IntroductIon
A major goal of neuroprosthetics is to design artificial limbs that are experienced (‘embodied’) like real limbs. However, despite important technological advances, this goal has not been reached and prosthesis embodiment is still very limited. Differently from our physical body, current bionic limbs do not provide the continuous multisensory feedback required for a limb to be experienced as one’s own. Here, we present a novel neuroprosthetic approach that combines peripheral neurotactile stimulation—inducing tactile sensation on the missing limb—and immersive digital technology—providing visual illumination of the prosthetic hand. We tested whether coherent multisensory visuo-tactile neural stimulation (VTNS)1 induced higher prosthesis embodiment and reduced the distorted perception of the phantom limb (telescoping, ie, the phantom limb is perceived as shorter than the intact limb).
Methods
Patient 1 and patient 2 are tranSRadial left forearm chronic amputees, who suffered upper limb telescoping. Patients were implanted with transverse intrafascicular multichannel electrodes (TIMEs), which induced the sensation of a vibration in a circumscribed skin region of the finger 2 via medial nerve stimulation in patient 1 (online supplementary figure 1A) and in a skin region of finger 5 via ulnar nerve stimulation in patient 2 (online supplementary figure 1B and material 1). Neurotactile stimulation2 was coupled with automatised visual illumination of a skin region on the patient’s prosthetic hand that corresponded to the somatotopic location of touch sensations experienced on the phantom hand (VTNS; online supplementary video 1, online supplementary figure 1, online supplementary material 1). VTNS was administered in two conditions, either with synchronous visual and neurotactile stimulation or in a control condition of asynchronous stimulation (1.5–2.5s delay).
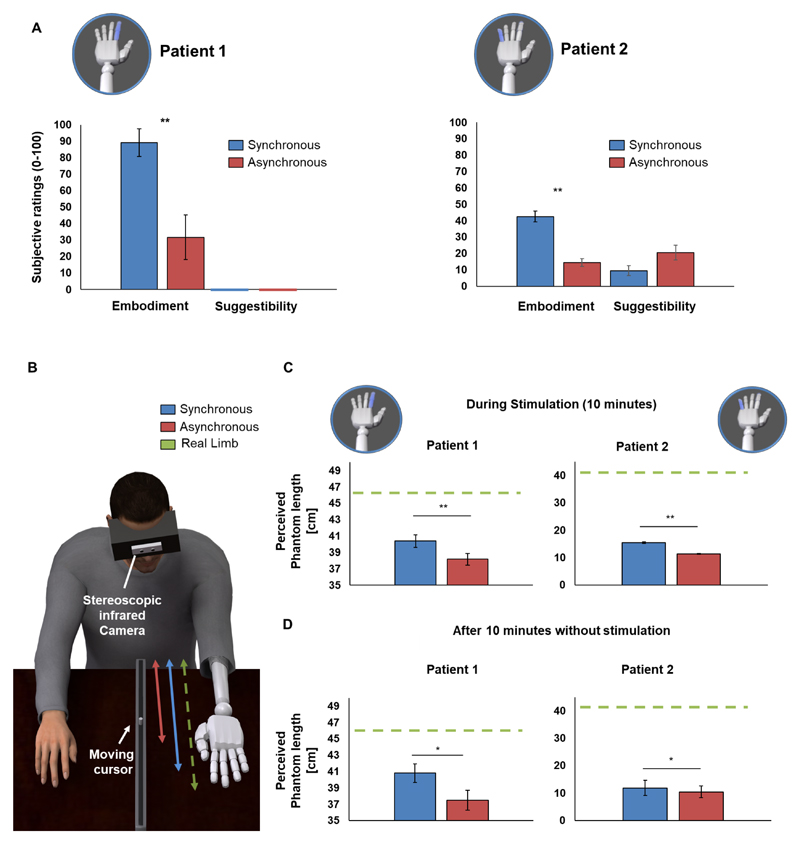
Prosthesis embodiment was measured via a questionnaire, whereas changes in phantom limb perception were tested via a body landmark task where patients indicated the perceived position of different parts of the phantom limb by moving a ruler in absence of visual stimulation (figure 1B). The experimental procedures were approved by the competent ethical committee.
Figure 1.
(A) Prosthesis embodiment. Average ratings of embodiment (Q1–3) and suggestibility items (Q4–5) are shown for all experimental conditions are shown for both patient 1 and patient 2 (see online supplementary material 1). embodiment was highest when VTNS was administered synchronously with illumination of the prosthetic hand (as compared with the asynchronous condition). the synchronous stimulation was characterised by a delay smaller than 10ms between the neurally induced tactile sensation and the visual illumination, whereas in the asynchronous condition the temporal mismatch (1.5–2.5s) between the neural stimulation and the visual illumination was randomly selected on each trial. In patient 1, suggestibility items were always rated 0 (shown as coloured lines). (B) reduction of abnormal phantom limb perceptions (telescoping). to measure the perceived length of the phantom limb, both patients were asked to operate a movable cursor inside a ruler with their right hand. the difference between the perceived position of this phantom finger and elbow was used to estimate the perceived length (in cm) of the phantom limb (average scores are reported). (C) During synchronous VTNS (blue) both perceived the tip of their phantom finger in a more distal position (vs asynchronous condition; p<0.01; red), compatible with an increase in the perceived length of their phantom limb (B; online supplementary video 2). (D) this condition-specific change in telescoping persisted, in both patients, 10min after VTNS had ended. error bars show Se of the mean. *P<0.05; **P<0.01. VTNS, visuo-tactile neural stimulation.
Results
Prosthesis embodiment
In both patients, embodiment ratings based on questionnaires revealed significantly higher scores during synchronous than asynchronous VTNS stimulation (ps<0.001; Fisher test; control items for suggestibility were not modulated: ps>0.073; figure 1A).
Reduction of abnormal phantom limb perception: telescoping
During the embodiment-inducing condition, VTNS improved telescoping (online supplementary video 1). Both patients perceived the phantom finger of the stimulated limb in a more distal position as compared with asynchronous stimulation (t=2.13, p<0.01, patient 1; t=3.6, p<0.001, patient 2; figure 1C), while there was no change in the perceived elbow position (control) between conditions (p=0.76, patient 1; p=0.099, patient 2). Thus, synchronous VTNS increased the perceived length of the phantom hand. Importantly, this effect persisted 10min after VTNS had ended (t=1.95, p=0.026, one-tailed, patient 1; t=1.94, p=0.029, one-tailed, patient 2; figure 1D). See online supplementary material 1 for extended results.
DiscussIon
By combining immersive digital technology, neuroprosthetics and paradigms from cognitive neuroscience, in two amputees, we administered direct tactile stimulation to the phantom limb via an intraneural implant into the residual limb nerves. Such stimulation, combined with personalised and coherent visual stimulation using immersive digital technology, VTNS, induced embodiment for the prosthetic hand, and importantly, reduced telescoping of the phantom limb thus improving abnormal phantom limb perceptions.
Our approach presents several advantages with respect to earlier therapeutic approaches aimed at inducing ownership in amputees,3–5 as those require the external application of tactile cues on skin regions of the residual limb, which had to be applied manually3 or via a robotic5 device as well as the concurrent application of a physical visual stimuli on the prosthetic device.3–5 This way, such procedures are difficult to apply during continuous prosthetic use in daily-life activities, thus limiting the intensity and duration of the induced prosthesis embodiment and thereby reducing their clinical relevance. Moreover, these previous studies did not test whether embodiment affected phantom limb sensations, that are critical for prosthesis acceptance.6 Our VTNS stimulation procedure shows that multisensory stimulation necessary to induce prosthesis embodiment does not need to be linked to realistic4 5 or functionally relevant interactions4 as long as VTNS respects the fundamental constraints of embodiment and multisensory integration (eg, synchronous multisensory stimulation).1
Abnormal phantom limb perceptions
Our results reveal another important clinical benefit: the reduction of telescoping. VTNS was able to reshape subjective sensations of upper limb dimensions, as to make it being perceived as more similar to the actual size of the missing limb and the prosthesis. Interestingly, previous research on phantom sensations in a large sample of amputee patients found that prosthesis embodiment sensations are more frequent in patients with an extended phantom as compared with patients with a telescoped phantom.6
Two theories have tried to account for cortical reorganisation and phantom sensations following amputation.7 8 On the one hand, the maladaptive plasticity theory posits that abnormal phantom sensations and phantom pain arise from maladaptive cortical reorganisation, triggered by loss of sensory input,7 thus associating greater pain with increased local cortical reorganisation. Recent data, on the other hand, suggest that cortical changes following limb amputation may also be due to a combination of loss/altered sensory inputs from the periphery and phantom pain experience, resulting in a maintained brain structural and functional representation of the missing limb in the sensorimotor cortices, but disturbed long-range connectivity.8 Our multisensory neuroprosthetic approach might act on either mechanism for the phantom limb syndrome and might be used to reduce telescoping, or in future studies to alleviate phantom pain, if such effects were to be found. Indeed, VTNS provides multisensory coherent bodily stimulation which may target central body representations, but also aims at restoring peripheral inputs from the residual nerves, in turn affecting long-range connectivity. At the moment, it is not possible to determine which mechanism is at the basis of the present effect.
Limitations of our study include the small number of participants tested, and the use of only one measure of embodiment (questionnaire). Future studies investigating prosthesis embodiment in amputees with peripheral neural implants should examine several aspects of embodiment in greater detail. Other limitations are the fact that the experimenters conducting the tests were not blinded to the experimental conditions and that the investigations were only carried out over a limited amount of time (ie, for several hours over different days).
Taken together, our results open up new opportunities to enhance prosthetic acceptance and advance the engineering of personalised artificial limbs that, by providing continuous multisensory feedback, might feel like real limbs.
Supplementary Material
Acknowledgements
The Authors are deeply grateful to the two patients who freely donated weeks of their life for the advancement of knowledge and for a better future of People with hand amputation. the Authors are also grateful to Professor Fernandez for the surgical implantation of the TIMEs.
Funding This work was partly supported by the EU Grant CP-FP-INFSO 224012 TIME project (transverse Intrafascicular Multichannel electrode system), by the EU Grant FET 611687 NEBIAS Project (Neurocontrolled BIdirectional Artificial upper limb and hand prosthesiS), by the EU Grant Health 602547 EPIONE project (Natural sensory feedback for phantom limb pain modulation and therapy), by the EU Grant ERC-STG 678908 rESHAPE project, by the project NEMESIS (Neurocontrolled mechatronic hand prosthesis) funded by the Italian Ministry of Health, by the Bertarelli Foundation, by the Wyss Center for Bio and Neuroengineering and by the Swiss National Competence Center in research (NCCR) in robotics.
Footnotes
Contributors GR designed the study, performed the experiments, analysed the data and wrote the paper. FMP and SR developed the software for the neural stimulation, contributed to the design of the study, performed the experiments and wrote the paper (GR, FMP and SR equally contributed to the work). GG, RDI, IS, GV and ED collaborated during the development of the neural stimulation software and during the experiment. MS designed, performed and analysed the telescoping experiment. RM developed the augmented reality software and integrated it with the neural stimulation device. JB-R and BH integrated the augmented reality software with the neural stimulation device, contributed to the design of the experiments and performed part of the experiments. GDP selected the patients and collaborated during the experiments. TS developed the TIME electrodes. DA and DG developed the device for the neural stimulation. PMR selected the patients and supervised the experiments. AS designed the study, performed the experiments, analysed the data and wrote the paper. SM and OB designed the study, supervised the experiments and wrote the paper (AS, SM and OB equally contributed to the work). All the authors read and approved the manuscript.
Competing Interests SM, SR, FMP hold shares of Sensars Neuroprosthetics, a company working to commercialise novel solutions for tranSRadial amputees.
Patient consent Not required.
Ethics approval The experimental procedures were approved by both the Institutional ethics Committees of Policlinic A Gemelli at Catholic University and the IRCCS S raffaele Pisana (rome). Informed consent was obtained from both patients.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Blanke O, Slater M, Serino A. Behavioral, neural, and computational principles of bodily self-consciousness. Neuron. 2015;88:145–66. doi: 10.1016/j.neuron.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 2.Raspopovic S, Capogrosso M, Petrini FM, et al. Restoring natural sensory feedback in real-time bidirectional hand prostheses. Sci Transl Med. 2014;6:ra219. doi: 10.1126/scitranslmed.3006820. [DOI] [PubMed] [Google Scholar]
- 3.Ehrsson HH, Rosén B, Stockselius A, et al. Upper limb amputees can be induced to experience a rubber hand as their own. Brain. 2008;131(Pt 12):3443–52. doi: 10.1093/brain/awn297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiefer M, Tan D, Sidek SM, et al. Sensory feedback by peripheral nerve stimulation improves task performance in individuals with upper limb loss using a myoelectric prosthesis. J Neural Eng. 2016;13 doi: 10.1088/1741-2560/13/1/016001. 016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marasco PD, Kim K, Colgate JE, et al. Robotic touch shifts perception of embodiment to a prosthesis in targeted reinnervation amputees. Brain. 2011;134(Pt 3):747–58. doi: 10.1093/brain/awq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giummarra MJ, Georgiou-Karistianis N, Nicholls ME, et al. Corporeal awareness and proprioceptive sense of the phantom. Br J Psychol. 2010;101(Pt 4):791–808. doi: 10.1348/000712610X492558. [DOI] [PubMed] [Google Scholar]
- 7.Flor H, Denke C, Schaefer M, et al. Effect of sensory discrimination training on cortical reorganisation and phantom limb pain. Lancet. 2001;357:1763–4. doi: 10.1016/S0140-6736(00)04890-X. [DOI] [PubMed] [Google Scholar]
- 8.Makin TR, Scholz J, Filippini N, et al. Phantom pain is associated with preserved structure and function in the former hand area. Nat Commun. 2013;4 doi: 10.1038/ncomms2571. 1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.