal F, Gómez-Cruz G, León-Ortiz P, Chakravarty MM, de la Fuente-Sandoval C, Graff-Guerrero A, 2016b. Glutamatergic Metabolites, Volume and Cortical Thickness in Antipsychotic-naive Patients with First-Episode Psychosis: Implications for Excitotoxicity. Neuropsychopharmacology 41, 2606–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poels EM, Kegeles LS, Kantrowitz JT, Javitt DC, Lieberman JA, Abi-Dargham A, Girgis RR, 2014. Glutamatergic abnormalities in schizophrenia: a review of proton MRS findings. Schizophr Res 152, 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port JD, Agarwal N, 2011. MR spectroscopy in schizophrenia. 34, 1251–1261. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R, Kaser M, Guloksuz S, 2017. The Link Between the Immune System, Environment, and Psychosis. Schizophr Bull 43, 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randau M, Oranje B, Miyakoshi M, Makeig S, Fagerlund B, Glenthoj B, Bak N, 2019. Attenuated mismatch negativity in patients with first-episode antipsychotic-naive schizophrenia using a source-resolved method. Neuroimage. Clinical 22, 101760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rass O, Forsyth J, Krishnan G, Hetrick WP, Klaunig M, Breier A, O’Donnell BF, Brenner CA, 2012. Auditory Steady State Response in the Schizophrenia, First-Degree Relatives, and Schizotypal Personality Disorder. Schizophr Res 136, 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MA, Salibi N, White DM, Gawne TJ, Denney TS, Lahti AC, 2019. 7T Proton Magnetic Resonance Spectroscopy of the Anterior Cingulate Cortex in First-Episode Schizophrenia. Schizophr Bull 45, 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes SA, Lockwood AH, Salvi RJ, Coad ML, Wack DS, Burkard RF, 2005. Mapping the 40-Hz auditory steady-state response using current density reconstructions. Hearing research 204, 1–15. [DOI] [PubMed] [Google Scholar]
- Reyes SA, Salvi RJ, Burkard RF, Coad ML, Wack DS, Galantowicz PJ, Lockwood AH, 2004. PET imaging of the 40 Hz auditory steady state response. Hearing research 194, 73–80. [DOI] [PubMed] [Google Scholar]
- Rissling AJ, Miyakoshi M, Sugar CA, Braff DL, Makeig S, Light GA, 2014. Cortical substrates and functional correlates of auditory deviance processing deficits in schizophrenia. Neuroimage. Clinical 6, 424–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LM, Summerfelt A, Wijtenburg SA, Du X, Chiappelli JJ, Krishna N, West J, Muellerklein F, Kochunov P, Hong LE, 2016. Frontal Glutamate and gamma-Aminobutyric Acid Levels and Their Associations With Mismatch Negativity and Digit Sequencing Task Performance in Schizophrenia. JAMA Psychiatry 73, 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW, 2007. Progressive and Interrelated Functional and Structural Evidence of Post-Onset Brain Reduction in Schizophrenia. Arch Gen Psychiatry 64, 521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, McCathern AG, Coffman BA, Murphy TK, Haigh SM, 2018. Complex mismatch negativity to tone pair deviants in long-term schizophrenia and in the first-episode schizophrenia spectrum. Schizophr Res 191, 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Polizzotto NR, Nestor PG, Haigh SM, Koehler J, McCarley RW, 2017. Pitch and Duration Mismatch Negativity and Premorbid Intellect in the First Hospitalized Schizophrenia Spectrum. Schizophrenia bulletin 43, 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, Griggs CB, Bonner-Jackson A, McCarley RW, 2002. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Archives of general psychiatry 59, 686–694. [DOI] [PubMed] [Google Scholar]
- Schwerk A, Alves FD, Pouwels PJ, van Amelsvoort T, 2014. Metabolic alterations associated with schizophrenia: a critical evaluation of proton magnetic resonance spectroscopy studies. J Neurochem 128, 1–87. [DOI] [PubMed] [Google Scholar]
- Sivarao DV, Chen P, Senapati A, Yang Y, Fernandes A, Benitex Y, Whiterock V, Li YW, Ahlijanian MK, 2016. 40 Hz Auditory Steady-State Response Is a Pharmacodynamic Biomarker for Cortical NMDA Receptors. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 41, 2232–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smesny S, Gussew A, Biesel NJ, Schack S, Walther M, Rzanny R, Milleit B, Gaser C, Sobanski T, Schultz CC, Amminger P, Hipler UC, Sauer H, Reichenbach JR, 2015. Glutamatergic dysfunction linked to energy and membrane lipid metabolism in frontal and anterior cingulate cortices of never treated first-episode schizophrenia patients. Schizophr Res 168, 322–329. [DOI] [PubMed] [Google Scholar]
- Stone JM, Bramon E, Pauls A, Sumich A, McGuire PK, 2010. Thalamic neurochemical abnormalities in individuals with prodromal symptoms of schizophrenia - relationship to auditory event-related potentials. Psychiatry Res 183, 174–176. [DOI] [PubMed] [Google Scholar]
- Stone JM, Morrison PD, Pilowsky LS, 2007. Glutamate and dopamine dysregulation in schizophrenia--a synthesis and selective review. Journal of psychopharmacology (Oxford, England) 21, 440–452. [DOI] [PubMed] [Google Scholar]
- Sullivan EM, Timi P, Hong LE, O'Donnell P, 2015. Effects of NMDA and GABA-A Receptor Antagonism on Auditory Steady-State Synchronization in Awake Behaving Rats. Int J Neuropsychopharmacol 18, pyu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Nagai T, Kirihara K, Koike S, Suga M, Araki T, Kobayashi T, Kasai K, 2016. Differential Alterations of Auditory Gamma Oscillatory Responses Between Pre-Onset High-Risk Individuals and First-Episode Schizophrenia. Cereb Cortex 26, 1027–1035. [DOI] [PubMed] [Google Scholar]
- Thune H, Recasens M, Uhlhaas PJ, 2016. The 40-Hz Auditory Steady-State Response in Patients With Schizophrenia: A Meta-analysis. JAMA Psychiatry 73, 1145–1153. [DOI] [PubMed] [Google Scholar]
- Tibbo PG, Bernier D, Hanstock CC, Seres P, Lakusta B, Purdon SE, 2013. 3-T proton magnetic spectroscopy in unmedicated first episode psychosis: a focus on creatine. Magn Reson Med 69, 613–620. [DOI] [PubMed] [Google Scholar]
- Todd J, Michie PT, Schall U, Ward PB, Catts SV, 2012. Mismatch negativity (MMN) reduction in schizophrenia-impaired prediction--error generation, estimation or salience? Int J Psychophysiol 83, 222–231. [DOI] [PubMed] [Google Scholar]
- Tse CY, Tien KR, Penney TB, 2006. Event-related optical imaging reveals the temporal dynamics of right temporal and frontal cortex activation in pre-attentive change detection. NeuroImage 29, 314–320. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Krljes S, 2005. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res 76, 1–23. [DOI] [PubMed] [Google Scholar]
- Umbricht DS, Bates JA, Lieberman JA, Kane JM, Javitt DC, 2006. Electrophysiological indices of automatic and controlled auditory information processing in first-episode, recent-onset and chronic schizophrenia. Biol Psychiatry 59, 762–772. [DOI] [PubMed] [Google Scholar]
- Veerman SR, Schulte PF, de Haan L, 2014. The glutamate hypothesis: a pathogenic pathway from which pharmacological interventions have emerged. Pharmacopsychiatry 47, 121–130. [DOI] [PubMed] [Google Scholar]
- Wacongne C, Changeux JP, Dehaene S, 2012. A neuronal model of predictive coding accounting for the mismatch negativity. J Neurosci 32, 3665–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijtenburg SA, Yang S, Fischer BA, Rowland LM, 2015. In vivo assessment of neurotransmitters and modulators with magnetic resonance spectroscopy: application to schizophrenia. Neuroscience and biobehavioral reviews 51, 276–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler I, Karmos G, Naatanen R, 1996. Adaptive modeling of the unattended acoustic environment reflected in the mismatch negativity event-related potential. Brain research 742, 239–252. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Berger GE, Wellard RM, Proffitt T, McConchie M, Velakoulis D, McGorry PD, Pantelis C, 2008. A 1H-MRS investigation of the medial temporal lobe in antipsychotic-naiïve and early-treated first episode psychosis. Schizophrenia Research 102, 163–170. [DOI] [PubMed] [Google Scholar]
- Woods SW, 2003. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 64, 663–667. [DOI] [PubMed] [Google Scholar]