KEY POINTS
New type 2 diabetes mellitus (T2DM) guidelines have moved from a glycemic-based to an outcome-based approach, recommending treatments based on patient comorbidities.
Sodium-glucose cotransporter-2 (SGLT2) inhibitors are now recommended as second-line treatment after metformin in patients with T2DM and prior atherosclerotic cardiovascular disease, heart failure or chronic kidney disease in American and European guidelines.
Sodium-glucose cotransporter-2 inhibitors have been shown to reduce substantially the risk of heart failure and progression of kidney disease in a wide range of patients with T2DM in large-scale randomized controlled trials.
Recognized adverse events with SGLT2 inhibitors include mycotic genital infections and volume depletion, but clinicians should be aware of other uncommon but potentially serious adverse effects, particularly diabetic ketoacidosis (which can occur in the presence of normal or only mildly elevated blood glucose) and possibly amputations.
In contemporary clinical practice, there are numerous glucose-lowering agents available for the treatment of type 2 diabetes mellitus (T2DM): from older drugs such as metformin and sulfonylureas to newer agents such as dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists and sodium-glucose cotransporter-2 (SGLT2) inhibitors. In the past decade, large-scale randomized controlled trials (RCTs) of glucose-lowering agents have shown varying benefits on cardiovascular and kidney outcomes among patients with T2DM.1 In particular, SGLT2 inhibitors are being prescribed increasingly by physicians in a wide range of specialties2 because of cardiovascular and kidney benefits reported in large RCTs, the findings of which are reflected in several major guideline updates in the past 18 months. We review the mechanism of action of SGLT2 inhibitors, summarizing data from major cardiovascular and kidney outcome trials underpinning current treatment recommendations (Box 1), and discuss the use of these agents in clinical practice, including important safety issues.
Box 1:
Evidence used in this review
We conducted a targeted search of MEDLINE to identify randomized, controlled, event-driven, cardiovascular or kidney outcome trials of sodium-glucose cotransporter-2 inhibitors using the medical subject heading terms “sodium-glucose transporter 2 inhibitors,” “diabetes mellitus, type 2” and “randomized controlled trial.” Our search yielded 4 randomized studies that investigated the effect of empagliflozin, canagliflozin or dapagliflozin on cardiovascular and kidney outcomes. In addition, we used evidence provided in 3 recently updated North American and European clinical practice guidelines: the 2019 American Diabetes Association standards of care, the 2018 consensus report of the American Diabetes Association and the European Association for the Study of Diabetes, and the Diabetes Canada 2018 guideline for the pharmacologic glycemic management of type 2 diabetes. These were supplemented by recent literature from our own collection.
How do SGLT2 inhibitors work?
Sodium-glucose cotransporter-2 inhibitors act by blocking the paired reuptake of sodium and glucose in the proximal tubule, thereby promoting urinary glucose excretion; these agents have been shown to lower glycated hemoglobin (HbA1c) by about 0.5%–0.7% in individuals with normal kidney function.3 Owing to the associated caloric losses, SGLT2 inhibitors have also been shown to reduce body weight modestly (about 2–3 kg).3 Beyond their glucosuria-mediated effects, SGLT2 inhibitors promote urinary excretion of sodium (i.e., natriuresis). The natriuretic effect of SGLT2 inhibitors contributes to intravascular volume contraction and influences intrarenal hemodynamics, yielding about 5 mm Hg reductions in systolic blood pressure and 30%–50% reductions in albuminuria in patients with micro- or macroalbuminuria.3–5 There is accumulating evidence that these nonglycemic, pleotropic effects are at least as important as glucose lowering in explaining the observed reduction in cardiovascular morbidity and mortality and protection against progression of diabetic kidney disease with SGLT2 inhibitors, both in RCTs and routine clinical practice.6,7
What have large-scale randomized controlled trials of SGLT2 inhibitors shown?
To date, there have been 4 large RCTs of SGLT2 inhibitors involving patients with T2DM, collectively enrolling 38 723 participants across 6 continents (Table 1). Three were cardiovascular outcome trials: EMPA-REG OUTCOME (empagliflozin),8 the CANVAS Program (CANVAS and CANVAS-R trials; canagliflozin)9 and DECLARE-TIMI 58 (dapagliflozin).10 These trials assessed the effect of SGLT2 inhibition on a primary outcome of major adverse cardiovascular events, defined as nonfatal myocardial infarction, nonfatal stroke or cardiovascular death. In contrast, the CREDENCE trial was specifically designed to test the effect of SGLT2 inhibition on kidney outcomes in patients with established diabetic kidney disease, with a primary outcome of doubling of serum creatinine, end-stage kidney disease or death caused by cardiovascular or kidney disease.11 All 4 studies were event-driven, double-blind, randomized, placebo-controlled trials with participants receiving guideline-directed care.
Table 1:
Summary of the major randomized controlled trials of sodium-glucose cotransporter-2 inhibitors
| Study characteristics | No. (%) of participants* | |||
|---|---|---|---|---|
| EMPA-REG OUTCOME8 n = 7020 |
CANVAS program9 n = 10 142 |
DECLARE-TIMI 5810 n = 17 160 |
CREDENCE11 n = 4401 |
|
| Drug | Empagliflozin | Canagliflozin | Dapagliflozin | Canagliflozin |
| Dose, mg | 10 or 25 | 100 or 300 | 10 | 100 |
| Age, mean ± SD; yr | 63.1 ± 8.7 | 63.3 ± 8.3 | 63.9 ± 6.8 | 63.0 ± 9.2 |
| Sex, female | 2004 (28.5) | 3633 (35.8) | 6422 (37.4) | 1494 (33.9) |
| Follow-up time, median; yr | 3.1 | 2.4 | 4.2 | 2.6 |
| History of cardiovascular disease | 7020 (100.0) | 6656 (65.6) | 6974 (40.6) | 2220 (50.4) |
| History of heart failure | 706 (10.1) | 1461 (14.4) | 1724 (10.0) | 652 (14.8) |
| eGFR < 60 mL/min/1.73 m2† | 1819 (25.9) | 2039 (20.1) | 1265 (7.4) | 2631 (59.8) |
| Micro- or macroalbuminuria | 2782 (39.6) | 3026 (29.8) | 5199 (30.3) | 4370 (99.3) |
| Primary outcome(s) | MACE | MACE | MACE and admission to hospital for heart failure or CV death | Doubling of serum creatinine level, ESKD, or CV or renal death |
Note: CKD-EPI = chronic kidney disease epidemiology collaboration equation, CV = cardiovascular, eGFR = estimate glomerular filtration rate, ESKD = end-stage kidney disease, MACE = major adverse cardiovascular events (defined as nonfatal myocardial infarction, nonfatal stroke or CV death), MDRD = modification of diet in renal disease equation.
Unless specified otherwise.
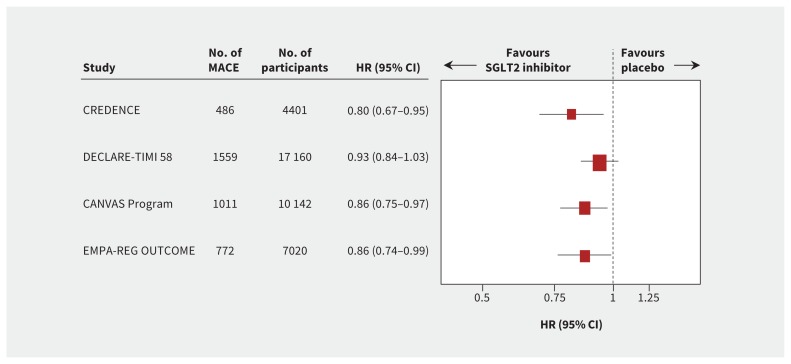
Overall evidence supports a moderate beneficial effect of SGLT2 inhibition on major adverse cardiovascular events, with proportional risk reductions of about 10% (Figure 1). The 3 cardiovascular outcome trials enrolled varying proportions of participants with a history of atherosclerotic cardiovascular disease (Table 1). About 60% of participants in DECLARE-TIMI 58 did not have prior cardiovascular disease and were thus at lower cardiovascular risk. In contrast, about two-thirds of those in the CANVAS Program and all participants in the EMPA-REG OUTCOME trial had a history of cardiovascular disease. A 2019 meta-analysis of these trials found that the reduction in major adverse cardiovascular events with SGLT2 inhibitors was primarily observed in those with established atherosclerotic cardiovascular disease (i.e., for the secondary prevention of cardiovascular events).6
Figure 1:
Major adverse cardiovascular events (MACE), defined as nonfatal myocardial infarction, nonfatal stroke or cardiovascular death (the size of each box is weighted using the inverse variance method). Note: CI = confidence interval, HR = hazard ratio, SGLT2 = sodium-glucose cotransporter-2.
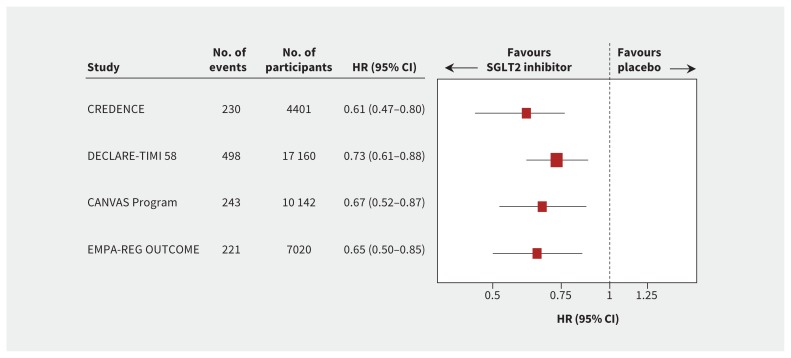
Perhaps more strikingly, SGLT2 inhibitors consistently reduced the risk of admission to hospital for heart failure by about 30%, individually and overall across the completed trials (Figure 2). In contrast to their effect on major adverse cardiovascular events, the benefits for heart failure were consistent regardless of a history of heart failure or atherosclerotic cardiovascular disease,6 highlighting the unique kidney and systemic hemodynamic effects of these drugs.
Figure 2:
Admission to hospital for heart failure (the size of each box is weighted using the inverse variance method). Note: CI = confidence interval, HR = hazard ratio, SGLT2 = sodium-glucose cotransporter-2.
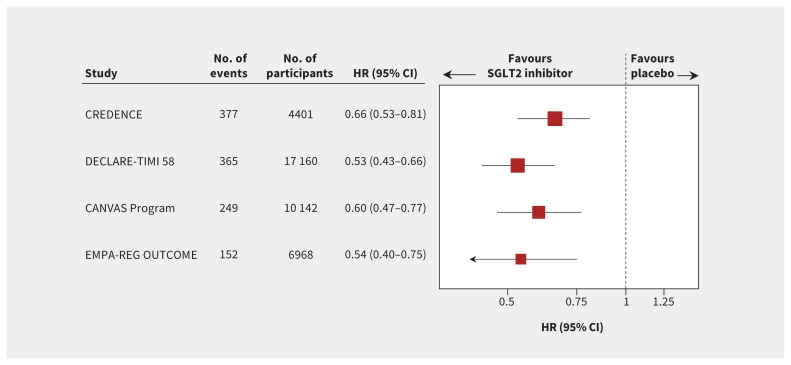
Although these trials were not primarily designed to assess the effect of SGLT2 inhibition on progression of diabetic kidney disease, all 3 trials reported prespecified or post hoc effects on a composite kidney outcome, defined as either doubling of serum creatinine or 40% decline in estimated glomerular filtration rate (eGFR), end-stage kidney disease or death caused by kidney disease.12–14 In secondary analyses, there was clear and consistent evidence of renoprotection with proportional risk reductions of greater than 30% in each trial and overall (Figure 3).
Figure 3:
Substantial loss of kidney function, ESKD or death from kidney disease. Substantial loss of kidney function was defined as doubling of serum creatinine level in the CREDENCE11 and EMPA-REG OUTCOME8 trials and sustained 40% decline in estimated glomerular filtration rate in the CANVAS Program9 and DECLARE-TIMI 58 trial.10 The size of each box is weighted using the inverse variance method. Note: CI = confidence interval, ESKD = end-stage kidney disease, HR = hazard ratio, SGLT2 = sodium-glucose cotransporter-2.
The CREDENCE trial was reported most recently and was explicitly designed to determine the effects of SGLT2 inhibition in patients at high risk of kidney failure.11 All participants had T2DM and macroalbuminuria and were required to be receiving maximum tolerated labelled dose of angiotensin-converting-enzyme (ACE) inhibitor or angiotensin II receptor blocker for at least 4 weeks before being randomly assigned. The trial found that canagliflozin reduced the risk of the primary composite outcome of doubling of serum creatinine, end-stage kidney disease or death caused by cardiovascular or kidney disease (hazard ratio [HR] 0.70, 95% confidence interval [CI] 0.59–0.82) with separate evidence of benefit for end-stage kidney disease alone (HR 0.68, 95% CI 0.54–0.86). Canagliflozin also reduced the risk of major adverse cardiovascular events (HR 0.80, 95% CI 0.67–0.95) and admission to hospital for heart failure (HR 0.61, 95% CI 0.47–0.80). In absolute terms, treatment with canagliflozin would be expected to prevent 47 primary composite outcomes and 24 end-stage kidney disease events for every 1000 “CREDENCE-like” patients treated over 2.5 years, translating into numbers needed to treat of 21 and 42, respectively.
By what mechanisms do SGLT2 inhibitors achieve their varied effects?
The glycemic effect of SGLT2 inhibitors is dependent on glomerular filtration and substantially diminishes as kidney function declines.15,16 This is their hallmark feature. In contrast, the effect of SGLT2 inhibitors on major adverse cardiovascular events does not appear to be influenced by kidney function, and relative (and absolute) effects on heart failure may even be greater in patients with an eGFR below 60 mL/min/1.73 m2.6,17 In the CREDENCE trial, canagliflozin provided renoprotection down to an eGFR of 30 mL/min/1.73 m2, despite markedly attenuated glycemic efficacy at lower ranges of eGFR,11 an observation reinforced by previous data showing that benefits for kidney outcomes are independent of HbA1c before and during therapy or by degree of reduction in HbA1c.18 Collectively, these data in patients with reduced kidney function raise an important question: To what extent are the cardiovascular and kidney benefits of SGLT2 inhibitors due to glucose lowering?
It is likely that several mechanisms (e.g., reduction in body weight, blood pressure and albuminuria) contribute to the observed reduction in cardiovascular and kidney outcomes with SGLT2 inhibitors. However, one of the most intriguing physiologic explanations is that SGLT2 inhibitors improve glomerular and systemic hemodynamics. It is well-established that chronic hyperglycemia promotes afferent arteriolar vasodilatation, which increases intraglomerular pressure — a crucial process in the pathogenesis of diabetic kidney disease.19 Sodium-glucose cotransporter-2 inhibitors are thought to lower intraglomerular pressure.20,21 By blocking proximal tubular sodium reuptake, these drugs increase distal sodium delivery to the macula densa, leading to afferent arteriolar vasoconstriction through a process called “tubuloglomerular feedback.” Clinically, this reduction in intraglomerular pressure is reflected in an acute “dip” in eGFR of about 5 mL/min/1.73 m2, followed by stabilization and preservation of kidney function, and a reduction in albuminuria over time.3
This characteristic acute eGFR response with SGLT2 inhibitors is similar to that seen with the only other drugs approved by regulatory agencies for the treatment of diabetic kidney disease — ACE inhibitors and angiotensin II receptor blockers. Although SGLT2 inhibitors reduce intraglomerular pressure by possibly enhancing afferent arteriolar vasoconstriction, ACE inhibitors and angiotensin II receptor blockers do so by promoting efferent arteriolar vasodilatation.22 Importantly, the parallel and complementary mechanisms of SGLT2 inhibitors and ACE inhibitors or angiotensin II receptor blockers appear to be additive, with no signal toward increased risk of acute kidney injury in any of the completed trials. Most participants (99.9%) in the CREDENCE and about 80% of participants in the 3 cardiovascular outcome trials were receiving ACE inhibitors or angiotensin II receptor blockers at baseline. The trials’ findings collectively suggest that these drugs might also reduce the risk of acute kidney injury, underscoring the importance of further mechanistic work to understand these drugs better.23,24
What are the adverse effects of SGLT2 inhibitors?
Safety data suggest that SGLT2 inhibitors are generally well tolerated, with some important caveats.
Osmotic diuresis and symptomatic volume depletion because of glucosuric and natriuretic effects can occur, but are generally of modest severity.25
There is about a threefold increased risk of mycotic genital infections, but not urinary tract infections,26 which in most cases does not require permanent discontinuation of the drug. Men and women are both at increased risk, but the absolute risk may be greater in women, given the greater frequency of mycotic infections.
Although the absolute risk is extremely low, the US Food and Drug Administration (FDA) recently issued a warning about the risk of Fournier gangrene, based on 12 cases involving patients taking SGLT2 inhibitors in postmarketing surveillance analyses.27 While this concern has been corroborated in a recent case series also using data from the FDA’s Adverse Event Reporting System,28 more cases occurred in participants treated with placebo than in the dapagliflozin arm in the DECLARE-TIMI 58 trial,10 underscoring the challenges of interpreting routinely collected data because of confounding by indication and variably quality of reports. Notwithstanding the extremely low incidence and limitations of current data, prescribers should be aware of this uncommon outcome, especially given the importance of early recognition and treatment.
Another uncommon but potentially serious adverse effect of SGLT2 inhibitors is ketoacidosis, which can occur even in the presence of normal or only mildly elevated blood glucose levels.29 This is because SGLT2 inhibitors stimulate lipolysis; the increased delivery of free fatty acids to the liver modestly increases circulating ketones.30 In most patients, this effect is not clinically significant. It may, however, contribute to an increased risk of ketoacidosis in those with pancreatic insufficiency requiring long-term treatment with insulin. In many instances, ketoacidosis has occurred in the context of a precipitating factor, such as infection, omitted insulin or prolonged fasting.31 Although only 74 cases occurred in 38 723 participants in the completed trials (event rates of 0.1–2.2 per 1000 patient-years),6,11 it is possible that the incidence might be higher as the use of these agents in wider clinical practice becomes more common. Thus, it is important to have a high index of suspicion for this uncommon but potentially life-threatening adverse effect.
An increased risk of amputation of the lower extremities, mainly at the level of the metatarsals, and a small increased risk of fracture were unexpected and concerning findings in the CANVAS Program.9 The amputation rates across the CANVAS Program were 6.3 and 3.4 per 1000 person-years with canagliflozin and placebo, respectively (HR 1.97, 95% CI 1.41–2.75). In light of data from the CANVAS Program, the US FDA issued a Drug Safety Communication for canagliflozin about the risk of amputation.32 The risk of amputations has not been observed in trials of empagliflozin or dapagliflozin, or in CREDENCE, despite these patients being at much higher risk of amputation. Additionally, the risk of fracture was observed only in 1 of the 2 companion trials in the CANVAS Program (CANVAS but not CANVAS-R), and not in any other SGLT2 trials, including CREDENCE. It remains unclear whether these differences are related to patient characteristics, trial protocols or chance.
How has the evidence on SGLT2 inhibitors influenced updated guidelines?
In 2018, several clinical practice guidelines for the treatment of T2DM were updated with new algorithms to reflect the latest evidence for cardiovascular and kidney protection with specific glucose-lowering agents. Major guideline updates included the American Diabetes Association standards of care,33 a consensus report by the American Diabetes Association and the European Association for the Study of Diabetes,34 the American College of Cardiology’s Expert Consensus Decision Pathway35 and Diabetes Canada’s clinical practice guideline.36 The American Diabetes Association’s standards of care were updated further in 2019 after the publication of findings from the CREDENCE trial.37
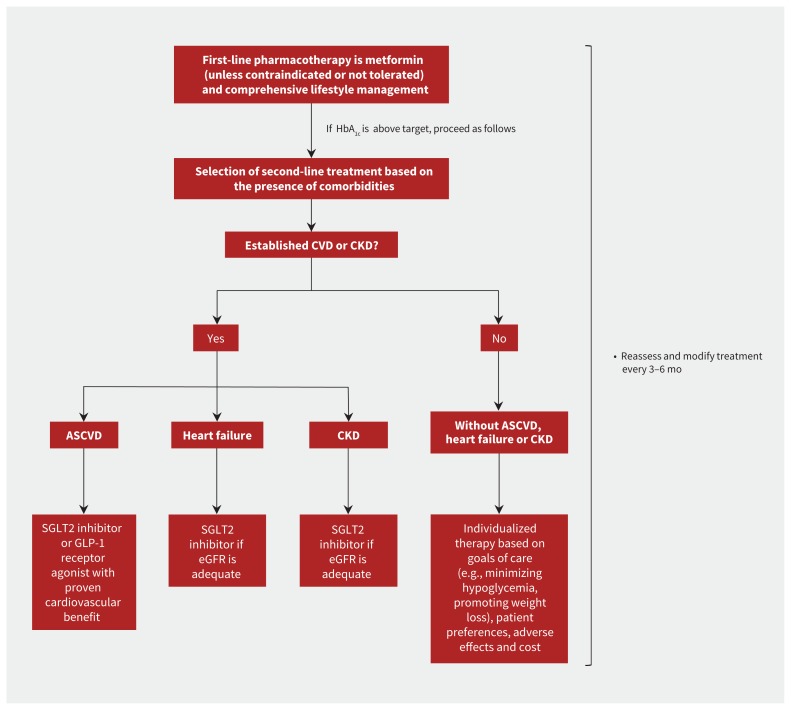
Largely because of its cost, tolerability and safety, metformin remains first-line pharmacotherapy, alongside comprehensive lifestyle management. However, there is limited evidence that metformin reduces the risk of cardiovascular outcomes or progression of kidney disease, with unclear effect on all-cause mortality.38 Because SGLT2 inhibitors and GLP-1 receptor agonists have been shown to reduce the risk of major adverse cardiovascular events in large trials of cardiovascular outcomes, both are now recommended as second-line agents in patients with T2DM and established cardiovascular disease (Figure 4).33–36
Figure 4:
Summary of the overall approach to glucose-lowering medications in type 2 diabetes as recommended by the 2018 consensus report of the American Diabetes Association and European Association for the Study of Diabetes.34 Note: ASCVD = atherosclerotic cardiovascular disease, CKD = chronic kidney disease, CVD = cardiovascular disease, eGFR = estimated glomerular filtration rate, GLP-1 = glucagon-like peptide-1, HbA1c = glycated hemoglobin, SGLT2 = sodium-glucose cotransporter-2.
The 2018 consensus report by the American Diabetes Association and European Association for the Study of Diabetes and the American Diabetes Association’s 2019 standards of care make additional specific recommendations on the use of SGLT2 inhibitors in patients with heart failure and chronic kidney disease.34,37 Because of the evidence that these drugs reduce the risk of admission to hospital for heart failure and clinically important kidney outcomes (Figures 2 and 3), SGLT2 inhibitors are now recommended as second-line treatment in people with T2DM and heart failure or chronic kidney disease in the American Diabetes Association and European Association for the Study of Diabetes guideline (Figure 4). In light of data from CREDENCE trial, the 2019 standards of care specifically recommended SGLT2 inhibitors for the prevention of kidney failure and cardiovascular events in patients with T2DM and an eGFR down to 30 mL/min/1.73 m2, particularly in those with macroalbuminuria.37
How should SGLT2 inhibitors be used in practice?
Based on current guideline recommendations,34 SGLT2 inhibitors should be considered as second-line treatment after metformin in patients with T2DM and atherosclerotic cardiovascular disease, heart failure or chronic kidney disease (Figure 4).
We suggest they be avoided in patients with a history of ketoacidosis, because this may identify a subgroup of patients with relative insulin deficiency or other physiologic predisposition to this condition. Dapagliflozin has been approved recently for the treatment of type 1 diabetes;39 however, treatment should be avoided generally outside RCTs other than after close consultation with specialist endocrinologists and a clear sick-day management plan, because of the much greater risk of ketoacidosis. Notwithstanding that no amputation signal was observed in CREDENCE, it would seem prudent to avoid these agents in patients with critical limb ischemia or a history of amputation while awaiting data from ongoing trials, given the adverse-effect profile observed in the CANVAS Program.
In Canada, there are 4 SGLT2 inhibitors approved for use in patients with T2DM: empagliflozin, canagliflozin, dapagliflozin and ertugliflozin. Both empagliflozin and canagliflozin have label indications for the prevention of cardiovascular events in patients with established cardiovascular disease.40,41 Because glycemic efficacy is tied to glomerular filtration, their use has been limited in patients with reduced kidney function; however, in Canada, these restrictions have been revised recently. Empagliflozin is now permitted for use in patients with an eGFR down to 30 mL/min/1.73 m2.40 Guidelines also recommend that canagliflozin may be considered for use down to the same eGFR threshold for cardiovascular and kidney protection.36,40 These recommendations are reinforced by the 2019 updates to the American Diabetes Association’s standards of care.37
Some other practical considerations are worth noting (Box 2). Patients taking concomitant diuretics may require dose adjustment to reduce the risk of volume depletion.42 Sodium-glucose cotransporter-2 inhibitors are insulin sparing and thus insulin doses should also be adjusted appropriately. At each consultation, patients should be carefully examined for any evidence of substantial ischemia in the lower extremities such as skin ulceration, and discontinuation of the drug in such cases may be prudent. Patients should be instructed to hold treatment if they are unable to tolerate oral intake, for example, owing to vomiting or diarrhea. These agents should also be withheld in the perioperative period to minimize the risk of ketoacidosis.43
Box 2:
Practical suggestions for the use of sodium-glucose cotransporter-2 inhibitors
Ensure patients are clinically stable and volume replete when commencing an sodium-glucose cotransporter-2 inhibitor.42
Consider reducing the dose of any concurrent loop diuretic (if euvolemic) and adjusting insulin dose appropriately.42
Emphasize the importance of personal hygiene to reduce the risk of mycotic infections.25
At each consultation, examine patients for signs of substantial lower extremity ischemia and consider drug withdrawal in such cases.37
Provide advice about withholding treatment if the patient is unable to eat or drink, such as during an acute illness.29
Temporarily withhold treatment before and after major surgery to reduce the risk of ketoacidosis.25
What research questions remain unanswered?
As these agents become increasingly used in a wider range of patients in routine practice, it will be important to continue to assess the generalizability of results from completed trials. A holistic assessment of absolute benefits, cost considerations, patient preferences and presence of multiple comorbidities is important to minimize harms and maximize benefits. These considerations might be particularly important in older, frail patients in whom the adverse effect profile may be prohibitive.
Several RCTs of SGLT2 inhibitors are expected to be completed and reported over the next 3–4 years and will provide additional important data. These include kidney outcome trials If HbA1c is above target, proceed as follows for empagliflozin (EMPA-KIDNEY, ClinicalTrials.gov No. NCT03594110)44 and dapagliflozin (DAPA-CKD, ClinicalTrials.gov No. NCT03036150), as well as trials of heart failure enrolling participants with reduced and preserved ejection fraction (i.e., EMPEROR-Reduced, ClinicalTrials.gov No. NCT03057977; EMPEROR-Preserved, ClinicalTrials.gov No. NCT03057951; and DELIVER, ClinicalTrials.gov No. NCT03619213). Because of the proposed nonglycemic mechanism of benefit, these trials are recruiting participants with and without diabetes. The recently completed DAPA-HF trial showed that dapagliflozin reduced the risk of heart failure in people with heart failure and reduced ejection fraction irrespective of diabetes status, providing further evidence that these drugs may benefit people without diabetes.45
Conclusion
Sodium-glucose cotransporter-2 inhibitors are a practice-changing development in the treatment of T2DM, and data to date from completed trials support their ability to provide cardiovascular and kidney protection in addition to current standard of care. In combination with ongoing trials, this class of medication is shifting the therapeutic paradigm in T2DM from a glycemic-based to an outcome-based approach, as shown by the number and scope of recent updates to treatment guidelines for T2DM. Treatments should be selected based on end-organ protection and patient comorbidities rather than focusing on lowering of glucose levels alone.
Footnotes
Competing interests: The George Institute for Global Health provides contract research services to Janssen for trials of SGLT2 inhibitors. Brendon Neuen has received travel support from Janssen. David Cherney has received honoraria from Boehringer Ingelheim, Eli Lilly, Merck, AstraZeneca, Sanofi, Merck, Mitsubishi Tanabe, AbbVie, Janssen, Bayer and Prometic, and has received operational funding for clinical trials from Boehringer Ingelheim, Eli Lilly, Merck, Janssen, Sanofi and AstraZeneca. Meg Jardine is responsible for research projects that have received unrestricted funding from Gambro, Baxter, CSL, Amgen, Eli Lilly and Merck; has served on advisory boards sponsored by Akebia, Baxter and Boehringer Ingelheim; and spoken at scientific meetings sponsored by Janssen, Amgen and Roche, with any consultancy, honoraria or travel support paid to her institution. Vlado Perkovic was the chair of a steering committee for a renal outcome study of an SGLT2 inhibitor (canagliflozin), serves on steering committees for AbbVie, Boehringer Ingelheim, GlaxoSmithKline, Janssen and Pfizer; serves on advisory boards or speaks at scientific meetings for AbbVie, Astellas, AstraZeneca, Bayer, Baxter, Bristol–Myers Squibb, Boehringer Ingelheim, Durect, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Novo Nordisk, Pfizer, Pharmalink, Relypsa, Roche, Sanofi, Servier and Vitae; and has received personal fees for consulting and scientific presentations related to canagliflozin from Mitsubishi Tanabe and Mundipharma.
This article has been peer reviewed.
Contributors: All of the authors contributed to the design of the review, analysis and interpretation of data; drafted the manuscript; revised the manuscript for important intellectual content; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding: Brendon Neuen is supported by an Australian National Health and Medical Research Council Postgraduate Scholarship, an Oxford Australia Clarendon Scholarship from the University of Oxford, the Graduate Research Fund at Lincoln College, University of Oxford, a John Chalmers PhD Scholarship from The George Institute for Global Health and a University Postgraduate Award from UNSW Sydney. David Cherney is supported by funding from the Canadian Institutes of Health Research, the Juvenile Diabetes Research Foundation, and the Banting & Best Diabetes Centre at the University of Toronto. David Cherney is supported in part by a University of Toronto Merit Award, and his trainees are supported by the Canadian Diabetes Association (Diabetes Canada) Postdoctoral Fellowship, and the University Health Network CaRE Fellowship Program. Meg Jardine is supported by a Medical Research Future Fund Next Generation Clinical Researchers Program Career Development Fellowship. Vlado Perkovic receives research support from the National Health and Medical Research Council (Senior Research Fellowship and Program Grant).
References
- 1.Cefalu WT, Kaul S, Gerstein HC, et al. Cardiovascular outcomes trials in type 2 diabetes: Where do we go from here? Reflections from a diabetes care editors’ expert forum. Diabetes Care 2018;41:14–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaduganathan M, Sathiyakumar V, Singh A, et al. Prescriber patterns of SGLT2i after expansions of U.S. Food and Drug Administration labeling. J Am Coll Cardiol 2018;72:3370–2. [DOI] [PubMed] [Google Scholar]
- 3.Heerspink HJ, Perkins BA, Fitchett DH, et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 2016; 134:752–72. [DOI] [PubMed] [Google Scholar]
- 4.Heerspink HJ, Kosiborod M, Inzucchi SE, et al. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int 2018;94:26–39. [DOI] [PubMed] [Google Scholar]
- 5.Neuen BL, Ohkuma T, Neal B, et al. Effect of canagliflozin on renal and cardiovascular outcomes across different levels of albuminuria: data from the CANVAS Program. J Amer Soc Nephrol 2019. Sept. 7 10.1681/ASN.2019010064. [DOI] [PMC free article] [PubMed]
- 6.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019; 393:31–9. [DOI] [PubMed] [Google Scholar]
- 7.Kosiborod M, Lam CSP, Kohsaka S, et al. CVD-REAL Investigators and Study Group. Lower cardiovascular risk associated with SGLT-2i in >400,000 patients: the CVD-REAL 2 study. J Am Coll Cardiol 2018;24748 10.1016/j.jacc.2018.03.009. [DOI] [Google Scholar]
- 8.Zinman B, Wanner C, Lachin JM, et al. EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28. [DOI] [PubMed] [Google Scholar]
- 9.Neal B, Perkovic V, Mahaffey KW, et al. CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–57. [DOI] [PubMed] [Google Scholar]
- 10.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–57. [DOI] [PubMed] [Google Scholar]
- 11.Perkovic V, Jardine MJ, Neal B, et al. CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380: 2295–306. [DOI] [PubMed] [Google Scholar]
- 12.Wanner C, Inzucchi SE, Lachin JM, et al. EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–34. 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 13.Perkovic V, de Zeeuw D, Mahaffey KW, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol 2018;6:691–704. [DOI] [PubMed] [Google Scholar]
- 14.Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE–TIMI 58 randomised trial. Lancet Diabetes Endocrinol 2019; 7: 606–17. [DOI] [PubMed] [Google Scholar]
- 15.Cherney DZI, Cooper ME, Tikkanen I, et al. Pooled analysis of Phase III trials indicate contrasting influences of renal function on blood pressure, body weight, and HbA1c reductions with empagliflozin. Kidney Int 2018;93:231–44. [DOI] [PubMed] [Google Scholar]
- 16.Toyama T, Neuen BL, Jun M, et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and meta-analysis. Diabetes Obes Metab 2019;21:1237–50. [DOI] [PubMed] [Google Scholar]
- 17.Neuen BL, Ohkuma T, Neal B, et al. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function: data from the CANVAS Program. Circulation 2018;138:1537–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper ME, Inzucchi SE, Zinman B, et al. Glucose control and the effect of empagliflozin on kidney outcomes in type 2 diabetes: an analysis from the EMPA-REG OUTCOME trial. Am J Kidney Dis 2019. June 26 [Epub ahead of print] 10.1053/j.ajkd.2019.03.432. [DOI] [PubMed] [Google Scholar]
- 19.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 2017;12:2032–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014;129:587–97. [DOI] [PubMed] [Google Scholar]
- 21.Kidokoro K, Cherney DZI, Bozovic A, et al. Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation 2019; 140:303–15. [DOI] [PubMed] [Google Scholar]
- 22.Tuttle KR. Back to the future: glomerular hyperfiltration and the diabetic kidney. Diabetes 2017;66:14–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilbert RE, Thorpe KE. Acute kidney injury with sodium-glucose co-transporter-2 inhibitors: a meta-analysis of cardiovascular outcome trials. Diabetes Obes Metab 2019;21:1996–2000. [DOI] [PubMed] [Google Scholar]
- 24.Neuen BL, Young T, Heerspink HJ, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2019. Sept. 5 10.1116/S2213-8587(19)30256-6. [DOI] [PubMed]
- 25.Fitchett D. A safety update on sodium glucose co-transporter 2 inhibitors. Diabetes Obes Metab 2019;21:34–42. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Li L, Li S, et al. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: a systematic review and meta-analysis. Sci Rep 2017;7:2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.FDA warns about rare occurrences of a serious infection of the genital area with SGLT2 inhibitors for diabetes; safety announcement. Silver Spring (MD): U.S. Food and Drug Administration; 2018. August 29 Available: www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-rare-occurrences-serious-infection-genital-area-sglt2-inhibitors-diabetes (accessed 2019 Sept. 30). [Google Scholar]
- 28.Bersoff-Matcha SJ, Chamberlain C, Cao C, et al. Fournier gangrene associated with sodium-glucose cotransporter-2 inhibitors: a review of spontaneous postmarketing cases. Ann Intern Med 2019;170:764–9. [DOI] [PubMed] [Google Scholar]
- 29.Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care 2015;38:1638–42. [DOI] [PubMed] [Google Scholar]
- 30.Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care 2016;39:1108–14. [DOI] [PubMed] [Google Scholar]
- 31.Meyer EJ, Gabb G, Jesudason D. SGLT2 inhibitor-associated euglycemic diabetic ketoacidosis: a South Australian clinical case series and Australian spontaneous adverse event notifications. Diabetes Care 2018;41:e47–9. [DOI] [PubMed] [Google Scholar]
- 32.FDA drug safety communication: interim clinical trial results find increased risk of leg and foot amputations, mostly affecting the toes, with the diabetes medicine canagliflozin (Invokana, Invokamet); FDA to investigate. Silver Spring (MD): U.S. Food and Drug Administration; updated 2017 May 9. Available: www.fda.gov/Drugs/DrugSafety/ucm500965.htm (accessed 2018 Dec. 11). [Google Scholar]
- 33.American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes—2018. Diabetes Care 2018;41(Suppl 1):S73–85. [DOI] [PubMed] [Google Scholar]
- 34.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018;61:2461–98. [DOI] [PubMed] [Google Scholar]
- 35.Das SR, Everett BM, Birtcher KK, et al. 2018 ACC Expert Consensus Decision Pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 2018;72:3200–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diabetes Canada Clinical Practice Guidelines Expert Committee Lipscombe L, Booth G, Butalia S, et al. Pharmacologic glycemic management of type 2 diabetes in adults [published erratum in Can J Diabetes 2018;42:336, 575]. Can J Diabetes 2018;42:S88–103. [DOI] [PubMed] [Google Scholar]
- 37.American Diabetes Association. 11. Microvascular complications and foot care: Standards of medical care in diabetes—2019. Diabetes Care 2019;42(Suppl 1): S124–38. [DOI] [PubMed] [Google Scholar]
- 38.Griffin SJ, Leaver JK, Irving GJ. Impact of metformin on cardiovascular disease: a meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia 2017;60:1620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adler AI, Ting S, Dent R, et al. NICE guidance on dapagliflozin with insulin for type 1 diabetes. Lancet Diabetes Endocrinol 2019;7:750–1. [DOI] [PubMed] [Google Scholar]
- 40.PrJardiance [product monograph]. Burlington (ON): Boehringer Ingelheim (Canada) Ltd.; revised 2019 Apr. 11. Available: www.boehringer-ingelheim.ca/sites/ca/files/documents/jardiancepmen.pdf (accessed 2018 Dec. 11). [Google Scholar]
- 41.PrInvokana [product monograph]. Toronto: Janssen Inc.; revised 2019 Apr. 26. Available: www.janssen.com/canada/sites/www_janssen_com_canada/files/prod_files/live/invokana_cpm.pdf (accessed 2018 Dec. 11). [Google Scholar]
- 42.Cherney DZ, Udell JA. Use of sodium glucose cotransporter 2 inhibitors in the hands of cardiologists: with great power comes great responsibility [published erratum in Circulation 2017;135:e49]. Circulation 2016;134:1915–7. [DOI] [PubMed] [Google Scholar]
- 43.Handelsman Y, Henry RR, Bloomgarden ZT, et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of SGLT-2 inhibitors and diabetic ketoacidosis. Endocr Pract 2016;22:753–62. [DOI] [PubMed] [Google Scholar]
- 44.Herrington WG, Preiss D, Haynes R, et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J 2018;11:749–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019. Sept. 19 [Epub ahead of print]. 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]