Abstract
Extensive atherosclerotic plaque burden in the lower extremities often leads to symptomatic peripheral artery disease (PAD) including impaired walking performance and claudication. Interleukin-1β (IL-1β) may play an important pro-inflammatory role in the pathogenesis of this disease. Interruption of IL-1β signaling was hypothesized to decrease plaque progression in the leg macrovasculature and improve the mobility of patients with PAD with intermittent claudication. Thirty-eight patients (mean age 65 years; 71% male) with symptomatic PAD (confirmed by ankle–brachial index) were randomized 1:1 to receive canakinumab (150 mg subcutaneously) or placebo monthly for up to 12 months. The mean vessel wall area (by 3.0 T black-blood magnetic resonance imaging (MRI)) of the superficial femoral artery (SFA) was used to measure plaque volume. Mobility was assessed using the 6-minute walk test. Canakinumab was safe and well tolerated. Markers of systemic inflammation (interleukin-6 and high-sensitivity C-reactive protein) fell as early as 1 month after treatment. MRI (32 patients at 3 months; 21 patients at 12 months) showed no evidence of plaque progression in the SFA in either placebo-treated or canakinumab-treated patients. Although an exploratory endpoint, placebo-adjusted maximum and pain-free walking distance (58 m) improved as early as 3 months after treatment with canakinumab when compared with placebo. Although canakinumab did not alter plaque progression in the SFA, there is an early signal that it may improve maximum and pain-free walking distance in patients with symptomatic PAD. Larger studies aimed at this endpoint will be required to definitively demonstrate this.
ClinicalTrials.gov Identifier:
Keywords: canakinumab, interleukin-1β, intermittent claudication, peripheral artery disease (PAD)
Introduction
Peripheral artery disease (PAD) affects > 8.5 million people in the United States1 and > 150 million worldwide.2 Current drug therapies aimed at improving PAD symptoms (intermittent claudication (IC)) or patient mobility have limited efficacy,3 and providing disease modifying medical therapies in addition to revascularization would have an impact.4 Exercise has been shown to improve walking performance in numerous studies;5 however, many patients do not have access to supervised exercise programs,6 and fewer still remain compliant with this therapy.7-9 Therefore, novel pharmacologic therapies to improve PAD symptoms and functional capacity are urgently needed.10
Antagonism of interleukin (IL)-1β is an attractive target for ameliorating vascular inflammation associated with atherosclerosis.10 Indeed, in a small trial of patients with atherosclerosis and type 2 diabetes or impaired glucose tolerance, patients treated with an anti-IL-1β neutralizing antibody (canakinumab) showed a trend toward reduction in plaque progression in the common carotid arteries.11 Reducing inflammation might improve limb skeletal muscle perfusion as well. The present proof of concept study (, ‘Safety, Tolerability and Efficacy of Canakinumab on Leg Artery Structure in Patients with Peripheral Artery Disease’) examined the ability of canakinumab to improve vascular structure using 3.0 T black-blood magnetic resonance imaging (MRI) of the superficial femoral artery (SFA) and functional capacity measures (6-minute walk test (6MWT)) in patients with PAD and IC.
Methods
Study design
This was a multicenter, prospective, randomized, double-blind, placebo-controlled clinical trial involving outpatients with PAD and IC. Patients were enrolled (randomized and treated) between February 2013 and August 2016, and 129 were screened at 16 centers in the United States (n = 8), Germany (n = 7), and Jordan (n = 1). All patients provided written informed consent for participation. The protocol was approved by an institutional review board for each participating site.
Participants were eligible for participation if they met all the following criteria: (1) provided written informed consent; (2) were between the ages of 18 and 85 years (inclusive); (3) had IC (including atypical symptoms as adjudicated by the investigator) AND met any one of the ankle–brachial index (ABI) criteria (described below); (4) were on stable statin and aspirin (or other anti-platelet) therapy for at least 6 weeks prior to screening (unless there was a documented statin or aspirin intolerance or contraindication); (5) had baseline acquisition of evaluable MRI images of the SFA (adjudicated by a central reading lab); and (6) met criteria for vital signs ranges (oral body temperature: 35–37.5°C; systolic blood pressure (BP): 90–170 mmHg; diastolic BP: 50–100 mmHg; pulse rate 40–100 beats per min).
ABI criteria for enrollment were met if any of the following were present at screening or had been documented within 3 months of screening (provided there had been no peripheral revascularization in the interim): (1) a resting ABI of 0.40–0.90 (inclusive) in at least one leg; (2) a resting ABI > 0.90 but ⩽ 1.0, with a decrease in ABI of ⩾ 20% after exercise or a decrease in ankle pressure of ⩾ 30 mmHg with exercise in at least one leg; or (3) a resting ABI > 0.90 and an abnormal toe–brachial index (TBI) < 0.70.
Exclusion criteria included: (1) use of other investigational drugs; (2) history of hypersensitivity to canakinumab or other drugs of a similar class; (3) pregnant or nursing women, or women of child-bearing potential (unless using specified methods of contraception during the study treatment); (4) inability to ambulate more than 15 m; (5) use of the following medications: chronic systemic steroids or other systemic immunosuppression, any biologics targeting the immune system, or more than one chronic opiate for pain; (6) presence of a non-healing wound or active infection; (7) critical limb ischemia; (8) recent significant illness including myocardial infarction, stroke or major surgical procedures; (9) significant concomitant diseases (NYHA class IV heart failure, aortic aneurysm > 5 cm, uncontrolled diabetes (HbA1C > 9% or fasting glucose > 240 mg/dL), significant kidney or liver disease, significant anemia (Hb < 10.6 g/dL)); (10) history of malignancy (except localized skin basal cell carcinoma) within the past 5 years; (11) live vaccinations planned during the study or within the past 3 months; (12) history of untreated or active tuberculosis; (13) history of immunodeficiency diseases (including HIV) or viral hepatitis (B surface antigen or C); or (14) contraindication to MRI (e.g. metal implants). The study consisted of an up to 21-day screening period, a 7-day run-in/baseline period, a 12-month treatment period, and a 1-month follow-up period. During the screening period, the patients were educated on a recommended home-based exercise program, which was to begin on the day of signing consent and followed throughout the study. No supervised exercise program was performed. The MRI of the SFA (Figure 1) was obtained during the run-in period (considered the ‘baseline’ image set), and after 3 and 12 months of treatment. Additional assessments included functional testing by 6MWT performed at baseline, and after 1, 2, 3, 6, 9, and 12 months of treatment.
Figure 1.

MRI proton-density black-blood plaque images of the superficial femoral artery (arrow) in a single matched slice in a placebo-treated patient at baseline (left panel) and 12-month follow-up (right panel). Extensive plaque with a lipid component (low signal) and small lumen is noted. No significant change is seen in the plaque area at 12 months.
MRI, magnetic resonance imaging.
Patients were randomly assigned at a 1:1 ratio to receive monthly either canakinumab (150 mg/1 mL) or placebo (1 mL) subcutaneously (sc) for a total of 12 doses. Study drugs were provided by Novartis as pre-filled syringes. (See the online supplementary material for details.)
Percutaneous interventions (including peripheral revascularization) were permissible during the trial and did not result in patient withdrawal. However, data from patients who underwent such interventions were excluded from further analysis if appropriate (see statistical analysis below). The study was permitted to stop based on futility at any interim analysis. This study was stopped after the third interim analysis based on futility of the primary endpoint. All randomized subjects received at least one dose of canakinumab or placebo and were included in the safety analysis set. At the time the study was stopped for futility, 13 of 18 subjects treated with canakinumab and eight of 20 placebo subjects had received all 12 doses of canakinumab or placebo.
Study endpoints
The MRI endpoint was adapted from the approach developed at 1.5 T for assessment of the SFA vasculature in PAD. The method was determined to be reliable and reproducible with a test-retest value of R = 0.996.12 In the present study, all sites utilized a high-field (3.0 T) MRI scanner and applied black-blood imaging of the vessel wall using a dedicated phased-array radio frequency coil (Machnet BV, Roden, The Netherlands) to assess the atherosclerotic plaque burden in the SFA bilaterally. The SFA was selected because it is the most common site of lower extremity atherosclerosis and because it ultimately supplies blood to the calf muscle, which is more typically symptomatic in patients with PAD. The 6MWT was performed in a flat corridor, 30–50 m in length. Maximum walking distance (MWD) and pain-free walking distance (PFWD) were recorded as previously described.13 Both assessments (MRI and walking performance) were performed by investigators blinded to study treatment.
The primary objective of the study was to assess the effect of canakinumab on peripheral artery morphometry using MRI techniques after 12 months of treatment. The primary efficacy variable analyzed was mean vessel wall area (mm2). Ratios of 3- and 12-month values to baseline values of the mean vessel wall area were analyzed in a linear mixed effect model for repeated measures (MMRM). The MMRM included treatment, visit, the treatment-by-visit interaction, baseline, and the visit-by-baseline interaction as fixed effects. The ratio and baseline mean vessel wall area were logarithmically transformed prior to analysis. An unstructured variance-covariance structure was used. The least-squares (LS) mean and associated 90% CIs were calculated for each treatment group and for the difference between the treatment groups at each visit. In addition, the p-value for the treatment comparison at 3 months and 12 months was calculated. Results were back-transformed and expressed as geometric means of ratio to baseline and relative ratios of geometric means between treatment groups. Because mean vessel wall area typically has a skewed rather than normal distribution, a log transformation was needed in order to apply models for normally distributed data. Fifteen study completers in each group would have provided 80% power to detect a 10% reduction in plaque volume based on the variability of log ratio to baseline estimated from the trial (SD = 0.111). Power is 67% based on the final number of completers (12 vs 9). Secondary objectives were (1) to assess the safety and tolerability of monthly sc doses of 150 mg canakinumab in patients with PAD and IC, and (2) to assess the effect of canakinumab on markers of systemic inflammation, including high-sensitivity C-reactive protein (hsCRP) and IL-6 levels. Exploratory objectives included assessment of the effects of canakinumab on functional capacity as assessed by 6MWT. Parameters measured and analyzed were MWD and PFWD.
Statistical analysis
A repeated measures MMRM model of the same form as for the primary endpoint was fit to the change from baseline for each functional capacity variable. The LS means and associated 80% CIs for each treatment group and the estimated mean treatment difference, the p-value, and the corresponding two-sided CIs were extracted from the model for each visit. For these exploratory endpoints, nominal p-values < 0.05 (*) are indicated in the figures below as appropriate. For MWD and PFWD, 80% CIs were produced. Post-intervention functional capacity data were excluded from the analysis for any patients who underwent peripheral vascular interventions before the end of treatment. The same analysis described above for MWD and PFWD were performed using the last observation carried forward (LOCF) approach of imputation for missing values as a sensitivity analysis. SAS 9.4 Mixed Procedures (SAS Institute Inc., Cary, NC, USA) was used for statistical analyis. Additional statistical methods are included in the online supplementary material.
Results
Patient characteristics
A total of 38 patients (18 in the canakinumab group; 20 in the placebo group) were enrolled in the study. There were 31 patients who completed their 3-month visit (15, canakinumab; 16, placebo), but only 26 (14, canakinumab; 12, placebo) who completed the full 12-month treatment. Of the 26 patients, seven had prior angioplasty (three in the canakinumab group, four in the placebo group), 10 had prior peripheral stenting at sites other than the SFA (four in the canakinumab group, six in the placebo group), one patient in the canakinumab group had a prior peripheral endarterectomy, and two in the placebo group had prior peripheral bypass surgery. There were no statistical differences in prior procedures between groups. Discontinuations were higher in the placebo group (40%) than in the canakinumab-treated (22%) group. Reasons for discontinuation included withdrawal of consent (one in each group); protocol deviations (one in the canakinumab group, two in the placebo group); adverse events (n = 6: five in the placebo group, including two peripheral stenting procedures and three with worsening claudication; one death after myocardial infarction in the canakinumab group).
The baseline demographics are shown in Table 1. Baseline systolic blood pressure was higher in the placebo group. There were no significant differences in baseline medical history parameters collected, with the exceptions of myocardial infarction (n = 6 in the canakinumab group and n = 1 in the placebo group, p < 0.05) and gastrointestinal disorders (n = 13 in the canakinumab group and n = 7 in the placebo group, p < 0.05). Four patients in the canakinumab group and two patients in the placebo group were also being treated with cilostazol or pentoxifylline. No significant between-group differences in use of statins (100% of patients were on statins) or anti-platelet agents (92% were on aspirin) were found during the study. Eight of 18 in the canakinumab group were on high potency statins and the other 10 were on high doses of intermediate potency statins. In the placebo group, half of the 20 patients were on high potency statins and the other half on high doses of intermediate potency statins.
Table 1.
Baseline demographic and clinical characteristics.
| Characteristic | Canakinumab (n = 18) |
Placebo (n = 20) |
Total (n = 38) |
|---|---|---|---|
| Age, years | |||
| Mean (SD) | 66.0 (8.64) | 63.5 (7.98) | 64.7 (8.29) |
| Range, min, max | 47, 79 | 49, 79 | 47, 79 |
| Sex, n (%) | |||
| Male | 14 (77.8) | 13 (65.0) | 27 (71.1) |
| Female | 4 (22.2) | 7 (35.0) | 11 (28.9) |
| Race, n (%) | |||
| Black | 3 (16.7) | 3 (15.0) | 6 (15.8) |
| Caucasian | 15 (83.3) | 17 (85.0) | 32 (84.2) |
| Max 6MWT distance, m, mean (SD) | 353.58 (132.822) | 357.30 (117.362) | 353.58 (132.822) |
| Resting ABI, mean (SD) | 0.76 (0.271) | 0.70 (0.149) | 0.76 (0.271) |
| SBP, mmHg, mean (SD) | 129.7 (14.29)* | 141.6 (17.87) | |
| DBP, mmHg, mean (SD) | 74.2 (8.35) | 76.2 (10.41) |
p < 0.05 vs placebo.
ABI, ankle-brachial index; DBP, diastolic blood pressure; SBP, systolic blood pressure; 6MWT, 6-minute walk test.
Superficial femoral artery plaque burden and biomarkers
There were no baseline differences in SFA atherosclerotic plaque burden (vessel wall area, mean ± SD) (canakinumab group, 115 ± 32 mm2; placebo, 116 ± 44 mm2), or normalized mean plaque area (canakinumab group, 0.63 ± 0.19; placebo, 0.69 ± 13). No measurable change in the mean SFA vessel wall area was seen during the 12-month follow-up period in either the placebo or canakinumab-treated groups (Table 2). Similarly, no change in mean lumen area was seen in either group over the 12-month follow-up period (Table 3).
Table 2.
Absolute values and changes in mean vessel wall area in the SFA.
| Canakinumab (mm2) Mean ± SD |
Placebo (mm2) Mean ± SD |
Canakinumab-adjusted ratio to baseline, mean ± SD |
Placebo-adjusted ratio to baseline, mean ± SD |
|
|---|---|---|---|---|
| Baseline | 115.2 ± 31.9 n = 17 |
116.1 ± 43.9 n = 19 |
||
| 3 Months | 117.3 ± 26.7 n = 17 |
117.8 ± 44.5 n = 15 |
1.04 ± 0.13 | 0.99 ± 0.09 |
| 12 Months | 119.5 ± 40.9 n = 12 |
98.9 ± 28.3 n = 9 |
1.05 ± 0.08 | 1.00 ± 0.16 |
Geo, geometric; SFA, superficial femoral artery.
Table 3.
Absolute values and changes in mean lumen area in the SFA.
| Canakinumab (mm2) Mean ± SD |
Placebo (mm2) Mean ± SD |
Canakinumab-adjusted ratio to baseline, mean ± SD |
Placebo-adjusted ratio to baseline, mean ± SD |
|
|---|---|---|---|---|
| Baseline | 70.2 ± 26.9 n = 17 |
54.0 ± 28.2 n = 15 |
||
| 3 Months | 71.8 ± 41.0 n = 17 |
54.3 ± 30.6 n = 15 |
1.01 ± 0.08 | 0.99 ± 0.08 |
| 12 Months | 73.5 ± 40.0 n = 12 |
49.3 ± 22.4 n = 9 |
0.99 ± 0.07 | 1.00 ± 0.08 |
Geo, geometric; SFA, superficial femoral artery.
Mean baseline hsCRP levels (Figure 2A) were elevated (> 2.0 mg/dL) in both groups (geometric mean: canakinumab, 2.62 mg/L; placebo, 2.54 mg/L). There was a decrease in hsCRP seen in the canakinumab-treated group as early as 1 month after starting treatment and was noted throughout the study. Baseline IL-6 levels (Figure 2B) were also similar between the groups (geometric mean: canakinumab, 3.97 pg/mL; placebo, 3.05 pg/mL). There was a significant and sustained decrease in IL-6 in the canakinumab versus placebo-treated group beginning at 1 month and lasting through 12 months of treatment.
Figure 2.
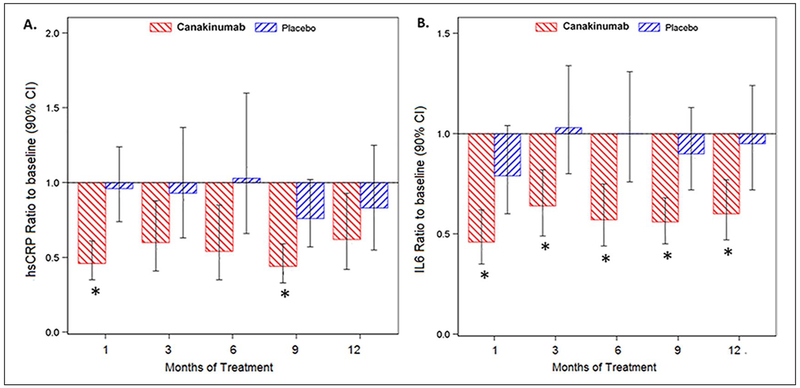
Canakinumab treatment leads to an overall decrease in inflammatory biomarkers. Patients were treated monthly with 150 mg canakinumab or placebo sc. There was a decrease (shown here as ratio to baseline) in hsCRP (A) and IL-6 (B) seen as early as 1 month of treatment that appears durable throughout the treatment period compared to placebo.
*p < 0.05 vs placebo.
hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; sc, subcutaneously.
Walking performance
There was no significant difference in the baseline MWD or PFWD in the patients randomized to canakinumab versus placebo (Table 1). Improvements in both MWD and PFWD were seen on the 6MWT in canakinumab as compared to placebo-treated patients (Figure 3). Significant differences were noted after 3 months of treatment, as canakinumab-treated patients walked 44 m (80% CI: 18.9, 69.1) further than placebo-treated patients (Figure 3A). This improvement continued at month 6 by 60 m (80% CI: 12.4, 107.9) and month 9 by 50 m (80% CI: 2.6, 97), but was not seen at later timepoints. There was a similar pattern in the improvement in PFWD seen in canakinumab versus placebo-treated patients, though the magnitude of effect was larger and there was a statistically significant improvement seen at both 3 and 6 months of treatment, with canakinumab-treated patients walking 58 m further than placebo controls at 3 months (80% CI: 30.4, 85.6) and 86 m further at 6 months (80% CI: 42.86, 128.20) (Figure 3B). There was no relationship between either hsCRP or IL-6 with walking performance at baseline or changes over time.
Figure 3.

Canakinumab treatment increases the 6MWT maximum and PFWD versus placebo. Patients were treated monthly with 150 mg canakinumab or placebo sc. MWD (A) and PFWD (B) were assessed at baseline and after 1, 2, 3, 6, 9, and 12 months of treatment. LS means (80% CI) of change from baseline in pain-free 6MWT distance are presented.
LS, least-squares; MWD, maximum walking distance; PFWD, pain-free walking distance; sc, subcutaneously; 6MWT, 6-minute walk test.
*p < 0.05 vs placebo.
Disproportionate dropout from the placebo group of patients with lower baseline MWD in the placebo group could result in apparent loss of efficacy (improvement over baseline) after 6 months. We observed baseline drift in the placebo group (see blue bars in Figure 3A, B) towards improved baseline MWD in the remaining patients at later timepoints, creating an imbalance in the baseline MWD between the two groups. We compared baseline MWDs in those patients who completed versus those who did not complete the full 12-month study (including 6MWT) for each treatment group (Table 4). To better understand how this confounding factor may have impacted the results, we performed a sensitivity analysis by carrying the last value measured forward (LOCF analysis) for any patient who did not complete the full 12-month follow-up (Figure 4).
Table 4.
Effects of dropout on baseline WD in canakinumab versus placebo groups.
| Canakinumab |
Placebo |
|||
|---|---|---|---|---|
| Completers | Non-completers | Completers | Non-completers | |
| n | 13 | 5 | 11 | 9 |
| Mean (SD) baseline WD, m | 364 (110) | 327 (193) | 416 (97) | 286 (103) |
| p-valuea | 0.610 | 0.009 | ||
Completers (those who completed the final 12-month 6MWT evaluation) versus non-completers in each treatment group.
WD, walking distance; 6MWT, 6-minute walk test.
Figure 4.
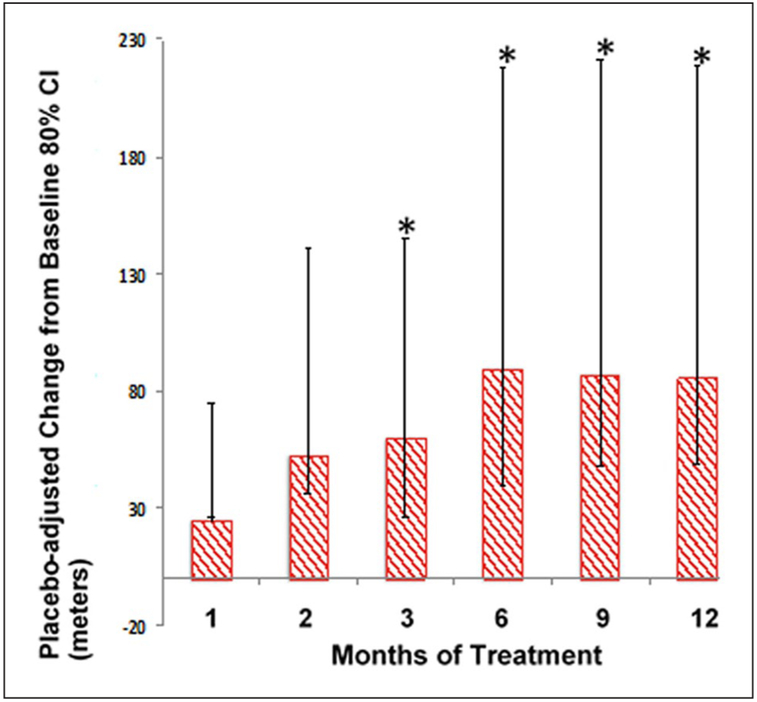
Sensitivity analysis (LOCF) supports improvement in PFWD with canakinumab treatment. LC means (80% CI) of placebo-adjusted change from baseline in maximum 6MWT distance are presented with the LOCF for any missing data.
*p < 0.05 versus placebo.
LC, least-squares; LOCF, last observation carried forward; PFWD, pain-free walking distance; 6MWT, 6-minute walk test.
Discussion
This study examined the effect of canakinumab compared to placebo on SFA plaque burden in patients with PAD on standard of care therapy including statins over a 1-year follow-up period by high-resolution 3.0 T MRI in a multicenter setting. No significant changes were observed in either group in SFA plaque burden during this time. The baseline normalized plaque areas seen in our PAD patient population are in keeping with data from the WALCS III study.14 These data suggest that interruption of IL-1β signaling is not sufficient to cause regression of plaque in the macrovasculature at this stage of symptomatic disease. Interestingly, plaque did not progress in the placebo group. This may be because of the guideline-directed therapy, including statins, with which the study patients were treated.
As an exploratory endpoint, we observed that canakinumab-treated patients demonstrated increasing MWD and PFWD, especially early in the course, in the absence of modulating SFA plaque burden. This raises the interesting hypothesis that anti-inflammatory therapy with canakinumab treatment might improve skeletal muscle perfusion and/or endothelial function, thus leading to improved walking performance. The most consistent change in inflammatory biomarkers in this study was a significant and sustained decrease in circulating IL-6 with canakinumab treatment. Patients with PAD with high IL-6 levels have more rapid functional declines (in 6MWT distance) than those with lower IL-6 levels.15 Interruption of IL-1β signaling has been shown to improve brachial forearm flow-mediated vasodilation in patients with rheumatoid arthritis.16 Further assessments of microvascular structure and function, particularly in the distal lower extremities, may provide more mechanistic insight into the observed functional performance responses for symptomatic patients with PAD with IC. Larger studies powered for this particular endpoint are needed to fully understand the potential role of canakinumab.
Overall, the effect of canakinumab on PFWD was greater than that on MWD. This may suggest that there is an additional effect on pain signal generation (perhaps by decreasing limb ischemia and lactate production) or pain perception. The idea that IL-1 modulates pain is supported by pre-clinical murine studies showing that chronic IL-1 blockade attenuated neuropathic pain.17
In the recently reported CANTOS study, which enrolled high risk patients with hsCRP > 2.0 mg/L (median ~4.1 mg/L), those patients whose hsCRP fell to or below this enrollment threshold of 2 mg/L at 3 months also appeared to have the most significant improvements in outcomes.18,19 Though we did not enrich our population for high hsCRP, the mean hsCRP at enrollment was still mildly elevated (mean ~2.5 mg/L), suggesting this is a high-inflammatory burden population as has been previously reported in other studies of PAD.20 Further study is required to test whether functional responses to canakinumab can be predicted by such a threshold response metric in patients with more elevated levels of baseline hsCRP.
Study limitations
This study was limited by its early termination, having not met its primary endpoint involving prevention of SFA plaque progression at the third interim analysis. Loss of patients, particularly in the placebo group, may have resulted in informative censoring, despite the use of a mixed model for analysis of the functional performance data. The discrepancy between the primary and sensitivity (LOCF) analyses suggests that the patients who completed the study may not completely reflect the entire cohort evaluated over the full study course. Therefore, effects of canakinumab might have been sustained if all patients were able to be followed throughout the entire treatment period. This study was not designed to assess the impact of exercise on PAD and claudication. Patients were given instructions on the importance of exercise prior to the study and encouraged to perform such exercise, though compliance with exercise was not specifically measured. There was substantial dropout at the later time points and thus the findings regarding walking performance should be viewed as preliminary.
Conclusions
In summary, no impact of canakinumab was noted on plaque burden in the SFA in patients with advanced PAD on guideline-directed therapy. However, in an exploratory analysis, patients showed improved MWD and PFWD with canakinumab treatment. Properly powered studies of canakinumab in PAD aimed at this particular endpoint are required to determine if pharmacologic inhibition of IL-1β has a role to improve exercise performance. Studies of skeletal muscle perfusion, energetics and endothelial function with anti-inflammatory therapies could add insight to any potential mechanistic benefit.
Supplementary Material
Acknowledgements
The authors wish to acknowledge the following contributions: Eric Svensson (manuscript reviewer) and Stephen Gleason (clinical trial leader).
Funding and Role of Sponsor
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the study was funded by Novartis Institutes for BioMedical Research. Novartis was involved with study design, interpretation, and manuscript preparation; data analysis was independent.
Footnotes
Declaration of conflicting interests
The authors declared the following potential conflict of interest with respect to the research, authorship, and/or publicaton of this article: Kerry S Russel, Denise Yates, Andrea Feller, Ping Mahling, Laurence Colin, Timothy Clough, Tianke Wang, and Craig T Basson are employees of Novartis. The other authors declared no potential conflicts of interest.
Supplemental material
The supplementary material is available online with the article.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: A report from the American Heart Association. Circulation 2017; 135: e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowkes FG, Aboyans V, Fowkes FJ, et al. Peripheral artery disease: Epidemiology and global perspectives. Nat Rev Cardiol 2017; 14: 156–170. [DOI] [PubMed] [Google Scholar]
- 3.Ratchford EV. Medical management of claudication. J Vasc Surg 2017; 66: 275–280. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch AT, Duval S. The global pandemic of peripheral artery disease. Lancet 2013; 382: 1312–1314. [DOI] [PubMed] [Google Scholar]
- 5.Murphy TP, Cutlip DE, Regensteiner JG, et al. CLEVER Study Investigators. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: Six-month outcomes from the Claudication: Exercise Versus Endoluminal Revascularization (CLEVER) study. Circulation 2012; 125: 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harwood AE, Smith GE, Cayton T, et al. A systematic review of the uptake and adherence rates to supervised exercise programs in patients with intermittent claudication. Ann Vasc Surg 2016; 34: 280–289. [DOI] [PubMed] [Google Scholar]
- 7.Mays RJ, Regensteiner JG. Exercise therapy for claudication: Latest advances. Curr Treat Options Cardiovasc Med 2013; 15: 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas TL, Lloyd PG, Yang HT, et al. Exercise training and peripheral arterial disease. Compr Physiol 2012; 2: 2933–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamburg NM, Balady GJ. Exercise rehabilitation in peripheral artery disease: Functional impact and mechanisms of benefits. Circulation 2011; 123: 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qamar A, Rader DJ. Effect of interleukin 1β inhibition in cardiovascular disease. Curr Opin Lipidol 2012; 23: 548–553. [DOI] [PubMed] [Google Scholar]
- 11.Choudhury RP, Birks JS, Mani V, et al. Arterial effects of canakinumab in patients with atherosclerosis and type 2 diabetes or glucose intolerance. J Am Coll Cardiol 2016; 68: 1769–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isbell DC, Meyer CH, Rogers WJ, et al. Reproducibility and reliability of atherosclerotic plaque volume measurements in peripheral arterial disease with cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2007; 9: 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montgomery PS, Gardner AW. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J Am Geriatr Soc 1998; 46: 706–711. [DOI] [PubMed] [Google Scholar]
- 14.McDermott MM, Liu K, Carr J, et al. Superficial femoral artery plaque, the ankle-brachial index, and leg symptoms in peripheral arterial disease: The Walking and Leg Circulation Study (WALCS) III. Circ Cardiovasc Imaging 2011; 4: 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDermott MM, Liu K, Ferrucci L, et al. Relation of interleukin-6 and vascular cellular adhesion molecule-1 levels to functional decline in patients with lower extremity peripheral arterial disease. Am J Cardiol 2011; 107: 1392–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikonomidis I, Lekakis JP, Nikolaou M, et al. Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation 2008; 117: 2662–2669. [DOI] [PubMed] [Google Scholar]
- 17.Gabay E, Wolf G, Shavit Y, et al. Chronic blockade of interleukin-1 (IL-1) prevents and attenuates neuropathic pain behavior and spontaneous ectopic neuronal activity following nerve injury. Eur J Pain 2011; 15: 242–248. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, Everett BM, Thuren T, et al. CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377: 1119–1131. [DOI] [PubMed] [Google Scholar]
- 19.Ridker PM, MacFadyen JG, Everett BM, et al. CANTOS Trial Group. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: A secondary analysis from the CANTOS randomised controlled trial. Lancet 2018; 391: 319–328. [DOI] [PubMed] [Google Scholar]
- 20.Crawford JR, Trial J, Nambi V, et al. Plasma levels of endothelial microparticles bearing monomeric C-reactive protein are increased in peripheral artery disease. J Cardiovasc Transl Res 2016; 9: 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


