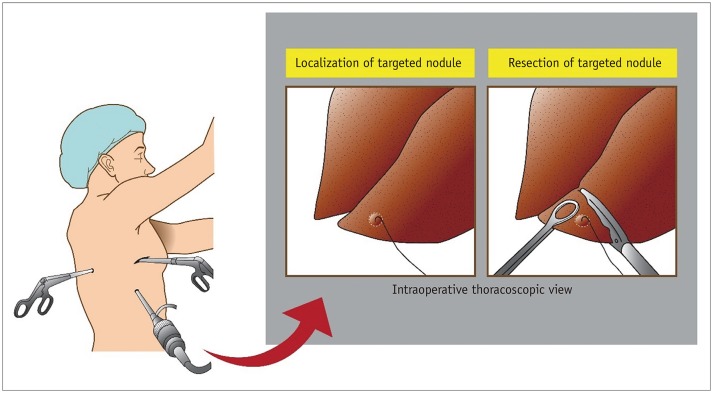
Abstract
Image-guided localization materials are constantly evolving, providing options for the localization of small pulmonary nodules to guide minimally invasive thoracic surgery. Several preoperative methods have been developed to localize small pulmonary lesions prior to video-assisted thoracic surgery. These localization techniques can be categorized into 4 groups according to the materials used: localization with metallic materials (hook-wire, microcoil, or spiral coil), localization with dye (methylene blue or indigo carmine), localization with contrast agents (lipiodol, barium, or iodine contrast agents), and radiotracers (technetium-99m). However, the optimal localization method has not yet been established. In this review article, we discuss the various localization techniques and the advantages and disadvantages of localization techniques as well as the available safety and efficacy data on these techniques.
Keywords: Localization; Tomography, X-ray computed; Ultrasonography; Coloring agents; Contrast media; Thoracic surgery, video-assisted
INTRODUCTION
The increasing use of low-dose chest computed tomography (CT) scans and the implementation of CT screening for lung cancer have made it increasingly important to diagnose and manage screen-detected solitary pulmonary nodules (SPNs) (1). Management of SPNs, especially subsolid nodules, is particularly challenging because of their malignant potential and heterogeneous characteristics (2,3). Minimally invasive tissue biopsies and the localization of SPNs for surgery are new and developing fields that will yield improvements in both diagnosis and treatment. Video-assisted thoracoscopic surgery (VATS) has become the optimal method for diagnosis and treatment of pulmonary nodules because of its minimal invasiveness and safety (4). Pulmonary nodules that are small or distant from the pleural surface may be difficult to detect during thoracoscopic surgery because they cannot be seen on the pleural surface or detected either by palpation or with endoscopic forceps. In addition, the geometric relationships of the target nodule to anatomic landmarks on the skin surface and within the chest, as previously determined by CT scanning, are distorted by the lung collapse and pneumothorax that occur during the procedure. In fact, failure to localize small and relatively deep pulmonary nodules often leads to conversion to open thoracotomy, and it is the most common cause of VATS failure (5). Therefore, accurate localization of small or deeply located pulmonary nodules before VATS is very important to prevent conversion to thoracotomy. The usefulness of preoperative localization has been well established in the context of a higher successful VATS rate, shorter operative time, and increased economic benefits (6,7). According to a meta-analysis comparing the effectiveness and safety of various lung localization techniques, the success rate of VATS was 96–99% when using lung localization methods such as hook-wire, microcoil, and lipiodol (8). Various preoperative localization techniques for small pulmonary nodules or subsolid nodules have been developed in an attempt to improve the accuracy of thoracoscopic resection. Image-guided localization methods primarily include three imaging tools: CT, bronchoscopy, and ultrasonography. These localization techniques can be categorized into 4 groups according to the materials used: localization with metallic materials (hook-wire, microcoil, or spiral coil), localization with dye (methylene blue or indigo carmine), localization with contrast agents (lipiodol, barium, or iodine contrast agents), and radiotracers (technetium-99m, 99mTc). Each procedure has different characteristics and shows both advantages and drawbacks related to their unique characteristics (Table 1). Currently, hook-wire localization is the most widely used method, whereas localization with lipiodol and microcoil has recently been reported to be accurate and safe.
Table 1. Summary of CT-Guided Localization Techniques according to Materials Used.
| Methods | Advantages | Disadvantages |
|---|---|---|
| Hook-wire | Simple and most commonly used methods | Patient discomfort or pain because of wire |
| No need for fluoroscopic guidance during surgery | Risk of wire dislodgement or migration | |
| Risk of air embolism | ||
| Microcoil or spiral coil | Can safely remain in body for relative long time | Need fluoroscopic guidance during surgery for coil detection |
| Reduced patient discomfort or pain compared to hook-wire | Risk of migration | |
| Does not interfere histopathologic examination | ||
| Lower procedure related complication rate compared to hook-wire | ||
| Dye (methylene blue or indigo carmine) | Simple and less costly methods than wire-related techniques | Rapid dye diffusion: Localization must be performed within 3 hours before surgery |
| No need for fluoroscopic guidance during surgery | Limited information on lesion depth | |
| Difficulty in identifying dye in case of extensive anthracotic pigmentation | ||
| Barium | Simple and less costly methods than wire or coil techniques | Can affect pathologic diagnosis because of inflammatory response |
| Remain stable in body for relative long time without diffusion | Need fluoroscopic guidance during surgery | |
| Provide information on lesion depth | ||
| Lipiodol | Simple and easy methods | Need fluoroscopic guidance during surgery |
| Remain stable in body for relative long time without diffusion | Risk of spillage to pleural space | |
| Do not cause inflammatory response in pathologic tissue | ||
| Provide information on lesion depth | ||
| Radiotracer (technetium-99m) | Simple and less costly methods | Short half-life (about 6 hours) |
| Remain stable in body for longer time (1 day) than dye | Needs gamma probe | |
| Radiation exposure | ||
| Risk of spillage to pleural space |
In this review article, we will focus on the various localization techniques based on CT and the advantages and disadvantages of these techniques as well as their available safety and efficacy data. Then, we will briefly review localization methods based on bronchoscopy and ultrasonography.
CT-Guided Techniques
Image-guided localization techniques can be categorized into 4 groups according to the materials used: localization with metallic materials (hook-wire, microcoil, or spiral coil), dyes (methylene blue or indigo carmine), contrast agents (lipiodol, barium, or iodine contrast agents), and radiotracers (99mTc).
Localization with Metallic Materials
Several guidewires, including hook-wires and microcoils, can be used for lung localization, and all of them have been shown to be effective in small pulmonary nodule localization. Hook-wire localization is the most commonly used localization method for VATS resection (9). The hook-wire is usually positioned in the lung parenchyma close to the targeted nodule. After administration of local anesthesia, the cannula needle of the hook-wire is inserted gradually into the lesion under CT guidance. When the cannula needle is inserted into the lesion, the outer cannula needle is removed, and the horn of the hook-wire is anchored to the lesion (Fig. 1). Once the hook-wire is in position, the part of it extending outside the chest wall is positioned on the skin under gauze dressings. Localization with the hook-wire is usually performed just before the patient is sent to the operating room in order minimize patient discomfort and complications (9). CT-guided hook-wire localization is considered to be both safe and effective for VATS resection. Nevertheless, the major drawbacks of hook-wires are that they should be positioned from the lung to the skin surface after the localization procedure until the operation, and dislodgement or migration could occur, which can hamper successful operative targeting (10). Moreover, there are some specific anatomic locations where this technique cannot be performed. For instance, if the small pulmonary nodule is in close proximity to the mediastinal great vessels, although it is technically feasible to perform CT-guided localization, the risk of hemorrhage is higher because of the sharp point of the hook-wire. Moreover, several studies have also reported that hook-wire localization can produce a massive air embolism (11,12).
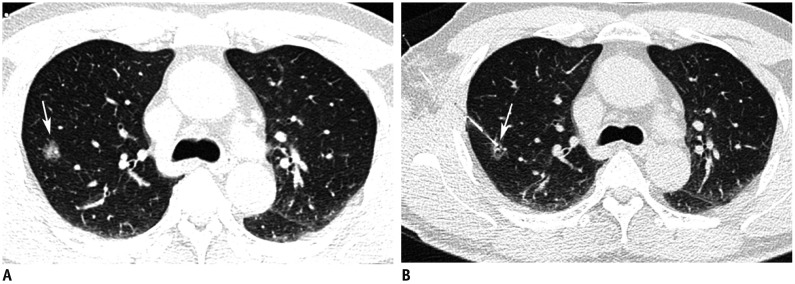
Fig. 1. Preoperative hook-wire localization for part-solid nodule in right upper lobe in 59-year-old man.
A. Axial CT image with lung window shows 15-mm part-solid nodule (arrow) in right upper lobe. B. Post-procedure axial CT image with lung window shows that horn of hook-wire is positioned in target nodule (arrow).
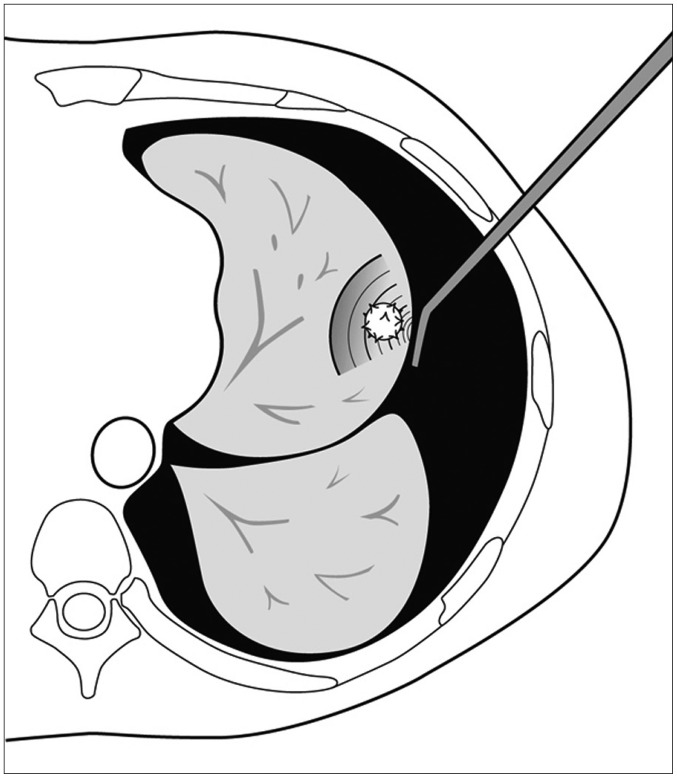
Microcoils, which are usually intended for embolization of selective vessels, have been used for localization since 1994 (13). For needle preparation, a fiber-coated platinum microcoil is pushed into the coaxial needle by using a stiff end of the guidewire. With CT guidance, a loaded coaxial needle is pushed 5 mm deep to the targeted nodule and a microcoil is deployed into the lung parenchyma. Two methods are used to deploy the microcoil (Fig. 2). In one method, the entire microcoil is inserted into the lung tissue. In the other method, the microcoil is partially inserted, and the end tail of the microcoil remains above the visceral pleura based on the distance from the lesion to the pleura. Su et al. (14) compared the entire microcoil implantation method with the leaving-microcoil-end implantation method and reported no significant difference in complications and efficacy between the two methods.
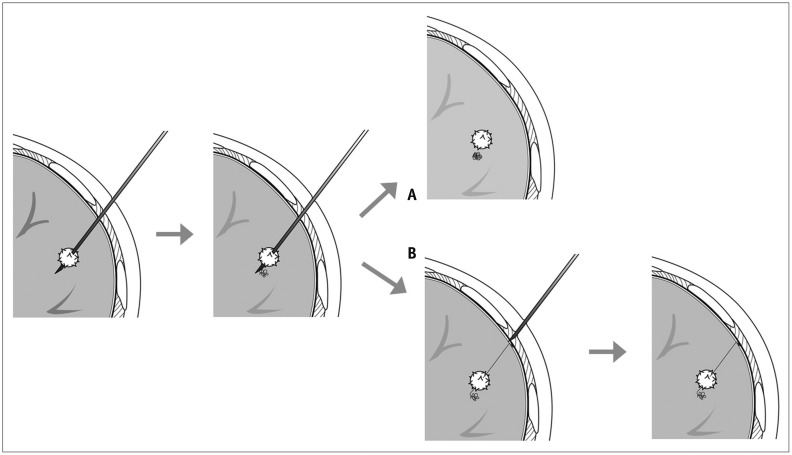
Fig. 2. Diagrams showing two localization methods using microcoil.
A. Drawing illustrates entire microcoil was inserted into lung tissue. B. Drawing illustrates that microcoil was partially inserted, and end tail of microcoil remained above visceral pleura.
Microcoils have several advantages. They are a clinically-proven material that can be safely retained in the human body for a long time and are not easily detached, permitting delayed surgery after the localization. Furthermore, microcoils do not interfere with histopathologic examination, even when they are passed through the nodule (15). Unlike localization with a hook-wire, no wire is left outside the chest wall after CT-guided localization, resulting in lower discomfort. Another advantage of microcoil localization was the lower rate of procedure-related complications such as pneumothorax and hemorrhage in comparison with hook-wire localization. The structural properties of a microcoil, which is composed of platinum and a thrombogenic fiber coating, might reduce the risk of procedure-related complications (16). One drawback of this localization technique is that it requires fluoroscopic guidance during the VATS procedure and results in radiation exposure for surgeons (9). In addition, migration of an implanted microcoil in the thoracic space is possible during an operation and could result in procedure failure; however, this occurs less frequently than hook-wire dislodgement (8).
Localization with Dye
Methylene blue or indigo carmine has been used alone or in combination with other localization techniques such as hook-wires or lipiodol (Fig. 3). Under CT guidance, the coaxial needle is placed into the nodule or as close to it as possible. After confirming the position of the needle tip, 0.3 mL of sterile dye is injected into the nodule and again during needle removal to stain the needle pathway and the visceral pleura (17). Dye localization shows high sensitivity for nodule localization, and the dye can be directly visualized during the VATS. Moreover, it is less costly than wire-related techniques and obviates wire-related complications. The accuracy of coloration of the targeted area is strongly influenced by the time between tumor localization and thoracoscopy. Rapid dye diffusion around the tissue after injection and limited information on lesion depth are the major drawbacks of this localization technique (18). Because of rapid dye diffusion, localization must be performed within 3 hours before surgery to enable the dye to be seen (19). Another drawback of methylene blue or indigo carmine is the difficulty in identifying the dye in patients with extensive anthracotic pigmentation (17).
Fig. 3. Preoperative localization with indigo carmine for part-solid nodule in left upper lobe in 60-year-old woman.
A. Axial CT image with lung window during localization shows 22-gauge Chiba needle introduced into nodule (arrow). B. Video-assisted thoracoscopic image shows deposition of indigo carmine as purple area around inserted area of needle (arrow).
Localization with Contrast Agents
Water-insoluble contrast agents, including barium sulfate and lipiodol, can be used to localize the lung nodule by CT-guided percutaneous needle injection or by CT-guided bronchoscopic injection (20). Several studies have reported that barium localization using bronchoscopy is an easy and accurate method for small lung nodule localization (21,22). With CT guidance, the needle tip is placed within 1 cm of the nodule, the inner stylet of the needle is withdrawn and a small amount (0.1–0.3 mL) of 140% w/v (weight-to-volume) barium sulfate suspension is injected through the needle (20). A localization technique using barium suspension can be much simpler to perform with no risk of dislodgement or migration compared to hook-wire localization. In addition, the barium ball appears white visually, is radiopaque on fluoroscopy, and can be palpated during the operation, making detection more accurate and easier. Another advantage of barium localization is that barium remains in the body for a relatively longer period of time with no diffusion, in comparison with dye localization. Therefore, this technique can allow flexible scheduling in the operation room. However, several studies have reported that barium induces mild acute inflammatory and edematous changes in the lung parenchyma and, therefore, can cause difficulty in the pathologic diagnosis of the target nodules (21,22,23). When using barium materials for lung nodule localization, care should be taken to avoid directly injecting the barium into the target nodule, especially in cases involving subsolid nodules or potential inflammatory lesions, as this may make the pathologic diagnosis of the target nodule difficult due to the acute inflammation associated with barium materials.
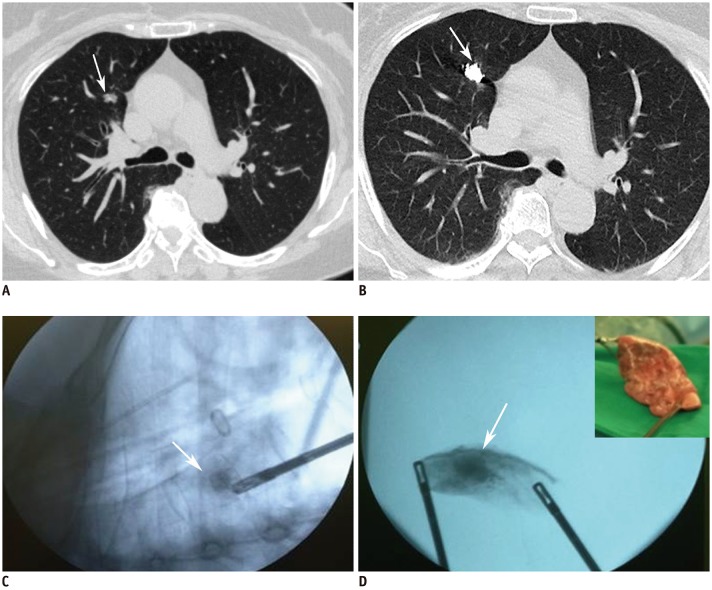
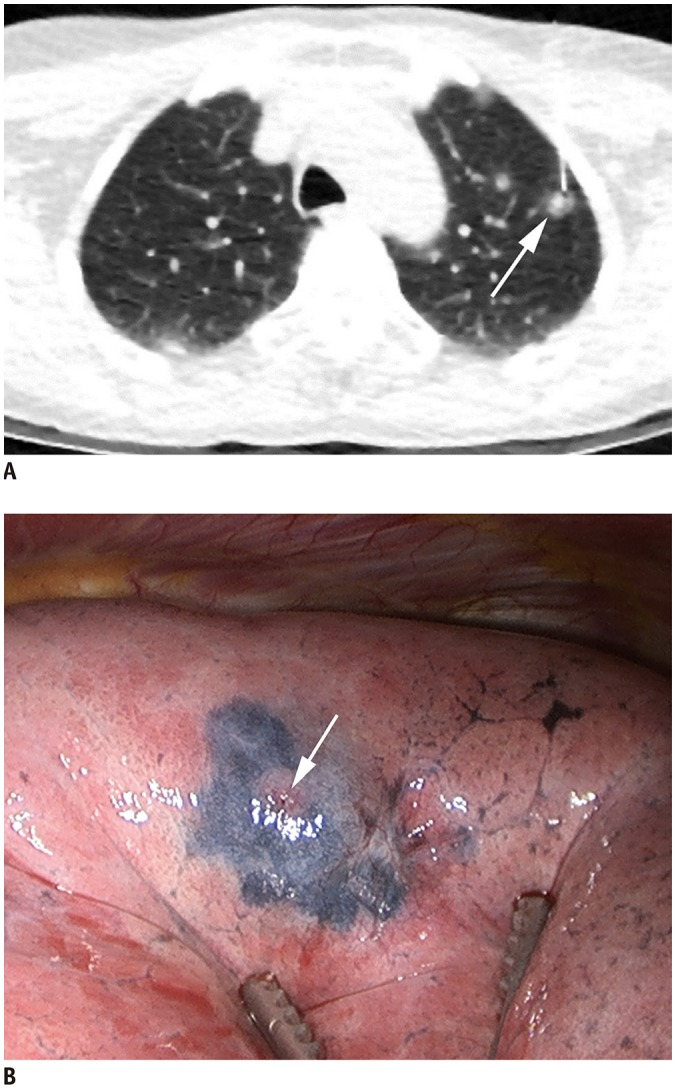
Lipiodol is a lipid-soluble radiopaque contrast medium that can be used for pulmonary nodule localization. After local anesthesia of the thoracic wall, a needle is introduced into the nodules. After drawing back on the syringe to confirm that blood does not flow backwards, a small amount (0.2–0.5 mL) of lipiodol is injected (Fig. 4) (24). Indigo carmine or colored collagen can be additionally used to mark the pleural surface (24,25). Lipiodol localization offers the following advantages. First, lipiodol does not trigger an inflammatory response, which could affect pathologic findings. Second, diffusion of lipiodol is minimal, and lipiodol localization persists for up to 3 months, which solves the problem of requiring both CT and the operating room simultaneously. Third, this is a simple and easy method that can also provide information about nodule depth (26). The limitations of water-insoluble agents such as barium and lipiodol include the fact that fluoroscopy is required to detect the location of them at the time of surgery. Moreover, embolism can occur because of the insolubility of the materials in water. To reduce the risk of embolism caused by lipiodol, after withdrawing the syringe to confirm that no blood has flowed backward, it is recommended that a minimal amount (not exceeding 0.5 mL) should be injected to avoid its spread into vessels (26). Lastly, spillage to the pleural space can occur. However, it does not cause surgical failure (Fig. 5) (27).
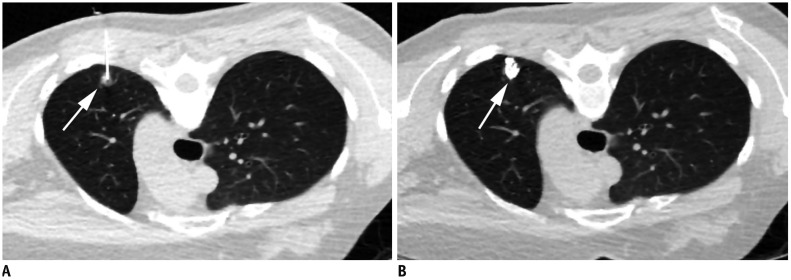
Fig. 4. Preoperative lipiodol localization for part-solid nodule in right upper lobe in 48-year-old woman.
A. Axial CT image with lung window during lipiodol localization in prone position shows 22-gauge Chiba needle introduced into nodule (arrow) in left upper lobe. B. Post-procedure axial CT image with lung window shows lipiodol accumulation (arrow) in target nodule after procedure.
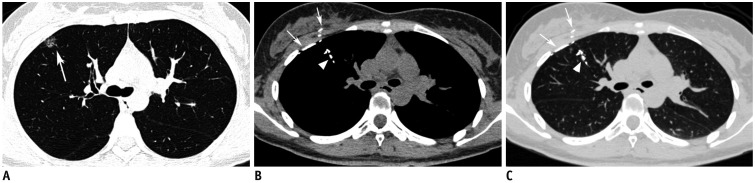
Fig. 5. Preoperative lipiodol localization for part-solid nodule in right upper lobe in 35-year-old woman.
A. Axial CT image with lung window shows 15-mm part-solid nodule (arrow) in right upper lobe. Post-procedure axial CT images with mediastinal (B) and lung (C) windows demonstrate lipiodol spillage to right pleural, right chest wall (arrows) and bronchi of right upper lobe (arrowheads). Thoracoscopic wedge resection was successfully performed.
Localization with Radiotracers
Gamma-emitting radioisotopes (99mTc) attached to large albumin molecules can be used for localization under CT guidance (28,29,30). On the day of the operation, 0.2 mL of 99mTc-labeled human serum albumin microspheres is injected as close to the lesion as possible through the thoracic wall with a 22-gauge needle under local anesthesia (30). Some centers add 0.1–0.2 mL of nonionic contrast to the radiotracer to confirm the presence of radiotracer within the nodule on post-procedure CT scans (28,31). During the operation, the radiotracer probe converts the gamma ray emissions into audiovisual signals proportional to the detected radioactivity. The strongest signal reflects the areas of the targeted nodule (9).
99mTc is a low-cost radiotracer and does not affect the pathologic findings. Although it remains stable in the body for a longer period of time (1 day) than dyes, its half-life of about 6 hours could lead to some difficulties in scheduling the localization procedure and the operation. This technique requires additional facilities such as gamma probe radiation protection equipment. The other disadvantages include radiation exposure and spillage (9).
Surgical Techniques after Localization
Thoracoscopic surgery is performed after localization under general anesthesia using single-lung ventilation via a double-lumen endobronchial tube. Thoracoscopic surgery is performed with a 3–5-cm utility incision at the anterior axillary line at the fourth or fifth intercostal space using endoscopic instruments without rib spreading and two or three additional ports for the camera, stapler insertion, and assistant. The operation is performed entirely with thoracoscopic visualization. In cases involving hook-wire localization, gauze dressings of the hook-wire are unpacked before collapse of the ipsilateral lung. After identification of the marked nodule either by thoracoscopic vision or by a C-arm fluoroscope, thoracoscopic wedge resection is performed using an endoscopic stapler with a 1–2-cm margin from the lesion. In cases involving hook-wire or dye localization, surgeons can visually identify the localized site directly without intraoperative fluoroscopy and radiation exposure (9). Using a hook-wire, light traction on the suture thread during VATS resection renders deep-seated lesions more superficial and facilitates complete excision by an endostapler (Fig. 6). When using contrast agents as localization materials, intraoperative fluoroscopy is used to detect the nodule. The nodules are visualized as radiopaque spots in multiple projections. After wedge resection or segmentectomy, successful resection is confirmed by inspecting resected specimens for the presence of radiopaque spots identified by intraoperative fluoroscopy (Fig. 7). All resected specimens are sent for frozen-section examination. If the pathological assessment of frozen sections reveals primary lung cancer, thoracoscopic lobectomy or segmentectomy with lymph node dissection is additionally performed. The prognosis of the patient is dependent on the surgical resection margin (32). Several studies have demonstrated that an increased margin distance is associated with a lower recurrence rate and longer survival in non-small-cell lung cancer (33,34). Therefore, the surgeons make the necessary efforts to secure a sufficient resection margin from the lesion. In cases involving hook-wire localization, it is difficult to determine the exact location of the lesion in the lung parenchyma, if the lesion is untouched, even though it is marked. Therefore, in such cases, the surgeon should resect the periphery of the marked position of the hook-wire by using the stapler along the imaginary margin. If the localization was not correctly performed or the wire migrated from the lesion, the surgical margin cannot be secured sufficiently. However, in cases involving localization with a contrast agent or microcoil, the procedure is performed while visualizing the exact location of the lesion using the C-arm in the operating field.
Fig. 6. Video-assisted thoracoscopic surgery after localization using hook-wire.
Thoracoscopic surgery is performed with 3–5-cm utility incision at anterior axillary line at fourth or fifth intercostal space using endoscopic instruments without rib spreading and additional 2 or 3 ports for camera, stapler insertion, and assistant. After identification of marked nodule by thoracoscopic vision, thoracoscopic wedge resection is performed using endoscopic stapler with 1–2-cm margin from lesion.
Fig. 7. Images in 65-year-old woman undergoing lipiodol localization for part-solid nodule in right upper lobe.
A. Axial CT image with lung window shows 11-mm part-solid nodule (arrow) in right upper lobe. B. Post-procedure axial CT image with lung window shows lipiodol accumulation (arrow) in right upper lobe. C. Intraoperative fluoroscopic images obtained during resection demonstrate radiopaque spot (arrow) representing lipiodol accumulation. D. After wedge resection, successful resection can be confirmed by identifying radiopaque spot (arrow) by intraoperative fluoroscopy.
Outcomes
Success Rate
Table 2 summarizes the success rates of various CT-guided localization techniques. The successful targeting rate using CT-guided hook-wire technique has been reported to range between 97.5% and 100%, and dislodgement was observed in 0.4–4.6% of patients who completed hook-wire localization (35,36,37,38,39,40,41,42,43,44,45) (Table 2). Wire dislodgement can occur in the following situations: during transportation of the patient to the operating room; during surgical deflation of the lung; or when the surgeon applies gentle retraction on the wire during the resection. Therefore, physicians should be careful in these situations (46). A previous study investigated and analyzed the factors related to the successful use of CT-guided hook-wire in 174 patients with small pulmonary nodules. According to their study, the distance between the wire tip and pleural surface was an independent factor for successful localization in multivariate analysis including demographic, nodule-related, and technical factors (38). Therefore, localization of a hook-wire with sufficient depth (more than 1 cm) from the pleural surface improves the success of VATS procedures, and CT confirmation before and after deployment of the hook-wire is important (35,38). When we used a short hook-wire, we could reduce the risk of dislodgement as well as pleural pain in comparison with procedures using a conventional long hook-wire (35,36). The success rate of VATS with a hook-wire ranged between 92.8% and 100%, with a thoracotomy conversion rate of up to 7.2% (Table 2) (35,36,37,38,39,40,41,42,43,44,45).
Table 2. Summary of Studies on CT-Guided Localization Techniques Since 2000.
| Methods | Studies | Nodule | Localization Success Rate (%) | Dislodgement, Migration, or Spillage Rate (%) | VATS Success Rate (%) | Conversion Rate to Thoracotomy (%) | Complications (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Diameter Number (mm) | Image | Surgical | Pneumothorax (Requiring Chest Tube) | Hemorrhage | Hemothorax | Air Embolism | |||||
| Hook-wire | Dendo et al. 2002 (35) | 168 | NS (2–30) | NS | 97.6 | 2.4 | 100.0 | 0 | 32.1 | 14.9 | 0.6 | 0 |
| Miyoshi et al. 2009 (36) | 125 | 9 (3–35) | 100.0 | 99.2 | 0.8 | 99.2 | 0.8 | NS (3.7) | NS | 0 | 0 | |
| Li et al. 2012 (37) | 107 | 13 (4–25) | 100.0 | 97.1 | 2.9 | 98.1 | 1.9 | 36.9 | 40.8 | 0 | 0 | |
| Seo et al. 2012 (38) | 174 | 10 (< 30) | NS | 95.4 | 4.6 | 98.3 | 1.7 | 39.7 | 35.6 | 0 | 0 | |
| Ichinose et al. 2013 (39) | 500 | NS | 100.0 | 99.6 | 0.4 | NS | NS | 68.0 (4.6) | 8.9 | 0 | 0.2 | |
| Suzuki et al. 2014 (44) | 161 | 10 (2–34) | 97.5 | 97.5 | 0.6 | 100.0 | 0 | 37.9 | 41.6 | 0 | 0.6 | |
| Hanauer et al. 2016 (40) | 187 | 10 (4–29) | 100.0 | 96.3 | 3.7 | 92.8 | 7.2 | 38.0 (2.1) | 5.9 | 0 | 0 | |
| Huang et al. 2017 (41) | 273 | 12 (4–30) | 100.0 | 97.8 | 2.2 | 99.6 | 0.4 | 5.9 | 27.1 | 0 | 0 | |
| Klinkenberg et al. 2017 (42) | 150 | 9* (4–24) | 100.0 | 99.3 | 0.7 | 95.3 | 4.7 | 29.3 (6.0) | 1.3 | 2.0 | 0 | |
| Yao et al. 2018 (43) | 105 | 10 (3–19) | 100.0 | 98.1 | 1.9 | 100.0 | 0 | 18.9 | 7.4 | 0 | 0 | |
| Microcoil | Mayo et al. 2009 (15) | 75 | 12 (4–24) | 100.0 | 97.3 | 2.7 | 97.3 | 2.7 | 74.6 (2.8) | 6.7 | 1.4 | 0 |
| Su et al. 2015 (14) | 101 | 9 (2–26) | 98.0 | 98.0 | 2.0 | 100.0 | 0 | 15.8 | 8.9 | 0 | 0 | |
| Sui et al. 2015 (16) | 98 | 10 (4–26) | 100.0 | 96.9 | 3.1 | 100.0 | 0 | 13.3 (1.0) | 4.1 | 0 | 0 | |
| Kha et al. 2016 (48) | 64 | 12 (NS) | 100.0 | 98.4 | 1.6 | 95.3 | 4.7 | 68.8 (1.6) | 45.3 | 0 | 0 | |
| Hajjar et al. 2017 (47) | 74 | 6 (< 20) | 100.0 | 97.3 | 2.7 | 100.0 | 0 | 4.1 | 0 | 0 | 0 | |
| Wang et al. 2018 (49) | 42 | NS (4–10) | 100.0 | 100.0 | 0 | 100.0 | 0 | 21.4 | 19.0 | 0 | 0 | |
| Methylene blue | Stephenson et al. 2015 (51) | 30 | 8 (4–18) | 100.0 | 93.3 | NS | 90.0 | 10.0 | 6.7 | 0 | 0 | 0 |
| Indigo carmine | Shimamura et al. 2018 (52) | 21 | 14 (6–27) | 100.0 | 100.0 | NS | 100.0 | 0 | 9.5 | 0 | 0 | 0 |
| Barium | Lee et al. 2012 (20) | 10 | 8 (3–14) | 100.0 | 100.0 | 0 | 100.0 | 0 | 20.0 (10.0) | 0 | 0 | 0 |
| Lipiodol | Watanabe et al. 2006 (56) | 174 | 10 (2–30) | 100.0 | 100.0 | 0 | 100.0 | 0 | 17.0 (6.0) | 0 | 0.6 | 0 |
| Kawanaka et al. 2009 (26) | 107 | 9 (3–27) | 100.0 | 100.0 | 0 | 100.0 | 0 | 30.8 (4.6) | 15.4 | 0 | 0 | |
| Kim et al. 2011 (27) | 68 | 11 (3–28) | 98.5 | 98.5 | 1.5 | 100.0 | 0 | 29.4 | 7.4 | 0 | 0 | |
| Mogi et al. 2015 (57) | 60 | 11 (3–20) | 100.0 | 98.2 | 0 | 98.2 | 1.8 | 37.5 (1.8) | 16.1 | 0 | 0 | |
| Technetium-99m | Chella et al. 2000 (29) | 39 | 8 (4–19) | 100.0 | 100.0 | NS | 100.0 | 0 | 15.4 | NS | 0 | 0 |
| Ambrogi et al. 2012 (28) | 211 | 8 (0.3–28) | 98.6 | 98.6 | 0 | 98.6 | 1.4 | 10.4 | 0 | 0 | 0 | |
All parameters recorded as reported percentage rates. If there are no reported percentages, parameters were defined as follows: Localization success rate (image) is defined as (number of successful targeting procedures during procedure/number of all localization procedures) × 100. Localization success rate (surgical) is defined as (number of visible target in operative field/number of all localization procedures) × 100. Dislodgement, migration, or spillage rate is defined as (number of dislodgements, migration, or spillage/number of all localization procedures) × 100. Conversion rate is defined as number of (conversion to thoracotomy/number of all localization procedures) × 100. Nodule diameter represents mean (range). *Median. NS = not stated, VATS = video-assisted thoracoscopic surgery
For microcoil localization, the rates of successful targeting during the localization procedure and successful localization in the operative field were 98–100% and 96.9–100%, respectively (Table 2) (14,15,47,48,49). The reported migration rate was 0%–3.1%. The insertion depth of the coaxial needle and absence of pneumothorax after localization were independent factors for successful microcoil localization (50). The success rate of VATS with microcoil localization was reported to be between 95.3–100%, with a thoracostomy conversion rate of 0–4.7% (14,15,47,48,49). A previous randomized controlled study investigated whether CT-guided microcoil localization decreases the need for thoracotomy for pulmonary lesion diagnosis. In this study, 60 patients with undiagnosed nodules ≤ 15 mm in diameter were randomized to either no localization or preoperative microcoil localization. According to this study, preoperative CT-guided microcoil localization increased the success rate of VATS excision of small lung nodules for pathologic diagnosis to 93% (27/29 patients), in comparison with a rate of 48% (13/27 patients) without guidance with no difference in total costs (7).
For dye localization, the rates of successful targeting during the localization procedure and successful localization in the operative field were 100% and 93.3–100%, respectively (Table 2) (51,52). In a study of 30 small metastatic nodules marked with methylene blue, Stephenson et al. (51) reported that 93.3% of the marked nodules could be detected in the operative field and thoracoscopic resection was possible in 90% of the nodules. The major disadvantage of dye localization is rapid diffusion of dye into the surrounding lung parenchyma. To avoid rapid dye diffusion, methylene blue-stained autologous blood or patent blue vital dye can be used with a high success rate (53,54). A study by Kleedehn et al. (55) comparing the success and complication rates of methylene blue injection and hook-wire insertion suggested that these two techniques are statistically equivalent for preoperative pulmonary nodule localization.
Lipiodol localization has been reported to be accurate and safe and is associated with a high success rate. Successful targeting rate of lipiodol localization was reported between 98.5–100% and successful localization rate and VATS success rate ranged between 98.2–100% (Table 2) (26,27,56,57,58). A recent meta-analysis that compared the effectiveness of three preoperative lung localization methods reported that the rates of successful targeting during the localization procedure were 96%, 98%, and 99% for the hook-wire, microcoil, and lipiodol techniques, respectively. In this study, the successful localization rate of 95% in the operative field of the hook-wire technique was relatively lower than that achieved with microcoil (97%) and lipiodol (99%) techniques because of dislodgement or migration (8). Only one study has reported the success rate of CT-guided barium localization. In this study, 10 patients with small pulmonary nodules (mean diameter, 7.6 mm) were included and CT-guided barium localization was performed before VATS resection. According to this study, CT-guided barium localization showed a 100% successful targeting rate and VATS success rate (20).
The rates of successful localization and successful VATS with radiotracers were reported to 98.6–100% and 98.6–100% (28,29). Doo et al. (59) attempted dual localization with radiotracer and hook-wire using CT fluoroscopy for 36 pulmonary nodules (mean diameter, 12.5 mm). Dual localization was successfully performed for all nodules without major complications, and all nodules were successfully resected without conversion to open thoracotomy.
Complications after Localization
CT-guided localization techniques using metallic materials, dye, and contrast media are relatively safe procedures with a low reported incidence of complications. Nevertheless, these techniques are associated with complications such as pneumothorax, parenchymal hemorrhage, hemothorax, and air embolism (Table 2).
Pneumothorax is the most common complication of CT-guided localization. The majority of the cases of pneumothorax are asymptomatic and 1.0–10% of cases require chest tube drainage (Table 2, Fig. 8) (15,16,20,35,36,39,40,42,45,48,56,57). Chest tube placement is indicated if a postbiopsy pneumothorax becomes symptomatic or continues to show enlargement on follow-up chest radiography, which is usually performed 1–3 hours after the procedure. According to a meta-analysis, the mean pneumothorax rate associated with hook-wire localization (35%) was higher than those associated with microcoil (16%) and lipiodol localization (31%). Microcoil localization is associated with the lowest rate of pneumothorax among the three localization techniques (8). The structural property of a microcoil, which is composed of a thrombogenic fiber coating, might reduce the risk of procedure-related complications. The thrombogenic coating of synthetic nylon fibers on the surface of the microcoil may promote blood coagulation of the surrounding lung tissues, block the needle pathway, and decrease the severity of pneumothorax (16). Iguchi et al. (60) evaluated the risk factors for pneumothorax in 267 short hook-wire placements and found that a transfissural approach and longer procedure duration were independent predictors of pneumothorax.
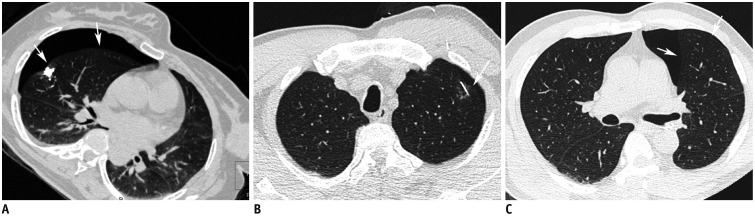
Fig. 8. Pneumothorax as complication after localization.
A. Lipiodol localization for right middle lobe part-solid nodule in 56-year-old woman. Post-procedure CT image shows right pneumothorax (arrows) after lipiodol localization. B, C. Hook-wire localization for left upper lobe part-solid nodule in 57-year-old man. Post-procedure CT image shows right pneumothorax (arrows) after hook-wire insertion. Thoracoscopic wedge resections were successfully performed in both patients.
Pulmonary hemorrhage manifests as a focal ground glass opacity observed on CT during localization, with an incidence of up to 45.3% (Table 2, Fig. 9) (48). A previous meta-analysis reported that the hemorrhage rate associated with microcoils (6%) was lower than those associated with hook-wires (16%) and lipiodol (12%) because of the structural properties of the microcoil. Ichinose et al. (39) analyzed the complications of CT-guided hook-wire localization in 417 patients. The authors reported that hemoptysis and pulmonary hematoma were observed in 10.3% of the cases, and an insertion distance greater than 25 mm was a risk factor for pulmonary hemorrhage and hemoptysis (39). A study of 650 patients with CT-guided lung biopsy evaluated the risk factors for pulmonary hemorrhage and demonstrated that small and basal lesions, increased lesion depth from the pleural surface, and increased length of the aerated lung parenchyma were risk factors for pulmonary hemorrhage (61). Patients with pulmonary hemorrhage should be placed in the lateral decubitus position with the localization site dependent to prevent spillage of blood into the contralateral lung. Excessive pulmonary hemorrhage is very rare. However, in cases of massive hemorrhage, intubation with a endotracheal tube is warranted to maintain the airway (62).
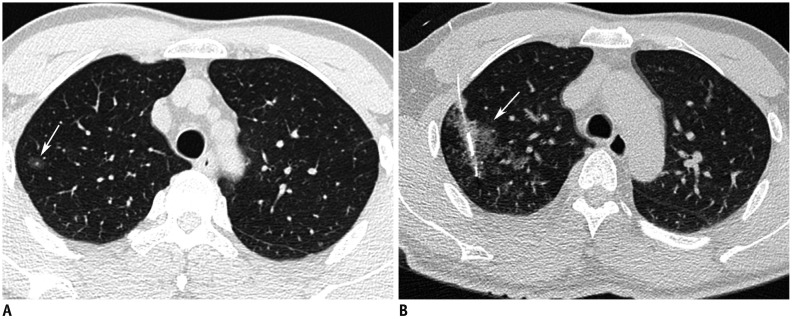
Fig. 9. Pulmonary hemorrhage as complication after hook-wire localization.
A. Axial CT image shows 10-mm ground glass nodules (arrow) in right upper lobe. B. Post-procedure axial CT image after hook-wire localization shows ground glass opacity around hook-wire, suggesting pulmonary hemorrhage (arrow) in right upper lobe. Patient was asymptomatic and required no treatment.
Hemothorax is observed in up to 2% of the patients who undergo CT-guided localization (Table 2). Significant chest wall hematoma and hemothorax are rare but may develop if the intercostal or internal mammary arteries are injured during the procedure (63). Therefore, care must be taken to avoid the internal mammary and intercostal arteries during the localization procedure.
Although relatively rare, a systemic air embolism is a possible serious complication during hook-wire localization. Systemic air embolism after CT-guided hook-wire localization has been reported in only 8 cases in the English literature (64). The mechanism underlying air embolisms during hook-wire localization involves temporary penetration between the airway and pulmonary vein and entry of air into the pulmonary vein during needle insertion. The proposed risk factors are lesions located above the left atrium, deep lesions far from the visceral pleura, prone position, patient movement during the procedure, repositioning after placement, and increased airway pressure due to cough, Valsalva maneuver, or positive pressure ventilation (64). Moreover, the target nodules are located in the lower lobes in most cases; this may be due to the fact that the lower lobe moves more than the upper lobe while breathing, which makes accurate localization difficult and increases the number of needle insertions by the introducer needle and the risk of penetration of vessels within the lung during puncture (64). There were no reports of air embolism during localization using other localization methods, including microcoil, dye, contrast agents, and radiotracers. Early detection and prevention of such embolisms are important, because the available treatment options are limited. To prevent air embolism, the introducer needle should always be occluded by the inner stylet, saline drops, or a finger. The patient should be instructed to avoid breathing deeply and coughing during the procedure (18). If an air embolism is recognized in the left heart or aorta during the procedure or is clinically suspected, the patient should be placed in the mild Trendelenburg position to prevent embolization of the air into the cerebral circulation; 100% oxygen should be administered immediately, which promotes the exchange of oxygen for nitrogen within the air bubbles and accelerates their resorption. Early hyperbaric oxygen therapy is recommended for patients with cerebral air embolism.
Localization Using Bronchoscopy
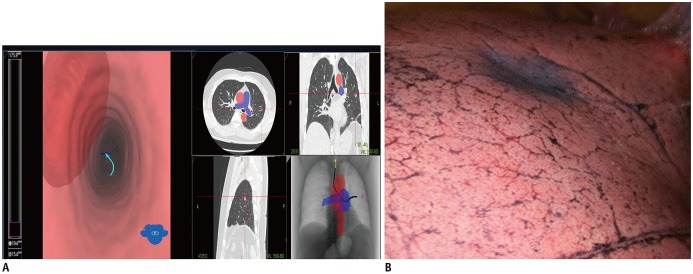
Transbronchial localization guided by fluoroscopy (65) or electromagnetic navigation bronchography (ENB) has been described in several studies (65,66,67,68,69). Among these, ENB-guided localization has been increasingly applied for preoperative localization. ENB is an image-based virtual endobronchial technique that allows the clinician to approach peripheral lung nodules, which are difficult to access using conventional bronchoscopy (69). ENB involves conversion of preoperative CT data into a virtual bronchial map, followed by the use of a steerable probe with a bronchoscope connected to an electromagnetic localization board. The steerable probe contains a position sensor and enables navigation of turns in the endobronchial tree (66). After guidance of the steerable probe tip to the targeted lung nodule, dye usually can be applied for location of the lung (Fig. 10). The successful localization rate of ENB-guided localization has been reported to range from 81% to 100% (65,66,67,68,69,70,71). The advantage of ENB-guided localization is the lower rate of pneumothorax and hemorrhage than those achieved with CT-guided localization (65,66,67,68,69,70,71). However, this technique needs additional general anesthesia when ENB-guided localization is not performed in the operating room on the day of surgery.
Fig. 10. ENB localization of lung nodule.
A. ENB can convert preoperative CT data into virtual bronchial map. Steerable probe contains position sensor and allows navigation of turns in endobronchial tree. After guidance of steerable probe tip to targeted lung nodule, dye is injected to target nodule. B. Video-assisted thoracoscopic image shows deposition of dye as purple area around targeted nodule position after ENB localization. ENB = electromagnetic navigation bronchography
Localization with Ultrasonography
Intraoperative thoracoscopic ultrasonography is an option for localization of non-visible, non-palpable small nodules since it is a real-time, nonionizing, and non-invasive method that does not need any additional preoperative procedure for localization of the nodules (72). In addition, ultrasonographic visualization is very helpful in planning the extent of the surgical resection that would be required and also in confirming the surgical margins. Using high-quality imaging data from ultrasonographic guidance to identify small and deep nodules, a strong correlation has been shown to exist between the resection margins measured using ultrasonography and the margins verified by histologic examination in the resected lung specimens (10). Intraoperative ultrasonography is performed under general anesthesia and one-lung ventilation. When the lung is collapsed completely, the probe is placed firmly in the area of the target lesion (Fig. 11). The pleural space is filled with 500 mL of warm saline to assist in obtaining a sonographic view with better definition, or conductive gel is applied to the parenchyma to facilitate localization of the lesion through the ultrasound device. The nodule of interest is identified by ultrasonographic visualization of a hyperechoic or hypoechoic nodule or by visualization of a hyperechoic shadow beneath the nodule. Many solid lung nodules show a hypoechoic pattern, with an associated acoustic enhancement of the posterior echo. Several single-center studies have demonstrated the feasibility of intraoperative ultrasound for localization of small solid nodules or subsolid nodules (73). The accurate detection rate of pulmonary nodules using intraoperative ultrasonography has been reported to be 92.9–100% (73,74,75,76). The main reasons for procedure failure were as follows: 1) Emphysematous or edematous lung parenchyma could interrupt ultrasonographic localization because of air, which can cause major reflection of ultrasound waves. 2) Small lesions or deeper lesions from the pleura are also major causes of procedure failure. In particular, lesions with a diameter smaller than 10 mm and located over 5 mm from the pleural surface are difficult to localize (74,75). The success rate of VATS using intraoperative thoracoscopic ultrasonography ranged between 97.8% and 100%, with a thoracotomy conversion rate of 0–14.2% (73,74,75,76).
Fig. 11. Diagram showing intraoperative ultrasonography localization for left upper lobe nodule.
Lung is collapsed and probe is placed firmly in area of target nodule.
Although intraoperative thoracoscopic ultrasonography has been shown to be effective and safe, this procedure has several limitations. First, this procedure requires complete lung collapse, because residual air in the lung causes an artifact and masks the nodule (73). A previous study reported that if the lung is not sufficiently deflated, the internal structures of the lungs were likely to display a “spotted hyperechoic pattern” owing to residual air echo artifacts, and this can mask the presence of a subsolid nodule (73). Therefore, complete collapse of the lung is necessary to ensure the reliability of this procedure. Furthermore, the presence of severe emphysema or asthma has been shown to cause errors in ultrasonographic evaluation. Second, this technique is operator-dependent and requires an experienced operator (77), and these limitations may preclude the wide use of thoracoscopic ultrasonography at present.
CONCLUSION
The need for localization procedures will increase, and these procedures will become more important because of the higher detection rates of small pulmonary nodules with CT screening. In particular, we think that the localization procedure will become more important as preliminary results of studies show that the outcomes of sublobar resection in some subsolid nodules are similar to those of lobectomy. For the practicing radiologist in the current era, it is important to understand the advantages and disadvantages of each procedure and to possess detailed knowledge of the developing fields of these techniques. In conclusion, we hope that this review article will contribute to broadening radiologists' understanding of the advantages and drawbacks related to various localization procedures for small pulmonary nodules before VATS resection.
Acknowledgments
We would like to thank Dr. Jung Seop Eom for providing images of ENB localization and Dr. Hyo Yeong Ahn for her academic advice.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.National Lung Screening Trial Research Team. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Godoy MC, Naidich DP. Subsolid pulmonary nodules and the spectrum of peripheral adenocarcinomas of the lung: recommended interim guidelines for assessment and management. Radiology. 2009;253:606–622. doi: 10.1148/radiol.2533090179. [DOI] [PubMed] [Google Scholar]
- 3.Infante M, Lutman RF, Imparato S, Di Rocco M, Ceresoli GL, Torri V, et al. Differential diagnosis and management of focal ground-glass opacities. Eur Respir J. 2009;33:821–827. doi: 10.1183/09031936.00047908. [DOI] [PubMed] [Google Scholar]
- 4.Mack MJ, Aronoff RJ, Acuff TE, Douthit MB, Bowman RT, Ryan WH. Present role of thoracoscopy in the diagnosis and treatment of diseases of the chest. Ann Thorac Surg. 1992;54:403–408. doi: 10.1016/0003-4975(92)90428-7. discussion 407–409. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki K, Nagai K, Yoshida J, Ohmatsu H, Takahashi K, Nishimura M, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest. 1999;115:563–568. doi: 10.1378/chest.115.2.563. [DOI] [PubMed] [Google Scholar]
- 6.Plunkett MB, Peterson MS, Landreneau RJ, Ferson PF, Posner MC. Peripheral pulmonary nodules: preoperative percutaneous needle localization with CT guidance. Radiology. 1992;185:274–276. doi: 10.1148/radiology.185.1.1523323. [DOI] [PubMed] [Google Scholar]
- 7.Finley RJ, Mayo JR, Grant K, Clifton JC, English J, Leo J, et al. Preoperative computed tomography-guided microcoil localization of small peripheral pulmonary nodules: a prospective randomized controlled trial. J Thorac Cardiovasc Surg. 2015;149:26–31. doi: 10.1016/j.jtcvs.2014.08.055. [DOI] [PubMed] [Google Scholar]
- 8.Park CH, Han K, Hur J, Lee SM, Lee JW, Hwang SH, et al. Comparative effectiveness and safety of preoperative lung localization for pulmonary nodules: a systematic review and meta-analysis. Chest. 2017;151:316–328. doi: 10.1016/j.chest.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Lin MW, Chen JS. Image-guided techniques for localizing pulmonary nodules in thoracoscopic surgery. J Thorac Dis. 2016;8(Suppl 9):S749–S755. doi: 10.21037/jtd.2016.09.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaman M, Bilal H, Woo CY, Tang A. In patients undergoing video-assisted thoracoscopic surgery excision, what is the best way to locate a subcentimetre solitary pulmonary nodule in order to achieve successful excision? Interact Cardiovasc Thorac Surg. 2012;15:266–272. doi: 10.1093/icvts/ivs068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horan TA, Pinheiro PM, Araújo LM, Santiago FF, Rodrigues MR. Massive gas embolism during pulmonary nodule hook wire localization. Ann Thorac Surg. 2002;73:1647–1649. doi: 10.1016/s0003-4975(01)03371-9. [DOI] [PubMed] [Google Scholar]
- 12.Sakiyama S, Kondo K, Matsuoka H, Yoshida M, Miyoshi T, Yoshida S, et al. Fatal air embolism during computed tomography-guided pulmonary marking with a hook-type marker. J Thorac Cardiovasc Surg. 2003;126:1207–1209. doi: 10.1016/s0022-5223(03)00821-3. [DOI] [PubMed] [Google Scholar]
- 13.Asamura H, Kondo H, Naruke T, Tsuchiya R, Wakao F, Kaneko M, et al. Computed tomography-guided coil injection and thoracoscopic pulmonary resection under roentgenographic fluoroscopy. Ann Thorac Surg. 1994;58:1542–1544. doi: 10.1016/0003-4975(94)91957-7. [DOI] [PubMed] [Google Scholar]
- 14.Su TH, Fan YF, Jin L, He W, Hu LB. CT-guided localization of small pulmonary nodules using adjacent microcoil implantation prior to video-assisted thoracoscopic surgical resection. Eur Radiol. 2015;25:2627–2633. doi: 10.1007/s00330-015-3676-5. [DOI] [PubMed] [Google Scholar]
- 15.Mayo JR, Clifton JC, Powell TI, English JC, Evans KG, Yee J, et al. Lung nodules: CT-guided placement of microcoils to direct video-assisted thoracoscopic surgical resection. Radiology. 2009;250:576–585. doi: 10.1148/radiol.2502080442. [DOI] [PubMed] [Google Scholar]
- 16.Sui X, Zhao H, Yang F, Li JL, Wang J. Computed tomography guided microcoil localization for pulmonary small nodules and ground-glass opacity prior to thoracoscopic resection. J Thorac Dis. 2015;7:1580–1587. doi: 10.3978/j.issn.2072-1439.2015.09.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenglinger FX, Schwarz CD, Artmann W. Localization of pulmonary nodules before thoracoscopic surgery: value of percutaneous staining with methylene blue. AJR Am J Roentgenol. 1994;163:297–300. doi: 10.2214/ajr.163.2.7518642. [DOI] [PubMed] [Google Scholar]
- 18.Keating J, Singhal S. Novel methods of intraoperative localization and margin assessment of pulmonary nodules. Semin Thorac Cardiovasc Surg. 2016;28:127–136. doi: 10.1053/j.semtcvs.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Vandoni RE, Cuttat JF, Wicky S, Suter M. CT-guided methylene-blue labelling before thoracoscopic resection of pulmonary nodules. Eur J Cardiothorac Surg. 1998;14:265–270. doi: 10.1016/s1010-7940(98)00160-2. [DOI] [PubMed] [Google Scholar]
- 20.Lee NK, Park CM, Kang CH, Jeon YK, Choo JY, Lee HJ, et al. CT-guided percutaneous transthoracic localization of pulmonary nodules prior to video-assisted thoracoscopic surgery using barium suspension. Korean J Radiol. 2012;13:694–701. doi: 10.3348/kjr.2012.13.6.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okumura T, Kondo H, Suzuki K, Asamura H, Kobayashi T, Kaneko M, et al. Fluoroscopy-assisted thoracoscopic surgery after computed tomography-guided bronchoscopic barium marking. Ann Thorac Surg. 2001;71:439–442. doi: 10.1016/s0003-4975(00)02378-x. [DOI] [PubMed] [Google Scholar]
- 22.Asano F, Shindoh J, Shigemitsu K, Miya K, Abe T, Horiba M, et al. Ultrathin bronchoscopic barium marking with virtual bronchoscopic navigation for fluoroscopy-assisted thoracoscopic surgery. Chest. 2004;126:1687–1693. doi: 10.1378/chest.126.5.1687. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi T, Kaneko M, Kondo H, Nakayama H, Asamura H, Tsuchiya R, et al. CT-guided bronchoscopic barium marking for resection of a fluoroscopically invisible peripheral pulmonary lesion. Jpn J Clin Oncol. 1997;27:204–205. doi: 10.1093/jjco/27.3.204. [DOI] [PubMed] [Google Scholar]
- 24.Nomori H, Horio H, Naruke T, Suemasu K. Fluoroscopy-assisted thoracoscopic resection of lung nodules marked with lipiodol. Ann Thorac Surg. 2002;74:170–173. doi: 10.1016/s0003-4975(02)03615-9. [DOI] [PubMed] [Google Scholar]
- 25.Moon SW, Wang YP, Jo KH, Kwack MS, Kim SW, Kwon OK, et al. Fluoroscopy-aided thoracoscopic resection of pulmonary nodule localized with contrast media. Ann Thorac Surg. 1999;68:1815–1820. doi: 10.1016/s0003-4975(99)00764-x. [DOI] [PubMed] [Google Scholar]
- 26.Kawanaka K, Nomori H, Mori T, Ikeda K, Ikeda O, Tomiguchi S, et al. Marking of small pulmonary nodules before thoracoscopic resection: injection of lipiodol under CT-fluoroscopic guidance. Acad Radiol. 2009;16:39–45. doi: 10.1016/j.acra.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Kim YD, Jeong YJ, I H, Cho JS, Lee JW, Kim HJ, et al. Localization of pulmonary nodules with lipiodol prior to thoracoscopic surgery. Acta Radiol. 2011;52:64–69. doi: 10.1258/ar.2010.100307. [DOI] [PubMed] [Google Scholar]
- 28.Ambrogi MC, Melfi F, Zirafa C, Lucchi M, De Liperi A, Mariani G, et al. Radio-guided thoracoscopic surgery (RGTS) of small pulmonary nodules. Surg Endosc. 2012;26:914–919. doi: 10.1007/s00464-011-1967-8. [DOI] [PubMed] [Google Scholar]
- 29.Chella A, Lucchi M, Ambrogi MC, Menconi G, Melfi FM, Gonfiotti A, et al. A pilot study of the role of TC-99 radionuclide in localization of pulmonary nodular lesions for thoracoscopic resection. Eur J Cardiothorac Surg. 2000;18:17–21. doi: 10.1016/s1010-7940(00)00411-5. [DOI] [PubMed] [Google Scholar]
- 30.Starnes SL, Wolujewicz M, Guitron J, Williams V, Scheler J, Ristagno R. Radiotracer localization of nonpalpable pulmonary nodules: a single-center experience. J Thorac Cardiovasc Surg. 2018;156:1986–1992. doi: 10.1016/j.jtcvs.2018.03.152. [DOI] [PubMed] [Google Scholar]
- 31.Bellomi M, Veronesi G, Trifirò G, Brambilla S, Bonello L, Preda L, et al. Computed tomography-guided preoperative radiotracer localization of nonpalpable lung nodules. Ann Thorac Surg. 2010;90:1759–1764. doi: 10.1016/j.athoracsur.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Koike T, Koike T, Yoshiya K, Tsuchida M, Toyabe S. Risk factor analysis of locoregional recurrence after sublobar resection in patients with clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg. 2013;146:372–378. doi: 10.1016/j.jtcvs.2013.02.057. [DOI] [PubMed] [Google Scholar]
- 33.El-Sherif A, Fernando HC, Santos R, Pettiford B, Luketich JD, Close JM, et al. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann Surg Oncol. 2007;14:2400–2405. doi: 10.1245/s10434-007-9421-9. [DOI] [PubMed] [Google Scholar]
- 34.Wolf AS, Swanson SJ, Yip R, Liu B, Tarras ES, Yankelevitz DF, et al. I-ELCAP Investigators. The impact of margins on outcomes after wedge resection for stage I non-small cell lung cancer. Ann Thorac Surg. 2017;104:1171–1178. doi: 10.1016/j.athoracsur.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 35.Dendo S, Kanazawa S, Ando A, Hyodo T, Kouno Y, Yasui K, et al. Preoperative localization of small pulmonary lesions with a short hook wire and suture system: experience with 168 procedures. Radiology. 2002;225:511–518. doi: 10.1148/radiol.2252011025. [DOI] [PubMed] [Google Scholar]
- 36.Miyoshi K, Toyooka S, Gobara H, Oto T, Mimura H, Sano Y, et al. Clinical outcomes of short hook wire and suture marking system in thoracoscopic resection for pulmonary nodules. Eur J Cardiothorac Surg. 2009;36:378–382. doi: 10.1016/j.ejcts.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 37.Li W, Wang Y, He X, Li G, Wang S, Xu L, et al. Combination of CT-guided hookwire localization and video-assisted thoracoscopic surgery for pulmonary nodular lesions: analysis of 103 patients. Oncol Lett. 2012;4:824–828. doi: 10.3892/ol.2012.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seo JM, Lee HY, Kim HK, Choi YS, Kim J, Shim YM, et al. Factors determining successful computed tomography-guided localization of lung nodules. J Thorac Cardiovasc Surg. 2012;143:809–814. doi: 10.1016/j.jtcvs.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 39.Ichinose J, Kohno T, Fujimori S, Harano T, Suzuki S. Efficacy and complications of computed tomography-guided hook wire localization. Ann Thorac Surg. 2013;96:1203–1208. doi: 10.1016/j.athoracsur.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 40.Hanauer M, Perentes JY, Krueger T, Ris HB, Bize P, Schmidt S, et al. Pre-operative localization of solitary pulmonary nodules with computed tomography-guided hook wire: report of 181 patients. J Cardiothorac Surg. 2016;11:5. doi: 10.1186/s13019-016-0404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang HZ, Wang GZ, Xu LC, Li GD, Wang Y, Wang YH, et al. CT-guided Hookwire localization before video-assisted thoracoscopic surgery for solitary ground-glass opacity dominant pulmonary nodules: radiologic-pathologic analysis. Oncotarget. 2017;8:108118–108129. doi: 10.18632/oncotarget.22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klinkenberg TJ, Dinjens L, Wolf RFE, van der Wauwer C, van der Wekken AJ, de Bock GH, et al. CT-guided percutaneous hookwire localization increases the efficacy and safety of VATS for pulmonary nodules. J Surg Oncol. 2017;115:898–904. doi: 10.1002/jso.24589. [DOI] [PubMed] [Google Scholar]
- 43.Yao F, Wang J, Yao J, Xu L, Wang J, Gao L. Reevaluation of the efficacy of preoperative computed tomography-guided hook wire localization: a retrospective analysis. Int J Surg. 2018;51:24–30. doi: 10.1016/j.ijsu.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki K, Shimohira M, Hashizume T, Ozawa Y, Sobue R, Mimura M, et al. Usefulness of CT-guided hookwire marking before video-assisted thoracoscopic surgery for small pulmonary lesions. J Med Imaging Radiat Oncol. 2014;58:657–662. doi: 10.1111/1754-9485.12214. [DOI] [PubMed] [Google Scholar]
- 45.Iguchi T, Hiraki T, Gobara H, Fujiwara H, Matsui Y, Sugimoto S, et al. Simultaneous multiple preoperative localizations of small pulmonary lesions using a short hook wire and suture system. Cardiovasc Intervent Radiol. 2015;38:971–976. doi: 10.1007/s00270-014-1028-5. [DOI] [PubMed] [Google Scholar]
- 46.Mullan BF, Stanford W, Barnhart W, Galvin JR. Lung nodules: improved wire for CT-guided localization. Radiology. 1999;211:561–565. doi: 10.1148/radiology.211.2.r99ma35561. [DOI] [PubMed] [Google Scholar]
- 47.Hajjar W, Al-Nassar S, Almousa O, Rahal S, Al-Aqeed A, Ahmed I, et al. Thoracoscopic resection of suspected metastatic pulmonary nodules after microcoil localization technique: a prospective study. J Cardiovasc Surg (Torino) 2017;58:606–612. doi: 10.23736/S0021-9509.16.07911-8. [DOI] [PubMed] [Google Scholar]
- 48.Kha LC, Hanneman K, Donahoe L, Chung T, Pierre AF, Yasufuku K, et al. Safety and efficacy of modified preoperative lung nodule microcoil localization without pleural marking: a pilot study. J Thorac Imaging. 2016;31:15–22. doi: 10.1097/RTI.0000000000000188. [DOI] [PubMed] [Google Scholar]
- 49.Wang ZX, Li L, Zhang Z, Wang GH, Kong DM, Wang XD, et al. High-resolution computed tomography features and CT-guided microcoil localization of subcentimeter pulmonary ground-glass opacities: radiological processing prior to video-assisted thoracoscopic surgery. J Thorac Dis. 2018;10:2676–2684. doi: 10.21037/jtd.2018.04.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sui X, Zhao H, Yang F, Liu G, Hu L, Chen C, et al. Analysis of factors affecting successful microcoil localization for pulmonary nodules. J Surg Res. 2018;224:193–199. doi: 10.1016/j.jss.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 51.Stephenson JA, Mahfouz A, Rathinam S, Nakas A, Bajaj A. A simple and safe technique for CT guided lung nodule marking prior to video assisted thoracoscopic surgical resection revisited. Lung Cancer Int. 2015;2015:235720. doi: 10.1155/2015/235720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimamura Y, Sasaki S, Shimohira M, Ogino H, Yuki D, Nakamae K, et al. New technique of percutaneous CT fluoroscopy-guided marking before video-assisted thoracoscopic surgery for small lung lesions: feasibility of using a 25-gauge needle without local anaesthesia. Br J Radiol. 2018;91:20170692. doi: 10.1259/bjr.20170692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin MW, Tseng YH, Lee YF, Hsieh MS, Ko WC, Chen JY, et al. Computed tomography-guided patent blue vital dye localization of pulmonary nodules in uniportal thoracoscopy. J Thorac Cardiovasc Surg. 2016;152:535–544.e2. doi: 10.1016/j.jtcvs.2016.04.052. [DOI] [PubMed] [Google Scholar]
- 54.McConnell PI, Feola GP, Meyers RL. Methylene blue-stained autologous blood for needle localization and thoracoscopic resection of deep pulmonary nodules. J Pediatr Surg. 2002;37:1729–1731. doi: 10.1053/jpsu.2002.36707. [DOI] [PubMed] [Google Scholar]
- 55.Kleedehn M, Kim DH, Lee FT, Lubner MG, Robbins JB, Ziemlewicz TJ, et al. Preoperative pulmonary nodule localization: a comparison of methylene blue and hookwire techniques. AJR Am J Roentgenol. 2016;207:1334–1339. doi: 10.2214/AJR.16.16272. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe K, Nomori H, Ohtsuka T, Kaji M, Naruke T, Suemasu K. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: experience with 174 nodules. J Thorac Cardiovasc Surg. 2006;132:320–324. doi: 10.1016/j.jtcvs.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 57.Mogi A, Yajima T, Tomizawa K, Onozato R, Tanaka S, Kuwano H. Video-assisted thoracoscopic surgery after preoperative CT-guided lipiodol marking of small or impalpable pulmonary nodules. Ann Thorac Cardiovasc Surg. 2015;21:435–439. doi: 10.5761/atcs.oa.15-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miura H, Yamagami T, Tanaka O, Yoshimatsu R, Ichijo Y, Kato D, et al. CT findings after lipiodol marking performed before video-assisted thoracoscopic surgery for small pulmonary nodules. Acta Radiol. 2016;57:303–310. doi: 10.1177/0284185115576047. [DOI] [PubMed] [Google Scholar]
- 59.Doo KW, Yong HS, Kim HK, Kim S, Kang EY, Choi YH. Needlescopic resection of small and superficial pulmonary nodule after computed tomographic fluoroscopy-guided dual localization with radiotracer and hookwire. Ann Surg Oncol. 2015;22:331–337. doi: 10.1245/s10434-014-3884-2. [DOI] [PubMed] [Google Scholar]
- 60.Iguchi T, Hiraki T, Gobara H, Fujiwara H, Matsui Y, Miyoshi S, et al. CT fluoroscopy-guided preoperative short hook wire placement for small pulmonary lesions: evaluation of safety and identification of risk factors for pneumothorax. Eur Radiol. 2016;26:114–121. doi: 10.1007/s00330-015-3815-z. [DOI] [PubMed] [Google Scholar]
- 61.Nour-Eldin NE, Alsubhi M, Naguib NN, Lehnert T, Emam A, Beeres M, et al. Risk factor analysis of pulmonary hemorrhage complicating CT-guided lung biopsy in coaxial and non-coaxial core biopsy techniques in 650 patients. Eur J Radiol. 2014;83:1945–1952. doi: 10.1016/j.ejrad.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 62.Wu CC, Maher MM, Shepard JA. Complications of CT-guided percutaneous needle biopsy of the chest: prevention and management. AJR Am J Roentgenol. 2011;196:W678–W682. doi: 10.2214/AJR.10.4659. [DOI] [PubMed] [Google Scholar]
- 63.Cham MD, Lane ME, Henschke CI, Yankelevitz DF. Lung biopsy: special techniques. Semin Respir Crit Care Med. 2008;29:335–349. doi: 10.1055/s-2008-1081278. [DOI] [PubMed] [Google Scholar]
- 64.Yi JH, Choi PJ, Bang JH, Jeong SS, Cho JH. Systemic air embolism after computed tomography-guided hook wire localization: two case reports and literature review. J Thorac Dis. 2018;10:E59–E64. doi: 10.21037/jtd.2017.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Endo M, Kotani Y, Satouchi M, Takada Y, Sakamoto T, Tsubota N, et al. CT fluoroscopy-guided bronchoscopic dye marking for resection of small peripheral pulmonary nodules. Chest. 2004;125:1747–1752. doi: 10.1378/chest.125.5.1747. [DOI] [PubMed] [Google Scholar]
- 66.Krimsky WS, Minnich DJ, Cattaneo SM, Sarkar SA, Harley DP, Finley DJ, et al. Thoracoscopic detection of occult indeterminate pulmonary nodules using bronchoscopic pleural dye marking. J Community Hosp Intern Med Perspect. 2014 Feb 17; doi: 10.3402/jchimp.v4.23084. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tay JH, Wallbridge PD, Larobina M, Russell PA, Irving LB, Steinfort DP. Electromagnetic navigation bronchoscopydirected pleural tattoo to aid surgical resection of peripheral pulmonary lesions. J Bronchology Interv Pulmonol. 2016;23:245–250. doi: 10.1097/LBR.0000000000000214. [DOI] [PubMed] [Google Scholar]
- 68.Kuo SW, Tseng YF, Dai KY, Chang YC, Chen KC, Lee JM. Electromagnetic navigation bronchoscopy localization versus percutaneous CT-guided localization for lung resection via video-assisted thoracoscopic surgery: a propensity-matched study. J Clin Med. 2019;8:E379. doi: 10.3390/jcm8030379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bolton WD, Howe H, 3rd, Stephenson JE. The utility of electromagnetic navigational bronchoscopy as a localization tool for robotic resection of small pulmonary nodules. Ann Thorac Surg. 2014;98:471–475. doi: 10.1016/j.athoracsur.2014.04.085. discussion 475–476. [DOI] [PubMed] [Google Scholar]
- 70.Marino KA, Sullivan JL, Weksler B. Electromagnetic navigation bronchoscopy for identifying lung nodules for thoracoscopic resection. Ann Thorac Surg. 2016;102:454–457. doi: 10.1016/j.athoracsur.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 71.Georgiou HD, Taverner J, Irving LB, Steinfort DP. Safety and efficacy of radial EBUS for the investigation of peripheral pulmonary lesions in patients with advanced COPD. J Bronchology Interv Pulmonol. 2016;23:192–198. doi: 10.1097/LBR.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 72.Wada H, Anayama T, Hirohashi K, Nakajima T, Kato T, Waddell TK, et al. Thoracoscopic ultrasonography for localization of subcentimetre lung nodules. Eur J Cardiothorac Surg. 2016;49:690–697. doi: 10.1093/ejcts/ezv124. [DOI] [PubMed] [Google Scholar]
- 73.Kondo R, Yoshida K, Hamanaka K, Hashizume M, Ushiyama T, Hyogotani A, et al. Intraoperative ultrasonographic localization of pulmonary ground-glass opacities. J Thorac Cardiovasc Surg. 2009;138:837–842. doi: 10.1016/j.jtcvs.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 74.Piolanti M, Coppola F, Papa S, Pilotti V, Mattioli S, Gavelli G. Ultrasonographic localization of occult pulmonary nodules during video-assisted thoracic surgery. Eur Radiol. 2003;13:2358–2364. doi: 10.1007/s00330-003-1916-6. [DOI] [PubMed] [Google Scholar]
- 75.Mattioli S, D'Ovidio F, Daddi N, Ferruzzi L, Pilotti V, Ruffato A, et al. Transthoracic endosonography for the intraoperative localization of lung nodules. Ann Thorac Surg. 2005;79:443–449. doi: 10.1016/j.athoracsur.2004.07.087. discussion 443–449. [DOI] [PubMed] [Google Scholar]
- 76.Khereba M, Ferraro P, Duranceau A, Martin J, Goudie E, Thiffault V, et al. Thoracoscopic localization of intraparenchymal pulmonary nodules using direct intracavitary thoracoscopic ultrasonography prevents conversion of VATS procedures to thoracotomy in selected patients. J Thorac Cardiovasc Surg. 2012;144:1160–1165. doi: 10.1016/j.jtcvs.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 77.Sortini D, Feo CV, Carcoforo P, Carrella G, Pozza E, Liboni A, et al. Thoracoscopic localization techniques for patients with solitary pulmonary nodule and history of malignancy. Ann Thorac Surg. 2005;79:258–262. doi: 10.1016/j.athoracsur.2004.06.012. discussion 262. [DOI] [PubMed] [Google Scholar]