Abstract
Research methods are needed that can predict whether the availability of potential modified risk tobacco products (MRTPs) may influence smokers’ quit-related motivation, choice, and behavior. This pilot study assessed the primary outcomes of feasibility and adherence to address this need using an electronic cigarette (ECIG) as a model MRTP. Cigarette smokers were randomly assigned to use only their own brand of cigarettes (OB-only) or a second-generation ECIG (18 ng/mL nicotine) plus their OB cigarettes (ECIG+OB) ad libitum for four weeks. Participants logged products using a mobile device, collected used cigarette filters, and provided saliva samples every day for analysis of cotinine. They returned to the lab once per week to provide a breath sample and accept or decline a choice to quit all tobacco products (i.e., cigarettes and/or ECIGs). They also returned for a one-month follow-up visit. Of those participants randomized (n=60), 56.7% completed the 4-week intervention and 40.0% completed the follow-up visit. The primary reason for withdrawal was poor adherence with mobile device use. Comparable numbers of participants in each group chose to make a quit attempt, although more OB-only participants chose to quit during the first two weeks and more ECIG+OB participants during the last two weeks. With protocol modifications to reduce participation burden, the current method might ultimately be used by regulators to predict how smokers’ quit-related motivation, choice, and behavior are influenced by current and future MRTPs.
Keywords: electronic cigarette (ECIG), quit attempt, tobacco, smoker, quit timing, feasibility
INTRODUCTION
The 2009 Family Smoking Prevention and Tobacco Control Act (“The Act”) calls for an evaluation of Modified Risk Tobacco Products (MRTPs), those purported to reduce the harms associated with use of traditional tobacco products. The Act specifically notes the need to understand “the increased or decreased likelihood that existing users of tobacco products who would otherwise stop using such products will switch” to an MRTP (H.R. 1256). The need for this directive is evidenced by the aftermath of “low yield” cigarette marketing in the 1950s that encouraged switching: “Considering all I heard, I decided to quit or smoke True®. I smoke True®”.1 Smokers who switched to a light or ultralight brand were shown more likely to make a quit attempt but less likely to achieve cessation than those who did not switch.2-4 This pattern, which was observed even among those who switched specifically “to quit smoking”,4 may have been due to an influence of product use on quit motivation. That is, an initially high motivation to quit smoking may have been diminished by the promise of a “healthier” cigarette.5 Of course, epidemiological work confirms that these switchers would have been exposed to the same level of harmful smoke toxicants as if they continued smoking regular cigarettes.6 To prevent a similar scenario, research methods are needed that can predict whether the availability of potential MRTPs may influence smokers’ motivation and choice to quit.
One potential MRTP is the electronic cigarette (ECIG), marketed previously to encourage switching rather than quitting: “Why quit? Switch to Bin…Nobody likes a quitter, so make the switch today7 Unlike with low yield cigarettes, switching from cigarettes to ECIGs may ultimately prove beneficial to smokers’ health.8-10 Unfortunately, however, the most common form of ECIG use is concurrently with cigarettes.11 At least some work suggests that smokers who use ECIGs are more likely to make a quit attempt than smokers who do not use ECIGs.12-15 However, there is mixed evidence regarding whether ECIG use facilitates16-18, 59-60 or hinders14-15,19-20 successful smoking cessation. One reason for this latter finding may be that the myriad of ECIG devices are poor substitutes for cigarettes because they fail to deliver sufficient nicotine and thus fail to suppress the withdrawal symptoms experienced during cigarette abstinence.21-25 Another possibility, however, is that ECIG use may diminish smokers’ motivation to quit cigarettes and ultimately postpone or terminate quit attempts. In turn, delays may further decrease motivation to quit over time52 and thus decrease the likelihood of success following an attempt to quit smoking.53 Currently, clinical laboratory studies that address this possibility suggest that ECIG use increases smokers’ motivation to quit smoking, but such studies are limited to single-arm pilot trials.26-28
Also relevant is that motivation to quit does not always predict cessation.29-31 The large majority of smokers report that they are thinking about quitting or are actively preparing to quit,32-33 though not all actually make a quit attempt and less than 10% of those who do are successful.34 Thus, methods are needed for evaluating how MRTP availability influences quit motivation, quit choice, and timing of quit attempts, and how these factors influence behavior long-term. In order to address this need, this study piloted a method for ultimate use in evaluating the influence of potential MRTPs on such outcomes using a simple randomized design. Cigarette smokers were randomly assigned to use their own brand of cigarettes only (OB-only) or in conjunction with an ECIG (ECIG+OB) ad libitum for four weeks. Once per week, they were asked to accept or reject a formal offer to quit all products (i.e., cigarettes and/or ECIGs); offer acceptance resulted in enrollment in a cessation program that included counseling and nicotine replacement therapy. The primary assessments of our proposed method were related to feasibility and protocol adherence. Secondary outcomes were quit motivation, quit choice, choice timing, product acceptability, and cessation behavior.
METHOD
Inclusion / Exclusion Criteria
Cigarette smokers were recruited from the community via fliers, online postings, and word of mouth. Flyers and online postings stated that the purpose of the study was “to examine electronic cigarettes’ influence on smoking cessation.” Participants who responded to these advertisements were told first via telephone that they may or may not be randomized to the ECIG condition. This fact was then reiterated at the in-person screening during informed consent.
Inclusion criteria included the following: 18 to 60 years of age; smoking ≥10 cigarettes per day for ≥ 1 year; exhaled air carbon monoxide (CO) level of ≥ 10 ppm (Micro+™basic monitor; CoVita; Haddonfield, NJ); and Contemplation or Preparation Stage of Change (indicating interest in a quit attempt within the next 1-6 months).35. Participants were excluded if they reported chronic health or psychiatric conditions, past month use of marijuana ≥ 5 days, past month use of any other illicit drugs, or regular use of ECIGs or other tobacco products (i.e., ≥ 1 day per week). Also excluded were individuals in the Precontemplation (no interest in quitting) or Action (actively trying to quit) Stage of Change.35 Individuals in the Precontemplation stage would be those least likely to join the cessation program, while individuals in the Action stage supposedly already initiated use of cessation support and/or made changes to their smoking patterns. Given the pilot nature of this study, we wanted to maximize the likelihood that participants would choose to make a quit attempt and enter the cessation program offered during the intervention phase. Females also were excluded if they were currently breast feeding or tested positive for pregnancy via urinalysis.
Materials
Mobile device hardware/software.
The mobile devices used were BLU Dash 5.0 smartphones (BLU Products; Doral, FL) with an Android operating system and software customized for this study (see https://www.utas.edu.au/health/research/groups/school-of-medicine/behavioural-and-situational-research-group-bsrg/hbart).
ECIGs.
The ECIG consisted of a Kanger mini Protank-II, which is a 1.5 ml Pyrex glass tank with a drip tip and atomizer head coils (KangerTech; China), and a 3.3 V constant output, 900 mAh, eGo-T battery (Joyetech; Irvine, CA). The liquid (The Vapor Room, Sky Vapors LLC, Frostburg, MD) was labeled as 70% propylene glycol and 30% vegetable glycerin, with a nicotine concentration requested of 18 mg/ml. Independent testing revealed the actual nicotine concentration to be M = 19.1 mg/ml (SD = 1.9, range = 16.3 to 22.0) (Bioanalytical Laboratory, Virginia Commonwealth University, Richmond, VA). Participants were given the option to choose between “TVR tobacco” (35.3%), “555 menthol” (11.8%), and “wild berry” flavors (23.5%), and also were permitted to switch flavors at each laboratory visit (29.4% switched from TVR tobacco to wild berry, or vice versa). A 20-mL bottle of the selected flavor was provided at the initial visit and additional 20-mL bottles were provided at in-person visits as needed (e.g., when switching flavors or when the amount in the current bottle was low). A second-generation tank-style ECIG was chosen because, relative to first-generation models, these models have shown to deliver nicotine more efficiently and are rated as more satisfying for withdrawal suppression among smokers.34,43
Saliva samples.
SalivaBio Oral Swabs (Salimetrics, State College, PA) were used for the collection of passive drool samples, which occurred nightly for the 4-week intervention period. Abstinence from food and drink was required for one hour prior to sample collection. During collection, participants were instructed to rinse their mouth with water, wait 10 minutes, and then place the cotton swab in their mouth for 2 minutes. The swab was then placed into a plastic vial and stored in the participants’ freezer until their next scheduled in-person visit. Once returned to the lab, saliva samples were stored at −80°C until assayed. Cotinine levels were determined by LC-MS/MS using extraction and processing methods described by Cappendijk and colleagues.44 The limit of quantification (LOQ) was 1 ng/mL.
Procedures
All study procedures were approved by the Institutional Review Board at West Virginia University (#1408391899). Using a simple randomized design, participants were assigned to one of two conditions: only their own brand cigarettes (OB-only) or an ECIG plus their OB cigarettes (ECIG+OB). For four weeks, participants used their condition-assigned product ad libitum and attended the laboratory weekly for assessments (Days 8, 15, 22, and 29). They also completed a follow-up visit at one month post-intervention. Participants were compensated $50 on Days 8 and 15, $75 on Days 22 and 29, and $25 on the follow-up visit; therefore, a total of $275 could be earned for completing the entire study.
Baseline (Day 1).
Following informed consent, participants completed several questionnaires: demographics, medical history, tobacco and other drug use history, Readiness to Quit Ladder,36 Stage of Change,35 and the Fagerstrom Test of Cigarette Dependence (FTCD).37 Eligible participants were then randomized to a condition; those assigned to the ECIG+OB condition were given the option to sample all ECIG liquid flavors available for take-home. Next, subjects received training on all study procedures. For those in the ECIG+OB group, research staff provided instructions on ECIG device operation (e.g., assembly, tank filling, charging) and then observed the participant modeling these same behaviors as well as puffing on the device. Instructions regarding expectations for ECIG and/or cigarette use mimicked real world conditions. That is, ECIG+OB participants were told that they could use the ECIG as much or as little as they wanted, and were not given specific instructions regarding their cigarette use. Staff instructed all participants to collect filters from all cigarettes smoked and to store those filters in containers that were pre-labeled for each day of the week. Third, participants were trained on the collection of saliva samples that were required each night. Finally, participants were given a tutorial on the use of a mobile device for real-time monitoring of affect and product use (see below). Participants left the laboratory with all relevant supplies as well as documents that reiterated the instructions given for these procedures.
Intervention (Days 1-29).
During the 4-week intervention period, participants used their condition-assigned product ad libitum and engaged with their monitoring device daily. Using this device, participants were required to log all cigarette and/or ECIG bouts immediately before the product was used. For a randomly selected portion of these logged products, participants were further prompted to complete questions that addressed mood, situational factors (e.g., location, activity), withdrawal symptoms (e.g., craving, irritability),38 and product-related effects (e.g., tastes good, coughing, throat irritation).39 The number of prompts randomly selected for these additional questions was based on participants’ self-reported number of cigarettes per day (CPD) at baseline in order to standardize the number of prompts across participants, which resulted in approximately 4-5 cigarettes per day sampled for assessment. All of the questions were measured using a visual analog scale with a range from 0 (not at all) to 100 (extremely). Participants also answered these same questions in response to random prompts that occurred independent of product use approximately 3-4 times per day. At the end of the day, participants again completed questionnaires (e.g., withdrawal symptoms, product-related effects), as well as tallied and logged any cigarettes and/or ECIGs that were used but had not been logged in real-time during the day. The time to complete each set of assessments varied across participants, though most required approximately 1-2 min. Thus, participants would have needed to engage with their mobile device ~ 15 minutes each day. Similar sampling procedures have been outlined in extensive detail elsewhere.40-42 Also required during this intervention period was the collection of all spent cigarette filters and of saliva samples each night before bedtime, both of which were returned to the laboratory at each in-person visit.
Study Visits (Days 8, 15, 22, and 29).
At the beginning of each weekly visit, participants returned their spent cigarette filters, saliva samples, and mobile devices. The devices were checked to evaluate compliance, with a minimum threshold of 80% set for responses to random prompts. If random-prompt compliance was < 80% for a given week, staff provided additional device training. Participants were withdrawn from the study if their compliance was < 80% for two consecutive weeks, if there were large discrepancies between logged CPD and returned filters for two consecutive weeks, or if they did not interact with the device for one week (i.e., did not respond to any random prompts or log any products used). Next, participants provided an expired air CO sample, completed questionnaires (e.g., Readiness to Quit, Stage of Change), and then indicated their choice to quit tobacco. That is, they formally rejected or accepted a written offer to enroll in our cessation program by checking a box on the form (“yes” or “no”). To prevent biased responses, participants completed this form in isolation (i.e., staff were not present in the room). Those who chose to make a quit attempt met immediately with a Certified Tobacco Treatment Specialist for a Motivational Interviewing-based session. They also were provided with the “My Path to a Smoke-Free Future” booklet (Mayo Clinic, Rochester, MN), a two-week supply of nicotine patch and/or gum, and a referral to the West Virginia QuitLine to receive additional counseling and pharmacotherapies. Participants assigned to the ECIG+OB condition who made the choice to quit were required to return their ECIG device and liquid. Regardless of the choice made, however, all participants were instructed to continue engaging with their monitoring device and left the laboratory with another week’s supply of materials.
One-Month Follow-up.
One month after the end of the 4-week intervention, participants returned to the laboratory for follow-up assessments. They reported on their current use of all nicotine/tobacco products, as well as their quit-related motivation, stage, and behavior. They also provided a breath sample to test CO level and completed questionnaires.
Data Considerations and Analyses
Analyses were conducted for those who completed the entire protocol (N=24; n=12 per group) as well as for those who completed the 4-week intervention but not the one-month follow-up visit (N=34). Importantly, the pattern of results for primary outcomes – method feasibility, protocol adherence, and ECIG acceptability – did not differ between these subgroups of participants; therefore, analyses are presented for the latter group of n=34 (henceforth referred to as “completers”) for these outcomes. For secondary outcomes, results are presented for both subgroups of participants.
Primary outcomes were those related to method feasibility and protocol adherence. Feasibility was assessed first by evaluating rates of randomization and attrition. Possible reasons for attrition were then evaluated by comparing completers and non-completers on baseline characteristics and frequency of negative side effects induced by nicotine/tobacco withdrawal and/or the ECIG (percentage of days reported) using chi-square and independent-samples t-tests for unequal sample sizes (p’s < .05). Comparisons between these same groups were conducted for those assigned to the ECIG+OB condition on mean ratings of ECIG acceptability and use rates (percentage of days reported out of completed days, and logs per day). Note that for ECIG use rates, those days after which a participant returned their device due to their choice to make a quit attempt were not considered in these calculations. Protocol adherence was evaluated by comparing completers’ logged cigarettes via the monitoring device to their returned cigarette filters using a Pearson’s correlation and a paired-samples t-test (p’s < .05). Also examined was participants’ engagement with their device via percent compliance with random prompts, including as a function of intervention group and study week.
Secondary outcome measures included quit choice, timing, and motivation (stage of change, readiness to change), as well as product use, acceptability, and biological indicators (expired air CO, salivary cotinine). Given the pilot nature of this work, and thus the lack of statistical power, these outcomes are summarized using descriptive statistics.
RESULTS
Primary Outcomes
Feasibility.
Feasibility was assessed via randomization and attrition rates. Of the 77 smokers who consented, 17 were disqualified because they failed to meet inclusion criteria: precontemplation stage of change (n=8), expired air CO level < 10 ppm (n=4), current ECIG or illicit drug use (n=3), current engagement in smoking cessation (n=1), and inconsistent reporting (n=1). The 60 remaining participants were randomized into the study groups (30 ECIG+OB and 30 OB-only). Of these participants, 34 (56.7%) completed the 4-week intervention; 24 (40.0%) also completed the one-month follow-up visit. Of the 26 participants who failed to complete the intervention period, the large majority was disqualified due to poor compliance with mobile device use. Specifically, 23.1% failed to respond reliably to random prompts and 53.8% failed to log their product use in real time. Moreover, nearly half (45.0%) of these 26 participants were disqualified in the first week of the intervention period. Attrition rates across the two intervention groups were 23.3% ECIG+OB vs. 20.0% OB-only for Week 1, 10.0% ECIG+OB vs. 10.0% OB-only for Week 2, 6.7% ECIG+OB vs. 16.7% OB-only for Week 3, 20.0% ECIG+OB vs. 13.3% OB-only for Week 4.
Non-completers did not differ significantly from completers on any baseline characteristic (see Table 1). The frequency of self-reported withdrawal symptoms and product-related side effects was also comparable between these groups; the percentage of study days on which these effects were reported ranged from 75.6% to 97.6% for non-completers and from 64.0% to 98.5% for completers (p’s > .05). Effects most commonly reported were craving, irritability, dry mouth, throat irritation, and cough. For those assigned to the ECIG+OB condition, completers and non-completers provided comparable ratings for items related to device acceptability, such as satisfying (53.2±3.8 vs. 50.1±9.0, respectively), pleasant (50.5±4.0 vs. 51.9±8.1, respectively), taste good (49.7±4.1 vs. 49.4±6.9, respectively), and liking (52.2±4.2 vs. 65.4±6.8, respectively). Also for ECIG+OB participants, ECIG use was logged on 81.2% of completed study days for completers and on 62.7% of completed study days for non-completers. On the days in which ECIG use was logged, the mean number of ECIG uses per day was 5.5 (SD = 4.2; median = 5.0; mode = 1.0) for completers and 4.5 (SD = 3.3; median = 4.5; mode = 1.0) for non-completers.
Table 1.
Baseline demographic and tobacco-related characteristics
| Completers | ||||||
|---|---|---|---|---|---|---|
| Completers (n=34) |
Non-completers (n=26) |
E-cigarette (n=18) |
Own Brand (n=16) |
Quitters (n=22) |
Non-Quitters (n=12) |
|
| M(SD) or % | M(SD) or % | M(SD) or % | M(SD) or % | M(SD) or % | M(SD) or % | |
| % White | 88.2% | 96.2% | 94.4% | 81.3% | 90.9% | 75.0% |
| % Non-Hispanic | 97.1% | 100.0% | 100.0% | 93.8% | 100.0% | 91.7% |
| % Male | 58.8% | 65.4% | 50.0% | 68.8% | 59.1% | 58.3% |
| Age (years) | 35.1 (11.0) | 36.8 (12.9) | 35.9 (9.5) | 34.1 (12.7) | 35.9 (11.7) | 34.5 (9.5) |
| Education (years) | 14.0 (1.8) | 12.7 (1.7) | 13.9 (1.8) | 14.0 (1.8) | 13.6 (1.5) | 14.8 (2.0) |
| Cigarettes/day | 16.7 (4.9) | 19.4 (6.1) | 17.1 (5.7) | 16.3 (4.1) | 16.1 (4.5) | 17.3 (6.2) |
| Years smoked | 13.2 (9.9) | 13.9 (11.0) | 12.4 (8.8) | 13.9 (11.4) | 13.0 (9.9) | 13.4 (10.5) |
| % Menthol | 20.6% | 34.6% | 16.7% | 25.0% | 28.6% | 8.3% |
| Expired air CO (ppm) | 24.2 (12.0) | 25.1 (14.6) | 24.9 (12.5) | 23.4 (11.8) | 22.5 (12.3) | 25.0 (10.3) |
| FTND Scorea | 5.3 (1.8) | 5.9 (1.9) | 5.2 (2.1) | 5.4 (1.4) | 5.0 (1.6) | 5.6 (2.3)* |
| Quit Ladder scorea | 6.6 (0.9) | 6.2 (1.1) | 6.4 (1.1) | 6.0 (1.1) | 6.6 (1.1) | 5.7 (0.9) |
| Baseline Stage of Change | ||||||
| Preparation | 44.0% | 56.0% | 47.1% | 41.2% | 50.0% | 66.7% |
| Contemplation | 52.9% | 47.1% | 52.3% | 58.8% | 50.0% | 33.3% |
Scale ranges for both Fagerstrom Test for Nicotine Dependence and Quit Ladder are 1-10
p < .05 compared to quitters
Protocol Adherence.
The average number of CPD between measurement methods of mobile device logs and returned filters was highly correlated (r = 0.70, p < .01) and not significantly different (t(918) = −0.12, p = .909). Collapsed across study days, mean (±SEM) CPD was 6.9±0.2 for logs and 6.9±0.2 for returned filters. Compliance with random prompts on the mobile device was 82.9% (SD = 27.6%) for completers; rates did not differ by randomization group or study week (p’s > .05). Compliance rates for completers ranged from 81.4% to 83.9% across weeks for OB-only participants, and from 81.0% to 86.2% across weeks for ECIG+OB participants.
Secondary Outcomes
Table 2 provides means (SDs) for secondary outcomes (except product acceptability) for participants who completed all study phases including follow-up (n=24), as well as for those who completed only the 4-week intervention (n=34). Results for categorical outcomes are further described below, as are results for product acceptability.
Table 2.
Means (SDs) or n (%) for secondary outcomes for Group x Quit Status x Time.
| OWN BRAND | ECIG | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total n=24 | BL | Week 1 | Week 2 | Week 3 | Week 4 | F-U | Total | BL | Week 1 | Week 2 | Week 3 | Week 4 | F-U | Total | |
| Quitters (n=14) | Returned Filters | 10.9 (4.5) | 9.1 (3.5) | 5.1 (4.1) | 4.3 (3.8) | 4.6 (3.3) | 3.4 (3.1) | 3.6 (3.8) | 1.6 (2.0) | ||||||
| Logged CPD | 11.4 (5.3) | 8.9 (4.0) | 4.0 (3.4) | 3.3 (3.1) | 8.0 (7.5) | 6.5 (8.5) | 5.9 (7.4) | 3.5 (3.5) | |||||||
| Logged ECIG | n/a | n/a | n/a | n/a | 7.5 (5.1) | 6.1 (2.6) | 4.8 (3.1) | 5.1 (2.3) | |||||||
| Cotinine | 541.5 (308.2) | 591.3 (345.8) | 529.1 (284.2) | 537.1 (297.1) | 463.4 (311.5) | 537.7 (337.1) | 497.1 (328.0) | 498.4 (310.9) | |||||||
| Expired air CO | 21.0 (10.1) | 19.3 (6.3) | 21.6 (10.6) | 19.1 (16.0) | 20.9 (17.2) | 18.0 (15.4) | 18.9 (14.4) | 11.0 (13.5) | 7.1 (10.7) | 6.4 (9.0) | 9.1 (12.3) | 8.6 (8.2) | |||
| Readiness to Quit | 6.1 (1.3) | 6.9 (0.9) | 7.3 (0.8) | 7.6 (1.1) | 7.9 (1.1) | 7.7 (1.3) | 7.0 (0.6) | 7.4 (0.8) | 7.7 (1.1) | 7.7 (1.0) | 7.7 (1.1) | 7.6 (1.3) | |||
| Quit choice (n and %) | 2 (16.7%) | 2 (16.7%) | 0 (0.0%) | 3 (25.0%) | 7 (58.3%) | 2 (16.7%) | 0 (0.0%) | 1 (8.3%) | 4 (33.3%) | 7 (58.3%) | |||||
| NonQuitters (n=10) | Returned Filters | 13.8 (7.1) | 13.2 (7.6) | 13.1 (8.1) | 11.7 (5.7) | 8.1 (5.1) | 6.3 (4.3) | 4.8 (3.6) | 4.9 (3.6) | ||||||
| Logged CPD | 11.2 (5.1) | 10.6 (6.7) | 11.3 (5.4) | 8.7 (3.3) | 8.7 (5.2) | 6.5 (4.0) | 4.5 (3.3) | 4.3 (3.1) | |||||||
| Logged ECIG | n/a | n/a | n/a | n/a | 3.7 (4.3) | 3.4 (3.2) | 3.7 (2.7) | 2.2 (2.4) | |||||||
| Cotinine | 681.7 (294.5) | 520.0 (343.1) | 539.9 (274.9) | 396.3 (283.5) | 561.9 (283.7) | 588.2 (318.2) | 602.4 (289.1) | 541.4 (280.8) | |||||||
| Expired air CO | 19.4 (12.2) | 18.8 (6.9) | 24.4 (8.4) | 18.6 (9.3) | 17.2 (5.9) | 18.6 (11.3) | 27.8 (9.8) | 21.2 (11.0) | 16.2 (12.3) | 22.8 (14.1) | 24.8 (23.3) | 22.0 (20.6) | |||
| Readiness to Quit | 5.8 (0.8) | 5.8 (1.0) | 5.8 (0.4) | 5.6 (0.9) | 5.8 (0.4) | 5.8 (0.4) | 5.6 (1.1) | 6.0 (0.8) | 6.3 (1.0) | 6.6 (1.1) | 6.8 (1.3) | 7.6 (1.7) | |||
| Total n=34 | BL | Week 1 | Week 2 | Week 3 | Week 4 | F-U | Total | BL | Week 1 | Week 2 | Week 3 | Week 4 | F-U | Total | |
| Quitters (n=20) | Returned Filters | 10.3 (4.5) | 8.2 (3.8) | 5.3 (3.9) | 4.6 (3.6) | 6.3 (4.7) | 5.0 (4.5) | 4.7 (4.5) | 2.8 (3.7) | ||||||
| Logged CPD | 12.1 (6.3) | 8.7 (4.0) | 4.3 (3.2) | 3.8 (2.9) | 8.3 (6.5) | 7.2 (8.6) | 5.3 (6.1) | 3.7 (3.9) | |||||||
| Logged ECIG | n/a | n/a | n/a | n/a | 5.4 (5.1) | 5.1 (5.4) | 3.5 (3.0) | 3.7 (2.9) | |||||||
| Cotinine | 494.3 (330.3) | 583.1 (355.1) | 549.7 (292.0) | 537.1 (297.1) | 463.4 (311.4) | 537.7 (337.1) | 497.1 (328.0) | 498.4 (310.9) | |||||||
| Expired air CO | 21.6 (9.4) | 20.9 (6.9) | 23.8 (10.9) | 19.3 (13.9) | 22.8 (16.6) | 22.5 (14.3) | 14.5 (12.8) | 11.5 (11.5) | 11.2 (12.1) | 13.9 (12.7) | |||||
| Readiness to Quit | 6.2 (1.4) | 6.8 (1.1) | 7.4 (0.7) | 7.6 (1.0) | 7.9 (0.9) | 7.1 (0.7) | 7.3 (0.8) | 7.6 (0.9) | 7.6 (0.8) | 7.6 (0.9) | |||||
| Quit choice (n and %) | 4 (25.0%) | 2 (12.5%) | 0 (0.0%) | 3 (18.8%) | 9 (56.3%) | 2 (11.1%) | 0 (0.0%) | 3 (16.7%) | 6 (33.3%) | 11 (61.1%) | |||||
| NonQuitters (n=14) | Returned Filters | 11.8 (6.9) | 11.6 (6.9) | 11.1 (7.6) | 9.6 (6.0) | 8.0 (4.9) | 6.3 (3.9) | 4.8 (3.4) | 4.7 (3.5) | ||||||
| Logged CPD | 9.8 (5.1) | 9.3 (6.2) | 9.4 (5.6) | 7.5 (3.8) | 7.8 (4.9) | 6.2 (3.7) | 4.4 (3.3) | 3.9 (2.9) | |||||||
| Logged ECIG | n/a | n/a | n/a | n/a | 4.8 (5.2) | 5.2 (4.2) | 4.9 (3.8) | 3.7 (3.7) | |||||||
| Cotinine | 654.1 (298.9) | 506.8 (349.2) | 578.9 (275.6) | 396.3 (281.5) | 616.9 (302.7) | 597.1 (319.9) | 617.9 (304.3) | 546.9 (288.5) | |||||||
| Expired air CO | 25.7 (14.8) | 20.4 (7.3) | 28.1 (12.6) | 19.1 (9.8) | 15.7 (7.2) | 28.9 (8.6) | 22.6 (10.4) | 22.3 (14.8) | 22.6 (11.9) | 25.1 (19.1) | |||||
| Readiness to Quit | 5.9 (0.7) | 5.7 (0.8) | 5.4 (0.8) | 5.7 (1.4) | 5.6 (0.5) | 6.1 (1.3) | 6.2 (0.8) | 6.0 (0.9) | 6.4 (1.0) | 6.9 (1.1) | |||||
| n=24 | BL | Week 1 | Week 2 | Week 3 | Week 4 | F-U | Total | BL | Week 1 | Week 2 | Week 3 | Week 4 | F-U | Total | |
| Quitters (n=14) | |||||||||||||||
| Returned Filters | 10.9 (4.5) | 9.1 (3.5) | 5.1 (4.1) | 4.3 (3.8) | 4.6 (3.3) | 3.4 (3.1) | 3.6 (3.8) | 1.6 (2.0) | |||||||
| Logged CPD | 11.4 (5.3) | 8.9 (4.0) | 4.0 (3.4) | 3.3 (3.1) | 8.0 (7.5) | 6.5 (8.5) | 5.9 (7.4) | 3.5 (3.5) | |||||||
| Logged ECIG | n/a | n/a | n/a | n/a | 7.5 (5.1) | 6.1 (2.6) | 4.8 (3.1) | 5.1 (2.3) | |||||||
| Cotinine | 541.5 (308.2) | 591.3 (345.8) | 529.1 (284.2) | 537.1 (297.1) | 463.4 (311.5) | 537.7 (337.1) | 497.1 (328.0) | 498.4 (310.9) | |||||||
| Expired air CO | 21.0 (10.1) | 19.3 (6.3) | 21.6 (10.6) | 19.1 (16.0) | 20.9 (17.2) | 18.0 (15.4) | 18.9 (14.4) | 11.0 (13.5) | 7.1 (10.7) | 6.4 (9.0) | 9.1 (12.3) | 8.6 (8.2) | |||
| Readiness to Quit | 6.1 (1.3) | 6.9 (0.9) | 7.3 (0.8) | 7.6 (1.1) | 7.9 (1.1) | 7.7 (1.3) | 7.0 (0.6) | 7.4 (0.8) | 7.7 (1.1) | 7.7 (1.0) | 7.7 (1.1) | 7.6 (1.3) | |||
| Quit choice (n and %) | 2 (16.7%) | 2 (16.7%) | 0 (0.0%) | 3 (25.0%) | 7 (58.3%) | 2 (16.7%) | 0 (0.0%) | 1 (8.3%) | 4 (33.3%) | 7 (58.3%) | |||||
| NonQuitters (n=10) | |||||||||||||||
| Returned Filters | 13.8 (7.1) | 13.2 (7.6) | 13.1 (8.1) | 11.7 (5.7) | 8.1 (5.1) | 6.3 (4.3) | 4.8 (3.6) | 4.9 (3.6) | |||||||
| Logged CPD | 11.2 (5.1) | 10.6 (6.7) | 11.3 (5.4) | 8.7 (3.3) | 8.7 (5.2) | 6.5 (4.0) | 4.5 (3.3) | 4.3 (3.1) | |||||||
| Logged ECIG | n/a | n/a | n/a | n/a | 3.7 (4.3) | 3.4 (3.2) | 3.7 (2.7) | 2.2 (2.4) | |||||||
| Cotinine | 681.7 (294.5) | 520.0 (343.1) | 539.9 (274.9) | 396.3 (283.5) | 561.9 (283.7) | 588.2 (318.2) | 602.4 (289.1) | 541.4 (280.8) | |||||||
| Expired air CO | 19.4 (12.2) | 18.8 (6.9) | 24.4 (8.4) | 18.6 (9.3) | 17.2 (5.9) | 18.6 (11.3) | 27.8 (9.8) | 21.2 (11.0) | 16.2 (12.3) | 22.8 (14.1) | 24.8 (23.3) | 22.0 (20.6) | |||
| Readiness to Quit | 5.8 (0.8) | 5.8 (1.0) | 5.8 (0.4) | 5.6 (0.9) | 5.8 (0.4) | 5.8 (0.4) | 5.6 (1.1) | 6.0 (0.8) | 6.3 (1.0) | 6.6 (1.1) | 6.8 (1.3) | 7.6 (1.7) | |||
| n=34 | BL | Week 1 | Week 2 | Week 3 | Week 4 | F-U | Total | BL | Week 1 | Week 2 | Week 3 | Week 4 | F-U | Total | |
| Quitters (n=20) | |||||||||||||||
| Returned Filters | 10.3 (4.5) | 8.2 (3.8) | 5.3 (3.9) | 4.6 (3.6) | 6.3 (4.7) | 5.0 (4.5) | 4.7 (4.5) | 2.8 (3.7) | |||||||
| Logged CPD | 12.1 (6.3) | 8.7 (4.0) | 4.3 (3.2) | 3.8 (2.9) | 8.3 (6.5) | 7.2 (8.6) | 5.3 (6.1) | 3.7 (3.9) | |||||||
| Logged ECIG | n/a | n/a | n/a | n/a | 5.4 (5.1) | 5.1 (5.4) | 3.5 (3.0) | 3.7 (2.9) | |||||||
| Cotinine | 494.3 (330.3) | 583.1 (355.1) | 549.7 (292.0) | 537.1 (297.1) | 463.4 (311.4) | 537.7 (337.1) | 497.1 (328.0) | 498.4 (310.9) | |||||||
| Expired air CO | 21.6 (9.4) | 20.9 (6.9) | 23.8 (10.9) | 19.3 (13.9) | 22.8 (16.6) | 22.5 (14.3) | 14.5 (12.8) | 11.5 (11.5) | 11.2 (12.1) | 13.9 (12.7) | |||||
| Readiness to Quit | 6.2 (1.4) | 6.8 (1.1) | 7.4 (0.7) | 7.6 (1.0) | 7.9 (0.9) | 7.1 (0.7) | 7.3 (0.8) | 7.6 (0.9) | 7.6 (0.8) | 7.6 (0.9) | |||||
| Quit choice (n and %) | 4 (25.0%) | 2 (12.5%) | 0 (0.0%) | 3 (18.8%) | 9 (56.3%) | 2 (11.1%) | 0 (0.0%) | 3 (16.7%) | 6 (33.3%) | 11 (61.1%) | |||||
| NonQuitters (n=14) | |||||||||||||||
| Returned Filters | 11.8 (6.9) | 11.6 (6.9) | 11.1 (7.6) | 9.6 (6.0) | 8.0 (4.9) | 6.3 (3.9) | 4.8 (3.4) | 4.7 (3.5) | |||||||
| Logged CPD | 9.8 (5.1) | 9.3 (6.2) | 9.4 (5.6) | 7.5 (3.8) | 7.8 (4.9) | 6.2 (3.7) | 4.4 (3.3) | 3.9 (2.9) | |||||||
| Logged ECIG | n/a | n/a | n/a | n/a | 4.8 (5.2) | 5.2 (4.2) | 4.9 (3.8) | 3.7 (3.7) | |||||||
| Cotinine | 654.1 (298.9) | 506.8 (349.2) | 578.9 (275.6) | 396.3 (281.5) | 616.9 (302.7) | 597.1 (319.9) | 617.9 (304.3) | 546.9 (288.5) | |||||||
| Expired air CO | 25.7 (14.8) | 20.4 (7.3) | 28.1 (12.6) | 19.1 (9.8) | 15.7 (7.2) | 28.9 (8.6) | 22.6 (10.4) | 22.3 (14.8) | 22.6 (11.9) | 25.1 (19.1) | |||||
| Readiness to Quit | 5.9 (0.7) | 5.7 (0.8) | 5.4 (0.8) | 5.7 (1.4) | 5.6 (0.5) | 6.1 (1.3) | 6.2 (0.8) | 6.0 (0.9) | 6.4 (1.0) | 6.9 (1.1) | |||||
Note. Of those participants randomized to study condition, n=34 completed the 4-week intervention while n=24 completed the 4-week intervention and the one-month follow-up visit. Within each condition, participants were those who did ("Quitters") or did not (“NonQuitters”) make a formal choice to quit tobacco and enter the cessation program during the 4-week intervention.
Quit choice and timing.
The number of participants within each group who chose or rejected a quit attempt are presented in Table 2. For n=24, 7 (50%) participants in both groups made the choice to quit. For n=34, 9 (45%) and 11 (55%) participants chose to quit for OB-only and ECIG+OB groups, respectively. For both n=24 and n=34 subgroups, most OB-only participants that chose to quit did so in the early weeks of the intervention (in weeks 1-2, 57.1% for n=24 and 66.7% for n=34) while most ECIG+OB participants that chose to quit did so in the later weeks (in weeks 3-4, 71.4% for n=24 and 81.8% for n=34).
Stage of change.
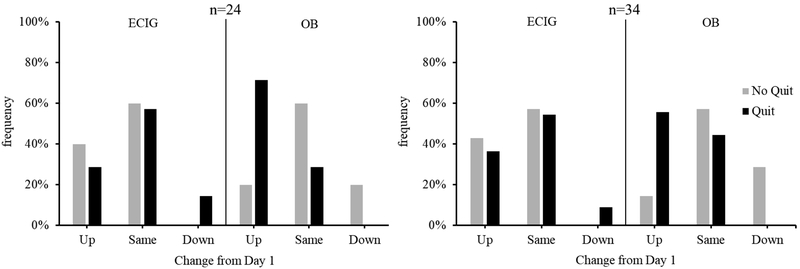
Figure 1 displays the proportion of participants who advanced toward or away from quitting, or who remained in the same stage, from Day 1 to Day 28 (n=34) or to the follow-up visit (n=24). As described previously, all participants were required to be in the Contemplation or Preparation stage on Day 1 for study enrollment. For the subgroup of n=34, the majority of participants remained in the same stage regardless of group or quit status except for OB-only participants who made the choice to quit. For this latter group, the majority of participants advanced at least one stage toward quitting. This same pattern was observed for the subgroup of n=24 participants. Also for both n=34 and n=24 subgroups, participants in the OB-only group who advanced toward quitting were primarily those who made the choice to quit (83.3% for n=24 and n=34), relative to those who rejected a quit attempt. For participants in the ECIG+OB group who advanced toward quitting, the number who accepted a quit attempt (50% for n=24 and 57.1% for n=34) was comparable to those who rejected a quit attempt.
Figure 1.
Frequency of participants who moved up or down at least one stage, or who remained in the same stage, from Day 1 to Day 28 (n=34) or to follow-up (n=24) as a function of group and quit status.
Product Acceptability.
The percentage of study days during which negative effects were reported was comparable between groups independent of quit choice status; rates ranged from 66.1% to 97.4% for OB-only, and from 61.3% to 97.3% for ECIG+OB, across items (p’s > .05). A similar pattern was observed when quit status within each group was considered, except for two items. The effects of nausea (56.2% vs. 65.8-75.3%, respectively) and dizziness (56.9% vs. 63.6-74.7%, respectively) were reported on slightly fewer days for ECIG+OB participants who made the choice to quit, relative to other subgroups (p’s < .05). Mean (±SD) ratings (collapsed across study days) for negative effects also were largely comparable when examined by group and quit choice status (p’s > .05). For instance, the two most commonly reported withdrawal-related symptoms were craving (OB-only quit: 75.5±22.7; OB-only no quit: 68.8±25.3; ECIG+OB quit: 67.7±25.6; ECIG+OB no quit: 70.9±25.4) and irritability (OB-only quit: 45.4±32.0; OB-only no quit: 26.6±28.1; ECIG+OB quit: 40.0±33.7; ECIG+OB no quit: 34.0±28.7). Also commonly reported were the side effects of throat irritation (OB-only quit: 38.0±31.3; OB-only no quit: 21.7±24.2; ECIG+OB quit: 36.4±33.8; ECIG+OB no quit: 38.9±32.1), cough (OB-only quit: 41.4±34.0; OB-only no quit: 38.6±32.6; ECIG+OB quit: 38.1±34.0; ECIG+OB no quit: 55.0±28.3), and dry mouth (OB-only quit: 46.4±32.2; OB-only no quit: 35.9±31.5; ECIG+OB quit: 48.5±31.9; ECIG+OB no quit: 44.4±29.8).
Participants in the ECIG+OB condition provided moderate ratings (collapsed across study days) for satisfying (53.2±32.3), pleasant (50.5±33.5), taste good (49.7±34.7), and liking (52.2±35.0). Ratings for these specific items were similar regardless of ECIG+OB participants’ quit choice status. For other items, however, lower ratings were observed among those that chose to quit compared to those that did not: harsh like OB (26.5±29.7 vs. 58.0±27.5, respectively), taste like OB (14.3±23.4 vs. 29.4±28.1, respectively), and feel like OB (17.8±25.0 vs. 30.2±29.0, respectively).
Expired air CO.
In addition to the mean (SD) values provided in Table 2, expired air CO levels also were categorized based on a pre-defined cutoff of ≤ 8 ppm. When considering n=24, ECIG+OB participants who met this cutoff at the follow-up visit were 57.1% (n=4) and 20.0% (n=1) for those that did or did not choose to quit, respectively. For OB-only participants, 28.6% (n=2) of those who did choose to quit, and 40.0% (n=2) of those that did not choose to quit, met this cutoff. When considering n=34, ECIG+OB participants who met this cutoff on Day 28 were observed for 45.5% (n=5) and 14.3% (n=1) for those who did or did not make the choice to quit, respectively. For OB-only participants, those who met this cutoff were 22.2% (n=2) and 14.3% (n=1) of those who did or did not make the choice to quit, respectively.
DISCUSSION
The purpose of this study was to pilot test a method for evaluating the effects of potential MRTPs, namely ECIGs in the current study, on quit-related motivation and behavior. While rates of randomization were nearly 80%, those for retention (40%) were on the lower end of the range reported previously for other smoking cessation studies (23-89.2%).45 Attrition occurred primarily due to poor mobile device adherence, with 76.9% of non-completers failing to record their product use and/or complete questionnaires prompted randomly. High attrition rates may be due to the burden placed on participants in terms of device use specifically, or in combination with the other daily requirements of cigarette filter and saliva sample collection. Importantly, attrition did not appear to be a function of product assignment, as rates were generally comparable between ECIG+OB and OB-only groups for each study week.
Adherence rates for mobile device use were between 81-86% for those that completed the 4-week intervention, which parallel those cited previously for smokers required to log their product use daily for several weeks (85-88%).41-42 Given the high rates of attrition in the present study due to low adherence with the mobile device, future work may reduce device-related burden by including more frequent contact with participants in the initial study week(s) to answer questions and assuage concerns; a compensation schedule designed to shape device use over time, particularly to increase retention in the early week(s); and/or short breaks from device use within the assessment period.46-50 Participants also returned a number of cigarette filters each day that was highly consistent with the number they logged in their device, providing additional support for the use of an electronic diary for real-time measurement of product use.51 Therefore, another idea to reduce participant burden might be to require cigarette filter collection less frequently or not at all given that product counts would be provided by the mobile device.
Product acceptability in regard to the ECIG device was evaluated to determine whether the ECIG was related to attrition rates. Importantly, the frequency and magnitude of reported aversive effects for the ECIG+OB group was comparable to that for OB-only cigarettes. Both groups commonly reported the nicotine/tobacco withdrawal symptoms of craving and irritability, and average ratings for such items were moderate-high across weeks. Also moderate were the average ratings for product-related items such as satisfying, pleasant, and taste good (~50 out of 100). Thus, this particular ECIG device was less than optimal in terms of withdrawal alleviation and product enjoyment relative to the OB-only condition. In turn, one potential limitation of the current study is the use of a specific ECIG model, with a single nicotine concentration of liquid (18 mg/ml) and limited flavor options. Each of these factors can influence product acceptability and nicotine delivery.54 Still, average ratings for withdrawal- and product-related effects were comparable between completers and non-completers within the ECIG+OB group, suggesting that the characteristics of the ECIG used here were not related to attrition.
An approximately equal number of participants in each group made the choice to quit in the present study. However, in the early weeks of the study, 2-3 times as many OB-only participants chose to quit as ECIG+OB participants. In the later weeks, the opposite pattern was observed with notably more ECIG+OB participants choosing to quit than OB participants. If reliable, this pattern of results may suggest that ECIG use delays a quit attempt, which may negatively impact cessation outcomes.52-53 More specifically, delays may decrease motivation to quit over time52 and thus decrease the likelihood of quit success. During the course of this study, very few participants moved away from quitting based on changes in their stage of change reports. Still, the number of participants who advanced toward quitting but rejected a quit attempt was two to three times higher for ECIG+OB than OB-only participants. On the other hand, a notable portion of ECIG+OB participants provided an expired air CO level at follow-up in support of smoking abstinence (i.e., 41.7% ECIG+OB and 33.3% OB-only). Of course, given the very small sample size available for these analyses, more work is needed to determine whether early measures of use and quit motivation predict follow-up outcomes.
In addition to high rates of attrition, one specific methodological feature should be considered when interpreting quit choice results. In the current study, ECIG+OB participants who made the choice to quit were required to forfeit their device upon entering the cessation program to prevent confounding the effects of the cessation program (NRT plus counseling) with those of continued ECIG use. Thus, a choice to quit for ECIG+OB participants was a choice to quit ECIGs in addition to cigarettes. Among those who rejected a formal quit attempt, the average CPD was lower for ECIG+OB participants than for OB-only (for both n=24 and n=34). While inferential statistics were not conducted for this secondary outcome measure, the pattern might suggest that ECIG+OB participants replaced some of their CPD with the ECIG.62 These same smokers may have delayed a choice to quit over concerns that their ECIG would no longer be available. Such a concern is unlikely in the real world; therefore, this design feature might be excluded and/or refined in future work to ensure external validity.
Another limitation is that, unlike the ECIG devices, cigarettes were not provided free-of-charge to participants in the OB-only (or ECIG+OB) condition. Similarly, OB-only participants did not receive an ECIG device. These differences may represent confounds between groups. Also, the measurement of cotinine prohibited differentiating between cigarette use and the other nicotine-containing products administered in this study. The inclusion of other biochemical measures (e.g., tobacco-specific nitrosamines) is thus warranted. Finally, one particular second-generation ECIG, with a single nicotine concentration and PG/VG ratio, was used as the exemplar MRTP. While the device/liquid combination was chosen based on previous work demonstrating significant nicotine delivery and withdrawal suppression in cigarette smokers,34,43 other combinations will need to be tested in future work. As has been proposed for MRTPs of the past,55 federal regulation of newer products should be based on science that employs a combination of pre-clinical, clinical, and epidemiological methods. Unlike pre-clinical methods, those that are clinical in nature are better able to consider the variability observed with MRTP use in the real world. Clinical methods also can be adapted quickly for different MRTPs (oral tobacco; IQOS),56-58 providing valuable information in a more rapid manner than can be obtained in epidemiological work. These strengths are highlighted with the specific clinical laboratory method proposed here, using an ECIG as the exemplar MRTP. Such a method may be preferred over larger-scale randomized control trials (RCTs) in which the timing of quit attempts is not necessarily considered. That is, our inclusion of weekly offers to make a quit attempt coupled with immediate entry into a cessation program allows for an evaluation of the timing of smokers’ choice to quit and how that timing influences actual behavior. With refinement, this method might ultimately be used by regulators to predict how smokers’ choice to quit and the timing of such choices may be influenced by current and future MRTPs.
HIGHLIGHTS.
Method to evaluate how MRTPs influence quit motivation, choice, and behavior.
ECIG availability may delay quit attempts.
ECIG availability may not impact cessation outcomes.
Results need to be replicated with larger sample sizes and a range of devices.
ACKNOWLEDGEMENTS
The authors thank Michael Phillips, Gemma David, and Colleen Warren for their diligent efforts with recruitment and data collection.
Role of Funding Sources
Financial support provided to MDB and GAD by WVU Senate Grant for Research, and to GAD, MDB, and NAT by Cooperative Agreement Number 1-U48-DP-005004 from the Centers for Disease Control and Prevention (CDC) to the West Virginia Prevention Research Center. Support provided to NJF and JEOH by the National Institute of General Medical Sciences (NIGMS T32 GM081741). Additional support provided by WV Tobacco Cessation QuitLine. The findings and conclusions in this report [journal article, etc.] are those of the author(s) and do not necessarily represent the official position of the CDC or the NIGMS. Funding sources had no role in the study design, collection, analysis, or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Conflict of Interest
Author SGF has consulted for various pharmaceutical companies on matters relating to smoking cessation. All other authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Pollay RW, Dewhirst T. The dark side of marketing seemingly “Light” cigarettes: successful images and failed fact. Tob Control. 2002;11(Suppl 1):I18–31. doi: 10.1136/tc.11.suppl_1.i18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haddock CK, Lando H, Klesges RC, et al. Modified tobacco use and lifestyle change in risk-reducing beliefs about smoking. Am J of Prev Med. 2004;27:35–41. doi: 10.1016/j.amepre.2004.03.010 [DOI] [PubMed] [Google Scholar]
- 3.Giovino GA, Tomar SL, Reddy MN, et al. (1996). Attitudes, knowledge, and beliefs about low-yield cigarettes among adolescents and adults Smoking and Tobacco Control Monograph No. 7. The FTC Cigarette Test Method for determining Tar, Nicotine, and Carbon Monoxide Yields of U.S. Cigarettes. Report of the NCI Expert Committee. 39–57. Bethesda, MD: National Cancer Institute. [Google Scholar]
- 4.Tindle HA, Shiffman S, Hartman AM, Bost JE. Switching to “lighter” cigarettes and quitting smoking. Tob Control. 2009;18:485–490. doi: 10.1136/tc.2008.029314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiffman S, Pillitteri JL, Burton SL, Di Marino ME. Smoker and ex-smoker reactions to cigarettes claiming reduced risk. Tob Control. 2004;13:78–84. doi: 10.1136/tc.2003.005272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hecht SS, Murphy SE, Carmella SG, et al. Similar uptake of lung carcinogens by smokers of regular, light, and ultralight cigarettes. Cancer Epidemiol Biomarkers Prev. 2005; 14:693–698. doi: 10.1158/1055-9965.EPI-04-0542 [DOI] [PubMed] [Google Scholar]
- 7.Campaign for Tobacco-Free Kids. 2013. Annual Report, www.tobaccofreekids.org
- 8.Campagna D, Amaradio MD, Sands MF, Polosa R. Respiratory infections and pneumonia: potential benefits of switching from smoking to vaping. Pneumonia (Nathan). 2016;8:4. doi: 10.1186/s41479-016-0001-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cibella F, Campagna D, Caponnetto P, et al. Lung function and respiratory symptoms in a randomized smoking cessation trial of electronic cigarettes. Clin Sci (Lond). 2016;130:1929–1937. doi: 10.1042/CS20160268 [DOI] [PubMed] [Google Scholar]
- 10.Veldheer S, Yingst J, Midya V, et al. Pulmonary and other health effects of electronic cigarette use among adult smokers participating in a randomized controlled smoking reduction trial. Addict Behav. 2018;S0306–4603:31250-31254. doi: 10.1016/j.addbeh.2018.10.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. 2016. Surgeon General’s Report: E-Cigarette Use Among Youth and Young Adults. Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion; https://www.cdc.gov/tobacco/data_statistics/sgr/e-cigarettes/index.htm [Google Scholar]
- 12.Nayak P, Pechacek TF, Weaver SR, Eriksen MP. Electronic nicotine delivery system dual use and intention to quit smoking: will the socioeconomic gap in smoking get greater? Addict Behav. 2016;61:112–116. doi: 10.1016/j.addbeh.2016.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutten LJ, Blake KD, Agunwamba AA, et al. Use of ECIGs among current smokers: associations among reasons for use, quit intentions, and current tobacco use. Nicotine Tob Res. 2015;17:1228–1234. doi: 10.1093/ntr/ntv003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Y, Pierce JP, White M, et al. E-cigarette use and smoking reduction or cessation in the 2010/2011 TUS-CPS longitudinal cohort. BMC Public Health. 2016;16:1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vickerman KA, Carpenter KM, Altman T, et al. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15:1787–1791. doi: 10.1093/ntr/ntt061 [DOI] [PubMed] [Google Scholar]
- 16.Beard E, West R, Michie S, Brown J. Association between electronic cigarette use and changes in quit attempts, success of quit attempts, use of smoking cessation pharmacotherapy, and use of stop smoking services in England: time series analysis of population trends. BMJ. 2016;13:354:i4645. doi: 10.1136/bmj.i4645 [DOI] [PubMed] [Google Scholar]
- 17.Brown J, Beard E, Kotz D, et al. Real-world effectiveness of e-cigarettes when used to aid smoking cessation: a cross-sectional population study. Addiction. 2014;109:1531–1540. doi: 10.1111/add.12623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine- replacement therapy. N Engl J Med. 2019;380:629–637. doi: 10.1056/nejmoa1808779 [DOI] [PubMed] [Google Scholar]
- 19.Weaver SR, Huang J, Pechacek TF, et al. Are electronic nicotine delivery systems helping smokers quit? Evidence from a prospective cohort study of U.S. adult smokers, 2015–2016. PLoS One. 2018;13:e0198047. doi: 10.1371/journal.pone.0198047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zawertailo L, Pavlov D, Ivanova A, et al. Concurrent ECIG use during tobacco dependence treatment in primary care settings: association with smoking cessation at three and six months. Nicotine Tob Res. 2017;19:183–189. doi: 10.1093/ntr/ntw218 [DOI] [PubMed] [Google Scholar]
- 21.Farsalinos KE, Spyrou A, Stefopoulos C, et al. Nicotine absorption from electronic cigarette use: comparison between experienced consumers (vapers) and naive users (smokers). Sci Rep. 2015;5:11269. doi: 10.1038/srep22169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fearon IM, Eldridge A, Gale N, et al. E-cigarette nicotine delivery: data and learnings from pharmacokinetic studies. Am J Health Behav. 2017;41:16–32. [DOI] [PubMed] [Google Scholar]
- 23.Lechner WV, Meier E, Wiener JL, et al. The comparative efficacy of first- versus second-generation electronic cigarettes in reducing symptoms of nicotine withdrawal. Addiction. 2015;110:862–867. doi: 10.1111/add.12870 [DOI] [PubMed] [Google Scholar]
- 24.Ruther T, Hagedorn D, Schiela K, et al. Nicotine delivery efficiency of first- and second-generation ECIGs and its impact on relief of craving during the acute phase of use. Int J Hyg Environ Health. 2018;221:191–198. doi: 10.1016/j.ijheh.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 25.Yan XS, D’Ruiz C. Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes. Regul Toxicol Pharmacol. 2015;71:24–34. doi: 10.1016/j.yrtph.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 26.Rohsenhow DJ, Tidey JW, Martin RA, et al. Effects of six weeks of electronic cigarette use on smoking rate, CO, cigarette dependence, and motivation to quit smoking: a pilot study. Addict Behav. 2018;80:65–70. doi: 10.1016/j.addbeh.2018.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valentine GW, Hefner K, Jatlow PI, et al. Impact of e-cigarettes on smoking and related outcomes in veteran smokers with psychiatric comorbidity. J Dual Diagn. 2018;14:2–13. doi: 10.1080/15504263.2017.1384877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagener TL, Meier E, Hale JJ, et al. Pilot investigation of changes in readiness and confidence to quit smoking after ECIG experimentation and 1 week of use. Nicotine Tobacco Res. 2014;16:108–114. doi: 10.1093/ntr/ntt138 [DOI] [PubMed] [Google Scholar]
- 29.Borland R, Yong HH, Balmfor J, et al. Motivational factors predict quit attempts but not maintenance of smoking cessation: findings from the International Tobacco Control Four country project. Nicotine Tob Res. 2010;12(Suppl S4): 11. doi: 10.1093/ntr/ntq050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smit ES, Hoving C, Schelleman-Offermans K, et al. Predictors of successful and unsuccessful quit attempts among smokers motivated to quit. Addict Behav. 2014;39:1318–1324. doi: 10.1016/j.addbeh.2014.04.017 [DOI] [PubMed] [Google Scholar]
- 31.Vangeli E, Stapleton J, Smit ES, et al. Predictors of attempts to stop smoking and their success in adult general population samples: a systematic review. Addiction. 2011;106:2110–2121. doi: 10.1111/j.1360-0443.2011.03565.x [DOI] [PubMed] [Google Scholar]
- 32.Danan ER, Joseph AM, Sherman SE, et al. Does motivation matter? Analysis of a randomized trial of proactive outreach to VA smokers. J Gen Intern Med. 2016;31:878–887. doi: 10.1007/s11606-016-3687-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Velicer WF, Redding CA, Sun X, Prochaska JO. Demographic variables, smoking variables,and outcome across five studies. Health Psychol. 2007;26:278–287. doi: 10.1037/0278-6133.26.3.278 [DOI] [PubMed] [Google Scholar]
- 34.Babb S, Malarcher A, Schauer G, et al. Quitting smoking among adults – United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017;65:1457–1464. doi: 10.15585/mmwr.mm6552a1 [DOI] [PubMed] [Google Scholar]
- 35.DiClemente CC, Prochaska JO, Fairhurst SK, et al. The process of smoking cessation: an analysis of precontemplation, contemplation, and preparation stages of change. J Consult Clin Psychol. 1991;59:295–304. [DOI] [PubMed] [Google Scholar]
- 36.Abrams DB, Niaura R, Brown RA, et al. The Tobacco Treatment Handbook: A Guide to Best Practices. NY: Guilford Press, 2003. [Google Scholar]
- 37.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br J Addict. 1991;86:1119–1127. [DOI] [PubMed] [Google Scholar]
- 38.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. [DOI] [PubMed] [Google Scholar]
- 39.Felicione NJ, Enlow P, Elswick D, et al. A pilot investigation of the effect of electronic cigarettes on smoking behavior among opioid-dependent smokers. Addict Behav. 2019;91:45–50. doi: 10.1016/j.addbeh.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 40.Ferguson SG, Shiffman S, Gwaltney CJ. Does reducing withdrawal severity mediate nicotine patch efficacy? A randomized clinical trial. J Consult Clin Psychol. 2006;74:1153–1161. doi: 10.1037/0022-006X.74.6.1153 [DOI] [PubMed] [Google Scholar]
- 41.Shiffman S, Dunbar MS, Li X, et al. Smoking patterns and stimulus control in intermittent and daily smokers. PLoS One. 2014;9:e89911. doi: 10.1371/journal.pone.0089911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiffman S, Dunbar MS, Tindle HA, Ferguson SG. Nondaily smokers’ experience of craving on days they do not smoke. J Abnorm Psychol. 2015;124:648–659. doi: 10.1037/abn0000063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen C, Zhuang YL, Zhu SH. ECIG design preference and smoking cessation: a U.S. population study. Am J Prev Med. 2016;51:356–363. doi: 10.1016/j.amepre.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cappendijik SL, Pirvan DF, Miller GL, et al. In vivo nicotine exposure in the zebra finch: a promising innovative animal model to use in neurodegenerative disorders related research. Pharmacol Biochem Behav. 2010;96:152–159. doi: 10.1016/j.pbb.2010.04.025 [DOI] [PubMed] [Google Scholar]
- 45.Belita E, Sidani S. Attrition in smoking cessation intervention studies: a systematic review.Can J Nurs Res. 2015;47:21–40. doi: 10.1177/084456211504700402 [DOI] [PubMed] [Google Scholar]
- 46.Asfar T, Al Ali R, Rastam S, et al. Behavioral cessation treatment of waterpipe smoking: the first pilot randomized controlled trial. Addict Behav. 2014;39:1066–1074. doi: 10.1016/j.addbeh.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burke LE, Shiffman S, Music E, et al. Ecological momentary assessment in behavioral research: addressing technological and human participant challenges. J Med Internet Res. 2017;19:e77. doi: 10.2196/jmir.7138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Comulada WS, Lightfoot M, Swendeman D, et al. Compliance to cell phone-based EMA among Latino youth in outpatient treatment. J Ethn Subst Abuse. 2015;14:232–250. doi: 10.1080/15332640.2014.986354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crooke AHD, Reid SC, Kauer SD, et al. Temporal mood changes associated with different levels of adolescent drinking. Drug Alcohol Rev. 2013;32:262–268. doi: 10.1111/dar.12034 [DOI] [PubMed] [Google Scholar]
- 50.Heron KE, Everhart RS, McHale SM, Smyth JM. Using mobile-technology-based ecological momentary assessment (EMA) methods with youth: a systematic review and recommendations. J Pediatr Psychol. 2017;42:1087–1107. doi: 10.1093/jpepsy/jsx078 [DOI] [PubMed] [Google Scholar]
- 51.Shiffman S Ecological momentary assessment (EMA) in studies of substance use. Psychol Assess. 2009;21:486–497. doi: 10.1037/a0017074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hughes JR, Solomon LJ, Livingston AE, et al. A randomized, controlled trial of NRT-aided gradual vs. abrupt cessation in smokers actively trying to quit. Drug Alcohol Depend. 2010;111:105–113. doi: 10.1016/j.drugalcdep.2010.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hughes JR, Callas PW. Is delaying a quit attempt associated with less success? Nicotine Tob Res. 2011;13:1228–1232. doi: 10.1093/ntr/ntr207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeVito EE, Krishnan-Sarin S. E-cigarettes: Impact of e-liquid components and device characteristics on nicotine exposure. Curr Neuropharmacol. 2018;16:438–459. doi: 10.2174/1570159X15666171016164430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hatsukami DK, Giovino GA, Eissenberg T, et al. Methods to assess potential reduced exposure products. Nicotine Tob Res. 2005;7:827–844. doi: 10.1080/14622200500266015 [DOI] [PubMed] [Google Scholar]
- 56.Lopez AA, Hiler M, Maloney S, et al. Expanding clinical laboratory tobacco product evaluation methods to loose-leaf tobacco vaporizers. Drug Alcohol Depend. 2016;169:33–40. doi: 10.1016/j.drugalcdep.2016.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vansickel AR, Weaver MF, Eissenberg T. Clinical laboratory assessment of the abuse liability of an electronic cigarette. Addiction. 2012;107(8):1493–500. doi: 10.1111/j.1360-0443.2012.03791.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Breland AB, Kleykamp BA, Eissenberg T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nic Tob Res. 2006;8:727–738. doi: 10.1080/14622200600789585 [DOI] [PubMed] [Google Scholar]
- 59.Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, Walker N. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629–1637. doi: 10.1016/S0140-6736(13)61842-5 [DOI] [PubMed] [Google Scholar]
- 60.Carpenter MJ, Heckman BW, Wahlquist AE, Wagener TL, Goniewicz ML, Gray KM,Froeliger B, Cummings KM. A naturalistic, randomized pilot trial of e-cigarettes: uptake, exposure, and behavioral effects. Cancer Epidemiol Biomarkers Prev. 2017;26:1795–1803. doi: 10.1158/1055-9965.EPI-17.0460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Donny EC, Griffin KM, Shiffman S, Sayette MA. The relationship between cigarette use,nicotine dependence, and craving in laboratory volunteers. Nicotine Tob Res. 2008;10:934–942. doi: 10.1080/14622200802133681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pasquereau A, Guignard R, Andler R, Nguyen-Thanh V. Electronic cigarettes, quit attempts and smoking cessation: a 6-month follow-up. Addiction. 2017;112:1620–1628. doi: 10.1111/add.13869 [DOI] [PubMed] [Google Scholar]
- 63.Felicione NJ, Enlow P, Elswick D, Long D, Sullivan CR, & Blank MD (2019). A pilot investigation of the effect of electronic cigarettes on smoking behavior among opioid-dependent smokers. Addictive Behaviors, 91, 45–50. doi: 10.1016/j.addbeh.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 64.Tseng TY, Ostroff JS, Campo A, Gerard M, Kirchner T, Rotrosen J, & Shelley D (2016). A randomized trial comparing the effect of nicotine versus placebo electronic cigarettes on smoking reduction among young adult smokers. Nicotine and Tobacco Research, 18, 1937–1943. doi: 10.1093/ntr/ntw017 [DOI] [PMC free article] [PubMed] [Google Scholar]