Abstract
The extent to which the increase in early-onset colorectal cancer (CRC) in the United States varies geographically is unknown. We analyzed changes in CRC incidence and risk factors among people aged 20–49 years by state using high-quality population-based cancer registry data provided by the North American Association of Central Cancer Registries and national survey data, respectively. Early-onset CRC incidence was mostly stable among blacks and Hispanics but increased in 40 of 47 states among non-Hispanic whites, most prominently in western states. For example, rates increased in Washington from 6.7 (per 100 000) during 1995–1996 to 11.5 during 2014–2015 (rate ratio = 1.73, 95% confidence interval = 1.48 to 2.01) and in Colorado from 6.0 to 9.5 (rate ratio = 1.57, 95% confidence interval = 1.30 to 1.91). Nevertheless, current CRC incidence was highest in southern states. From 1995 to 2005, increases occurred in obesity prevalence in all states and heavy alcohol consumption in one-third of states, but neither were correlated with CRC incidence trends. Early-onset CRC is increasing most rapidly among whites in western states. Etiologic studies are needed to explore early life colorectal carcinogenesis.
Early-onset colorectal cancer (CRC) is increasing in the United States for unknown reasons (1–3). Geographic differences in the trend could contribute to etiologic hypotheses but are unknown. We analyzed changes in CRC incidence and risk factors among adults younger than 50 years during 1995–2015 by state and race and ethnicity.
High-quality data on invasive CRC (n = 240 915) in adults 20–49 years during 1995–2015 were obtained from the North American Association of Central Cancer Registries for 47 states and the District of Columbia (DC) (4). Data were missing for some state-years. Tumor subsite was classified according to International Classification of Diseases for Oncology, Third Edition, as colon (C18.0, C18.2–18.9, C26.0) or rectum (C19.9, C20.9) (5). Race and ethnicity were categorized as non-Hispanic white (white), non-Hispanic black (black), or Hispanic.
Age-standardized (2000 US standard population) incidence rates per 100 000 person-years and incidence rate ratios (RRs) were calculated using SEER*Stat software (6). The Joinpoint Regression Program (version 4.6.0.0; National Cancer Institute) was used to compare temporal trends across states and to estimate average annual percent change (AAPC). Data were reported for sexes combined to improve statistical power because AAPCs were not statistically significantly different in men and women. States with fewer than 6 cases per year and/or fewer than 25 cases during 2011–2015 were suppressed in trend and cross-sectional analyses, respectively. Maps were created using Jenks natural breaks optimization in ArcGIS (7). Prevalence of obesity (body mass index > 30 kg/m2) and heavy alcohol consumption (>14 drinks per week for males and >7 for females) were estimated among adults 20–49 years from 1995–2010 Behavioral Risk Factor Surveillance System surveys (8). Ratios comparing risk factor prevalence over time were computed from logistic regression models with predicted marginal probabilities in SAS-Callable SUDAAN version 9.4, Research Triangle Park, NC (9). The relationship between CRC risk factors and incidence was assessed using the Pearson correlation coefficient. P values were two-tailed (α = 0.05) based on the permutation method (incidence) or Wald χ2 test (risk-factor prevalence).
Based on cancer registries representing 95% of the US population, early-onset CRC incidence increased over the most recent 10 data years (2006–2015) by 1.1% per year (95% confidence interval [CI] = 0.3 to 2.0%), including 0.7% per year (95% CI = 0.5 to 0.9%) for colon tumors and 1.7% per year (95% CI = 1.4 to 2.0%) for rectal tumors. Incidence increased in 36 of 47 states for CRC, 22 of 47 states for colon cancer, 34 of 43 states for rectal cancer; and was otherwise stable (Supplementary Table 1, available online).
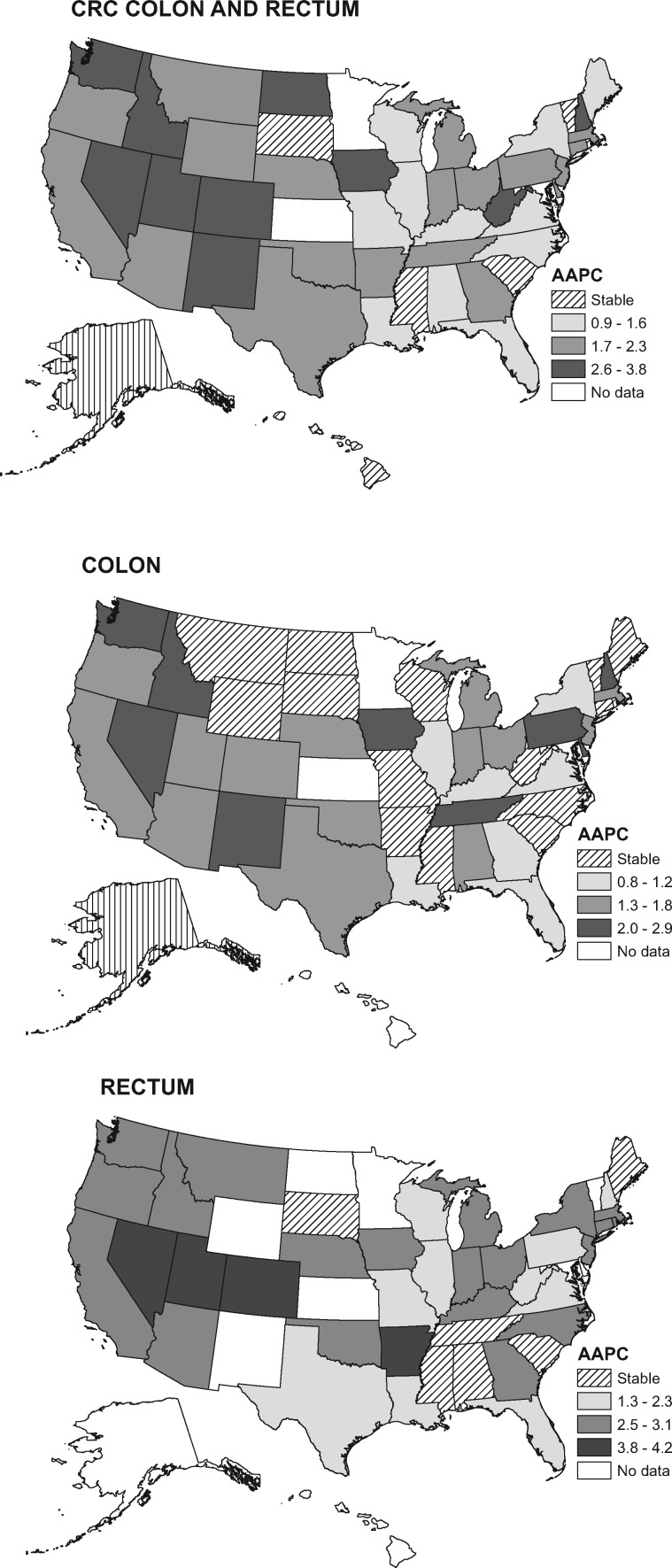
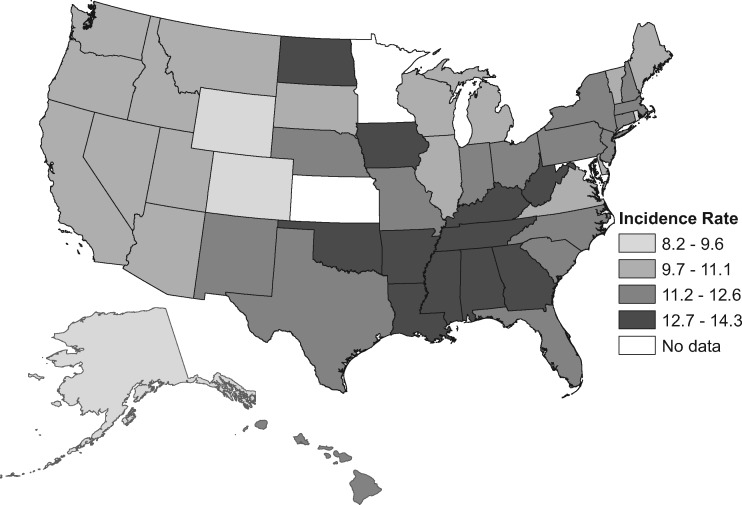
Increased incidence varied statistically significantly in magnitude between states and was mostly confined to whites, among whom rates rose in 40 of 47 states, with AAPCs greater than 2.5 in 10 states, 6 of which are in the West (Figure 1; Supplementary Tables 2 and 3, available online). For example, CRC incidence increased in Washington from 6.7 (per 100 000) during 1995–1996 to 11.5 during 2014–2015 (RR = 1.73, 95% CI = 1.48 to 2.01) and in Colorado from 6.0 to 9.5 (RR = 1.57, 95% CI = 1.30 to 1.91). Increases were generally steeper for rectal than for cancer colon, with AAPCs at least 3.0 in seven states and more than 4.0 in Utah, Nevada, and Colorado. Rectal cancer rates doubled in some states (eg, in Colorado, from 1.9 to 4.2), converging with those for colon cancer (RR rectum vs colon = 0.81, 95% CI = 0.63 to 1.03). Nevertheless, during 2011–2015, CRC incidence rates were generally lowest in western states and highest in southern states (Figure 2; Supplementary Table 4, available online) ranging from 8.7 (per 100 000) in Alaska to 14.3 in Kentucky.
Figure 1.
Average annual percent change (AAPC) in colon and rectal cancer incidence rates during 2006–2015 among non-Hispanic white adults aged 20 to 49 years by state. Trend was designated as “stable” if the AAPC was not statistically significantly different from zero. Because of missing data years, AAPC is based on 2001–2010 for Nevada and 2003–2012 for New Mexico. AAPC could not be calculated for states with fewer than 6 cases per year. CRC = colorectal cancer.
Figure 2.
Colorectal cancer incidence rates during 2011–2015 among non-Hispanic white adults aged 20 to 49 years by state. Rates are per 100 000 population and age-standardized to the 2000 US standard population. Incidence rate is based on data during 2011–2014 for the District of Columbia, 2011–2012 for New Mexico, and 2006–2010 for Nevada.
Among blacks, early-onset CRC incidence was stable in 21 of 27 states; declined in Florida, North Carolina, and Virginia; and increased in Alabama, New York, and Pennsylvania (Supplementary Table 5, available online). Increasing trends were confined to rectal cancer, which also increased in Louisiana. Among Hispanics (13 states), rates were stable except for increases in California, New Mexico, and New York, and a decline in Florida (Supplementary Table 6, available online).
Obesity among adults aged 20–49 years increased in every state during 1995–2005, with the largest absolute changes in southern states, where prevalence was highest (Supplementary Table 7, available online). Relative changes showed a less distinct geographic pattern. Prevalence ratios (PRs) among whites ranged from 1.23 (95% CI = 1.01 to 1.50) in Florida (obesity prevalence from 15.7% to 19.4%) to 2.25 (95% CI = 1.74 to 2.90) in Massachusetts (from 9.1% to 20.4%; Supplementary Table 8, available online). Heavy alcohol consumption increased during 1995–2005 in 19 of 49 geographically dispersed states (17 of 49 among whites) and was otherwise stable (Supplementary Tables 9 and 10, available online). Prevalence nationally was 5.9% in 2005. There was no association between trends in obesity or heavy alcohol consumption during 1995–2005 and CRC incidence during 2006–2015 based on Pearson correlation coefficients (r = 0.1, P= .47 and r = 0.1, P= .45, respectively; Supplementary Figure 1, available online).
CRC incidence increased in more than four of five states among young whites but was mostly stable among blacks and Hispanics. The trend was steepest in some western states, where rectal cancer rates became comparable to those for colon cancer (data not shown). Lower baseline rates may contribute to these relatively rapid increases, although the AAPC for rectal cancer in Kentucky (2.8, 95% CI = 2.0 to 3.6), where CRC incidence was highest, was the same as that in Washington.
Established CRC risk factors include obesity, excess alcohol consumption, a diet high in red and/or processed meat and low in fiber, physical inactivity, and smoking (10). Whereas lower CRC incidence in western than southern states likely reflects less obesity and physical inactivity (11), racial and geographic differences in temporal trends are inconsistent with risk factor patterns. Obesity prevalence has risen nationwide and more rapidly in blacks than in whites or Hispanics (12). The prevalence of smoking and heavy alcohol use among young adults is low, and trends do not appear to explain the CRC incidence trajectory (13).
Our study is strengthened by use of high-quality, nationwide population-based incidence data but cannot provide evidence for causation because of the ecologic design. Changes in tumor-site coding by clinicians (ie, coding rectosigmoid tumors as rectal instead of colon) may contribute to increased rectal cancer rates, although this is unlikely given the parallel increase in colon cancer. Additional limitations include biases in self-reported health information from the Behavioral Risk Factor Surveillance System (14) and lack of state-level colonoscopy data for adults younger than 50 years, which may help explain variations in trends.
Early-onset CRC is rising most rapidly among whites in western states, where healthy behaviors are prominent. Differences in the trend by subsite, race, and state implicate factors in addition to the “usual suspects” and highlight the need for etiologic studies to explore early life colorectal carcinogenesis.
Funding
No funding to list for this study.
Notes
Affiliations of authors: Surveillance and Health Services Research, Intramural Research Department, American Cancer Society, Atlanta, GA (RLS, SAF, AJ); Food Animal Health Research Program, Department of Population Medicine, Ohio Agricultural Research and Development Center, The Ohio State University, Wooster, OH (GAM).
The American Cancer Society had no role in this study other than the employment of the researchers. RLS and GAM contributed equally to this work.
The authors have no conflicts of interest to disclose.
Supplementary Material
References
- 1. Austin H, Henley SJ, King J, Richardson LC, Eheman C.. Changes in colorectal cancer incidence rates in young and older adults in the United States: what does it tell us about screening. Cancer Causes Control. 2014;252:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 2015;1501:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. J Natl Cancer Inst. 2017;1098:djw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.North American Association of Central Cancer Registries, SEER*Stat Database: NAACCR Incidence Data—CiNA Analytic File, 1995–2015, Public Use, accessed October 18, 2018.
- 5. Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology. 3rd ed. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 6. SEER*Stat Software, Version 8.3.5. Statistical Research and Applications Branch. Bethesda, MD: National Cancer Institute; 2018. [Google Scholar]
- 7. Environmental Systems Research Institute. ArcGIS and ArcMap [Software], Version 10.2.1 Redlands, CA; 2017.
- 8. Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Data. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention 2011.
- 9. Bieler GS, Brown GG, Williams RL, Brogan DJ.. Estimating model-adjusted risks, risk differences, and risk ratios from complex survey data. Am J Epidemiol. 2010;1715:618–623. [DOI] [PubMed] [Google Scholar]
- 10. Aleksandrova K, Pischon T, Jenab M, et al. Combined impact of healthy lifestyle factors on colorectal cancer: a large European cohort study. BMC Med. 2014;12(1):168.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fedewa SA, Sauer AG, Siegel RL, Jemal A.. Prevalence of major risk factors and use of screening tests for cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2015;244:637–652. [DOI] [PubMed] [Google Scholar]
- 12. Ljungvall A, Zimmerman FJ.. Bigger bodies: long-term trends and disparities in obesity and body-mass index among U.S. adults, 1960–2008. Soc Sci Med. 2012;751:109–119. [DOI] [PubMed] [Google Scholar]
- 13. American Lung Association Research and Program Services. Trends in Tobacco Use American Lung Association, Epidemiology and Statistics Unit July. 2011. http://www.lung.org/assets/documents/research/tobacco-trend-report.pdf. Accessed January 26, 2018.
- 14. Stommel M, Schoenborn CA.. Accuracy and usefulness of BMI measures based on self-reported weight and height: findings from the NHANES & NHIS 2001-2006. BMC Public Health. 2009;9(1):421.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.