Abstract
Substituted nitrobenzothiazinones (BTZs) are potent antituberculosis prodrugs that are reductively activated to produce nitroso moieties that form covalent adducts with a cysteine residue of decaprenylphosphoryl-β-d-ribose-2′-oxidase (DprE1) of Mycobacterium tuberculosis (Mtb). The resulting cell wall synthesis inhibition is lethal to Mtb, leading to consideration of development of BTZs for clinical use. The hydride-induced reduction of the nitroaromatic proceeds by reversible formation of the corresponding Meisenheimer complex. Herein we demonstrate that chemical reduction of BTZ043 with NaBD4 followed by reoxidation incorporates deuterium into the core nitro aromatic warhead. Subsequent reduction of the deuterated species is not affected, but, as expected, reoxidation is slowed by the deuterium isotope effect, thus prolonging the lifetime of the active nitroso oxidation state.
Keywords: Deuterium isotope effect, Meisenheimer complex, nitrobenzothiazinone, nitroso, antituberculosis
Replacement of carbon–hydrogen bonds with isotopic carbon–deuterium bonds results in a kinetic isotope effect that is reflected in a change in the rate of chemical reactions involving those bonds. Studies of isotope effects are useful for testing proposed reaction mechanisms including enzymatic and metabolic processes in biological systems. Also, deuterium incorporation can sometimes significantly alter the metabolic profile of a molecule, thereby resulting in changes in the ratio of parent drug to metabolites and changes in the amounts of metabolites formed.1 Thus, substitutions of metabolically labile C–H bonds for stronger C–D bonds can improve pharmacodynamics, tolerability, and efficacy of a therapeutic agent.2 While several deuterated analogs of drugs have been described and found to have improved properties relative to their C–H analogs, syntheses of specifically deuterated compounds often involve extensive modifications of the synthetic procedures used to obtain the parent drugs. Herein, we describe facile incorporation of deuterium into the core, electrophilic warhead of the nitrobenzothiazinone BTZ043 (2), a potent antituberculosis prodrug, and its consequent influence on the enzymatically active nitroso oxidation state.
Tuberculosis (TB) now ranks above HIV/AIDS as the leading cause of death from a single infectious agent and is the ninth leading cause of death worldwide.3 Approximately two billion people are currently infected with Mycobacterium tuberculosis (Mtb), the bacterium that causes TB. While most infections are latent, people with weakened immune systems are exceptionally prone to develop active cases. The problem is worldwide and approximately 10.4 million people become ill and 1.7 million die from TB annually. Treatment of active TB requires committed and diligent use of multidrug therapy over 6–30 months, and success is declining due to the emergence of multidrug resistant (MDR), extensive-drug resistant (XDR), and even totally drug resistant (TDR) strains.4 While it is unlikely that a single stand-alone drug will be effective against resistant strains of TB, new drugs and combinations with new modes of action are desperately needed.
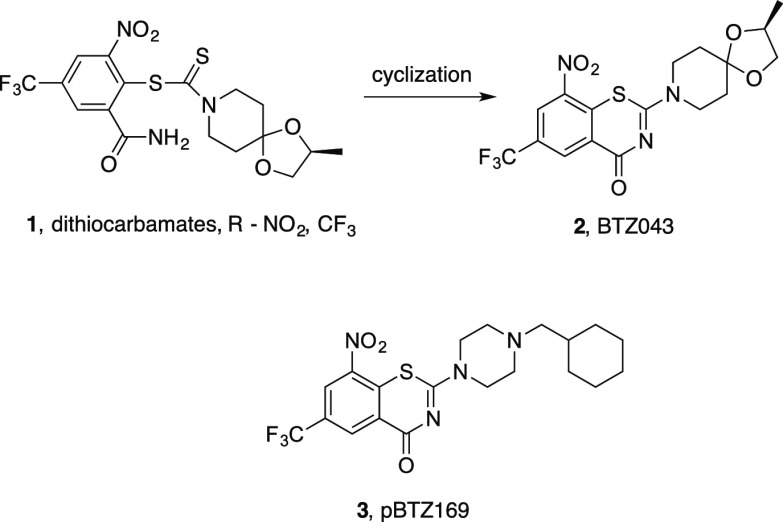
In 2012, bedaquiline (TMC207), the first new anti-TB agent after a multidecade drought, was approved for use in treatment of MDR-TB. It is a diarylquinoline that inhibits mycobacterial adenosine triphosphate (ATP) synthase.5,6 This approval stimulated further interest in discovery and development as well as clinical evaluation of potential new therapies for treatment of TB. The combined efforts of academic, institutional, government, industrial groups, and regulators have enhanced the pipeline related to new anti-TB agents,7−9 yet extensive efforts are still needed. The recent shift from target-based approaches to those based on phenotypic screens has accelerated discovery and development of new anti-TB lead compounds.10 Among the most potent classes of investigational anti-TB agents are the nitro-substituted benzothiazinones, represented by BTZ043 (2; Figure 1),11 which recently successfully finished Phase I clinical trials. The related pBTZ169 (3; Figure 1)12 is in Phase II trials in Russia.13 The first reported nitro-substituted benzothiazinone, BTZ043, was discovered during studies of nitroaryl dithiocarbamates (DTCs, 1)14 that had moderate anti-TB activity but, during cultivation of mycobacteria in the presence of DTCs, cyclized to the benzothiazinones that have low nanomolar anti-Mtb activity in whole cell assays.
Figure 1.
Discovery of BTZ043 and structure of pBTZ169.
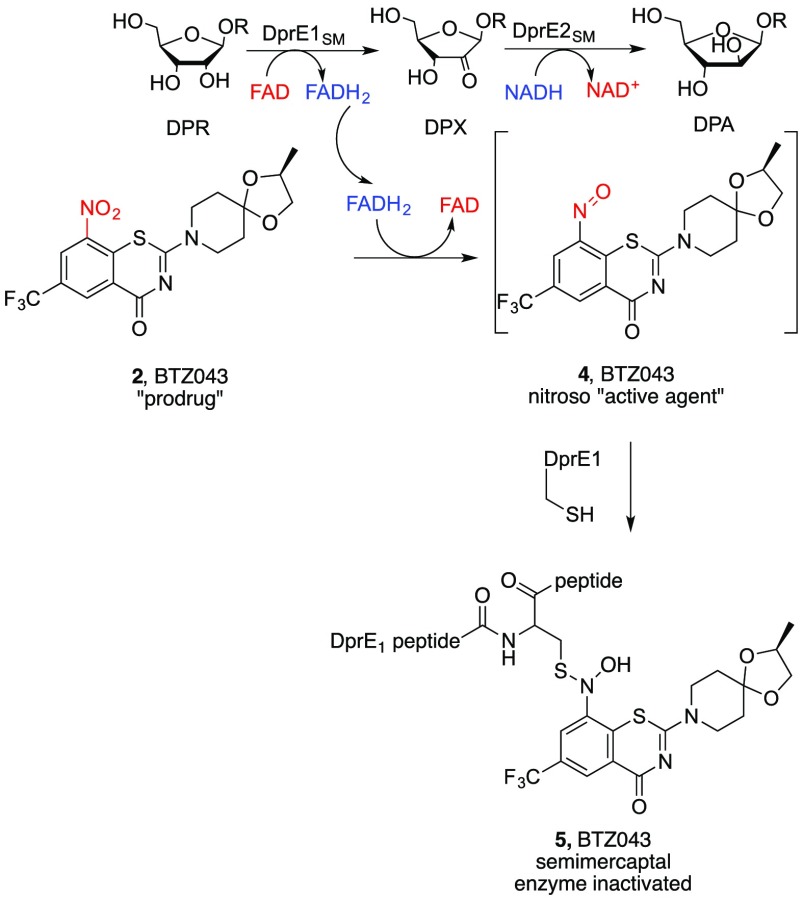
The nitrobenzothiazinone (BTZ) compounds act as prodrugs, which, upon activation by reduction of the nitro substituent to an electrophilic nitroso moiety, 4, induce suicide inhibition of the enzyme decaprenylphosphoryl-β-d-ribose-2′-oxidase (DprE1) of Mtb.15 DprE1 mediates biosynthesis of decaprenylphosphoryl-β-d-arabinose (DPA), which is needed for assembly of the complex cell wall of mycobacteria.16 Previous studies revealed formation of a “semimercaptal adduct” (5) at the active site of DprE1 from reaction of the active-site cysteine with the nitroso intermediate (4) derived from BTZ043.17 Resistant mutants and other forms of mycobacteria that are less susceptible to the benzothiazinones contain active site alanine or serine instead of the active site cysteine.18 Separate reduction of the nitro group to the corresponding hydroxylamine and amine are also detrimental to anti-TB activity. While the mechanistic details related to the reduction of the nitro group of BTZ043 to the nitroso intermediate are yet to be fully elucidated, the DprE1-mediated process does require biological reductants (Scheme 1). The ultimate result is that the nitrobenzothiazinones have low nanomolar activity against mycobacteria, including Mtb, that have the essential cysteine-containing DprE1 enzyme.
Scheme 1. Formation of a Nitroso Moiety from BTZ043 and Reaction with the Target DprE1 Enzyme.
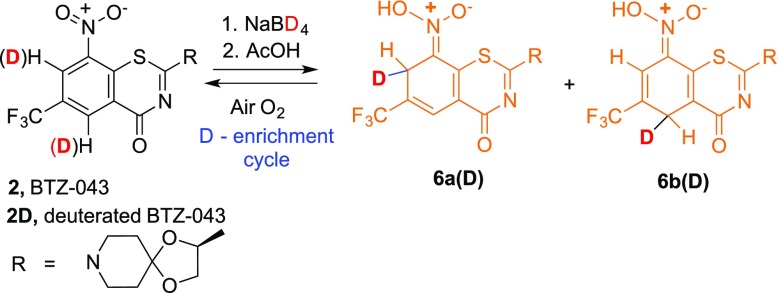
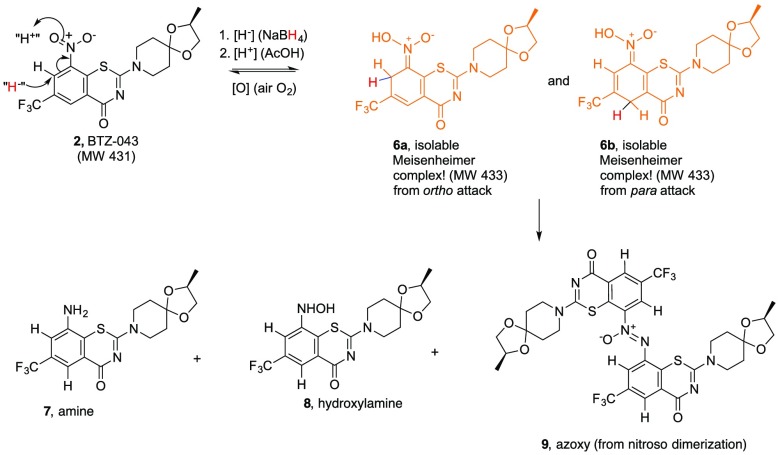
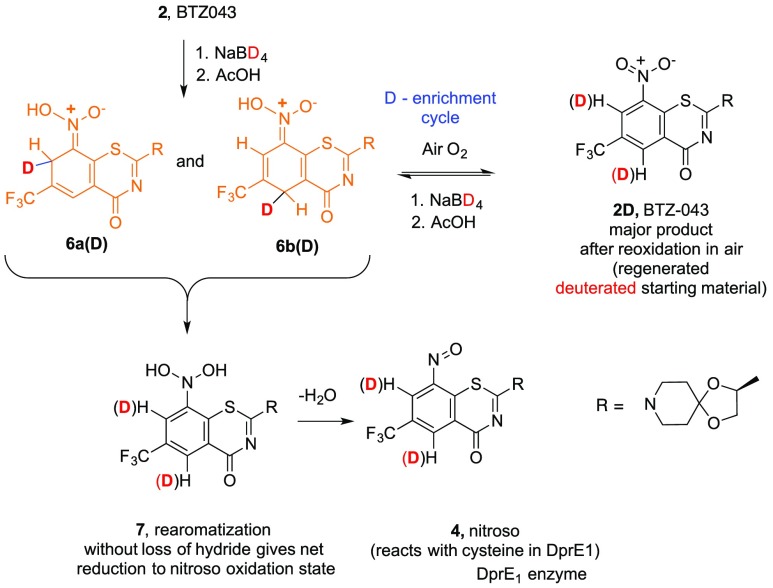
We previously reported that thiolates and other nucleophiles,19 including hydride,20 react directly and cleanly with BTZ and related nitroaromatic compounds through addition ortho and/or para to the essential nitro group with concomitant reduction of the nitro group. The initial products from chemical hydride reduction are orange/red dearomatized Meisenheimer complexes (6a and 6b) that can be isolated and fully characterized. Interestingly, metabolically only the Meisenheimer complex (6b) corresponding to the para attack of hydride is formed.21 Dehydration and rearomatization of the Meisenheimer complexes can provide the enzymatically reactive nitroso moiety, 4 (Scheme 2).
Scheme 2. Hydride-induced Formation of Meisenheimer Complexes and Subsequent Reactions.
As indicated in Scheme 2, the hydride reduction is reversible. Thus, while Meisenheimer complexes 6a and 6b can be isolated and stored as solids under inert atmosphere, solutions of the mixture exposed to air slowly (overnight at room temperature) oxidize to regenerate the starting BTZ043 (2) cleanly. Interestingly, in argon sparged solvents, the Meisenheimer complexes also react, but LC/MS of the reaction mixture not only shows regenerated BTZ043 (2) but also significant amounts of the corresponding amine (7), hydroxylamine (8), and azoxyarene (9). It is well-known that hydride reduction of aromatic nitro groups can generate azoxyarenes that form from the corresponding nitroso intermediates,21,22 which would correspond to nitrosoarene 4 in this case. We also have previously demonstrated that nitrosoarene 4 and the corresponding azoxyarene 9 can be generated from reactions of BTZ043 (2) with other nucleophiles.19 These results are consistent with the hydride-generated Meisenheimer complexes being key intermediates to the nitroso moieties that are responsible for DprE1 inactivation. Thus, we were interested in determining if replacement of the ortho and para hydrogens with deuterium in the electrophilic nitro-substituted “warhead” core could extend the lifetime of these important Meisenheimer complexes by deuterium isotope effect slowing the reoxidation process.
Addition of NaBD4 to BTZ-043 (2, MW 431; LC/MS M + 1, 432) in MeOH or THF/MeOH instantly generated the deep orange solution characteristic of the previously reported Meisenheimer complexes. After 10 min, the reaction was quenched by addition of excess acetic acid relative to the amount of NaBD4 used. LC/MS analysis of an aliquot of the resulting solution revealed clean formation of a mixture of partially deuterated Meisenheimer derivatives 6a(D) and 6b(D) with a major M + 1 peak at m/z = 435 (Scheme 3). The solution was then allowed to stir at room temperature exposed to air and was periodically monitored by LC/MS, which indicated slow (24 h), but clean, oxidation of Meisenheimer complexes 6a(D) and 6b(D) to partially deuterated BTZ043, 2D. Extractive workup after 24 h afforded 91% of deuterated BTZ043 (2D). Proton NMR analysis revealed partial deuterium substitution ortho (57% D) and para (24% D) to the nitro group reflecting the differential reactivity at the two positions. A portion of the isolated partially deuterated material was resubjected to the reaction with NaBD4 to again give a dark orange solution of Meisenheimer complexes. After 5 min, the reaction was quenched with acetic acid and allowed to stir in air. As expected, reoxidation to BTZ043 with enhanced deuterium incorporation was slower. After 3 days, workup gave a 95% recovery of D-BTZ043 (2D). Proton NMR analysis indicated additional deuterium incorporation at the ortho and para positions. Repetition of the reduction/reoxidation process further enhanced the deuterium incorporation but since the reoxidation process in air required several days, alternative reoxidation methods were sought.
Scheme 3. Reduction of BTZ043 with NaBD4 Incorporates Deuterium into the BTZ Core.
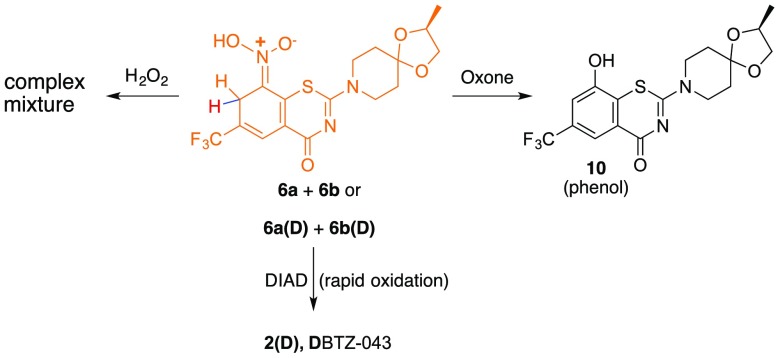
Treatment of the mixture of Meisenheimer complexes (6a + 6b) with hydrogen peroxide resulted in decomposition. Interestingly, treatment of 6a + 6b with oxone generated the corresponding phenol (10, Scheme 4) rather than reforming the aromatic nitro compound (2). However, reaction of 2 (BTZ043) with NaBD4 as before but with subsequent addition of diisopropylazodicarboxylate (DIAD) induced immediate loss of the orange color characteristic of the mixture of Meisenheimer complexes. LC/MS and HNMR analyses revealed formation of partially deuterated BTZ (2D) (Scheme 4). Thus, rather than waiting days at room temperature for the slow reoxidation process, the reoxidation could be completed quickly. In fact, it was possible to repeat the reduction/oxidation sequence 10 times within a single day to give 2D (D-BTZ043) in a 45% overall yield and enhanced deuterium incorporation (94% ortho and 83% para) for the 10-fold process.
Scheme 4. Alternative Reoxidation of Meisenheimer Complexes.
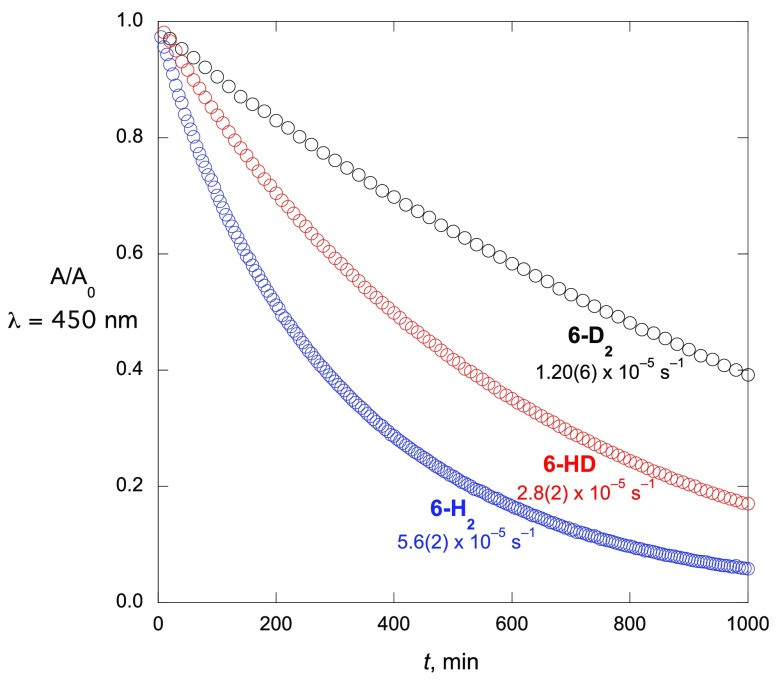
In order to probe the isotope effect on the reoxidation of BTZ043, varying isotopomers of the Meisenheimer complexes 6 were generated and the kinetics of their decomposition studied by UV–visible spectroscopy. The protio compound 6-H2 was generated in methanol by reduction of 2 with NaBH4, followed by quenching with acetic acid, and then allowed to react at 36 °C while its optical spectrum was monitored. A compound with one deuterium and one protium at the sp3-hybridized carbon, 6-HD, was generated by analogous reduction of 2D with NaBH4, while compounds with two deuterium atoms at the sp3-hybridized carbon, 6-D2, were prepared from 2D and NaBD4. In all cases, reactions were carried out under air, though control experiments showed that reactions were only about 30% faster under air than under argon at this temperature.
Reactions in all cases displayed clean first-order kinetics with no sign of biphasic behavior (Figure 2). This suggests that rate differences between 6a and 6b under these conditions are not large. As expected, increasing deuteration leads to significantly decreased rates of reaction, with the kinetic isotope effect (KIE) on monodeuteration kHH/kHD = 2.00 ± 0.15 and the KIE on dideuteration kHH/kDD = 4.7 ± 0.3. The magnitude of the latter KIE indicates the presence of a primary isotope effect, confirming that the C–H bond to the sp3-hybridized carbon in 6 is broken in the rate-determining step of rearomatization. However, the monodeutero compound reacts appreciably more slowly than expected if only a primary isotope effect were present (as an isotope effect kHH/kHD can only be as high as 2.00 if the primary isotope effect were so large that essentially only the C–H bond, not the C–D bond, were ever broken on reaction of 6). This suggests that there must be a significant normal secondary isotope effect in the reaction, and indeed normal secondary isotope effects are often observed in reactions where the reactive carbon is rehybridized from sp3 to sp2 in the transition state of the rate-determining step.23 The experimental data are consistent with a primary KIE of 3.2 ± 0.6 and a secondary KIE of 1.46 ± 0.26 in the rearomatization reaction. While primary KIEs have garnered the majority of attention in modulating drug properties, the existence of a significant secondary KIE is of potential interest as well, particularly for a drug like BTZ043, where protium is introduced in vivo in a critical reactive position, so the effect of deuteration may well be carried to a significant extent by its secondary, rather than primary, isotope effect. In this case, for example, the 2-fold longer lifetime of 6-HD compared to 6-H2 is 69% due to the primary KIE, but 31% of the increase in lifetime is due to the secondary KIE.
Figure 2.
Reactions of isotopomers of 6 monitored at 450 nm (CH3OH, 36 °C).
Both origina1 BTZ043 (2) and the final deuterium enriched sample (2D) were tested for antituberculosis activity and, as expected, had identical potent activity in whole cell assays (<0.004 μM each in MABA media and 0.02–0.03 μM in GAS media using H37RV cells).
In conclusion, reduction of the potent anti-TB agent, BTZ043, with deuteride followed by reoxidation incorporates deuterium into the nitroaromatic warhead. The deuterated products are rapidly reduced by hydride to form Meisenheimer complexes that more slowly regenerate the deuterated aromatic core/warhead.
Acknowledgments
This work was supported in part by NIH grants R01 AI054193 and R37 AI054193 and the University of Notre Dame (George & Winifred Clark professorship, to M.J.M.). We gratefully acknowledge Prof. Scott Franzblau, University of Illinois, Chicago, for the anti-TB assays.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.9b00308.
General experimental details, kinetic studies, and 1HNMR and HRMS data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Harbeson S. L.; Tung R. D. Deuterium Medicinal Chemistry: An New Approach to Drug Discovery and Development. MedChem. News 2014, 2, 8–22. [Google Scholar]
- Harbeson S. L.; Tung R. D. (ed. , Chapter 24: Deuterium in Drug Discovery and Development in Annu. Rep. Med. Chem.; Macor J. E., Ed.; Elsevier: Oxford, UK, 2011; Vol. 46, pp 403–417. [Google Scholar]
- World Health Organization . Global Tuberculosis Report 2017. [Google Scholar]
- https://www.tballiance.org/why-new-tb-drugs/global-pandemic (accessed June 6, 2019).
- Diacon A. H.; Pym A.; Grobusch M.; Patientia R.; Rustomjee R.; Page-Shipp L.; Pistoriius C.; Krause R.; Bogoshi M.; Churchyard G.; Venter A.; Allen J.; Palomino J. C.; De Marez T.; van Heeswijk R. P. G.; Lounis N.; Meyvisch P.; Verbeeck J.; Parys W.; de Beule K.; Andries K.; McNeeley D. F. The Diarylquinoline TMC207 for Multidrug-Resistant Tuberculosis. N. Engl. J. Med. 2009, 360, 2397–2405. 10.1056/NEJMoa0808427. [DOI] [PubMed] [Google Scholar]
- Andries K.; Verhasselt P.; Guillemont J.; Gohlmann H. W. H.; Neefs J. M.; Winkler H.; Van Gestel J.; Timmerman P.; Zhu M.; Lee E.; Williams P.; de Chaffoy D.; Huitric E.; Hoffner S.; Cambau E.; Truffot-Pernot C.; Lounis N.; Jarlier V. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 2005, 307, 223–227. 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- Furin J.; Diacon A. H.; Andries K. Combatting drug-resistant tuberculosis: the unexpected benefits of bedaquiline. Int. J. Tuberc. Lung Dis 2017, 21, 4–5. 10.5588/ijtld.16.0768. [DOI] [PubMed] [Google Scholar]
- Horsburgh C. R. Jr; Haxaire-Theeuwes M.; Lienhardt C.; Wingfield C.; McNeeley D.; Pyne-Mercier L.; Keshavjee S.; Varaine F. Research Excellence To Stop TB Resistance (RESIST-TB); Critical Path to TB Drug Regimens’ Access and Appropriate Use Workgroup. Compassionate use of and expanded access to new drugs for drug-resistant tuberculosis. Int. J. Tuberc Lung Dis 2013, 17, 146–152. 10.5588/ijtld.12.0017. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Policy implementation package for new TB drug introduction, 2014. http://www.who.int/tb/PIPnewTBdrugs.pdf?ua1/41 (accessed June 10, 2019).
- Mikusova K.; Ekins S. Learning from the past for TB drug discovery in the future. Drug Discovery Today 2017, 22, 534–545. 10.1016/j.drudis.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarov V.; Manina G.; Mikusova K.; Möllmann U.; Ryabova O.; Saint-Joanis B.; Dhar N.; Pasca M. R.; Buroni S.; Lucarelli A. P.; Milano A.; De Rossi E.; Belanova M.; Bobovska A.; Dianiskova P.; Kordulakova J.; Sala C.; Fullam E.; Schneider P.; McKinney J. D.; Brodin P.; Christophe T.; Waddell S.; Butcher P.; Albrethsen J.; Rosenkrands I.; Brosch R.; Nandi V.; Bharath S.; Gaonkar S.; Shandil R. K.; Balasubramanian V.; Balganesh T.; Tyagi S.; Grosset J.; Riccardi G.; Cole S. T. Benzothiazinones kill Mycobacterium tuberculosis by blocking arabinan synthesis. Science 2009, 324, 801–804. 10.1126/science.1171583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarov V.; Lechartier B.; Zhang M.; Neres J.; van der Sar A. M.; Raadsen S. A.; Hartkoorn R. C.; Ryabova O. B.; Vocat A.; DecosterdL A.; Widmer N.; Buclin T.; Bitter W.; Andries K.; Pojer F.; Dyson P. J.; Cole S. T. Towards a new combination therapy for tuberculosis with next generation benzothiazinones. EMBO Mol. Med. 2014, 6, 372–383. 10.1002/emmm.201303575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://clinicaltrials.gov/ct2/show/NCT03334734 (accessed May 24, 2019).
- Makarov V.; Riabova O. B.; Yuschenko A.; Urlyapova N.; Daudova A.; Zipfel P. F.; Möllmann U. Synthesis and antileprosy activity of some dialkyldithiocarbamates. J. Antimicrob. Chemother. 2006, 57, 1134–1138. 10.1093/jac/dkl095. [DOI] [PubMed] [Google Scholar]
- Neres J.; Pojer F.; Molteni E.; Chiarelli L. R.; Dhar N.; Boy-Rottger S.; Buroni S.; Fullam E.; Degiacomi G.; Lucarelli A. P.; Read R. J.; Zanoni G.; Edmondson D. E.; De Rossi E.; Pasca M. R.; McKinney J. D.; Dyson P. J.; Riccardi G.; Mattevi A.; Cole S. T.; Binda C. Structural basis for benzothiazinone-mediated killing of Mycobacterium tuberculosis. Sci. Transl. Med. 2012, 4, 150ra121. 10.1126/scitranslmed.3004395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecik M.; Centárová I.; Mukherjee R.; Kolly G. S.; Huszár S.; Bobovská A.; Kilacsková E.; Mokošová V.; Svetlíková Z.; Šarkan M.; Neres J.; Korduláková J.; Cole S. T.; Mikušová K. DprE1 is a vulnerable tuberculosis drug target due to its cell wall localization. ACS Chem. Biol. 2015, 10, 1631–1636. 10.1021/acschembio.5b00237. [DOI] [PubMed] [Google Scholar]
- Trefzer C.; Rengifo-Gonzalez M.; Hinner M. J.; Schneider P.; Makarov V.; Cole S. T.; Johnsson K. Benzothiazinones: prodrugs that covalently modify the decaprenylphosphoryl-β-Dribose 2′-epimerase DprE1 of Mycobacterium tuberculosis. J. Am. Chem. Soc. 2010, 132, 13663–13665. 10.1021/ja106357w. [DOI] [PubMed] [Google Scholar]
- de Jesus Lopes Ribeiro A. L.; Degiacomi G.; Ewann F.; Buroni S.; Incandela M. L.; Chiarelli L. R.; Mori G.; Kim J.; Contreras-Dominguez M.; Park Y.-S.; Han S.-J.; Brodin P.; Valentini G.; Rizzi M.; Riccardi G.; Pasca M. R. Analogous mechanisms of resistance to benzothiazinones and dinitrobenzamides in Mycobacterium smegmatis. PLoS One 2011, 6, e26675 10.1371/journal.pone.0026675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari R.; Moraski G. C.; Krchnak V.; Miller P. A.; Colon-Martinez M.; Herrero E.; Oliver A. G.; Miller M. J. Thiolates chemically induce redox activation of BTZ043 and related potent nitroaromatic anti-tuberculosis agents. J. Am. Chem. Soc. 2013, 135, 3539–3549. 10.1021/ja311058q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloss F.; Krchnak V.; Krchnakova A.; Schieferdecker S.; Dreisbach J.; Krone V.; Möllmann U.; Hoelscher M.; Miller M. J. In Vivo Dearomatization of the Potent Antituberculosis Agent BTZ043 via Meisenheimer Complex Formation. Angew. Chem., Int. Ed. 2017, 56, 2187–2191. 10.1002/anie.201609737. [DOI] [PubMed] [Google Scholar]
- Errede L. A.; Davis H. R. The Anomalous behavior of m-trifluroomethylnitrosobenzene dimer. J. Org. Chem. 1963, 28, 1430–1431. 10.1021/jo01040a534. [DOI] [Google Scholar]
- Suwiński J.; Wagner P.; Holt E. M. Reduction of aromatic nitrocompounds by sodium borohydride in methanol in the presence of sodium methoxide. Tetrahedron 1996, 52, 9541–9552. 10.1016/0040-4020(96)00491-7. [DOI] [Google Scholar]
- Carey F. A.; Sundberg R. J.. Advanced Organic Chemistry Part A: Structure and Mechanisms, 5th ed.; Springer: New York, 2007; Chapter 3.5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.