Cell death is a fundamental step in progression of various hepatic diseases. Necroptosis, an apoptosis alternate cell death, has been documented in various hepatic diseases. Just like apoptosis, necroptosis modulation could also halt disease progression and could be beneficial. Generally, necroptosis is thought to mediated by receptor-interacting serine/threonine-protein kinase-1 (RIP1), RIP3 and mixed lineage kinase domain-like pseudokinase (MLKL) (1).
Seehawer et al. made an important contribution in the field of cell death and hepatology by publishing an interesting study in Nature Journal (2). The study addressed how hepatic microenvironment could influence the type of hepatic tumor and elaborates that oncogenically activated hepatocytes if within apoptotic microenvironment opt for hepatocellular carcinoma (HCC), while if within necroptotic environment opt for intracellular cholangiocarcinoma (ICC). Overall, the study is elegantly designed and explicitly explains the underlying mechanism.
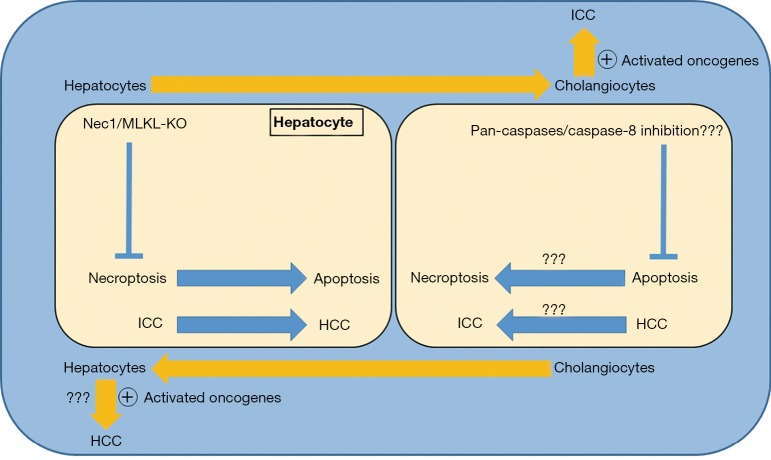
To develop multifocal HCC, transposable elements were delivered to hepatocytes of p19Arf-/- mice via hydrodynamic tail vein injection (HDTV) and to develop unifocal HCC, transposable elements were delivered via electroporation (Epo) which, surprisingly, led to development of ICC, HCC or combined tumors at electroporation site. In HDTV group, apoptosis was increased while in Epo group, necroptosis was increased. Interestingly, ICC was originating from differentiated hepatocytes. Surprisingly, the use of mock Epo + transposon via HDTV, resulted in development of ICC or combined ICC-HCC at Epo site, while HDTV induced HCC development at other sites.
Further analysis found no role of mutation, stellate cells, immune cells or overall death in modulating hepatic microenvironment for Epo induced ICC development. The cytokines expression analysis revealed differential release of cytokines in HDTV and Epo groups. In Epo group, administration of Nec1 not only reduced cell death, inhibited necroptosis but also reduced Epo induced cytokines release and shifted death from necroptosis to apoptosis. Interestingly, Epo induced ICC was also switched to HCC with Nec-1 treatment. Similar results were observed with hepatocytes-specific MLKL-KO mice. Of note, almost same cytokines were suppressed with RIP1, MLKL and toll-like receptors inhibition. Similar to in vivo findings, in primary human hepatic cancer, necroptosis signature was increased in ICC while apoptosis was increased in HCC. The study also found that Tbx3 and Prdm5 genes were epigenetically regulated and act synergistically in determining the lineage. Reciprocal pattern of Tbx3 and Prdm5 genes expression was observed with HCC and ICC (2).
In common bile duct (CBD) ligation induced hepatic injury necroptosis is identified (3); however, HDTV treatment in CBD induced liver injury increased K19 positive cells, suggesting the development of ICC (2). Recent studies suggest hepatocytes can differentiate into cholangiocytes and vice versa (4,5). During chronic liver injuries, cellular plasticity could also modulate hepatic microenvironment. The possibility exists that cholangiocytes would also be contributing/responding to hepatic microenvironment changes and would be differentially responding to death associated paracrine effects of either the apoptosis or necroptosis. In future, it would be more interesting to elaborate if cholangiocytes also responded in similar way as hepatocytes.
Although, both the HCC and ICC can potentially originate from hepatocytes. However, the underlying mechanism which determining the hepatocytes preference for apoptosis or necroptosis is still largely unknown. Moreover, the shift in cell death type from necroptosis to apoptosis or vice versa is well-known (6,7). It is of interest that as the type of cell death switched from necroptosis to apoptosis, the primary liver cancer type also changed from ICC to HCC which was independent of oncogenic drive and was dependent on microenvironment dictated by either the apoptosis or the necroptosis (2). Importantly, the shift happened during tumorigenesis phase not when the tumor has already developed. Although, inhibition of necroptosis shifted ICC to HCC; however, inhibiting apoptosis, by targeting pan-caspases or capase-8, with delivery of transposable elements with HDTV injection would have provided further interesting outcomes, probably ICC.
The study by Seehawer et al. (2) also highlights a number of remarkable dynamic changes within liver microenvironment which could potentially direct the overall fate of disease condition. First, hepatocytes potential to differentiate into cholangiocytes or vice versa, which has already been observed (4,5). Second, switching of cell death from necroptosis to apoptosis or vice versa. Third, switching of cell death also has the potential to impact the type of primary liver cancer (Figure 1). Of note, shift of cell death mostly does not occur by itself. Usually, it has to be pharmacologically or genetically induced. Although, modulation of hepatic microenvironment and cell death can now be achieved genetically or pharmacologically. However, whether or not the change in cell death type and thus the change in type of primary liver cancer also occurs during natural course of disease progression is yet to be clarified.
Figure 1.
Necroptosis changes hepatic microenvironment.
To be HCC or ICC, the author elegantly elaborated that it is hepatic microenvironment which could modulate the type of primary liver cancer (2). The hepatic microenvironment itself is modulated by type of cell death, apoptosis or necroptosis. If hepatocytes die by apoptosis, then the apoptosis induced microenvironment promotes the development of HCC, whereas if hepatocytes die by necroptosis then the necroptosis induced microenvironment encourages the development of ICC (2). This also implies that during chronic liver disease conditions, not only the shift towards less fatal condition with improved prognosis could possibly be made but also the natural course of disease progression could be changed. Importantly, tissue demise during chronic diseases is mostly a mixture of different death types. Therefore, even if apoptosis shifts to necroptosis or necroptosis shifts to apoptosis, it is the cell death which still remains the constant and would result not only in loss of functional tissue parenchyma but also changes in hepatic microenvironment. It might be a matter of choosing lesser evil among the many. The mystery still remains to a great extent that why and how a cell opts for a particular death flavor, be it the apoptosis, necroptosis or others. Later, the after effects of that particular death choice, mainly in the form of cytokines release, directs neighboring tissue response.
Acknowledgments
Funding: This work was supported by grants from the National Research Foundation of Korea (NRF-2017M3A9C8028794).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Saeed WK, Jun DW. Necroptosis: an emerging type of cell death in liver diseases. World J Gastroenterol 2014;20:12526-32. 10.3748/wjg.v20.i35.12526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seehawer M, Heinzmann F, D'Artista L, et al. Necroptosis microenvironment directs lineage commitment in liver cancer. Nature 2018;562:69-75. 10.1038/s41586-018-0519-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afonso MB, Rodrigues PM, Simao AL, et al. Activation of necroptosis in human and experimental cholestasis. Cell Death Dis 2016;7:e2390. 10.1038/cddis.2016.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng X, Zhang X, Li W, et al. Chronic Liver Injury Induces Conversion of Biliary Epithelial Cells into Hepatocytes. Cell Stem Cell 2018;23:114-22.e3. 10.1016/j.stem.2018.05.022 [DOI] [PubMed] [Google Scholar]
- 5.Schaub JR, Huppert KA, Kurial SNT, et al. De novo formation of the biliary system by TGFbeta-mediated hepatocyte transdifferentiation. Nature 2018;557:247-51. 10.1038/s41586-018-0075-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han W, Xie J, Li L, et al. Necrostatin-1 reverts shikonin-induced necroptosis to apoptosis. Apoptosis 2009;14:674-86. 10.1007/s10495-009-0334-x [DOI] [PubMed] [Google Scholar]
- 7.Zhang DW, Shao J, Lin J, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009;325:332-6. 10.1126/science.1172308 [DOI] [PubMed] [Google Scholar]