Abstract
Objectives
To determine whether synthetic phosphorylated hexaacyl disaccharides (PHADs) provide antimicrobial protection in clinically relevant models of bacterial infection.
Design
Laboratory study.
Setting
University laboratory.
Subjects
BALB/c, C57BL/10J, and C57BL/10ScNJ mice.
Interventions
Mice were treated with Lactated Ringer’s (vehicle) solution, monophosphoryl lipid A (MPLA) or PHADs at 48 and 24 hours prior to intraperitoneal (IP) P. aeruginosa or intravenous (IV) S. aureus infection. Leukocyte recruitment, cytokine production, and bacterial clearance were measured 6 hours after P. aeruginosa infection. In the systemic S. aureus infection model, one group of mice was monitored for 14-day survival and another for S. aureus tissue burden at 3 days post-infection. Duration of action for 3D 6-Acyl PHAD was determined at 3, 10, and 14 days using a model of IP P. aeruginosa infection. Effect of 3D 6-Acyl PHAD on in vivo leukocyte phagocytosis and respiratory burst was examined. Leukocyte recruitment, cytokine production, and bacterial clearance were measured after P. aeruginosa infection in wildtype and TLR4 knockout mice treated with 3D 6-Acyl PHAD or vehicle to assess receptor specificity.
Measurements and Main Results
During IP P. aeruginosa infection, PHADs significantly attenuated infection-induced hypothermia, augmented leukocyte recruitment and bacterial clearance and decreased cytokine production. At 3 days post S. aureus infection, bacterial burden in lungs, spleen, and kidneys were significantly decreased in mice treated with MPLA or PHADs, which was associated with improved survival. Leukocyte phagocytosis and respiratory burst functions were enhanced after treatment with MPLA or PHADs. A time course study showed MPLA- and 3D 6-Acyl PHAD-mediated protection against P. aeruginosa lasts for up to 10 days. Partial loss of augmented innate antimicrobial responses was observed in TLR4 knockout mice treated with 3D 6 Acyl PHAD.
Conclusions
PHADs significantly augment resistance against clinically relevant Gram negative and positive infections via enhanced leukocyte recruitment, phagocytosis and respiratory burst functions of innate leukocytes. Improved antimicrobial protection persists for up to 10 days and is partially mediated through TLR4.
Keywords: TLR4 agonist, LPS, MPLA, PHADs, P. aeruginosa, S. aureus, antimicrobial
INTRODUCTION
Critically ill patients are at increased risk of acquiring nosocomial infections due to development of injury- and illness-induced immune alterations that prevent mounting of proper immune responses against pathogens (1). Prophylactic antibiotics remain the primary prevention strategy against nosocomial infections; but with the escalating threat of antibiotic resistance, alternative therapies to circumvent or improve the efficacy of antibiotics are needed (2, 3).
We demonstrated that the TLR4 agonist and vaccine adjuvant monophosphoryl lipid A (MPLA) improves resistance to Gram positive, Gram negative, fungal and polymicrobial sepsis and that resistance persists for at least 15 days (4–6).
Despite abundant work demonstrating the antimicrobial benefits of MPLA in experimental animals, application in humans remains limited to vaccine adjuvants, without prospects for development as an independent antimicrobial agent. MPLA is only available as a component of a proprietary vaccine adjuvant system and not as a standalone drug. That prompted our search for synthetic MPLA analogs that augment innate antimicrobial immunity.
Phosphorylated hexaacyl disaccharides (PHADs) are the result of that search. Three variants, termed PHAD, 3-deacyl PHAD (3D PHAD) and 3D 6-Acyl PHAD, have been developed with varying acyl side chain conformations. We hypothesized that priming with PHADs would augment antimicrobial resistance to common Gram negative and Gram positive nosocomial pathogens. In the present study, we observed that PHADs are potent TLR4 agonists that bolster host resistance to common hospital-acquired pathogens by facilitating recruitment of neutrophils and monocytes to sites of infection and augmenting their antimicrobial functions. To our knowledge, these are the first studies to characterize the immunomodulatory functions of ultra-pure, synthetic TLR4 agonists with clinically translatable potential as stand-alone antimicrobial agents.
MATERIALS AND METHODS
Mice
Studies were approved by the Institutional Animal Care and Use Committee at Vanderbilt University Medical Center and complied with the National Institutes of Health Guide for the Care and Use of Experimental Animals. Male 8- to 12-week-old BALB/c, C57BL/10J, and C57BL/10ScNJ mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and Charles River (Hollister, CA). Mouse strain C57BL/10ScNJ was derived following a spontaneous, loss of function mutation of TLR4 in C57BL/10J mice, and thus served as the model for TLR4 KO mice.
Monophosphoryl lipid A (MPLA), Monophosphoryl Lipid A Synthetic
phosphorylated hexaacyl disaccharide (PHAD), 3-deacyl PHAD (3D PHAD), and 3D 6-Acyl PHAD. MPLA was purchased from Sigma-Aldrich Chemical and is produced by acid hydrolysis of lipid A from S. minnesota (St. Louis, MO). PHAD, 3D PHAD, and 3D 6-Acyl PHAD were synthesized de novo by Avanti Polar Lipids (Alabaster, AL). Comparison of the structures of each of the PHADs with native lipid A and MPLA is shown in Supplemental Figure 1. These three analogs are collectively referred as PHADs throughout the paper. All TLR4 agonists were dissolved in endotoxin-free water containing 0.2% trimethylamine and sonicated in a 40oC water bath for 1 hour. Dilutions of MPLA and PHADs were prepared for injection using Lactated Ringers (LR) solution.
TLR4 agonist priming experiments
BALB/c mice underwent intravenous (IV) priming with vehicle, MPLA, PHAD, 3D PHAD, or 3D 6-Acyl PHAD. Priming is defined as administration (1 mg/kg; 20 μg in 0.2 ml LR) at 48 and 24 hours prior to infection.
Bacterial infection
Intraperitoneal Pseudomonas aeruginosa Infection
After priming with vehicle, MPLA, or PHADs, mice were challenged with 1 ×108 colony forming units (CFU) of P. aeruginosa (American Type Culture and Collection, Manassas, VA; ATCC 19660) in 0.5 mL of 0.9% normal saline by IP injection. Rectal temperature was measured hourly and peritoneal lavage was performed at 6 hours after infection to measure bacterial and leukocyte counts. Plasma and peritoneal lavage fluid were harvested at 6 hours after infection for measurement of cytokine concentrations.
Systemic Staphylococcus aureus Infection
After priming with vehicle, MPLA or PHADs, mice were infected with S. aureus (American Type Culture and Collection, Manassas, VA; ATCC 25923), 1 × 108 CFU in 0.2 mL of sterile saline via IV injection. At 72 hours post-infection, mice were euthanized for harvest of blood, lung, spleen, and kidney. A second cohort of mice were monitored for survival for 14 days.
Measurement of bacterial counts
Serial dilutions of peritoneal lavage fluid obtained after P. aeruginosa infection, and blood and tissue homogenates obtained after systemic S. aureus infection were grown on tryptic soy agar overnight. Colony counts were performed to determine CFU per milliliter of P. aeruginosa in peritoneal fluid and S. aureus per gram of tissue homogenate (7).
Flow Cytometry
Leukocytes obtained by peritoneal lavage were suspended in phosphate buffered saline (PBS) (1 × 107 cells/ml) and incubated with anti-mouse CD16/32 (eBioscience, 1 μl/ml) for 5 minutes to block nonspecific Fc receptor–mediated antibody binding. One million cells were then transferred into polystyrene tubes, incubated with fluorochrome-conjugated antibodies or isotype controls (0.5 μg /tube), washed with 2 ml of cold PBS, centrifuged at 300xg for 10 minutes and resuspended in 250 μl cold PBS. Samples were run immediately on an Accuri C6 flow cytometer (BD Biosciences, San Diego, CA)(8). Data were analyzed using Accuri C6 software.
Antibodies included anti-Ly6G-PE, anti-Ly6C-PE Cy5.5, anti-F4/80-FITC, and appropriate isotype controls (EBioscience, San Diego, CA). Neutrophils were identified as F4/80−Ly6G+, macrophages as F4/80+Ly6C−, and monocytes as F4/80+Ly6C+.
Cytokine/chemokine measurements
Blood and peritoneal lavage fluid were harvested 6 hours after P. aeruginosa infection for cytokine measurements. Plasma was collected after centrifugation of blood (2119 x g for 10 minutes at 4°C) and retained for cytokine analysis. Peritoneal lavage was performed with 5 ml of cold PBS. Concentrations of granulocyte-colony stimulating factor (G-CSF), chemokine ligand 1 (CXCL-1; KC), chemokine ligand 2 (CXCL-2; MIP-2), interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ) were measured using a Bio-Plex mouse bead array according to the manufacturer’s directions (Bio-Rad). A MagPix MultiPlex Reader (Bio-Rad) was used to analyze plasma and peritoneal fluid samples.
Phagocytosis assay
Mice were primed with 3D 6-Acyl PHAD at 48 and 24 hours prior to an IP injection of 500 μg pHrodo-tagged S. aureus bioparticles in 0.2 mL of LR solution (Life Technologies, Carlsbad, CA). Six hours after injection, the peritoneal cavity was lavaged with 5 mL of PBS. Leukocytes obtained by peritoneal lavage were prepared for flow cytometry as described above and stained with anti-Ly6G-FITC, anti-F4/80-FITC, and anti-Ly6C-PE-Cy5.5 antibodies. Cellular pHrodo mean fluorescence intensity (MFI) and cell type were determined by flow cytometry.
Respiratory burst assay
Mice were primed with 3D 6-Acyl PHAD at 48 and 24 hours prior to euthanasia, followed by peritoneal lavage with 5 mL of PBS. Peritoneal leukocytes were prepared for flow cytometry as described above. Cells were resuspended at 1 × 106 cells/mL, incubated with dihydrorodamine 123 for 15 minutes, followed by incubation with phorbol myristate acetate (PMA, 200 nM) for 45 minutes to provoke generation of reactive oxygen species, according to manufacturer’s recommendations (Cayman Chemical, Ann Arbor, MI). After stimulation, dihydrorhodamine 123 MFI was measured by flow cytometry. Forward versus side scatter was used to identify neutrophils, and monocytes.
Generation of bone marrow-derived macrophages (BMDM)
The marrow cavities of femurs from C57BL/10J and C57BL/10ScNJ mice were flushed with 5mL PBS. Cells were washed and re-suspended in Roswell Park Memorial Institute (RPMI) 1640 medium (Corning) supplemented with 10% fetal bovine serum, 1% Antibiotic-Antimycotic, (Gibco® Antibiotic-Antimycotic; penicillin, streptomycin, Fungizone® Antimycotic) and 10 ng/mL recombinant macrophage-colony stimulating factor (M-CSF, R&D Systems, Minneapolis, MN) (M-RPMI). One million cells were cultured in 150mm petri dishes (Falcon by Corning) at 37°C and 5% CO2. On day 7 after harvest, cells were washed and re-suspended in fresh M-RPMI. BMDM purity was confirmed to be >98% F4/80+ CD11b+ CD11c− by flow cytometry on day 8 and were resuspended at 1×106 cells/mL in 6-well plates. Cells were allowed to attach for 2 hours and then stimulated with vehicle or PHADs (1μg/mL) for 45 minutes.
Western Blotting
BMDM were washed twice with Hanks Buffered Saline Solution (HBSS) and lysed with radioimmunoprecipitation assay (RIPA) buffer (Sigma) containing PhosStop and complete Protease Inhibitor tablets (Roche). Protein samples were mixed 1:1 with Laemmli buffer (Bio-Rad) and separated by gel electrophoresis on MINI-PROTEAN precast 4–20% tris-glycine gels (Bio-Rad). Protein was transferred to nitrocellulose membranes (Perkin-Elmer). Membranes were blocked with 5% fraction V bovine serum albumin (BSA) (RPI Corp.) for one hour and incubated with primary antibodies from Cell Signaling Technology (1:1000 dilution; phospho-IKK-alpha/beta [Clone 16A6], Cat:2697S; IKK-beta [clone 2C8], Cat: 2370S; Phospho-IRF-3 [clone S396], Cat: 4947S; IRF-3 [clone D83B9], Cat: 4302S) in 5% BSA at 4°C overnight. Membranes were washed 3 times for 5 minutes in tris-buffered saline with 0.1% Tween-20 (TBST). Protein bands were detected with HRP-conjugated secondary antibodies (1:2000 dilution; anti-Rabbit IgG, HRP-linked, Cat: 7074S) in 5% BSA, washed 3 times for 5 minutes in TBST, and incubated for 60 seconds with electrochemiluminescence (ECL) reagent. Images were cropped using ImageJ 1.52a software from the NIH (http://imagej.nih.gov/ij/).
Statistics
All data were analyzed using GraphPad Prism software (GraphPad Software, San Diego, CA, USA). Data from multiple group experiments were analyzed using one-way ANOVA followed by Tukey post hoc multiple comparison test and Kruskal-Wallis test for comparison of the median. Survival data was analyzed using the Log-rank test. Values are reported as the mean ± SEM. A value of p<0.05 was considered statistically significant.
RESULTS
PHADs protect against P. aeruginosa IP infection via enhanced bacterial clearance
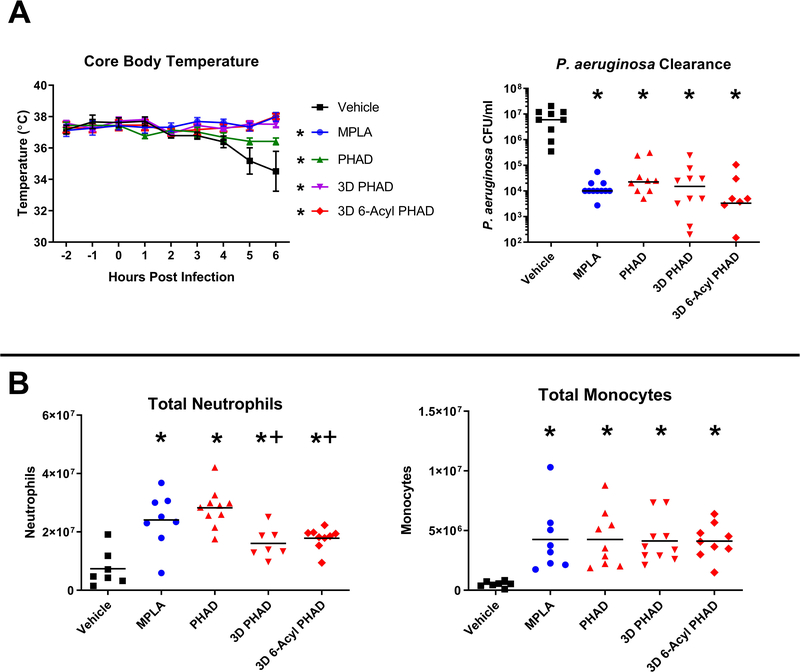
MPLA or PHADs protected mice from infection-induced hypothermia (Figure 1A). Intraperitoneal bacterial burden in MPLA- and PHAD-primed mice was significantly lower than in vehicle-treated mice, and no significant differences were observed among the PHADs or when comparing each of the PHADs to MPLA (Figure 1A).
Figure 1. PHADs protect against infection-induced hypothermia, mediate bacterial clearance, and enhance recruitment of leukocytes after P. aeruginosa infection.
Mice received vehicle or 20 μg of MPLA or PHADs via IP injection 48 and 24 hours prior to IP injection with 1 × 108 CFU of P. aeruginosa. Six hours after infection, blood was collected for plasma extraction and peritoneal lavage was performed. (A) Hourly core body temperature was measured beginning 2 hours prior to infection and continued until euthanasia. P. aeruginosa numbers were quantified in peritoneal lavage fluid as a measure of bacterial burden. (B) Peritoneal cells were stained for F4/80, Ly6G, and Ly6C. Neutrophils were identified as F4/80−Ly6G+. Monocytes were identified as F4/80+/Ly6C+. Total neutrophils and monocytes were measured from peritoneal lavage fluid. *p<0.05 compared to vehicle, +p<0.05 compared to PHAD. Results represent 2 separate experiments, n=7–10 per group.
PHADs facilitate rapid recruitment of neutrophils and monocytes to the site of infection
We measured the total number of neutrophils (F4/80−Ly6G+) and monocytes (F4/80+Ly6C+) in the peritoneal cavity after P. aeruginosa infection (Figure 1B). The total numbers of neutrophils and monocytes in the peritoneal cavity were higher in mice treated with MPLA and PHADs compared to control (Figure 1B). 3D PHAD induced a higher proportion of monocytes as compared to PHAD. Both 3D PHAD and 3D 6-Acyl PHAD induced significantly fewer total numbers of neutrophils as compared to PHAD, although numbers remained significantly elevated above other treatment groups and vehicle-treated mice.
PHADs attenuate cytokine production during infection
We measured cytokine concentrations in the plasma after PHADs priming and subsequent infection. We observed lower plasma and peritoneal lavage concentrations of CXCL-1, IL-6, and TNF-α in mice treated with MPLA or PHADs compared to vehicle control (Supplemental Figure 2).
PHADs enhance bacterial clearance and survival during systemic S. aureus infection
Mice were primed with vehicle, MPLA or PHADs at 48 and 24 hours prior to infection (Figure 2A). Mice treated with MPLA or PHADs had significantly fewer S. aureus colonies in lung, spleen and kidney (Figure 2B). Priming with MPLA or PHADs resulted in >50% survival at two weeks after infection compared to 100% mortality in vehicle treated mice (Figure 2C). No significant difference in survival was observed amongst PHADs or when comparing PHADs to MPLA.
Figure 2. PHADs induce bacterial clearance and promote survival after systemic S. aureus infection.
(A) Mice received vehicle or 20 μg of MPLA or PHADs via IV injection 48 and 24 hours prior to IV injection with 1 × 108 CFU of S. aureus. (B) In one set of experiments, mice were euthanized at 72 hours after infection and lung, spleen, and kidney tissues were harvested to measure bacterial burden. (C) In a separate set of experiments, mice underwent the same treatment with MPLA or PHADs, followed by IV infection with S. aureus and were monitored for survival over 14 days. *p<0.05 compared to vehicle. Results represent 4 separate experiments, n=13–16 per group.
3D 6-Acyl PHAD does not induce the host inflammatory response
Due to similar antimicrobial properties amongst all PHADs, we focused the remaining mechanistic studies on 3D 6-Acyl PHAD, which is most structurally similar to MPLA. To examine the potential of 3D 6-Acyl PHAD to induce the host inflammatory response independent of infection, we measured pro-inflammatory cytokine production at 3 and 6 hours after 3D 6-Acyl PHAD treatment. LPS treatment served as a positive control. Our results demonstrate that treatment with 20 μg of LPS induced significant elevation of CXCL-1, CXCL-2, G-CSF, IL-6, and TNF-α at 3 and 6 hours after treatment. In contrast, with the exception of G-CSF, treatment with 20 μg of 3D 6-Acyl PHAD did not elevate cytokine production (Supplemental Figure 3).
3D 6-Acyl PHAD enhances phagocytosis and respiratory burst
Mice were primed with 3D 6-Acyl PHAD followed by IP challenge with S. aureus particles conjugated to pH-rodo red. Peritoneal leukocytes were isolated 6 hours after particle injection and pH-rodo MFI was measured by flow cytometry. Neutrophils (F4/80−Ly6G+), monocytes (F4/80+Ly6C+), and macrophages (F4/80+Ly6C−) from 3D 6-Acyl PHAD-treated mice demonstrated enhanced phagocytosis. (Figure 3A). We also identified enhanced respiratory burst, as measured by DHR-123 MFI, in neutrophils and monocytes from 3D 6-Acyl PHAD-treated mice (Figure 3B).
Figure 3. 3D 6-Acyl PHAD enhances phagocytosis and respiratory burst in primary innate leukocytes.
Mice received vehicle or 20 μg of 3D 6-Acyl PHAD via IP injection 48 and 24 hours prior to assessing phagocytic function and respiratory burst. (A) Phagocytic function was assessed by IP injection of 500 μg pH Rodo Red S. aureus particles, followed by a 6 hour incubation period. Peritoneal lavage cells were examined using flow cytometry. pH Rodo MFI represents the degree of phagocytosis per cell. Results represent 4 separate experiments, n=16–20 per group. (B) Respiratory burst function of peritoneal lavage cells was assessed following induction of respiratory burst with PMA. Respiratory burst capacity was assessed by DHR 123 MFI. Results represent 2 separate experiments, n=4 per group.
The protective effects of 3D 6-Acyl PHAD persist for up to 10 days
We primed mice with 3D 6-Acyl PHAD on 3, 10, and 14 days prior to infection (Figure 4A). After infection, core temperature was recorded hourly for 6 hours at which time bacterial burden was assessed in peritoneal lavage fluid. Priming with 3D 6-Acyl PHAD attenuated infection-induced hypothermia when given 3, 10 and 14 days prior to infection (Figure 4B). Bacterial clearance was augmented at 3 and 10 days after 3D 6-Acyl PHAD treatment (Figure 4C).
Figure 4. 3D 6-Acyl PHAD protects against sepsis-induced hypothermia for up to 14 days, and enhances bacterial clearance in an IP P. aeruginosa infection for up to 10 days.
(A) Mice received 2 doses of 3D 6-Acyl PHAD at either 4 and 3, 11 and 10, or 15 and 14 days prior to IP infection with 1 × 108 CFU P. aeruginosa followed by euthanasia 6 hours after infection. (B) Hourly core body temperature was measured beginning at time of infection and continued until euthanasia. (C) P. aeruginosa numbers were quantified in peritoneal lavage fluid as a measure of bacterial burden. Results represent 4 separate experiments, n=10 per treatment group and n=23 for vehicle group.
PHADs are selective TLR4 agonists that induce partial immune protection in the absence of functional TLR4 receptors
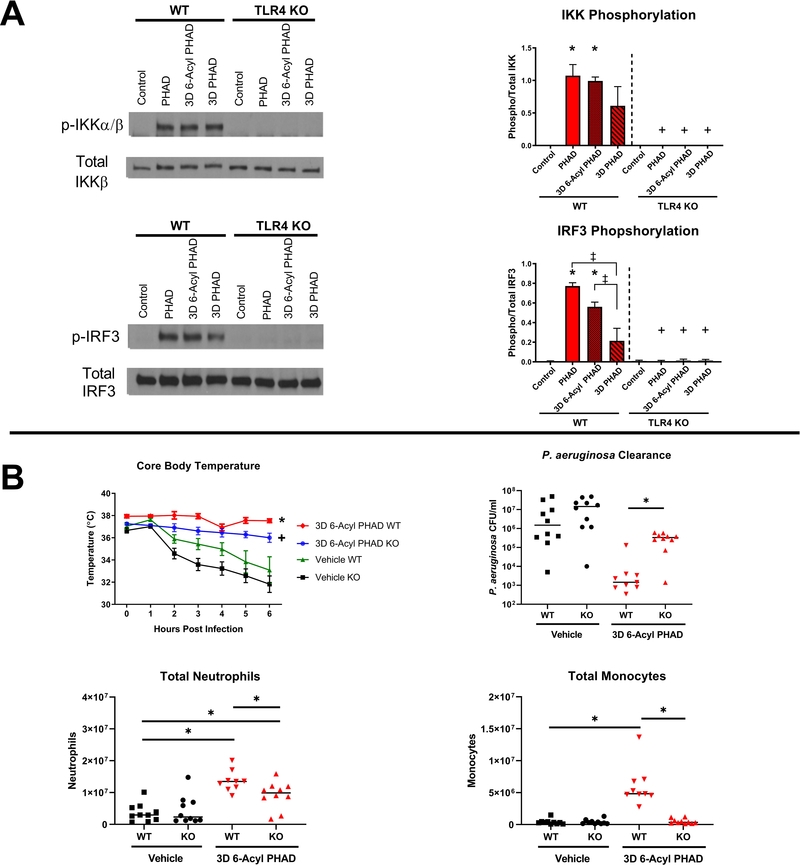
All 3 PHADs activated MyD88- and TRIF-dependent signaling pathways as indicated by IKK and IRF3 phosphorylation, respectively, in wild type BMDM. PHAD-induced IKK and IRF3 phosphorylation was ablated in TLR4 KO BMDM. 3D PHAD induced significantly lower IRF3, but not IKK, phosphorylation in wild type BMDM, as compared to PHAD and 3D 6-Acyl PHAD (Figure 5A). To assess whether loss of TLR4 ablates immune protection afforded by PHADs in vivo, wildtype (WT) or TLR4 KO mice were treated by intraperitoneal injection with 3D 6-Acyl PHAD or vehicle control at 48 and 24 hours prior to intraperitoneal challenge with P. aeruginosa. Core body temperature was monitored hourly for 6 hours after infection. P. aeruginosa CFU, peritoneal leukocyte numbers and plasma cytokine levels were measured at 6 hours post-infection. Core body temperature was maintained in both WT and KO mice treated with 3D 6-Acyl PHAD, while vehicle treated mice of both genotypes became hypothermic by 6 hours post-infection (Figure 5B). P. aeruginosa CFU in the peritoneal lavage fluid was significantly decreased in WT mice treated with 3D 6-Acyl PHAD. However, the bacterial clearance benefit provided by 3D 6-Acyl PHAD treatment was ablated in the KO mice. Notably, neutrophil numbers were significantly elevated in both WT and KO mice that received 3D 6-Acyl PHAD, although not to the same degree as in the WT mice. However, monocytes were significantly elevated only in the WT mice after 3D 6-Acyl PHAD treatment, and not in 3D 6-Acyl PHAD-treated-KO mice (Figure 5B). Finally, the plasma pro-inflammatory cytokines IL-6, TNF-α, and IFN-γ were significantly attenuated 6 hours post-infection in both WT and KO mice treated with 3D 6-Acyl PHAD (Supplemental Figure 4).
Figure 5. PHADs induce TLR4-dependent MyD88- and TRIF-pathway signaling in bone marrow derived macrophages, and 3D 6-Acyl PHAD mediates partial immune function in TLR4 knockout mice.
(A) Bone marrow derived macrophages from wild type and TLR4 knockout mice were incubated with vehicle or PHADs for 45 minutes. IKK phosphorylation and total IKK, and IRF3 phosphorylation and total IRF3 were determined by Western blotting. IKK and IRF3 phosphorylation was quantified via densitometry (ImageJ 1.52a). (B) Wild type or knockout mice received vehicle or 20 μg of 3D 6-Acyl PHAD or vehicle via IP injection 48 and 24 hours prior to IP injection with 1 × 108 CFU of P. aeruginosa. Six hours after infection, blood was collected for plasma extraction and peritoneal lavage was performed. Hourly core body temperature was measured after infection and continued until euthanasia. P. aeruginosa numbers were quantified in peritoneal lavage fluid as a measure of bacterial burden. (B) Peritoneal cells were stained for F4/80, Ly6G, and Ly6C. Neutrophils were identified as F4/80−Ly6G+. Monocytes were identified as F4/80+/Ly6C+. Total neutrophils and monocytes were measured from peritoneal lavage fluid*p<0.05 compared to control, ‡p<0.05 compared to 3D PHAD, and +p<0.05 compared to wild type. Results represent 3 separate experiments for Western blots, n=6, and 2 separate experiments for infection studies, n=10.
DISCUSSION
The major finding of our study is that PHADs, when given as a prophylactic treatment, enhance the innate antimicrobial response against P. aeruginosa and S. aureus, two common nosocomial pathogens. Our previous studies indicate that the TLR4 agonist MPLA is capable of enhancing innate immune responses and improving survival in an immunocompromised severe burn injury and subsequent lethal murine model of wound infection (4, 9). To provide proof of concept data for the PHAD compounds, we examined antimicrobial responses in healthy immunocompetent mice. Prophylaxis with any of the PHADs reduced bacterial burden and inflammatory cytokine concentrations and augmented innate leukocyte recruitment during infection. Protection was associated with enhanced respiratory burst and phagocytosis by neutrophils and macrophages. In addition, we identified the duration of efficacy for 3D 6-Acyl PHAD, showing protection against hypothermia lasting up to 14 days after treatment and improved bacterial clearance up to 10 days after treatment.
PHADs have significant potential for clinical development since they are synthetic and ultrapure. PHADs are structurally similar to MPLA – all agonists lack the C1 phosphate that is present on native lipid A, which renders these agonists equipotent with lipid A in regard to immunotherapeutic properties but with 0.1% of the immunotoxicity (10–12). Differences among the three PHADs involve modifications of the location of the 5th acyl chain (Supplemental Figure 1). 3D 6-Acyl PHAD has the strongest resemblance to MPLA, having the 5th acyl chain attached to the hydroxyl group of the 6th acyl chain. In the case of 3D PHAD, the 5th acyl chain is absent rendering a compound with 5, rather than 6, acyl chains. However, all PHADs were equipotent immunomodulators when compared to MPLA. Numerous investigators have examined the impact of acyl chain number and conformation on the potency of lipid A analogs (13–17). MPLA and PHADs, even the hexaacylated forms, are weak inducers of cytokine production due to loss of the C1 phosphate group (11). Our study indicates that both penta- and hexa-acylated MPLA analogs retain potent capacity to augment innate antimicrobial functions beyond cytokine production.
PHAD-induced activation of MyD88- and TRIF-dependent signaling pathways in macrophages is TLR4 selective (Figure 5). We noted 3D PHAD was less potent at inducing phosphorylation of IRF3 (indicator of TRIF signaling) than other agonists. However, 3D PHAD induced IKK phosphorylation (indicator of MyD88 signaling) to the same degree as PHAD and 3D 6-Acyl PHAD. 3D PHAD is the only compound that is penta-acylated, lacking a single carbon chain, which may explain the weaker ability to induce IRF3 phosphorylation. The impact of structural difference among the PHADs and the effect of those differences on activation of TLR4 signaling pathways warrants further investigation.
Mice lacking a functional TLR4 receptor showed significant loss of several innate immune enhancements afforded by PHAD treatment. After 3D 6-Acyl PHAD treatment, infected TLR4 KO mice lost the ability to effectively clear P. aeruginosa from the peritoneal cavity. The ability to recruit greater numbers of monocytes by 3D 6-Acyl PHAD treatments was completely ablated in TLR4 KO mice. However, neutrophil recruitment was only slightly impaired. Interestingly, protection against infection-induced hypothermia and attenuation of inflammatory cytokine production was also maintained in TLR4 KO mice after treatment with 3D 6-Acyl PHAD. These findings suggest that TLR4 is critical for PHAD-augmented bacterial clearance and monocyte recruitment, but less important for PHAD-induced neutrophil recruitment and the global physiologic response to sepsis. This raises the concept that there may be additional TLR4-independent mechanisms by which 3D 6-Acyl PHAD can mediate host immune and physiologic responses to infection. It has been shown that the immune system can be activated by LPS though TLR4-independent pathways, including sensing of LPS intracellularly by caspases (18–20). Notably, both penta- and hexa-acyl lipid A components of LPS can activate immune responses independently of TLR4 through activation of cytosolic caspase-11 signaling (19). Future studies will aim at identifying alternate pathways by which PHADs can activate innate immune responses, independent of TLR4, which may be responsible for mediating partial immune protection in our model.
Based on our results, PHADs are optimal candidates for clinical development as antimicrobial agents. Recently, the CDC upgraded the threat level of both antibiotic-resistant P. aeruginosa and S. aureus to “Serious,” and there is increasing evidence of rising antibiotic resistance (3). TLR4 agonists are an experimentally validated prospect to meet this need (4, 21, 22). However, there is currently no opportunity for the use of MPLA outside of its role as a vaccine adjuvant. Our study identifies PHADs as novel, synthetic TLR4 agonists with high prospect for clinical development as stand-alone or adjunct antimicrobial agents.
Our study has limitations. Although different mouse strains were employed to enhance rigor, we recognize this may not readily translate to human subjects. Testing in clinically-relevant large animal models of infection is required for clinical translation. Furthermore, we demonstrated priming with PHADs is an effective means of immunoprophylaxis, but therapeutic efficacy remains unclear for an active infection. Our studies involved healthy mice which underwent a controlled infection, rather than immunosuppressed mice that underwent secondary infections, which are more common in critically ill patients. The impact of PHADs on critically ill patients may require complex models of infection that represent vulnerable patient populations such as burn patients who often develop secondary infections commonly plagued with antibiotic resistance. Studies are currently underway in our laboratory to further assess the efficacy of PHADs in clinically relevant models of post-injury infection.
CONCLUSIONS
Our findings indicate that PHADs share many of the immunogenic properties that MPLA possesses. PHADs enhanced innate leukocyte recruitment and bacterial clearance in clinically relevant models of infection involving common Gram negative and Gram positive pathogens. Thus, PHADs are synthetic, ultrapure TLR4 agonists with high potential for use as immunotherapeutic drugs in the clinical setting, and warrant further investigation and development for clinical application.
Supplementary Material
Supplemental Figure 1. PHADs have structural similarities to MPLA and differences from E. coli Lipid A. (A) The native diphosphoryl Lipid A portion of lipopolysaccharide from E. coli is illustrated here showing two phosphate groups. The second phosphate group is highlighted with a circle, illustrating the phosphate group that is cleaved in MPLA and PHADs. (B) The structure of MPLA is illustrated, where the 5th acyl chain is bound to the distal hydroxyl group of the 6th acyl 14 carbon chain. (C) The three different Phosphorylated Hexaacyl Disaccharides are illustrated, noting the different bonding location of the 5th acyl 14 carbon chain. The 5th acyl chain is bound to disaccharide ring in PHAD, absent in 3D PHAD, and bound to the distal hydroxyl group of the 6th acyl chain in 3D 6-Acyl PHAD, similar to MPLA.
Supplemental Figure 2. PHADs attenuate plasma cytokine production after P. aeruginosa infection. Mice received vehicle or 20 μg of MPLA or PHADs via IP injection 48 and 24 hours prior to IP injection with 1 × 108 CFU of P. aeruginosa. Six hours after infection, blood was collected for plasma extraction. Plasma was analyzed for cytokine concentrations. *p<0.05 compared to vehicle. Results represent 2 separate experiments, n=7–10 per group.
Supplemental Figure 3. 3D 6-Acyl PHAD does not induce inflammatory cytokine production. Mice received vehicle or 20 μg of LPS or 3D 6-Acyl PHAD via IP injection. Blood was harvested at 3 or 6 hours after treatment, processed for plasma extraction, and analyzed for cytokine concentrations. *p<0.05 compared to LPS. Results represent 2 separate experiments, n=5–10 per group.
Supplemental Figure 4. 3D 6-Acyl PHAD does not induce inflammatory cytokine production in TLR KO mice. Wild type or knockout mice received vehicle or 20 μg of 3D 6-Acyl PHAD or vehicle via IP injection 48 and 24 hours prior to IP injection with 1 × 108 CFU of P. aeruginosa. Six hours after infection, blood was collected for plasma extraction. Plasma was analyzed for cytokine concentrations. *p<0.05 compared to control. Results represent 2 separate experiments, n=10.
Acknowledgements
This study was supported by U.S. National Institute of Health, Institute of General Medicine Grants R01 GM104306 to ERS, R01 GM12171 to JKB, and K08 GM123345 to AH, and T32 grant, 5T32GM108554–05 to NKP.
Supported by NIH Grants K08 GM123345 (AH), R01 GM121711 (JKB), R01 GM104306 (ERS), and 5T32GM108554–05 (NKP).
All authors acknowledge there are no financial or commercial conflicts of interest.
Copyright form disclosure: Drs. Hernandez, Luan, Stothers, Patil, Fensterheim, Guo, Sherwood, and Bohannon received support for article research from the National Institutes of Health (NIH). Dr. Sherwood’s institution received funding from the NIH. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Abbreviations
- BMDM
bone marrow–derived macrophage
- CFU
colony forming unit
- CXCL-1
chemokine ligand 1
- CXCL-2
chemokine ligand 2
- G-CSF
granulocyte colony stimulating factor
- IKK
IkB kinase
- IRF
IFN regulator factor
- IL-6
interleukin 6
- INF-γ
interferon-gamma
- KO
knockout
- LR
lactated Ringer’s
- MPLA
monophosphoryl lipid A
- MyD88
myeloid differentiation primary response gene 88
- PHADs
phosphorylated hexaacyl disaccharides
- TNF-α
tumor necrosis factor-alpha
- TRIF
Toll/IL-1 receptor (TIR) domain containing adaptor-inducing IFN-β
- lMFI
mean fluorescence intensity
Footnotes
Conflict of Interest and Disclosures
The authors declare no conflict of interest.
References
- 1.Gentile LF, Cuenca AG, Efron PA, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg 2012;72(6):1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta Y, Gupta A, Todi S, et al. Guidelines for prevention of hospital acquired infections. Indian J Crit Care Med 2014;18(3):149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hampton T Novel Programs and Discoveries Aim to Combat Antibiotic Resistance. JAMA 2015;313(24):2411–2413. [DOI] [PubMed] [Google Scholar]
- 4.Romero CD, Varma TK, Hobbs JB, et al. The Toll-like receptor 4 agonist monophosphoryl lipid a augments innate host resistance to systemic bacterial infection. Infect Immun 2011;79(9):3576–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fensterheim BA, Guo Y, Sherwood ER, et al. The Cytokine Response to Lipopolysaccharide Does Not Predict the Host Response to Infection. J Immunol 2017;198(8):3264–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fensterheim BA, Young JD, Luan L, et al. The TLR4 Agonist Monophosphoryl Lipid A Drives Broad Resistance to Infection via Dynamic Reprogramming of Macrophage Metabolism. J Immunol 2018;200(11):3777–3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patil NK, Bohannon JK, Luan L, et al. Flt3 Ligand Treatment Attenuates T Cell Dysfunction and Improves Survival in a Murine Model of Burn Wound Sepsis. Shock 2017;47(1):40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez A, Bohannon JK, Luan L, et al. The role of MyD88- and TRIF-dependent signaling in monophosphoryl lipid A-induced expansion and recruitment of innate immunocytes. J Leukoc Biol 2016;100(6):1311–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohannon JK, Luan L, Hernandez A, et al. Role of G-CSF in monophosphoryl lipid A-mediated augmentation of neutrophil functions after burn injury. J Leukoc Biol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qureshi N, Takayama K, Ribi E. Purification and Structural Determination of Nontoxic Lipid-a Obtained from the Lipopolysaccharide of Salmonella-Typhimurium. Journal of Biological Chemistry 1982;257(19):1808–1815. [PubMed] [Google Scholar]
- 11.Bentala H, Verweij WR, Huizinga-Van der Vlag A, et al. Removal of phosphate from lipid A as a strategy to detoxify lipopolysaccharide. Shock 2002;18(6):561–566. [DOI] [PubMed] [Google Scholar]
- 12.Pichichero ME. Improving vaccine delivery using novel adjuvant systems. Hum Vaccin 2008;4(4):262–270. [DOI] [PubMed] [Google Scholar]
- 13.Berezow AB, Ernst RK, Coats SR, et al. The structurally similar, penta-acylated lipopolysaccharides of Porphyromonas gingivalis and Bacteroides elicit strikingly different innate immune responses. Microb Pathog 2009;47(2):68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dixon DR, Darveau RP. Lipopolysaccharide heterogeneity: innate host responses to bacterial modification of lipid a structure. J Dent Res 2005;84(7):584–595. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Powell DA, Shaffer SA, et al. LPS remodeling is an evolved survival strategy for bacteria. Proc Natl Acad Sci U S A 2012;109(22):8716–8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yun J, Wang X, Zhang L, et al. Effects of lipid A acyltransferases on the pathogenesis of F. novicida. Microb Pathog 2017;109:313–318. [DOI] [PubMed] [Google Scholar]
- 17.Masihi KN, Lange W, Brehmer W, et al. Immunobiological activities of nontoxic lipid A: enhancement of nonspecific resistance in combination with trehalose dimycolate against viral infection and adjuvant effects. Int J Immunopharmacol 1986;8(3):339–345. [DOI] [PubMed] [Google Scholar]
- 18.Kayagaki N, Wong MT, Stowe IB, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 2013;341(6151):1246–1249. [DOI] [PubMed] [Google Scholar]
- 19.Hagar JA, Powell DA, Aachoui Y, et al. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 2013;341(6151):1250–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmid-Burgk JL, Gaidt MM, Schmidt T, et al. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur J Immunol 2015;45(10):2911–2917. [DOI] [PubMed] [Google Scholar]
- 21.Chase JJ, Kubey W, Dulek MH, et al. Effect of monophosphoryl lipid A on host resistance to bacterial infection. Infect Immun 1986;53(3):711–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Astiz ME, Galera A, Saha DC, et al. Monophosphoryl lipid A protects against gram-positive sepsis and tumor necrosis factor. Shock 1994;2(4):271–274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. PHADs have structural similarities to MPLA and differences from E. coli Lipid A. (A) The native diphosphoryl Lipid A portion of lipopolysaccharide from E. coli is illustrated here showing two phosphate groups. The second phosphate group is highlighted with a circle, illustrating the phosphate group that is cleaved in MPLA and PHADs. (B) The structure of MPLA is illustrated, where the 5th acyl chain is bound to the distal hydroxyl group of the 6th acyl 14 carbon chain. (C) The three different Phosphorylated Hexaacyl Disaccharides are illustrated, noting the different bonding location of the 5th acyl 14 carbon chain. The 5th acyl chain is bound to disaccharide ring in PHAD, absent in 3D PHAD, and bound to the distal hydroxyl group of the 6th acyl chain in 3D 6-Acyl PHAD, similar to MPLA.
Supplemental Figure 2. PHADs attenuate plasma cytokine production after P. aeruginosa infection. Mice received vehicle or 20 μg of MPLA or PHADs via IP injection 48 and 24 hours prior to IP injection with 1 × 108 CFU of P. aeruginosa. Six hours after infection, blood was collected for plasma extraction. Plasma was analyzed for cytokine concentrations. *p<0.05 compared to vehicle. Results represent 2 separate experiments, n=7–10 per group.
Supplemental Figure 3. 3D 6-Acyl PHAD does not induce inflammatory cytokine production. Mice received vehicle or 20 μg of LPS or 3D 6-Acyl PHAD via IP injection. Blood was harvested at 3 or 6 hours after treatment, processed for plasma extraction, and analyzed for cytokine concentrations. *p<0.05 compared to LPS. Results represent 2 separate experiments, n=5–10 per group.
Supplemental Figure 4. 3D 6-Acyl PHAD does not induce inflammatory cytokine production in TLR KO mice. Wild type or knockout mice received vehicle or 20 μg of 3D 6-Acyl PHAD or vehicle via IP injection 48 and 24 hours prior to IP injection with 1 × 108 CFU of P. aeruginosa. Six hours after infection, blood was collected for plasma extraction. Plasma was analyzed for cytokine concentrations. *p<0.05 compared to control. Results represent 2 separate experiments, n=10.