Important Compound Classes
Title
IRAK Degraders and Uses Thereof
Patent Application Number
WO 2019/133531 A1
Publication Date
July 04, 2019
Priority Application
US 62/712,377
Priority Date
July 31, 2018
Inventors
Mainolfi, N.; Ji, N.; Kluge, A. F.; Weiss, M. M.; Zhang, Y.
Assignee Company
Kymera Therapeutics, Inc.; 400 Technology Square, 10th Floor, Cambridge, Massachusetts 02139 (US).
Disease Area
Cancer
Biological Target
Interleukin-1 Receptor-Associated Kinase (IRAK)
Summary
The interleukin-1 receptor-associated kinase 4 (IRAK4) is a serine/threonine kinase that performs scaffolding and phosphorylation in toll-like receptor (TLR) and interleukin-1 receptor (IL-1R) signaling pathways. TLR and IL-1R are examples of transmembrane pattern recognition that are critical in the detection of pathogen-associated molecular pattern and form a first line of response to infection in mammals, which helps to distinguish between self- and nonself-antigens. Ligand binding to either IL-1R or TLR leads to dimerization and recruitment of adaptor molecules that form a conserved motif on the cytoplasmic domain that enables the recruitment of MyD88 and the formation of myddosome complex such as the interleukin-1 receptor activated kinase (IRAK) family. The first step in the signal transduction of propagating the innate immune response of these kinases is the autophosphorylation of IRAK4, which leads to IRAK1 phosphorylation, consequently leading to downstream signaling through multiple cascades. IRAK2 and IRAK3 are catalytically inactive kinases. Individuals who lack IRAK4 show impaired activation of the innate immune response, however showed no increase in susceptibility to viral or fungal infection. Selective small molecule inhibitors of IRAK4 may have anti-inflammatory activity given the high degree of sequence homology between the ATP binding sites of IRAK1 and IRAK4. The dysregulation of TLR signaling has been implicated in autoinflammatory diseases such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). Further discussion of IRAK4 also can be found in a recent ACS Med. Chem. Lett. Patent Highlight (https://pubs.acs.org/doi/10.1021/acsmedchemlett.9b00385).
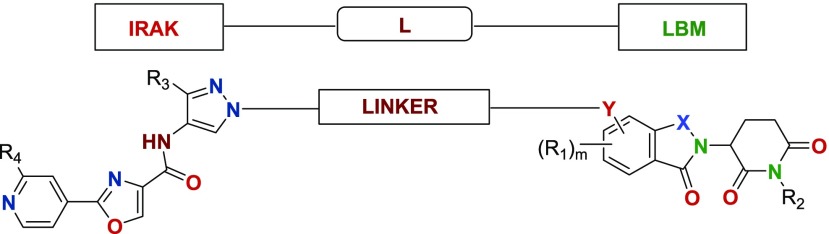
The ubiquitin-proteasome pathway (UPP) is a critical pathway that regulates regulatory proteins and degrades misfolded or abnormal proteins. The defectiveness or imbalanced of UPP leads to pathogenesis of a variety of diseases. The covalent attachment of ubiquitin to specific protein substrates is achieved via the action of the E3 ubiquitin ligases. Recent drug development has focused on E3 ubiquitin ligases as more attractive therapeutic targets with specific ligand binding motifs. There are over 600 E3 ubiquitin ligases that facilitate the ubiquitination of different proteins in vivo, and they comprise four families: U-box E3s, HECT-domain, monomeric RING, and multisubunits E3s. Small molecule therapeutic agents that utilize E3 ligase mediated protein degradation to target cancer-associated proteins such as IRAK provide promising therapeutic agents. This Patent Highlight consists of bifunctional compounds, which function to recruit IRAK kinases to E3 ubiquitin ligase for degradation, which may provide a broad range of pharmacological activities, including the treatment or amelioration of a disease or conditions associated with regulation of signaling pathways implicating IRAK kinases, such as cancer. IRAK represents an IRAK binding moiety capable of binding to one or more of IRAK-1, -2, -3, or -4. L is a bivalent or linker moiety that connects IRAK to a ligase binding moiety (LBM).
Key Structures
Definitions
X = NH or O;
Y = CH2, O, NH, alkynyl;
R1 = (C1–C4) alkyl, (C3–C6) cycloalkyl, heterocyclyl;
R2 = H, CH3;
R3 = CONH2; CF3, CHF2, CH2NH2;
R4 = H, CH3, NHCH2CF3; NHCH2cyclopropyl;
n = 0, 1, 2, 3 or 4.
Biological Assay
The human peripheral blood mononuclear cells (PMBCs) degradation assay. THP-1 and OCI-LY-10 degradation assay.
Biological Data
The compounds in this Patent Highlight
shows % IRAK4 degradation, where percentage of IRAK4 degraded after
4 h across three different concentrations. The Table below shows degradation
in which A = >50%, B = >20–50%; C = <20%.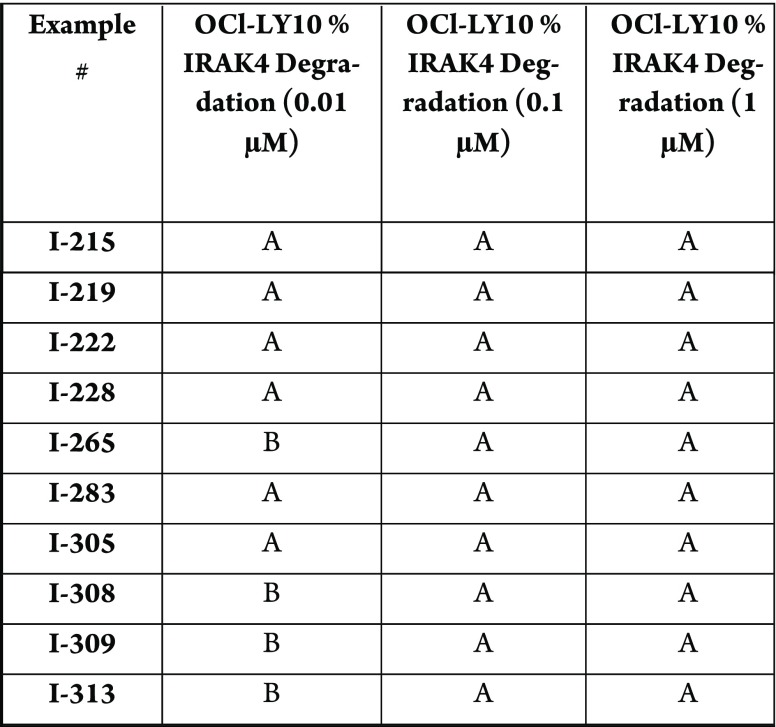
Recent Review Articles
-
1.
McElroy W. T.Expt. Opin. Ther. Pat. 2019, 29, 243.
-
2.
Mortaz E.; Adcock I. M.; Tabarsi P.; Darazam I. A.; Movassaghi M.; Garssen J.; Jamaati H.; Velayati A.. Eur. J. Pharmacol. 2017, 808, 49.
-
3.
Patra M. C.; Choi S.. Molecules. 2016, 21, 1529.
-
4.
Chaudhary D.; Robinson S.; Romero D. L.. J. Med. Chem. 2015, 58, 96.
-
5.
Jain A.; Kaczanowska S.; Davila E.. Front. Immunol. 2014, 5, 1.
The author declares no competing financial interest.