Abstract
Objective:
As intensive care units (ICUs) are increasingly a site of end-of-life (EOL) care, many have adopted EOL care resources. We sought to determine the association of such resources with outcomes of ICU patients.
Design:
Retrospective cohort study.
Setting:
Pennsylvania ICUs.
Patients:
Medicare fee-for-service beneficiaries.
Exposures:
Availability of any of one hospital-based resource (palliative care consultants) or four ICU-based resources (protocol for withdrawal of life-sustaining therapy, triggers for automated palliative care consultation, protocol for family meetings, and palliative care clinicians embedded in ICU rounds).
Measurements and Main Results:
In mixed-effects regression analyses, admission to a hospital with EOL resources was not associated with mortality, length of stay, or treatment intensity (mechanical ventilation, hemodialysis, tracheostomy, gastrostomy, artificial nutrition, or cardiopulmonary resuscitation); however, it was associated with a higher likelihood of discharge to hospice (OR = 1.58; 95% CI = 1.11 to 2.24), an effect that was driven by ICU-based resources (OR = 1.37; 95% CI = 1.04 to 1.81) rather than hospital-based resources (OR = 1.19; 95% CI = 0.83 to 1.71). Instrumental variable analysis using differential distance (defined as the additional travel distance beyond the hospital closest to a patient’s home needed to reach a hospital with EOL resources) demonstrated that among those for whom differential distance would influence receipt of EOL resources, admission to a hospital with such resources was not associated with any outcome.
Conclusions:
ICU-based EOL care resources do not appear to change mortality but are associated with increased hospice utilization. Given that this finding was not confirmed by the instrumental variable analysis, future studies should attempt to verify this finding, and identify specific resources or processes of care that impact the care of ICU patients at the end of life.
Keywords: palliative care, intensive care units, hospice, hospital mortality, medicare
Introduction
Medicare beneficiaries are increasingly admitted to an intensive care unit (ICU) in the last 30 days of life, estimated at nearly 30% in 2015.1 Consequently, many ICUs have adopted end-of-life (EOL) resources to facilitate concurrent delivery of critical care and palliative care.2 In a 2014 survey, 52% of ICUs in Pennsylvania reported having access to a hospital-based palliative care clinician, and 49% had a protocol for withdrawal of life-sustaining therapy.3
The impact of such EOL resources among critically ill patients is not yet known. Several studies have reported that palliative care consultation increases rates of discharge to hospice and may reduce hospital length of stay (LOS); however, these studies include data only from single centers or exclude palliative care provided by ICU-based clinicians rather than consultative teams.4–13
The goal of our study was to determine the association of EOL care resources, including ICU-based resources, and outcomes of ICU patients in the state of Pennsylvania. We hypothesized that exposure to EOL resources would be associated with higher in-hospital mortality and rates of discharge to hospice, and lower treatment intensity and hospital LOS.
Materials and Methods
The University of Pennsylvania Institutional Review Board approved the study. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines in preparing this manuscript.14
Study population and data sources
We performed a retrospective study of Medicare fee-for-service beneficiaries who were admitted to a Pennsylvania acute care hospital on or after January 1st, 2014 and discharged on or before December 31st, 2015, and whose admissions included an ICU stay, defined as the presence of an ICU revenue center code but not including intermediate care.15 We used the Centers for Medicare and Medicaid Services (CMS) Medicare Provider Analysis and Review (MedPAR) file, which includes inpatient hospital final action stay records, and the Master Beneficiary Summary File, which includes death dates, to ascertain patient-level outcomes.
We linked patient-level data with the results of a 2014 organizational survey that was completed by nurse managers from 136 of 223 (61%) ICUs in Pennsylvania.3 Organizational characteristics of hospitals with responding and non-responding ICUs did not vary significantly.3 The survey included questions about the availability of five EOL care resources, including one hospital-based resource (palliative care consultants), and four ICU-based resources (protocol for withdrawal of life-sustaining therapy, triggers for automated palliative care consultation, protocol for family meetings, and palliative care clinicians embedded in ICU rounds). As CMS data did not allow reliable identification of the admitting ICU in hospitals with multiple ICUs, we defined EOL resources at the hospital level. Hospitals in which all ICUs did not respond to the survey were excluded from the study sample. We included information about hospital organizational factors from the American Hospital Association’s 2014 Annual Survey Database.
We excluded patients younger than 18 years. To minimize the risk of exposure misclassification, we also excluded any admissions resulting in or originating from an inter-hospital transfer as these patients were exposed to EOL resources in more than one ICU. We restricted the study sample to the first admission for each patient during the study period to avoid non-independence of subsequent admissions. To ensure accurate ascertainment of the exposure, we further restricted the sample to patients who had an index admission in a hospital where all ICUs responded to the survey.
Study variables
We defined hospitals as having EOL resources if they had at least one of the five resources in at least one of their ICUs. The primary outcome was in-hospital mortality. Secondary outcomes included death within 30, 60, or 90 days of hospital admission, as well as hospital LOS, discharge to hospice, and treatment intensity. Treatment intensity has been previously defined as receipt of one or more of the following: mechanical ventilation, hemodialysis, tracheostomy placement, gastrostomy placement, enteral or parenteral nutrition, or cardiopulmonary resuscitation.16
We identified potential confounders a priori. Patient characteristics included age, gender, race or ethnicity, location prior to admission to the hospital (i.e. outpatient or other), admission day of week (i.e. weekday or weekend), severity of illness, and comorbid illness. Severity of illness was measured using the components of the Acute Organ Failure Score (AOFS), a claims-based risk adjustment methodology for ICU admissions, and comorbidities were measured according to Elixhauser present on admission diagnoses.17–19 Hospital characteristics included patient-to-nurse ratio (averaged across all ICUs), number of hospital beds, core-based statistical area (e.g., metropolitan or rural), whether the hospital had an Accreditation Council for Graduate Medical Education (ACGME) training program, whether the hospital was church-operated, and whether the following were present in at least one ICU in the hospital: daily team-based rounds,20 daytime intensivist physician staffing,21,22 and nighttime intensivist physician staffing.23
Statistical analyses
We limited all analyses to hospitals with at least 100 study patients to exclude hospitals with limited experience caring for critically ill patients. We compared baseline patient and hospital characteristics using the chi-squared test and t-test for categorical and continuous data, respectively. To test the independent association of admission to a hospital with EOL resources and outcomes, we fit multivariable regression models, including a random intercept for hospitals and all patient and hospital characteristics described above as potential confounders. Linear and logistic mixed-effects regression were used for continuous outcomes and binary outcomes, respectively. All analyses were performed with Stata/IC Version 14.2 (StataCorp LLC, College Station, TX).
Secondary, subgroup, and sensitivity analyses
Given the heterogeneity of the five EOL resources, we performed two secondary analyses – analyzing hospital- and ICU-based resources separately, and each resource as an independent exposure. We also performed three subgroup analyses. First, patients admitted to surgical ICUs are less likely to receive palliative care than patients admitted to medical ICUs;24 therefore, we repeated all analyses on a population restricted to medical admissions according to the Medicare Severity-Diagnosis Related Group.17 Second, we repeated analyses in a subgroup of patients with metastatic cancer, as determined by the Elixhauser comorbidity groupings, who may be more likely to benefit from EOL care.25 Third, because previous studies have demonstrated that higher institutional volume of critically ill patients is associated with lower mortality,26 we repeated analyses among patients admitted to hospitals with 200 or more beds. Finally, to minimize bias stemming from exposure misclassification, we performed a sensitivity analysis including only hospitals with one ICU.
Instrumental variable analysis
As this is an observational study, there may be unmeasurable differences among patients within different hospitals that are associated with the outcomes of interest, which could lead to an errant result that these outcomes are associated with exposure to EOL resources. Therefore, we performed an instrumental variable (IV) analysis, a statistical method that simulates random assignment of patients to the exposure to account for unmeasured confounding. An ideal instrumental variable is a variable that causes the exposure under study (in this case, admission to a hospital with EOL resources), but is not associated with unmeasured confounders or the outcome of interest, except through its effect on the exposure variable.27,28 We used “differential distance” as the instrumental variable, defined as the difference between 1) the distance between a patient’s home and the nearest hospital with EOL resources, and 2) the distance between a patient’s home and the nearest hospital, as in previous studies of hospital-level exposures.28,29 In other words, patients who live closer to hospitals with EOL resources are more likely to be exposed to such resources. However, the location of their residence relative to nearby hospitals is unlikely to be associated with confounders (e.g. comorbidities or preferences for EOL care), nor is it likely to cause any outcomes of interest (e.g. mortality) except that it affects their likelihood of being exposed to EOL resources. We calculated geodetic distances between zip code centroids using the “geonear” package (StataCorp LLC, College Station, TX).
We tested instrument validity using three conditions.27 First, we tested the strength of the relationship between the IV and exposure by calculating the proportion of variability in EOL resources explained by the instrument as the ratio of the variance of EOL resources predicted given the instrument and the total variance of EOL resources.30 Second, as it is not possible to verify a lack of unmeasured confounding, we instead examined the balance of measured patient confounders across dichotomized levels of the IV. Third, although untestable, we believe that the IV affects the outcome only through its effect on the exposure, and in support of this assumption, differential distance has previously been used in studies with similar outcomes.28,29
We excluded patients if the hospital closest to their home had an unknown exposure status, as it would not have been possible to calculate the value of the IV under these circumstances. We performed 2-stage predictor substation regression, adjusting for hospital-level clustering and the confounders described previously. In the first stage, we used differential distance to predict the probability of being admitted to a hospital with EOL resources. In the second stage, we tested the association of this predicted probability and patient outcomes. The results of the analysis allowed us to estimate the effect of the exposure among marginal patients, those for whom differential distance would influence receipt of EOL resources.27,28
Results
Unadjusted patient and hospital comparisons
The final study sample included 78 hospitals in which all ICUs responded to the survey. Table 1 summarizes hospital characteristics. Fifty-four hospitals (69.2%) had at least one EOL resource, most frequently a protocol for withdrawal of life-sustaining therapy or a palliative care consultative service (Table E1). Hospitals with EOL resources were more likely to have 200 or more beds, be in a metropolitan area, and have an ACGME training program. Hospitals with EOL resources were also more likely to have daily team-based rounds in the ICU and employ intensivists.
Table 1.
Characteristics of hospitals without and with end-of-life resources.
| Hospital characteristics | No EOL resources | At least one EOL resource |
|---|---|---|
| Hospitals, No. | 24 | 54 |
| Bed size, % | ||
| 0–99 | 33.3 | 15.1 |
| 100–199 | 45.8 | 26.4 |
| 200+ | 20.8 | 58.5 |
| Metropolitan CBSA, % | 54.2 | 84.9 |
| ACGME site, % | 12.5 | 54.7 |
| Church-operated, % | 4.2 | 11.3 |
| Daily rounds, % | 33.3 | 63.0 |
| Daytime intensivist staffing, % | 54.2 | 77.8 |
| Nighttime intensivist staffing, % | 4.2 | 24.1 |
| Number of patients per nurse, average (SD) | 3.0 (0.7) | 2.8 (0.5) |
Definition of abbreviations: ACGME = Accrediation Council for Graduate Medical Education; CBSA = core-based statistical area; EOL = end-of-life; SD = standard deviation.
Among the 63,645 patients in the study cohort, 54,911 (86.3%) were admitted to hospitals with EOL resources. Table 2 summarizes patient characteristics. There were racial and ethnic differences, with more non-Hispanic black patients being treated at hospitals with EOL resources than without (14.1% vs 4.1%). In addition, patients admitted to hospitals with EOL resources were less likely to be admitted for medical than surgical diagnoses, and had more chronic diseases and higher rates of some acute organ failures.
Table 2.
Characteristics of patients admitted to hospitals without and with end-of-life resources.
| Patient characteristics | Admitted to hospital without EOL resources | Admitted to hospital with at least one EOL resource |
|---|---|---|
| Patients, No. | 8,734 | 54,911 |
| Age, mean (SD) | 74.8 (12.1) | 74.0 (12.1) |
| Age categories, % | ||
| 18–44 | 2.0 | 2.2 |
| 45–54 | 4.1 | 4.3 |
| 55–64 | 8.9 | 9.3 |
| 65–74 | 31.5 | 33.6 |
| >75 | 53.5 | 50.5 |
| Female, % | 48.9 | 49.9 |
| Race or ethnicity, % | ||
| Non-Hispanic white | 94.1 | 83.1 |
| Non-Hispanic black | 4.1 | 14.1 |
| Other | 1.8 | 2.8 |
| Medical patient, % | 64.6 | 57.0 |
| Admission source, % | ||
| Outpatient | 97.4 | 96.5 |
| Other or unknown | 2.5 | 3.5 |
| Weekday admission, % | 74.6 | 75.9 |
| Number of Elixhauser comorbidities, % | ||
| 0 | 1.7 | 1.4 |
| 1 | 7.3 | 5.9 |
| 2 | 15.5 | 12.0 |
| 3 | 19.3 | 17.0 |
| >3 | 56.2 | 63.8 |
| Acute organ failure, % | ||
| Respiratory | 15.0 | 15.4 |
| Renal | 22.5 | 23.6 |
| Hematologic | 5.5 | 6.5 |
| Metabolic | 9.6 | 11.2 |
| Neurologic | 7.1 | 7.4 |
| Hepatic | 1.4 | 1.7 |
Definition of abbreviations: EOL = end-of-life; SD = standard deviation.
Results of the unadjusted analyses described in Table 3 demonstrate that patients admitted to hospitals with EOL resources were more likely to die in the hospital, enter hospice upon discharge from the hospital, receive intensive therapies, and have a longer hospital stay.
Table 3.
Unadjusted analyses of outcomes of patients admitted to hospitals without and with end-of-life resources.
| Outcome | Univariable analysis | ||
|---|---|---|---|
| No EOL resources | At least one EOL resource | P value | |
| In-hospital mortality, % | 9.6 | 11.2 | <0.001 |
| Discharge to hospice, % | 3.8 | 5.5 | <0.001 |
| 30-day mortality, % | 17.8 | 18.4 | 0.18 |
| 60-day mortality, % | 21.6 | 22.2 | 0.27 |
| 90-day mortality, % | 23.9 | 24.4 | 0.33 |
| Treatment intensity, % | 18.4 | 26.3 | <0.001 |
| Outcome | No EOL resources | At least one EOL resource | P value |
| LOS (days), mean (SD) | 6.5 (5.9) | 8.3 (8.2) | <0.001 |
Definition of abbreviations: EOL = end-of-life; LOS = length of stay; SD = standard deviation.
Adjusted comparisons
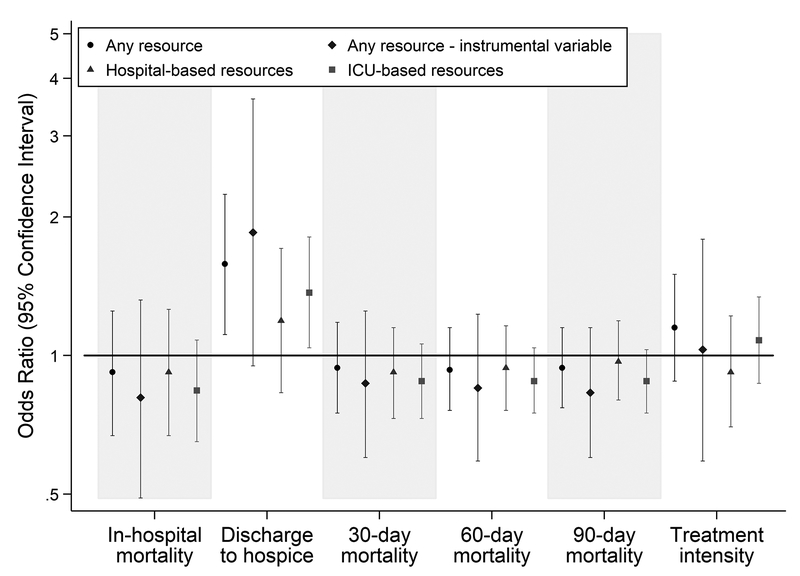
After adjustment for patient severity of illness and chronic comorbidities, and hospital characteristics, admission to a hospital with EOL resources was not associated with in-hospital mortality, 30-day mortality, 60-day mortality, or 90-day mortality, or treatment intensity (Figure 1), nor with hospital LOS (beta coefficient = 0.18 days; 95% CI = −0.27 to 0.63). However, patients admitted to hospitals with EOL resources were more likely to be discharged to hospice (odds ratio [OR] = 1.58; 95% confidence interval [CI] = 1.11 to 2.24) (Figure 1). In secondary analyses, this effect was driven by ICU-based resources (OR = 1.37; 95% CI = 1.04 to 1.81), specifically availability of a protocol for withdrawal of life-sustaining therapy (OR = 1.36; 95% CI = 1.01 to 1.83) (Figure 1 and Table E2).
Figure 1. Multivariable analyses of outcomes of patients admitted to hospitals with end-of-life resources compared to patients admitted to hospitals without end-of-life resources.
Multivariable regression analyses included all covariates included in Tables 1 and 2. Four hospital covariates, ACGME training program site, church-operated facility, number of hospital beds, and core-based statistical area were missing for 402 patients. Complete-case analysis was used to account for missing data, thus the regressions included 62,523 of 62,925 (99.4%) cases. Results of analyses using the following four exposure definitions are shown above: (1) the presence of any end-of-life (EOL) resource, (2) the differential distance instrumental variable, (3) hospital-based EOL resources, and (4) ICU-based EOL resources.
In subgroup analyses, medical patients exposed to EOL resources were more likely to be discharged to hospice (OR = 1.58; 95% CI = 1.09 to 2.28). Patients admitted to hospitals with 200 or more beds and exposed to EOL resources also had a higher likelihood of being discharged to hospice (OR = 2.70; 95% CI = 1.48 to 4.91), as well as a longer LOS (beta coefficient = 1.18 days; 95% CI = 0.83 to 1.53). Admission to a hospital with EOL resources was not associated with other outcomes among these subgroups of patients, or with any outcomes among patients with metastatic cancer (Table 4). The results of the sensitivity analysis were similar to those of the primary analysis (Table E3).
Table 4.
Subgroup analyses of outcomes of patients admitted to hospitals with end-of-life resources compared to patients admitted to hospitals without end-of-life resources.
| Medical patients (N = 36,045) |
Patients with metastatic cancer (N = 2,774) |
Patients admitted to hospitals with ≥200 beds (N = 48,435) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value |
| In-hospital mortality | 0.87 | 0.63 to 1.21 | 0.42 | 1.05 | 0.65 to 1.70 | 0.83 | 0.68 | 0.40 to 1.17 | 0.16 |
| Discharge to hospice | 1.58 | 1.09 to 2.28 | 0.01 | 1.28 | 0.79 to 2.08 | 0.31 | 2.70 | 1.48 to 4.91 | <0.001 |
| 30-day mortality | 0.90 | 0.71 to 1.15 | 0.42 | 0.88 | 0.61 to 1.26 | 0.48 | 0.74 | 0.50 to 1.11 | 0.15 |
| 60-day mortality | 0.92 | 0.74 to 1.14 | 0.47 | 0.98 | 0.68 to 1.42 | 0.93 | 0.80 | 0.56 to 1.15 | 0.24 |
| 90-day mortality | 0.92 | 0.75 to 1.13 | 0.46 | 0.87 | 0.59 to 1.28 | 0.50 | 0.85 | 0.60 to 1.20 | 0.37 |
| Treatment intensity | 1.08 | 0.81 to 1.46 | 0.57 | 0.96 | 0.62 to 1.49 | 0.86 | 0.85 | 0.52 to 1.40 | 0.53 |
| Outcome | Beta | 95% CI | P value | Beta | 95% CI | P value | Beta | 95% CI | P value |
| LOS (days) | 0.22 | −0.23 to 0.67 | 0.34 | 0.50 | −0.64 to 1.65 | 0.38 | 1.18 | 0.83 to 1.53 | <0.001 |
Definition of abbreviations: Beta = beta coefficient; CI = confidence interval; LOS = length of stay; OR = odds ratio. Patients were classified as medical (versus surgical) based on Medicare Severity-Diagnosis Related Groups. Patients with metastatic cancer were identified according to Elixhauser comorbidity definitions. The beta coefficient represents the increase in LOS in days.
Instrumental variable analysis
The instrumental variable analysis included 59,174 patients with a median differential distance of zero miles (mean = 3.00 miles, standard deviation = 8.15), which indicates that patients live in the same zip code as the hospital nearest to their home. Differential distance explained 27.3% of the variance in the exposure. Measured patient characteristics appeared balanced across levels of the IV, except for nonwhite patient race and ethnicity, which was more prevalent among patients with a differential distance of zero than those with greater differential distances (16.8% vs 5.2%). In addition, the prevalence of the exposure was 96.0% among patients with a differential distance of zero and 53.7% among those with a larger differential distance, indicating that 42.3% of the study population would be considered marginal patients (Table E4).28,31 After adjusting for patient and hospital characteristics, admission to a hospital with EOL resources among marginal patients was not associated with mortality at any time point or treatment intensity (Figure 1), nor with hospital LOS (beta coefficient = 0.19 days; 95% CI = −0.67 to 1.06). The likelihood of discharge to hospice was higher in those admitted to hospitals with EOL resources (OR = 1.85; 95% CI = 0.95 to 3.61) (Figure 1).
Discussion
In a state-wide sample of ICU patients, we found that the availability of EOL resources was not associated with increased in-hospital mortality, mortality up to 90 days after hospital admission, or increased resource utilization at the end of life. We found that admission to hospitals with such resources, specifically ICU-based resources, was associated with increased likelihood of discharge to hospice in the multivariable analysis. However, this association was not reproducible using the instrumental variable analysis among the subset of patients for whom differential distance would influence their receipt of EOL resources.
That the availability of ICU EOL resources does not seem to be associated with increased mortality by any definition is an important finding, particularly for those ICU clinicians, surrogates, and patients who may be hesitant to engage in care planning or attend to the burdensome physical symptoms that manifest at the end of life due to concern that such care may hasten death. The observation that admission to a hospital with EOL resources does not reliably reduce resource utilization, specifically treatment intensity or LOS, was consistent with prior multi-center studies evaluating the impact of palliative care consultation on outcomes of seriously ill patients.10,32 This suggests that ICU-based EOL care may not have an additive effect to palliative care consultation, possibly because these resources are not utilized together for any given patient or the additional impact of such care is limited. Another potential explanation may be that these resources are deployed late in the course of a patient’s hospitalization, once intensive therapies have already been instituted, thus limiting their potential to reduce treatment intensity or the length of a patient’s hospital stay.9,33
Finally, in adjusted analyses, the odds of being discharged to hospice were 58% higher among patients admitted to hospitals with EOL resources. Increasing hospice enrollment is a meaningful outcome, as it has previously been associated with improved quality of life among dying patients and their caregivers.34–36 In contrast to a similar study using New York state data,10 this finding was not driven by the availability of hospital-based palliative care clinicians but was rather associated with the availability of ICU-based resources, specifically protocols for withdrawal of life-sustaining therapy, although it is not clear whether the presence of any individual resource is meaningful or serves as a proxy for an ICU-level culture that prioritizes EOL care. This finding was robust in subgroup analyses of patients admitted with medical diagnoses and those admitted to hospitals with ≥200 beds, but it was not reproducible among patients with metastatic cancer or in the instrumental variable analysis of marginal patients. The null finding in the latter two groups is likely due to loss of precision resulting from a smaller sample size, particularly as all point estimates in the IV analysis were similar to the corresponding point estimates from the standard regression analysis but had wider confidence intervals. Alternatively, since the instrumental variable analysis is meant to approximate random assignment of patients to the exposure, the standard regression analysis may have been influenced by residual patient-level confounding, which was attenuated in the instrumental variable analysis, thus minimizing the possibility of a type I error and disclosing a true null result.
This study has several strengths. It includes a large and organizationally diverse population of ICUs. Additionally, unlike prior studies that have included only hospital-based EOL care resources, this study includes hospital- and ICU-based EOL care resources, thus providing a more holistic and nuanced assessment of the care that ICU patients may receive near the end of life.
Our study also has some key limitations. First, although the exposure was defined according to a survey about organizational resources, availability and utilization of a resource may not be highly correlated, potentially leading to a type II error. In addition, the inability to measure patient-level EOL care precludes definitive identification of the resources that lead to differences in patient outcomes, hindering implementation efforts by health system administrators and potentially explaining why our results are discordant with prior data that demonstrated associations between hospital-based palliative care resources and likelihood of discharge to hospice.10 However, in light of the current paucity of multi-center data evaluating the role of EOL care resources in ICU patient outcomes, we believe that this study meaningfully improves our understanding of the impact of such care, and provides rationale for future studies involving analysis of detailed clinical data to isolate the impact of specific processes of care. Second, the availability of EOL resources may have changed after the survey was administered in 2014. We limited the study period to one year after survey administration to minimize the impact of potential exposure misclassification. Third, risk adjustment models that use administrative claims data to predict mortality among critically ill patients do not perform as well as those that rely on physiologic data; therefore, patient-level residual confounding is possible.17 To mitigate this bias and approximate random assignment of patients to the exposure, we performed an instrumental variable analysis. However, given that the results of our standard regression analyses were not definitively confirmed by the instrumental variable analyses, possibly due to limitations of instrument strength and power, future randomized trials may be necessary to conclusively address concerns about both patient- and hospital-level confounding. Fourth, our results may not be generalizable to other states, as there are considerable regional variations in EOL practices.37 Fifth, the exclusion of admissions involving an inter-hospital transfer may have led to a loss of seriously ill patients for whom EOL care may be particularly relevant. However, due to the difficulty with accurately assigning an exposure to patients who have received care in more than ICU, we believe this exclusion was necessary to produced less biased results.
Conclusions
In conclusion, in this study examining the association of EOL care resources, including ICU-based resources, and outcomes of ICU patients in 78 hospitals in the state of Pennsylvania, we found no evidence to suggest that such care influences mortality, hospital LOS, or treatment intensity; however, patients admitted to hospitals possessing these resources were more likely to be discharged to hospice in some analyses. These results may provide further support for the integration of palliative care and critical care, as EOL care delivered in the ICU does not appear to hasten death but is associated with increased enrollment in hospice. Future studies should attempt to identify specific resources or processes of care that meaningfully change the care of patients at end of life. In addition, they should focus on the timing of EOL care relative to the institution of intensive therapies, as this may have significant implications for the potential impact on acute care resource utilization, including avoiding ICU admissions among patients who are unlikely to benefit from ICU-level care.38,39
Supplementary Material
Supplemental Digital Content
Tables
Table E1. Distribution of end-of-life care resources among study hospitals and patients.
Table E2. Secondary analyses of outcomes of patients admitted to hospitals with end-of-life resources compared to patients admitted to hospitals without end-of-life resources with each end-of-life resource individually.
Table E3. Sensitivity analyses of outcomes of patients admitted to hospitals with end-of-life resources compared to patients admitted to hospitals without end-of-life resources restricted to patients admitted to hospitals with one intensive care unit.
Table E4. Measured patient characteristics across levels of the instrumental variable.
Acknowledgements
We acknowledge Dylan Small, PhD and Vicky Tam, MA for their assistance with the instrumental variable analysis.
Conflicts of interest and source of funding: This project was funded by the NIH/NHLBI (DCA supported by T32 HL007891; MOH supported by K99 HL141678; and MPK supported by K08 HL116771). The authors have no conflicts of interest to disclose.
Copyright form disclosure: Dr. Ashana’s institution received funding from the National Institutes of Health (NIH)/NHLBI T32 HL007891. Drs. Ashana, Kohn, Madden, Harhay, and Kerlin received support for article research from the NIH. Dr. Umscheid’s institution received funding from AHRQ EPC contracts and the FDA, and he received funding from PCORI Advisory Panel. Dr. Stephens-Shields received funding from Juniper Pharmaceuticals, Oct 2016-Mar 2017. Dr. Harhay’s institution received funding from the NIH/NHLBI K99 HL141678. Dr. Kerlin’s institution received funding from the NIH/NHLBI K08 HL116771. Dr. Chen disclosed that he does not have any potential conflicts of interest.
Footnotes
This work was performed at the Perelman School of Medicine at the University of Pennsylvania in Philadelphia, Pennsylvania.
References
- 1.Teno JM, Gozalo P, Trivedi AN, et al. Site of Death, Place of Care, and Health Care Transitions Among US Medicare Beneficiaries, 2000–2015. JAMA. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook D, Rocker G. Dying with Dignity in the Intensive Care Unit. The New England Journal of Medicine. 2014;370(26):2506–2514. [DOI] [PubMed] [Google Scholar]
- 3.Kohn R, Madden V, Kahn JM, et al. Diffusion of Evidence-based Intensive Care Unit Organizational Practices. A State-Wide Analysis. Annals of the American Thoracic Society. 2017;14(2):254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lustbader D, Pekmezaris R, Frankenthaler M, et al. Palliative medicine consultation impacts DNR designation and length of stay for terminal medical MICU patients. Palliative & Supportive Care. 2011;9(4):401–406. [DOI] [PubMed] [Google Scholar]
- 5.Campbell ML, Guzman JA. Impact of a Proactive Approach to Improve End-of-Life Care in a Medical ICU. Chest. 2003;123(1):266–271. [DOI] [PubMed] [Google Scholar]
- 6.Norton SA, Hogan LA, Holloway RG, et al. Proactive palliative care in the medical intensive care unit: Effects on length of stay for selected high-risk patients. Critical Care Medicine. 2007;35(6):1530. [DOI] [PubMed] [Google Scholar]
- 7.Braus N, Campbell TC, Kwekkeboom KL. Prospective study of a proactive palliative care rounding intervention in a medical ICU. Prospective study of a proactive palliative care rounding intervention in a medical ICU. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyeremanteng K, Gagnon L-PP, Thavorn K, et al. The Impact of Palliative Care Consultation in the ICU on Length of Stay: A Systematic Review and Cost Evaluation. Journal of intensive care medicine. 2016. [DOI] [PubMed] [Google Scholar]
- 9.Zalenski RJ, Jones SS, Courage C, et al. Impact of Palliative Care Screening and Consultation in the ICU: A Multihospital Quality Improvement Project. Journal of pain and symptom management. 2017;53(1):5–12000. [DOI] [PubMed] [Google Scholar]
- 10.Hua M, Ma X, Morrison SR, et al. Association between the Availability of Hospital-Based Palliative Care and Treatment Intensity for Critically Ill Patients. Annals of the American Thoracic Society. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aslakson R, Cheng J, Vollenweider D, et al. Evidence-based palliative care in the intensive care unit: a systematic review of interventions. Journal of palliative medicine. 2014;17(2):219–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtis RJ, Nielsen EL, Treece PD, et al. Effect of a Quality-Improvement Intervention on End-of-Life Care in the Intensive Care Unit. American journal of respiratory and critical care medicine. 2011;183(3):348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khandelwal N, Kross EK, Engelberg RA. Estimating the effect of palliative care interventions and advance care planning on ICU utilization: a systematic review. Estimating the effect of palliative care interventions and advance care planning on ICU utilization: a systematic review. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. [DOI] [PubMed] [Google Scholar]
- 15.Weissman GE, Hubbard RA, Kohn R, et al. Validation of an Administrative Definition of ICU Admission Using Revenue Center Codes. Crit Care Med. 2017;45(8):e758–e762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnato AE, Farrell MH, Chang C-CH, et al. Development and Validation of Hospital “End-of-Life” Treatment Intensity Measures. Medical Care. 2009;47(10):1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Courtright KR, Halpern SD, Bayes B, et al. Adaptation of the Acute Organ Failure Score for Use in a Medicare Population. Critical care medicine. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quan H, Sundararajan V, Halfon P, et al. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Medical Care. 2005;43(11):1130. [DOI] [PubMed] [Google Scholar]
- 19.Elias KM, Moromizato T, Gibbons FK, et al. Derivation and Validation of the Acute Organ Failure Score to Predict Outcome in Critically Ill Patients: A Cohort Study. Critical Care Medicine. 2015;43(4):856. [DOI] [PubMed] [Google Scholar]
- 20.Kim MM, Barnato AE, Angus DC, et al. The effect of multidisciplinary care teams on intensive care unit mortality. Arch Intern Med. 2010;170(4):369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa DK, Wallace DJ, Kahn JM. The Association Between Daytime Intensivist Physician Staffing and Mortality in the Context of Other ICU Organizational Practices: A Multicenter Cohort Study. Crit Care Med. 2015;43(11):2275–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilcox ME, Chong CA, Niven DJ, et al. Do intensivist staffing patterns influence hospital mortality following ICU admission? A systematic review and meta-analyses. Crit Care Med. 2013;41(10):2253–2274. [DOI] [PubMed] [Google Scholar]
- 23.Kerlin MP, Adhikari NK, Rose L, et al. An Official American Thoracic Society Systematic Review: The Effect of Nighttime Intensivist Staffing on Mortality and Length of Stay among Intensive Care Unit Patients. Am J Respir Crit Care Med. 2017;195(3):383–393. [DOI] [PubMed] [Google Scholar]
- 24.Kross EK, Engelberg RA, Downey L, et al. Differences in End-of-Life Care in the ICU Across Patients Cared for by Medicine, Surgery, Neurology, and Neurosurgery Physicians. Chest. 2014;145(2):313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 26.Kahn JM, Goss CH, Heagerty PJ, et al. Hospital Volume and the Outcomes of Mechanical Ventilation. The New England Journal of Medicine. 2006;355(1):41–50. [DOI] [PubMed] [Google Scholar]
- 27.Hernán MA, Robins JM. Instruments for Causal Inference: An Epidemiologist’s Dream? Epidemiology. 2006;17(4):360. [DOI] [PubMed] [Google Scholar]
- 28.Valley TS, Sjoding MW, Ryan AM, et al. Association of Intensive Care Unit Admission With Mortality Among Older Patients With Pneumonia. JAMA. 2015;314(12):1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClellan M, McNeil BJ, Newhouse JP. Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. JAMA. 1994;272(11):859–866. [PubMed] [Google Scholar]
- 30.Rassen JA, Brookhart MA, Glynn RJ, et al. Instrumental variables II: instrumental variable application-in 25 variations, the physician prescribing preference generally was strong and reduced covariate imbalance. J Clin Epidemiol. 2009;62(12):1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newhouse JP, McClellan M. Econometrics in outcomes research: the use of instrumental variables. Annu Rev Public Health. 1998;19:17–34. [DOI] [PubMed] [Google Scholar]
- 32.Horton JR, Morrison SR, Capezuti E, et al. Impact of Inpatient Palliative Care on Treatment Intensity for Patients with Serious Illness. Journal of Palliative Medicine. 2016;19(9):936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Mahony S, Blank AE, Zallman L, et al. The Benefits of a Hospital-Based Inpatient Palliative Care Consultation Service: Preliminary Outcome Data. Journal of Palliative Medicine. 2005;8(5):1033–1039. [DOI] [PubMed] [Google Scholar]
- 34.Teno JM, Gozalo PL, Lee IC, et al. Does Hospice Improve Quality of Care for Persons Dying from Dementia? Journal of the American Geriatrics Society. 2011;59(8):1531–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallston KA, Burger C, Smith R, et al. Comparing the Quality of Death for Hospice and Non-Hospice Cancer Patients. Medical Care. 1988;26(2):177. [DOI] [PubMed] [Google Scholar]
- 36.Wright AA, Keating NL, Balboni TA, et al. Place of Death: Correlations With Quality of Life of Patients With Cancer and Predictors of Bereaved Caregivers’ Mental Health. Journal of Clinical Oncology. 2010;28(29):4457–4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnato AE, Herndon BM, Anthony DL, et al. Are Regional Variations in End-of-Life Care Intensity Explained by Patient Preferences?: A Study of the US Medicare Population. Medical Care. 2007;45(5):386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El Majzoub I, Qdaisat A, Chaftari PS, et al. Association of emergency department admission and early inpatient palliative care consultation with hospital mortality in a comprehensive cancer center. Support Care Cancer. 2018. [DOI] [PubMed] [Google Scholar]
- 39.Murphy K, Cooksley T, Haji-Michael P. Short- and long-term outcomes of patients with solid tumours following non-surgical intensive care admission. QJM. 2018;111(6):379–383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content
Tables
Table E1. Distribution of end-of-life care resources among study hospitals and patients.
Table E2. Secondary analyses of outcomes of patients admitted to hospitals with end-of-life resources compared to patients admitted to hospitals without end-of-life resources with each end-of-life resource individually.
Table E3. Sensitivity analyses of outcomes of patients admitted to hospitals with end-of-life resources compared to patients admitted to hospitals without end-of-life resources restricted to patients admitted to hospitals with one intensive care unit.
Table E4. Measured patient characteristics across levels of the instrumental variable.