Abstract
Background:
Data are limited on cumulative impacts of depression on engagement in care and HIV outcomes in women living with HIV (WLWH) during the era of universal antiretroviral therapy (ART). Understanding the relationship of accumulated depression with HIV disease management may help identify benefits of interventions to reduce severity and duration of depressive episodes.
Setting:
A cohort of WLWH (N=1,491) from the Women’s Interagency HIV Study (WIHS) at nine sites across the US.
Methods:
This longitudinal observational cohort study (2013-2017) followed WLWH for a maximum of nine semi-annual visits. Depression was quantified as a time-updated measure of percent of days depressed (PDD) created from repeated assessments using the Center for Epidemiologic Studies Depression (CES-D) scale. Marginal structural Poisson regression models were used to estimate the effects of PDD on the risks of missing an HIV care appointment, <95% ART adherence, and virological failure (≥200 copies/mL).
Results:
The risk of missing an HIV care appointment [risk ratio (RR)=1.16, 95% confidence interval (CI)=0.93 to 1.45; risk difference (RD)=0.01, −0.01 to 0.03], being <95% ART adherent (RR=1.27, 1.06 to 1.52; RD=0.04, −0.01 to 0.07), and virological failure (RR=1.09, 1.01 to 1.18; RD=0.01, −0.01 to 0.03) increased monotonically with increasing PDD (comparing those with 25 to those with 0 PDD). The total effect of PDD on virological failure was fully (%100) mediated by being <95% ART adherent.
Conclusions:
Time spent depressed increases the risk of virological failure through ART adherence, even in the era of universal ART regimes forgiving of imperfect adherence.
Keywords: HIV, Mental Illness, Cumulative Depression, HIV Care Continuum
INTRODUCTION
Approximately 20%-40% of people living with HIV (PLWH) experience clinical depression with even higher prevalence (30–60%) among women living with HIV (WLWH).1-3 In addition to being extremely common, depression complicates management of HIV disease throughout the HIV care continuum. Depression has been linked to missed HIV care appointments,4-6 reduced adherence to antiretroviral therapy (ART),7-9 and increased levels of HIV viremia. 4,9-11
The vast majority of past literature examining the impacts of depression on HIV care outcomes has used standardized binary thresholds to define depression at a single point in time. This approach does not fully capture the chronic and cyclical nature of depression and thus may not account for the condition’s potential cumulative impacts on health behaviors and clinical outcomes. More recently, two studies published results that used the cumulative burden of depression approach to explore the relationships between depression and HIV care continuum outcomes.12,13 These studies found that relatively modest increases in time spent depressed led to increases in the risks of missing HIV care appointments, having a detectable HIV viral load, and mortality. However, both studies include data that pre-date the recommendation for treatment of all people with HIV infection regardless of CD4 cell count known as universal ART14,15 and the availability ART regimens more forgiving of moderate nonadherence,16,17 and may not fully reflect how depression is currently impacting health behaviors and HIV care outcomes.
The purpose of this paper is to expand upon prior research using a cumulative burden of depression approach by using data from a national cohort of WLWH during the period following recommendations for universal ART (≥2013).15,18,19 We use data from the period of universal ART to estimate the effects of cumulative depression on the risks of missing an HIV care appointment, reporting <95% ART adherence, and virological failure. Using the cumulative approach in conjunction with recent data ensure the results of this study are reflective of and informative to the current landscape of managing HIV disease.
METHODS
Study Design and Population
We used data from the Women’s Interagency HIV Study (WIHS), a longitudinal cohort of US women with or at risk for HIV. 20-22 The WIHS collects sociodemographic, clinical, healthcare utilization, and health behavior data at semiannual visits. For the present analysis, women from all four enrollment waves (1994–1995, 2001–2002, 2011–2012, 2013-present) recruited at nine sites (Bronx, NY; Brooklyn, NY; Washington, DC; Chicago, IL; San Francisco, CA; Atlanta, GA; Chapel Hill, NC; Miami, FL; Birmingham AL/Jackson MS) were eligible to participate. We limited our analysis to visits between 2013 and 2017 in order to generate results reflective of the current era of universal ART. Follow-up continued from the analysis baseline until the earliest of the following events: a maximum of nine visits (the last available visit in WIHS at time of this analysis), death, or loss to follow-up (defined as two consecutive missed visits).
At the time of this analysis, there were 3,704 women with HIV infection (prevalent at enrollment or seroconverted during follow-up) enrolled in WIHS. Of these women, 1,946 were excluded because they died prior to 2013 (n=1,114) or due to non-participation during the analysis period (n=832). Of the remaining 1,758 women, we excluded 14 women who had missing data on important potential confounders at their analysis baseline visit. Among the 1,744 women with complete data at their analysis baseline visit, 92 women were immediately lost to follow-up (meaning they missed their next two consecutive visits) and therefore were excluded from the analysis. Finally, we excluded 161 women who reported not receiving regular HIV care at the analysis baseline visit. These criteria left a total of 1,491 women for analysis. Supplemental Materials Table S4 compares participants excluded to those included in the analysis on selected baseline characteristics. All participants provided written informed consent and local institutional review boards reviewed and approved all study protocols.
Exposure measure: Percent of days depressed
We defined percent of days depressed (PDD) as the estimated time-updated proportion of days a participant experienced symptoms consistent with clinical depression over the follow-up period. The method used to calculate PDD is based on previous studies employing the cumulative burden of depression approach and is described in detail elsewhere.12,13 Briefly, PDD was calculated using serial participant CES-D scores (range: 0–60) observed at semi-annual WIHS visits. The CES-D is a previously validated instrument for measuring depressive symptoms.23,24 Based on prior research, we converted consecutive CES-D scores into depression values ranging from 0 to 1. A score of 0 was assigned to CES-D values ≤9 indicating no depression symptoms, while a score of 1 was assigned to CES-D values ≥33 indicating fully symptomatic depression 12,25. CES-D scores falling between 10 and 32 were assigned a prorated value between 0 and 1 indicating partial depressive symptoms. 12,26 After conversion, each pair of consecutive depression values was averaged and multiplied by the number of days between the two visits. This produced an estimated number of days with depression experienced in each interval. We then calculated a time-updated days with depression value representing the running sum of days with depression experienced from analysis baseline through the end of each interval. Finally, we calculated time-updated PDD as the quotient of the running total of days with depression and total days observed from analysis baseline to the end of each interval.
Outcome measures
We considered three repeated measure outcomes in our analysis that reflect important steps along the HIV continuum of care:27, missed HIV care appointments, <95% ART adherence, and virological failure. We defined missed HIV care appointments as a binary outcome indicating whether or not the participant reported missing any scheduled regular HIV care appointments in the past six months without rescheduling.28 ART adherence was measured via participant self-report of how often she took ART as prescribed since her last visit. As a binary variable, a self-report of taking ART as prescribed <95% of the time was given a value of 1, while a response indicating ≥95% was assigned a value of 0. Follow-up visits where the participant indicated they were no longer taking ART (9%, n=766) were assigned a value of 1. We defined virological failure as a binary outcome indicating whether or not the participant had an HIV-1 RNA viral load measure of ≥200 copies/mL.29 For all three outcomes, the observed value was related to the time updated exposure (PDD) as of the most recent prior visit (approximately six month gap).
Other Covariates
We selected several time-fixed and time-varying covariates to control for potential confounding based on past literature, clinical expertise and directed acyclic graphs. Time-fixed covariates were those observed at the baseline visit of this analysis and included: age, race (white non-Hispanic, black non-Hispanic, other non-Hispanic, Hispanic), education (<12 years, 12 years, >12 years), employment status (yes/no), stable housing (yes/no - defined as yes if renting, owning or staying with family/friends), health insurance status (yes/no), WIHS site, WIHS enrollment wave, ART regimen type (definition provided in Supplemental Materials), CD4 count and self-reported substance use [yes/no – defined as yes if self-reported alcohol consumption met the definition of at-risk drinking (>7 drinks per week) and/or self-reported illicit drug use, injected or otherwise.
Time-varying covariates were: CD4 count, time under observation (measured in years), employment status, stable housing, health insurance status, self-reported substance use, diabetes and hypertension. As dichotomous variables, diabetes and hypertension were set to a value of 0 at analysis baseline. Once the participant met the criteria as determined by WIHS procedures (See Table 1) for either condition (either at baseline or during follow-up), the value was set to 1 and remained as such for all remaining visits. Given 91% of the follow-up visits had complete data, we used a last observation carried forward approach to address missing values for all time-varying variables. We also report descriptive data on receipt of mental health treatment in order to place the results of the main analysis into the context of psychiatric care coverage.
TABLE 1.
Characteristics of Study Participants in The Women's Interagency HIV Study from 2013-2017 (N=1,491)
| Characteristic | Baseline (N = 1,491), N (%) |
Follow-up Visits* (N=8,624), N (%) |
|---|---|---|
| Outcomes of interest | ||
| Missed HIV care appointment | 184 (12) | 928 (11) |
| <95% ART adherent (ART nonadherence) | 312 (21) | 1,735 (20) |
| Virological failure (≥200 copies/mL) | 250 (17) | 1,468 (17) |
| Depression | ||
| CES-D score, median (IQR) | 8 (3 to 18) | 8 (3 to 18) |
| Cumulative PDD, median (IQR) | --- | 10 (0 to 36) |
| Covariates | ||
| Site | ||
| San Francisco, CA | 187 (13) | --- |
| Southern** | 526 (35) | --- |
| Bronx, NY | 211 (14) | --- |
| Brooklyn, NY | 216 (14) | --- |
| Wsshington DC | 177 (12) | --- |
| Chicago, IL | 174 (12) | --- |
| Age, median (IQR) | 48 (42 to 54) | 50 (44 to 56) |
| Race | ||
| White non-Hispanic | 147 (10) | --- |
| Black non-Hispanic | 1,080 (72) | --- |
| Other non-Hispanic | 45 (3) | --- |
| Hispanic | 219 (15) | --- |
| Education | ||
| Less than 12 years | 500 (34) | --- |
| 12 years | 473 (32) | --- |
| More than 12 years | 518 (35) | --- |
| Employed at visit | 490 (33) | 2,989 (35) |
| Stable housing since last visit | 1,310 (88) | 7,763 (90) |
| Health insurance at visit | 1,290 (87) | 7,754 (90) |
| WIHS enrollment wave | ||
| 1: 1994-1995 | 470 (32) | --- |
| 2: 2001-2002 | 315 (21) | --- |
| 3: 2011-2012 | 188 (13) | --- |
| 4: 2013-present | 518 (35) | --- |
| CD4 T-cell count, median (IQR), cells/mm3 | 594 (391 to 792) | 638 (435 to 859) |
| Diabetesa | 310 (21) | 1,978 (23) |
| Hypertensionb | 1,065 (71) | 6,548 (76) |
| Substance usec | 395 (26) | 2,211 (26) |
| ART Regimen | ||
| HAARTd | 1,322 (89) | 7,753 (90) |
| Integrase Inhibitor | 370 (25) | 3,416 (40) |
| Entry Inhibitor | 5 (0) | 32 (0) |
| Boosted protease Inhibitor | 415 (28) | 1,626 (19) |
| Unboosted protease Inhibitor | 41 (3) | 274 (3) |
| NNRTI and/or NRTI | 491 (33) | 2,405 |
| Mono or dual therapye | 19 (1) | 104 (1) |
| Unknown or Not on ARTf | ||
| Received mental health treatmentg | 564 (42) | 3,254 (41) |
| Received mental health treatment and CES-D ≥ 16h | 262 (46) | 1,487 (46) |
Abbreviations: HIV, human immunodeficiency virus; ART, antiretroviral therapy; IQR, interquartile range; CES-D, Center for Epidemiologic Studies-Depression; PDD, Percent of days depressed; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor.
4,303 person-years; median follow-up: 3.3 years (IQR, 2.4-3.5); maximum follow-up: 3.9 years; women lost due to missed visits: 23 (2% of participants); women died: 53 (4% of participants).
Includes Atlanta, GA; Chapel Hill, NC; Miami, FL; Birmingham AL/Jackson MS
Diabetes defined as, when nonpregnant, self-reported antidiabetic medication, 2 fasting glucose measurements ≥126 mg/dL, or concurrent measurements of hemoglobin A1c ≥6.5% and fasting glucose ≥126 mg/dL.
Hypertension defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, self-reported hypertension, or use of antihypertensive medications.
Defined as self-reported alcohol consumption that met the definition of at-risk drinking (>7 drinks per week) and/or self-reported drug illicit drug use, injected or otherwise.
HAART is defined as the reported use of three or more antiretroviral medications, one of which has to be a PI, an NNRTI, one of the NRTIs abacavir or tenofovir, an integrase inhibitor (e.g., raltegravir), or an entry inhibitor (e.g., Maraviroc or enfuvirtide). Subcategories defined by the ART class that anchors the regimen which has met the WIHS definition of HAART.
Regimens not meeting the definition of HAART including reported use of only one or two ART medications.
Self-reported receiving regular HIV care at baseline but either indicated not taking ART medication or did not provide ART medication information.
Defined as self-reported receipt of antidepressant medication or care from a psychiatrist, counselor or other mental health professional since the last visit; missing 132 at baseline and 765 during follow-up
Percentage out of the number of participants receiving mental health treatment (baseline: n=564; follow-up: n=3,254)
Statistical Analysis
We used inverse-probability-of-exposure weights (IPEW) to fit marginal structural models to address time-fixed and time-varying confounding in our analysis.30 IPEWs were calculated by dividing PDDs into deciles, 31 followed by fitting a pooled ordinal logistic regression model to predict the probability the participant would experience the PDD decile they actually experienced conditional upon the time-fixed and time-varying covariates described above. Time-varying covariates were lagged by one visit, 30 meaning they were taken from observations made at the visit immediately preceding the most recent visit. Continuous data covariates were specified either as linear terms or restricted cubic splines as appropriate based on linearity assessments of the covariates relation to PDD decile. After running the pooled ordinal logistic regression model, we calculated IPEWs for all participant visits as the inverse of the predicted probability of the PDD decile experienced. Next, in order to account for the participant’s entire exposure history, we multiplied IPEWs across all visits. Finally, we stabilized IPEWs with time-fixed (baseline) covariates and trimming at the 5th and 95th percentiles, resulting in a well behaved set of weights.
To estimate the effects of PDD, we used the IPEWs to fit three pooled marginal structural Poisson regression models with a robust variance estimator for repeated measures. The exponentiated coefficients generated from these models with binary outcomes are interpretable as risk ratios (RR).32 We also produced risk differences (RDs) using post-estimation commands available in Stata Version 15.1 (StataCorp, College Station, Texas). We assessed the assumption of a linear relationship between PDD and outcomes on the log risk scale by comparing linear, quadratic, and restricted cubic spline specifications using Akaike’s information criterion (AIC) and visual plots. For the missed HIV care appointment and <95% ART adherence models, evidence supported a non-linear relationship with PDD, and the best fitting model in both cases was a restricted cubic spline using three knots.33 For the virological failure models, a simple linear relationship with PDD was supported. To facilitate interpretation, for all models we produced graphs depicting weighted predicted RRs and RDs and associated 95% confidence interval (CI) estimates for all observed values of PDD relative to 0 PDD. Additionally, we estimated RRs and RDs for 25 and 100 PDD relative to 0 PDD. All analyses were completed in Stata Version 15.1 (StataCorp, College Station, Texas) and SAS version 9.4 (SAS Institute, Cary, North Carolina).
Exploratory mediation analysis
We conducted an exploratory analysis to test the hypothesis that the total effect of PDD on the risk of virological failure would be partially or fully mediated by missing any HIV care appointment and/or being <95% ART adherent. To test the hypothesis, we compared the point estimate in our primary model assessing the total effect of PDD on virological failure to the point estimate after adding the hypothesized mediators to the outcome model. If our hypothesis is correct, we would expect to observe partial or complete attenuation of the coefficient for PDD after adding the mediators.13,34.
Secondary Analysis
In a secondary analysis, we fit three covariate--adjusted models for each outcome in order to assess the impact of additional factors relative to depression. The covariate adjustment set mirrored the variables used to derive each outcome’s IPEWs.
RESULTS
The baseline and follow-up characteristics of the study sample are provided in Table 1. At baseline, the median age of the 1,491 women was 48 years [interquartile range (IQR]), 42 to 54], 21% indicated <95% ART adherence and 17% had an HIV-1 RNA viral load ≥200 copies/mL. We observed a total of 4,303 person-years (8,624 visits) during which 53 women died and 23 were lost to follow-up. The median length of follow-up was 3.3 years (IQR, 2.4 to 3.5). Over follow-up, participants reported missing an HIV care appointment, <95% adherence to ART, and had an HIV-1 RNA viral load ≥200 copies/mL for 11%, 20% and 17% of the observed visits, respectively. At 41% of the observed visits, participants reported receiving mental health treatment. Among the visits where the participant reported receiving mental health treatment, 46% of the women scored ≥ 16 on the CES-D, a traditional cutoff point indicative of depressive symptoms.23 At the last observed visit, the median PDD was 10 (IQR, 0 to 36). WIHS participants included in this study were less likely to be <95% ART adherent and have a HIV-1 RNA viral load ≥200 copies/mL compared to those excluded from the analysis due to non-participation, missing data and immediate loss to follow-up (See Supplemental Materials Table S4).
Missing an HIV care appointment
The risk of missing an HIV care appointment increased monotonically, if slightly nonlinearly, with increasing PDD (Figures 1.A. and 2.A.). For example, women who spent 25% of their observed time depressed (25 PDD) had a 16% [RR=1.16, 95% CI (0.93 to 1.45); RD=0.01, 95% CI (−0.01 to 0.03)] greater risk of missing an HIV care appointment compared to those with 0 PDD (Table 2). Women who spent 100% of their observed time depressed had an 82% greater risk of missing an HIV care appointment compared to those with 0 PDD [RR=1.82 (1.35 to 1.96); RD=0.07 (0.03 to 0.11)].
FIGURE 1.
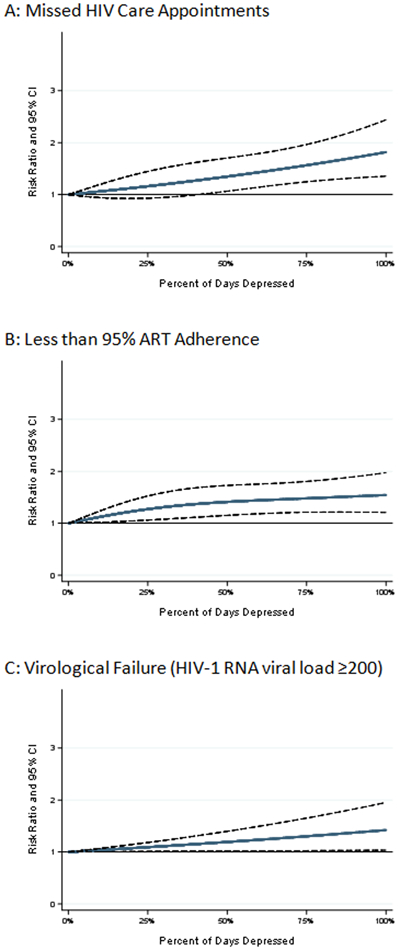
Weighted predicted risk ratios and 95% confidence intervals (CI) for each observed value of percent of days depressed with 0 percent of days depressed serving as the reference point for A) Missed HIV Care Appointments, B) Being <95% ART Adherent and C) Virological Failure.
FIGURE 2.
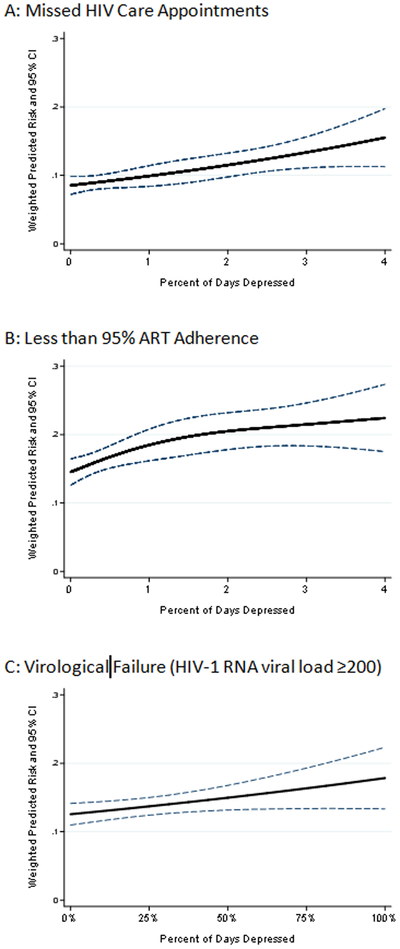
Weighted predicted risk and 95% confidence intervals (CI) for each observed value of percent of days depressed with 0 percent of days depressed serving as the reference point for A) Missed HIV Care Appointments, B) Being <95% ART Adherent and C) Virological Failure.
TABLE 2.
Results of Marginal Structural Poisson Regression Models (N=1,491)*
| 25% of days depressed compared to never depressed |
100% of days depressed compared to never depressed |
|||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | RR | 95% CI | RD | 95% CI | RR | 95% CI | RD | 95% CI |
| Missed HIV care appointment** | 1.16 | 0.93 to 1.45 | 0.01 | −0.01 to 0.03 | 1.82 | 1.35 to 1.96 | 0.07 | 0.03 to 0.11 |
| <95% ART adherence** | 1.27 | 1.06 to 1.52 | 0.04 | 0.01 to 0.07 | 1.54 | 1.21 to 1.97 | 0.08 | 0.03 to 0.13 |
| Virological failure (≥200 copies/mL) | 1.09 | 1.01 to 1.18 | 0.01 | −0.01 to 0.03 | 1.42 | 1.03 to 1.95 | 0.05 | 0.01 to 0.10 |
Abbreviations: ART, antiretroviral therapy; RR, risk ratio; CI, confidence interval; RD, risk difference.
Marginal structural Poisson regression models weighted for time-fixed (baseline) values of race, education, stable housing, employment status, health insurance status, WIHS site, WIHS enrollment wave, age, ART regimen type, virological failure and CD4 count, and time-varying CD4 count, days observed, diabetes, hypertension, stable housing, employment status, health insurance status, and substance use. Time-varying virological failure was included in the weight models in the ART adherence and missed appointment analyses.
Percent of days depressed specified as restricted cubic spline with 3 knots.
Less than 95% ART Adherent
Similarly, the risk of being <95% ART adherent also increased monotonically with increasing PDD (Figures 1.B. and 2.B.). For example, women who spent 25% of their observed time depressed had a 27% greater risk of reporting <95% ART adherence [RR=1.27 (1.06 to 1.52); RD=0.04 (0.01 to 0.07)] compared to those with 0 PDD (Table 2). Additionally, compared to women who reported 0 PDD, women who experienced depression 100% of the time had a 54% increase in the risk of reporting <95% ART adherence [(RR=1.54 (1.21 to 1.97); RD=0.08 (0.03 to 0.13)].
Virological Failure
Figures 1.C. and 2.C. present the weighted predicted RR and risks and associated 95% CIs for virological failure for each observed PDD value. This figure shows that the risk of virological failure increases in a linear fashion with time spent depressed. For example, women experience an 8% increase in the risk of virological failure for each additional 25 PDD [RR=1.09 (1.01 to 1.18); RD=0.01 (−0.01 to 0.03).
Exploratory mediation analysis
The additions of missed HIV care appointments and <95% ART adherence, both individually and in combination, caused the estimate for PDD to be attenuated towards the null, suggesting that these two variables explain part or all of the relationship between PDD and virological failure (Table 3).
TABLE 3.
Results of Marginal Structural Poisson Regression Models Exploratory Mediation Analysis (N=1,491)*
| Total effect of PDD on risk of virological failure |
Direct effect of PDD on risk of virological failure controlling for mediator: missed HIV care appointment |
Direct effect of PDD on risk of virological failure controlling for mediator: <95% ART adherence |
Direct effect of PDD on risk of virological failure controlling for both mediators: Missed HIV care appointment and <95% ART adherence |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | RR | 95 % CI | RR | 95% CI | % Change in RR Point Estimate *** |
RR | 95% CI | % Change in RR Point Estimate *** |
RR | 95% CI | % Change in RR Point Estimate *** |
| 25 PDD** | 1.09 | 1.01 to 1.18 | 1.07 | 0.99 to 1.16 | −21% | 1.00 | 0.93 to 1.07 | −100% | 1.00 | 0.93 to 1.07 | −100% |
| Mediators | |||||||||||
| Missed HIV care appointment | --- | --- | 1.90 | 1.62 to 2.23 | --- | --- | --- | 1.37 | 1.19 to 1.60 | --- | |
| <95% ART adherent | --- | --- | --- | --- | --- | 4.32 | 3.60 to 5.18 | --- | 4.12 | 3.42 to 4.96 | --- |
Abbreviations: PDD, Percent of days depressed; ART, antiretroviral therapy; RR, risk ratio; CI, confidence interval.
Marginal structural Poisson regression models weighted for time-fixed (baseline) values of race, education, stable housing, employment status, health insurance status, WIHS site, WIHS enrollment wave, age, ART regimen type, virological failure and CD4 count, and time-varying CD4 count, days observed, diabetes, hypertension, stable housing, employment status, health insurance status, and substance use.
25% of days depressed compared to never depressed, or 25 PDD represents a 1 unit change
% change in RR on the log scale for the direct effect of PDD on risk of having a virological failure compared to the effect without controlling for mediators.
Secondary Analysis
In covariate-adjusted models, PDD remained one of the strongest predictors of each outcome relative to other factors included as control variables (Supplemental Materials Tables S1, S2 and S3).
DISCUSSION
In this multi-site longitudinal cohort study of WLWH, we used a cumulative burden of depression approach to estimate the impacts of percent of days depressed (PDD) on three important outcomes along the HIV care continuum: HIV care appointment attendance, <95% ART adherence and virological failure. We found that a greater proportion of time spent depressed increased the risk of missing an HIV care appointment, being <95% ART adherent and virological failure in a dose-response fashion. In addition, our results suggest that the relationship between PDD and virological failure is largely mediated by being <95% ART adherent. Our findings are consistent with past literature examining the relationships between depression and HIV care appointment attendance5,13, ART adherence7-9 and viral load.4,9-11,13 Additionally, the present study’s findings on HIV care appointment attendance and viral load are consistent with a recent publication using the cumulative depression approach in a national clinical cohort of PLWH in the United States.13
These findings have several important implications. First, our data suggest that even modest amounts of time spent depressed (25 PDD, equating to 1 in 4 days of full depression or persistent mild-moderate levels of depressive symptoms) can have a negative impact on treatment engagement, which in turn leads to an increased risk of virological failure. Secondly, our findings suggest that even in the modern era of universal ART with regimens that are more forgiving of moderate nonadherence35-37, greater than 95% adherence to ART remains important for maintaining viral suppression. Third, our descriptive data revealed a potential gap in mental health care for depression among this study population. The existence of unmet depression treatment needs in conjunction with the negative consequences found to be associated with cumulative depression highlight the necessity for and potential clinical benefits of implementing and evaluating enhanced depression care models38,39 that incorporate an HIV treatment engagement support component 39 into routine HIV clinical care settings. Finally, because PDD incorporates the cumulative history of depression, our data indicate that past exposure to depression, in addition to current symptomology, is an important predictor of negative health outcomes for WLWH. Thus, clinicians should consider a patient’s entire history of exposure to depressive symptoms when evaluating present-day risks to HIV treatment success.
There are several limitations that must be considered when interpreting our results. Given that this is an observational study, lack of randomized assignment to depression status threatens effect estimates’ internal validity. To address this threat, we used marginal structural models to approximate randomization on several important covariates. However, marginal structural models do not account for unmeasured confounders. Of particular concern in this study is co-occurring post-traumatic stress disorder, anxiety disorder, bipolar disorder, and schizophrenia. These conditions often present in conjunction with depression among PLWH40 and have been shown to affect the outcomes of interest in this study.41-44 Thus the estimates we report here may reflect the impact not just of depression alone, but potentially of depression in conjunction with other co-occurring psychiatric disorders on HIV care outcomes, suggesting the importance of coordinated responses to co-occurring mental health conditions among PLWH. This study’s results were also subject to bias from participant exclusions due to missing data, immediate loss to follow-up and non-participation. However, excluded WIHS participants experienced greater depressive symptoms along with higher likelihoods of poor engagement in care and virological failure (See Supplemental Materials Table S4). As such, our findings are likely conservative estimates of the impact depression has on this study’s outcomes.
An additional limitation is potential measurement error stemming from unobserved fluctuations in depressive symptoms that occurred between visits. Specifically, CES-D scores are collected every six months in the WIHS; therefore, 22 weeks of depressive symptoms were not directly observed within each visit interval. In a recent study, Kinyanda and colleagues (2018) found that 67% of the major depressive disorder cases resolved in less than six months among a cohort of people with HIV (PWH) from Uganda.45 In such cases, assessments every six months may not fully capture average depression experience. However, in contrast to the Uganda study, two recent investigations of large cohorts of PWH in the US reported stable depressive symptoms over time for a vast majority of participants.46,47 In fact, Kelso-Chichetto and colleagues (2017) used data from the WIHS and observed stable depressive symptom patterns over a 10-year follow-up period for 93% of WLWH. This finding suggests semiannual assessments should provide a relatively accurate reflection of our study sample’s average depression experience. Moreover, the method used to calculate PDD is built upon the metric depression-free days26,48 which has been shown to perform just as effectively with semiannual assessments compared to more frequent measurements.26 Additional limitations includes social desirability and error recall bias in self-reported outcomes along with limited generalizability to the general population of WLWH, many of whom do not have access to study related resources.
Despite these limitations, this study makes important contributions to the literature investigating factors that impede progress along the HIV care continuum. First, the present study expands upon past research that has employed the cumulative burden approach to quantifying depression by assessing this measure’s impact on a range of important outcomes along the HIV care continuum. One specific contribution is the inclusion of ART adherence as an outcome measure, which had previously not been explored in relationship to cumulative depression. We also provide additional support for the conceptual framework that ART adherence remains an important pathway through which depression impacts HIV disease management. Specifically, we show that time spent depressed increases the risk for virological failure, even in the era of universal ART regimens thought to be forgiving of less than perfect adherence. Finally, we were able to employ sophisticated quantitative causal inference methods to produce robust effect estimates that accounted for important time-fixed and time-varying confounders.
CONCLUSIONS
To the best of our knowledge, this is the first study to employ the cumulative burden of depression approach to analyze the impacts of time spent depressed on engagement in HIV care and virological failure, with a focus on the era of universal ART. Even in the era of universal ART, with potent and durable antiretroviral regimens, our evidence indicates that time spent depressed poses a significant threat to viral suppression, largely through the mediating effect of ART adherence. Therefore, comprehensive approaches that simultaneously reduce severity and time spent depressed and barriers to treatment engagement are in need of evaluation to guide treatment decisions in the context of routine HIV clinical care. This line of work will be important to uncovering the most effective approaches for addressing the negative effects of time spent depressed on treatment engagement and viral suppression in WLWH.
Supplementary Material
ACKNOWLEDGEMENTS:
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (Principal Investigators): UAB-MS WIHS (Mirjam-Colette Kempf and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos and Anjali Sharma), U01-AI-035004; Brooklyn WIHS (Deborah Gustafson and Tracey Wilson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Seble Kassaye and Daniel Merenstein), U01-AI-034994; Miami WIHS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (Bradley Aouizerat and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I – WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR000454 (Atlanta CTSA), P30-AI-050410 (UNC CFAR), and P30-AI-027767 (UAB CFAR).
SOURCES OF FUNDING:
This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health [T32 AI007001 to A.A.]; and the Women’s Interagency HIV Study, a National Institutes of Health funded program made possible by the National Institute of Allergy and Infectious Diseases; Eunice Kennedy Shriver National Institute of Child Health and Human Services; National Cancer Institute, National Institute on Drug Abuse; and the National Institute on Mental Health [grant numbers U01-AI-103401 to M.K.; U01-AI-103408; U01-AI-035004; U01-AI-031834; U01-AI-034993 to M.C.; U01-AI-034994 to S.K.; U01-AI-103397 to M.F.; U01-AI-103390 to A.A.; U01-AI-034989; U01-AI-042590; U01-HD-032632]. Targeted supplemental funding for specific projects is also provided by the National Institutes of Health at the National Institute of Dental and Craniofacial Research; the National Institute on Alcohol Abuse and Alcoholism; the National Institute on Deafness and other Communication Disorders; and the Office of Research on Women’s Health. Women’s Interagency HIV Study data collection is also supported by UCSF CTSA [UL1-TR000004]; Atlanta CTSA [UL1-TR000454]; University of North Carolina at Chapel Hill Center for AIDS Research [P30-AI-050410]; and the University of Alabama at Birmingham Center for AIDS Research [P30-AI-027767].
Footnotes
CONFERENCE PRESENTATIONS: None to report
CONFLICTS OF INTEREST:
The authors have no conflicts of interest to report.
Contributor Information
Jon C. Mills, Institute for Global Health and Infectious Diseases, University of North Carolina at Chapel Hill, McGavran-Greenberg Hall 2103B, Chapel Hill, NC 27599.
Brian W. Pence, Department of Epidemiology, The University of North Carolina at Chapel Hill, Gillings School of Global Public Health, Chapel Hill, United States.
Andrew Edmonds, Department of Epidemiology, The University of North Carolina at Chapel Hill, Gillings School of Global Public Health, Chapel Hill, United States.
Adebola Adedimeji, Department of Epidemiology & Population Health, Albert Einstein College of Medicine, Bronx, United States.
Rebecca M. Schwartz, Department of Occupational Medicine, Epidemiology and Prevention, Zucker School of Medicine at Hofstra/Northwell, Great Neck, United States.
Seble Kassaye, Department of Infectious Diseases, Georgetown University, Georgetown University Medical Center, Washington, DC, United States.
Jennifer Cocohoba, Department of Clinical Pharmacy, University of California San Francisco, School of Pharmacy, San Francisco, United States.
Mardge H. Cohen, Department of Medicine, John H. Stroger, Jr. Hospital of Cook County, Chicago, United States.
Gretchen Neigh, Department of Anatomy and Neurobiology, Virginia Commonwealth University, School of Medicine, Richmond, United States.
Margaret A. Fischl, Department of Medicine/Infectious Diseases, Miami Center for AIDS Research, University of Miami, Miller School of Medicine, Miami, United States.
Mirjam-Colette Kempf, Schools of Nursing, Public Health and Medicine, University of Alabama at Birmingham, Birmingham, AL, United States.
Adaora A. Adimora, Institute for Global Health and Infectious Diseases, University of North Carolina at Chapel Hill, Chapel Hill, United States.
References:
- 1.Bing EG, Burnam MA, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58(8):721–728. [DOI] [PubMed] [Google Scholar]
- 2.Orlando M, Burnam MA, Beckman R, et al. Re-estimating the prevalence of psychiatric disorders in a nationally representative sample of persons receiving care for HIV: results from the HIV Cost and Services Utilization Study. Int J Methods Psychiatr Res. 2002;11(2):75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ickovics JR, Hamburger ME, Vlahov D, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. Jama. 2001;285(11):1466–1474. [DOI] [PubMed] [Google Scholar]
- 4.Bengtson AM, Pence BW, Mimiaga MJ, et al. Depressive Symptoms and Engagement in HIV Care following ART Initiation. Clin Infect Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuniga JA, Yoo-Jeong M, Dai T, Guo Y, Waldrop-Valverde D. The Role of Depression in Retention in Care for Persons Living with HIV. AIDS Patient Care STDS. 2016;30(1):34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Traeger L, O’Cleirigh C, Skeer MR, Mayer KH, Safren SA. Risk factors for missed HIV primary care visits among men who have sex with men. J Behav Med. 2012;35(5):548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58(2):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–2107. [DOI] [PubMed] [Google Scholar]
- 9.Horberg MA, Silverberg MJ, Hurley LB, et al. Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;47(3):384–390. [DOI] [PubMed] [Google Scholar]
- 10.Ironson G, O’Cleirigh C, Fletcher MA, et al. Psychosocial factors predict CD4 and viral load change in men and women with human immunodeficiency virus in the era of highly active antiretroviral treatment. Psychosom Med. 2005;67(6):1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pence BW, Miller WC, Gaynes BN, Eron JJ Jr. Psychiatric illness and virologic response in patients initiating highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;44(2):159–166. [DOI] [PubMed] [Google Scholar]
- 12.Mills JC, Pence BW, Todd JV, et al. Cumulative Burden of Depression and All-Cause Mortality in Women Living with HIV. Clin Infect Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pence BW, Mills JC, Bengtson AM, et al. Association of Increased Chronicity of Depression With HIV Appointment Attendance, Treatment Failure, and Mortality Among HIV-Infected Adults in the United States. JAMA Psychiatry. 2018;75(4):379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallant JE, Mehta SH, Sugarman J. Universal antiretroviral therapy for HIV infection: should US treatment guidelines be applied to resource-limited settings? Clin Infect Dis. 2013;57(6):884–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. Jama. 2012;308(4):387–402. [DOI] [PubMed] [Google Scholar]
- 16.Nelson M, Girard PM, Demasi R, et al. Suboptimal adherence to darunavir/ritonavir has minimal effect on efficacy compared with lopinavir/ritonavir in treatment-naive, HIV-infected patients: 96 week ARTEMIS data. J Antimicrob Chemother. 2010;65(7):1505–1509. [DOI] [PubMed] [Google Scholar]
- 17.Parienti JJ, Ragland K, Lucht F, et al. Average adherence to boosted protease inhibitor therapy, rather than the pattern of missed doses, as a predictor of HIV RNA replication. Clin Infect Dis. 2010;50(8):1192–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58(1):1–10. [DOI] [PubMed] [Google Scholar]
- 19.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV: Department of Health and Human Services; 2018.
- 20.Bacon MC, von Wyl V, Alden C, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12(9):1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9(2):117–125. [PubMed] [Google Scholar]
- 22.Adimora AA, Ramirez C, Benning L, et al. Cohort Profile: The Women’s Interagency HIV Study (WIHS). Int J Epidemiol. 2018;47(2):393–394i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106(3):203–214. [DOI] [PubMed] [Google Scholar]
- 24.Kalichman SC, Rompa D, Cage M. Distinguishing between overlapping somatic symptoms of depression and HIV disease in people living with HIV-AIDS. J Nerv Ment Dis. 2000;188(10):662–670. [DOI] [PubMed] [Google Scholar]
- 25.Choi SW, Schalet B, Cook KF, Cella D. Establishing a common metric for depressive symptoms: linking the BDI-II, CES-D, and PHQ-9 to PROMIS depression. Psychol Assess. 2014;26(2):513–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vannoy SD, Arean P, Unutzer J. Advantages of using estimated depression-free days for evaluating treatment efficacy. Psychiatr Serv. 2010;61(2):160–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mugavero MJ, Amico KR, Horn T, Thompson MA. The state of engagement in HIV care in the United States: from cascade to continuum to control. Clin Infect Dis. 2013;57(8):1164–1171. [DOI] [PubMed] [Google Scholar]
- 28.Mugavero MJ, Lin HY, Willig JH, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009;48(2):248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV: Department of Health and Human Services; 2018:H-2.
- 30.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. [DOI] [PubMed] [Google Scholar]
- 31.Naimi AI, Moodie EE, Auger N, Kaufman JS. Constructing inverse probability weights for continuous exposures: a comparison of methods. Epidemiology. 2014;25(2):292–299. [DOI] [PubMed] [Google Scholar]
- 32.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 33.Harrell FE. J. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 34.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. [DOI] [PubMed] [Google Scholar]
- 35.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43(7):939–941. [DOI] [PubMed] [Google Scholar]
- 36.Shuter J, Sarlo JA, Kanmaz TJ, Rode RA, Zingman BS. HIV-infected patients receiving lopinavir/ritonavir-based antiretroviral therapy achieve high rates of virologic suppression despite adherence rates less than 95%. J Acquir Immune Defic Syndr. 2007;45(1):4–8. [DOI] [PubMed] [Google Scholar]
- 37.Shuter J Forgiveness of non-adherence to HIV-1 antiretroviral therapy. J Antimicrob Chemother. 2008;61(4):769–773. [DOI] [PubMed] [Google Scholar]
- 38.Pence BW, Gaynes BN, Adams JL, et al. The effect of antidepressant treatment on HIV and depression outcomes: results from a randomized trial. Aids. 2015;29(15):1975–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Safren SA, Bedoya CA, O’Cleirigh C, et al. Cognitive behavioural therapy for adherence and depression in patients with HIV: a three-arm randomised controlled trial. Lancet HIV. 2016;3(11):e529–e538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaynes BN, O’Donnell J, Nelson E, et al. Psychiatric comorbidity in depressed HIV-infected individuals: common and clinically consequential. Gen Hosp Psychiatry. 2015;37(4):277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Machtinger EL, Haberer JE, Wilson TC, Weiss DS. Recent trauma is associated with antiretroviral failure and HIV transmission risk behavior among HIV-positive women and female-identified transgenders. AIDS Behav. 2012;16(8):2160–2170. [DOI] [PubMed] [Google Scholar]
- 42.Bengtson AM, Pence BW, Moore R, et al. Relationship between ever reporting depressive symptoms and all-cause mortality in a cohort of HIV-infected adults in routine care. Aids. 2017;31(7):1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shacham E, Onen NF, Donovan MF, Rosenburg N, Overton ET. Psychiatric Diagnoses among an HIV-Infected Outpatient Clinic Population. J Int Assoc Provid AIDS Care. 2016;15(2):126–130. [DOI] [PubMed] [Google Scholar]
- 44.Blank MB, Himelhoch S, Walkup J, Eisenberg MM. Treatment considerations for HIV-infected individuals with severe mental illness. Curr HIV/AIDS Rep. 2013;10(4):371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kinyanda E, Levin J, Nakasujja N, et al. Major Depressive Disorder: Longitudinal Analysis of Impact on Clinical and Behavioral Outcomes in Uganda. J Acquir Immune Defic Syndr. 2018;78(2):136–143. [DOI] [PubMed] [Google Scholar]
- 46.Kelso-Chichetto NE, Okafor CN, Cook RL, Abraham AG, Bolan R, Plankey M. Association Between Depressive Symptom Patterns and Clinical Profiles Among Persons Living with HIV. AIDS Behav. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bengtson AM, Pence BW, Powers KA, et al. Trajectories of Depressive Symptoms Among a Population of HIV-Infected Men and Women in Routine HIV Care in the United States. AIDS Behav. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mallick R, Chen J, Entsuah AR, Schatzberg AF. Depression-free days as a summary measure of the temporal pattern of response and remission in the treatment of major depression: a comparison of venlafaxine, selective serotonin reuptake inhibitors, and placebo. J Clin Psychiatry. 2003;64(3):321–330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.