Abstract
We describe a 70 year-old independently ambulatory man with a 10-year history of progressive axial and limb-girdle weakness, hyperCKemia, and a 5-year history of dyspnea requiring nocturnal ventilatory support due to a known c.1309C>T (p.Arg437Cys) variant and a novel in-frame deletion of exons 17–19 in the calpain-3 encoding gene (CAPN3). Pulmonary function tests revealed neuromuscular respiratory weakness. Biceps femoris biopsy showed chronic myopathic changes, numerous lobulated fibers, and reduced calpain-3 immunoreactivity. Muscle immunoblot showed markedly reduced calpain-3 expression. Respiratory insufficiency is uncommon in autosomal recessive calpainopathy, and generally develops in the advanced stages of the disease when individuals become wheelchair-dependent. Our patient broadens the phenotypic spectrum of recessive calpainopathy to include early respiratory insufficiency and also further expands its molecular spectrum.
Keywords: Axial myopathy, Calpain-3, CAPN3, LGMD2A, Limb-girdle muscular dystrophy, Early respiratory insufficiency
1. Case report
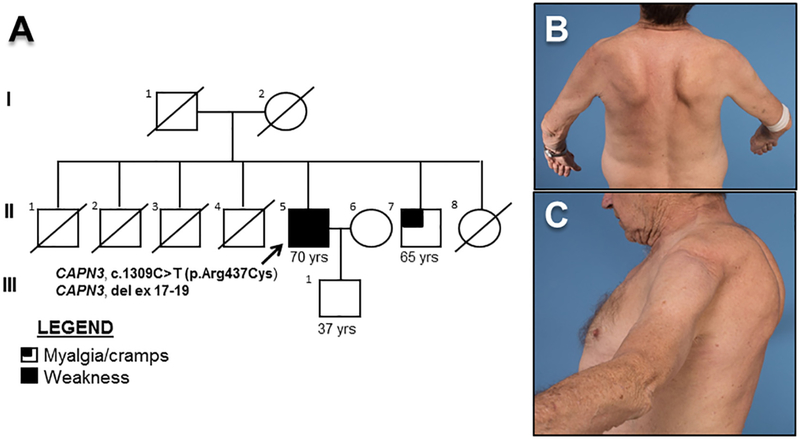
A 70-year old man developed gait difficulty at age 60 with subsequent lower limb weakness and upper limb weakness in his late-60’s. He described a 5-year history of exertional dyspnea requiring nocturnal oxygen for two years. He ambulated independently. He had no bulbar symptoms or rhabdomyolysis. His parents were asymptomatic (Fig. 1A).
Fig. 1.
Family pedigree and proband. (A) The arrow indicates the proband (II-5). A brother (II-7) developed myalgia and thigh cramping in his 60’s. A sister (II-8) died in her 60’s from unclear causes. Four brothers had coronary artery disease but none had a pacemaker/defibrillator. Photographs of the proband depicting (B) asymmetric scapular winging with arm flexion and (C) periscapular atrophy.
Neurologic examination was remarkable for a waddling, severely hyperlordotic gait, trace orbicularis oculi weakness, and severe symmetric shoulder and pelvic girdle weakness, including the thigh adductors and hamstrings with sparing of thigh abductors and quadriceps. There was mild intrinsic hand and toe extensor weakness. He had scapular winging and periscapular muscular atrophy (Fig. 1B,C).
Creatine kinase (CK) was 1338 U/L (normal < 232 U/L) five years ago and 253 U/L (normal < 336 U/L) on our evaluation. Acid alpha-glucosidase level was normal. Needle electromyography showed small motor unit potentials in proximal and axial muscles with rare fibrillation potentials. His vital capacity was 62% of the predicted value and maximal respiratory pressures were reduced. Echocardiogram and Holter monitor were normal.
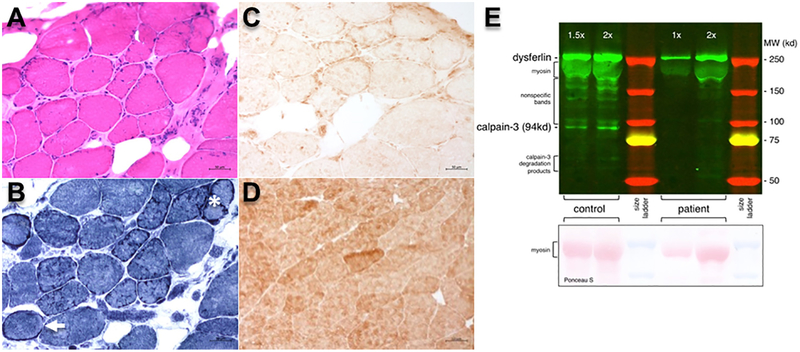
Biceps femoris biopsy showed numerous lobulated fibers and attenuated calpain-3 immunoreactivity (Fig. 2A–D). Muscle immunoblot showed markedly reduced calpain-3 expression (Fig. 2E).
Fig. 2.
Muscle histological and western blot findings. Biceps femoris muscle biopsy of the proband showing muscle fiber variation and an increase in fibrous and fatty connective tissue (A, hematoxylin and eosin). NADH dehydrogenase reacted sections (B) show numerous lobulated fibers (asterisk) or ring fibers (arrowhead). (C) Calpain-3 immunoreactivity (anti-calpain-3 2C4 antibodies) is reduced in the proband. (D) A normal muscle section with preserved calpain-3 immunoreactivity is shown for comparison. (E) Control muscle was compared to the muscle biopsy from the proband. While dysferlin appears normal in size and amount for the proband, full-length (94kd) calpain-3 and calpain-3 degradation products are greatly reduced. The antibodies used for Western blotting were Hamlet (anti-dysferlin) and 12A2 (anti–calpain-3), both purchased from Leica Biosystems.
2. Molecular genetic studies
Facioscapulohumeral dystrophy type 1 and 2 genetic testing was unremarkable (University of Iowa Diagnostic Laboratories). Next generation sequencing of 120 genes causative of myopathies and congenital myasthenic syndromes (Invitae; supplementary material) showed a known c.1309C>T variant in exon 10 (p.Arg437Cys) and a novel in-frame deletion of exons 17–19 in the calpain-3 encoding gene (CAPN3).
3. Discussion
Our patient had clinical and histopathologic features compatible with adult-onset calpainopathy, except for early respiratory insufficiency [1]. He carried compound heterozygous CAPN3 mutations, a known pathogenic mutation (c.1309C>T) and a novel deletion of exons 17–19. Calpain-3 is a muscle specific non-lysosomal cysteine protease involved in muscle regeneration, sarcolemmal remodeling, cytoskeleton regulation, and calcium homeostasis [2]. The c.1309C>T variant has been reported in homozygotes and compound heterozygotes, but genotypic association with respiratory status has not been described [3]. The exon 17–19 deletion removes a calcium-binding EF hand domain believed important for protease function [4]. Functional studies have not been performed for this deletion, but a missense mutation within the deleted region was reported as pathogenic in mouse models [5]. Furthermore, the greatly reduced amount of calpain-3 detected by immunostaining and immunoblotting strongly supports the pathogenicity of this novel deletion of exons 17–19.
Recessive CAPN3 mutations have been long known to cause a common limb-girdle muscular dystrophy, LGMD2A, featuring adolescent-onset, hip-girdle and axial weakness with wide inter- and intra-familial phenotypic variability. Scapular winging and hip extensor, thigh adductor and hamstring involvement is common [1]. Recently, a heterozygous 21-base pair in-frame deletion (c.643_663del21) in CAPN3 was described in autosomal dominant LGMD of milder phenotype [6,7]. While we cannot determine if the patient’s CAPN3 variants are heteroallelic, asymptomatic parents support autosomal recessive inheritance.
While calpainopathy is typically associated with minimal respiratory involvement [8,9], Mori-Yoshimura et al. reported moderate-to-severe respiratory insufficiency in 20% of Japanese calpainopathy patients, whom were 65 and older, had longer disease duration, were non-ambulatory, and had lower CK levels than patients with normal respiratory function. There was no genotypic correlation with respiratory dysfunction in this cohort, and mutations reported in these cases did not overlap with our patient [10]. Pollitt et al. reported a non-ambulatory individual with respiratory insufficiency requiring non-invasive ventilation, who carried a c.566 T > C variant in exon 4 and a splice site variant in exon 18 [11], the latter which overlaps with our patient’s deletion. Respiratory involvement in our patient is unique as it occurred early in the course of the disease while he remained ambulatory.
Our findings further expand the spectrum of recessive calpainopathy to include early respiratory insufficiency and highlight another calpain-3 region necessary for protein function. Whether mutations affecting a calcium-binding EF hand domain are associated with respiratory insufficiency remains to be elucidated.
Acknowledgments
The authors thank Mary Cox, Department of Pathology, University of Iowa, who performed the western blots.
Funding
Ms. Cox and Dr. Moore are supported by the Iowa Wellstone Muscular Dystrophy Cooperative Research Center, U54, NS053672.
Abbreviations:
- CAPN3
calpain-3
- AR
autosomal recessive
- CK
creatine kinase
- LGMD
limb girdle muscular dystrophy
- LGMD2A
limb-girdle muscular dystrophy type 2A
- del
deletion
- Arg
arginine
- Cys
cysteine
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jocn.2018.04.025.
Disclosures
The authors have no conflicts of interest or disclosures to report.
References
- [1].Fanin M, Angelini C. Protein and genetic diagnosis of limb girdle muscular dystrophy type 2A: the yield and the pitfalls. Muscle Nerve 2015;52 (2):163–73. [DOI] [PubMed] [Google Scholar]
- [2].Ono Y, Ojima K, Shinkai-Ouchi F, Hata S, Sorimachi H. An eccentric calpain, CAPN3/p94/calpain-3. Biochimie 2016;122:169–87. [DOI] [PubMed] [Google Scholar]
- [3].Richard I, Hogrel JY, Stockholm G, Payan CA, Fougerousse F, The Calpainopathy Study Group, et al. Natural history of LGMD2A for delineating outcome measures in clinical trials. Ann Clin Transl Neurol 2016;3(4):248–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Partha SK, Ravulapalli R, Allingham JS, Campbell RL, Davies PL. Crystal structure of calpain-3 penta-EF-hand (PEF) domain- a homodimerized PEF family member with calcium bound at the fifth EF-hand. FEBS J 2014;281 (14):3138–49. [DOI] [PubMed] [Google Scholar]
- [5].Ermolova N, Kudryashova E, DiFranco M, Vergara J, Kramerova I, Spencer MJ. Pathogenicity of some limb girdle muscular dystrophy mutations can results from reduced anchorage to myofibrils and altered stability of calpain 3. Hum Mol Genet 2011;20(17):3331–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vissing J, Barresi R, Witting N, Van Ghelue M, Gammelgaard L, Bindoff LA, et al. A heterozygous 21-bp deletion in CAPN3 causes dominantly inherited limb girdle muscular dystrophy. Brain 2016;139:2154–63. [DOI] [PubMed] [Google Scholar]
- [7].Martinez-Thompson JM, Niu Z, Tracy JA, Moore SA, Swenson A, Wieben ED, Milone M. Autosomal dominant calpainopathy due to heterozygous CAPN3 c.643_663del21. Muscle Nerve 2017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fardeau M, Eymard B, Mignard C, Tome FM, Richard I, Beckman JS. Chromosome 15-linked limb-girdle muscular dystrophy: clinical phenotypes in Reunion Island and French metropolitan communities. Neuromuscul Disord 1996;6:447–53. [DOI] [PubMed] [Google Scholar]
- [9].Groen EJ, Charlton R, Barresi R, Anderson LV, Eagle M, Hudson J, et al. Analysis of the UK diagnostic strategy for limb girdle muscular dystrophy 2A. Brain 2007;130(12):3237–9. [DOI] [PubMed] [Google Scholar]
- [10].Mori-Yoshimura M, Segawa K, Minami N, Oya Y, Komaki H, Nonaka I, et al. Cardiopulmonary dysfunction in patients with limb-girdle muscular dystrophy 2A. Muscle Nerve 2017;55:465–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pollitt C, Anderson LVB, Pogue R, Davison K, Pyle A, Bushby KMD. The phenotype of calpainopathy: diagnosis based on a multidisciplinary approach. Neuromusc Disord 2001:287–96. [DOI] [PubMed] [Google Scholar]