Abstract
Objective
Hospitalization places patients at elevated risk for the development of “nosocomial” or hospital acquired complications, ranging from multidrug resistant infections to delirium and physical deconditioning. Adverse nosocomial psychological effects of hospitalization may also exist. This paper introduces a nosocomial based stress model, conceptualizing hospitalization as a unique period of biopsychosocial vulnerability, due to physiologic effects of acute illness and psychosocial variables of the hospital experience.
Method:
A research synthesis and narrative review was performed to evaluate evidence supporting this model, integrating existing knowledge of the psychological and physiological effects of acute life threatening events, with known sequelae associated with hospitalization.
Result:
Psychosocial factors during hospitalization may act as independent predictors of recovery following hospitalization, moderating variables impacting ongoing physiologic changes due to acute illness, and/or dynamic bidirectional elements, influencing medical and psychological outcomes in the near and long-term setting.
Conclusion:
The Nosocomial Stress model provides a novel framework to understanding the biopsychosocial interactions between the psychological and physiologic processes associated with illness and hospitalization. Based on this model, a research agenda is proposed to assess the contributions of acute illness, the hospital experience, and their interactions on the recovery of patients following hospitalization.
Keywords: Nosocomial Stress, Hospitalization, Hospital Stressors
1. Introduction
While hospitals have long served as specialized centers for the care of the ill, hospitalization is not without risks.[1] Clinicians have noted that hospitalization exposes patients to a broad range of hospital acquired or nosocomial (latin for “hospital”, itself tracing from Greek nosos for disease)[2] complications. The risks that patients encounter with hospitalization are diverse, ranging from increased exposure to multidrug resistant infections,[3] acute changes in mental status (e.g. delirium),[4] and generalized deconditioning.[5] The prevalence of these medically adverse events associated with hospitalization has led some researchers to warn of the “hazards of hospitalization”, cautioning that aspects of the hospital environment itself may be associated with deleterious outcomes in patients.[6]
Aspects of the hospital environment may also have unique nosocomial effects on the psychological outcomes of patients. For many patients, the psychological experience of being treated for an acute medical event is a frightening and stressful experience associated with short and long-term adverse psychological and health outcomes. While clinicians, dating back to the earliest healers, have observed that patients treated for acute illness often suffer from significant levels of psychological duress,[7] the last two decades have witnessed a flourishing of research into the near and long-term consequences of such stress, and the variables that may influence it. For example, nearly 1 in 8 survivors of an acute coronary syndrome (ACS) event go onto to develop symptoms of posttraumatic stress disorder (PTSD)[8, 9], with similar incidence of PTSD noted in survivors of other life threating conditions such as stroke[10] and physical trauma.[11, 12]
The impact of these persistent psychological symptoms following acute illness is broad and associated with long-term functional impairment, such as chronic pain,[13] as well as increased overall mortality in patients with life threatening illness such as ACS.[14, 15] The psychophysiological stress associated with hospitalization may also lead to impaired short term recovery and increased long-term healthcare utilization. Following hospital discharge, nearly 1 in 3 patients return for an unscheduled acute evaluation, and more than 1 in 6 are readmitted, often with diagnoses different from their previous admitting diagnosis.[16, 17] Surprisingly, this increased vulnerability for readmission is not age restricted (e.g. younger patients 18–64 may be at even higher risk for readmission)[18] and is seen following a broad range of medical conditions from heart failure, ACS, and pulmonary disease.[19, 20] The “Posthospital Syndrome” has been used to describe this period of vulnerability, with risk differing as a function of the psychological experience during and immediately after hospitalization. In these recently hospitalized patients, for whom perceived psychological stress was high, a nearly 3 fold increase in risk for 30-day all cause rehospitalization exists.[5] [15]
Despite the wealth of evidence linking psychological distress during an acute life threatening event with downstream health outcomes, the etiology of the relationship between psychological distress during hospitalization and these near and long-term adverse health outcomes is currently not well understood. What pathophysiologic pathways link psychological stress experienced during hospitalization with sustained psychological distress and functional decline following hospitalization? Additionally, while the experience of an acute life threatening event is stressful, in and of itself, there is evidence that aspects of the clinical environments in which these events are managed may themselves exacerbate patients’ psychological outcomes.[21, 22] How do external clinical factors, such as hospital crowding and clinician-patient interactions, contribute to and/or moderate any observed effects from psychological stress? Finally, do these psychosocial effects also moderate and interact with the physiological changes and effects due to the acute illness experienced by patients? The answer to these questions has significant implications, not only for the well-being of survivors of acute life threatening events, but for our general understanding of the interrelationships between the psychological perception of illness and its impact on the body and mind.
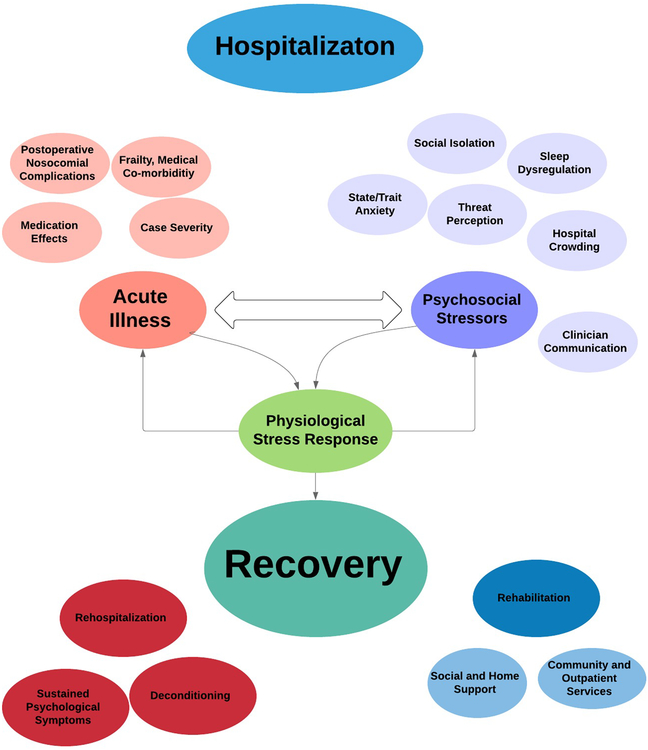
This paper introduces a nosocomial based stress model, proposing that the effects of acute illness and psychosocial variables of the hospitalization together contribute to a unique period of biopsychosocial vulnerability (Figure 1). Integrating existing knowledge of the psychophysiological effects of acute medical events, with past work on environmental effects of the hospital, this model provides a theoretical foundation for a multidisciplinary research agenda focused on identifying testable hypotheses to improve our understanding of the relationship between acute disease, the hospital experience, and their influence on patient recovery.
Figure 1:
Conceptual Map of the Nosocomial Stress Model
2.1. Acute illness represents a period of increased physiologic stress reactivity, adversely affecting recovery during hospitalization.
Acute medical events, such as acute coronary syndrome, ischemic stroke, and physical trauma are marked by an acute activation of the body’s neuroendocrine system, notably the hypothalamic-pituitary-adrenal (HPA) axis and Autonomic Nervous System (ANS).[23, 24] This activation results in cardiovascular stimulation and a surge in circulating stress chemicals including cortisol,[25, 26], norepinephrine[27] and hyperglycemia.[28] Alongside this neuroendocrine reaction, immune responses generated through natural immunity cells (e.g. neutrophils, macrophages), and cytokines, induce systemic responses including fever and inflammation during acute illness.[29] Cytokines encompass a diverse group of molecules, with some (e.g. IL-12), stimulating immune activity,[30] while others, such as IL-10 and transforming growth factor β, blunting immunological responses [31]
Although a balance between activation and suppression of the inflammatory and immune response permits a dynamic response to medical illness, dysregulation in this homeostasis may contribute to impaired recovery during hospitalization following acute illness. Past work in trauma surgery and critical care has noted a period of increased risk for the development of systemic infections in patients immediately following acute surgical and non-operative traumatic events, thought to be driven in part by dysregulation within immune and inflammatory pathways. [32, 33] In both patients and experimental animal models, blunted immune cell production (e.g. Th1 immunity) frequently occurs, characterized by disruptions in cell production and immune stimulating cytokine production.[34–37] Additional work has found that HPA axis associated catecholamines and glucocorticoids, (e.g. norepinephrine, cortisol and ACTH) down-regulate the expression of IL-12, a cytokine critical for immune cell production.[38, 39] In physical trauma patients managed operatively and nonoperatively, decreased IL-12 levels have been associated with increased risk for the development of systemic infection in the post-acute recovery phase.[40, 41] This pattern of systemic immunosuppression following an acute medical event is not limited to physical trauma patients. Similar blunted immune responses have been noted in other acute conditions, including immediately following ischemic stroke [42, 43] and cardiac disease, suggesting a period of inflammatory and immunologic dysregulation following many acute disease states that may contribute to the timely recovery of patients following acute illness. [44–46]
2.2. Psychosocial Contributors to Stress during Hospitalization adversely affects the psychological and health recovery of patients following acute illness
In addition to the physiological stressors due to acute illness, patients also experience psychological distress and fear about their disease. In some patients, the fear experienced during their acute hospitalization may be marked by excessive “threat perception”: an elevated sense of vulnerability, distress, and helplessness.[47] Elevated threat perception is characterized by a state of prolonged hypervigilance and sensitivity to potential life threat and risk of disease recurrence, impacting not only a patient’s immediate response to an acute medical event, but leading to sustained negative effects after hospitalization. The Enduring Somatic Threat Model, proposed by Edmondson, conceptualizes acute illness as a potentially traumatic event, akin to other psychological stressors such as sexual assault or conflict exposure.[48] However, unlike external traumatic stressors, the source of the traumatic stressors following an acute medical event resides from “within” patients themselves. These internal or “interoceptive” symptoms, such as an irregular heartbeat or sensation of numbness during ACS or stroke, may give rise to a cognitive schema marked by a sense of hypervigilance and hypersensitivity to internal/interoceptive cues.[48] Viewed through the lens of these maladaptive cognitive schema, benign interoceptive cues such as a change in heart rate or breathing, may be seen as a potential life threatening event, evoking fear and psychological distress. The consequence of this mindset leads to both an elevated sense of life threat during hospitalization, but also to durable patterns of fear and distress for future symptoms long removed from the acute event. Ultimately, such schema may help give rise to the presence of psychological symptoms such as PTSD. Indeed, a substantial body of literature supports this hypothesis and has found that increased threat perceptions during a traumatic experience is one of the most powerful predictors of future acute stress and PTSD symptoms following an acute medical event.[9, 49–53]
The maladaptive cognitive schema associated with psychological distress experienced in the acute setting are influenced by dispositional and trait factors, such as pre-existing anxiety and depression, and trauma related disorders like PTSD,[54, 55] as well as state related variables such as current mood.[56] However, excessive threat perception among patients during hospitalization does not appear to be driven solely by dispositional factors. Aspects of the care environment significantly influence the degree to which patients experience threat. For example, Emergency Department (ED) crowding and hallway care, have been separately associated with increased threat perception in patients evaluated for acute cardiovascular events such as ACS, even accounting for pre-existing depression and anxiety.[22, 57] Other aspects of the acute care environment including sleep dysregulation, excessive noise and uncomfortable lighting has also been found to negatively impact perceptions of stress in the ED.[58, 59] Finally, clinicians themselves play an important role in influencing perceived stress of patients during hospitalization. Perceived clinician-patient communication is a vital part of the patient care experience during hospitalization and has been associated with acute threat perception levels and acute stress symptoms. [21] Collectively, the evidence from these studies suggests that psychosocial variables experienced during hospitalization play an important role in patient recovery following hospitalization for acute illness.
2.3. The physiological effects of acute illness and the psychosocial factors of the hospital environment may both act as independent predictors of hospital recovery, while also interacting and moderating the effects of each other.
Hospitalization may represent a unique period of generalized vulnerability for patients, due to the physiologic demands of acute illness and the concomitant psychosocial stressors of the hospital environment. These separate effects of acute illness and psychosocial stress in turn, may share a common psychophysiological pathway. A long tradition of research has recognized the deep inter-connections between psychosocial stress and the neuroendocrine and immune stress response.[60–62] Perceived acute psychological stress has been associated with activation of the ANS and HPA axis, including changes in heart rate variability, [63], circulating cortisol[64], norepinephrine,[65] and immune suppressing cytokines including Il-10 and transforming growth factor β.[66]. The associations between psychological stress and the neuroendocrine stress system have been associated with numerous adverse long-term health effects related to recovery, including increased infection risk[67], cardiac disease,[68] and poor wound recovery.[69, 70] In the setting of the neuroendocrine physiologic stress responses associated with acute disease, the additional adverse effects on the body’s neuroendocrine and immune reactions brought on by psychosocial stress may become amplified. The net impact of both psychological stress and acute disease may lead to an exaggerated period of disruption of the body’s stress response system, ultimately impairing the timely recovery of patients following illness.
3. Future Directions: Establishing a biopsychosocial Research Agenda for acute hospitalization and recovery
A research program focused on elucidating the biopsychosocial relationships of acute hospitalization can use the nosocomial stress model to systematically test hypotheses regarding disease pathophysiology and psychosocial stress during hospitalization. Broadly, the nosocomial stress model aims to clarify: 1) the unique contributions of the physiological stress associated with acute illness towards hospital recovery, 2) the mechanisms by which psychosocial variables act as an independent predictor of hospital recovery, and 3) whether illness driven and hospital psychosocial effects also serve as moderating factors, interacting with each other and influencing the recovery process.
Given the potential contributions of a multitude of patient, clinician, and environmental variables to psychological and physiological activation in the acute setting, a multi-modal and interdisciplinary approach is crucial. Preliminary work should be based on validating the mechanistic pathways linking physiological and psychological stress responses in hospital care settings, such as the ED, across a range of disease states and patient populations. Studying psychosocial and physiological responses across the age spectrum, may reveal covariates related to frailty, advanced age, and polypharmacy. These variables, in turn, may interact in a complex and bidirectional fashion with other factors, such as the magnitude of index event and postoperative complications (e.g. delirium) to influence inflammatory pathways and recovery.
Research should clarify whether neuroendocrine/physiological relationships to psychosocial and recovery outcomes are disease-specific versus representative of a more generalized nonspecific threat state in the hospitalization period. For example, while the EST model may be most relevant for somatic cue driven diseases such as cardiac, neurologic or pulmonary conditions, psychosocial cues may be less important for somatic based conditions such as orthopedic trauma or other surgical diseases.
Work into the pathophysiology of recovery should also systematically describe the pattern of any potential neuroendocrine/immunological mediators for the development of generalized deconditioning and psychological risk during hospitalization. Serum or saliva assays of candidate stress molecules, such as cortisol and IL-10, could be assessed across patients in the hospital setting to evaluate any associations with subjective stress, as well as future risk for recovery outcomes such as 30 day rehospitalization or PTSD development. Other recent work has explored the relationship between cellular level changes at the mitochondrial level to psychological stress.[71] Coupled with the known association of mitochondrial dysregulation following acute illnesses such as ischemic stroke,[72] the psychological stress experienced by patients experiencing an acute medical event may lead to an amplifying or interaction of any existing dysregulation at the cellular level due to the medical event. Theses relationship between potential pathophysiological mediators can be explored across a range of levels of analysis. The association between trait pre-existing depression or anxiety levels and state levels of stress biomarkers would allow researchers and clinicians to assess the relative contribution of state vs trait factors in the development of psychiatric disease, such as depression or PTSD, following an acute medical event. These relationships should be studied to understand if any associations vary dynamically and the degree to which they are stable or modifiable in response to not just internal but external factors such as ED crowding, noise, sleep disruptions, or enhanced clinician-patient communication.
The individual effects of acute disease and psychosocial stress on the neuroendocrine stress systems proposed by the nosocomial stress model may also explain, in part, the observations noted in the Post Hospital Syndrome which have found that patients acutely hospitalized are at risk for generalized functional decline.[5] In the setting of impaired neuroendocrine stress responses and blunted ability to mount an effective inflammatory or immune response, recently hospitalized patients may acquire a transient state of generalized vulnerability. Prospective cohort studies of hospitalized patients, could follow these samples and track whether assessments of psychosocial stress, in addition to inflammatory and immunological candidate markers are associated with overall hospital recovery and unanticipated rehospitalization risk. Additional work could focus on common hospital issues such as social isolation, malnutrition, and sleep dysregulation, and evaluated whether such factors impair neuroendocrine functioning and increase patient vulnerability to general debilitation and infection risk.[73, 74] Lastly, one could study the impact of psychosocial settings from acute to subacute and chronic care. Post-hospitalization programs aimed at increasing behavioral and physical engagement (e.g. rehabilitation, short stay care facilities) play an important role in the recovery of patients following acute illness.[75–77] These support systems may influence the psychophysiological recovery of patients following illness and help moderate any deleterious effects following hospitalization.
The identification of any patterns of association with hospital environment and neuroendocrine outcomes would have significant implications regarding our understanding of the links between the acute care environment and the psychological experience of acutely ill patients, as well as its impact on stress physiology. Such a program of research would inform not only biopsychosocial mechanisms of stress and disease, but also guide the identification of potential modifiable variables in the hospital care environment or patient experience that could be changed to improve patient outcomes.
Finally, a broader question regarding disease phenomenology can also be explored by this nosocomial stress model. In clinical practice, providers encounter a number of patients falling under the rubric of the “worried well”; individuals who may be anxious about a condition but have no discernable or identifiable pathophysiologic correlate on evaluation.[78, 79] While providers may attribute much of complaints of these patients to somatization or generalized anxiety, this subgroup of patients may still share many of the same psychological schema and experiences as those with diagnosed acute illness. Recent work studying the prevalence of PTSD symptoms in ED patients evaluated for potential ACS events, surprisingly found that prevalence of PTSD symptoms in patients whose discharge diagnosis was not ACS was similar to the prevalence of PTSD symptoms in patients with actual diagnosed ACS.[80] This suggests that the perceived level of disease in patients may be a powerful influence on the subsequent development of adverse psychological and health events, regardless of acute disease. In comparison to individuals with elevated threat perception with an actual diagnosis of acute medical event, the study of stress biomarkers in a cohort of the “worried well” would provide researchers a naturalistic comparison group to tease apart potential pathways and the individual contributions of psychological stress and acute medical illness to neuroendocrine stress systems. The results of this research would have significant potential implications for how clinicians communicate and manage patients for whom, providers have deemed to be not in imminent danger of an acute medical event. If data supports an association between stress activation and perceived threat regardless of actual diagnosis, then the cohort of patients who are the “worried well” may very well be at risk for some potential adverse psychophysiological outcomes during their acute evaluation.
4. Conclusion
For many patients, hospitalization represents a period of significant psychological duress and physiologic stress that may present unique risks for patients. Future efforts aimed at a multi-disciplinary approach to the treatment of acute disease, factoring in the unique psychological experience of patients during hospitalization, may result not only in clinically significant improvements in patient experience, but in sustained positive long-term health outcomes for survivors of acute illness.
Acknowledgements:
The author wishes to thank Dr. Donald Edmondson for his mentorship and review of the manuscript, along with Dr. Gabriel Brat for his surgical content expertise, review of the manuscript, and friendship.
Funding: This work was supported by a grant by the National Institutes of Health (R01HL141811)
Footnotes
Conflict of Interest: BPC does not have any conflict of interest to declare
REFERENCES
- [1].Carstens HR. The history of hospitals, with special reference to some of the world’s oldest institutions. Annals of Internal Medicine 1936;10:670–682. [Google Scholar]
- [2].Mullan RJ and Frazier TM. “NOSOCOMIAL”: A BROADER PERSPECTIVE? American journal of epidemiology 1986;124:342–342. [DOI] [PubMed] [Google Scholar]
- [3].Garner JS, Jarvis WR, Emori TG, Horan TC and Hughes JM. CDC definitions for nosocomial infections, 1988. American journal of infection control 1988;16:128–140. [DOI] [PubMed] [Google Scholar]
- [4].Inouye SK, Schlesinger MJ and Lydon TJ. Delirium: a symptom of how hospital care is failing older persons and a window to improve quality of hospital care. The American journal of medicine 1999;106:565–573. [DOI] [PubMed] [Google Scholar]
- [5].Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. New England Journal of Medicine 2013;368:100–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schimmel EM. The hazards of hospitalization. Annals of internal Medicine 1964;60:100–110. [DOI] [PubMed] [Google Scholar]
- [7].Porter R. The Greatest Benefit to Mankind: A Medical History of Humanity (The Norton History of Science). WW Norton & Company, 1999. [Google Scholar]
- [8].Kubzansky LD, Koenen KC, Jones C and Eaton WW. A prospective study of posttraumatic stress disorder symptoms and coronary heart disease in women. Health Psychol 2009;28:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Edmondson D, Richardson S, Falzon L, Davidson KW, Mills MA and Neria Y. Posttraumatic stress disorder prevalence and risk of recurrence in acute coronary syndrome patients: a meta-analytic review. PloS one 2012;7:e38915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Edmondson D, Richardson S, Fausett JK, Falzon L, Howard VJ and Kronish IM. Prevalence of PTSD in survivors of stroke and transient ischemic attack: a meta-analytic review. PloS one 2013;8:e66435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Blanchard EB, Hickling EJ, Taylor AE and Loos W. Psychiatric morbidity associated with motor vehicle accidents. Journal of Nervous and Mental Disease 1995. [DOI] [PubMed] [Google Scholar]
- [12].Blanchard EB, Hickling EJ, Taylor AE, Loos WR, Forneris CA and Jaccard J. Who develops PTSD from motor vehicle accidents? Behaviour research and therapy 1996;34:1–10. [DOI] [PubMed] [Google Scholar]
- [13].Brennstuhl MJ, Tarquinio C and Montel S. Chronic pain and PTSD: evolving views on their comorbidity. Perspectives in Psychiatric Care 2015;51:295–304. [DOI] [PubMed] [Google Scholar]
- [14].Edmondson D, Rieckmann N, Shaffer JA, Schwartz JE, Burg MM, Davidson KW, et al. Posttraumatic stress due to an acute coronary syndrome increases risk of 42-month major adverse cardiac events and all-cause mortality. J Psychiatr Res 2011;45:1621–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Edmondson D, Green P, Ye S, Halazun HJ and Davidson KW. Psychological stress and 30-day all-cause hospital readmission in acute coronary syndrome patients: an observational cohort study. PloS one 2014;9:e91477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. Jama 2013;309:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jencks SF, Williams MV and Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. New England Journal of Medicine 2009;360:1418–1428. [DOI] [PubMed] [Google Scholar]
- [18].Dharmarajan K, Hsieh A, Dreyer RP, Welsh J, Qin L and Krumholz HM. Relationship between age and trajectories of rehospitalization risk in older adults. Journal of the American Geriatrics Society 2017;65:421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Krumholz HM, Hsieh A, Dreyer RP, Welsh J, Desai NR and Dharmarajan K. Trajectories of risk for specific readmission diagnoses after hospitalization for heart failure, acute myocardial infarction, or pneumonia. PloS one 2016;11:e0160492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ranasinghe I, Wang Y, Dharmarajan K, Hsieh AF, Bernheim SM and Krumholz HM. Readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia among young and middle-aged adults: a retrospective observational cohort study. PLoS medicine 2014;11:e1001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chang BP, Sumner JA, Haerizadeh M, Carter E and Edmondson D. Perceived clinician–patient communication in the emergency department and subsequent post-traumatic stress symptoms in patients evaluated for acute coronary syndrome. Emerg Med J 2016:emermed-2015–205473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chang BP, Carter E, Suh EH, Kronish IM and Edmondson D. Patient treatment in emergency department hallways and patient perception of clinician-patient communication. The American journal of emergency medicine 2016;34:1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fassbender K, Schmidt R, Mössner R, Daffertshofer M and Hennerici M. Pattern of activation of the hypothalamic-pituitary-adrenal axis in acute stroke. Relation to acute confusional state, extent of brain damage, and clinical outcome. Stroke 1994;25:1105–1108. [DOI] [PubMed] [Google Scholar]
- [24].Brydon L, Magid K and Steptoe A. Platelets, coronary heart disease, and stress. Brain, behavior, and immunity 2006;20:113–119. [DOI] [PubMed] [Google Scholar]
- [25].Hellhammer DH, Wüst S and Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 2009;34:163–171. [DOI] [PubMed] [Google Scholar]
- [26].Kirschbaum C, Pirke K-M and Hellhammer DH. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 1993;28:76–81. [DOI] [PubMed] [Google Scholar]
- [27].Tsigos C and Chrousos GP. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. Journal of psychosomatic research 2002;53:865–871. [DOI] [PubMed] [Google Scholar]
- [28].Marik PE and Bellomo R. Stress hyperglycemia: an essential survival response! Critical Care 2013;17:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lowry SF. Cytokine mediators of immunity and inflammation. Arch Surg 1993;128:1235–1241. [DOI] [PubMed] [Google Scholar]
- [30].Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nature Reviews Immunology 2003;3:133. [DOI] [PubMed] [Google Scholar]
- [31].de Vries JE. Immunosuppressive and anti-inflammatory properties of interleukin 10. Annals of medicine 1995;27:537–541. [DOI] [PubMed] [Google Scholar]
- [32].Lenz A, Franklin GA and Cheadle WG. Systemic inflammation after trauma. Injury 2007;38:1336–1345. [DOI] [PubMed] [Google Scholar]
- [33].Napolitano LM, Ferrer T, McCarter RJ Jr and Scalea TM. Systemic inflammatory response syndrome score at admission independently predicts mortality and length of stay in trauma patients. Journal of Trauma and Acute Care Surgery 2000;49:647–653. [DOI] [PubMed] [Google Scholar]
- [34].Lederer JA, Rodrick ML and Mannick JA. The effects of injury on the adaptive immune response. Shock (Augusta, Ga) 1999;11:153–159. [DOI] [PubMed] [Google Scholar]
- [35].Faist E, Kupper T, Baker CC, Chaudry I, Dwyer J and Baue A. Depression of cellular immunity after major injury. Arch Surg 1986;121:1000–1005. [DOI] [PubMed] [Google Scholar]
- [36].Decker D, Schöndorf M, Bidlingmaier F, Hirner A and von Ruecker AA. Surgical stress induces a shift in the type-1/type-2 T-helper cell balance, suggesting down-regulation of cell-mediated and up-regulation of antibody-mediated immunity commensurate to the trauma. Surgery 1996;119:316–325. [DOI] [PubMed] [Google Scholar]
- [37].Gaudillière B, Fragiadakis GK, Bruggner RV, Nicolau M, Finck R, Tingle M, et al. Clinical recovery from surgery correlates with single-cell immune signatures. Science translational medicine 2014;6:255ra131–255ra131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Franchimont D, Galon J, Gadina M, Visconti R, Zhou Y-J, Aringer M, et al. Inhibition of Th1 immune response by glucocorticoids: dexamethasone selectively inhibits IL-12-induced Stat4 phosphorylation in T lymphocytes. The Journal of Immunology 2000;164:1768–1774. [DOI] [PubMed] [Google Scholar]
- [39].Elenkov IJ. Glucocorticoids and the Th1/Th2 balance. Ann N Y Acad Sci 2004;1024:138–146. [DOI] [PubMed] [Google Scholar]
- [40].O’sullivan ST, Lederer JA, Horgan AF, Chin D, Mannick JA and Rodrick ML. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Annals of surgery 1995;222:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hensler T, Heidecke C-D, Hecker H, Heeg K, Bartels H, Zantl N, et al. Increased susceptibility to postoperative sepsis in patients with impaired monocyte IL-12 production. The Journal of Immunology 1998;161:2655–2659. [PubMed] [Google Scholar]
- [42].Iadecola C and Anrather J. The immunology of stroke: from mechanisms to translation. Nature medicine 2011;17:796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Prass K, Meisel C, Höflich C, Braun J, Halle E, Wolf T, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1–like immunostimulation. J Exp Med 2003;198:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nature Reviews Immunology 2010;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lange LG and Schreiner GF. Immune mechanisms of cardiac disease. New England Journal of Medicine 1994;330:1129–1135. [DOI] [PubMed] [Google Scholar]
- [46].Weyand CM, Goronzy JJ, Liuzzo G, Kopecky SL, Holmes DR Jr and Frye RL. T-cell immunity in acute coronary syndromes. Mayo Clinic Proceedings. Elsevier, 2001. p. 1011–1020. [DOI] [PubMed] [Google Scholar]
- [47].Cornelius T, Agarwal S, Garcia O, Chaplin W, Edmondson D and Chang BP. Development and Validation of a Measure to Assess Patients’ Threat Perceptions in the Emergency Department. Academic Emergency Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Edmondson D. An enduring somatic threat model of posttraumatic stress disorder due to acute life‐threatening medical events. Social and personality psychology compass 2014;8:118–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].White M, Edmondson D, Umland R, Sanchez G and Chang BP. Erratum to “Patient perceptions of stress during evaluation for ACS in the ED”, YAJEM 35/2 (2017) 351–352. The American Journal of Emergency Medicine 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].White M, Edmondson D, Umland R, Sanchez G and Chang BP. Patient perceptions of stress during evaluation for acute coronary syndrome in the emergency department. The American Journal of Emergency Medicine 2017;35:351–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chang BP, Sumner JA, Haerizadeh M, Carter E and Edmondson D. Perceived clinician–patient communication in the emergency department and subsequent post-traumatic stress symptoms in patients evaluated for acute coronary syndrome. Emerg Med J 2016;33:626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Meli L, Kautz M, Julian J, Edmondson D and Sumner JA. The role of perceived threat during emergency department cardiac evaluation and the age-posttraumatic stress disorder link. Journal of behavioral medicine 2017:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ozer EJ, Best SR, Lipsey TL and Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychological bulletin 2003;129:52. [DOI] [PubMed] [Google Scholar]
- [54].Muris P, Kindt M, Bögels S, Merckelbach H, Gadet B and Moulaert V. Anxiety and threat perception abnormalities in normal children. Journal of Psychopathology and Behavioral Assessment 2000;22:183–199. [Google Scholar]
- [55].Connell CM, Davis WK, Gallant MP and Sharpe PA. Impact of social support, social cognitive variables, and perceived threat on depression among adults with diabetes. Health Psychol 1994;13:263. [DOI] [PubMed] [Google Scholar]
- [56].Johnson EJ and Tversky A. Affect, generalization, and the perception of risk. Journal of personality and social psychology 1983;45:20. [Google Scholar]
- [57].Edmondson D, Shimbo D, Ye S, Wyer P and Davidson KW. The association of emergency department crowding during treatment for acute coronary syndrome with subsequent posttraumatic stress disorder symptoms. JAMA Intern Med 2013;173:472–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gulrajani R. Physical environmental factors affecting factors affecting patients’ stress in the accident and emergency department. Accident and Emergency Nursing 1995;3:22–27. [DOI] [PubMed] [Google Scholar]
- [59].Short AE, Short KT, Holdgate A, Ahern N and Morris J. Noise levels in an Australian emergency department. Australasian Emergency Nursing Journal 2011;14:26–31. [Google Scholar]
- [60].Negrao A, Deuster P, Gold P, Singh A and Chrousos G. Individual reactivity and physiology of the stress response. Biomedicine & pharmacotherapy 2000;54:122–128. [DOI] [PubMed] [Google Scholar]
- [61].Burke HM, Davis MC, Otte C and Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology 2005;30:846–856. [DOI] [PubMed] [Google Scholar]
- [62].Segerstrom SC and Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychological bulletin 2004;130:601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Thayer JF, Åhs F, Fredrikson M, Sollers JJ III and Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews 2012;36:747–756. [DOI] [PubMed] [Google Scholar]
- [64].Dickerson SS and Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological bulletin 2004;130:355. [DOI] [PubMed] [Google Scholar]
- [65].Carrasco GA and Van de Kar LD. Neuroendocrine pharmacology of stress. European journal of pharmacology 2003;463:235–272. [DOI] [PubMed] [Google Scholar]
- [66].Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, et al. Immune and Clinical Correlates of Psychological Stress–Induced Production of Interferon–γ and Interleukin–10 in Humans Cytokines. CRC Press, 2002. p. 48–59. [Google Scholar]
- [67].Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T and Howes OD. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology 2016;233:1637–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lagraauw HM, Kuiper J and Bot I. Acute and chronic psychological stress as risk factors for cardiovascular disease: Insights gained from epidemiological, clinical and experimental studies. Brain, behavior, and immunity 2015;50:18–30. [DOI] [PubMed] [Google Scholar]
- [69].Bonneau RH, Sheridan JF, Feng N and Glaser R. Stress-induced modulation of the primary cellular immune response to herpes simplex virus infection is mediated by both adrenal-dependent and independent mechanisms. Journal of neuroimmunology 1993;42:167–176. [DOI] [PubMed] [Google Scholar]
- [70].Burns VE, Drayson M, Ring C and Carroll D. Perceived stress and psychological well-being are associated with antibody status after meningitis C conjugate vaccination. Psychosomatic medicine 2002;64:963–970. [DOI] [PubMed] [Google Scholar]
- [71].Picard M and McEwen BS. Psychological Stress and Mitochondria: A Conceptual Framework. Psychosomatic medicine 2018;80:126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Chan PH. Mitochondrial dysfunction and oxidative stress as determinants of cell death/survival in stroke. Ann N Y Acad Sci 2005;1042:203–209. [DOI] [PubMed] [Google Scholar]
- [73].Creditor MC. Hazards of hospitalization of the elderly. Annals of internal medicine 1993;118:219–223. [DOI] [PubMed] [Google Scholar]
- [74].Covinsky KE, Pierluissi E and Johnston CB. Hospitalization-associated disability:“She was probably able to ambulate, but I’m not sure”. Jama 2011;306:1782–1793. [DOI] [PubMed] [Google Scholar]
- [75].Ades PA. Cardiac rehabilitation and secondary prevention of coronary heart disease. New England Journal of Medicine 2001;345:892–902. [DOI] [PubMed] [Google Scholar]
- [76].Wang Q, Suo J, Jiang J, Wang C, Zhao YQ and Cao X. Effectiveness of fast‐track rehabilitation vs conventional care in laparoscopic colorectal resection for elderly patients: a randomized trial. Colorectal disease 2012;14:1009–1013. [DOI] [PubMed] [Google Scholar]
- [77].Ottenbacher KJ and Jannell S. The results of clinical trials in stroke rehabilitation research. Archives of neurology 1993;50:37–44. [DOI] [PubMed] [Google Scholar]
- [78].Miller D, Acton T and Hedge B. The worried well: their identification and management. J R Coll Physicians Lond 1988;22:158. [PMC free article] [PubMed] [Google Scholar]
- [79].Musey PI Jr, Patel R, Fry C, Jimenez G, Koene R and Kline JA. Anxiety Associated With Increased Risk for Emergency Department Recidivism in Patients With Low-Risk Chest Pain. The American journal of cardiology 2018;122:1133–1141. [DOI] [PubMed] [Google Scholar]
- [80].Velazquez Z, Edmondson D, Moise N, Chang B, Wei Y, Veneros DL, et al. Are patients who rule out for acute coronary syndrome at risk for posttraumatic stress disorder? ANNALS OF BEHAVIORAL MEDICINE. SPRINGER; 233 SPRING ST, NEW YORK, NY 10013 USA, 2017. p. S1907–S1908. [Google Scholar]