Abstract
Background
Colorectal cancer remains a common cause of cancer death in the UK, with surgery being the mainstay of treatment. An objective measurement of the suitability of each patient for surgery, and their risk–benefit calculation, would be of great utility. We postulate that sarcopenia (low muscle mass) could fulfil this role as a prognostic indicator. The aim of this study was to determine the relationship between sarcopenia and long-term outcomes in patients undergoing elective bowel resection for colorectal cancer.
Methods
One hundred and sixty-three consecutive patients who had elective curative colorectal resection for cancer were eligible for inclusion in the study. Psoas muscle mass was assessed on preoperative computed tomography scan at the level of the L3 vertebra and standardised for patient height (total psoas index, TPI). Sarcopenia (low muscle mass) was defined as < 524 mm2/m2 in males and 385 mm2/m2 in females. In addition to clinical–pathological parameters, postoperative complications were recorded and patients were followed up for mortality for 1 year after surgery.
Results
Sarcopenia was present in 19.6% of the study participants and was significantly related to body mass index (p = 0.007), 30-day mortality (p = 0.042) and 1-year mortality (p = 0.046). In univariate analysis, American Society of Anesthesiologists grade (p = 0.016), tumour stage (p = 0.018) and sarcopenia (p = 0.043) were found to be significant independent predictors of 1-year mortality.
Conclusions
This study has found sarcopenia to be prevalent in patients with colorectal cancer having elective surgery. Independent of age, sarcopenia was associated with poorer 30-day mortality and survival at 1 year. Measurement of muscle mass preoperatively could be used to stratify a patient’s risk, allowing targeted strategies such as prehabilitation, to be implemented to modify sarcopenia and improve long-term outcomes for patients.
Keywords: Colorectal cancer, Sarcopenia, One-year survival
Introduction
Colorectal cancer is the fourth most common cancer in the UK, with over 41,000 new diagnoses in 2014 [1]. With incidence increasing with age, and the ageing population expanding, there is a clinical need for new prognostic knowledge-aided decision making to improve long-term outcomes [2, 3]. One potential prognostic marker is sarcopenia. Sarcopenia was defined in 2010 as ‘a syndrome characterised by progressive and generalised loss of skeletal muscle mass and strength’ (the European Working Group on Sarcopenia in Older People (EWGSOP) [4]. There are various techniques available to measure sarcopenia, varying from simple to complex, but computed tomography (CT) measurement of the psoas muscle, with established cutoffs defining sarcopenia, is a widely accepted technique [4].
There have been numerous studies that have assessed the relationship between sarcopenia and surgical outcomes in patients having various intra-abdominal operations, including radical cystectomy, liver transplantation and emergency general surgery, with their findings pointing towards an association between sarcopenia and mortality [5–8].
Studies specifically analysing the influence of sarcopenia on postoperative elective colorectal cancer patients have found that the presence of sarcopenia results in an increased risk of postoperative complications, increased length of hospital stay and increased cost of care [9–15]. A study published in 2019 showed that sarcopenia is highly predictive of serious postoperative complications in colorectal cancer patients [15]. In contrast, there are only a few studies focusing on the relationship between sarcopenia and long-term mortality [16–19]. Although all suggested a negative influence of sarcopenia on 1-year survival, drawing conclusions is difficult due to the different patient populations studied and varying methodologies.
The primary aim of this study was to determine the relationship between preoperative sarcopenia and mortality at 1 year in patients who had elective colorectal cancer resection.
Materials and methods
Data sources and study population
The study was registered with the NHS Greater Glasgow and Clyde Clinical Effectiveness Department (August 2016). Analysis was undertaken of data extracted from the prospective enhanced recovery after surgery (ERAS) database at the Royal Alexandra Hospital, Paisley, for the period January 2015–December 2016. This database has been set up as a part of the National Enhanced Recovery Colorectal Initiative (NERCI) supported by the Whole System Patient Flow Improvement Programme as part of the Scottish Government’s Health Performance and Delivery Directorate. Briefly, data from all elective colorectal surgery (both benign and malignant pathology) are submitted from each surgical unit in Scotland every month with regular feedback after central analysis of the data.
Inclusion criteria extended to any patient who had elective colorectal resection with curative intent, where pathology had confirmed colonic or rectal adenocarcinoma. Stage 4 patients with resectable liver and lung metastases, as adjudged by the relevant clinical specialists, were included. Patients were excluded if: they had received neo-adjuvant therapy; had surgery to treat any condition other than colorectal cancer; had widespread unresectable metastatic disease; had surgery as an emergency; if no preoperative CT scan was available; or if their most recent CT scan was performed more than 4 months before surgery.
Demographic, anthropometric and clinical data including height, weight, body mass index (BMI) [weight (kg)/height (m)2], American Society of Anesthesiologists (ASA) score, operative procedure, American Joint Committee on Cancer (AJCC) TNM stage [20], length of hospital stay (days), postoperative complications and grade [21] and re-admission to the hospital within 30 days were analysed. In addition, patients’ electronic records were followed up for mortality for 1 year after the date of surgery.
Sarcopenia measurement: CT scan analysis
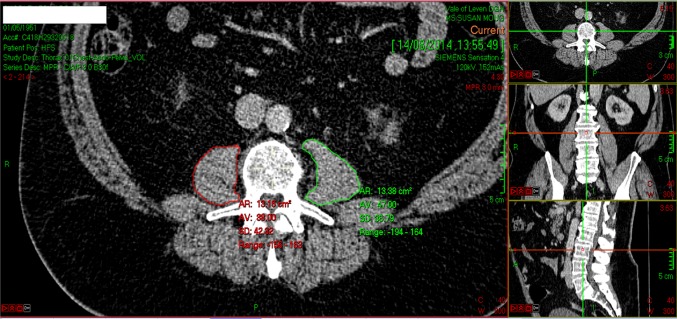
To measure sarcopenia in each patient, the total cross-sectional area of the psoas muscles (total psoas area, TPA) was measured using a manual technique at the level of the L3 vertebra on preoperative CT (median interval between CT scan and surgery was 38 days, range 2–119 days) [22]. To ensure standardisation, the exact level of measurement was defined as the CT slice in which both L3 transverse processes were maximally in view. Area was measured using a free-hand drawing technique on Picture Archiving and Communication System (PACS) software (Fig. 1). The outline of each individual psoas muscle was traced, the area of each calculated, and summated to provide the TPA (mm2). The TPA was then standardised for patient height using the formula: TPA (mm2)/height (m2). This provided the total psoas index (TPI) for each patient.
Fig. 1.
CT image at the level of the L3 vertebra, demonstrating the method of outlining the left (green) and right (red) psoas muscle and measuring their respective areas
For the purposes of this study, the threshold values used for the diagnosis of sarcopenia are the same as those used by Prado et al. in their widely cited 2008 paper [23]: 524 mm2/m2 for males and 385 mm2/m2 for females. All individuals with a TPI below this threshold for their gender were classified as sarcopenic. For the purposes of this study, sarcopenia is defined as an absolute variable; patients were either sarcopenic or non-sarcopenic.
To ensure reliability of our technique, 20 scans were randomly selected and measured for TPI by blindly trained investigators to allow calculation of inter- and intra-class correlation coefficients (ICCC). The ICCC values for inter- and intra-class reliability were 0.94 and 0.99, respectively (values close to 1 indicate excellent agreement).
Statistical analysis
Relevant variables were transcribed to SPSS version 21 [24] to enable statistical analysis. Descriptive statistics were used to characterise the patient population, with continuous variables summarised by median and interquartile range and categorical variables presented in tabulated form with percentages. Continuous variables were tested using non-parametric methods.
To determine the primary outcome of the influence of sarcopenia on survival at 1 year, survival analysis using log-rank testing was performed. Further exploration of variables influencing mortality was performed using univariate analysis and a Cox proportional hazards model. The level of significance was set at 5%.
Results
Patient demographics, physiological and pathological characteristics
Over the study period, 331 patients had elective colorectal surgery, with 163 (49.2%) eligible for inclusion in the study (Fig. 2). The median age of the cohort was 70 years (IQR 61–75) and 99 (60.7%) were male. The majority of surgery was for patients with rectal cancer who had an ASA of 2 and 112 (68.7%) were overweight or obese. One hundred and forty-nine (91.4%) had a pathological R0 resection with only 8.6% of patients having a major complication leading to a median length of the hospital stay of 8 days (IQR 6–12) [Table 1]. Eleven patients had stage 4 tumours, however, these patients had lung/liver metastases that were considered resectable and so they were operated on with curative intent.
Fig. 2.
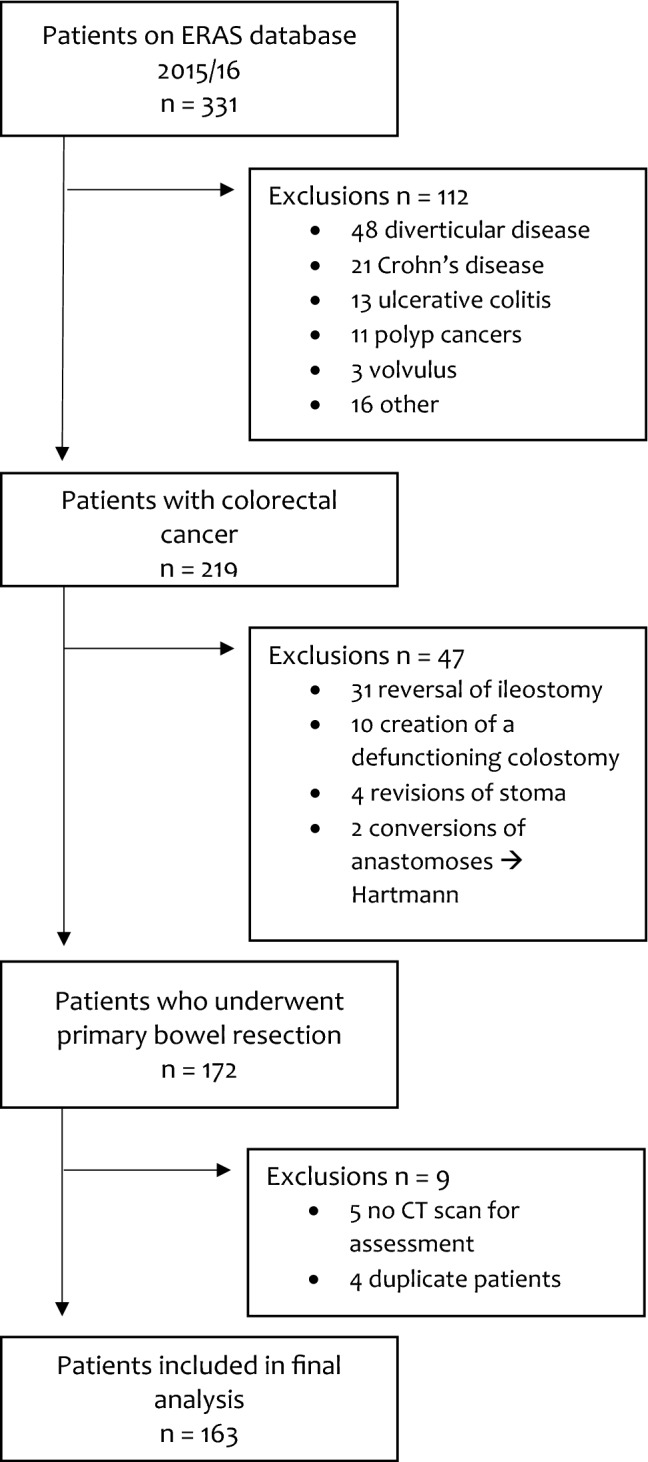
Patient flowchart for the study and reasons for exclusion of individuals. ERAS enhanced recovery after surgery
Table 1.
Baseline demographics, clinical, and pathological characteristics of the patients underdoing elective surgery for colorectal cancer
| Characteristic | n (%) |
|---|---|
| Sex | |
| Male | 99 (60.7) |
| Female | 64 (39.3) |
| Median age, years (IQR) | |
| 70 (61–75) | |
| Age category, years | |
| < 65 | 57 (35) |
| 65–74 | 59 (36.2) |
| ≥ 75 | 47 (28.8) |
| BMI category kg/m2 | |
| Underweight | 4 (2.5) |
| Normal | 47 (28.8) |
| Overweight | 67 (41.1) |
| Obese | 45 (27.6) |
| ASA grade | |
| 1 | 4 (2.5) |
| 2 | 101 (62) |
| 3 | 56 (34.4) |
| 4 | 2 (1.2) |
| TNM stage | |
| 0 | 8 (4.9) |
| 1 | 34 (20.9) |
| 2 | 57 (35) |
| 3 | 53 (32.5) |
| 4 | 11 (6.7) |
| Site of cancer | |
| Colon | 72 (44.2) |
| Rectum | 91 (55.8) |
| Procedure | |
| Right hemicolectomy | 52 (31.9) |
| Left hemicolectomy or sigmoid colectomy | 15 (9.2) |
| Total or sub-total colectomy | 6 (3.7) |
| Anterior resection | 65 (39.9) |
| APER | 25 (15.3) |
| Resection margin | |
| R0 | 149 (91.4) |
| R1 | 14 (8.6) |
| In-hospital complication | |
| Yes | 91 (55.8) |
| No | 72 (44.2) |
| Major in-hospital complication* | |
| Yes | 14 (8.6) |
| No | 149 (91.4) |
| Median length of stay (IQR), days | |
| 8 (6–12) | |
| Re-admitted within 30 days | |
| Yes | 21 (12.9) |
| No | 142 (87.1) |
| Sarcopenic | |
| Yes | 32 (19.6) |
| No | 131 (80.4) |
*Complications classified as 3 or greater on the Clavien–Dindo scale
ASA American Society of Anesthesiologists, AJCCTNM American Joint Committee on Cancer TNM stage, BMI body mass index
Sarcopenia
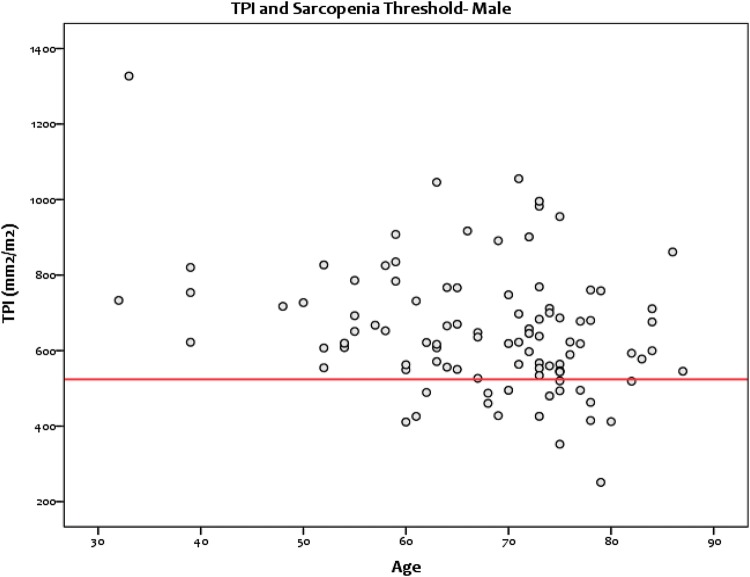
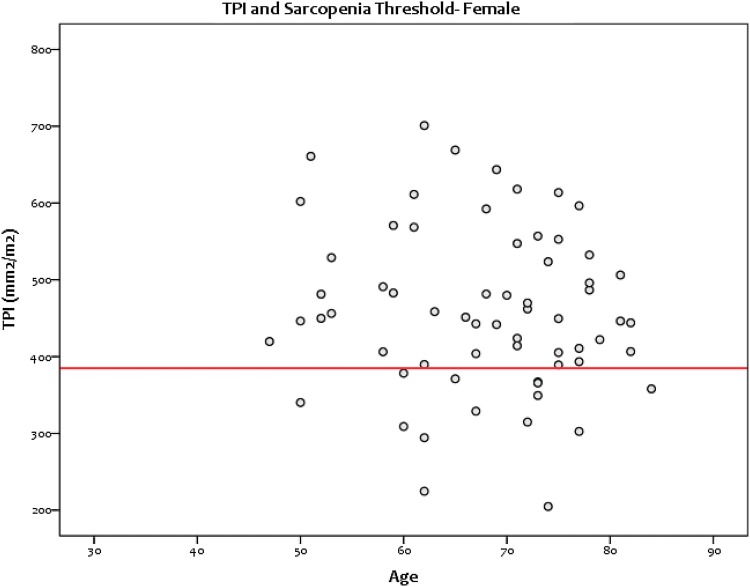
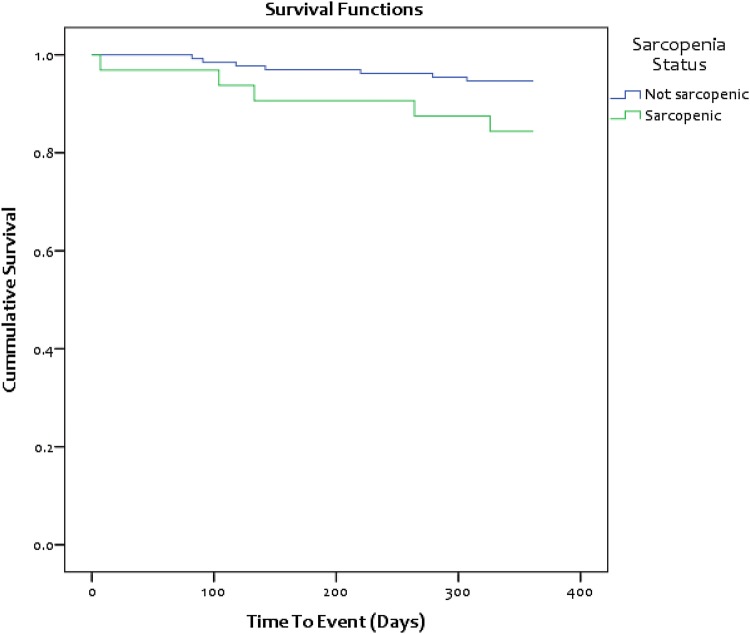
Overall, 32 (19.6%) patients having elective colorectal cancer surgery were sarcopenic: 18/99 males (18.2%) (Fig. 3) and 14/64 females (21.9%) (Figs. 3, 4). The characteristics of the sarcopenic and non-sarcopenic groups are compared in Table 2. Significant differences were noted between the two groups in the categories: BMI (p = 0.007), 30-day mortality (p = 0.042) and 1-year mortality (p = 0.046). Here, the sarcopenic patients were more likely to be classified as underweight and have increased postoperative mortality at 30 days and 1 year [Fig. 5].
Fig. 3.
Scatterplot showing the spread of TPI values for male patients and the threshold for sarcopenia
Fig. 4.
Scatterplot showing the spread of TPI values for female patients and the threshold for sarcopenia
Table 2.
Demographics, clinical, and pathological characteristics of patients with sarcopenia compared to patients without sarcopenia
| Sarcopenic (n = 32) | Non-sarcopenic (n = 131) | p value | |
|---|---|---|---|
| Sex | |||
| Male | 18 (56.3) | 81 (61.8) | 0.350 |
| Female | 14 (43.8) | 50 (38.2) | |
| Age category, years | |||
| < 65 | 8 (25.0) | 49 (37.4) | 0.412 |
| 65–74 | 13 (40.6) | 46 (35.1) | |
| ≥ 75 | 11 (34.4) | 36 (27.5) | |
| BMI category kg/m2 | |||
| Underweight | 3 (9.4) | 1 (0.8) | 0.007* |
| Normal | 13 (40.6) | 34 (26.0) | |
| Overweight | 11 (34.4) | 56 (42.7) | |
| Obese | 5 (15.6) | 40 (30.5) | |
| Median ASA (SD) | |||
| 2 (0.56) | 2 (0.55) | 0.542 | |
| TNM stage | |||
| 0 | 3 (9.4) | 5 (3.8) | 0.194 |
| 1 | 3 (9.4) | 31 (23.7) | |
| 2 | 12 (37.5) | 45 (34.4) | |
| 3 | 12 (37.5) | 41 (31.3) | |
| 4 | 2 (6.3) | 9 (6.9) | |
| Site of cancer | |||
| Colon | 17 (53.1) | 55 (42.0) | 0.174 |
| Rectum | 15 (46.9) | 76 (58.0) | |
| Resection margin | |||
| R0 | 30 (93.8) | 119 (90.8) | 0.598 |
| R1 | 2 (6.3) | 12 (9.2) | |
| In-hospital complication | |||
| Yes | 14 (43.8) | 73 (55.7) | 0.957 |
| No | 18 (56.3) | 60 (44.3) | |
| Major in-hospital complication | |||
| Yes | 2 (6.3) | 12 (9.2) | 0.598 |
| No | 30 (93.8) | 119 (90.8) | |
| Median length of stay (IQR), days | |||
| 8 (6–12) | 8 (6–12) | 0.567 | |
| Re-admitted within 30 days | |||
| Yes | 3 (9.4) | 18 (13.7) | 0.509 |
| No | 28 (90.6) | 113 (86.3) | |
| Alive at 30 days | |||
| Yes | 31 (96.9) | 131 (100) | 0.042* |
| No | 1 (3.1) | 0 (0) | |
| Alive at 90 days | |||
| Yes | 31 (96.9) | 130 (0.8) | 0.277 |
| No | 1 (3.1) | 1 (99.2) | |
| Alive at 1 year | |||
| Yes | 27 (84.4) | 124 (94.7) | 0.046* |
| No | 5 (15.6) | 7 (5.3) | |
Complications classified as 3 or greater on the Clavien–Dindo scale
ASA American Society of Anesthesiologists, AJCCTNM American Joint Committee on Cancer TNM stage, BMI body mass index
*P < 0.05: level of significance
Fig. 5.
Comparison of 1-year mortality between colorectal cancer patients with sarcopenia and those without sarcopenia
Survival at 1 year
For the whole patient cohort, univariate analysis found sarcopenia (p = 0.043), tumour stage (p = 0.018), ASA grade (p = 0.016) and major complications (p = 0.021) to be significantly associated with survival at 1 year. Multivariable analysis revealed that only ASA grade and tumour stage were significantly independent predictors of mortality at 1 year (p = 0.042 and p = 0.007, respectively) (Table 3).
Table 3.
Univariate and multivariable analyses of factors influencing survival at 1 year in patients who have undergone elective surgery for colorectal cancer
| Univariate analysis | Multivariable analysisa | |||
|---|---|---|---|---|
| Variable | Log-rank p value | Hazard ratio | 95% CI | p value |
| Sarcopenia | 0.043 | 2.233 | 0.665–7.504 | 0.194 |
| TNM stage | 0.018 | 2.609 | 1.307–5.208 | 0.007 |
| ASA | 0.016 | 2.861 | 1.037–7.892 | 0.042 |
| Major complication | 0.021 | – | – | – |
| BMI category | 0.225 | – | – | – |
| Age group | 0.546 | – | – | – |
| Gender | 0.418 | – | – | – |
ASA American Society of Anesthesiologists, BMI body mass index
aIncluded preoperative variables that had been identified as significant in univariate analysis
Discussion
This study demonstrated that sarcopenia is prevalent, occurring in nearly one-fifth of the patients having elective colorectal cancer surgery. In addition to confirming that sarcopenia places patients at greater risk of postoperative complications, this work has found sarcopenia also negatively influences survival at 1 year after curative surgery.
These results have strengths over the previous publications looking at 1 year survival. It is prospective and contains both colon and rectal cancer patient populations, not just rectal [18]. In addition, two of the previous studies assessed sarcopenia in Asian populations, and members of these populations have accepted differences in lifestyle and body habitus when compared to the western population [18, 19]. For example, in the paper by Choi et al., mean age of the patients was 61.3 years and mean BMI was 23.8 kg/m2. These figures are both significantly lower than in the cohort of patients included in this study. Overall, our findings are more applicable to the western colorectal cancer patients, adding to the evolving evidence that sarcopenia is a negative prognostic factor for patients having elective gastrointestinal oncological surgery, and more specifically, colorectal resection.
The mechanism underlying the significant relationship between sarcopenia and 1-year mortality requires discussion. It may be indicative of the presence of sub-clinical synchronous metastasis, which is subsequently picked up during follow-up after surgery. If this is the case, then sarcopenia may highlight patients who would benefit from more extensive staging investigations, such as positron emission tomography (PET) scanning. This may lead to the consideration of preoperative chemotherapy and/or more frequent clinical and radiological follow-up with the aim of improving outcomes. An alternative explanation is that sarcopenia indicates that patients have poorer physical capacity, making them less resilient to the physiological stresses of surgery and more at risk of complication [15]. Indeed, the relationship between sarcopenia and frailty is well documented [25]. This raises the possibility that, unlike other preoperative prognostic markers (e.g. TNM staging), sarcopenia could be modified via prehabilitation. This individualised physical activity-centred intervention is delivered in the period between diagnosis and the commencement of treatment for cancer and has been shown to be feasible, safe and reduce postoperative complications in patients treated for colorectal cancer [26, 27], although may not always produce improvements in fitness. Furthermore, a recent systematic review has demonstrated that the positive effects of prehabilitation can be seen after as little as 2 weeks [28]. Therefore, the pretreatment measurement of sarcopenia could offer an effective way of selecting patients who would benefit the most from such targeted, individualised prehabilitation [29]. Individualised prehabilitation programmes will require adequate funding to introduce; however, we do not expect interventions to be particularly costly, and would hope that the institution of prehabilitation would lead to savings in resources due to reduced morbidity and shortened stays in hospital.
The strengths of our study were that the data were collected prospectively and are reviewed centrally on a monthly basis, minimising the likelihood of any inaccuracy in the measurement or recording of variables. The method used to measure sarcopenia is simple and easy to learn, low cost, valid, and requires no investment from healthcare providers, meaning it could be seamlessly integrated into the care pathway for colorectal cancer patients. This is in contrast to other methods of skeletal muscle measurement that use costly specialist software packages that require additional training.
It is important to acknowledge several limitations of this work. First, this is a single-centre study and future work should be multi-centred to allow greater number of patients with colorectal cancer to be included. A multi-centre study would also allow the inclusion of a more ethnically diverse population and subsequent subgroup analysis. Second, although all scans were performed within 4 months of surgery, it is possible that the skeletal muscle mass could have changed before surgery. Furthermore, the use of predefined cutoffs for sarcopenia has been defined in populations, which are not necessarily homogenous to the population included in this study. For example, the Prado sarcopenia cutoffs used in this study were defined in an obese population in Canada, with tumours of the respiratory and gastrointestinal tracts. However, using predefined cutoffs is preferable to arbitrarily concluding that patients in the lowest quartile for muscle mass are sarcopenic. Lastly, 30-day mortality was related to sarcopenia in this study. However, this finding was driven by a single death in the cohort and should be interpreted with caution.
Conclusions
This study has found sarcopenia to be prevalent in colorectal cancer patients having elective surgery, resulting in poorer long-term survival. CT measurement of total psoas mass is a valid and simple technique for diagnosing sarcopenia that could be used to augment existing methods of patient-risk stratification prior to surgery. Such sarcopenic patients could then undergo targeted strategies such as prehabilitation, to improve both their short- and long-term outcomes.
Acknowledgements
We gratefully acknowledge support from The Carnegie Trust of Scotland who awarded the lead author a Vacation Scholarship to allow completion of this work.
Author contributions
SJM conceived and designed the study. DRD and KAK co-ordinated the data collection. SM and DRD performed the sarcopenia analysis. DRD, KAK and SJM interpreted the data. All the authors revised manuscript drafts, approved the final manuscript, and contributed intellectually important content.
Funding
This study received no funding.
Compliance with ethical standards
Conflict of interest
All the authors declare no conflicts of interest and have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf.
Ethical approval
No additional ethical approval was required. The National Patient Flow Database is registered with the Scottish Government with Caldicott approval.
Informed consent
For this study informed consented was not needed.
Transparency declaration
The corresponding author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Footnotes
Accepted for presentation as a poster at: National Cancer Research Institute Conference 2018, Glasgow.
Accepted for presentation as a poster at: European Society of Surgical Research 2019, Geneva.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cancer Research UK. Statistics by cancer type. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer. Accessed 30 Nov 2017
- 2.Ait Ouakrim D, Pizot C, Boniol M, Malvezzi M, Boniol M, Negri E, et al. Trends in colorectal cancer mortality in Europe: retrospective analysis of the WHO mortality database. BMJ. 2015;351:h4970. doi: 10.1136/bmj.h4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Office for National Statistics. What does the 2011 Census tell us about older people? 6th September 2013. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/ageing/articles/whatdoesthe2011censustellusaboutolderpeople/2013-09-06. Accessed 04 Mar 2019
- 4.Ruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmadi H, Montie JE, Weizer AZ, et al. Patient psoas muscle mass as a predictor of complications and survival after radical cystectomy. Curr Urol Rep. 2015;16:79. doi: 10.1007/s11934-015-0548-0. [DOI] [PubMed] [Google Scholar]
- 6.Gani F, Buettner S, Margonis GA, Sasaki K, Wagner D, Kim Y, Hundt J, Kamel IR, Pawlik TM. Sarcopenia predicts costs among patients undergoing major abdominal operations. Surgery. 2016;160(5):1162–1171. doi: 10.1016/j.surg.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Englesbe MJ, Patel SP, He K, et al. Sarcopenia and post-liver transplant mortality. J Am Coll Surg. 2010;211(2):271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang D, Karvellas CJ, Baracos V, Williams DC, Khadaroo RG. Sarcopenia is a predictor of outcomes in very elderly patients undergoing emergency surgery. Surgery. 2014;156(3):521–527. doi: 10.1016/j.surg.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 9.Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107:931–936. doi: 10.1038/bjc.2012.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reisinger KW, van Vugt JL, Tegels J, Snijders C, Hulsewe KW, Hoofwijk AG, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg. 2015;261(2):345–352. doi: 10.1097/sla.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 11.Huang DD, Wang SL, Zhuang CL, Zheng BS, Lu JX, Chen FF, et al. Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after surgery for colorectal cancer. Colorectal Dis. 2015;17(11):O256–O264. doi: 10.1111/codi.13067. [DOI] [PubMed] [Google Scholar]
- 12.Reisinger KW, Derikx JP, van Vugt JL, Von Meyenfeldt MF, Hulsewe KW, Olde Damink SW, et al. Sarcopenia is associated with an increased inflammatory response to surgery in colorectal cancer. Clin Nutr. 2016;35(4):924–927. doi: 10.1016/j.clnesp.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Nakanishi R, Oki E, Sasaki S, Hirose K, Jogo T, Edahiro K, Korehisa S, Taniguchi D, Kudo K, Kurashige J, Sugiyama M, Nakashima Y, Ohgaki K, Saeki H, Maehara Y. Sarcopenia is an independent predictor of complications after colorectal cancer surgery. Surg Today. 2018;48(2):151–157. doi: 10.1007/s00595-017-1564-0. [DOI] [PubMed] [Google Scholar]
- 14.Gani F, Buettner S, Margonis GA, Sasaki K, Wagner D, Kim Y, Hundt J, Kamel IR, Pawlik TM. Sarcopenia predicts costs among patients undergoing major abdominal operations. Surgery. 2016;160(5):1162–1171. doi: 10.1016/j.surg.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Herrod PJJ, Boyd-Carson H, Doleman B, Trotter J, Schlichtemeier S, Sathanapally G, Somerville J, Williams JP, Lund JN. Quick and simple; psoas density measurement is an independent predictor of anastomotic leak and other complications after colorectal resection. Tech Coloproctol. 2019;23(2):129–134. doi: 10.1007/s10151-019-1928-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malietzis G, Currie A, Athanasiou T, Johns N, Anyamene N, Glynne-Jones R, et al. Influence of body composition profile on outcomes following colorectal cancer surgery. Br J Surg. 2016;103(5):572–580. doi: 10.1002/bjs.10075. [DOI] [PubMed] [Google Scholar]
- 17.Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J, et al. Sarcopenia is a negative prognostic factor after curative resection of colorectal cancer. Ann Surg Oncol. 2015;22(8):2663–2668. doi: 10.1245/s10434-014-4281-6. [DOI] [PubMed] [Google Scholar]
- 18.Choi M, Oh S, Lee I, Oh S, Won D. Sarcopenia is negatively associated with long-term outcomes in locally advanced rectal cancer. J Cachexia Sarcopenia Muscle. 2017;9(1):53–59. doi: 10.1002/jcsm.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hopkins J, Reif R, Bigam D, Baracos V, Eurich D, Sawyer M. The impact of muscle and adipose tissue on long-term survival in patients with stage I to III colorectal cancer. Dis Colon Rectum. 2019;62(5):549–560. doi: 10.1097/DCR.0000000000001352. [DOI] [PubMed] [Google Scholar]
- 20.https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/staged.html. Accessed 17 Apr 2018
- 21.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones KI, Doleman B, Scott S, Lund JN, Williams JP. Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Dis. 2015;17:O20–O26. doi: 10.1111/codi.12805. [DOI] [PubMed] [Google Scholar]
- 23.Prado C, Lieffers J, McCargar L, Reiman T, Sawyer M, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 24.American Cancer Society. AJCC staging of colorectal cancer. https://www-01.ibm.com/support/docview.wss?uid=swg21608060. Accessed 17 Apr 2018
- 25.Cesari M, Landi F, Vellas B, Bernabei R. Marzetti E. Sarcopenia Phys Frailty: Two sides of the same coin. frontiers in aging neuroscience; 2014. p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moug S, Mutrie N, Barry S, Mackay G, Steele R, Boachie C, et al. Prehabilitation is feasible in patients with rectal cancer undergoing neoadjuvant chemoradiotherapy and may minimize physical deterioration: results from the REx trial. Colorectal Dis. 2019;21(5):548–562. doi: 10.1111/codi.14560. [DOI] [PubMed] [Google Scholar]
- 27.Barberan-Garcia A, Ubré M, Roca J, Lacy A, Burgos F, Risco R, et al. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery. Ann Surg. 2018;267(1):50–56. doi: 10.1097/SLA.0000000000002293. [DOI] [PubMed] [Google Scholar]
- 28.Faithfull S, Turner L, Poole K, Joy M, Manders R, Weprin J, et al. Prehabilitation for adults diagnosed with cancer: a systematic review of long-term physical function, nutrition and patient-reported outcomes. Eur J Cancer Care (Engl) 2019;28(4):e13023. doi: 10.1111/ecc.13023. [DOI] [PubMed] [Google Scholar]
- 29.Buffart L, Sweegers M, May A, Chinapaw M, van Vulpen J, Newton R, et al. Targeting exercise interventions to patients with cancer in need: an individual patient data meta-analysis. J Natl Cancer Inst. 2018;110(11):1190–1200. doi: 10.1093/jnci/djy161. [DOI] [PMC free article] [PubMed] [Google Scholar]