Abstract
Objectives
To report our institutional experience, management, and outcomes of cutaneous periauricular squamous cell carcinoma (SCC).
Study Design
Retrospective chart review.
Setting
Tertiary academic center.
Subjects
Patients undergoing treatment of cutaneous periauricular SCC from 2000 to 2016.
Results
A total of 112 patients had a median follow-up of 24.5 months, a mean ± SD age of 75.7 ± 10.6 years, and a strong male predominance (93.8%). Site distribution shows 87 (77.7%) auricular, 26 (23.2%) preauricular, and 10 (8.8%) postauricular lesions. Of auricular lesions, tumors involved the tragus (n = 3, 3.4%), helix/antihelix (n = 47, 54.0%), conchal bowl (n = 31, 35.6%), external auditory canal (n = 18, 16.1%), and lobule (n = 3, 3.4%). Most patients presented at stage I (52.7%) versus stages II (28.6%), III (6.3%), and IV (12.5%). Patients were largely treated surgically with primary tumor resection ranging from wide local excision to lateral temporal bone resection (± parotidectomy and neck dissection), with 17.0% and 5.4% receiving adjuvant radiation and chemoradiation, respectively. Metastatic spread was seen to the parotid (25.9%) and neck (26.8%), with most common cervical spread to level II. Overall survival, disease-specific survival, and disease-free survival at 3 years were 62%, 89%, and 56%, respectively. Nodal disease was associated with worse disease-specific survival (P < .001) and disease-free survival (P = .042). Pre- and postauricular sites were associated with worse overall survival (P = .007) relative to auricular sites.
Conclusion
Among cutaneous SCC, periauricular subsites pose treatment challenges related to surrounding anatomy and represent a unique tumor population. The reported propensity toward recurrence and patterns of metastasis may better guide treatment of aggressive tumors to include regional nodal dissection.
Keywords: cutaneous, squamous cell carcinoma, periauricular, preauricular
The incidence of cutaneous squamous cell carcinoma (SCC) in the United States continues to rise, with as many as 400,000 new cases diagnosed annually.1 Greater than 50% of new SCC lesions arise on chronically sun-exposed skin of the head and neck, including the scalp, auricles, nose, and lips.2,3 The mainstay of treatment for these lesions is surgical extirpation with adjuvant radiotherapy reserved for high-risk pathologic features or regional nodal metastases.4
SCC of the external auditory canal (EAC), external ear, or periauricular skin poses unique challenges for definitive surgical treatment and reconstruction, as the lesion may deeply invade the lateral skull base,5 abut or infiltrate the facial nerve (cranial nerve: CN VII),6 compromise hearing, and metastasize to nodal basins in the parotid and neck.7,8 There remains considerable controversy in the literature regarding the appropriate workup, staging, surgical terminology, and adjuvant treatment for SCC arising from these auricular and periauricular skin sites. Disparate staging systems (ie, Pittsburgh vs American Joint Committee on Cancer [AJCC])9,10 and heterogeneous surgical practices (ie, ear canal sleeve resections vs lateral vs total temporal bone resection)11 complicate evidence-based management of these aggressive cancers.
Many published series describing management and outcomes of EAC, external ear, or periauricular SCC are hampered by low numbers and incomplete data. Herein, we describe our robust, single-center experience with diagnosis, workup, and management of a large patient cohort with SCC of the EAC, external ear, or periauricular skin. Furthermore, we emphasize rates of local and regional disease control stratified by stage and treatment modality.
Materials and Methods
Data Source and Study Population
Following approval by the University of Michigan Institutional Review Board (HUM00115814), a retrospective medical record review was conducted of all patients diagnosed with nonmelanoma cutaneous cancer of the head and neck and treated at our institution from 2000 to 2016. DataDirect8 and EMERSE9 search functions were utilized to identify patients from the electronic health medical record for inclusion in our study. A total of 112 patients were identified as meeting inclusion criteria of cutaneous SCC of the periauricular region, defined as biopsy-proven lesions of the EAC, auricle, immediate pre-, or postauricular regions.
Measures and Statistical Analysis
Comprehensive data were collected on patient demographics, tumor characteristics (ie, detailed anatomic location, size), radiographic and pathologic findings, treatment rendered (surgery with or without radiation and/or chemotherapy), clinical follow-up, rates of recurrence, and survival. Tumors were classified as superficial (involving the pinna or skin only) or deep (extension to EAC or underlying EAC bone, parotid, or neck). Superficial tumors were staged according to the AJCC seventh edition for cutaneous SCC,10 while deep tumors were staged according to the Pittsburgh system for SCC of the temporal bone to allow better comparison by T-classification prognostication.11 Descriptive statistics and inferential statistics, including Student’s t test (2-tailed, α = 0.05), were performed with SPSS 22.0 (IBM, Chicago, Illinois). Survival is reported with Kaplan-Meier methodology.
Results
Demographics
The 112 patients meeting inclusion criteria had a median follow-up of 24.5 months (range, 1.4-208.9 months). The mean age was 75.7 years (SD, 10.6 years), with a strong predominance of male (93.8%) and Caucasian (97.2%) patients. Many patients exhibited known risk factors for carcinoma, including smoking status at diagnosis (current, 9.0%; former, 41.4%) and a positive history of significant sun exposure (90.9%). Further comorbidities included diabetes, immunosuppression, transplant history, and chronic lymphocytic leukemia ( Table 1 ). A majority (67.9%) of patients also showed a propensity toward SCC lesions at other anatomic sites.
Table 1.
Demographics and Tumor Characteristics for Patients with Periauricular SCC (N = 112).
| n (%)a | |
|---|---|
| Patient characteristics | |
| Sex | |
| Male | 105 (93.8) |
| Female | 7 (6.3) |
| Ethnicity | |
| White | 106 (97.2) |
| Black | 2 (1.8) |
| Asian | 1 (0.9) |
| Tobacco use | |
| Current | 10 (9.0) |
| Former | 46 (41.4) |
| Never | 55 (49.5) |
| Comorbidities | |
| Diabetes | 29 (25.6) |
| Immunosuppression | 13 (11.6) |
| Transplant history | 14 (12.5) |
| CLL | 9 (8.0) |
| History of sun exposure | |
| Yes | 60 (90.9) |
| No | 6 (9.1) |
| History of SCC at other sites | |
| Yes | 76 (67.9) |
| No | 36 (32.1) |
| Tumor characteristics | |
| Age at initial diagnosis, y | 75.8 ± 10.6b |
| Initial site | |
| Preauricular | 20 (17.9) |
| Postauricular | 7 (6.3) |
| Auricular | 85 (75.9) |
| Auricular subsite (n = 85 tumors) | |
| Tragus | 3 (3.5) |
| Helix/antihelix | 47 (55.3) |
| Conchal bowl | 31 (36.5) |
| Lobule | 3 (3.5) |
| External auditory canal | 18 (21.2) |
| Extension to preauricular | 8 (9.4) |
| Extension to postauricular | 3 (3.5) |
| Staging system | |
| Superficial (AJCC 7th ed.) | 94 (83.9) |
| Deep (Pittsburgh) | 18 (16.1) |
| T classification | |
| T1 | 59 (52.7) |
| T2 | 44 (39.3) |
| T3 | 2 (1.8) |
| T4 | 7 (6.3) |
| N classification | |
| N0 | 96 (85.7) |
| N+ | 16 (14.3) |
| Overall stage | |
| I | 59 (52.7) |
| II | 32 (28.6) |
| III | 7 (6.3) |
| IV | 14 (12.5) |
| Tumor differentiation | |
| Well (low grade) | 24 (21.4) |
| Moderate | 52 (46.4) |
| Poor (high grade) | 18 (16.1) |
| Pathologic characteristics | |
| Perineural invasion | 24 (21.4) |
| Lymphovascular invasion | 17 (15.2) |
| Cartilage invasion | 18 (16.1) |
| Bone invasion | 6 (5.3) |
| Positive margin (resected) | 19 (17.0) |
Abbreviations: AJCC, American Joint Committee on Cancer; CLL, chronic lymphocytic leukemia; SCC, squamous cell carcinoma.
Unweighted number and weighted percentage.
Mean ± SD.
Tumor Characteristics
Tumor site distribution shows 87 (77.7%) auricular, 26 (23.2%) preauricular, and 10 (8.8%) postauricular lesions ( Figure 1 ). Of the 87 auricular lesions, tumors involved the tragus (n = 3, 3.4%), helix/antihelix (n = 47, 54.0%), conchal bowl (n = 31, 35.6%), EAC (n = 18, 16.1%), and lobule (n = 3, 3.4%). Nineteen (17.0%) tumors involved >1 subsite of the auricle or exhibited extension to the preauricular skin (n = 8, 9.4%) or postauricular skin (n = 3, 3.5%). The majority of patients presented at stage I (52.7%) versus stages II (28.6), III (6.3), and IV (12.5%).
Figure 1.
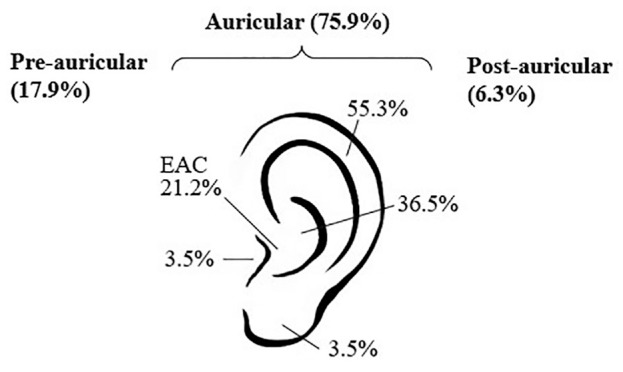
Periauricular subsite distribution of primary tumors. EAC, external auditory canal.
Treatment of Primary Tumors
Patients were largely treated surgically (n = 107, 95.5%). Extent of primary tumor resection ranged from Moh’s excision (n = 10, 9.3%) to wide local excision (WLE; n = 80, 74.8%), additional sleeve resection of EAC skin (n = 8, 7.5%), or lateral temporal bone resection (n = 9, 8.4%; Table 2 ). Additional dissections indicated at the time of first surgery included superficial parotidectomy (n = 20, 18.7%), total parotidectomy (n = 8, 7.5%), and selective neck dissection (n = 23, 21.1%). In 5 cases, parotidectomy was performed with WLE; in the remainder of cases, parotidectomy (superficial or total) was performed concurrently with selective neck dissection of levels II to V. When indicated and performed, 53.6% of parotid specimens and 34.7% of neck dissections were positive for nodal disease. In the context of the whole cohort, metastatic disease was observed to lymph nodes in the parotid basin (25.9%) and the neck (26.8%). Metastatic lymph nodes in the neck were observed most commonly in level II (50% of neck metastases), followed by levels III, V, IV, and I ( Figure 2 ).
Table 2.
Treatment Characteristics for Primary and Recurrent Tumors.
| Tumors, n (%)a | ||
|---|---|---|
| Treatment | Primary (N = 112) | Recurrent (n = 34) |
| Initial treatment type | ||
| Surgery | 107 (95.5) | 26 (76.5) |
| RT alone | 2 (1.8) | 2 (5.9) |
| CRT | 2 (1.8) | 1 (2.9) |
| None | 1 (0.9) | 5 (14.7) |
| Surgery performed (n = 107) | ||
| Moh’s excision of primary | 10 (9.3) | 0 (0.0) |
| WLE of primary (incudes partial/total auriculectomy) | 80 (74.8) | 24 (70.6) |
| WLE including sleeve resection | 8 (7.5) | 1 (2.9) |
| Lateral temporal bone resection | 9 (8.4) | 5 (14.7) |
| Additional procedures | ||
| Neck dissection | 23 (21.1) | 17 (50.0) |
| Superficial parotidectomy | 20 (18.7) | 8 (23.5) |
| Total parotidectomy | 8 (7.5) | 8 (23.5) |
| Sentinel lymph node biopsy | 14 (13.1) | 0 |
| Nerve sacrifice | 1 (0.9) | 5 (14.7) |
| Adjuvant treatment | ||
| Radiation alone | 19 (17.0) | 14 (41.2) |
| Chemoradiation | 6 (5.4) | 2 (5.9) |
| Type of recurrence | ||
| Local | 24 (70.6) | |
| Regional | 16 (47.1) | |
| Distant | 3 (8.8) | |
Abbreviations: CRT, chemoradiation; RT, radiation; WLE, wide local excision.
Patient number and weighted percentage of column.
Figure 2.
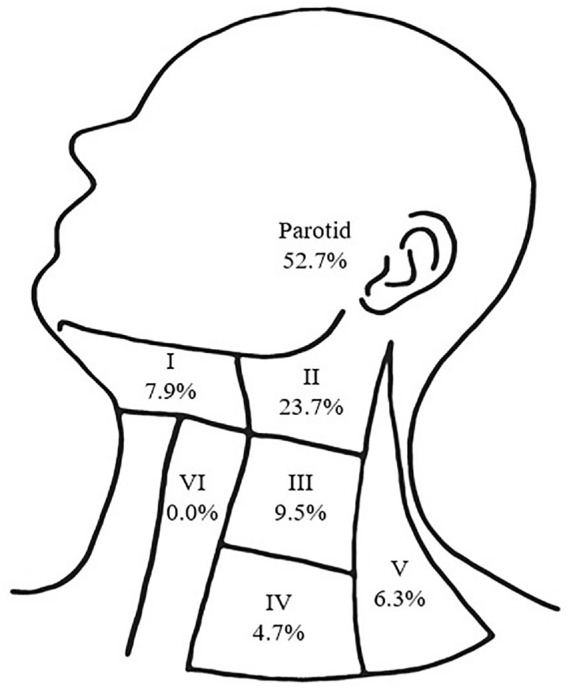
Pattern of metastatic spread to parotid and cervical lymph node basins among patients with positive nodal disease.
Facial nerve sacrifice was required in only 1 patient’s primary tumor resection. Fourteen patients underwent sentinel lymph node biopsy (SLNB) at the time of WLE. All SLNB procedures identified at least 1 sentinel node (median, 2; range, 1-10), with 2 procedures identifying positive sentinel nodes to guide subsequent completion nodal dissection (1 parotid, 1 level IIB). Sites of the 34 sentinel nodes in 14 procedures were level II (n = 18), level V (n = 6), intraparotid (n = 5), external jugular (n = 4), and postauricular (n = 1). Following primary tumor resection, 17.0% and 5.4% of patients received adjuvant radiation and concurrent chemoradiation, respectively. Forty-three (38.4%) patients had cross-sectional imaging of the neck as part of their initial workup, most commonly computed tomography (35.7%), followed by positron emission tomography / computed tomography (10.7%) and magnetic resonance imaging (8.9%). Thirty-six (32.1%) advanced-stage cases had a multidisciplinary tumor board involved in their plan of care.
Recurrence
Thirty-four patients exhibited local (21.4% of total cohort), regional (14.3%), and/or distant (2.7%) recurrences. Mean time to recurrence was 9.8 months (SD, 5.76; median, 12.5). Locoregional recurrences were largely managed with surgical re-excision of recurrent primary tumor or metastatic nodal disease (n = 26), with adjuvant radiation (n = 17, 65.4% of surgical patients) or chemoradiation (n = 1, 3.8%; Table 2 ). Beyond repeat WLE, surgical treatments for recurrent SCC included neck dissection (n = 17), superficial parotidectomy (n = 8), total parotidectomy (n = 8), and lateral temporal bone resection (n = 5). Facial nerve sacrifice was required in 5 of 18 (27.8%) patients who required that the parotid gland or temporal bone be addressed due to recurrent disease. Recurrent tumors exhibited perineural invasion (10 of 26), lymphovascular invasion (6 of 26), cartilage invasion (4 of 26), bone invasion (2 of 26), and positive margins (5 of 26). Twelve patients had >1 recurrence. Of 3 patients with distant recurrence, 1 received chemotherapy, 1 palliative radiation, and 1 declined further treatment.
Survival Analysis
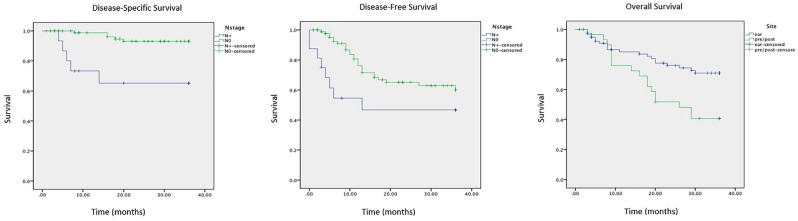
Median follow-up for this cohort was 24.5 months. Overall survival (OS), disease-specific survival (DSS), and disease-free survival (DFS) at 3 years were 62% (95% CI, 57%-67%), 89% (95% CI, 86%-92%), and 56% (95% CI, 49%-63%), respectively. Nodal disease was associated with worse DSS (P < .001) and DFS (P = .042). Pre-/postauricular site was associated with worse OS (P = .007). Overall stage was a poor predictor of DFS, DSS, and OS. Kaplan-Meier survival analyses are shown in Figure 3 .
Figure 3.
Kaplan-Meier curves showing estimated survival at 3 years. Disease-specific survival (left) and disease-free survival (center) for groups with and without nodal disease. Overall survival for pre- and postauricular sites versus auricular site (right).
Discussion
Our large retrospective cohort suggests that cutaneous SCC of the external ear (auricle), EAC, and periauricular skin represents a unique subset of cutaneous malignancies often requiring extensive and multimodality treatment. Furthermore, these neoplasms harbor aggressive local growth patterns and are quite prone to nodal spread and locoregional recurrence.12 With this study, we aimed to report our single-center experience in the diagnosis and treatment of these tumors to inform optimal surgical management and prognostic counseling.
The majority (93.8%) of patients in our cohort were male. This finding is concordant with recent population-based studies showing clear sex disparities in anatomic distribution of cutaneous SCC.3,13 Cutaneous SCC of the scalp and ears is 10 times more prevalent in males versus females, a disparity likely explained by patterns of hair growth in females that shield scalp and ear skin from damaging ultraviolet light. Similar to established epidemiologic patterns, immunosuppression (ie, transplant history, chronic lymphocytic leukemia, and/or diabetes) and synchronous or metachronous lesions of other subsites were quite common in our cohort.14
Approximately 76% of primary SCC tumors in our cohort were located on the auricle. Of these, there was a clear predilection of tumors for the helix/antihelix and conchal bowl ( Figure 1 ). A significant proportion of auricular tumors involved multiple subsites of the auricle, pre-, and/or postauricular skin, posing unique aesthetic challenges for skin graft or local flap reconstruction after excision. Notably, 21% of the auricular tumors in our cohort arose directly from the skin of the EAC, a subsite largely shielded from direct ultraviolet light exposure. This poses an intriguing and unaddressed question whether cutaneous SCC of the EAC is genetically and biologically distinct from “typical” cutaneous SCC driven by ultraviolet damage.15 Future studies investigating the genetic makeup of these tumors is required to potentially identify molecular features that may account for the aggressive nature of these tumors.
We staged our tumors in accordance with the criteria of the AJCC seventh edition10 and the Pittsburgh staging system11 for superficial versus deep lesions (ie, EAC vs bone involvement), respectively. The majority of tumors (81.3%) were of early stage I/II ( Table 2 ). The reported survival outcomes are commensurate with other series of SCC of the ear or temporal bone and indeed are poorer than survival for all-comers with cutaneous SCC when all body sites are included. We found that nodal disease was associated with worse DSS and DFS. A lack of association with OS may be explained by the advanced age and relative comorbidities within the cohort. Pre- and postauricular sites were associated with worse OS relative to auricular sites. For cutaneous SCC of all sites, clinically useful staging systems with groups that are discrete, discriminatory, and highly predictive of metastasis and survival remain elusive.16 To our knowledge, criteria of the AJCC eighth edition17 have yet to be validated for periauricular SCC. It remains to be seen whether this staging system or additional variables, such as immunosuppression, in-transit metastasis, or genetic profiles, may improve prognostic stratification in patients with these tumors.18
In our cohort, the vast majority of periauricular cutaneous SCC was managed surgically, and >20% of patients had concomitant nodal dissection of the parotid and/or ipsilateral neck ( Table 2 ). When indicated, nodal dissection resulted in a high rate of pathologic confirmation of metastases, particularly within the parotid gland and ipsilateral level II neck ( Figure 2 ). The high propensity for periauricular cutaneous SCC to metastasize and recur locoregionally, relative to other facial subsites, heralds the importance of addressing clinically and/or radiographically evident nodal disease concomitant with the primary tumor.19
Typical management of SCCs of the EAC and temporal bone consist of en bloc surgical resection. In addition to lateral temporal bone resection, the intimate relationship of the temporal bone to the parotid gland and the potential spread to the lymph nodes often mandate parotidectomy and selective neck dissection. While the data are not clear regarding a surgical standard of care in terms of SCC of the EAC and temporal bone, it is our experience to include the parotid and nodes of the upper neck (levels II-V) with the primary resection. This complete resection is often accompanied by a free tissue transfer closure of the defect given the wide and deep excision that is required to clear this aggressive disease. In addition to surgical resection, postoperative radiotherapy is utilized for more aggressive tumors (T3/T4) or for close surgical margins, perineural spread, or lymphovascular invasion. The indication for chemotherapy, in our experience, is based on the final pathology after surgical resection and is typically reserved for metastatic disease.
Given that most patients with temporal bone and EAC SCC present with advanced disease, we have found that multidisciplinary head and neck tumor board input is vital and provides ideal insight into surgical and postsurgical adjuvant therapy recommendations. These discussions are tailored to the individual patient; therefore, it is challenging to completely standardize management of temporal bone and EAC SCCs.
For small primary SCC tumors treated with WLE, SLNB was occasionally employed for nodal staging. SLNB for periauricular SCC resulted in a high rate of sentinel lymph node identification, most commonly in the ipsilateral level II neck. Consistent with previous reports,20 roughly 15% of sentinel lymph nodes were positive, leading to completion nodal dissection. While the exact role of SLNB for cutaneous SCC of the head and neck remains undefined, ours and other retrospective studies demonstrate the potential utility of SLNB in the surgical management of these cancers, particularly in the presence of high-risk features.20,21
In our cohort, adjuvant radiation or chemoradiation was reserved for patients with high-risk disease that was locally invasive (necessitating lateral temporal bone resection), metastatic to nodal basins in the parotid and/or neck, or recurrent ( Table 2 ). Adjuvant radiation has a clearly defined role for management of high-risk cutaneous SCC of the face, although the long-term risk of ototoxicity after treatment of periauricular tumors is a concern.22
Locoregional recurrence of periauricular cutaneous SCC after definitive treatment was quite high in our cohort, with 30.4% of patients experiencing recurrent disease and with a 3-year DFS of 56% (95% CI, 49%-63%). Our recurrence rate is higher than previous reports estimating any recurrence of periauricular SCC as roughly 10%.23 In our study, small tumors arising within previous periauricular surgical beds were classified as recurrent disease, though it is possible that these in fact were new primaries. As expected, extent and morbidity of reoperation for treatment of locoregionally recurrent disease were considerable. Patients more frequently required lateral temporal bone resection, neck dissection, superficial or total parotidectomy, and/or CN VII sacrifice for complete extirpation of locoregionally recurrent disease ( Table 2 ). Our data illustrate the importance of aggressive treatment of primary periauricular SCC and the need to appropriately counsel patients of the risk and morbidity of recurrent disease.
Estimated 3-year OS for our cohort was 62% (95% CI, 57%-67%). Despite the high recurrence rate of periauricular SCC seen in our study, 3-year DFS was favorable at 89% (95% CI, 86%-92%). Cutaneous SCC is often a disease of elderly individuals with multiple comorbidities, and this is clearly reflected in our survival data.24 Given the high rate of locoregional and distant recurrences seen in our study, it is likely that the current study would benefit from longer follow-up duration to capture all disease-related deaths. While stage did not predict survival, positive nodes with primary disease was significantly predictive of shortened DFS (P = .042) and DSS (P < .001), consistent with previous reports.25 We did find that patients with cutaneous SCC of pre- or postauricular skin had significantly worse OS (P = .007) but comparable DFS and DSS relative to those with primary tumors of the auricle. A plausible explanation for this finding outside of a cohort selection bias is challenging to ascertain. It is possible that the anatomic structure of the ear may provide some anatomic barrier to spread of tumors more distally located on the auricle, as there is more tissue to traverse prior to extension directly to deeper structures.
Our study is limited by inherent biases of retrospective studies. Analysis of variables, including margin of excision or pathologic margin, and intraoperative clinical details (eg, gross involvement of the parotid) were limited by inconsistencies in documentation and missing data. However, our large number of patients with periauricular cutaneous SCC adds to empiric knowledge of the clinical management and natural history of this unique and challenging cohort.
Conclusion
This study of our institutional experience highlights the unique challenges of treating cutaneous SCC at periauricular subsites. The reported propensity toward recurrence and patterns of metastasis may better guide treatment of aggressive tumors to include regional nodal dissection in addition local extirpation, and consultation of a multidisciplinary tumor board is valuable in selected cases. Future studies of periauricular cutaneous SCC should focus on predictive prognostic factors and personalized management of these cancers via elucidation of genetic and radiographic markers of aggressive disease.
Author Contributions
Kevin J. Kovatch, study design, authoring of manuscript, critical review and revision of manuscript, approval of manuscript as submitted, agreement to be accountable for all aspects of the work; Joshua D. Smith, study design, authoring of manuscript, critical review and revision of manuscript, approval of manuscript as submitted, agreement to be accountable for all aspects of the work, Andrew C. Birkeland, study design, critical review and revision of manuscript, approval of manuscript as submitted, agreement to be accountable for all aspects of the work; John E. Hanks, study design, authoring of manuscript, critical review and revision of manuscript, approval of manuscript as submitted, agreement to be accountable for all aspects of the work; Rasha Jawad, study design, critical review and revision of manuscript, approval of manuscript as submitted, agreement to be accountable for all aspects of the work; Scott A. McLean, study design, critical review and revision of manuscript, approval of manuscript as submitted, agreement to be accountable for all aspects of the work; Alison B. Durham, study design, critical review and revision of manuscript, approval of manuscript as submitted, agreement to be accountable for all aspects of the work; Ashok Srinivasan, study design, critical review and revision of manuscript, approval of manuscript as submitted, agreement to be accountable for all aspects of the work; Jonathan B. McHugh, study design, critical review and revision of manuscript, approval of manuscript as submitted, agreement to be accountable for all aspects of the work; Gregory J. Basura, study design, authoring of manuscript, critical review and revision of manuscript, approval of manuscript as submitted, agreement to be accountable for all aspects of the work.
Disclosures
Competing interests: None.
Sponsorships: Author Kevin J. Kovatch is supported by National Institutes of Health grant T32 DC005356 (T-32 Training Grant).
Funding source: None.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
This article was presented at the AAO-HNSF 2018 Annual Meeting & OTO Experience; October 7-10, 2018; Atlanta, Georgia.
References
- 1. Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957-966. [DOI] [PubMed] [Google Scholar]
- 2. English DR, Armstrong BK, Kricker A, Winter MG, Heenan PJ, Randell PL. Demographic characteristics, pigmentary and cutaneous risk factors for squamous cell carcinoma of the skin: a case-control study. Int J Cancer. 1998;76:628-634. [DOI] [PubMed] [Google Scholar]
- 3. Subramaniam P, Olsen CM, Thompson BS, et al. Anatomical distributions of basal cell carcinoma and squamous cell carcinoma in a population-based study in Queensland, Australia. JAMA Dermatol. 2017;153:175-182. [DOI] [PubMed] [Google Scholar]
- 4. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: management of advanced and high-stage tumors. J Am Acad Dermatol. 2018;78:249-261. [DOI] [PubMed] [Google Scholar]
- 5. Lassig AA, Spector ME, Soliman S, El-Kashlan HK. Squamous cell carcinoma invading the temporal bone: lateral temporal bone resection as primary intervention. Otol Neurotol. 2013;34:141-150. [DOI] [PubMed] [Google Scholar]
- 6. Sweeny L, Zimmerman T, Carroll WR, Schmalbach CE, Day KE, Rosenthal EL. Head and neck cutaneous squamous cell carcinoma requiring parotidectomy: prognostic indicators and treatment selection. Otolaryngol Head Neck Surg. 2014;150:610-617. [DOI] [PubMed] [Google Scholar]
- 7. Kadakia S, Saman M, Gordin E, Marra D, Ducic Y. The role of parotidectomy in the treatment of auricular squamous cell carcinoma. Otolaryngol Head Neck Surg. 2015;152:1048-1052. [DOI] [PubMed] [Google Scholar]
- 8. Kheterpal S. DataDirect: a self-serve tool for data retrieval. https://datadirect.med.umich.edu/. Published 2015. Accessed October 1, 2018.
- 9. Hanauer DA, Mei Q, Law J, Khanna R, Zheng K. Supporting information retrieval from electronic health records: a report of University of Michigan’s nine-year experience in developing and using the Electronic Medical Record Search Engine (EMERSE). J Biomed Inform. 2015;55:290-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- 11. Arriaga MA, Curtin H, Takahashi H, Hirsch BE, Kamerer DB. Staging proposal for external auditory meatus carcinoma based on preoperative clinical examination and computed tomography findings. Ann Otol Rhinol Laryngol. 1990;99(9, pt 1):714-721. [DOI] [PubMed] [Google Scholar]
- 12. Gal TJ, Futran ND, Bartels LJ, Klotch DW. Auricular carcinoma with temporal bone invasion: outcome analysis. Otolaryngol Head Neck Surg. 1999;121:62-65. [DOI] [PubMed] [Google Scholar]
- 13. Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690. [DOI] [PubMed] [Google Scholar]
- 14. Muzic JG, Schmitt AR, Wright AC, et al. Incidence and trends of basal cell carcinoma and cutaneous squamous cell carcinoma: a population-based study in Olmsted County, Minnesota, 2000-2010. Mayo Clin Proc. 2017;92:890-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dotto GP, Rustgi AK. Squamous cell cancers: a unified perspective on biology and genetics. Cancer Cell. 2016;29:622-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roscher I, Falk RS, Vos L, et al. Validating 4 staging systems for cutaneous squamous cell carcinoma using population-based data: a nested case-control study. JAMA Dermatol. 2018;154:428-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Amin MB, Edge SB, Greene FL, et al. , eds. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017. [Google Scholar]
- 18. Xu MJ, Lazar AA, Garsa AA, et al. Major prognostic factors for recurrence and survival independent of the American Joint Committee on Cancer eighth edition staging system in patients with cutaneous squamous cell carcinoma treated with multimodality therapy. Head Neck. 2018;40:1406-1414. [DOI] [PubMed] [Google Scholar]
- 19. Clark RR, Soutar DS. Lymph node metastases from auricular squamous cell carcinoma: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2008;61:1140-1147. [DOI] [PubMed] [Google Scholar]
- 20. Durham AB, Lowe L, Malloy KM, et al. Sentinel lymph node biopsy for cutaneous squamous cell carcinoma on the head and neck. JAMA Otolaryngol Head Neck Surg. 2016;142:1171-1176. [DOI] [PubMed] [Google Scholar]
- 21. Seim NB, Wright CL, Agrawal A. Contemporary use of sentinel lymph node biopsy in the head and neck. World J Otorhinolaryngol Head Neck Surg. 2016;2:117-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bhandare N, Jackson A, Eisbruch A, et al. Radiation therapy and hearing loss. Int J Radiat Oncol Biol Phys. 2010;76(3):S50-S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gaudet JE, Walvekar RR, Arriaga MA, et al. Applicability of the Pittsburgh Staging System for advanced cutaneous malignancy of the temporal bone. Skull Base. 2010;20:409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Connolly KL, Jeong JM, Barker CA, Hernandez M, Lee EH. A systematic review of comorbidity indices used in the nonmelanoma skin cancer population. J Am Acad Dermatol. 2017;76:344-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmults CD, Karia PS, Carter JB, Han J, Qureshi AA. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: a 10-year, single-institution cohort study. JAMA Dermatol. 2013;149:541-547. [DOI] [PubMed] [Google Scholar]