Abstract
An investigation of the yeast cell resealing process was performed by studying the absorption of the tetraphenylphosphonium (TPP+) ion by the yeast Saccharomyces cerevisiae. It was shown that the main barrier for the uptake of such TPP+ ions is the cell wall. An increased rate of TPP+ absorption after treatment of such cells with a pulsed electric field (PEF) was observed only in intact cells, but not in spheroplasts. The investigation of the uptake of TPP+ in PEF treated cells exposed to TPP+ for different time intervals also showed the dependence of the absorption rate on the PEF strength. The modelling of the TPP+ uptake recovery has also shown that the characteristic decay time of the non-equilibrium (PEF induced) pores was approximately a few tens of seconds and this did not depend on the PEF strength. A further investigation of such cell membrane recovery process using a florescent SYTOX Green nucleic acid stain dye also showed that such membrane resealing takes place over a time that is like that occurring in the cell wall. It was thus concluded that the similar characteristic lifetimes of the non-equilibrium pores in the cell wall and membrane after exposure to PEF indicate a strong coupling between these parts of the cell.
Subject terms: Biophysics, Biological physics
Introduction
The molecules, which enter cells such as yeast or bacteria, must pass through the cell wall, a periplasmic space and then obtain access to the transporters located in the plasma membrane. The porosity of the yeast cell wall is highly regulated and is dependent on the cell cycle. This is important for water retention in the periplasmic space, where some important proteins, such invertase1, alpha galactosidase2 and acid trehalase3 are located. The cell wall is considered to be a sieve-like structure and does not present an absolute obstacle for solutes since it is being highly charged negatively by the phosphate and carboxyl groups in the cell wall with mananns playing the role of an ion exchanger4. Hence, the cell wall acts as the first selective and primary barrier for nutrients, biomolecules and ions5. It isn’t clear, but there is some evidence that molecules such as bleomycin and tetraphenylphosphonium may also be suspended by this biosorption process6–9. There are many ways to regulate (mainly to increase) the rate at which these various molecules can pass the cell wall barrier. This can be done by using chemical treatment with organic solvents and detergents10 or by mechanical shearing or treatment with a pulsed electric field (PEF), thus generating pores11.
The other very important barrier for molecules diffusion is the plasma membrane. Besides simple diffusion, when gasses and small lipophilic molecules are being transported, yeast cells have many instruments (transporters and channels) to preserve cell homeostasis12. This is the reason why the lipid bilayer is always confronted with hyper osmotic pressure at the interior of the cell due to the impermeable intracellular compounds. This osmotic pressure is the main mechanical force that stretches the plasma membrane and this can be at times the reason for cell lysis13. It is known that there exists a relationship between the turgor pressure and cell wall pathway integrity14. Moreover, there is evidence that the plasma membrane fluidity changes the mechanical properties of the cell wall due to the fact that some of the cell wall proteins are linked to the membrane via the GPI (glycosylphosphatidylinositol) anchor15. However, the actual molecular mechanism of such a connection is not clear.
Our previous work demonstrated that the permeability to the lipophilic ion tetraphenylphosphonium (TPP+) across the yeast cell membrane and cell wall may be reversibly changed by their exposure to PEF of microsecond duration8. Moreover, the accumulation of these TPP+ ions by the yeast is not affected by an electric field when they reach a steady-state after 90–120 min, while exposure to a high (≈100 kV/cm) nanosecond duration PEF speeds this process up to 65 times16. As far as we know, the influence of post-pulse resealing on the elevated TPP+ absorption rate has not been investigated. Moreover, as was noted, the duration and character of the transient pore decay is essential for understanding the mechanism of PEF induced yeast cell permeabilization17.
In this paper, we investigated yeast cell electrical permeabilization by applying a PEF regime, which does not affect the viability of the cells, i.e. most of the cells can recover to their initial (non-permeabilized) state. These investigations were performed by applying two different methods. One method was based on the study of the TPP+ ion absorption kinetics using a potentiometric ion selective electrode, which provides data about the yeast cell wall permeabilization. Another method, based on fluorescent dye measurements, showed the permeabilized state of the cell membrane. These experimental results were analyzed by applying the mathematical model, which took into consideration the decay of the post-pulse transient pores. Experiments and modeling have shown a strong link between the yeast cell membrane and the wall resealing process after PEF action.
Materials and Methods
Yeast strain and cultivation
Saccharomyces cerevisiae SEY6210 (MATα, leu2-3, leu2-112, ura3-52, his3- Δ200, trp1-Δ901, lys2-801, suc2-Δ9, GAL) yeast cells were grown to their early exponential growth phase in a complete medium (YPD) containing 1% (w/v) yeast extract, 2% (w/v) peptone and 2% (w/v) glucose (Merck, Darmstadt, Germany). The yeast cells were then re-suspended in 1 ml of a cold (4 °C) electroporation buffer (EPB) containing 1 M sorbitol and a 20 mM Tris-HCl buffer, pH 7.4 (Applichem, Darmstadt, Germany) while keeping the final concentration of the yeasts at the (4–6) × 109 colony forming unit (CFU)/mL. Cell density was determined by measuring the optical density (OD) using an optical absorption spectrophotometer (Halo RB-10, Dynamica Scientific, UK) operating at a wavelength of 600 nm.
Spheroplasts preparation
After the modification cells were pelleted, washed twice and suspended in a lyticase specific buffer, additional control samples of non-modified cells were prepared in parallel. The lyticase buffer containing 1.2 M of sorbitol, 0.5 mM of MgCl2 and 35 mM of K2HPO4 (pH 6.0) was adjusted for this solution according to our previous report18. The final optical density of the diluted suspension was 1.5 OD (600 nm). A further 50 U/ml of lyticase were added to each cell sample and then incubated at 30 °C for 2 hours. To prove that the lyticase enzyme was active during the experiment, its optical density was measured at 600 nm before and after the incubation cycles of the sample suspensions. After such digestion, both cell samples were inspected with a bright field optical microscope. For spheroplasts preparation, the yeast cells (≈1 · 107 cells/ml) were suspended and washed with sterile water. After that, the cells were harvested by centrifugation and resuspended in a SCEM buffer (1 M sorbitol, 0.1 M sodium citrate (pH = 5.6), 10 mM EDTA and 30 mM 2-mercaptoethanol (Merck KGaA, Germany)). Then 30 U/mL of lyticase (Sigma-Aldrich, USA) was added for digestion by the cell walls. After a 2-hour incubation at 30 °C with occasional inversions, the cells were then collected, washed and re-suspended using a STC buffer (1 M sorbitol, 10 mM Tris-HCl (pH = 7.5), 10 mM CaCl2 (Merck KGaA, Germany)) by centrifugation at 200·g 5 min.
Pulsed electric field treatment technique
A programmable electroporator for the generation of high-voltage square-wave electric pulses (developed at the Center for Physical Sciences and Technology, Vilnius, Lithuania) was used for the PEF experiments. This electroporator has a variable energy storage capacitor, thus accumulation of high energy is avoided. It generates a single or a sequence of single electrical pulses with widths from 3 μs to 10 ms and voltages up to 3.5 kV. It has an LCD screen for the monitoring of the input parameters and an additional LCD screen for the display of the pulse waveform (the current passing through the cuvette with the yeast sample) during the experiment. When experiments are performed with a 2 mm gap electroporation cuvette (VWR International, Taiwan) connected to the device, the electric field strength across the cuvette can reach up to about 16 kV/cm.
Cell wall permeability measurements
For the investigation of the permeability of the yeast cell wall, the concentration changes of TPP+ ions as a function of time was measured. A custom-build mini-potentiostat with a special high sensitivity electronic circuit, protected against static electrical interference, was used for the measurements19. The electrode with the selective membrane was fabricated according to the protocol found in the literature20. During all measurements of the changes of the lipophilic tetraphenylphosphonium (TPP+) ion concentrations, the TPP+ chloride (Acros Organics, Belgium) was used. For all the measurements, the intact yeast cell and spheroplasts suspension concentrations were the 4–6 × 109 colony forming unit (CFU)/mL. For the evaluation of the changes of the TPP+ concentration in both yeast suspensions, 200 μL of the yeast suspension was added to 2 mL of a 1 μM concentration TPP+ solution and the potentiometric changes were recorded. The concentration of TPP+ ions was calculated from the calibration curve19.
To evaluate the role of the cell wall and cell membrane in the TPP+ absorption process, square waveform electrical pulses of 150 μs duration and amplitudes of electric field strength (E) (up to 4.2 kV/cm) were applied to the intact yeast cells and spheroplasts.
To investigate the resealing of the yeast cell walls after such PEF treatment, the TPP+ was added to the electroporation cuvette with the yeast suspension. The initial TPP+ concentration (Nis) of 1 μM was added to the cuvette at different time intervals (Δt = 5, 10, 20, 30, 40, 50, 60, 80, 120 and 180 seconds) after the PEF treatment (E = 5.85, 4.38 and 2.93 kV/cm, tp = 150 μs). It was then incubated for t = 3 min. at 20 °C in each case and centrifuged in order to get cell free supernatant. These experiments were repeated three times and presented as means values. The quantity of TPP+ (N) absorbed by the yeast cells (N = Nis − [TPP+]supernatant) was expressed as the accumulation ratio N/Nm, where Nm is the maximal concentration of TPP+ that can be accumulated in yeast8.
Investigation of the resealing of the cell membrane
The resealing of the cell membrane after treatment with PEF (square waveform electric pulses with amplitude of 5.85 kV/cm and duration of 150 μs) was evaluated using fluorescent dye21 (by loading the cell with Fluorescent SYTOX Green nucleic acid stain (Thermofisher Scientific, USA)). During the investigation, the yeast cell suspension was kept on ice. The fluorescent dye was added to the suspension of up to a 125 nM final concentration at different time intervals in the range from 10 s to 600 s after exposure to the PEF. After incubation for one minute, the specimen was placed into the fluorescence measurements cuvette (light path of 1 cm) of spectrometer LS50B (Perkin Elmer, USA) and exposed to 505 ± 5 nm wavelength light. The cell membrane permeability was evaluated as the fluorescence spectrum intensity at a peak wavelength equal to 529 nm.
Results
TPP+ absorption by yeast cells
The ability of the yeast cells to accumulate the TPP+ ions was investigated by measuring the kinetics of the TPP+ absorption by the cells not treated with the electric field. For this purpose, 200 μL of the yeast suspension was injected into a 2 mL solution having different concentrations of TPP+ ions. The typical signal of the response from the TPP+ selective electrode vs. time dependence reflecting the amount of absorbed TPP+ ions is shown in Fig. 1. As it can be seen, the TPP+ ions uptake kinetics consisted of three stages. These were (i) stage I, lasting ~10 s. This was the fast stage, which appeared immediately after the injection of the yeast cells into the TPP+ solution; (ii) stage II, or the delay stage, which lasted approximately 3–5 minutes after the fast stage, when the concentration of the TPP+ changes became no more than 10% (see inset in Fig. 1) and (iii) stage III, when the slow TPP+ absorption took place and saturation was reached after ~2 hours.
Figure 1.
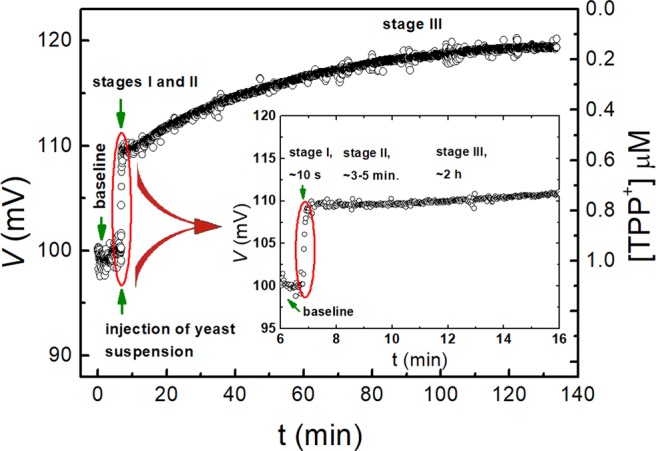
The response signal from the TPP+ selective electrode vs. time dependence in the yeast suspension. The stages of the TPP+ ions absorption kinetics are outlined in the inset.
PEF influence on TPP+ absorption
The influence of the PEF on the kinetics of the TPP+ uptake is shown in Fig. 2. The circular symbols (black) in Fig. 2 correspond to the TPP+ absorption by the yeast cells, which were not affected by the PEF and all show the three TPP+ uptake stages as was described in previous section. The triangular symbols (red) show the kinetics of the TPP+ absorption by the yeast after exposure to PEF of 6 kV/cm strength and 150 μs duration. As can be seen in the figure, stage II, i.e. the delay of the TPP+ uptake is absent and the fast TPP+ concentration decrease (response to the voltage increase corresponding to stage I), is changed to a slower one (stage III). In both cases, (the cells affected and not affected by the PEF), the fast stage was of the same rate, but of a slightly different amplitude. It was thus obvious that the PEF significantly changes the TPP+ absorption rate, i.e. it eliminates the delay stage.
Figure 2.
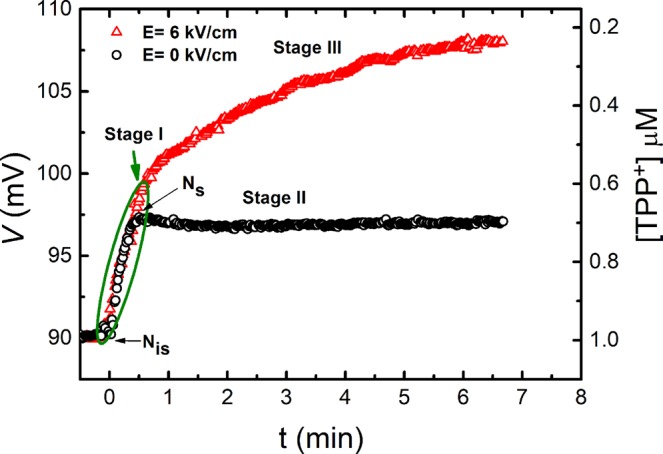
The response of the signal the from TPP+ selective electrode vs. time dependence in the yeast suspension, when E = 0 kV/cm (circles), and 6 kV/cm (triangles).
TPP+ absorption by spheroplasts
For a comparison of the PEF action on intact yeast cells containing wall and wall-free spheroplasts, the TPP+ absorption measurements were performed at different electric field strengths. Figure 3 presents the ratio N/Nm dependence on E, where N is the TPP+ amount accumulated by yeast cells after a 3 min. exposure to 1 µM of TPP+.
Figure 3.
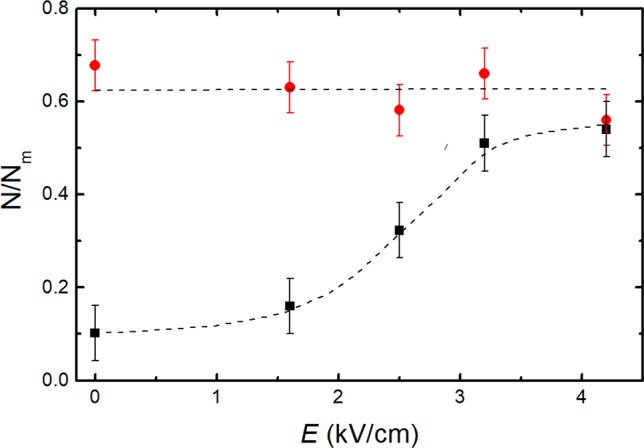
A comparison of the relative change of the TPP+ concentration (N) normalized to the maximal value (Nm) accumulated by intact yeast cells (square dots) and wall-free spheroplasts (circular dots) 3 min. after PEF treatment (150 μs pulse duration, up to 4.2 kV/cm electric field strength).
As it can be seen from Fig. 3, the influx of TPP+ in the case of the intact yeast increased with an increase of the PEF strength. However, there were no changes of the TPP+ influx in the case of wall-free spheroplasts. It is thus evident that PEF can affect the TPP+ influx only in the yeast cells having walls and that the cell walls are mainly responsible for the changes of the TPP+ uptake rates of the yeast cells.
The recovery of cell membrane and wall barrier function after PEF
Our previous investigations demonstrated that high amplitude PEF is able to increase the rate of TPP+ ions uptake by several times and that saturation is reached in relatively short period of time. As it was found that PEF amplitude not exceeding 6 kV/cm with pulse duration of 150 μs does not affect yeast cells viability8, thus these PEF parameters were applied in this study.
Cell membrane resealing after PEF
To study the permeability of the cell membrane after treatment with PEF, the fluorescent dye SYTOX Green was used. The fluorescent dye was added to the cell suspension with different time delays (Δt = 10, 30, 90, 180, 360 and 600 seconds) after treating the cells with PEF (150 μs pulse duration 5.85 kV/cm electric field strength). Figure 4 shows the intensity of the fluorescent spectrum peak value (529 nm) vs. time (Δt) of the fluorescent dye added to the cells after PEF.
Figure 4.
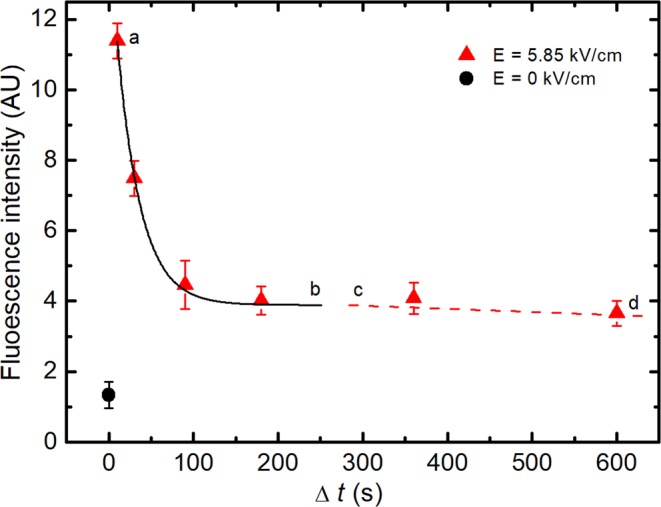
Fluorescence spectrum peak (at 529 nm) intensity vs. time (Δt) of the dye added to the cells after PEF treatment (triangles). The black circle shows the intensity of the fluorescence in the case of the control (untreated) cells. The solid curve (a,b) obtained using Eq. (1), adj. R2 is 0.99394. The dashed line (c,d) represents the extended membrane resealing processes.
The experimental data presented in Fig. 4 show that the fluorescence intensity drops with the increase of time after cell treatment by PEF down to the level of the residual intensity (Ir). The total decay time showing cell membrane relaxation to their initial state after PEF action is approximately 100 seconds and the fluorescence intensity decay can be well fitted by the exponential expression:
| 1 |
here I0 is the fluorescence intensity at Δt = 0 and τl is the characteristic decay time of the lipidic pores. This model describes that part of the resealing process, where the change of fluorescence intensity is fast (see Fig. 4 solid curve a,b). The fitting results show that permeability of a significant part of the cell membrane recovers to its initial state in a characteristic time τl ≈ 20 s. The difference between fluorescence intensity of untreated cells (Fig. 4, black circle) and the residual intensity (Fig. 4, dashed line c,d) might be related to the extended membrane resealing processes due to the experimental setup where the cells were kept on ice22.
Cell wall recovery after PEF
The recovery of the barrier function of the cells walls was investigated by analyzing the TPP+ absorption process of the yeast cells after PEF action with pulse amplitudes of three different electric field strengths. The results of this investigation are presented in Fig. 5.
Figure 5.
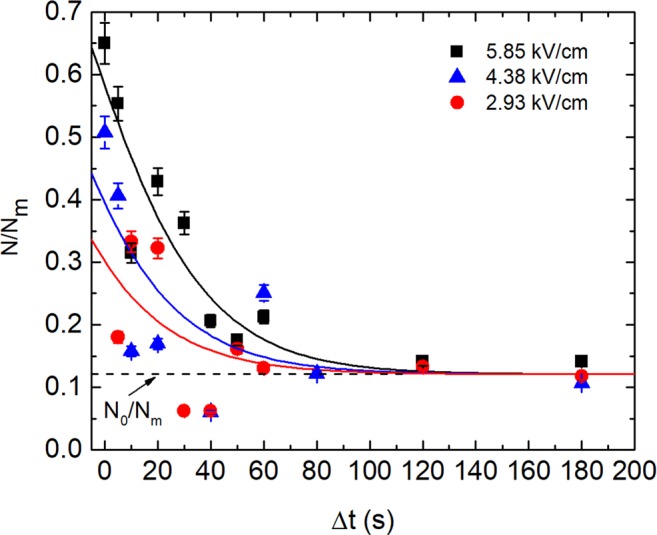
The dependence of TPP+ absorption by yeast cells over time after PEF treatment. The y axis represents the relative TPP+ amount accumulated in the yeast cells; the x axis represents the TPP+ injection time after PEF treatment. The dashed line represents the full recovery of the TPP+ absorption level by the yeast cells (PEF = 0) after 3 minutes of incubation. The solid curves were obtained by using Eq. (10), adj. R2 values are 0.854 (rectangles), 0.582 (triangles) and 0.341 (circles).
Here N/Nm is the ratio between the TPP+ amount (N) accumulated inside the yeast cells after 3 min. exposure in a TPP+ solution and the maximal amount (Nm) of TPP+ which can be accumulated in the tested volume of yeast (without PEF action). The dashed line (N0/Nm) represents the full recovery of the TPP+ absorption process which corresponds to the relative amount of TPP+ (N0/Nm ≈ 0.12) absorbed during 3 min. of incubation. With the rise of the electric field strength from 2.93 kV/cm to 5.85 kV/cm, the relative TPP+ amount absorbed by cells drops to the full recovery level in approximately 90–110 seconds. The solid curves in Fig. 5 represent fitting results using model described in the next Section. It has to be noted that the characteristic time evaluated from modelling results was obtained similar for all three electric field strengths.
The modelling of cell wall recovery process after PEF
The experimental results presented in Fig. 5 show that the recovery of the yeast cell wall after PEF action takes place in a relatively short time when compared to the duration of the whole 1.5 h-long TPP+ ions absorption process (without PEF) until the steady state is reached (see Fig. 1). This means that the post-pulse transient pores in cell wall responsible for the increase of TPP+ absorption rate exist only for a few minutes after the electric pulse action. At high electric field strength (more than 6 kV/cm), the TPP+ ions uptake by the yeast significantly increases and saturation is reached in a relatively short time8. In such case, the TPP+ ions absorption process was described while ignoring the cell wall recovery after the PEF action. Thus for the description of TPP+ absorption kinetics, the following pseudo-second order differential equation was used8:
| 2 |
here N(t) is the time dependent concentration of the TPP+ ions in the supernatant, Nm is maximal change of the TPP+ concentration during the slow period of TPP+ accumulation by the yeast and Ns is the concentration of TPP+ ions at the beginning of stage II (corresponding to TPP+ concentration at the time instant when the absorption process starts (see Fig. 2)), kα is absorption rate coefficient. It was assumed, that kα depends on the electric field strength as follows8:
| 3 |
here b is the empirical constant, which shows the effectiveness of the electric field on permeabilization, f is the cell shape factor (1.5 for spheres), Ep is the amplitude of electric field strength, Eth.p is the threshold electric field strength at which the TPP+ absorption rate begins to be dependent on the electric field, tp is the pulse duration and tch is the characteristic time required to charge the membrane as a capacitor.
However, at lower amplitudes of electric field strength (less than 6 kV/cm in our case), the decay of the post-pulse transient pores in the cell walls can significantly change the kinetics of the TPP+ ions adsorption by the yeast cells. For this reason, the coefficient ka in Eq. (2) was modified by considering that it was not only a function of the pulsed electric field strength and duration, but also depended on the resealing of the transient pores after PEF action.
In order to obtain a mathematical expression of coefficient’s ka dependence on time, we made the following assumptions: (a) that the PEF permeabilization which facilitated the TPP+ ions diffusion is proportional to the number of pores in the cell wall; (b) that the generation of the additional post-pulse non-equilibrium pores is the result of PEF action and that the number of these pores depends on the PEF energy (amplitude and duration of the square waveform pulses); (c) that the characteristic lifetime of the post-pulse transient non-equilibrium pores and the naturally existing equilibrium pores is different. Based on these assumptions the following expression of ka was suggested:
| 4 |
here ka0 is the absorption coefficient at E = 0 kV/cm, ΔMp(E, t) = Mp − Mp,eq is the difference between the whole amount of post-pulse transient non-equilibrium pores (Mp) and the amount of naturally existing equilibrium pores (Mp,eq), and C is a constant. The dependence of ΔMp on time can be obtained from the following first order differential equation:
| 5 |
here k = 1/τd (τd is the characteristic lifetime of the PEF induced pore). The solution of the Eq. (5) is the following:
| 6 |
here ΔMp0 is the initial amount of post-pulse transient non-equilibrium pores generated after the PEF action. Taking into account Eqs (3, 4), one can express ΔMp0 as a function of electric field strength:
| 7 |
The solution of Eq. (2) using Eqs (4) and (6) is the following:
| 8 |
| 9 |
We considered the TPP+ ions absorption delay at the time intervals Δt after the PEF action (Fig. 5). This could be described by Eq. (8) using modified expression (9). It has to be noted that Eqs (8 and 9) represent the solution of Eq. (2) in which the time t starts from the moment when TPP+ was added to the cuvette with the yeast suspension. In order to investigate the absorption delay, an experiment was performed by adding TPP+ with a Δt delay after PEF action. Therefore, ΔMp0 in Eq. (9) had to be replaced by ΔMp0 exp(−Δt/τd), because the concentration of the non-equilibrium pores had exponentially decreased during the time Δt. In such case, the S in Eq. (9) is modified as follows:
| 10 |
The solid curves in Fig. 5 show the fitting of the experimental data using modified Eq. (8), when SΔt instead of S (see Eq. (9)) was used:
| 11 |
During fitting the incubation time t, after which the absorption of the TPP+ was measured, was kept constant t = 3 min. The level N0/Nm marked by the dashed line in Fig. 5 corresponds to the full recovery of the cell wall, where N0 is the amount of the TPP+ absorbed by untreated yeast cells:
| 12 |
here S0 is:
| 13 |
Thus, the N/Nm − N0 /Nm in Fig. 5 shows the difference between the TPP+ amount absorbed by the PEF affected cells (N) at Δt and the amount of TPP+ absorbed by the untreated yeast cells (N0) relative to maximal amount Nm. The data when Δt = 0 in Fig. 5 represent the N/Nm ratio immediately after the PEF action. One can see that at Δt = 0 for E = 5.85 kV/cm, the ratio N/Nm ≈ 0.65, which is in good agreement with the results presented in8, where the absorption coefficient’s ka dependence on E was expressed as Eq. (3) without considering its dependence on delay time Δt. The fitting results using this model (solid curves in Fig. 5) show that the characteristic time (τd) of the wall recovery almost does not depend on the PEF electric field strength. Due to scatter of experimental data the fitting procedure resulted in τd values in the range of (21–27) s, thus the solid curves in Fig. 5 were shown for average τd = 24 s.
Discussion
Tetraphenylphosphonium (TPP+), a lipophilic cation is widely used as a probe for various cells as well as an artificial membrane potential measurements20,23–26. The ability of the aromatic groups to delocalize and shield the electric charge and to increase the lipid solubility facilitates their translocation across the hydrophobic barrier27,28. The accumulation of TPP+ ions in yeast cells strongly depends on various factors, such as the yeast strain, the properties of the cell wall, the yeast growth phase, the growing conditions and mutations determining the structure of the cell wall and others25,29–31. Unlike structurally similar compound dibenzyldimethylammonium, TPP+ is not transported via the thiamine transport system, nor via another inducible translocation mechanism. As described in the literature, the uptake of TPP+ ions into the yeast cells has distinct stages. Two groups of authors, Eraso et al. and Slayman et al. observed the biphasic uptake of TPP+ when the yeast was collected in the logarithmic phase of growth25,32. In both cases, the exponential and quasi-linear stages were outlined. Ketteter et al. experimented with artificial lipid bilayer membranes, which showed a three-stage uptake: (1) adsorption to the membrane-solution interface; (2) passage of the ion to the opposite interface; and (3) desorption into the aqueous solution. The rate-determining step is the jump from the adsorption site into the aqueous phase24. For lipophilic molecules such as TPP+, it usually takes from 0.5 up to 2 or more hours to reach the steady state25,32. In our experiments, three stages of TPP+ uptake was observed (Fig. 1). Stage I was due to the injection of yeast suspension into the sample; stage II, when the TPP+ concentration changes were delayed for several minutes. As far as we know, these were absent in the works of other authors due to the different experimental setups. The last stage, i.e. stage III, consisting of slow TPP+ absorption and a plateau, is similar to biphasic TPP+ absorption, which is outlined in the works of Eraso et al. and Slayman et al.25,32.
Stage I in our experiments could be explained by TPP+ ions adsorption on the surfaces of negatively charged yeast cells4 and the interaction between it and the positive TPP+ ions (i.e. sorbate). A similar behavior of the sorbate was observed during the investigation of the yeast cells, when the fast, reversible and metabolically-independent adsorption step was attributed to the cell wall surface complexation with metal cation uptake33. The stage II, i.e. delay stage, could be explained by the additional time needed for the TPP+ ions to reach the surface of the plasma membrane through the cell wall and periplasmic space. The slow stage reflects the process of TPP+ absorption deeper inside the yeast cell and through the membrane. It should be noted that due to the large TPP+ concentration at the surface of the cell, the expulsion of TPP+ ions from the cell to the solution has to be negligible. Thus, the yeast cells accumulate TPP+ ions mainly during the last slow stage. As is known, the main ion transport systems into the yeast cell include ion pumps, several transporters, a potassium channel and a non-selective cation current. The presence of a complex structure cell wall adds more complexity for ion transport. Thus, the TPP+ absorption experiments were performed in cell wall-free spheroplasts, which were obtained by degrading the wall with the enzyme lyticase. Such spheroplasts provide an easy means to manipulate the experimental system for studies of whole genome transferring34, cell fusion35 or toxin transport36. In our experiments, the system of spheroplasts served for the clarification of the role of the cell wall in TPP+ absorption. As seen from Fig. 3 (circular dots), the PEF strength had no effect on TPP+ absorption for spheroplasts, which was almost the same at all applied electric field strengths including E = 0. This result showed that the main barrier for TPP+ ion absorption is the cell wall, not the membrane.
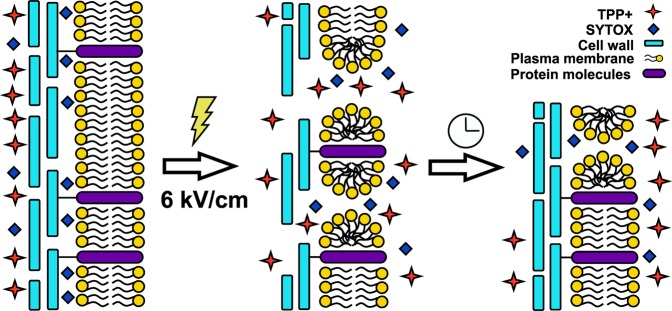
The experimental results presented in Figs 4 and 5 demonstrated that both the membrane and the wall relaxation process after PEF lasted for approximately 100 s. Moreover, the characteristic time of these relaxations evaluated by means of mathematical simulations was also of the same order. Good fitting was also obtained at all the different electric field strengths, which had been applied, of the same characteristic lifetime of the post-pulse transient pores in the cell walls. This was τd = 24 s, which was close enough to the characteristic lifetime of lipidic pores, which was τl ≈ 20 s (see Fig. 4). However, as is known from molecular dynamics related with thermal processes, the post-pulse lipidic pore lifetime is in the range from 100 ns17 to 3·ms37,38, which is drastically shorter than the characteristic relaxation time observed in our experiments. Moreover, experimental investigation showed that this relaxation time could range from milliseconds39 to several hours if the cells are cooled down after electroporation by putting them on ice22. It is evident, that the lifetimes of the lipid pores may vary along a broad range and depend on the experimental setup or the object40. In our case, however, this cannot be attributed to the pores, which disappear via spontaneous thermal fluctuations. Yeast treatment with PEF simultaneously induces permeabilization of the whole cell barrier, i.e. both the cell wall and the membrane. The recovery of these parts to a non-permeabilized state takes a similar time (Scheme 1).
Scheme 1.
Schematic overview of the cell wall and the resealing of the cell membrane after the PEF treatment.
The strong connection between the resealing of the yeast cell membrane and the wall recovery allows one to assume that this recovery process is “mechanical” in nature, which could be related to changes of osmotic pressure during electroporation and the processes taking place after the PEF. The verification of this hypothesis could be an object of future investigations.
In conclusion, our investigations confirmed that the rate of absorption of the TPP+ ions into the yeast cells is stimulated by the action of the pulsed electric field (PEF). Moreover, as the main barrier to the influx of TPP+ ions is the cell wall and the PEF stimulates the generation of transient (non-equilibrium) pores in this wall, this is responsible for the increase of the TPP+ ions absorption rate. It was demonstrated that the treatment of the yeast by the PEF simultaneously induces permeabilization of both parts of this cell barrier, i.e. the cell wall and the membrane. The characteristic time of recovery of these parts to a non-permeabilized state takes several tens of seconds, what demonstrates a strong coupling between these processes.
Acknowledgements
This research was funded by the European Regional Development Fund according to the supported activity ‘Research Projects Implemented by World-class Researcher Groups’ under Measure No. 01.2.2-LMT-K-718-01-0063.
Author Contributions
A.S. performed research; analyzed data; drafted the manuscript, R.C.-G. analyzed and interpreted the data, drafted the manuscript, A.Z., P.S. and A.D. performed the research. A.R. and N.Z. designed the research and contributed the analytic tools, S.B. designed the research and approved the final version of the manuscript to be submitted.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Carlson M, Botstein D. Two differentially regulated mRNAs with different 5′ ends encode secreted and intracellular forms of yeast invertase. Cell. 1982;28:145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- 2.Sumner-Smith M, Bozzato RP, Skipper N, Davies RW, Hopper JE. Analysis of the inducible MEL1 gene of Saccharomyces carlsbergensis and its secreted product, alpha-galactosidase (melibiase) Gene. 1985;36:333–340. doi: 10.1016/0378-1119(85)90188-X. [DOI] [PubMed] [Google Scholar]
- 3.Parrou JL, Jules M, Beltran G, François J. Acid trehalase in yeasts and filamentous fungi: Localization, regulation and physiological function. FEMS Yeast Res. 2005;5:503–511. doi: 10.1016/j.femsyr.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Orlean P. Architecture and biosynthesis of the Saccharomyces cerevisiae cell wall. Genetics. 2012;192:775–818. doi: 10.1534/genetics.112.144485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francois, J. M. In Yeast Membrane Transport (eds José Ramos, Hana Sychrová, & Maik Kschischo) 11–31 (Springer International Publishing, 2016).
- 6.Aouida M, Tounekti O, Belhadj O, Mir LM. Comparative roles of the cell wall and cell membrane in limiting uptake of xenobiotic molecules by Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 2003;47:2012–2014. doi: 10.1128/aac.47.6.2012-2014.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yasuda K, Ohmizo C, Katsu T. Potassium and tetraphenylphosphonium ion-selective electrodes for monitoring changes in the permeability of bacterial outer and cytoplasmic membranes. Journal of Microbiological Methods. 2003;54:111–115. doi: 10.1016/S0167-7012(02)00255-5. [DOI] [PubMed] [Google Scholar]
- 8.Stirke A, et al. Electric field-induced effects on yeast cell wall permeabilization. Bioelectromagnetics. 2014;35:136–144. doi: 10.1002/bem.21824. [DOI] [PubMed] [Google Scholar]
- 9.Ballarin-Denti A, Slayman C, Kuroda H. Small lipid-soluble cations are not membrane voltage probes for Neurospora or Saccharomyces. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1994;1190:43–56. doi: 10.1016/0005-2736(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 10.Jamur MC, Oliver C. Permeabilization of cell membranes. Methods in molecular biology (Clifton, N.J.) 2010;588:63–66. doi: 10.1007/978-1-59745-324-0_9. [DOI] [PubMed] [Google Scholar]
- 11.Ganeva V, Galutzov B, Teissie J. Evidence that Pulsed Electric Field Treatment Enhances the Cell Wall Porosity of Yeast Cells. Appl. Biochem. Biotechnol. 2014;172:1540–1552. doi: 10.1007/s12010-013-0628-x. [DOI] [PubMed] [Google Scholar]
- 12.José Ramos, H. S. a. M. K. Yeast Membrane Transport. Vol. 892 (Springer Nature Switzerland AG, 2016).
- 13.Cohen, B. E. Membrane Thickness as a Key Factor Contributing to the Activation of Osmosensors and Essential Ras Signaling Pathways. Frontiers in Cell and Developmental Biology6, 10.3389/fcell.2018.00076 (2018). [DOI] [PMC free article] [PubMed]
- 14.Levin DE. Cell Wall Integrity Signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2005;69:262. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupont S, Rapoport A, Gervais P, Beney L. Survival kit of Saccharomyces cerevisiae for anhydrobiosis. Appl. Microbiol. Biotechnol. 2014;98:8821–8834. doi: 10.1007/s00253-014-6028-5. [DOI] [PubMed] [Google Scholar]
- 16.Stirke A, et al. Permeabilization of yeast Saccharomyces cerevisiae cell walls using nanosecond high power electrical pulses. Applied Physics Letters. 2014;105:253701. doi: 10.1063/1.4905034. [DOI] [Google Scholar]
- 17.Weaver, J. C. a. V. & Thomas, P. Pore lifetimes in cell electroporation: Complex dark pores? ARXIV eprint arXiv:1708.07478 (2017).
- 18.Kawai S, Hashimoto W, Murata K. Transformation of Saccharomyces cerevisiae and other fungi: Methods and possible underlying mechanism. Bioengineered Bugs. 2010;1:395–403. doi: 10.4161/bbug.1.6.13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balevicius Z, et al. Compact high-sensitivity potentiometer for detection of low ion concentrations in liquids. Review of Scientific Instruments. 2018;89:044704. doi: 10.1063/1.5023443. [DOI] [PubMed] [Google Scholar]
- 20.Moreno AJ, Santos DL, Magalhães-Novais S, Oliveira PJ. Measuring Mitochondrial Membrane Potential with a Tetraphenylphosphonium-Selective Electrode. Current Protocols in Toxicology. 2015;65:25.25.21–25.25.16. doi: 10.1002/0471140856.tx2505s65. [DOI] [PubMed] [Google Scholar]
- 21.Garcia PA, Ge Z, Moran JL, Buie CR. Microfluidic Screening of Electric Fields for Electroporation. Sci. Rep. 2016;6:21238. doi: 10.1038/srep21238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yano, K. I. et al. Bioelectrics: Biological Responses. In Bioelectrics, 155–274 (2017).
- 23.Boxman AW, Barts PWJA, Borst-Pauwels GWFH. Some characteristics of tetraphenylphosphonium uptake into Saccharomyces cerevisiae. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1982;686:13–18. doi: 10.1016/0005-2736(82)90146-8. [DOI] [PubMed] [Google Scholar]
- 24.Ketterer B, Neumcke B, Läuger P. Transport mechanism of hydrophobic ions through lipid bilayer membranes. The Journal of Membrane Biology. 1971;5:225–245. doi: 10.1007/bf01870551. [DOI] [PubMed] [Google Scholar]
- 25.Eraso P, Mazón MJ, Gancedo JM. Pitfalls in the measurement of membrane potential in yeast cells using tetraphenylphosphonium. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1984;778:516–520. doi: 10.1016/0005-2736(84)90402-4. [DOI] [Google Scholar]
- 26.Labajova A, et al. Evaluation of mitochondrial membrane potential using a computerized device with a tetraphenylphosphonium-selective electrode. Analytical Biochemistry. 2006;353:37–42. doi: 10.1016/j.ab.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 27.Altenbach C, Seelig J. Binding of the lipophilic cation tetraphenylphosphonium to phosphatidylcholine membranes. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1985;818:410–415. doi: 10.1016/0005-2736(85)90016-1. [DOI] [Google Scholar]
- 28.Ross MF, et al. Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochemistry. Biokhimiia. 2005;70:222–230. doi: 10.1007/s10541-005-0104-5. [DOI] [PubMed] [Google Scholar]
- 29.De Nobel JG, Barnett JA. Passage of molecules through yeast cell walls: A brief essay-review. Yeast. 1991;7:313–323. doi: 10.1002/yea.320070402. [DOI] [PubMed] [Google Scholar]
- 30.De Nobel JG, Klis FM, Priem J, Munnik T, Van Den Ende H. The glucanase-soluble mannoproteins limit cell wall porosity in Saccharomyces cerevisiae. Yeast. 1990;6:491–499. doi: 10.1002/yea.320060606. [DOI] [PubMed] [Google Scholar]
- 31.Daugelavicius R, Buivydas A, Sencilo A, Bamford DH. Assessment of the activity of RND-type multidrug efflux pumps in Pseudomonas aeruginosa using tetraphenylphosphonium ions. International journal of antimicrobial agents. 2010;36:234–238. doi: 10.1016/j.ijantimicag.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 32.Slayman CL, Kuroda H, Ballarin-Denti A. Cation effluxes associated with the uptake of TPP+, TPA+, and TPMP+ by Neurospora: evidence for a predominantly electroneutral influx process. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1994;1190:57–71. doi: 10.1016/0005-2736(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 33.Fomina M, Gadd GM. Biosorption: current perspectives on concept, definition and application. Bioresource technology. 2014;160:3–14. doi: 10.1016/j.biortech.2013.12.102. [DOI] [PubMed] [Google Scholar]
- 34.Karas BJ, et al. Transferring whole genomes from bacteria to yeast spheroplasts using entire bacterial cells to reduce DNA shearing. Nature Protocols. 2014;9:743. doi: 10.1038/nprot.2014.045. [DOI] [PubMed] [Google Scholar]
- 35.Russell I, Stewart GG. Spheroplast Fusion of Brewers Yeast Strains. Journal of the Institute of Brewing. 1979;85:95–98. doi: 10.1002/j.2050-0416.1979.tb06834.x. [DOI] [Google Scholar]
- 36.Becker B, Schmitt MJ. Adapting Yeast as Model to Study Ricin Toxin A Uptake and Trafficking. Toxins. 2011;3:834–847. doi: 10.3390/toxins3070834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasilkoski Z, Esser AT, Gowrishankar TR, Weaver JC. Membrane electroporation: The absolute rate equation and nanosecond time scale pore creation. Physical review. E, Statistical, nonlinear, and soft matter physics. 2006;74:021904. doi: 10.1103/PhysRevE.74.021904. [DOI] [PubMed] [Google Scholar]
- 38.Son RS, Smith KC, Gowrishankar TR, Vernier PT, Weaver JC. Basic features of a cell electroporation model: illustrative behavior for two very different pulses. The Journal of membrane biology. 2014;247:1209–1228. doi: 10.1007/s00232-014-9699-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melikov KC, et al. Voltage-induced nonconductive pre-pores and metastable single pores in unmodified planar lipid bilayer. Biophysical journal. 2001;80:1829–1836. doi: 10.1016/s0006-3495(01)76153-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pakhomov AG, et al. Long-lasting plasma membrane permeabilization in mammalian cells by nanosecond pulsed electric field (nsPEF) Bioelectromagnetics. 2007;28:655–663. doi: 10.1002/bem.20354. [DOI] [PubMed] [Google Scholar]