Summary
Transcription activator‐like effectors (TALEs) are important effectors of Xanthomonas spp. that manipulate the transcriptome of the host plant, conferring susceptibility or resistance to bacterial infection. Xanthomonas citri ssp. citri variant AT (X. citri AT) triggers a host‐specific hypersensitive response (HR) that suppresses citrus canker development. However, the bacterial effector that elicits this process is unknown. In this study, we show that a 7.5‐repeat TALE is responsible for triggering the HR. PthA4AT was identified within the pthA repertoire of X. citri AT followed by assay of the effects on different hosts. The mode of action of PthA4AT was characterized using protein‐binding microarrays and testing the effects of deletion of the nuclear localization signals and activation domain on plant responses. PthA4AT is able to bind DNA and activate transcription in an effector binding element‐dependent manner. Moreover, HR requires PthA4AT nuclear localization, suggesting the activation of executor resistance (R) genes in host and non‐host plants. This is the first case where a TALE of unusually short length performs a biological function by means of its repeat domain, indicating that the action of these effectors to reprogramme the host transcriptome following nuclear localization is not limited to ‘classical’ TALEs.
Keywords: citrus, hypersensitive response (HR), Nicotiana benthamiana, transcription activator‐like (TAL) effectors, Xanthomonas citri
Introduction
Understanding how plants perceive and hamper the attack by potential pathogens has been a fundamental question for plant biologists in recent decades. We now know that bacterial infection normally fails because plants carry genes whose products detect conserved pathogen‐associated molecular patterns (PAMPs) and trigger a complex defence response (PAMP‐triggered immunity, PTI) that restricts pathogen growth (Macho and Zipfel, 2014). Evolution has provided many bacteria with the ability to suppress this basal plant immune system and hence facilitate infection through the action of a battery of proteins called effectors (Macho, 2016). These effectors are expressed during the course of the infection and are translocated into the host mesophyll cells by means of the type III secretion system (T3SS) where they interact with specific plant components. As a countermeasure, some plants have evolved the ability to detect specific bacterial effectors, restricting effector‐mediated bacterial infection by triggering a second layer of defence responses called effector‐triggered immunity (ETI) (Toruño et al., 2016). The ability of the pathogen to avoid plant recognition relies then on effector evolution, in a never‐ending struggle between both organisms (Carella et al., 2018).
Transcription activator‐like effectors (TALEs), belonging to the Xanthomonas AvrBs3/PthA family of T3SS effectors, function as eukaryotic transcription factors in plant cells, playing a central role in promoting bacterial disease by the induction of host susceptibility (S) genes. Contrary to the mode of action of other families of bacterial effectors that exert their function in the cytoplasm, TALEs are translocated into the plant cell nucleus and bind to the promoter regions of plant target genes whose transcription facilitates bacterial colonization and spread (Boch and Bonas, 2010; Bogdanove et al., 2010). The specificity of action of different TALEs is determined by the structure of these proteins. They contain T3SS translocation signals in the N‐terminal region, a central DNA‐binding domain and a C‐terminal region with nuclear localization signals (NLS) followed by an acidic activation domain (AD). TALEs vary mostly in their central region, which consists of a number of tandemly arranged repeats of 33–34 amino acids with hypervariable di‐amino acids at positions 12 and 13, termed the repeat‐variable diresidue (RVD). Each repeat interacts with a nucleotide in the DNA, and these two residues determine the specificity of each repeat for a particular nucleotide. Thus, the RVD sequence of each TALE defines the effector‐binding element (EBE) in the promoter region of the target genes (Boch et al., 2009; Moscou and Bogdanove, 2009). Over the course of evolution, some plants have evaded the activation of susceptibility genes by modification of their promoters (Bogdanove et al., 2010; Hutin et al., 2015), or co‐opted the TALE mechanism of gene activation for resistance, by means of gene traps (executor resistance (R) genes) that contain EBEs in their promoter regions, and whose activation by the corresponding TALE triggers a host resistance response (Römer et al., 2007; Zhang et al., 2015).
Xanthomonas citri ssp. citri (X. citri) is the causal agent of citrus canker, a serious economic disease that provokes losses worldwide. Infected fruits have decreased commercial quality, compromising their acceptance by most markets (Canteros et al., 2017; Ference et al., 2018). Outbreaks occur sporadically and copper‐based products and eradication of infected trees are the strategies employed to control the disease so far (Behlau et al., 2010; Favaro et al., 2017). Canker lesions are characterized by raised spongy eruptions caused by bacterial‐induced cell hypertrophy and hyperplasia in leaves and fruits (Graham et al., 2004). The pathogenic reference strain X. citri 306 contains four TALE genes (pthA1, pthA2, pthA3 and pthA4 harbouring 16.5, 15.5, 15.5 and 17.5 repeats, respectively) located on plasmids pXAC33 (pthA1/pthA2) and pXAC64 (pthA3/pthA4) (da Silva et al., 2002). TALE PthA4 promotes the expression of citrus lateral organ boundaries gene (CsLOB1), a transcription factor involved in hypertrophy and hyperplasia of the cells (Duan et al., 1999; Hu et al., 2014; Pereira et al., 2014). Moreover, TALE PthA1 and PthA3 also contribute to canker symptoms in a host‐dependent manner (Yukari Abe and Benedetti, 2015).
Several reports suggest specific recognition of effectors by host gene products that render plants resistant to X. citri, i.e. interactions in which the effector acts as an avirulence factor (Chen et al., 2012; Deng et al., 2010; Khalaf et al., 2011; Lee et al., 2009). Recently, we have isolated a X. citri strain (X. citri AT) that triggers a host‐specific defence response in Citrus limon that is associated with the interference of biofilm development and arrest of bacterial growth (Chiesa et al., 2013; Roeschlin et al., 2017). The occurrence of an oxidative burst, the accumulation of salicylic acid and phenolic compounds, and the hypersensitive response (HR) together suggest that it is an ETI response. However, the avirulence effector that elicits these processes is unknown.
In this study, we demonstrate that the causal agent of the triggering of the defence response to X. citri AT strain is a short TALE containing 7.5 repeats. In nature, the number of TALEs in a single bacterial strain is highly variable and the RVDs range from 1.5 to 33.5 (Boch and Bonas, 2010; Cox et al., 2017; Denancé et al., 2018). However, the optimal length for specificity ranges between 15.5 and 19.5 repeats (Rinaldi et al., 2017). The screening of X. citri diversity in different citrus‐growing regions has identified TALEs ranking from 6.5 to 29 repeats, which likely contain different RVDs. It is suggested that the diversification is driven by recombination, facilitated by the repetitive structure of the central region, leading to variants differing in the number of repeats (Al‐Saadi et al., 2007; Gochez et al., 2018; Lee et al., 2008; Shiotani et al., 2007; Ye et al., 2013). It has been demonstrated that TALEs shorter than 6.5 repeats are non‐functional in terms of gene activation; these just could be by‐products of recombination events (Boch et al, 2009). It is not known, however, if TALEs of intermediate length are functional for gene activation.
Here we show that this 7.5‐repeat TALE of the X. citri AT strain is necessary and sufficient to induce HR in C. limon and C. sinensis. The protein is able to bind DNA in vitro and to activate transcription in an EBE‐dependent manner. The HR is triggered only if the TALE protein reaches the plant cell nucleus, and the resistance requires transcriptional activation, suggesting a classical TALE mode of action via activation of executor gene(s).
Results
A new repertoire of pthA genes is present in the X. citri AT strain
Previous reports have shown a great variability in the size of the pthA genes from different X. citri strains (Al‐Saadi et al., 2007; Gochez et al., 2018; Lee et al., 2008; Ye et al., 2013). Given the mode of action of these TALE proteins, it is expected that this variability will contribute to explain differences in pathogenicity among the strains. To define the pthA ‘repertoire’ of the X. citri AT strain, Southern blot assays using a pthA probe over EcoRI‐ and BamHI‐digested DNA of the two X. citri plasmids were performed. EcoRI digestion of X. citri AT showed the presence of four bands, similar to the reference strain X. citri 306, indicating the presence of four pthA genes. However, after BamHI digestion a new pattern of three discrete 3.8‐, 3.2‐ and 2.4‐kb bands was observed in X. citri AT that differed to the one previously reported for X. citri 306 (3.4‐kb for pthA4, 3.3‐kb for pthA1 and 3.2‐kb for pthA2/pthA3 genes; da Silva et al., 2002) (Fig. 1a). To further investigate the functionality of the pthA genes of X. citri AT, the BamHI bands, revealing the number of repetitions, were cloned and sequenced. The 3.2‐kb band was in fact a double‐band containing the central region of two different pthA genes of the same size that were identical to the pthA2 and pthA3 genes of X. citri 306. However, the two other pthA genes differed in the number of central repeats in the coded proteins compared with those of X. citri 306. The 3.8‐kb band corresponds to a pthA gene encoding a TALE containing 21.5 repeats. The smallest 2.4‐kb BamHI band contains a pthA gene encoding a TALE with 7.5 repeat domains. Sequence analysis revealed that the 21.5‐repeat protein is identical to other members of the PthA1 group, differing only in three RVDs in positions 13–15 (Figs 1b and S1). The new 7.5‐repeat protein is identical to PthA4 from X. citri 306 in the N‐ and C‐terminal regions, but has an internal deletion of 10 central repeats (Figs 1b and S2). We named these proteins PthA1AT and PthA4AT, respectively.
Figure 1.
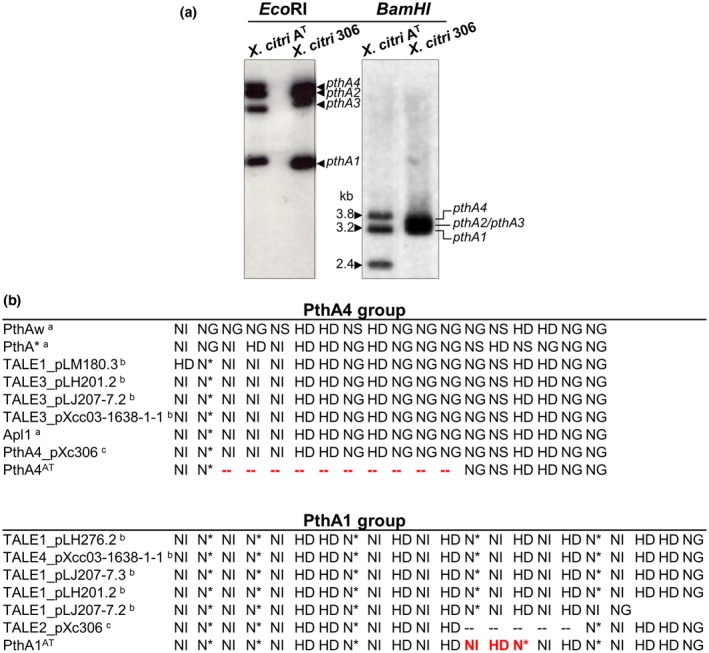
Identification of variants of pthA genes in Xanthomonas citri AT. (a) Southern blot analysis of X. citri AT and X. citri 306 pthA genes. Plasmids were digested with EcoRI or BamHI and probed with a pthA PCR fragment. Fragments of 3.8, 3.2 and 2.4 kb correspond to pthA1, pthA2/3 and pthA4 from X. citri AT, respectively. (b) Alignment of repeat variable di‐residue (RVD) sequences of PthA4 and PthA1. RVD sequences from X. citri isolated TALEs were compared with PthA4 and PthA1 from X. citri AT. Dashed red lines show RVD deleted motif on PthA4AT. The residues highlighted in red show the RVDs that differ in positions 13–15 on PthA1AT. aAl‐Saadi et al. (2007), bGochez et al. (2018), cda Silva et al. (2002).
PthA4AT is necessary and sufficient for the host‐specific induction of hypersensitive response in citrus
Although many genes from X. citri 306 contribute to pathogenicity, the pthA4 17.5‐repeat TALE gene is a major effector, promoting canker disease symptoms (Duan et al., 1999; Da Silva et al., 2002; Yukari Abe and Benedetti 2015). To determine if the immune response triggered by X. citri AT is still induced in the presence of a full‐length pthA4 gene, a pBBR plasmid expressing PthA4 from X. citri 306 was transferred to X. citri AT and the expression was evaluated by western blot (Fig. S3). The pthA4–expressing X. citri AT was still able to triggered HR in C. limon (Fig. S4), indicating that an active PthA4 does not suppress HR.
To evaluate whether any of the two new pthA genes found in X. citri AT play any role in this HR, the gene for each TALE was cloned in a pBBR plasmid and expressed in X. citri 306 (Fig. S3). No difference in symptoms was observed in C. limon after infection with the natural pathogenic X. citri 306 or the pthA1AT‐expressing X. citri 306. However, pthA4AT‐expressing X. citri 306 was unable to cause canker symptoms and induced an HR on C. limon (Fig. 2a). Similar results were obtained when these two pthA genes were expressed in the Argentinian pathogenic T strain, which is related to X. citri 306 (Fig. S5). To confirm these results, each of three X. citri 306 mutant strains (ΔpthA1, ΔpthA4 or ΔpthA1,4) were transformed with either pthA1AT or pthA4AT‐expressing plasmids (Fig. S3). The X. citri 306 mutant strains expressing pthA1AT exhibited the same symptoms in C. limon as the wild‐type 306 strain (Fig. 2b). By contrast, the presence of PthA4AT either in ΔpthA4 or ΔpthA1,4 caused an HR similar to that caused by X. citri AT (Fig. 2c). These results suggest that the presence of PthA4AT is necessary for the HR observed in C. limon. In further experiments, the pthA4AT gene was cloned under the control of the CaMV 35S promoter and transiently expressed in planta. Agroinfiltration assays showed that transient expression of PthA4AT triggered an HR (Fig. 2d), suggesting that the presence of this protein is necessary and sufficient for the induction of this response in C. limon.
Figure 2.
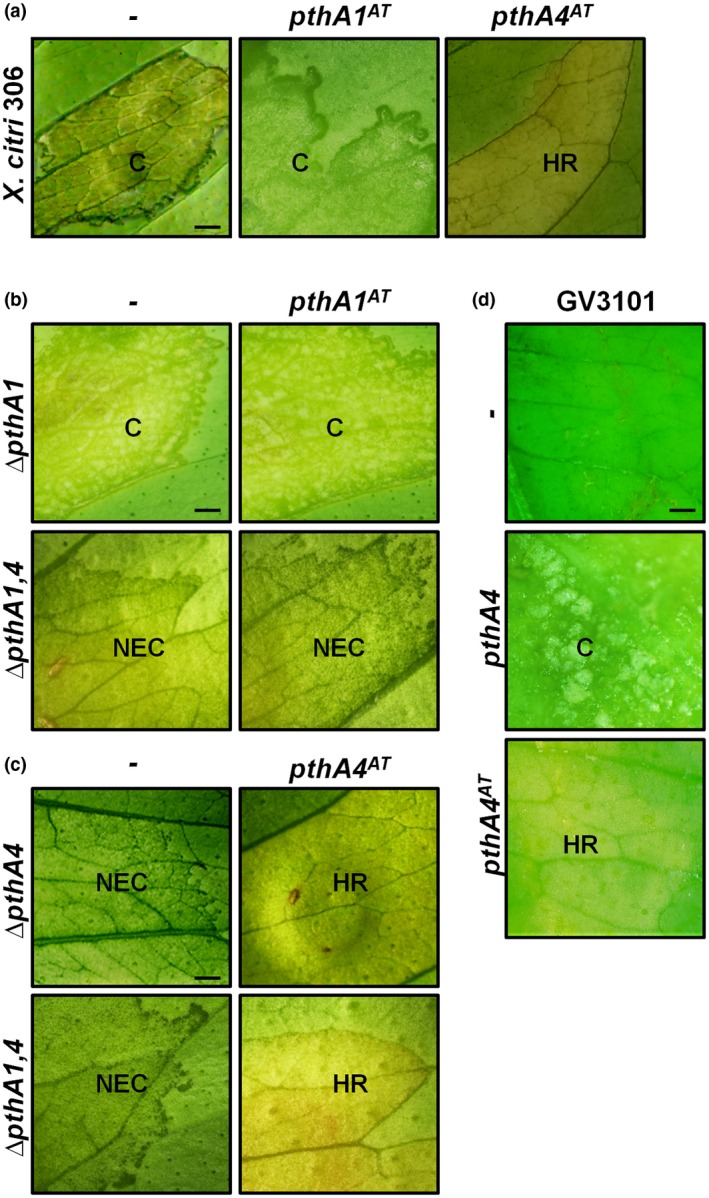
PthA4AT triggers host defence response in Citrus limon leaves. (a) Symptoms induced by PthA1AT and PthA4AT on Xanthomonas citri 306. (b) and (c) Complementation of X. citri mutant strains (ΔpthA1, ΔpthA1,4 and ΔpthA4) with PthA1AT or PthA4AT. Leaves were infiltrated with the corresponding bacterial suspension and photographed at 15 days post‐inoculation (dpi). (d) Agrobacterium tumefaciens GV3101‐mediated transient expressions of PthA4AT and PthA4 on C. limon leaves. Leaves were agroinfiltrated and symptoms were photographed at 10 dpi. HR, hypersensitive response; C, canker; NEC, non‐eruptive canker. Scale bar: 10 mm.
Nuclear localization of PthA4AT is needed to trigger host defense response in Citrus limon
The results presented above suggest that PthA4AT recognition by the host plant is the initial step triggering the HR observed in C. limon. In order to assess if nuclear localization of PthA4AT is necessary for recognition, two PthA4AT‐modified proteins were designed: one with an 87‐amino acid deletion in the C‐terminal region, eliminating the three conserved nuclear localization signals (∆NLSAT), and a second one with point mutations in the same three NLS (mutNLSAT) (Fig. S6). The constructs were introduced into ΔpthA4 (Fig. S3) and assayed in C. limon following pressure infiltration into the leaves or swab inoculation of the leaf surface. As observed in Fig. 3, none of the two mutant versions of the protein impaired in nuclear localization was able to trigger an HR. Similar results were obtained when X. citri 306 was transformed with these constructs (Fig. S7). Moreover, when the NLS of the SV40 (Kalderon et al., 1984; Szurek et al., 2001) was fused to the impaired ∆NLSAT protein (Fig. S6), the HR was restored (Fig. 3). Taken together, these results indicate that PthA4AT must be localized in the plant cell nucleus to trigger the defence response.
Figure 3.
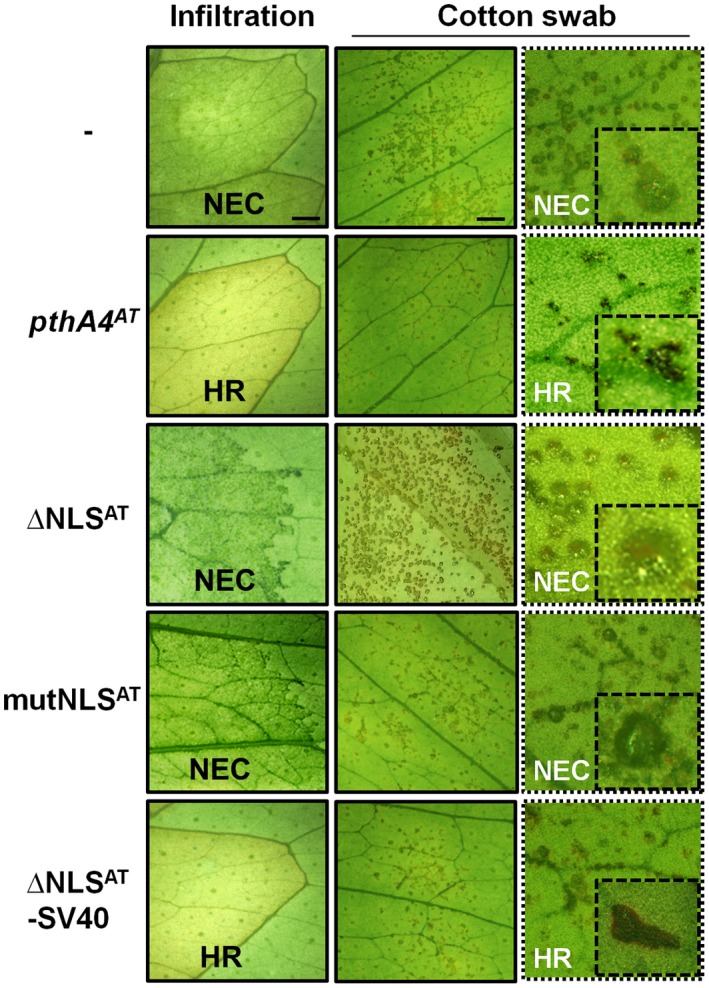
Nuclear localization of PthA4AT is needed to trigger host defence response in Citrus limon. Phenotypic response of Xanthomonas citri 306 ∆pthA4 transformed with the different constructs was evaluated in C. limon leaves inoculated by pressure infiltration or cotton swab. Photographs were taken 15 days post‐inoculation and the insets show amplification of symptoms. NEC, non‐eruptive canker; HR, hypersensitive response. ∆NLSAT:PthA4AT derivative, with an 83‐amino acid deletion in the C‐terminal region, eliminating the three conserved nuclear localization signals (NLS); mutNLSAT:PthA4AT derivative mutated in the three NLS; ∆NLSAT‐SV40:∆NLSAT derivative containing the NLS of SV40. Scale bar: 10 mm.
PthA4AT is able to bind DNA following the TALE code and to activate transcription in an EBE‐dependent manner
To elucidate whether the 7.5‐repeat PthA4AT TALE was able to bind to DNA in vitro, and to determine the specificity of binding, a PBM11 protein‐binding microarray was employed (Godoy et al., 2011) that contains all possible double‐stranded 11‐mers (approximately 4.2 million sequences) in approximately 180 000 oligonucleotides of 35 bp. Probing PBM11 with PthA4AT for determination of DNA‐binding specificity yielded an E‐score of 0.48 to the TATTACCTT sequence, with similar affinity to variants of this motif containing one mismatch (Table S6). Considering that it is generally assumed that motifs with E‐score > 0.45 reflect high binding specificity (Berger and Bulyk, 2009; Weirauch et al., 2013, 2014), we obtained the consensus recognition sequence for PthA4AT from DNA motifs with E‐scores > 0.45 (Fig. 4a). This motif matches quite well with the expected binding domain for PthA4AT according to its TALE code (Boch et al., 2009).
Figure 4.
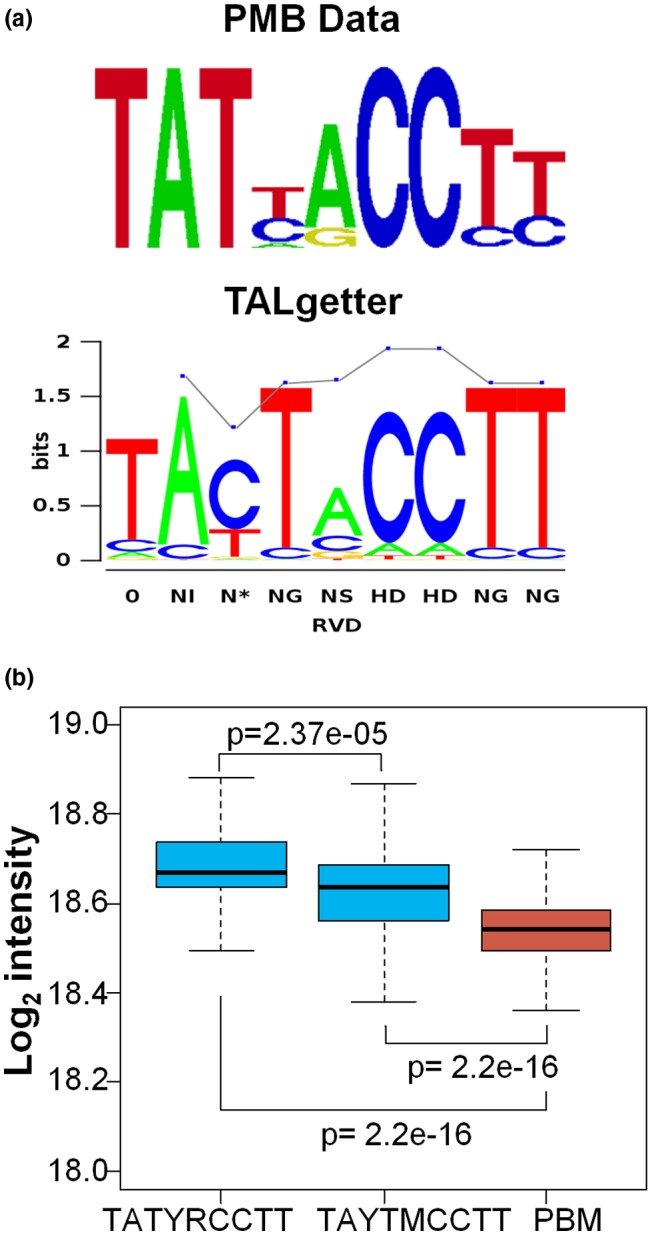
DNA‐binding of PthA4AT follows the TALE code. (a) Consensus predicted sequences for PthA4AT obtained after protein binding microarray (PBM) assay (E‐score > 0.45) and TALgetter using Citrus sinensis promotorome for the analysis. (b) Relative binding of PthA4AT to different DNA motifs. The box plot represents the distribution of intensities of the DNA probes containing the sequence element bound by PthA4AT with the highest affinity (TATYRCCTT) and the intensities of probes covering the motif predicted by TALgetter (TAYTMCCTT), both in light blue. PthA4AT recognizes both DNA elements, but binding to PBM‐derived motif is higher than to that from TALgetter. Red box corresponds to the distribution of intensities of all the probes in the PBM array. Statistical differences between the different distributions were calculated with the Wilcox exact test. Nucleotide codes are as follows: Y, C or T; M, A or C; R, A or G.
X. citri AT generates HR in C. sinensis similarly to generation in C. limon and X. citri 306 ΔpthA4, and ΔpthA1,4‐expressing PthA4AT also behaves similarly in C. sinensis and C. limon, indicating that the same mechanisms of defence are triggered in both species (Fig. S8). Since the C. sinensis genome sequence is available (Wu et al., 2014; Xu et al., 2013) we used C. sinensis for molecular analysis of predicted targets (Grau et al., 2013). TALgetter consensus‐predicted sequences for PthA4AT using C. sinensis promotorome are shown in Fig. 4a.
Although both consensus sequences described above are very similar, it is interesting to note that the second repeat of PthA4AT (N*) binds preferably to T in the protein binding microarray (PBM) assay, whereas this position is predicted C/T by TALgetter. Moreover, the third repeat (NG), predicted T by TALgetter, binds equally to C and T in the PBM assay. In order to quantify the subtle differences in both consensus sequences in our PBM assay, we extracted signal intensities for the probes containing the sequence determined in PBM (TATYRCCTT) and the predicted by TALgetter (TAYTMCCTT). This analysis revealed a significantly higher binding of PthA4AT to the sequence determined in PBM (TATYRCCTT). Nevertheless, both groups of sequences performed much better binding than the average array probes (Fig. 4b). The second repeat (N*) is shared between PthA4 and PthA4AT. Interestingly, the two known targets of PthA4 (LOB1 and SWEET1; Hu et al., 2014) contain a T in this position, reinforcing the use of this high‐throughput methodology for experimental determination of DNA motifs recognized by TALEs.
To estimate the ability of PthA4AT to activate transcription in planta, five putative target promoters of C. sinensis genes containing an EBE in their proximal region (400 bp upstream + 200 bp downstream of the transcription start site) were fused to the uidA (β‐glucuronidase [GUS]) reporter gene and transiently co‐expressed with the 35S promoter‐driven pthA4AT via Agrobacterium into N. benthamiana leaves. According to the different behaviour of X. citri AT in C. sinensis and C. clementina (Chiesa et al., 2013), the five genes were selected to fulfil the criteria of being predicted targets of PthA4AT by TALgetter (P value < 10−5) in C. sinensis but not in C. clementine and the consensus sequences obtained for PMB assay (Fig. 5a). As shown in Fig. 5b, transient expression assays indicate that PthA4AT was able to activate GUS expression when this reporter was under the control of the Cs4‐promoter, indicating that the 7.5‐repeat TALE is able to bind DNA and activate transcription in planta. The other four promoters were not able to activate GUS PthA4AT‐dependent transcription, indicating that not all predicted binding sites are functional in planta. Next, to clarify if the PthA4AT‐dependent transcription of GUS under the Cs4 promoter was dependent on the EBE, we mutated this box in the C. sinensis promoter. As shown in Fig. 5c, the construct with the mutated EBE lost the ability to activate transcription of the uidA gene when co‐delivered with the 35S promoter‐driven pthA4AT, indicating that the PthA4AT‐mediating transcription observed for the Cs4 promoter is EBE‐dependent.
Figure 5.
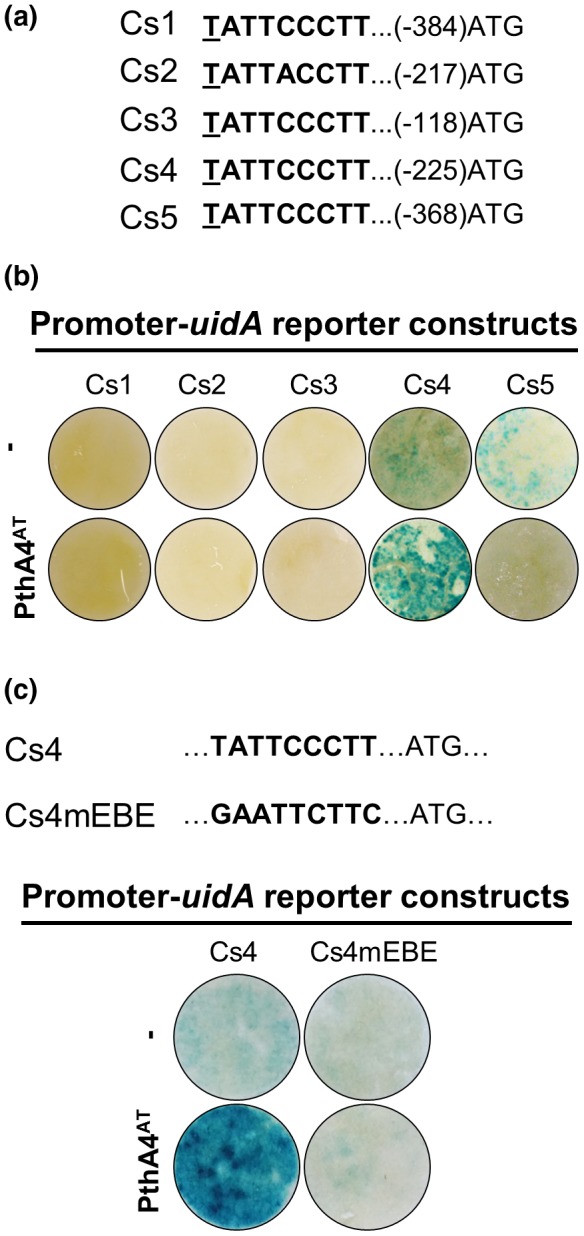
PthA4AT activates transcription in planta. (a) Effector‐binding element (EBE) site of five Citrus sinensis (Cs) selected promoters. Underlined nucleotide indicates the 0 position of the EBE and the number in brackets indicates the position of last T nucleotide from the putative ATG. (b) GUS assay results after Agrobacterium co‐infiltration of Cs‐uidA promoters and 35S::PthA4AT. (c) EBE site and base mutation (mEBE) on Cs4. GUS staining in Nicotiana benthamiana leaves was performed 48 h post‐inoculation. Cs1, Cs2, Cs3, Cs4 and Cs5 correspond to C. sinensis IDs orange1.1g019568, orange1.1g047725, orange1.1g038742, orange1.1g048430 and orange1.1g048684, respectively.
HR development is mediated by PthA4AT‐dependent transcriptional activation
The PthA4AT protein presents an activation domain (AD) in its C‐terminal region, identical to the one in AvrBs3 protein from Xanthomonas campestris pv. vesicatoria (Szurek et al., 2001). To ascertain the relevance of transcriptional activation on the PthA4AT‐mediated defence response, a PthA4AT deletion construct without the 27 amino acids marked in Szurek et al. (2001) as AD was generated (ΔADAT). The expression of ΔADAT was unable to prevent canker development by X. citri wild‐type strains (Figs 6a and S9), suggesting that this activation domain is necessary for triggering an HR.
Figure 6.
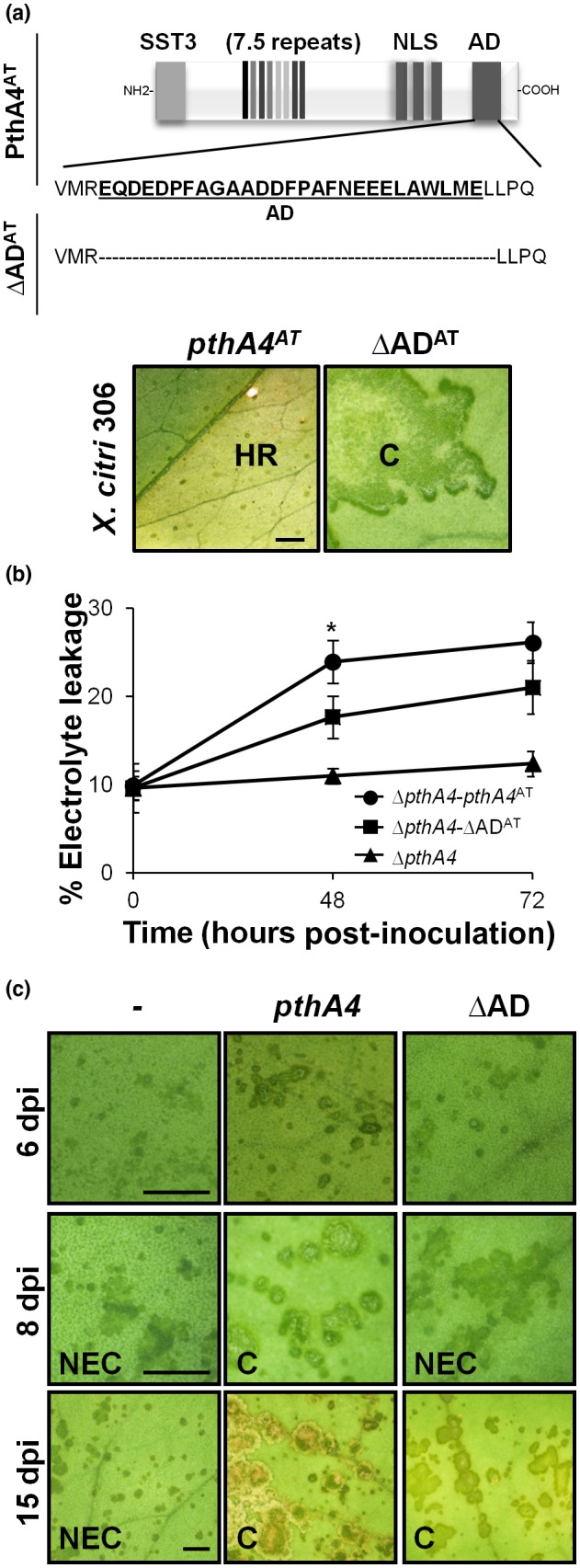
PthA4AT‐mediated resistance depends on transcriptional activation. (a) PthA4AT variant harbouring a deletion of 27 amino acids on the activation domain (∆ADAT). Symptoms induced on Citrus limon leaves inoculated with X. citri 306 transformed with PthA4AT or ∆ADAT. HR: hypersensitive response: C, canker; NLS, nuclear localization signal; AD, activation domain. (b) Percentage of electrolytic leakage at 0, 48 and 72 h post‐inoculation of C. limon inoculated with ∆pthA4, transformed with PthA4AT or ∆ADAT. Values are expressed as means ±SD of three independent biological replicates. The dataset marked with an asterisk is significantly different as assessed by Tukey’s test (P < 0.05). (c) Symptoms induced on C. limon leaves inoculated with ∆pthA4 transformed with PthA4 or ∆AD from Xanthomonas citri 306 observed at different days post‐inoculation (dpi). ∆AD:PthA4 from X. citri 306 harbouring a deletion of 27 amino acids on the activation domain. NEC, non‐eruptive canker; C, canker. Scale bar: 10 mm.
Interestingly, the expression of ΔADAT in ΔpthA4‐X. citri 306 strain showed a delay in the manifestation of HR after C. limon leaves inoculation, as assessed by conductivity assays. As shown in Fig. 6b, a significant reduction in electrolyte leakage was observed at 48 h after inoculation with ΔADAT‐expressing ΔpthA4 as compared with inoculation with ΔpthA4 strain expressing the full‐length pthA4AT gene. As for the control, no cell death was observed after ΔpthA4 inoculation. To establish whether the action of PthA4 as a virulence factor is similarly dependent on the activation domain, a deletion derivative of the X. citri 306 pthA4 lacking the 27 amino acids of the activation domain (ΔAD) was generated and transformed into ΔpthA4 mutant of X. citri 306. Interestingly, canker development was delayed after infection with ΔpthA4‐expressing ΔAD as compared with that containing the full‐length pthA4 gene, as shown in Fig. 6c. Taken together, these results suggest that the deletion of AD in both pthA4 and pthA4AT genes reduces, but does not completely abolish, gene activation, suggesting that HR development is mediated by PthA4AT‐dependent transcriptional activation, as a canonical TAL effector, presumably of an unknown executor R gene.
PthA4AT triggers hypersensitive response on non‐host Nicotiana benthamiana
To study the effect of the expression of PthA4AT on the activation of HR in non‐host plants, the TAL effector was analysed via Agrobacterium‐mediated transient expression in leaves of Arabidopsis thaliana, Solanum tuberosum and N. benthamiana, and plant reactions were scored over a 5‐day period. As shown in Fig. 7a, PhA4AT was able to trigger a macroscopic cell death only on N. benthamiana leaves, suggesting that PthA4AT induces an HR in this non‐host plant. To determine if this response required the C‐terminal domains of the protein, transient expression of PthA4AT derivatives (∆NLSAT, mutNLSAT, ∆NLSAT‐SV40, ∆ADAT) was assessed. Similar to citrus, ∆NLSAT and mutNLSAT constructs did not develop a visible HR and ∆NLSAT‐SV40 partially restored the cell death response (Fig. 7b). This suggests that, as with citrus, PthA4AT action in N. benthamiana requires localization to the nuclei. Moreover, the full HR manifestation required the presence of a functional AD (Fig. 7b), suggesting that in this non‐host plant PthA4AT is acting as a canonical TAL effector.
Figure 7.
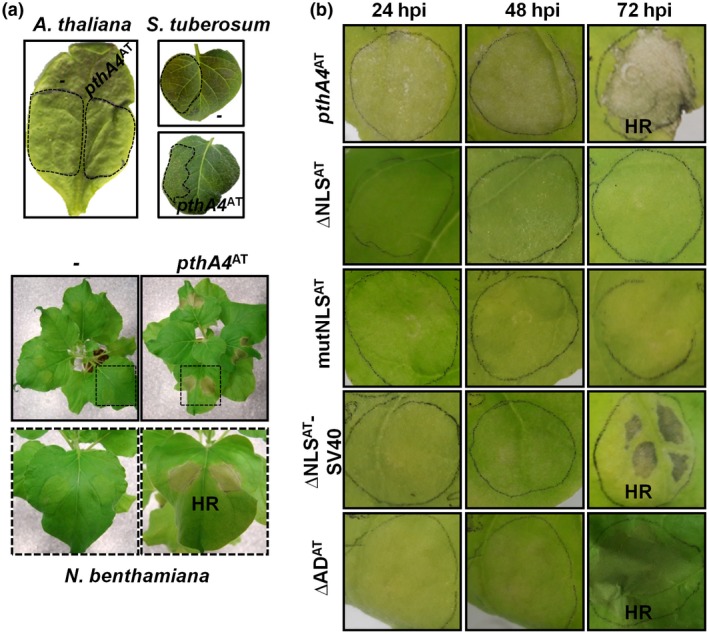
Hypersensitive response (HR) induced by PthA4AT in non‐host Nicotiana benthamiana model plant. (a) Agrobacterium tumefaciens GV3101‐mediated transient expressions of PthA4AT on Arabidopsis thaliana, Solanum tuberosum and N. benthamiana leaves. Leaves were agroinfiltrated and symptoms were photographed 72 h post‐inoculation (hpi). (b) Phenotypic response of the transient expression of the different PthA4AT constructs evaluated in N. benthamiana leaves. Photographs were taken at 24, 48 and 72 hpi. ∆NLSAT:PthA4AT derivative with an 83‐amino acid deletion in the C‐terminal region, eliminating the three conserved nuclear localization signals (NLS); mutNLSAT:PthA4AT derivative mutated in the three NLS; ∆NLSAT‐SV40:∆NLSAT derivative containing the NLS of SV40. ∆ADAT:PthA4AT derivative with a 27 amino acid deletion in the activation domain (AD) region.
Discussion
Surveys of the size of TALEs in nature indicate that the great majority of them contain a mean of 18.5 RVDs, indicating that this length has been selected by evolution to achieve high target specificity (Boch and Bonas, 2010; Rinaldi et al., 2017). The presence of very short TALEs in some bacterial strains has been documented, as exemplified by the 1.5‐repeat AvrXa3 of Xanthomonas oryzae pv. oryzae (Xoo) (Wu et al., 2006), but they are likely non‐functional for transcriptional activation (Boch et al., 2009). These short TALEs are probably by‐products of the complex sequence rearrangement that shapes the TALomes of the different strains (Denancé et al., 2018). For TALEs containing 6.5 to 9.5 RVDs, transcriptional activation is still possible, although it is much weaker than for longer RVDs (Boch et al., 2009). This raises the question of whether these intermediate‐length TALEs are biologically functional or not, given that their shorter length will translate into an increased number of potential targets, which in addition will presumably not be highly expressed (Richter et al., 2016). Tn5‐based mutagenesis in Xoo generated a mutant with an insertion in a gene encoding a TALE with 8.5 RVDs; the mutation conferred increased avirulence to rice cultivars carrying the Xa3 R gene (Li et al., 2004). However, the effect was maintained in a second mutant expressing a 124 amino acid open reading frame containing only the NLS and the C‐terminal activation domains. These results suggested that the effect was not dependent on the RVD, but rather on the recognition of the C‐terminal part of the protein by Xa3, in a similar way to Bs4 and Xo1 R proteins in pepper and rice, respectively (Schornack et al., 2004; Triplett et al., 2016). In the current study, the evidence suggests that the avirulence of PthA4AT on C. limon and C. sinensis is dependent upon the protein entering the nucleus activating transcription of an executor R gene. As was demonstrated, nuclear localization and transcriptional activation are necessary for triggering a proper HR (Figs 3 and 6). To our knowledge, this is the first case where a ‘short’ 7.5‐repeat TALE exerts a biological function via the RVD and transcriptional activation, i.e. in the manner of a classical TALE.
The C‐terminal region of all members of the PthA/AvrBs3 family contains an acidic amino acid domain, a property typical of eukaryotic acidic transcription AD. This motif is required for avirulence activity of the AvrXa10 protein in rice (Zhu et al., 1998, 1999) and of the AvrBs3 protein in pepper (Szurek et al., 2001). When the same 27 amino acids critical for avirulence of AvrBs3 were removed from PthA4AT (to give the ΔADAT derivative), its ability to restrict canker development was lost, indicating the importance of this activation domain for PthA4AT avirulence (Fig. 6b). However, when ΔADAT is expressed into the ΔpthA4 mutant strain, an HR is still developed, although it is retarded, suggesting that the deletion in the activation domain did not completely abolish gene activation. Interestingly, similar results were obtained in the non‐host N. benthamiana (Fig. 7b). Although activation domains are characterized by the presence of acidic amino acids, acidity is not the sole characteristic of an activating region and the net negative charge does not strictly correlate with the efficiency (Gill et al., 1990). Powerful artificially generated transcriptional activation domain completely devoid of acidic residues has been generated (Lu et al., 2000). It is thought that the role of these domains is merely to stick to the transcriptional machinery and thereby to recruit it to DNA (Ptashne and Gann, 1997; Yuan et al., 2016). There are data from other TALEs with nearly identical C‐terminal regions that support our results. In AvrBs3, the deletion of the 27 amino acids AD does not avoid transcriptional activation in yeast (Szurek et al., 2001) and similar results were observed for the AvrXa10 protein of Xoo (Zhu et al., 1998). Moreover, only 50% reduction in transcriptional activity was observed when the 28 amino acids immediately upstream of the NLS and AD domain of X. campestris pv. armoraciae Hax3 protein were truncated (Zhang et al., 2011). In these three truncated‐AD TALEs, and in ∆AD pthAs as well (Fig. 6), this stretch still contains 21% of acidic amino acids that could make the protein stick to the transcriptional machinery.
The C‐terminal portion of PthA also encodes three NLS that are critical for localization to the host cell nucleus (Yang and Gabriel, 1995). It was demonstrated that PthA4 TALE targeted to the nucleus by interaction with different host nuclear factors (Domingues et al., 2010; Soprano et al., 2013; de Souza et al., 2012). Using a dominant‐negative strategy, Yang et al. (2011) generated transgenic C. sinensis resistant to canker by overexpressing the NLS of PthA4, this NLS playing an importin‐binding interference role. It could be argued that the mechanism by which PthA4AT exerts its role in pathogenic X. citri strains is similar, and that ∆NLSAT and mutNLSAT derivatives lost their ability to generate the HR only because they cannot interfere anymore with the host importins. However, this is unlikely, as expression of PthA1AT (with identical NLS region) on X. citri strains does not influence canker development after infection of C. limon, and PthA1AT expression on the ΔpthA1,4 or ΔpthA1 mutants did not cause HR (Fig. 2). All this evidence suggests that canker protection caused by PthA4AT goes via a different mechanism, presumably via the activation of an R executor gene. Until now, only five of those genes have been identified (Zhang et al., 2015). They trigger host responses associated with HR, similarly to classical R genes like receptor like‐kinases (RLK) and nucleotide binding site‐leucine‐rich repeat (NBS‐LRR) genes, but if the resistance pathways triggered by the TALE‐mediated executor R genes intersect with those of the other R genes remain unknown. Agrobacterium‐mediated delivery of a 35S promoter‐driven executor Bs3 coding‐region did not cause a visible HR in citrus (Shantharaj et al., 2017). The functionality of the other known executor R proteins in citrus is unknown. We could not find homologous to the known executor R genes on the TALgetter predicted PthA4AT target genes list (Table S3). This would suggest a different mechanism for PthA4AT‐induced HR activation in citrus.
X. citri AT can still develop canker lesions without the presence of the pthA4 virulence gene in C. clementina and X. aurantifolia (Chiesa et al., 2013). This indicates that the absence of canker in X. citri AT‐infected C. limon is not solely an effect of the absence of the pthA4 gene. In support of this contention, reintroduction of the pthA4 virulence gene into X. citri AT does not restore canker development in C. limon. Finally, the host specificity observed for X. citri AT weakens the hypothesis that PthA4AT could be acting indiscriminately over many gene targets, generating a cytotoxic effect by the serendipitous activation of some or many of them, leading to metabolic perturbation as suggested by Reyon et al. (2012). This is unlikely, as predictions by TALgetter indicate that the number of potential targets for such a short TALE is huge not only in C. sinensis, where the HR is observed, but also in the susceptible C. clementine; many of the targets are coincident (Tables S3 and S4). As expected, we could determine that not all the potential targets will be activated by PthA4AT (Hummel et al., 2012; Pereira et al., 2014). In fact, we could obtain transcriptional activation in only one out of the five promoters tested (Fig. 5b). This confirms that PthA4AT‐dependent transcriptional activation is possible, but suggests a gene (or genes)‐specific activation preference that requires a more thorough approach to decipher. The generation of artificial PthA4AT derivatives, using combinatorial cloning (Geiβler et al., 2011) coupled with pathogenicity tests on C. limon and non‐host N. benthamiana, could help to define PthA4AT specificity and thereafter to narrow down the number of targets in the search for those triggering the defence response. Transcriptome profiling using X. citri strains containing the different derivatives of PthA4AT generated in this study will support the criterion for the identification of the R genes (Boch et al., 2014; Strauss et al., 2012). Given the short length of PthA4AT, a specific pattern of activation across multiple targets, as proposed for the 18‐repeat Tal2a gene that elicits HR in rice (Hummel et al., 2017), is plausible.
Any selective advantage was found for X. citri AT in any of the hosts assayed (Chiesa et al., 2013). The existence of the pthA4AT gene is perhaps just an accident, a by‐product of a recombination event in the course of effector evolution. Interestingly, Ye and colleagues (2013) showed that two further strains of X. citri (029‐2 and 049) with attenuated virulence and reduced bacterial growth in planta were characterized by the absence of the 3.4‐kb band characteristic of the pthA4 gene, and by the appearance of a smaller band of around 2.4 kb, in a similar way to X. citri AT (Fig. 1). It would be interesting to isolate and sequence those genes, and test if they also can confer avirulence on citrus hosts. Recently, Pacbio sequencing of X. citri strains also revealed a 6.5‐repeat pthA1 variant in the LM180 strain, obtained from infected grapefruit samples (Gochez et al., 2018), but the biological relevance of this TALE gene is unknown. The discovery of a TALE conferring canker resistant in C. limon and C. sinensis paves the way to identify citrus R genes involved in this important aspect of citriculture.
Experimental Procedures
Bacterial strains growth conditions, plant material and inoculation assays
The strains and plasmids used in this study are listed in Tables S1 and S2, respectively. X. citri strains were cultured at 28 °C with shaking in peptone yeast and malt extract (PYM) medium (Cadmus et al., 1976). Escherichia coli strains DH5α and BL21 and Agrobacterium tumefaciens GV3101 were grown in Luria‐Bertani (LB) medium at 37 °C and 28 °C, respectively. Recombinants plasmids were introduced into the X. citri and Agrobacterium strains by electroporation (Roeschlin et al., 2017). When required, the antibiotics ampicillin (100 µg/mL), kanamycin (50 µg/mL), rifampicin (50 µg/mL) or spectinomycin (100 µg/mL) were added to the growth media.
‘Eureka’ lemon [C. limon (L.) Burm. f.] grafted onto Troyer citrange, ‘Valencia Late’ sweet orange [C. sinensis (L.) Osbeck] grafted onto citrange Carrizo, Arabidopsis thaliana ecotype Col‐0, Solanum tuberosum ‘Spunta’ and N. benthamiana plants were grown under controlled conditions in a growth chamber with a temperature of 25–27 °C and photoperiod of 16 h light/8 h dark. For pathogenicity assays on Citrus spp., new shoots were selected according to Roeschlin et al. (2017). Bacterial suspensions of 107 or 109 colony‐forming units (cfu) per millilitre were prepared in 10 mM MgCl2 and inoculated by infiltration or cotton swab onto 20‐day‐old leaves of the new shoots, respectively. The plants were maintained for 15 days in a growth chamber. Symptoms progression was phenotypically monitored using an MVX10 stereomicroscope as reported in Roeschlin et al. (2017) and conductivity was measured according to Chiesa et al. (2019). For Agrobacterium‐mediated transient expression, strains preparation and induction were conducted as described by Enrique et al. (2011).
Southern hybridization analysis
Plasmid DNA from X. citri strains was isolated according to the manufacturer's instructions (QIAGEN Plasmid Midi Kit; QIAGEN, Mainz, Germany). DNA (10 μg) was digested with EcoRI or BamHI overnight at 37 °C and subjected to electrophoresis on 0.8% agarose gel. DNAs were transferred to Hybond N+ nylon membrane (Amersham International, UK) using standard protocols (Sambrook et al., 1989). The blots were hybridized with a non‐radioactive labelled DNA probe (pthA), generated by a polymerase chain reaction (PCR) product, using the primer pairs Jpth1/Jpth2 (Cubero and Graham, 2002). Probe labelling and signal detection were performed according to the manufacturer's instructions (AlkPhos DIRECT kit, GE Healthcare UK Limited Amersham, Little Chalfont, Buckinghamshire, UK).
Cloning and sequencing of TALE genes
The repeat region of pthA genes was cloned as a BamHI fragment into pUC18 vector (ThermoFisher Scientific, Waltham, MA, USA), screened by PCR with Jpth1/Jpth2 primers and Sanger sequenced (DNA Sequencing Facility, University of Maine, USA). To obtain the 5ʹ and 3ʹ terminal regions of PthAs, plasmids from X. citri AT were sequenced using Roche (454) pyrosequencing technology. Reads were assembled with Newbler v. 2.6 software (Roche 454 Life Sciences, Branford, CT, USA). The final sequence of the two circular plasmids was identical to the reference plasmids (pXAC64 and pXAC33) from X. citri strain 306 (da Silva et al., 2002). PthAs sequences were deposited at GenBank under the accession numbers MK425208, MK425209, MK425210 and MK425211.
To obtain full‐length pthA genes, BamHI‐fragments were subcloned into pUC57‐RR linearized BamHI vector (Data S1) and then screened by PCR and sequenced to check orientation (pUC57RR‐pthA). Constructs with NLS deletion (∆NLS), NLS mutations (mutNLS), SV40 insertion (SV40) and AD deletion (∆AD) were obtained by replacing the BclI/HindIII 3ʹ terminal sequence of pUC57RR‐pthA with the corresponding fragments obtained by gene synthesis (Data S1).
For X. citri and A. tumefaciens expression, pthA constructs from pUC57RR were subcloned into the pBBR1‐MCS2 or pCHF3 vectors using the restriction sites XhoI/HindIII or XbaI/HindIII, respectively.
Immunoblot analysis
Xanthomonas citri overnight cultures (200 μL) were centrifuged and resuspended in protein sample buffer using a volume equal to the optical density at 600 nm 10‐1. Equal volumes (15 µL) of these whole‐cell extracts were separated by 10% SDS‐PAGE and transferred to polyvinylidene fluoride membranes (PVDF‐Immun‐Blot®, BioRad, Hercules, CA, USA). Immunoblotting was performed using 3×FLAG antibodies (1:5000 dilution, Cat# F3165, Sigma‐Aldrich, St Louis, MO, USA) and visualized using a peroxidase‐conjugated goat anti‐rabbit IgG (1:6000 dilution) and Pierce™ ECL Western Blotting Substrate (ThermoFisher Scientific, Waltham, MA, USA) according to manufacturer’s instructions.
Protein binding microarrays
DNA corresponding to full‐length pthA4AT was transferred to pMAL‐c2 vector (New England Biolabs, Inc., MA, USA), yielding maltose binding protein (MBP) N‐terminal fusions. MBP–pthA4AT constructs were transformed into E. coli BL‐21 strains and selected with the corresponding antibiotic. Cultures with an optical density at 600 nm of 0.8 were incubated at 37 °C for 15 min with 2 mM betaine monohydrate (Sigma‐Aldrich) and then induced for 2 h with 0.5 mM isopropyl β‐d‐1‐thiogalactopyranoside (Sigma‐Aldrich). Expression of MBP‐pthA4AT was analysed by Coomassie blue staining of standard SDS‐PAGE and recombinant protein extracts were obtained from 25 mL of induced cultures. The PBM was performed as described previously (Godoy et al., 2011). Normalization of probe intensities and calculation of E‐scores and Z‐scores of all of the possible 8‐mers were carried out with the PBM Analysis Suite. To obtain a single representative motif, we followed the method ‘PWM_align_E’, in which the 9‐mer motifs with E‐score > 0.45 were aligned and each sequence in the alignment is first weighted by the E‐score of the corresponding sequence. Then, the positions present in at least half of the sequences in the alignment were considered, and the resulting alignment is converted to a position frequency matrix with Enologos (http://www.benoslab.pitt.edu/cgi-bin/enologos/enologos.cgi).
TALgetter predictions in Citrus spp. and GUS assay
PthA4AT binding elements were searched in C. sinensis and C. clementina promoterome (400 bp upstream + 200 bp downstream of the transcription start site) by using TALgetter software and considering both strands (http://galaxy2.informatik.uni-halle.de:8976/; Grau et al., 2013; Streubel et al., 2017). The candidate promoters are listed in Tables S3 and S4. Selected promoter regions were amplified from the corresponding genomic DNA using designed primers (Table S5). The promoter fragments were digested with the corresponding restriction enzyme (BamHI or XbaI with NcoI) and fused with uidA (GUS) gene in pCAMBIA1303 expression vector. Positive clones were analysed by PCR amplification and DNA sequencing, and transformed into A. tumefaciens GV3101. EBE‐mutated Cs4 promoter was generated by overlapping PCR using primers incorporating mutations in the EBE site (Table S5).
For GUS expression in N. benthamiana leaves, two Agrobacterium suspensions were mixed in a ratio of 1:1. Forty‐eight hours post‐infiltration, leaf disks were stained on GUS staining buffer (50 mM NaPO4 (pH 7.0), 0.1% Triton X‐100, 10 mM EDTA, 1 mM K3Fe(CN)6, 1 mM K4Fe(CN)6, 0.5 mg/mL X‐gluc] and incubated at 37 °C for 3 h. Then, the disks were cleared in ethanol and photographed.
Accession Numbers
Supporting information
Fig. S1 Alignment of PthA1 from X. citri AT and X. citri 306.
Fig. S2 Alignment of PthA4 from X. citri AT and X. citri 306.
Fig. S3 Western‐blot analysis of PthA expression on X. citri strains.
Fig. S4 Macroscopic symptoms developed by X. citri AT and X. citri 306 ∆pthA4 mutant expressing pthA4.
Fig. S5 Phenotypic response of PthA1AT and PthA4AT expressed in Argentinian X. citri T strain in Citrus limon leaves.
Fig. S6 Mutations in the PthA4AT C‐terminal region.
Fig. S7 Phenotypic response of X. citri 306 strain expressing mutant version in nuclear localization of PthA4AT.
Fig. S8 PthA4AT triggers host defense response in Citrus sinensis leaves.
Fig. S9 Phenotypic response on C. limon leaves inoculated with X. citri T transformed with ∆ADAT.
Table S1. Bacterial strains.
Table S2. Plasmids used in this study.
Table S3. TALgetter results of candidate promoters to PthA4AT on C. clementine.
Table S4. TALgetter results of candidate promoters to PthA4AT on C. sinensis.
Table S5. List of oligonucleotide primers used in this study.
Table S6. List of motifs variants obtained with PBM11 protein‐binding microarrays for PthA4AT.
Data S1. Design sequences for synthesis and cloning of the full‐length of pthAs and mutant construction.
Acknowledgements
This work was mainly supported by the Agencia Nacional de Promoción Cientifica y Tecnologica (PICT‐2016‐1222) to M.R.M. and by a Programa de Cooperacion Bilateral CONICET‐CSIC PCB II 2013 to M.R.M. and J.G. M.R.M and M.A.C. were Career Investigators of CONICET; R.A.R., L.G., M.A.F. and F.U., C.M., S.T. were supported by postdoctoral and doctoral scholarships, respectively, from CONICET. This article is based upon work from COST Action CA16107 EuroXanth, supported by COST (European Cooperation in Science and Technology). We thank Celso E. Benedetti for providing the pthA‐deletions mutants of X. citri and the Bioinformatics Core Service of the IBMCP (UPV‐CSIC) for its help in bioinformatic analyses. The authors thank J. M. Dow and J. Boch for critical review of the manuscript.
Contributor Information
José Gadea, Email: jgadea@ibmcp.upv.es.
María Rosa Marano, Email: marano@ibr-conicet.gov.ar.
References
- Al‐Saadi, A. , Reddy, J.D. , Duan, Y.P. , Brunings, A.M. , Yuan, Q. and Gabriel, D.W. (2007) All five host‐range variants of Xanthomonas citri carry one pthA homolog with 17.5 repeats that determines pathogenicity on citrus, but none determine host‐range variation. Mol. Plant‐Microbe Interact. 20, 934–943. [DOI] [PubMed] [Google Scholar]
- Behlau, F. and Belasque, J. , Graham, J.H. and Leite, R.P. (2010) Effect of frequency of copper applications on control of citrus canker and the yield of young bearing sweet orange trees. Crop Prot. 29, 300–305. [Google Scholar]
- Berger, M.F. and Bulyk, M.L. (2009) Universal protein‐binding microarrays for the comprehensive characterization of the DNA‐binding specificities of transcription factors. Nat. Protoc. 4, 393–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boch, J. and Bonas, U. (2010) Xanthomonas AvrBs3 family‐type III effectors: discovery and function. Annu. Rev. Phytopathol. 48, 419–436. [DOI] [PubMed] [Google Scholar]
- Boch, J. , Bonas, U. and Lahaye, T. (2014) TAL effectors‐pathogen strategies and plant resistance engineering. New Phytol. 204, 823–832. [DOI] [PubMed] [Google Scholar]
- Boch, J. , Scholze, H. , Schornack, S. , Landgraf, A. , Hahn, S. , Kay, S. , Lahaye, T. , Nickstadt, A. and Bonas, U. (2009) Breaking the code of DNA binding specificity of TAL‐type III effectors. Science, 326, 1509–1512. [DOI] [PubMed] [Google Scholar]
- Bogdanove, A.J. , Schornack, S. and Lahaye, T. (2010) TAL effectors: finding plant genes for disease and defense. Curr. Opin. Plant Biol. 13, 394–401. [DOI] [PubMed] [Google Scholar]
- Cadmus, M.C. , Rogovin, S.P. , Burton, K.A. , Pittsley, J.E. , Knutson, C.A. and Jeanes, A. (1976) Colonial variation in Xanthomonas campestris NRRL B‐1459 and characterization of the polysaccharide from a variant strain. Can. J. Microbiol. 22, 942–948. [DOI] [PubMed] [Google Scholar]
- Canteros, B.I. , Gochez, A.M. and Moschini, R.C. (2017) Management of citrus canker in Argentina, a success story. Plant Pathol. J. 33, 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carella, P. , Evangelisti, E. and Schornack, S. (2018) Sticking to it: phytopathogen effector molecules may converge on evolutionarily conserved host targets in green plants. Curr. Opin. Plant Biol. 44, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, P.S. , Wang, L.Y. , Chen, Y.J. , Tzeng, K.C. , Chang, S.C. , Chung, K.R. and Lee, M.H. (2012) Understanding cellular defence in kumquat and calamondin to citrus canker caused by Xanthomonas citri subsp. citri . Physiol. Mol. Plant Pathol. 79, 1–12. [Google Scholar]
- Chiesa, M.A. , Roeschlin, R.A. , Favaro, M.A. , Uviedo, F. , Campos‐Beneyto, L. , D’Andrea, R. , Gadea, J. and Marano, M.R. (2019) Plant responses underlying nonhost resistance of Citrus limon against Xanthomonas campestris pv. campestris . Mol. Plant Pathol. 20, 254–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa, M.A. , Siciliano, M.F. , Ornella, L. , Roeschlin, R.A. , Favaro, M.A. , Delgado, N.P. , Sendín, L.N. , Orce, I.G. , Ploper, L.D. , Vojnov, A.A. and Vacas, J.G. (2013) Characterization of a variant of Xanthomonas citri subsp. citri that triggers a host‐specific defense response. Phytopathology, 103, 555–564. [DOI] [PubMed] [Google Scholar]
- Cox, K.L. , Meng, F. , Wilkins, K.E. , Li, F. , Wang, P. , Booher, N.J. , Carpenter, S.C.D. , Chen, L.Q. , Zheng, H. , Gao, X. , Zheng, Y. , Fei, Z. , Yu, J.Z. , Isakeit, T. , Wheeler, T. , Frommer, W.B. , He, P. , Bogdanove, A.J. and Shan, L. (2017) TAL effector driven induction of a SWEET gene confers susceptibility to bacterial blight of cotton. Nat. Commun. 10.1038/ncomms15588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubero, J. and Graham, J.H. (2002) Genetic relationship among worldwide strains of Xanthomonas causing canker in citrus species and design of new primers for their identification by PCR. Appl. Environ. Microbiol. 68, 1257–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denancé, N. , Szurek, B. , Doyle, E.L. , Lauber, E. , Fontaine‐Bodin, L. , Carrère, S. , Guy, E. , Hajri, A. , Cerutti, A. , Boureau, T. and Poussier, S. (2018) Two ancestral genes shaped the Xanthomonas campestris TAL effector gene repertoire. New Phytol. 219, 391–407. [DOI] [PubMed] [Google Scholar]
- Deng, Z.N. , Xu, L. , Li, D.Z. , Long, G.Y. , Liu, L.P. , Fang, F. and Shu, G.P. (2010) Screening citrus genotypes for resistance to canker disease (Xanthomonas axonopodis pv. citri). Plant Breed. 129, 341–345. [Google Scholar]
- Domingues, M.N. , De Souza, T.A. , Cernadas, R.A. , de Oliveira, M.L. , Docena, C. , Farah, C.S. and Benedetti, C.E. (2010) The Xanthomonas citri effector protein PthA interacts with citrus proteins involved in nuclear transport, protein folding and ubiquitination associated with DNA repair. Mol. Plant Pathol. 11, 663–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, Y.P. , Castaneda, A. , Zhao, G. , Erdos, G. and Gabriel, D.W. (1999) Expression of a single, host‐specific, bacterial pathogenicity gene in plant cells elicits division, enlargement, and cell death. Mol. Plant‐Microbe Interact. 12, 556–560. [Google Scholar]
- Enrique, R. , Siciliano, F. , Favaro, M.A. , Gerhardt, N. , Roeschlin, R. , Rigano, L. , Sendin, L. , Castagnaro, A. , Vojnov, A. and Marano, M.R. (2011) Novel demonstration of RNAi in citrus reveals importance of citrus callose synthase in defence against Xanthomonas citri subsp. citri . Plant Biotech. J. 9, 394–407. [DOI] [PubMed] [Google Scholar]
- Favaro, M.A. , Roeschlin, R.A. , Ribero, G.G. , Maumary, R.L. , Fernandez, L.N. , Lutz, A. , Sillon, M. , Rista, L.M. , Marano, M.R. and Gariglio, N.F. (2017) Relationships between copper content in orange leaves, bacterial biofilm formation and citrus canker disease control after different copper treatments. Crop Prot. 92, 182–189. [Google Scholar]
- Ference, C.M. , Gochez, A.M. , Behlau, F. , Wang, N. , Graham, J.H. and Jones, J.B. (2018) Recent advances in the understanding of Xanthomonas citri ssp. citri pathogenesis and citrus canker disease management. Mol. Plant Pathol. 19, 1302–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiβler, R. , Scholze, H. , Hahn, S. , Streubel, J. , Bonas, U. , Behrens, S.‐E. and Boch, J. (2011) Transcriptional activators of human genes with programmable DNA‐specificity. PLoS ONE, 6, e19509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, G. , Sadowski, I. and Ptashne, M. (1990) Mutations that increase the activity of a transcriptional activator in yeast and mammalian cells. Proc. Natl. Acad. Sci. USA. 87, 2127–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gochez, A.M. , Huguet‐Tapia, J.C. , Minsavage, G.V. , Shantaraj, D. , Jalan, N. , Strauß, A. , Lahaye, T. , Wang, N. , Canteros, B.I. , Jones, J.B. and Potnis, N. (2018) Pacbio sequencing of copper‐tolerant Xanthomonas citri reveals presence of a chimeric plasmid structure and provides insights into reassortment and shuffling of transcription activator‐like effectors among X. citri strains. BMC Genom. 10.1186/s12864-017-4408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy, M. , Franco‐Zorrilla, J.M. , Pérez‐Pérez, J. , Oliveros, J.C. , Lorenzo, O. and Solano, R. (2011) Improved protein‐binding microarrays for the identification of DNA‐binding specificities of transcription factors. Plant J. 66, 700–711. [DOI] [PubMed] [Google Scholar]
- Graham, J.H. , Gottwald, T.R. , Cubero, J. and Achor, D.S. (2004) Xanthomonas axonopodis pv. citri: factors affecting successful eradication of citrus canker. Mol. Plant Pathol. 5, 1–15. [DOI] [PubMed] [Google Scholar]
- Grau, J. , Wolf, A. , Reschke, M. , Bonas, U. , Posch, S. and Boch, J. (2013) Computational predictions provide insights into the biology of TAL effector target sites. PLoS Comput. Biol. 9, e1002962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Y. , Zhang, J. , Jia, H. , Sosso, D. , Li, T. , Frommer, W.B. , Yang, B. , White, F.F. , Wang, N. and Jones, J.B. (2014) Lateral organ boundaries 1 is a disease susceptibility gene for citrus bacterial canker disease. Proc. Natl. Acad. Sci. USA. 111, 521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel, A.W. , Doyle, E.L. and Bogdanove, A.J. (2012) Addition of transcription activator‐like effector binding sites to a pathogen strain‐specific rice bacterial blight resistance gene makes it effective against additional strains and against bacterial leaf streak. New Phytol. 195, 883–893. [DOI] [PubMed] [Google Scholar]
- Hummel, A.W. , Wilkins, K.E. , Wang, L. , Cernadas, R.A. and Bogdanove, A.J. (2017) A transcription activator‐like effector from Xanthomonas oryzae pv. oryzicola elicits dose‐dependent resistance in rice. Mol. Plant Pathol. 18, 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutin, M. , Pérez‐Quintero, A. , Lopez, C. and Szurek, B. (2015) MorTAL Kombat: the story of defense against TAL effectors through loss‐of‐susceptibility. Front. Microbiol. 10.3389/fpls.2015.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon, D. , Richardson, W.D. , Markham, A.F. and Smith, A.E. (1984) Sequence requirements for nuclear location of simian virus 40 large‐T antigen. Nature, 311, 33–38. [DOI] [PubMed] [Google Scholar]
- Khalaf, A.A. , Gmitter, F.G. Jr , Conesa, A. , Dopazo, J. and Moore, G.A. (2011) Fortunella margarita transcriptional reprogramming triggered by Xanthomonas citri subsp. citri . BMC Plant Biol. 10.1186/1471-2229-11-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S.D. , Lee, J.H. , Lee, D.H. and Lee, Y.H. (2008) Diversity of pthA gene of Xanthomonas strains causing citrus bacterial canker and its relationship with virulence. Plant Pathol. J. 10.5423/PPJ.2008.24.3.357. [DOI] [Google Scholar]
- Lee, I.J. , Kim, K.W. , Hyun, J.W. , Lee, Y.H. and Park, E.W. (2009) Comparative ultrastructure of nonwounded Mexican lime and Yuzu leaves infected with the citrus canker bacterium Xanthomonas citri pv. citri . Microsc. Res. Tech. 72, 507–516. [DOI] [PubMed] [Google Scholar]
- Li, P. , Long, J. , Huang, Y. , Zhang, Y. and Wang, J. (2004) AvrXaS: a novel member of avrBs3 gene family from Xanthomonas oryzae pv. oryzae has a dual function. Prog. Nat. Sci. 14, 774–780. [Google Scholar]
- Lu, X. , Ansari, A.Z. and Ptashne, M. (2000) An artificial transcriptional activating region with unusual properties. Proc. Natl. Acad. Sci. USA. 97, 1988–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macho, A.P. (2016) Subversion of plant cellular functions by bacterial type‐III effectors: beyond suppression of immunity. New Phytol. 210, 51–57. [DOI] [PubMed] [Google Scholar]
- Macho, A.P. and Zipfel, C. (2014) Plant PRRs and the activation of innate immune signaling. Mol. Cell, 54, 263–272. [DOI] [PubMed] [Google Scholar]
- Moscou, M.J. and Bogdanove, A.J. (2009) A simple cipher governs DNA recognition by TAL effectors. Science, 326, 1501–1502. [DOI] [PubMed] [Google Scholar]
- Pereira, A.L. , Carazzolle, M.F. , Abe, V.Y. , de Oliveira, M.L. , Domingues, M.N. , Silva, J.C. , Cernadas, R.A. and Benedetti, C.E. (2014) Identification of putative TAL effector targets of the citrus canker pathogens shows functional convergence underlying disease development and defense response. BMC Genom. 10.1186/1471-2164-15-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne, M. and Gann, A. (1997) Transcriptional activation by recruitment. Nature, 386, 569–577. [DOI] [PubMed] [Google Scholar]
- Reyon, D. , Tsai, S.Q. , Khayter, C. , Foden, J.A. , Sander, J.D. and Joung, J.K. (2012) FLASH assembly of TALENs for high‐throughput genome editing. Nature Biotech. 30, 460–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter, A. , Streubel, J. and Boch, J. (2016) TAL effector DNA‐binding principles and specificity. Meth. Mol. Biol. 1338, 9–25. [DOI] [PubMed] [Google Scholar]
- Rinaldi, F.C. , Doyle, L.A. , Stoddard, B.L. and Bogdanove, A.J. (2017) The effect of increasing numbers of repeats on TAL effector DNA binding specificity. Nucl. Acids Res. 45, 6960–6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeschlin, R.A. , Favaro, M.A. , Chiesa, M.A. , Alemano, S. , Vojnov, A.A. , Castagnaro, A.P. , Filippone, M.P. , Gmitter, F.G. Jr , Gadea, J. and Marano, M.R. (2017) Resistance to citrus canker induced by a variant of Xanthomonas citri ssp. citri is associated with a hypersensitive cell death response involving autophagy‐associated vacuolar processes. Mol. Plant Pathol. 18, 1267–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römer, P. , Hahn, S. , Jordan, T. , Strauß, T. , Bonas, U. and Lahaye, T. (2007) Plant pathogen recognition mediated by promoter activation of the pepper Bs3 resistance gene. Science, 318, 645–648. [DOI] [PubMed] [Google Scholar]
- Sambrook, J. , Fritsch, E.F. and Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual, 2nd edition. Cold Spring Harbor: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Schornack, S. , Ballvora, A. , Gürlebeck, D. , Peart, J. , Baulcombe, D. , Ganal, M. , Baker, B. , Bonas, U. and Lahaye, T. (2004) The tomato resistance protein Bs4 is a predicted non‐nuclear TIR‐NB‐LRR protein that mediates defense responses to severely truncated derivatives of AvrBs4 and overexpressed AvrBs3. Plant J. 37, 46–60. [DOI] [PubMed] [Google Scholar]
- Shantharaj, D. , Römer, P. , Figueiredo, J.F.L. , Minsavage, G.V. , Krönauer, C. , Stall, R.E. , Moore, G.A. , Fisher, L.C. , Hu, Y. , Horvath, D.M. and Lahaye, T. (2017) An engineered promoter driving expression of a microbial avirulence gene confers recognition of TAL effectors and reduces growth of diverse Xanthomonas strains in citrus. Mol. Plant Pathol. 18, 976–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiotani, H. , Fujikawa, T. , Ishihara, H. , Tsuyumu, S. and Ozaki, K. (2007) A pthA homolog from Xanthomonas axonopodis pv. citri responsible for host‐specific suppression of virulence. J. Bacteriol. 189, 3271–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva, A.C. , Ferro, J.A. , Reinach, F.C. , Farah, C.S. , Furlan, L.R. , Quaggio, R.B. , Monteiro‐Vitorello, C.B. , Van Sluys, M.A. , Almeida, N.F. , Alves, L.M. and Do Amaral, A.M. (2002) Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature, 417, 459–463. [DOI] [PubMed] [Google Scholar]
- Soprano, A.S. , Yukari Abe, V. , Smetana, J.H.C. and Benedetti, C.E. (2013) Citrus MAF1, a repressor of RNA Polymerase III, binds the Xanthomonas citri canker elicitor PthA4 and suppresses citrus canker development. Plant Physiol. 163, 232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza, T.A. , Soprano, A.S. , de Lira, N.P.V. , Quaresma, A.J.C. , Pauletti, B.A. , Leme, A.F.P. and Benedetti, C.E. (2012) The TAL effector PthA4 interacts with nuclear factors involved in RNA dependent processes including a HMG protein that selectively binds poly(U) RNA. PLoS ONE. 10.1371/journal.pone.0032305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss, T. , van Poecke, R.M. , Strauss, A. , Römer, P. , Minsavage, G.V. , Singh, S. , Wolf, C. , Strauss, A. , Kim, S. , Lee, H.A. and Yeom, S‐I. (2012) RNA‐seq pinpoints a Xanthomonas TAL‐effector activated resistance gene in a large‐crop genome. Proc. Natl. Acad. Sci. USA. 109, 19480–19485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streubel, J. , Baum, H. , Grau, J. , Stuttman, J. and Boch, J. (2017) Dissection of TALE‐dependent gene activation reveals that they induce transcription cooperatively and in both orientations. PLoS ONE, 12, e0175653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurek, B. , Marois, E. , Bonas, U. and Van den Ackerveken, G. (2001) Eukaryotic features of the Xanthomonas type III effector AvrBs3: protein domains involved in transcriptional activation and the interaction with nuclear import receptors from pepper. Plant J. 26, 523–534. [DOI] [PubMed] [Google Scholar]
- Toruño, T.Y. , Stergiopoulos, I. and Coaker, G. (2016) Plant‐pathogen effectors: cellular probes interfering with plant defenses in spatial and temporal manners. Annu. Rev. Phytopathol. 54, 419–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett, L.R. , Cohen, S.P. , Heffelfinger, C. , Schmidt, C.L. , Huerta, A.I. , Tekete, C. , Verdier, V. , Bogdanove, A.J. and Leach, J.E. (2016) A resistance locus in the American heirloom rice variety Carolina Gold Select is triggered by TAL effectors with diverse predicted targets and is effective against African strains of Xanthomonas oryzae pv. oryzicola . Plant J. 87, 472–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weirauch, M.T. , Cote, A. , Norel, R. , Annala, M. , Zhao, Y. , Riley, T.R. , Saez‐Rodriguez, J. , Cokelaer, T. , Vedenko, A. , Talukder, S. and Agius, P. (2013) Evaluation of methods for modeling transcription factor sequence specificity. Nature Biotech. 31, 126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weirauch, M.T. , Yang, A. , Albu, M. , Cote, A.G. , Montenegro‐Montero, A. , Drewe, P. , Najafabadi, H.S. , Lambert, S.A. , Mann, I. , Cook, K. and Zheng, H. (2014) Determination and inference of eukaryotic transcription factor sequence specificity. Cell, 158, 1431–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X. , Li, R. , Zou, L. and Chen, G. (2006) Gene‐for‐gene relationships between rice and diverse avrBs3/pthA avirulence genes in Xanthomonas oryzae pv. oryzae . Plant Pathol. 56, 26–34. [Google Scholar]
- Wu, G.A. , Prochnik, S. , Jenkins, J. , Salse, J. , Hellsten, U. , Murat, F. , Perrier, X. , Ruiz, M. , Scalabrin, S. , Terol, J. and Takita, M.A. (2014) Sequencing of diverse mandarin, pummelo and orange genomes reveals complex history of admixture during citrus domestication. Nature Biotech. 32, 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Q. , Chen, L.L. , Ruan, X. , Chen, D. , Zhu, A. , Chen, C. , Bertrand, D. , Jiao, W.B. , Hao, B.H. , Lyon, M.P. and Chen, J. (2013) The draft genome of sweet orange (Citrus sinensis). Nature Genet. 45, 59–66. [DOI] [PubMed] [Google Scholar]
- Yang, Y. and Gabriel, D.W. (1995) Intragenic recombination of a single plant pathogen gene provides a mechanism for the evolution of new host specificities. J. Bacteriol. 177, 4963–4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, L. , Hu, C. , Li, N. , Zhang, J. , Yan, J. and Deng, Z. (2011) Transformation of sweet orange [Citrus sinensis (L.) Osbeck] with pthA‐nls for acquiring resistance to citrus canker disease. Plant Mol. Biol. 75, 11–23. [DOI] [PubMed] [Google Scholar]
- Ye, G. , Hong, N. , Zou, L.F. , Zou, H.S. , Zakria, M. , Wang, G.P. and Chen, G.Y. (2013) TALE based genetic diversity of Chinese isolates of the citrus canker pathogen Xanthomonas citri subsp. citri . Plant Dis. 97, 1187–1194. [DOI] [PubMed] [Google Scholar]
- Yuan, M. , Ke, Y. , Huang, R. , Ma, L. , Yang, Z. , Chu, Z. , Xiao, J. , Li, X. and Wang, S. (2016) A host basal transcription factor is a key component for infection of rice by TALE carrying bacteria. eLife. 10.7554/eLife.19605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukari Abe, V. and Benedetti, C.E. (2015) Additive roles of PthAs in bacterial growth and pathogenicity associated with nucleotide polymorphisms in effector‐binding elements of citrus canker susceptibility genes. Mol. Plant Pathol. 10.1111/mpp.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, F. , Cong, L. , Lodato, S. , Kosuri, S. , Church, G.M. and Arlotta, P. (2011) Efficient construction of sequence‐specific TAL effectors for modulating mammalian transcription. Nature Biotech. 29, 149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Yin, Z. and White, F. (2015) TAL effectors and the executor R genes. Front. Plant Sci. 10.3389/fpls.2015.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, W. , Yang, B. , Chittoor, J.M. , Johnson, L.B. and White, F.F. (1998) AvrXa10 contains an acidic transcriptional activation domain in the functionally conserved C terminus. Mol. Plant‐Microbe Interact. 11, 824–832. [DOI] [PubMed] [Google Scholar]
- Zhu, W. , Yang, B. , Wills, N. , Johnson, L.B. and White, F.F. (1999) The C terminus of AvrXa10 can be replaced by the transcriptional activation domain of VP16 from the herpes simplex virus. Plant Cell, 11, 1665–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Alignment of PthA1 from X. citri AT and X. citri 306.
Fig. S2 Alignment of PthA4 from X. citri AT and X. citri 306.
Fig. S3 Western‐blot analysis of PthA expression on X. citri strains.
Fig. S4 Macroscopic symptoms developed by X. citri AT and X. citri 306 ∆pthA4 mutant expressing pthA4.
Fig. S5 Phenotypic response of PthA1AT and PthA4AT expressed in Argentinian X. citri T strain in Citrus limon leaves.
Fig. S6 Mutations in the PthA4AT C‐terminal region.
Fig. S7 Phenotypic response of X. citri 306 strain expressing mutant version in nuclear localization of PthA4AT.
Fig. S8 PthA4AT triggers host defense response in Citrus sinensis leaves.
Fig. S9 Phenotypic response on C. limon leaves inoculated with X. citri T transformed with ∆ADAT.
Table S1. Bacterial strains.
Table S2. Plasmids used in this study.
Table S3. TALgetter results of candidate promoters to PthA4AT on C. clementine.
Table S4. TALgetter results of candidate promoters to PthA4AT on C. sinensis.
Table S5. List of oligonucleotide primers used in this study.
Table S6. List of motifs variants obtained with PBM11 protein‐binding microarrays for PthA4AT.
Data S1. Design sequences for synthesis and cloning of the full‐length of pthAs and mutant construction.


