Abstract
Purpose
To compare the efficacy and safety of Botox and Neuronox in the management of benign essential blepharospasm (BEB).
Methods
We performed a triple-masked, randomized control study to compare Botox and Neuronox in 48 eyes of 24 patients with BEB. All 24 patients randomly received Botox or Neuronox in the periorbital region in a masked, randomized split-face manner, keeping the injection sites and doses uniform. The toxin preparation, injection, and clinical evaluations were done by three independent observers. Objective outcome measures included improvement in the severity of spasm, grading of the functional visual status, changes in palpebral fissure height, lagophthalmos, superficial punctate keratitis and Schirmer's test at 2 weeks, 6 weeks, and upon conclusion of the effect of the toxin. Subjective outcome measures included duration of the effect and a forced choice stating which half of the face was better. Evaluations were performed through clinical measurements, external digital photography, and high-definition videography.
Results
The mean duration of relief from spasms was 3.78 months (standard deviation, 1.58 months; range, 1 to 6 months). The improvement in the objective parameters like severity of spasm and functional visual status was statistically significant at the 2-week and 6-week follow-up visits (p < 0.001). The changes in palpebral fissure height, lagophthalmos, and superficial punctate keratitis were equally observed in both groups. At 2 and 6 weeks, three of 24 (12.5%) and one of 24 (4%) patients, respectively, reported an unequal effect between the two sides of the face, but this difference was not statistically significant. At final follow-up (conclusion of the toxin effect), patients reported equal effect with no preference for either hemiface. No statistically significant differences were found in the comparative analysis between the Neuronox and Botox groups.
Conclusions
Neuronox and Botox are comparable in terms of their safety and efficacy in the management of BEB.
Keywords: Blepharospasm, Botulinum toxins, Botulinum toxin type A
Botulinum toxin type A (BTX-A) injection is the mainstay of management for patients with benign essential blepharospasm (BEB) owing to its proven safety and efficacy. BTX-A preparations available in various countries are Botox (Allergan, Irvine, CA, USA), Dysport (Ipsen, Slough, UK) [1,2,3], Xeomin (Merz Pharmaceuticals, Frankfurt am Main, Germany), and Prosigne (Lanzhou Biological Products, Lanzhou, China) [4,5]. Neuronox (Medytox, Seoul, Korea) is a recently introduced BTX-A preparation. Botox and Neuronox are purified extracts of BTX type A available as vacuum-dried and a freeze-dried lyophilized preparations, respectively [6,7]. Though both products are nearly similar, Neuronox is cheaper. This has a bearing on the choice of the product, especially for a repetitive disorder like BEB.
There are very limited data comparing the two products. Stone et al. [8] studied the dose-response relationship for inhibition of muscle force of Botox and Neuronox in a murine model and characterized the time course of recovery from the toxin-induced muscle paralysis. Gastrocnemius muscle force generation was examined following stimulation of the tibial nerve. Botox and Neuronox produced nearly equivalent decrements in muscle force (30% to 90%) at 4 days after toxin injection. At 28 days after injection (1 U/kg), muscle force had recovered from the effects of both toxin preparations. For facial spasms, only one double-blind, prospective, randomized control study has been conducted in humans to compare Botox and Neuronox in two separate cohorts of BEB patients in Korea [9]. The study concluded that both preparations are comparable in terms of their safety and efficacy. However, more studies are needed to consolidate the comparative data.
We therefore utilized the bilaterally symmetrical nature of BEB to conduct a split-face, prospective, randomized control study to compare safety and efficacy of the two BTX-A preparations in the management of BEB. A split face study eliminates any confounding factors that might be at play when two separate cohorts are studied.
Materials and Methods
This split-face prospective randomized control study was conducted in accordance with the principles of the declaration of Helsinki. The study protocol as well as patient consent were approved by the institution review board of LV Prasad Eye Institute, Hyderabad, India (LEC 11-067). All patients consented to participation in the study before enrollment. The study was conducted at the LV Prasad Eye Institute, Hyderabad, India. All patients diagnosed with BEB and fulfilling the inclusion and exclusion criteria (Table 1) were included in this split-face study.
Table 1. Inclusion and exclusion criteria for the split-face study.
Since there was no prior split-face study to compare the two drugs, we decided to perform this as a pilot study enrolling a minimum of 20 patients. Each vial of Botox or Neuronox contained 100 units (U) of Clostridium Botulinum type A neurotoxin complex, 0.5 mg of human albumin, and 0.9 mg of sodium chloride in a sterile, dried solid without preservatives. Each 100-U vial was carefully reconstituted with 4 mL of sterile, non-preserved 0.9% normal saline to achieve a concentration of 2.5 U/0.1 mL. The reconstituted solution was stored in a refrigerator at 4℃ until needed. A 30-gauge half-inch needle was used for all injections after topical point-application of Prilocaine-Lidocaine cream. The study methodology was divided into the following three phases.
Pre-injection phase
Written informed consent was obtained from the appropriately included participants. Severity of spasm and functional visual status were graded using the mentioned grading system (Table 2). The grading was done at the pre-injection visit and at subsequent follow-up assessments. Palpebral fissure height, lagophthalmos, and Schirmer's test without topical anaesthetic were documented at the pre-injection visit and at subsequent follow-up visits. Digital external photographs of the upper half of the face and a high-definition video clip were recorded to further document the measured parameters.
Table 2. Grading of severity of spasm and functional visual status in the pre-injection and post-injection period for patients with benign essential blepharospasm.
Injection phase
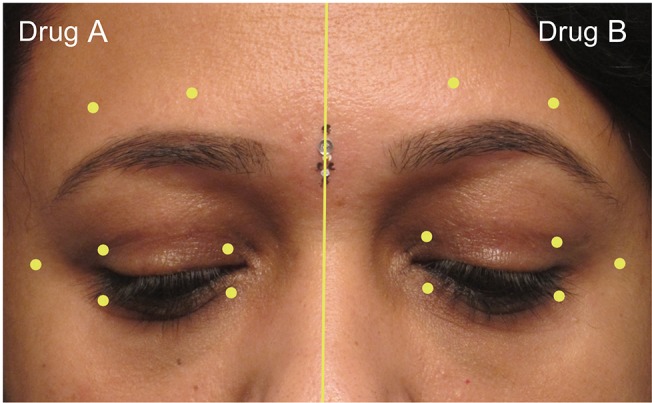
The injector was provided with two identical syringes containing equal amounts of toxin. To mask the identity of the toxin from the injector and the patient, the syringes were labeled as drug A and drug B. These syringes were loaded and provided to the injector by a single observer (MJA) who was blinded to the injection techniques and the post-injection evaluations. The right side of the face was injected first with either drug A or drug B, selected randomly by secret ballot method. An equal dose of the remaining drug was injected on the left side. All patients received 17.5 to 20 U of a given drug on each half side of the face. The identity of the drugs (drugs A and B) was concealed until the end of the statistical analysis. A standard technique was employed by a single injector (MNN) to inject 0.1 mL aliquots (2.5 U) per site with a 30-gauge needle over a Tuberculin syringe at seven prefixed sites in the periorbital area (Fig. 1). The sites were identical on both sides. The patients were prescribed preservative-free lubricant eye drops in both eyes.
Fig. 1. The seven sites of Botulinum toxin injection used in this study. Site 1, 4 mm above the upper punctum; site 2, 4 mm above the lateral canthus; site 3, 4 mm below the lower punctum; site 4, 4 mm below the lateral canthus; site 5, 1 cm lateral to the lateral canthus; site 6, 8 mm above the brow hair at the junction of the medial and middle third of the brow; site 7, 8 mm above the brow hair at the junction of the lateral and middle third of the brow. Written informed consent for publication was obtained from the participant.
Post-injection follow-up phase
At the 2- and 6-week post-injection follow-up visits, all the aforementioned clinical parameters were documented. Patients were encouraged to comply with follow-up visits by telephonic reminders. Objective outcome measures included improvement in the grades of severity of spasm and functional visual scale, as well as the occurrence of side effects like ptosis, lagophthalmos, and superficial punctuate keratitis.
Subjective outcome measures included duration of symptomatic relief, and a subjective feeling of a ‘better’ effect between the two sides of the face. To assess the latter, the patient was asked to choose which side (right or left) felt better than the other. Photographic and video graphic documentation was obtained at each follow-up visit. All the pre- and post-injection questionnaires and evaluations were performed by a single masked observer (SS).
Beyond the 6-week follow-up visit, patients were asked to report when the action of the toxin wore off, and they felt the need for the next injection. The patient was asked to report the subjective duration of the effect after complete washout of the action of the toxin, as well as any inequality in the effect between the two sides of the face.
Statistical analysis was performed using the SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA). For pre- and post-injection analysis, the paired t-test was used. For comparative analysis between drug A and drug B, independent t-tests were used.
Results
Twenty-four patients were enrolled in this prospective study. Fifteen were males, and nine females. The mean age was 52 years (SD, 11.1 years). The analysis of parameters such as severity of spasm, functional visual scale and forced choice of the better side was done considering the total number of patients as a denominator (n = 24) while for all other parameters, the denominator was taken as the total number of eyes (n = 48).
The most common presentation was involuntary closure of the eyes and/or increased rate of blinking noted in all patients. Two out of 24 patients also had spasmodic contractions of the lower face and neck region (lower facial dystonia), suggestive of Meige syndrome. The mean duration of the symptoms was 24.7 months.
Twenty-one patients received 17.5 U of the drug on each side (total 35 U), and three patients received 20 U (total 40 U). The identity of the drug was disclosed only after the statistical analysis was completed. Drug A was revealed to be Neuronox and drug B was Botox.
Five eyes had lagophthalmos ranging from 1 to 3 mm, and eight eyes had superficial punctuate keratopathy.
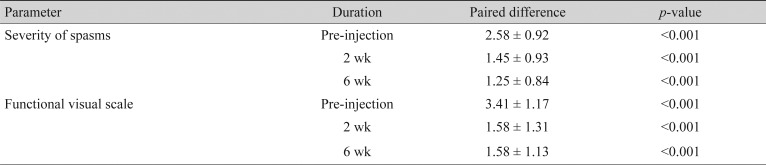
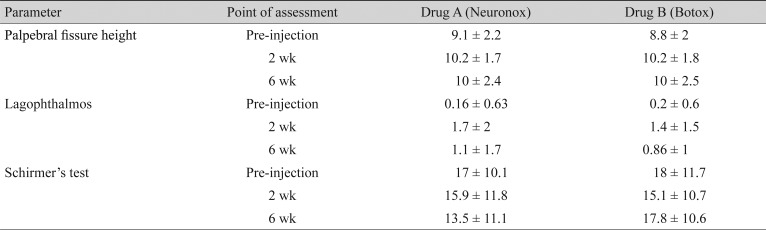
Among the objective outcome measures, the severity of spasm and the functional visual status showed statistically significant improvement (Table 3). Individual objective outcome measures like palpebral fissure height, induced lagophthalmos, and Schirmers test showed statistically significant differences, but the results were comparable in the Neuronox and Botox groups (Table 4).
Table 3. Pre-injection and post-injection analysis of the change in severity of spasm and functional visual scale at 2 and 6 weeks.
Values are presented as mean ± standard deviation.
Table 4. Objective outcome measures analyzed in the Neuronox and Botox groups before, 2 weeks, and 6 weeks after injection.
Values are presented as mean ± standard deviation.
At the 2- and 6-week follow-up, the difference in occurrence of superficial punctate keratopathy was not statistically significant in any of the groups.
Among the subjective outcome measures, the mean duration of the symptomatic relief was 3.8 months (SD, 1.6 months; range, 1 to 6 months). At the 2-week post-injection follow-up, all the patients showed symptomatic improvement. At the 2-week visit, three out of 24 (12.5%; 95% CI, 0 to 25.7) patients noted that the effect of the treatment was better on one side compared to the other, where the better side had received Neuronox. At the 6-week visit, one patient (4%) reported more activity on one half of the face compared to the other, where the better side had received Botox. These differences in their forced choice at 2 and 6 weeks were not statistically significant.
When asked to make a forced choice between the two sides about the benefit and the duration of the effect, all 24 patients felt that the effect of the treatment was similar on both sides of the face.
Discussion
Botulinum toxin type A is widely used in several specialties of medicine. It is used as the first-line treatment in the management of BEB. Botox is the oldest BTX-A preparation, and hence is time-tested and efficacious in the management of BEB. Neuronox is a recently introduced BTX-A preparation, and is structurally similar to Botox. The only difference between the two products is that Botox is a vacuum-dried preparation, whereas, Neuronox is a freeze-dried preparation.
Though animal studies have proven Neuronox to be effective and safe, only one study is available in the literature that compares it with Botox in humans. This study by Yoon et al. [9] compared the two preparations in two separate cohorts of patients of BEB, and found them to be comparable in safety and efficacy.
There are several merits to our study. We utilized the bilateral symmetric nature of BEB to conduct a split-face comparative study. In our study, all the participating patients received both preparations in a ‘split-face’ manner on either side of the face. In our study, both the preparations were tested and compared in the same individuals, at the same time. We attempted to eliminate confounding factors related to intrinsic mechanisms, differences in the sensitivity to the drug, and genetic variability among different individuals, which could be potential causes of the differences in response to the drug. A single trained physician did the subjective assessment of the response to both the drugs.
The mean age at presentation and the chief presenting complaints in our study were similar to other reports. Half of our patients had received Botulinum toxin injection (Botox) in the past, and hence were well versed with its benefits and side effects. Following injection, there was a statistically significant improvement in the severity of spasm and the mean functional visual score. This indicates that both the drugs were equally effective. Among the subjective outcome measures, though the patients did report a difference between the two sides at the 2- and 6-week follow-up visit (three patients at 2 weeks reported that the Neuronox side was better, and one patient at 6 weeks reported that the Botox side was better), their final assessment at the conclusion of the toxin effect was that both sides were comparable.
The mean increase in palpebral fissure height, lagophthalmos (all eyes), and punctate keratopathy was significant in both groups. However, there were no statistically significant differences between the two when Botox was compared with Neuronox. This indicates that the side-effect profile was comparable for both drugs. This is expected, since the side-effect profile was directly comparable to the actual effect.
Our study is the first study to compare two Botulinum toxin products in a split-face manner. This avoids the inherent disadvantages of studying two drugs in two different cohorts of patients. The split-face study could be the best study model to compare two different drugs in the management of facial spasms.
In conclusion, our study indicates that Botox and Neuronox are equally effective in the management of BEB. The induced improvements in palpebral fissure height, visual impairment scale, and grade of severity of spasm are comparable. The side-effect profile (lagophthalmos and punctuate keratopathy) was also comparable.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Sampaio C, Ferreira JJ, Simoes F, et al. DYSBOT: a single-blind, randomized parallel study to determine whether any differences can be detected in the efficacy and tolerability of two formulations of Botulinum toxin type A--Dysport and Botox--assuming a ratio of 4:1. Mov Disord. 1997;12:1013–1018. doi: 10.1002/mds.870120627. [DOI] [PubMed] [Google Scholar]
- 2.Nussgens Z, Roggenkamper P. Comparison of two Botulinum-toxin preparations in the treatment of essential blepharospasm. Graefes Arch Clin Exp Ophthalmol. 1997;235:197–199. doi: 10.1007/BF00941758. [DOI] [PubMed] [Google Scholar]
- 3.Truong D, Comella C, Fernandez HH, et al. Efficacy and safety of purified Botulinum toxin type A (Dysport) for the treatment of benign essential blepharospasm: a randomized, placebo-controlled, phase II trial. Parkinsonism Relat Disord. 2008;14:407–414. doi: 10.1016/j.parkreldis.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Roggenkamper P, Jost WH, Bihari K, et al. Efficacy and safety of a new Botulinum toxin type a free of complexing proteins in the treatment of blepharospasm. J Neural Transm (Vienna) 2006;113:303–312. doi: 10.1007/s00702-005-0323-3. [DOI] [PubMed] [Google Scholar]
- 5.Rieder CR, Schestatsky P, Socal MP, et al. A double-blind, randomized, crossover study of Prosigne versus Botox in patients with blepharospasm and hemifacial spasm. Clin Neuropharmacol. 2007;30:39–42. doi: 10.1097/01.WNF.0000236771.77021.3C. [DOI] [PubMed] [Google Scholar]
- 6.Botox. [Package insert] Irvine: Allergan Pharmaceuticals; 2010. [Google Scholar]
- 7.Neuronox. [Package insert] Seoul: Medytox; 2005. [Google Scholar]
- 8.Stone AV, Ma J, Whitlock PW, et al. Effects of Botox and Neuronox on muscle force generation in mice. J Orthop Res. 2007;25:1658–1664. doi: 10.1002/jor.20450. [DOI] [PubMed] [Google Scholar]
- 9.Yoon JS, Kim JC, Lee SY. Double-blind, randomized, comparative study of Meditoxin versus Botox in the treatment of essential blepharospasm. Korean J Ophthalmol. 2009;23:137–141. doi: 10.3341/kjo.2009.23.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]