Abstract
The green peach aphid, Myzus persicae Sulzer (Hemiptera: Aphididae), is an important agricultural pest with a wide range of host plants. To study effects of host species on the life history traits of M. persicae, aphids were individually reared on five host plants: Brassica campestris L. (Brassicales: Brassicaceae), Capsicum annuum L. (Tubiflorae: Solanaceae), Nicotiana tabacum L. (Tubiflorae: Solanaceae), Raphanus sativus L. (Brassicales: Brassicaceae), and Vicia faba L. (Rosales: Leguminosae). TWOSEX-MSchart software was used for the statistical analysis according to the age-stage, two-sex life table theory. The results showed that the shortest preadult stage and adult/total prereproductive period of M. persicae were 6.48, 0.19, and 6.67 d on V. faba, respectively. While the adult and total longevity of M. persicae on R. sativus (25.00 and 31.62 d) and N. tabacum (24.40 and 30.56 d) were significantly longer than that on the other three hosts, as was the reproductive period. The fecundity of M. persicae on R. sativus (80.83 nymphs per female), N. tabacum (71.72 nymphs per female), and V. faba (70.39 nymphs per female) was also greater than that on B. campestris and C. annuum. It was demonstrated that V. faba, R. sativus, and N. tabacum were more suitable plants for the growth of M. persicae exhibiting a shorter preadult stage, longer longevity, and greater fecundity than the remaining two species, as confirmed by the higher intrinsic rate of increase and net reproductive rate.
Keywords: Myzus persicae, host species, life table, population characteristics
Ecological patterns may often be determined by the physiological constraints of interacting organisms (Singer 2001). One of the most important factors affecting the fitness of insect herbivores is the quality of the host plant. Compounds from host plants, such as carbon, nitrogen, and defense metabolites, directly affect the potential and achieved herbivore fecundity, and the responses of insect herbivores to changes in host plant quality vary within and between feeding guilds (Awmack and Leather 2002, Hosseini et al. 2019). Polyphagous herbivores are particularly challenged, as feeding on different plant species can result in differences in life history traits (Ojala et al. 2005). It is estimated that 15% of crop yield worldwide is lost to herbivore pests in spite of plant breeding and pest control efforts (Van Der Meijden 2015). However, plant diversity also promotes herbivore suppression through movement patterns, host associations, and predation promises a potential alternative to pesticide-intensive monoculture crop production (Letourneau et al. 2011). The fitness of a pest population is represented by its damage capacity to the host plant. That fitness can be properly evaluated using a life table, as it provides an integrated and comprehensive description of the survival, development, and reproduction of a population (Tuan et al. 2016).
The life table is a powerful and necessary tool for analyzing and understanding the effect of external factors and host plants on the growth, survival, reproduction, and intrinsic rate of increase of insect populations (Chi and Su 2006). A life table study is fundamental for population ecology research and provides a complete description of the survivorship, development, stage differentiation, and reproduction of a population as well as basic population growth parameters (Hu et al. 2010, Yousaf et al. 2018). Using a life table, population projections can be made by means of computer simulations (Chi 1990). Life tables have been used in a variety of studies related to population ecology, such as the effect of temperature on population growth of Hyalopterus pruni Geoffroy (Hemiptera: Aphididae) (Atlihan and Chi 2008), climate change/global warming studies (Kanle Satishchandra et al. 2019), host plant resistance on Lipaphis erysimi Kaltenbach (Hemiptera: Aphididae) (Qayyum et al. 2018), and harvesting theory (Yu et al. 2018).
As an economically important pest, the green peach aphid, Myzus persicae Sulzer (Hemiptera: Aphididae), attacks over 400 species in more than 50 plant families, including Solanaceae, Cruciferae, and Leguminosae (Weber 1985, Tang et al. 2019). Its polyphagy is attributed to its ecological and phenotypic plasticity and physiological adaptability to a large array of primary and secondary compounds from diverse host species. To quantitatively analyze the effect of different host plants on the life history of M. persicae, we constructed several life tables of M. persicae using the age-stage, two-sex life table theory. The current study provides a comprehensive insight into the host preference of M. persicae. Our findings can be used for developing targeted strategies for prevention of the green peach aphid.
Materials and Methods
Aphid and Host Plant Culture
Adult M. persicae samples were collected from Nicotiana tabacum L. (Tubiflorae: Solanaceae) plants in Chongqing, China, and then maintained on the same host species in a climate incubator at 23 ± 1°C, 50 ± 5% RH, and a photoperiod of 16:8 (L:D) h. Five host plants commonly found in Chongqing were used for this study, including rapeseed [Brassica campestris L. (Brassicales: Brassicaceae)], pepper [Capsicum annuum L. (Tubiflorae: Solanaceae)], tobacco (N. tabacum), radish [Raphanus sativus L. (Brassicales: Brassicaceae)], and faba bean [Vicia faba L. (Rosales: Leguminosae)]. All tested plants were grown in growth chambers with Pindstrup substrate as media and were watered as required.
Life Table Construction
For the life table study, more than 100 M. persicae adults were transferred onto tobacco leaves. After 12 h, newborn nymphs were individually transferred to the leaf discs (2 cm of diameter) of five host plants. The leaf disc was placed upside down on a water-saturated sponge pad in a plastic dish (8.5 cm of diameter, and 1.5 cm of height). A piece of filter paper with a hole (2 cm of diameter at the center) was placed onto the leaf disc to form a water fence. The molting and reproduction of M. persicae were observed and recorded every 12 h until all aphids were dead. All studies were carried out in climate incubators (MLR-351H, Panasonic, Matsuyama, Japan) at 23 ± 1°C, 70 ± 5% RH, and a photoperiod of 16:8 (L:D) h (Tang et al. 2017).
Statistical Analysis
TWOSEX-MSchart software was used for analysis of the life history based on the age-stage, two-sex life table theory (Chi and Liu 1985). The means of developmental periods for each stage and total longevity of M. persicae reared on different host plants were calculated as well as the age-stage-specific survival rate (sxj). sxj represents the probability that a newborn aphid will survive to age x and stage j. The age-stage life expectancy (exj) was also calculated, which is defined as the length of duration or time that a green peach aphid of x and j is predicted to live (Chi and Su 2006).
where represents the probability that individuals of age = x and stage = j will survive to age = i and stage = y.
Additionally, the means of prereproductive period and female fecundity were also calculated. The adult prereproductive period (APRP) was defined as the duration of time from the emergence of the adult female to its initial reproduction, and the total prereproductive period (TPRP) was the total duration of time from the beginning of the life table study to the female's initial reproduction. After that, we calculated the reproductive value (vxj) to evaluate the contribution of an individual at age x in stage j to the future population (Rostami et al. 2018).
The population parameters (R0, net reproductive rate; r, intrinsic rate of increase; λ, finite rate of increase; and T, the mean generation time) of M. persicae on different host plants were estimated accordingly. R0 is defined as the total offspring produced by an individual during its life span. r is the rate at which a population increases in size without density-dependent forces. λ indicates the number of times the population multiplies in a unit of time. T is the time required for a population to increase its size by R0-fold in the stable age distribution (Akköprü et al. 2015). These parameters were calculated as:
The means and standard errors of the developmental time, longevity, fecundity, and population parameters were estimated using the bootstrap technique. To reduce the variability of the results, we used 200,000 bootstrap replications in this study. The differences among the five host treatments were analyzed by the paired bootstrap test with a P value of less than 0.05.
Results
Effect of Host Species on the Development of M. persicae
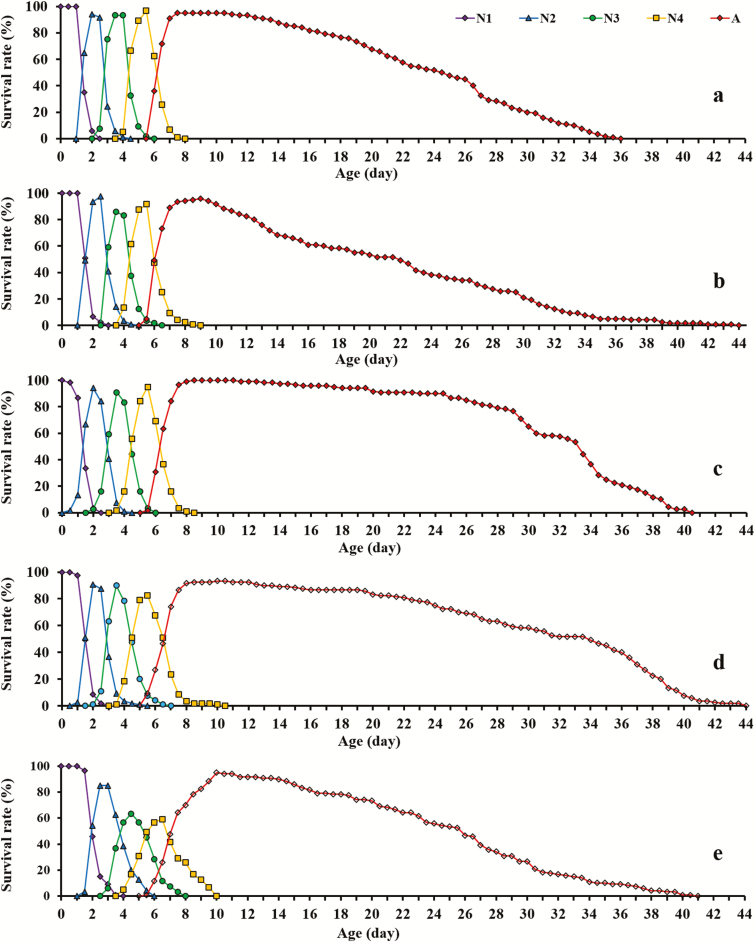
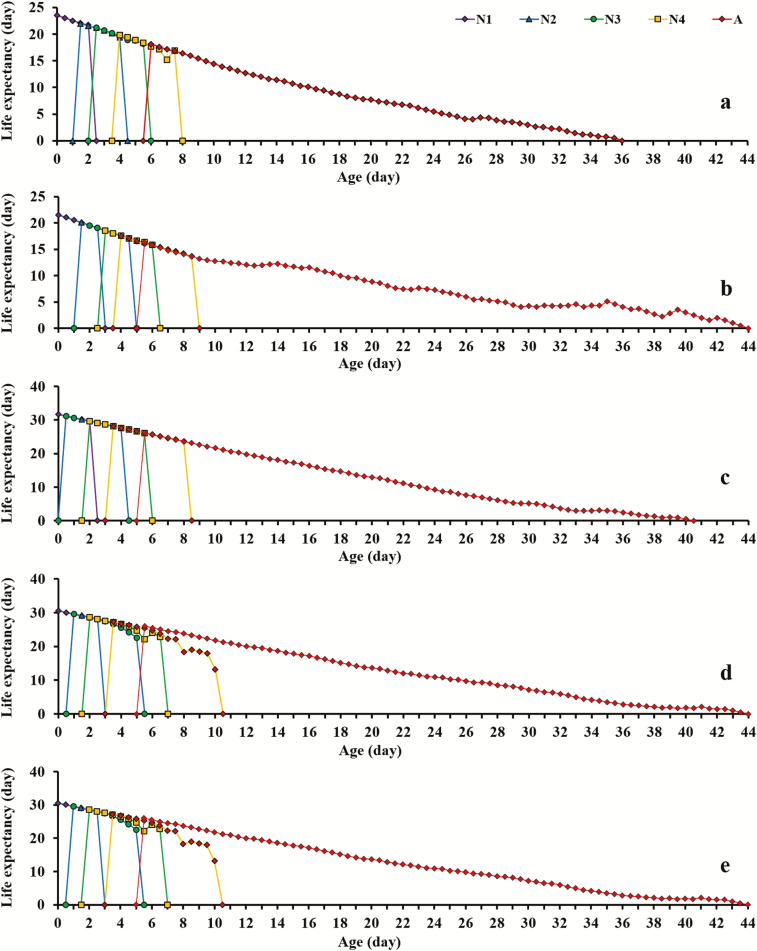
The durations of the four nymph stages for the aphids fed with five plant species all differed significantly from each other (Table 1). The preadult durations for M. persicae reared on B. campestris, C. annuum, N. tabacum, R. sativus, and V. faba were 7.54 ± 0.11, 6.44 ± 0.06, 6.77 ± 0.08, 6.62 ± 0.05, and 6.48 ± 0.04 d, respectively. The shortest preadult durations were observed on C. annuum and V. faba, and the curves of the age-stage-specific survival rate (sxj) showed that the survival rates of mature aphids on C. annuum and B. campestris were lower than those on N. tabacum and R. sativus (Fig. 1; Supp Fig. 1 [online only]). The adult longevity and total longevity of the aphids reared on C. annuum and B. campestris were also significantly shorter than those reared on N. tabacum and R. sativus. The age-stage life expectancy (exj) showed the same trend (Fig. 2; Supp Fig. 2 [online only]). The life expectancy of the new born nymph at age = 0 and stage = 1 (e01 value) was exactly the mean longevity of 21.55 ± 0.83 d on C. annuum, 23.51 ± 0.68 d on V. faba, 24.69 ± 0.75 d on B. campestris, 30.56 ± 0.85 d on N. tabacum, and 31.62 ± 0.56 d on R. sativus, respectively (Table 1).
Table 1.
Effect of host plants on the development of Myzus persicae
| Statistics | Host species | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vicia faba | Capsicum annuum | Raphanus sativus | Nicotiana tabacum | Brassica campestris | ||||||
| n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | |
| Preadult duration (d) | 116 | 6.48 ± 0.04c | 118 | 6.44 ± 0.06c | 120 | 6.62 ± 0.05b | 117 | 6.77 ± 0.08b | 116 | 7.54 ± 0.11a |
| First instar (d) | 120 | 1.70 ± 0.03c | 120 | 1.80 ± 0.03b | 120 | 1.61 ± 0.03d | 120 | 1.78 ± 0.03bc | 120 | 2.34 ± 0.05a |
| Second instar (d) | 119 | 1.42 ± 0.02d | 120 | 1.50 ± 0.03bc | 120 | 1.55 ± 0.03b | 120 | 1.42 ± 0.03cd | 116 | 1.78 ± 0.07a |
| Third instar (d) | 118 | 1.57 ± 0.02a | 120 | 1.42 ± 0.03b | 120 | 1.57 ± 0.02a | 119 | 1.63 ± 0.03a | 116 | 1.63 ± 0.05a |
| Fourth instar (d) | 116 | 1.79 ± 0.03b | 118 | 1.71 ± 0.03b | 120 | 1.89 ± 0.02a | 117 | 1.97 ± 0.05a | 116 | 1.81 ± 0.09ab |
| Adult longevity (d) | 116 | 17.66 ± 0.62b | 118 | 15.37 ± 0.82c | 120 | 25.00 ± 0.56a | 117 | 24.40 ± 0.81a | 116 | 17.80 ± 0.71b |
| Total longevity (d) | 120 | 23.51 ± 0.68bc | 120 | 21.55 ± 0.83c | 120 | 31.62 ± 0.56a | 120 | 30.56 ± 0.85a | 120 | 24.69 ± 0.75b |
Means in the same row followed by different letters are significantly different (P < 0.05) using bootstrap test.
Fig. 1.
The age-stage-specific survival rate (sxj) of Myzus persicae on five different host plants. N1, first instar nymph, purple curve; N2, second instar nymph, blue curve; N3, third instar nymph, green curve; N4, fourth instar nymph, yellow curve; A, adult aphid, red curve. Each panel represents Vicia faba (a), Capsicum annuum (b), Raphanus sativus (c), Nicotiana tabacum (d), Brassica campestris (e), respectively.
Fig. 2.
The age-stage-specific life expectancy (exj) of Myzus persicae on five different host plants. N1, first instar nymph, purple curve; N2, second instar nymph, blue curve; N3, third instar nymph, green curve; N4, fourth instar nymph, yellow curve; A, adult aphid, red curve. Each panel represents Vicia faba (a), Capsicum annuum (b), Raphanus sativus (c), Nicotiana tabacum (d), Brassica campestris (e), respectively.
Effect of Host Species on the Fecundity of M. persicae
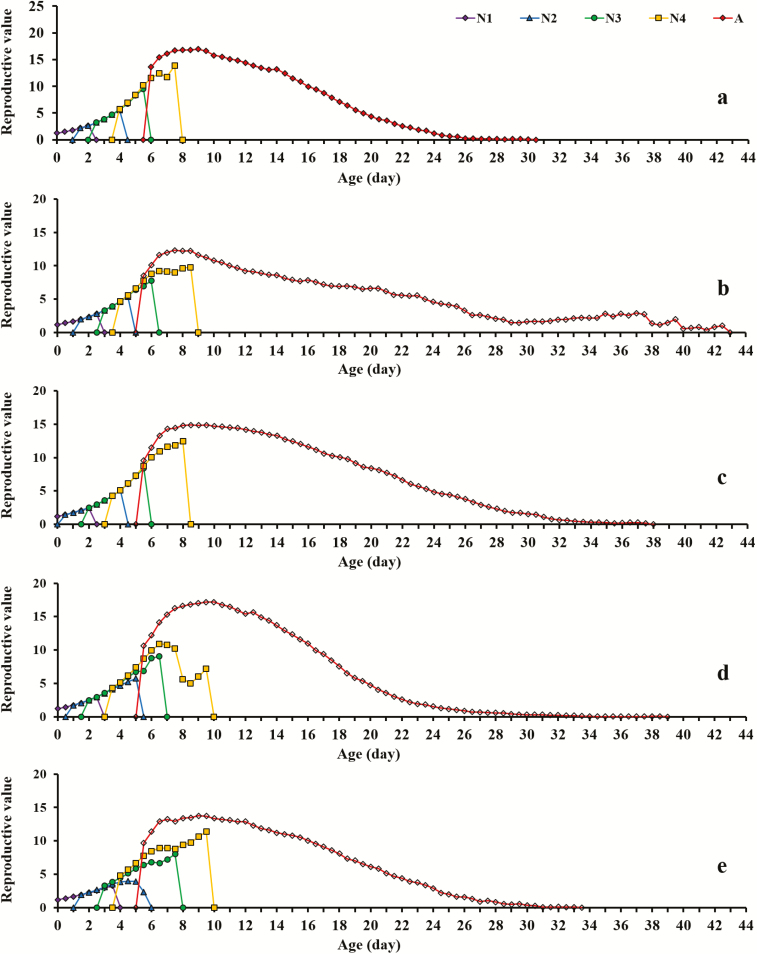
Means of TPRP and APRP of M. persicae reared on different host plants also differed significantly (Table 2). Mature aphids and newborn aphids on V. faba both took the least time to produce offspring (APRP = 0.19 ± 0.03 d and TPRP = 6.67 ± 0.05 d), whereas the greatest APRP and TPRP were, respectively, observed in newborn aphids on C. annuum (APRP = 0.61 ± 0.04 d) and mature aphids on B. campestris (TPRP = 7.99 ± 0.13 d). Conversely, the reproductive period of M. persicae on C. annuum and B. campestris was shortest. Upon comparison of aphids' fecundity on different host species, we found that the mean nymph-laying per female on C. annuum and B. campestris was also significantly lower than on the other three host plants. There were no significant differences among the vxj of the M. persicae females reared on various host species, but the curves for aphids on C. annuum and B. campestris showed lower peaks than the others (Fig. 3; Supp Fig. 3 [online only]), which further supported the weak fecundities of the two host populations.
Table 2.
Effect of host plants on the fecundity (nymphs per female) of Myzus persicae
| Statistics | Host species | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vicia faba | Capsicum annuum | Raphanus sativus | Nicotiana tabacum | Brassica campestris | ||||||
| n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | |
| APRP (d)a | 114 | 0.19 ± 0.03c | 116 | 0.61 ± 0.04a | 120 | 0.41 ± 0.03b | 112 | 0.64 ± 0.07a | 115 | 0.44 ± 0.08ab |
| TPRP (d)b | 114 | 6.67 ± 0.05d | 116 | 7.04 ± 0.08c | 120 | 7.03 ± 0.06c | 112 | 7.37 ± 0.09b | 115 | 7.99 ± 0.13a |
| Reproductive period (d) | 114 | 12.96 ± 0.36c | 116 | 11.05 ± 0.59d | 120 | 18.30 ± 0.43a | 112 | 14.03 ± 0.34b | 115 | 12.26 ± 0.44cd |
| Fecundity (nymphs per female) | 114 | 70.39 ± 2.26b | 116 | 47.05 ± 2.54d | 120 | 80.83 ± 2.09a | 112 | 71.72 ± 2.12b | 115 | 53.71 ± 2.23c |
Means in the same row followed by different letters are significantly different (P < 0.05) using bootstrap test.
aAPRP = adult prereproductive period.
bTPRP = total prereproductive period.
Fig. 3.
The age-stage-specific reproductive value (vxj) of Myzus persicae on five different host plants. N1, first instar nymph, purple curve; N2, second instar nymph, blue curve; N3, third instar nymph, green curve; N4, fourth instar nymph, yellow curve; A, adult aphid, red curve. Each panel represents Vicia faba (a), Capsicum annuum (b), Raphanus sativus (c), Nicotiana tabacum (d), Brassica campestris (e), respectively.
Life Table Parameters
Table 3 presents the age-stage, two-sex life table parameters of M. persicae reared on different host species, all of which showed significant differences among these populations. Aphids on V. faba exhibited the highest intrinsic rate (r = 0.3831 ± 0.0037 d−1) and finite rate of increase (λ = 1.4669 ± 0.0054 d−1), and the shortest mean generation time (T = 11.0139 ± 0.0842 d). Correspondingly, the aphids on B. campestris exhibited the lowest r (0.3207 ± 0.0052 d−1) and λ (1.3781 ± 0.0072 d−1), and the longest T (12.3146 ± 0.1360 d). The largest net reproductive rate R0 was detected in aphids on R. sativus (80.8317 ± 2.0886 offspring per individual), while aphids on C. annuum and B. campestris produced the least total offspring (46.2691 ± 2.5565 offspring per individual for C. annuum, and 51.9251 ± 2.3313 offspring per individual for B. campestris) during their life spans.
Table 3.
Life table parameters of Myzus persicae on five different host plants
| Statistics | Host species | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vicia faba | Capsicum annuum | Raphanus sativus | Nicotiana tabacum | Brassica campestris | ||||||
| n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | |
| r (d−1) | 120 | 0.3831 ± 0.0037a | 120 | 0.3422 ± 0.0045c | 120 | 0.3616 ± 0.0034b | 120 | 0.3595 ± 0.0045b | 120 | 0.3207 ± 0.0052d |
| λ (d−1) | 120 | 1.4669 ± 0.0054a | 120 | 1.4080 ± 0.0063c | 120 | 1.4356 ± 0.0049b | 120 | 1.4327 ± 0.0065b | 120 | 1.3781 ± 0.0072d |
| R 0 (offspring per individual) | 120 | 68.0439 ± 2.4674b | 120 | 46.2691 ± 2.5565c | 120 | 80.8317 ± 2.0886a | 120 | 67.5278 ± 2.5226b | 120 | 51.9251 ± 2.3313c |
| T (d) | 120 | 11.0139 ± 0.0842c | 120 | 11.2025 ± 0.1346c | 120 | 12.1484 ± 0.1041a | 120 | 11.7156 ± 0.0890b | 120 | 12.3146 ± 0.1360a |
r = intrinsic rate of increase; λ = finite rate of increase; R0 = net reproductive rate; T = mean generation time. Means in the same row followed by different letters are significantly different (P < 0.05) using bootstrap test.
Discussion
The parameter r (intrinsic rate of increase) is regarded as a useful concept in demographic analyses of insect populations (Birch 1948). This parameter (r) summarizes the physiological qualities of an animal relative to its capacity of increase and is often used to compare the fitness of populations across diverse climatic and food-related conditions (Tsai and Wang 2001, Saeed et al. 2010). The r values for M. persicae were reported as 0.160–0.256 d−1 a decade ago on solanaceous and cruciferous vegetables and crops (Boughton et al. 2006, Chi and Su 2006, Davis et al. 2006, Ribeiro et al. 2006), which is significantly lower than in the present study. This might be attributed to the enhanced adaptability of M. persicae to a complex environment. Life history theory is based on the assumption that evolution is constrained by trade-offs among different traits that contribute to fitness, and life history costs play a central role in explaining the evolution of resistance (Carriere et al. 1994). Fitness costs include reduced survival, increased developmental time, and reduced fecundity (Sayyed et al. 2008). Therefore, the stronger the adaptability, the lower the fitness cost, and the higher the r value.
The age at first nymph-laying is critical for r value calculation. If fecundity remains the same, early reproduction will be associated with a high r value (Huang and Chi 2012). Both the APRP and TPRP of M. persicae on V. faba were shortest, resulting in the highest r value of increase, which is consistent with the aforementioned theory. Conversely, the two statistics, APRP and TPRP do not represent the actual beginning of reproduction, and thus their effects on the r value should not be overemphasized (Jha et al. 2012). Barlow (1962) found that offspring produced after peak fecundity have little influence on the r value of the population when the fecundity rate is more or less sharply defined in time. Therefore, the number of offspring produced by aphids on the five host species before peak fecundity was analyzed and indicated the same r value order. It does not necessarily follow that the higher the r value is, the more successful the species will be.
Most species exist in saturated environments and abundance fluctuates in response to changes in the conditions of survival and growth as a result of changes in factors such as weather (Cole 1954). One can examine the potential importance of life history parameters in adapting to perturbations by determining the effect of change in the life history parameters on the net reproductive rate R0. In our study, aphids reared on R. sativus exhibited a higher R0 than that on the other plant species, while the aphids on C. annuum and B. campestris performed poorly. To determine the effective factors, correlation analyses were carried out with a correlation coefficient equal to 0.05 between R0 and the means of each developmental period, reproductive period, female fecundity, age-stage-specific survival rate sxj, and the female age-stage-specific fecundity fx,female. With the exception of reproductive period, sxj, and female fecundity, the remainder of the indices showed no significant correlation with R0 value. Female fecundities showed a similar tendency as the R0 value, as preadult survival rates of the M. persicae females on the five host plants were quite similar in terms of the equation R0 = sα·F, where sα represents the preadult survival rate and F is the mean female fecundity. However, the female age-stage-specific fecundity fx,female was not found to be closely related to the R0 value, which indicated that the net reproductive rate of a population is associated with the adult number and the total offspring but not the adult age or the daily fecundity. Though the reproductive period and sxj of the aphids on V. faba were both lower than those on N. tabacum, the R0 value for M. persicae on V. faba was similar to that on N. tabacum. Nitrogen application rates are known to affect the individual size, survival, and r value of several species of rice pest (Jahn et al. 2005). It was concluded that the nitrogen-rich legumes improved the fertility of the parasitic aphid and further affected the population development.
In conclusion, V. faba, R. sativus, and N. tabacum constituted more suitable host plants for M. persicae and were associated with a shorter preadult stage, longer longevity, and stronger fecundity in comparison to the remaining species. Furthermore, the intrinsic rate r of the aphids was also significantly higher on V. faba, which depended on their ages at first nymph-laying and the number of offspring produced before peak fecundity. Another important life history parameter, R0 was found to be higher in aphids reared on R. sativus than that on the other plant species, proving that the adult number was more meaningful to the population growth of M. persicae than the adult age. Laboratory colonies of M. persicae are necessary for experimental study of its biology, behavior, and insect–virus interactions. In our study, R. sativus was proved to be the most favorable cultivar for the green peach aphid in lab which exhibited the longest longevity and strongest fecundity. If mass-rearing of this species, V. faba will be the best choice because of the short prereproductive period and the strong fecundity.
Supplementary Data
Supplementary data are available at Journal of Insect Science online.
Figure S1. The age-specific survival rate (sxj) of adult Myzus persicae on five different host plants. Purple curve, Vicia faba; blue curve, Capsicum annuum; green curve, Raphanus sativus; yellow curve, Nicotiana tabacum; red curve, Brassica campestris.
Figure S2. The age-specific life expectancy (exj) of adult Myzus persicae on five different host plants. Purple curve, Vicia faba; blue curve, Capsicum annuum; green curve, Raphanus sativus; yellow curve, Nicotiana tabacum; red curve, Brassica campestris.
Figure S3. The age-specific reproductive value (vxj) of adult Myzus persicae on five different host plants. Purple curve, Vicia faba; blue curve, Capsicum annuum; green curve, Raphanus sativus; yellow curve, Nicotiana tabacum; red curve, Brassica campestris.
Acknowledgments
We thank Dr. Hsin Chi (National Chung Hsing University, China) for technical assistance. This research was funded by the National Key Research and Development Project (2018YFD0200300) and the Ph.D. Research Funding of Southwest University (SWU118110).
References Cited
- Akköprü E. P., Atlıhan R., Okut H., and Chi H.. 2015. Demographic assessment of plant cultivar resistance to insect pests: a case study of the dusky-veined walnut aphid (Hemiptera: Callaphididae) on five walnut cultivars. J. Econ. Entomol. 108: 378–387. [DOI] [PubMed] [Google Scholar]
- Atlihan R., and Chi H.. 2008. Temperature-dependent development and demography of Scymnus subvillosus (Coleoptera: Coccinellidae) reared on Hyalopterus pruni (Homoptera: Aphididae). J. Econ. Entomol. 101: 325–333. [DOI] [PubMed] [Google Scholar]
- Awmack C. S., and Leather S. R.. 2002. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 47: 817–844. [DOI] [PubMed] [Google Scholar]
- Barlow C. A. 1962. Influence of temperature on growth of experimental populations of Myzus persicae (Sulzer) and Macrosiphum Euphorbiae (Thomas) (Aphididae). Can. J. Zool. 40: 145–156. [Google Scholar]
- Birch L. C. 1948. The intrinsic rate of natural increase of an insect population. J. Anim. Ecol. 17: 15–26. [Google Scholar]
- Boughton A. J., Hoover K., and Felton G. W.. 2006. Impact of chemical elicitor applications on greenhouse tomato plants and population growth of the green peach aphid, Myzus persicae. Entomol. Exp. Appl. 120: 175–188. [Google Scholar]
- Carriere Y., Deland J. P., Roff D. A., and Vincent C.. 1994. Life-history costs associated with the evolution of insecticide resistance. Proc. R. Soc. Lond B-Biol. Sci. 258: 35–40. [Google Scholar]
- Chi H. 1990. Timing of control based on the stage structure of pest populations - a simulation approach. J. Econ. Entomol. 83: 1143–1150. [Google Scholar]
- Chi H., and Liu H.. 1985. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 24: 225–240. [Google Scholar]
- Chi H., and Su H. Y.. 2006. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 35: 10–21. [Google Scholar]
- Cole L. C. 1954. The population consequences of life history phenomena. Q. Rev. Biol. 29: 103–137. [DOI] [PubMed] [Google Scholar]
- Davis J. A., Radcliffe E. B., and Ragsdale D. W.. 2006. Effects of high and fluctuating temperatures on Myzus persicae (Hemiptera: Aphididae). Environ. Entomol. 35: 1461–1468. [Google Scholar]
- Hosseini A., Hosseini M., Michaud J. P., Awal M. M., and Ghadamyari M.. 2019. Life history responses of Hippodamia variegata (Coleoptera: Coccinellidae) to changes in the nutritional content of its prey, Aphis gossypii (Hemiptera: Aphididae), mediated by nitrogen fertilization. Biol. Control. 130: 27–33. [Google Scholar]
- Hu L. X., Chi H., Zhang J., Zhou Q., and Zhang R. J.. 2010. Life-table analysis of the performance of Nilaparvata lugens (Hemiptera: Delphacidae) on two wild rice species. J. Econ. Entomol. 103: 1628–1635. [DOI] [PubMed] [Google Scholar]
- Huang Y. B., and Chi H.. 2012. Age-stage, two-sex life tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Sci. 19: 263–273. [Google Scholar]
- Jahn G. C., Almazan L. P., and Pacia J. B.. 2005. Effect of nitrogen fertilizer on the intrinsic rate of increase of Hysteroneum setariae (Thomas) (Homoptera: Aphididae) on rice (Oryza sativa L.). Environ. Entomol. 34: 938–943. [Google Scholar]
- Jha R. K., Chi H., and Tang L.. 2012. A comparison of artificial diet and hybrid sweet corn for the rearing of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) based on life table characteristics. Environ. Entomol. 41: 30–39. [DOI] [PubMed] [Google Scholar]
- Kanle Satishchandra N., Chakravarthy A. K., Ozgökçe M. S., and Atlihan R.. 2019. Population growth potential of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on tomato, potato, and eggplant. J. Appl. Entomol. 143: 518–526. [Google Scholar]
- Letourneau D. K., Armbrecht I., Rivera B. S., Lerma J. M., Carmona E. J., Daza M. C., Escobar S., Galindo V., Gutiérrez C., López S. D., . et al. 2011. Does plant diversity benefit agroecosystems? A synthetic review. Ecol. Appl. 21: 9–21. [DOI] [PubMed] [Google Scholar]
- Ojala K, Julkunen-Tiito R., Lindstrom L., and Mappes J.. 2005. Diet affects the immune defence and life-history traits of an arctiid moth Parasemia plantaginis. Evol. Ecol. Res. 7: 1153–1170. [Google Scholar]
- Qayyum A., Aziz M. A., Iftikhar A., Hafeez F., and Atlihan R.. 2018. Demographic parameters of Lipaphis erysimi (Hemiptera: Aphididae) on different cultivars of Brassica vegetables. J. Econ. Entomol. 111: 1885–1894. [DOI] [PubMed] [Google Scholar]
- Ribeiro A. P. O., Pereira E. J. G., Galvan T. L., Picanco M. C., Picoli E. a. T., Da Silva D. J. H., Fari M. G., and Otoni W. C.. 2006. Effect of eggplant transformed with oryzacystatin gene on Myzus persicae and Macrosiphum euphorbiae. J. Appl. Entomol. 130: 84–90. [Google Scholar]
- Rostami N., Maroufpoor M., Sadeghi A., Ghazi M. M., and Atlıhan R.. 2018. Demographic characteristics and population projection of Phytonemus pallidus fragariae reared on different strawberry cultivars. Exp. Appl. Acarol. 76: 473–486. [DOI] [PubMed] [Google Scholar]
- Saeed R., Sayyed A. H., Shad S. A., and Zaka S. M.. 2010. Effect of different host plants on the fitness of diamond-back moth, Plutella xylostella (Lepidoptera: Plutellidae). Crop Prot. 29: 178–182. [Google Scholar]
- Sayyed A. H., Ahmad M., and Crickmore N.. 2008. Fitness costs limit the development of resistance to indoxacarb and deltamethrin in Heliothis virescens (Lepidoptera: Noctuidae). J. Econ. Entomol. 101: 1927–1933. [DOI] [PubMed] [Google Scholar]
- Singer M. S. 2001. Determinants of polyphagy by a woolly bear caterpillar: a test of the physiological efficiency hypothesis. Oikos. 93: 194–204. [Google Scholar]
- Tang Q. L., Ma K. S., Hou Y. M., and Gao X. W.. 2017. Monitoring insecticide resistance and diagnostics of resistance mechanisms in the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae) in China. Pestic. Biochem. Physiol. 143: 39–47. [DOI] [PubMed] [Google Scholar]
- Tang Q., Ma K., Chi H., Hou Y., and Gao X.. 2019. Transgenerational hormetic effects of sublethal dose of flupyradifurone on the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae). PLoS One. 14: e0208058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J. H., and Wang J. J.. 2001. Effects of host plants on biology and life table parameters of Aphis spiraecola (Homoptera: Aphididae). Environ. Entomol. 30: 44–50. [Google Scholar]
- Tuan S. J., Yeh C. C., Atlihan R., Chi H., and Tang L. C.. 2016. Demography and consumption of Spodoptera litura (Lepidoptera: Noctuidae) reared on cabbage and taro. J. Econ. Entomol. 109: 732–739. [DOI] [PubMed] [Google Scholar]
- Van Der Meijden E. 2015. Herbivorous insects - a threat for crop production, pp. 103–114. In Lugtenbergs B. (ed.), Principles of plant-microbe interactions. Springer International Publishing, Cham. [Google Scholar]
- Weber G. 1985. Genetic-variability in host plant adaptation of the green peach aphid, Myzus persicae. Entomol. Exp. Appl. 38: 49–56. [Google Scholar]
- Yousaf H. K., Shan T., Chen X., Ma K., Shi X., Desneux N., Biondi A., and Gao X.. 2018. Impact of the secondary plant metabolite Cucurbitacin B on the demographical traits of the melon aphid, Aphis gossypii. Sci. Rep. 8: 16473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J. Z., Chen B. H., Güncan A., Atlihan R., Gökçe A., Smith C. L., Gümüs E., and Chi H.. 2018. Demography and mass-rearing Harmonia dimidiata (Coleoptera: Coccinellidae) using Aphis gossypii (Hemiptera: Aphididae) and eggs of Bactrocera dorsalis (Diptera: Tephritidae). J. Econ. Entomol. 111: 595–602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.