Abstract
Background/Objective:
Staging and type of resection for rectal neuroendocrine tumors (R-NETS) relies on preoperative identification of lymph node (LN) involvement. Study objective was to develop a Preoperative Rectal Stratification Score (PReSS) for LN-positivity and to assess the association of PReSS with overall survival (OS).
Methods:
All patients in the National Cancer Database (2004–2014) with non-metastatic/nonfunctional R-NETS were included. Tumor size was divided into three categories (<1, 1–2, and ≥2 cm).
Results:
Among 383 patients, median age was 57 years, 52% were male (n = 200), median tumor size was 1.4 cm, 43% had positive LNs (n = 163). On univariate analysis, age > 60, poorly differentiated grade, depth of invasion past submucosa, and size >1 cm were associated with LN positivity. On multivariable analysis, depth of invasion past submucosa, and increasing tumor size >1 cm remained associated with LN positivity. As these can be determined preoperatively, incidence of LN positivity was determined for each combination of tumor size and depth of invasion. Each variable was assigned a score to create a PReSS of four groups (0–3) associated with an increasing rate of LN-positivity (PReSS group 0: 11%, 1: 38%, 2: 50%, 3: 78%, P < .01). PReSS correlated with 10-year OS (PReSS 0: 90%; 1: 81%; 2: 59%; 3: 41%).
Conclusion:
For R-NETS, depth of invasion and tumor size predict LN positivity and both can be obtained preoperatively. PReSS incorporates both variables and stratifies tumors into four risk groups of progressively increasing LN positivity and should be used to guide surgical approach.
Keywords: endoscopic resection, endoscopic ultrasound, low anterior resection, lymph node metastasis, rectal neuroendocrine tumor
1 |. INTRODUCTION
The biologic behavior of neuroendocrine tumors and their associated outcomes vary widely based on the anatomic location of the primary tumor. As a result, guidelines regarding management are site-specific. For rectal neuroendocrine tumors, resection is routinely recommended for all tumors, but the type of resection depends predominantly on tumor size given its previously established concordance with lymph node status which has historically been shown to predict worse tumor biology.1–5 Consequently, successful staging and selection for type of resection for rectal neuroendocrine tumors relies on preoperative identification of lymph node involvement. However, accurate preoperative evaluation of lymph node status remains difficult in clinical practice.
Multiple modalities are currently available for the preoperative evaluation and staging of rectal tumors.6 Endorectal ultrasound (ERUS) is the most commonly used modality, and its accuracy for assessing depth of invasion ranges from 75% to 90%.7–10 In regard to nodal status, the accuracy of ERUS is equally variable ranging from 75% to 88% due to its potential inability to assess involved nodes that may exist higher or deeper in the mesorectum. Similarly, magnetic resonance imaging (MRI) has demonstrated accuracy rates of up to 80% for depth of invasion, but only approximately 60% for nodal status.11,12 Due to this potential low accuracy in assessing nodal status, and in the absence of other specific biomarkers, clinicians and expert consensus guidelines routinely rely on tumor size for recommendations regarding operative management.
Indeed, current guidelines from the National Comprehensive Cancer Network (NCCN) recommend endoscopic resection of tumors smaller than 1 cm and radical resection with a low anterior resection or an abdominoperineal resection for tumors larger than 2 cm. For tumors 1 to 2 cm in size, controversy remains regarding their optimal management and current guidelines recommend preoperative staging with ERUS or MRI to assess for the depth of invasion and evaluate candidacy for an endoscopic versus formal, anatomic resection.13 The European Neuroendocrine Tumor Society and The North American Neuroendocrine Tumor Society propose similar size-based guidelines.14,15
Given the potential morbidity and long-term effects on quality of life associated with a formal resection of a rectal tumor, accurately predicting nodal status based on other preoperatively available pathologic variables without compromising long-term outcomes is paramount. Therefore, the primary aim of this study was to devise a clinically applicable risk score for lymph node positivity for rectal neuroendocrine tumors using other preoperatively known clinicopathologic factors that better discriminate lymph node involvement rather than tumor size alone.
2 |. METHODS
2.1 |. Data source and study variables
The National Cancer Database (NCDB) is a hospital-based registry, a joint program of the American College of Surgeons Committee on Cancer and the American Cancer Society, with data sources from more than 1500 Commission on Cancer-accredited hospitals.16 A query of the NCDB registry from 2004 to 2014 was performed to identify patients with non-functional rectal neuroendocrine tumors according to International Classification of Diseases for Oncology-3 codes including 8240 (carcinoid not otherwise specific) and 8246 (neuroendocrine carcinoma). The analysis excluded patients with metastatic disease, palliative resections, or 30-day mortality. Patients were further excluded if they had missing data with regard to tumor size, pathologic T-stage, and pathologic lymph node status. Resection type was categorized as local resection (excisional biopsy in combination with polypectomy, curette/fulguration, and electrocautery) and formal/anatomic resection (low anterior resection, abdominoperineal resection, and Hartmann procedure). Tumor size was divided into three categories (<1, 1–2, and ≥2 cm). Patient demographics, clinicopathologic variables, and survival data were extracted. Tumor staging was based on the American Committee on Cancer (AJCC) 6th and 7th edition guidelines. The primary outcome was lymph node positivity after surgery. The secondary outcome was overall survival.
2.2 |. Statistical analysis
Descriptive statistics for each variable were reported. The χ2 test was used for comparison of discrete variables, and the analysis of variance test was used for comparison of continuous variables between the two cohorts. A Cox proportional hazards model was used to assess the association between clinicopathologic variables and survival. To create the Preoperative Rectal Stratification Score (PReSS), univariate logistic regression analysis was used to determine the association of clinicopathologic factors with lymph node positivity. Given the high accuracy of ERUS and MRI to assess depth of invasion preoperatively, pathologic T‐stage was utilized as a surrogate for this information that would otherwise normally be readily available preoperatively. A multivariable model was then constructed using sequential backward selection. Variables statistically significantly associated with lymph node positivity (P < .05) on multivariable analysis were used to create PReSS. Each selected variable was assigned a score, from 0 to 2, based on the magnitude of the model coefficient. The incidence of lymph node positivity was determined for each combination of variables and groups were combined based on similar rates of lymph node involvement. These were incorporated to create a PReSS of four groups (0–3) associated with an increasing rate of lymph node positivity.
Kaplan‐Meier analysis and Cox‐regression analysis were used to determine the association of PReSS with overall survival. Statistical significance was pre‐defined as two‐tailed P < .05. All statistical analyses were conducted using SAS, version 9.4 (SAS Institute), and SAS macros developed at the Biostatistics and Bioinformatics at Winship Cancer Institute.17
3 |. RESULTS
3.1 |. Demographic and clinicopathologic characteristics
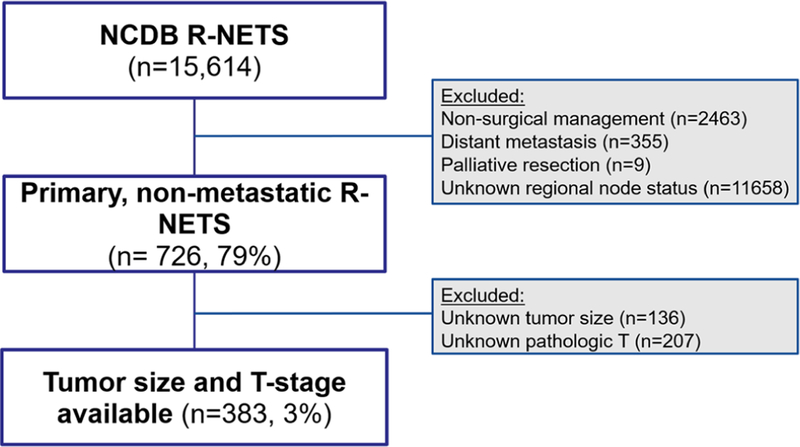
Among the 12384 patients with nonmetastatic rectal neuroendocrine tumors in the NCDB, a total of 383 patients met inclusion criteria (Figure 1). Demographic and histopathologic data are listed in Table 1. Mean patient age at diagnosis was 58 ± 12.4 years with a similar distribution of female (n = 183, 48%) and male (n = 200, 52%) patients. Mean tumor size was 2.1 ± 2.2 cm with 44% (n = 169) tumors measuring <1 cm, 16% (n = 61) tumors measuring 1–2 cm, and 40% (n = 153) tumors measuring ≥2 cm. On final pathologic analysis, the majority of patients had well‐differentiated tumors (39%, n = 149), and pathologic T1 (submucosa) stage tumors (52%, n = 198). Median follow‐up for the entire cohort was 45.3 months. Data on complications and recurrence were not available from the NCDB source to evaluate.
FIGURE 1.
Flow diagram of inclusion and exclusion criteria of patients in the National Cancer Database (NCDB) diagnosed with rectal neuroendocrine tumors. R‐NETS, rectal neuroendocrine tumors
TABLE 1.
Demographic and clinicopathologic factors of the entire cohort and comparing lymph node‐negative vs lymph node‐positive cohorts
| All patients n = 383 (%) | LN negative n = 220 (%) | LN positive n = 163 (%) | LN negative vs LN positive P value | |
|---|---|---|---|---|
| Demographic variables | ||||
| Age at diagnosis (mean ± std) | 58 ± 12.4 | 57 ± 11.9 | 59 ± 12.9 | 0.11 |
| Sex | ||||
| Female | 183 (48) | 103 (47) | 80 (49) | 0.66 |
| Male | 200 (52) | 117 (53) | 83 (51) | |
| Race | ||||
| White | 244 (64) | 149 (68) | 95 (58) | 0.06 |
| Non-white | 183 (48) | 71 (32) | 68 (42) | |
| Charlson-Deyo score | ||||
| 0 | 297 (78) | 169 (77) | 128 (79) | 0.89 |
| 1 | 70 (18) | 42 (19) | 28 (17) | |
| 2+ | 16 (4) | 9(4) | 7(4) | |
| Histopathologic factors Tumor size, cm | ||||
| <1 | 169 (44) | 144 (65) | 25 (15) | <0.01 |
| 1–1.99 | 61 (16) | 28 (13) | 33 (20) | |
| ≥2 | 153 (40) | 48 (22) | 105 (64) | |
| Tumor differentiation | ||||
| Well | 149 (39) | 97 (44) | 52 (32) | <0.01 |
| Moderate | 35 (9) | 15 (7) | 20 (12) | |
| Poor/undifferentiated | 86 (22) | 29 (13) | 57 (35) | |
| Not determined | 113 (30) | 70 (32) | 34 (21) | |
| AJCC pathologic T stage | ||||
| T1 (submucosa) | 198 (52) | 160 (73) | 38 (23) | <0.01 |
| T2 (muscularis propria) | 65 (17) | 34 (15) | 31 (19) | |
| T3 (through muscular propria) | 97 (25) | 19 (9) | 78 (48) | |
| T4 (adjacent organs) | 23 (6) | 7 (3) | 16 (10) | |
| Type of Resection | ||||
| Local | 77 (20) | 72 (33) | 5 (3) | <0.01 |
| Anatomic | 306 (80) | 148 (67) | 158 (97) | |
| Surgical margins | ||||
| Negative | 323 (84) | 191 (87) | 132 (81) | <0.01 |
| Positive | 48 (13) | 17 (8) | 31 (19) | |
| Unknown | 12 (3) | 12 (5) | 0(0) | |
| Follow-up (median, mo) | 45.3 | 39 | 51.6 | <0.001 |
Note: Percentages in parentheses are based on cohort size.
Abbreviations: AJCC, American Committee on Cancer; LN, lymph node.
Bold indicates statistical significance.
Among the entire cohort, 43% of patients had positive lymph nodes (n = 163). There were no significant differences between lymph node‐negative and lymph node‐positive patients when comparing patient characteristics including age at diagnosis, sex, race, and Charlson‐Deyo score (all P > .05, Table 1). Compared to patients with negative lymph nodes, patients with positive lymph nodes had a larger proportion of ≥2 cm tumors (153 patients (40%) vs 48 patients (22%); P < .01), more poorly or undifferentiated tumors (57 patients (35%) vs 29 patients (13%); P < .01), a higher proportion of pathologic T3 (through muscularis propria) stage tumors (78 patients (48%) vs 19 patients (9%); P < .01), and were more likely to undergo an anatomic resection (158 patients (97%) vs 148 patients (67%); P < .001). On Cox regression for overall survival, lymph node positivity was associated with worse overall survival (HR 2.55, 95% CI 1.48–4.39, P < .01), even when accounting for other negative prognostic factors such as age older than 60 years and poorly or undifferentiated tumor grade.
3.2 |. Prognostic factors for lymph node positivity
Clinicopathologic factors associated with lymph node positivity are listed in Table 2 and include age older than 60, tumor size ≥1 cm, moderate, poor or undifferentiated tumor grade, and pathologic T‐stage past the submucosa. On multivariable binary logistic regression, two factors persisted as being associated with lymph node positivity and include tumor size ≥1 cm (1–2 cm: HR 4.24, 95% CI 2.08–8.65, P < .01; ≥ 2 cm: HR 3.61 95% CI 1.64–7.92, P < .01), and pathologic T‐stage past the submucosa (T2—muscularis propria: HR 2.03, 95% CI 0.97–4.23, P < .01; T3—through muscularis propria: HR 7.41, 95% CI 3.28–16.83, P = .06, T4—adjacent organs: HR 4.08, 95% CI 1.29–12.91, P = .02)
TABLE 2.
Binary logistic regression: clinicopathologic factors associated with positive lymph node status
| Variable | Univariable logistic regression | Multivariable logistic regression | ||
|---|---|---|---|---|
| OR (95% CI) |
P value |
OR (95% CI) |
P value |
|
| Age at diagnosis, y | ||||
| ≤60 | Reference | - | ||
| >60 | 2.15 (1.42–3.26) | <.01 | - | |
| Sex | ||||
| Male | Reference | - | ||
| Female | 1.09 (0.73–1.64) | .66 | - | |
| Race | ||||
| White | Reference | - | ||
| Non-White | 1.50 (0.99–2.29) | .06 | - | |
| Charlson-Deyo score | ||||
| 0 | Reference | - | ||
| 1 | 1.03 (0.37–2.83) | .64 | - | |
| 2+ | 0.88 (0.52–1.50) | .96 | - | |
| Tumor size, cm | ||||
| <1 | Reference | Reference | ||
| 1–1.99 | 6.79 (3.51–13.12) | <.01 | 4.24 (2.08–8.65) |
<.01 |
| ≥2 | 12.60 (7.31–21.73) |
<.01 | 3.61 (1.64–7.92) |
<.01 |
| Tumor differentiation | ||||
| Well | Reference | - | ||
| Moderate | 2.49 (1.18–5.26) | .02 | - | |
| Poor/ | 3.67 (2.09–6.42) | <.01 | - | |
| undifferentiated | ||||
| Not determined | 0.80 (0.48–1.36) | .41 | - | |
| AJCC pathologic T | ||||
| T1 (submucosa) | Reference | Reference | ||
| T2 (muscularis propria) | 3.84 (2.10–7.01) | <.01 | 2.03 (0.97–4.23) |
.06 |
| T3 (through muscular propria) | 17.28 (9.36–31.93) |
<.01 | 7.41 (3.26–16.83) |
<.01 |
| T4 (adjacent organs) | 9.62 (3.70–25.03) | <.01 | 4.08 (1.29–12.91) |
.02 |
Note: Number of observations in the original data set = 383. Number of observations used = 383. Bold indicates statistical significance.
The logistic regression modeled the probability of LN = positive. Backward selection with an α level of removal of 0.05 was used. The following variables were removed from the model: age, sex, Charlson‐Deyo score, and tumor differentiation.
Abbreviations: AJCC, American Committee on Cancer; CI, confidence interval; OR, odds ratio.
3.3 |. PReSS
When creating the PReSS, the two factors associated with increased odds of lymph node positivity were considered including tumor size (<1, 1–1.99, and ≥2 cm), and pathologic T‐stage (T1: submucosa, T2: muscularis propria, T3: through muscularis propria, T4: adjacent organs) as each of these tumor characteristics can be determined pre‐operatively by initial endoscopic evaluation and/or MRI. The incidence of lymph node positivity was determined for each combination of tumor size and depth of invasion and groups were then combined based on similar rates of lymph node involvement (Table 3). Each factor was assigned a score from a scale of 0 to 2 points to create four risk groups 0 to 3 (tumor size: <1 cm—0 points, 1–1.99 cm—1 point, ≥2 cm—1 point, pathologic T‐stage: T1 submucosa—0 points, T2 muscularis propria—1 point, T3 through muscularis propria—2 points, T4 adjacent organs—2 points). The percentage of patients with positive lymph nodes increased with increasing risk score: risk group 0 (0 points): 13% (n = 114/383), risk group 1 (1 point): 33%–42% (n = 54/383), risk group 2 (2 points): 50%–54% (n = 42/383), risk group 3 (3 points): 70%–83% (n = 110/383). Only two patients with invasion through the muscularis propria had tumors that were <1 cm, both of whom were lymph node negative, and no tumors invading into adjacent organs were <2 cm. Notably, when evaluating the entire cohort of 12 172 patients, there were only 12 patients that had <1 cm tumors invading through the muscularis propria, and 0 patients that had <2 cm tumors invading into adjacent organs, making these groups negligible.
TABLE 3.
Preoperative Rectal Stratification Score (PReSS) for lymph node positivity
| <1cm (0 points) | 1–1.99 cm (1 point) | ≥2 cm (1 point) | |
|---|---|---|---|
| Submucosa (0 points) | 13% (0 points) | 42% (1 point) | 33% (1 point) |
| Muscularis propria (1 point) | 36% (1 point) | 54% (2 points) | 50% (2 points) |
| Through muscularis propria (2 points) | N/A | 80% (3 points) | 83% (3 points) |
| Adjacent organs (2 points) | N/A | N/A | 70% (3 points) |
3.4 |. Association of PReSS with overall survival
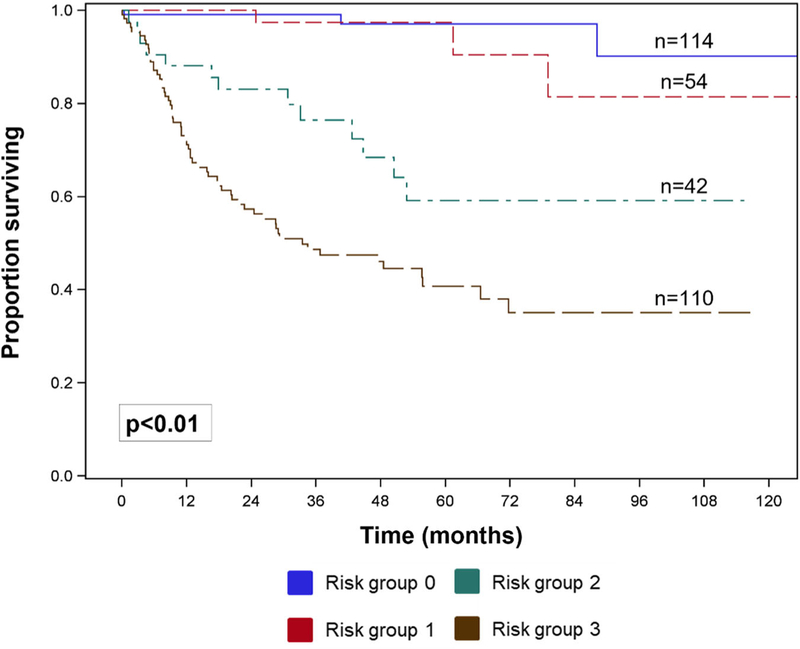
On Kaplan‐Meier analysis, there was a decreased 10‐year overall survival with increase PReSS group (PReSS group 0: 90%; group 1: 81%; group 2: 59%; group 3: 41%, P < .01, Figure 2). Clinicopathologic factors significantly associated with worse overall survival are listed in Table 4 and include age older than 60 years, Charlson‐Deyo score ≥2, poorly or undifferentiated tumor grade, positive resection margin status, and PReSS groups 2 and 3. On multivariable analysis, three factors persisted as being associated with worse overall survival and include age older than 60 years (HR 1.91, 95% CI 1.17–3.12, P < .01), poorly or undifferentiated tumor grade (HR 3.65, 95% CI 1.87–7.11, P < .01), PReSS group 2 (HR 5.47, 95% CI 1.50–19.98, P = .01), and PReSS group 3 (HR 8.61, 95% CI 2.53–29.22, P < .01).
FIGURE 2.
Overall survival by PReSS, Preoperative Rectal Stratification Score risk groups
TABLE 4.
Clinicopathologic factors associated with overall survival for entire cohort
| Variable | Univariable Cox regression | Multivariable Cox regression | ||
|---|---|---|---|---|
| HR (95% CI) |
P value |
HR (95% CI) |
P value |
|
| Age at diagnosis, y | ||||
| ≤60 | Reference | Reference | ||
| >60 | 3.07 (1.92–4.90) | <.01 | 1.91 (1.17–3.12) |
<.01 |
| Sex | ||||
| Male | Reference | - | ||
| Female | 1.13 (0.73–1.76) | .59 | - | |
| Race | ||||
| White | Reference | - | ||
| Non - White | 2.06 (1.23–3.44) | .06 | - | |
| Charlson-Deyo score | ||||
| 0 | Reference | - | ||
| 1 | 1.25 (0.72–2.19) | .43 | - | |
| 2+ | 2.51 (1.14–5.51) | .02 | - | |
| Tumor differentiation | ||||
| Well | Reference | Reference | ||
| Moderately | 1.48 (0.48–4.58) | .05 | 1.32 (0.42–4.14) |
.63 |
| Poorly/ | 9.26 | <.01 | 3.65 | <.01 |
| undifferentiated | (4.96–17.30) | (1.87–7.11) | ||
| Not determined | 0.55 (0.22–1.39) | .21 | 0.51 (0.20–1.31) |
.16 |
| Margin status | ||||
| Negative | Reference | - | ||
| Positive | 2.75 (1.63–4.63) | <.01 | - | |
| PReSS risk group | ||||
| Group 0 | Reference | Reference | ||
| Group 1 | 1.97 (0.40–9.76) | 0.41 | 1.35 (0.27–6.74) |
.72 |
| Group 2 | 11.70 (3.33–41.06) |
<.01 | 5.47 (1.50–19.98) |
.01 |
| Group 3 | 25.49 (7.99–81.31) |
<.01 | 8.61 (2.53–29.22) |
<.01 |
Note: Number of observations in the original data set = 383. Number of observations used = 320. Bold indicates statistical significance. Backward selection with an α level of removal of .05 was used. The following variables were removed from the model: sex, Charlson‐Deyo score, margin status.
Abbreviaitons: CI, confidence interval; HR, hazard ratio; PReSS, Pre-operative Rectal Stratification Score.
4 |. DISCUSSION
The rectum is one of the most common sites of gastrointestinal neuroendocrine tumors, and rectal neuroendocrine tumors have increased in incidence over the past decades, largely due to widespread use of screening colonoscopies.18 Historically, rectal neuroendocrine tumors have demonstrated the best prognosis of all gastrointestinal neuroendocrine tumors with a 5‐year survival of 96%.19 However, it has been recognized recently that not all rectal neuroendocrine tumors behave in an indolent fashion, and prognosis is largely dependent on stage. Indeed, the AJCC staging classifies all node‐positive colorectal neuroendocrine tumors as stage III, and according to a population‐based study, the 5‐year survival of stage III, node‐positive tumors is only 35%.2,20 Therefore, staging patients with the appropriate surgical resection is paramount to adequately educate patients on their prognosis and guide further treatment and surveillance strategies. Preoperative knowledge of lymph node positivity would serve to accurately select patients for either local or anatomic resection. Therefore, the aim of this study was to devise a clinically applicable risk score for lymph node positivity using other pre‐operatively known clinicopathologic factors that better discriminate lymph node involvement rather than size alone. Our results are in accord with previous studies that have demonstrated the negative prognostic value on survival of lymph node positivity in rectal neuroendocrine tumors (HR 2.55, 95% CI 1.48–4.39, P < .01; Table 2). In the current study, two factors were strongly associated with lymph node positivity and included tumor size and depth of invasion. These two variables, which can be easily and accurately assessed preoperatively with an endorectal ultrasound or MRI, were incorporated into the PReSS which stratifies tumors into four risk groups (0–3) of progressively increasing lymph node positivity ranging from 11% to as high as 78% (PReSS group 0: 11%, group 1: 38%, group 2: 50%, group 3: 78%, P < .01, Table 4). Importantly, PReSS is also able to predict 10‐year overall survival (PReSS group 0: 90%; group 1: 81%; group 2: 59%; group 3: 41%, Figure 2).
As lymph node metastases have demonstrated a negative prognostic role across most neuroendocrine tumor sites, including pancreas, and small bowel, various other studies have sought to predict nodal positivity with pre‐operatively available clinicopathologic variables to guide patient management.21 Our group recently demonstrated that for pancreatic neuroendocrine tumors, node positivity is associated with a worse 5‐year recurrence‐free survival with a minimum of seven lymph nodes required for adequate staging.22 Similarly, small bowel neuroendocrine tumors have demonstrated aggressive behavior, and guidelines recommend radical resection for all tumors with routine lymphadenectomy.23,24 Conversely, the role of lymphadenectomy for duodenal neuroendocrine tumors remains ill‐defined and although regional nodal involvement may be common with increasing tumor size, the predictive value of lymph node metastases on long‐term outcomes has not been proven.25 In fact, the extent of resection for patients with duodenal neuroendocrine tumors remains controversial.26 The ability to find an association between nodal status and long‐term survival for this particular anatomic location may be hindered by this tumor’s low incidence and indolent nature. Among nonfunctional rectal neuroendocrine tumors, the association between nodal involvement and poor outcomes has been clearly established with a recent study demonstrating worse survival with an increasing number of positive lymph nodes.27,28 The current study supports these previous findings, with node‐positive patients displaying a three‐fold hazard ratio for overall survival compared to node‐negative patients even when accounting for other negative prognostic factors. Currently, surgical resection represents the first‐line therapy for rectal neuroendocrine tumors, but the extent of surgery is based solely on tumor size and further remains ambiguous for intermediate size tumors of 1 to 2 cm. Indeed, according to the NCCN guidelines, an endoscopic technique can be used for resection of tumors smaller than 1 cm while a radical resection is warranted for tumors larger than 2 cm.13
Unlike tumors of the midgut in which resection and lymphadenectomy may be performed with relatively minimal risk and morbidity, radical resection of rectal neuroendocrine tumors usually necessitates a low anterior resection or abdominoperineal resection, which can both be associated with much higher morbidity and decreased quality of life when compared to local resection alone. Indeed, leak rates after low anterior resection range from 10% to 36% in some studies, and this complication can further result in longer hospital length of stay and requirement for permanent stoma creation.29,30 Similarly, abdominoperineal resection is associated with a high incidence of perineal wound complications which may result in chronic perineal fistulae, prolonged pain and wound care, and decreased quality of life.31 Furthermore, the sequelae of both of these procedures in regard to urological and sexual dysfunction have been described in several studies.32,33 Consequently, accurate preoperative assessment of nodal status is paramount, and the findings from this study further highlight the importance of high quality ERUS and pelvic MRI for careful sizing and local staging of these tumors.
Previous data have attempted to identify prognostic tumor‐ specific factors to help guide operative management of rectal neuroendocrine tumors, however, studies have been limited by small cohorts and no consensus has been reached regarding the most accurate approach to risk‐stratify these tumors.28,34–36 Our data is powered by a larger sample, and although only 3% of patients had lymph nodes harvested, this highlights the inherent selection bias or clinical practice pattern to approach rectal neuroendocrine tumors in a non‐oncologic manner. Indeed, a recent Surveillance, Epidemiology, and End Results Program study demonstrated that the majority of patients with rectal neuroendocrine tumors are undergoing local resection, with only 5% undergoing formal, anatomic excision.37 According to PReSS, the incidence of lymph node positivity ranged from 11% to 78% which was higher than expected, but PReSS is able to accurately stratify patients as seen by each group’s association with overall survival. Clearly, this risk score is identifying biologically aggressive tumors, regardless of size by taking into account depth of invasion. Specifically, PReSS identifies small‐sized tumors with an increased depth that have higher rates of lymph node positivity than expected, and larger tumors with minimal penetration that have lower than expected lymph node‐positive disease.
In the absence of data from randomized control trials, this risk score should be applied with various objectives. It should help guide discussions with patients regarding their risk of lymph node metastases, and options for excision including local vs formal resection in the context of each procedure’s advantages and disadvantages when taking into account each patient’s comorbidities. Additionally, this risk score should be applied when developing surveillance strategies after surgery as higher risk tumors that undergo local resection should be surveyed more closely.
The limitations of this study include those related to retrospective analysis and use of large databases such as potential coding errors, missing data, and the absence of several variables within the NCDB including mitotic rate, Ki‐67 index, disease recurrence, and disease‐specific survival. The lack of recurrence data poses a challenge when studying an indolent disease in which overall survival may not be an ideal outcome to evaluate its natural history. Additionally, our analysis only includes patients who had lymph nodes pathologically assessed after surgery, which introduces selection bias. Thirdly, while our analysis uses pathologic T‐stage as a surrogate for preoperative ERUS/MRI depth of invasion, previous studies have demonstrated an accurate correlation.6 Lastly, although the risk groups adequately predict overall survival, this risk score has not been externally validated and this poses an area of future study.
5 |. CONCLUSION
For rectal neuroendocrine tumors, depth of invasion and tumor size predict lymph node positivity and both clinicopathologic variables can be readily obtained with a preoperative endoscopic ultrasound and/or MRI. This novel PReSS incorporates both variables and stratifies tumors into four risk groups of progressively increasing lymph node positivity. Rather than tumor size alone, this score should be used to guide surgical approach as local resection alone will not yield lymph nodes and may lead to under‐staging and anatomic resection may be preferred in patients with a higher risk for lymph node positivity.
ACKNOWLEDGMENTS
Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The data used in the study are derived from a de‐identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Funding information
National Center for Advancing Translational Sciences, Grant/Award Number: UL1TR002378/TL1TR002382
Footnotes
MEETING PRESENTATION
This manuscript was presented at the 2019 American Society of Colon and Rectal Surgeons annual meeting in Cleveland, OH.
DATA ACCESSIBILITY
The data that support the findings of this study are available from the National Cancer Database. Restrictions apply to the availability of these data, which were used under license for this study. Data are available authors with the permission of the National Cancer Database.
REFERENCES
- 1.Mani S, Modlin IM, Ballantyne G, Ahlman H, West B. Carcinoids of the rectum. J Am Coll Surg. 1994;179(2):231–248. [PubMed] [Google Scholar]
- 2.Chagpar R, Chiang YJ, Xing Y, et al. Neuroendocrine tumors of the colon and rectum: prognostic relevance and comparative performance of current staging systems. Ann Surg Oncol. 2013;20(4):1170–1178. [DOI] [PubMed] [Google Scholar]
- 3.Wang M, Peng J, Yang W, Chen W, Mo S, Cai S. Prognostic analysis for carcinoid tumours of the rectum: a single institutional analysis of 106 patients. Colorectal Dis. 2011;13(2):150–153. [DOI] [PubMed] [Google Scholar]
- 4.Li P, et al. Analysis of the factors affecting lymph node metastasis and the prognosis of rectal neuroendocrine tumors. Int J Clin Exp Pathol. 2015;8(10):13331–13338. [PMC free article] [PubMed] [Google Scholar]
- 5.Concors SJ, Sinnamon AJ, Folkert IW, et al. Predictors of metastases in rectal neuroendocrine tumors: results of a National Cohort Study. Dis Colon Rectum. 2018;61(12):1372–1379. [DOI] [PubMed] [Google Scholar]
- 6.Schaffzin DM, Wong WD. Endorectal ultrasound in the preoperative evaluation of rectal cancer. Clin Colorectal Cancer. 2004;4(2):124–132. [DOI] [PubMed] [Google Scholar]
- 7.Landmann RG, Wong DW, Hoepfl J, et al. Limitations of early rectal cancer nodal staging may explain failure after local excision. Dis Colon Rectum. 2007;50(10):1520–1525. [DOI] [PubMed] [Google Scholar]
- 8.Saitoh N, Okui K, Sarashina H, Suzuki M, Arai T, Nunomura M. Evaluation of echographic diagnosis of rectal cancer using intrarectal ultrasonic examination. Dis Colon Rectum. 1986;29(4):234–242. [DOI] [PubMed] [Google Scholar]
- 9.Garcia–Aguilar J, Pollack J, Lee SH, et al. Accuracy of endorectal ultrasonography in preoperative staging of rectal tumors. Dis ColonRectum. 2002;45(1):10–15. [DOI] [PubMed] [Google Scholar]
- 10.Beynon J An evaluation of the role of rectal endosonography in rectal cancer. Ann R Coll Surg Engl. 1989;71(2):131–139. [PMC free article] [PubMed] [Google Scholar]
- 11.Thaler W, Watzka S, Martin F, et al. Preoperative staging of rectal cancer by endoluminal ultrasound vs. magnetic resonance imaging. Dis Colon Rectum. 1994;37(12):1189–1193. [DOI] [PubMed] [Google Scholar]
- 12.Starck M, Bohe M, Fork FT, Lindström C, Sjöberg S. Endoluminal ultrasound and low‐field magnetic resonance imaging are superior to clinical examination in the preoperative staging of rectal cancer. Eur J Surg. 1995;161(11):841–845. [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology (NCCN Guidelines): Neuroendocrine Tumors, Rectal Cancer. May 17, 2019; Available from: https://www.nccn.org/professionals/physician_gls/default.aspx [DOI] [PubMed]
- 14.Anthony LB, Strosberg JR, Klimstra DS, et al. The NANETS consensus guidelines for the diagnosis and management of gastrointestinal neuroendocrine tumors (nets): well‐differentiated nets of the distal colon and rectum. Pancreas. 2010;39(6):767–774. [DOI] [PubMed] [Google Scholar]
- 15.Ramage JK, Goretzki PE, Manfredi R, et al. Consensus guidelines for the management of patients with digestive neuroendocrine tumours: well‐differentiated colon and rectum tumour/carcinoma. Neuroendocrinology. 2008;87(1):31–39. [DOI] [PubMed] [Google Scholar]
- 16.American College of Surgeons. National Cancer Database. 2018. https://www.facs.org/quality‐programs/cancer/ncdb. Accessed October 17, 2018
- 17.Liu Y, Nickleach DC, Chao Z, Switchenko JM, Kowalski J. Carrying out streamlined routine data analyses with reports for observational studies: introduction to a series of generic SAS® macros. F1000 Res. 2018;7:1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsikitis VL, Wertheim BC, Guerrero MA. Trends of incidence and survival of gastrointestinal neuroendocrine tumors in the United States: a seer analysis. J Cancer. 2012;3:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population‐based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. [DOI] [PubMed] [Google Scholar]
- 21.Partelli S, Gaujoux S, Boninsegna L, et al. Pattern and clinical predictors of lymph node involvement in nonfunctioning pancreatic neuroendocrine tumors (NF‐PanNETs). JAMA Surg. 2013;148(10):932–939. [DOI] [PubMed] [Google Scholar]
- 22.Lopez‐Aguiar AG, Zaidi MY, Beal EW, et al. Defining the role of lymphadenectomy for pancreatic neuroendocrine tumors: An Eight‐Institution Study of 695 patients from the US Neuroendocrine Tumor Study Group. Ann Surg Oncol. 2019;26:2517–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howe JR, Cardona K, Fraker DL, et al. The surgical management of small bowel neuroendocrine tumors: consensus guidelines of the North American Neuroendocrine Tumor Society. Pancreas. 2017;46(6):715–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niederle B, Pape UF, Costa F, et al. ENETS consensus guidelines update for neuroendocrine neoplasms of the Jejunum and Ileum. Neuroendocrinology. 2016;103(2):125–138. [DOI] [PubMed] [Google Scholar]
- 25.Dogeas E, Cameron JL, Wolfgang CL, et al. Duodenal and ampullary carcinoid tumors: size predicts necessity for lymphadenectomy. J Gastrointest Surg. 2017;21(8):1262–1269. [DOI] [PubMed] [Google Scholar]
- 26.Jensen RT, Rindi G, Arnold R, et al. Well‐differentiated duodenal tumor/carcinoma (excluding gastrinomas). Neuroendocrinology.2006;84(3):165–172. [DOI] [PubMed] [Google Scholar]
- 27.Fields AC, McCarty JC, Ma‐Pak L, et al. New lymph node staging for rectal neuroendocrine tumors. J Surg Oncol. 2019;119(1):156–162. [DOI] [PubMed] [Google Scholar]
- 28.Shields CJ, Tiret E, Winter DC. Carcinoid tumors of the rectum: a multi‐institutional international collaboration. Ann Surg. 2010;252(5):750–755. [DOI] [PubMed] [Google Scholar]
- 29.Caulfield H, Hyman NH. Anastomotic leak after low anterior resection: a spectrum of clinical entities. JAMA Surg. 2013;148(2):177–182. [DOI] [PubMed] [Google Scholar]
- 30.Phillips BR, Harris LJ, Maxwell PJ, Isenberg GA, Goldstein SD. Anastomotic leak rate after low anterior resection for rectal cancer after chemoradiation therapy. Am Surg. 2010;76(8):869–871. [PubMed] [Google Scholar]
- 31.Musters GD, Klaver CEL, Bosker RJI, et al. Biological mesh closure of the pelvic floor after extralevator abdominoperineal resection for rectal cancer: A multicenter randomized controlled trial (the BIOPEX‐study). Ann Surg. 2017;265(6):1074–1081. [DOI] [PubMed] [Google Scholar]
- 32.Ho VP, Lee Y, Stein SL, Temple LKF. Sexual function after treatment for rectal cancer: a review. Dis Colon Rectum. 2011;54(1):113–125. [DOI] [PubMed] [Google Scholar]
- 33.Kasparek MS, Hassan I, Cima RR, Larson DR, Gullerud RE, Wolff BG. Long‐term quality of life and sexual and urinary function after abdominoperineal resection for distal rectal cancer. Dis Colon Rectum. 2012;55(2):147–154. [DOI] [PubMed] [Google Scholar]
- 34.Weinstock B, Ward SC, Harpaz N, Warner RRP, Itzkowitz S, Kim MK. Clinical and prognostic features of rectal neuroendocrine tumors. Neuroendocrinology. 2013;98(3):180–187. [DOI] [PubMed] [Google Scholar]
- 35.Fahy BN, Tang LH, Klimstra D, et al. Carcinoid of the rectum risk stratification (CaRRs): a strategy for preoperative outcome assessment. Ann Surg Oncol. 2007;14(5):1735–1743. [DOI] [PubMed] [Google Scholar]
- 36.Tsang ES, McConnell YJ, Schaeffer DF, Yin Y, Speers CH, Kennecke HF. Prognostic factors for locoregional recurrence in neuroendocrine tumors of the rectum. Dis Colon Rectum. 2018;61(2):187–192. [DOI] [PubMed] [Google Scholar]
- 37.McConnell YJ. Surgical management of rectal carcinoids: trends and outcomes from the surveillance, epidemiology, and end results database (1988 to 2012). Am J Surg. 2016;211(5):877–885. [DOI] [PubMed] [Google Scholar]