Abstract
Purpose
To estimate the proportion of children with trachomatous inflammation—follicular (TF) and adults with trachomatous trichiasis (TT) in internally displaced persons (IDP) camps in the Darfur States of Sudan and to evaluate associated risk factors.
Methods
IDP camps were identified from government census data. We conducted a subanalysis of data collected in these camps during 2014–2015 as part of surveys covering 37 districts of the Darfur States within the Global Trachoma Mapping Project. A random-effects hierarchical model was used to evaluate factors associated with TF in children or TT in adults.
Results
Thirty-six IDP camps were represented in the survey data, in which 1926 children aged 1–9 y were examined, of whom 38 (8%) had TF. Poor sanitation, younger age and living in a household that purchased water from a vendor were associated with TF in children aged 1–9 y. Of 2139 individuals examined aged ≥15 y, 16 (0.7%) had TT. TT was strongly independently associated with being older and living alone.
Conclusion
Trachoma is found at low levels in these camps, but still at levels where intervention is needed. Disease elimination in conflict-related settings presents a unique challenge for the trachoma community, and may require an innovative approach. Understanding how best to undertake trachoma elimination interventions in these areas should be prioritized.
Keywords: Darfur, Global Trachoma Mapping Project, prevalence, Sudan, trachoma, trichiasis
Introduction
The United Nations Office for Coordination of Humanitarian Affairs (UNOCHA) estimates that in 2014 more than 59 million people worldwide were displaced from their homes due to conflict or insecurity.1 Over 38 million of these were internally displaced persons (IDPs) still living within the borders of their own country.2 Displaced persons present unique challenges to health systems, having an increased risk of infectious disease,3–5 malnutrition related to food and water insecurity,6–8 trauma-related psychiatric disorders9–11 and maternal mortality.12,13 Even where healthcare needs could otherwise be managed, large-scale interventions are made difficult by ongoing risks of violence3,14,15 and the itinerant nature of the population.
Trachoma is an eye disease that blinds through recurrent16,17 conjunctival infection with the bacterium Chlamydia trachomatis. It is the most common infectious cause of blindness worldwide,18 affecting the world’s poorest and most vulnerable populations.19 Infection is spread from eye to eye directly by touch, or indirectly through fomites or eye-seeking flies of the Musca genus. Female Musca spp. flies preferentially lay eggs on human feces left exposed on the soil.20 Trachoma is associated with low levels of access to water, sanitation and hygiene (WASH), being found in areas of extreme poverty across the globe.21
WHO has targeted trachoma for elimination by 202022 using the SAFE strategy. This consists of Surgery for those with advanced disease, Antibiotic treatment, Facial cleanliness and Environmental improvement in endemic areas.23 Recommendations for the A, F and E interventions are stratified by the prevalence of the sign trachomatous inflammation—follicular (TF), with (for example) areas having a prevalence of TF ≥10% in children aged 1–9 y requiring mass drug administration (MDA) of antibiotics, plus implementation of the F and E components of SAFE for at least 3 y before review.21,24 In theory, F reduces the community-level volume of infected secretions available for transfer from eye to eye25; E, which involves increasing access to water and sanitation, facilitates facial cleanliness and reduces the number of breeding sites for Musca spp.26 However, the evidence base for the F and E interventions is significantly weaker than that for S and A.27–29
The Darfur States in Sudan have been affected by conflict and population displacement since the outbreak of civil war in 2003. UNOCHA estimates that as of December 2015 there were 2.7 million displaced people in Darfur out of a total population of 8.8 million.30,31 Darfur comprises five states in the West of Sudan: North, East, West, South and Central Darfur.
Recent trachoma surveys conducted in the Darfur States justify antibiotic MDA in five districts found to have a high prevalence of TF in children. These surveys included IDP camps, and found that living in an IDP camp was a strong independent risk factor for TF, with children living in these camps having odds of TF 2.6 times higher than non-IDP camp-resident children.32 In this paper, we conduct a subanalysis of this IDP camp population to identify independent risk factors that might explain this increased risk of trachoma in the IDP camp population. We report the trachoma prevalence and between-camp risk factors associated with disease.
Methods
Study design
We conducted a secondary analysis of data from 27 cross-sectional population-based trachoma prevalence surveys carried out in the Darfur States during 2014–2015. Full details of the methodologies used in the original surveys are presented elswhere.32–34 IDP camps were identified from these primary data sets by cross-referencing government census data.
Water and sanitation access
Household-level data on access to water supply for drinking and washing, and sanitation facilities, were collected by field teams at the time of the survey by a focused interview with the head of the household and by direct observation. Water and sanitation access was recorded by a combination of direct observation of structures and responses to questions, depending on whether local or remote structures were used; reported use was obtained by interviewing the head of the household. Recorders were trained to identify water and sanitation infrastructure and access categories based on the WHO/UNICEF Joint Monitoring Programme for Water Supply, Sanitation and Hygiene definitions, which were used up to 2015.35,36 GPS coordinates were recorded at the front entrance to each household.
Climate variables
Data on local climate (at 2.5 arc-min resolution ~5 km) were downloaded from WorldClim BioClim variables (worldclim.org). Annual mean rainfall and mean temperature, maximum temperature of the hottest month, and major landcover type, were considered as variables that could potentially influence infection transmission and therefore disease prevalence. Values were extracted from available rasters at the cluster level, defined as the mean easting and mean northing GPS coordinates of all households in a given cluster.
Statistical analysis
Multilevel logistic regression was used in order to account for a change in the variance in the outcome variable between the different population levels.37 Data were collected from units at three different levels (state, camp and household). Outcomes were evaluated for potential clustering of both TF and trachomatous trichiasis (TT) at state, camp and household levels. If no clustering was identified, a standard logistic regression model was used. Univariable associations were considered for inclusion in the multivariate model if p≤0.05 (Wald’s χ2 test). A stepwise-inclusion approach was used, with variables retained in the model if significant at the p≤0.05 level (likelihood ratio test). All risk factor analysis was carried out in Stata 10.2 (Stata Corp LP, College Station, TX, USA).
Results
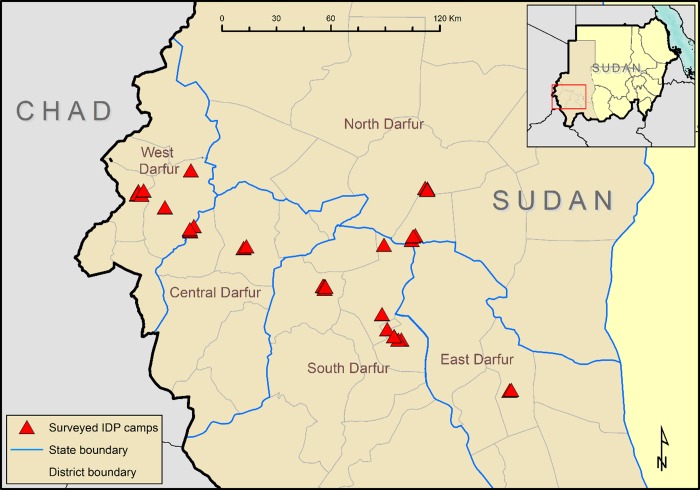
A total of 36 IDP camp clusters within 11 districts were identified in 27 surveys in the Darfur States (Table 1, Figure 1). In principle, an IDP camp should be a transient place of residence for individuals who have been forced to migrate due to (for example) civil unrest or famine. In reality, some camps here were settled decades ago, with a proportion inhabited by those who migrated from what is now South Sudan, and they may have little hope of return. Because of the method of selection we used, all included data were by definition from IDP camps that were formally recognized by the government of Sudan, and so were not likely to be recent settlements.
Table 1.
Numbers sampled, examined, absent, refused and showing trachomatous inflammation—follicular (TF) or trachomatous trichiasis (TT), internally displaced persons camps, Darfur States, Sudan, 2014–2015
| State | District (camp#) | Households | Examined | Absent | Refused/other | Total | 1–9-y-olds | 10–14-y-olds | ≥15-y-olds | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TF | TT | Examined | TF | TT | Examined | TF | TT | Examined | |||||||
| Central Darfur | Azoom | 30 | 103 | 3 | 0 | 106 | 0 | 0 | 45 | 0 | 0 | 6 | 0 | 4 | 55 |
| Zalinji (1) | 30 | 115 | 38 | 1 | 154 | 0 | 0 | 48 | 0 | 0 | 20 | 0 | 0 | 86 | |
| Zalinji (2) | 30 | 93 | 26 | 0 | 119 | 11 | 0 | 49 | 0 | 0 | 19 | 0 | 0 | 51 | |
| East Darfur | El Daein (East) (1) | 30 | 130 | 0 | 0 | 130 | 4 | 0 | 76 | 0 | 0 | 13 | 0 | 0 | 41 |
| El Daein (East) (2) | 30 | 94 | 19 | 2 | 115 | 0 | 0 | 47 | 0 | 0 | 15 | 0 | 0 | 53 | |
| El Daein (East) (3) | 29 | 97 | 56 | 0 | 153 | 0 | 0 | 63 | 0 | 0 | 21 | 0 | 0 | 69 | |
| North Darfur | El Fashir (1) | 30 | 102 | 14 | 0 | 116 | 7 | 0 | 62 | 1 | 0 | 21 | 1 | 1 | 54 |
| El Fashir (2) | 31 | 109 | 0 | 0 | 109 | 2 | 0 | 54 | 0 | 0 | 1 | 0 | 0 | 61 | |
| El Fashir (3) | 31 | 112 | 2 | 0 | 114 | 0 | 0 | 43 | 0 | 0 | 12 | 0 | 0 | 55 | |
| El Fashir (4) | 29 | 98 | 9 | 0 | 107 | 2 | 0 | 47 | 0 | 0 | 15 | 0 | 0 | 54 | |
| Dar El Salam (1) | 30 | 109 | 28 | 0 | 137 | 4 | 1 | 39 | 0 | 0 | 26 | 0 | 1 | 44 | |
| Dar El Salam (2) | 30 | 104 | 12 | 0 | 116 | 14 | 0 | 57 | 0 | 0 | 11 | 0 | 1 | 46 | |
| Dar El Salam (3) | 30 | 101 | 9 | 0 | 110 | 9 | 0 | 48 | 0 | 0 | 2 | 0 | 1 | 57 | |
| South Darfur | Kas (1) | 30 | 114 | 22 | 0 | 136 | 1 | 0 | 47 | 0 | 0 | 9 | 0 | 0 | 53 |
| Kas (2) | 31 | 101 | 29 | 0 | 130 | 7 | 0 | 43 | 1 | 0 | 15 | 0 | 0 | 80 | |
| Kas (3) | 31 | 140 | 0 | 0 | 140 | 6 | 0 | 24 | 1 | 0 | 12 | 0 | 0 | 66 | |
| Kas (4) | 30 | 144 | 4 | 0 | 148 | 1 | 0 | 56 | 0 | 0 | 9 | 0 | 0 | 52 | |
| Kas (5) | 30 | 147 | 15 | 0 | 162 | 13 | 0 | 72 | 0 | 0 | 8 | 0 | 1 | 56 | |
| Kas (6) | 30 | 135 | 16 | 0 | 151 | 6 | 0 | 50 | 0 | 0 | 13 | 0 | 1 | 67 | |
| Nyala City (1) | 30 | 116 | 33 | 0 | 149 | 6 | 0 | 69 | 0 | 0 | 8 | 0 | 1 | 63 | |
| Belale (1) | 30 | 109 | 0 | 0 | 109 | 6 | 0 | 67 | 0 | 0 | 11 | 0 | 2 | 70 | |
| Belale (2) | 31 | 113 | 25 | 0 | 138 | 0 | 0 | 69 | 1 | 0 | 29 | 0 | 0 | 64 | |
| Belale (3) | 29 | 82 | 20 | 0 | 102 | 1 | 0 | 74 | 0 | 0 | 11 | 0 | 0 | 66 | |
| Belale (4) | 30 | 117 | 0 | 0 | 117 | 1 | 0 | 69 | 0 | 0 | 16 | 0 | 1 | 64 | |
| Unitty (1) | 30 | 122 | 8 | 1a | 131 | 0 | 0 | 47 | 0 | 0 | 22 | 0 | 0 | 62 | |
| Unitty (2) | 30 | 98 | 19 | 0 | 117 | 0 | 0 | 43 | 0 | 0 | 15 | 0 | 0 | 59 | |
| West Darfur | El Jinaina (1) | 30 | 72 | 40 | 0 | 112 | 1 | 0 | 36 | 0 | 0 | 6 | 0 | 0 | 70 |
| El Jinaina (2) | 30 | 139 | 26 | 0 | 165 | 1 | 0 | 74 | 0 | 0 | 19 | 0 | 0 | 72 | |
| El Jinaina (3) | 30 | 77 | 21 | 0 | 98 | 8 | 0 | 41 | 0 | 0 | 9 | 0 | 0 | 48 | |
| El Jinaina (4) | 30 | 96 | 40 | 0 | 136 | 21 | 0 | 59 | 0 | 0 | 14 | 0 | 0 | 63 | |
| El Jinaina (5) | 30 | 75 | 38 | 0 | 113 | 3 | 0 | 37 | 0 | 0 | 13 | 0 | 1 | 63 | |
| El Jinaina (6) | 29 | 85 | 23 | 0 | 108 | 3 | 0 | 41 | 0 | 0 | 17 | 0 | 0 | 50 | |
| Kreanik (1) | 29 | 133 | 8 | 0 | 141 | 0 | 0 | 71 | 0 | 0 | 16 | 0 | 0 | 54 | |
| Kreanik (2) | 30 | 103 | 36 | 0 | 139 | 0 | 0 | 65 | 0 | 0 | 13 | 0 | 0 | 61 | |
| Kreanik (3) | 30 | 96 | 29 | 0 | 125 | 0 | 0 | 49 | 0 | 0 | 20 | 0 | 1 | 56 | |
| Kreanik (4) | 30 | 96 | 7 | 0 | 103 | 0 | 0 | 45 | 0 | 0 | 4 | 0 | 0 | 54 | |
| Total | 1080 | 3877 | 675 | 4 | 4556 | 138 | 1 | 1823 | 4 | 0 | 362 | 1 | 16 | 1692 | |
aOther: reason not specified
Figure 1.
Internally displaced persons (IDP) camps surveyed as part of the Global Trachoma Mapping Project, Darfur States, Sudan, 2014–2015.
A total of 1080 households and 4556 individuals were enumerated in the 36 IDP camp clusters (Table 2), of whom 3877 individuals (85%) were present and consented to examination. A total of 1926 children aged 1–9 y were enumerated over all identified IDP camps, with 1823 (95%) present and consenting to examination. The median age of participants of all ages was 13 y (range 1–100 y) and 56% were female.
Table 2.
Study population characteristics
| Parameter | Number, N |
|---|---|
| States | 5 |
| Districts | 13 |
| Internally displaced persons camps | 36 |
| Households | 1080 |
| Population sampled | 4556 |
| Female (%) | 2548 (56%) |
| Children aged 1–9 y | 1926 |
| Median individuals examined/camp (IQR) | 111.5 (98–122) |
| Median children aged 1–9 y/camp (IQR) | 42.5 (23–47.5) |
| Camp land cover grassland (%) | 28 (78%) |
| Median annual rainfall (mm) | 453 |
| Median annual mean temperature (°C) | 25.5 |
| Median temperature of hottest month (°C) | 38.6 |
| Median individuals/household | 3 |
| Median number of 1–9-y-olds/household | 2 |
Proportion of TF
Of 1823 children aged 1–9 y examined, 138 (8%) had TF and 4 (0.2%) had trachomatous inflammation—intense (TI). All four cases of TI also had TF. The proportion of children with TF aged 1–9 y in each camp ranged from 0 to 40%. No cases of TF were found in 11 of the 36 camps.
Clustering of TF
A null model for TF adjusted for age and gender showed statistically significant clustering at state, camp and household levels. The adjusted standard deviation in the odds due to between-state clustering was 0.38 (SE 0.20, p=0.01), due to between-camp clustering was 1.54 (SE 0.28, p<0.0001), and due to between-household clustering was 2.35 (SE 0.31, p<0.0001). The model adjusting for clustering at household level only was a better fit than models also accounting for state and camp clustering. All subsequent analyses on TF presented in this paper are from two-level hierarchical models with adjustment for household-level clustering.
Proportion of TT
Of 2139 individuals examined aged ≥15 y, 16 (0.7%) had TT. The camp-level prevalence of TT ranged from 0% up to a maximum of 7.3% (4/55) of those aged ≥15 y in an IDP camp in the Azoom district of Central Darfur.
Clustering of TT
A null model for TT adjusted for age and gender showed no statistically significant clustering at state, camp or household levels. All subsequent analyses on TT presented in this paper are from logistic regression models.
Factors associated with TF in children aged 1–9 y
Univariable associations of TF are presented in Table 3. In the final multivariable model, TF was independently associated with being aged 1–4 y (OR 1.7, 95% CI 1.1 to 2.7); practicing open defecation (OR 3.1, 95% CI 1.1 to 8.6) and accessing a shared (as opposed to a private household) latrine (OR 3.0, 95% CI 1.1 to 8.5). There was also a strong independent association between TF and obtaining household drinking water from a water vendor (OR 9.9, 95% CI 2.1 to 46.8) compared with obtaining water from an improved source (Table 4).
Table 3.
Univariable associations of trachomatous inflammation–follicular (TF) in children aged 1–9 y, internally displaced persons camps, Darfur States, Sudan, 2014–2015
| Individual | TF (%) | N (%) | OR (95% CI)a | p-valueb |
|---|---|---|---|---|
| Age | ||||
| 1–4 y | 81 (8.9) | 912 (50.0) | 1.71 (1.07–2.72) | <0.0001 |
| 5–9 y | 57 (6.3) | 911 (50.0) | ||
| Gender | ||||
| Male | 68 (7.4) | 923 (50.6) | 1 | 0.754 |
| Female | 70 (7.8) | 900 (49.4) | 1.07 (0.69–1.68) | |
| Household | ||||
| Household size | ||||
| ≥8 members | 19 (8.0) | 236 (13.0) | 1.28 (0.49–3.33) | 0.613 |
| 1–7 members | 119 (7.5) | 1587 (87.0) | 1 | |
| Number of resident children aged 1–9 y | ||||
| ≥5 | 12 (6.0) | 200 (11.0) | 0.61 (0.19–1.91) | 0.395 |
| 1–4 | 126 (7.8) | 1623 (89.0) | 1 | |
| Water access c | ||||
| Main source of water for drinking | ||||
| Improved sourced | 69 (5.7) | 1210 (66.4) | 1 | 0.0095 |
| Unimproved sourcee | 12 (3.5) | 343 (18.8) | 0.4 (0.1–2.3) | |
| Water vendor | 57 (21.1) | 270 (14.8) | 7.8 (1.7–34.9) | |
| Time to main source of drinking water | ||||
| Up to 30 min round-trip | 103 (8.0) | 3245 (71.2) | 1 | 0.2088 |
| ≥30 min | 35 (6.5) | 1311 (28.8) | 0.4 (0.1–1.8) | |
| Main source of water for washing | ||||
| Improved sourced | 69 (5.7) | 1209 (66.3) | 1 | 0.0094 |
| Unimproved sourcee | 12 (3.5) | 344 (18.9) | 0.4 (0.1–2.2) | |
| Water vendor | 57 (21.1) | 270 (14.8) | 7.8 (1.7–34.8) | |
| Time to main source of washing water | ||||
| Up to 30 min round-trip | 103 (8.0) | 1284 (70.4) | 1 | 0.2514 |
| ≥30 minutes | 35 (6.5) | 539 (29.6) | 0.4 (0.1–2.0) | |
| Latrine access c | ||||
| Private latrine | 38 (5.2) | 744 (40.8) | 1 | 0.0357 |
| Latrine facilities absent (open defecation) | 43 (6.4) | 674 (37.0) | 2.86 (1.02–8.05) | |
| Shared latrine access | 57 (14.1) | 405 (22.2) | 3.27 (1.15–9.25) | |
| Latrine typef | ||||
| Pit latrine with slab (improved pit latrine) | 20 (4.7) | 425 (23.3) | 1 | 0.1691 |
| Pit latrine without slab (unimproved pit latrine) | 73 (10.0) | 728 (39.9) | 1.3 (0.4–4.0) | |
| No facilities, bush or field | 45 (6.8) | 660 (36.2) | 2.9 (0.9–9.5) | |
| Otherg | 0 (0.0) | 10 (0.5) | - | |
| Camp | ||||
| Climate/environment k | ||||
| Rainfalll | ||||
| Desert (annual rainfall <500 mm) | 95 (8.2) | 1153 (63.3) | 1.5 (0.7–3.2) | 0.2445 |
| ≥500 mm | 43 (6.4) | 670 (36.8) | 1 | |
| Mean temperature annuallyl | ||||
| ≥25°C | 95 (7.6) | 1256 (68.9) | 1.1 (0.2–5.9) | 0.9067 |
| <25°C | 43 (7.6) | 567 (31.1) | 1 | |
| Maximum temperature of hottest monthl | ||||
| ≥40°C | 15 (8.9) | 169 (9.3) | 1.3 (0.4–3.9) | 0.6945 |
| <40°C | 123 (7.4) | 1654 (90.7) | 1 | |
| Major land cover type | ||||
| Open shrubland | 34 (14.1) | 241 (13.2) | 4.1 (0.6–29.5) | 0.4371 |
| Grassland | 100 (6.9) | 1444 (79.2) | 1 | |
| Croplands/natural vegetation mosaic | 2 (2.1) | 94 (5.2) | 0.3 (0.1–8.9) | |
| Barren or sparsely vegetated | 2 (4.6) | 44 (2.4) | 1.3 (0.1–87.8) |
aUnadjusted OR and 95% CI from two-level mixed effects logistic regression
bp-value from Wald’s χ2, statistically significant associations highlighted in bold (p≤0.05)
cfrom focused interview with head of household
dImproved water source—piped water into dwelling, piped water to yard/plot, public tap or standpipe, tubewell or borehole, protected dug well, protected spring, rainwater; 15 households had different washing/drinking water sources
eUnprotected spring, unprotected dug well, surface water
fdirect observation by researchers
gLatrine subtype not specified further
kextracted from 2.5 arc-min (~5 km at the equator) raster data from worldclim.org
iNo association in linear model, categorical data presented here
hOpen shrublands, cropland/natural vegetation mosaic, barren or sparsely vegetated
Table 4.
Multivariable logistic regression model of trachomatous inflammation—follicular (TF) in children aged 1–9 y, internally displaced persons camps, Darfur States, Sudan, 2014–2015
| Variable | OR (95% CI)a | p-valueb |
|---|---|---|
| Individual | ||
| Age 1–4 y (compared with 5–9 y) | 1.7 (1.0–2.7) | 0.027 |
| Household | ||
| Main source of water for drinkingc | ||
| Improved sourced | 1 | 0.0136 |
| Unimproved sourcee | 0.4 (0.2–1.2) | |
| Water vendor | 8.5 (4.1–21.0) | |
| Latrine access d | ||
| Private latrine | 1 | 0.024 |
| Latrine facilities absent (use of open defecation) | 3.1 (1.1–8.6) | |
| Shared latrine access | 3.0 (1.1–8.5) |
aAdjusted OR and 95% CI from two-level mixed effects logistic regression; sex included in model a priori
bp-value from likelihood ratio test for variable nested in full model
cfrom focused interview with head of household
dPiped water into dwelling, piped water to yard/plot, public tap or standpipe, tubewell or borehole, protected dug well, protected spring, rainwater; 15 households had different washing/drinking water sources.
eUnprotected spring, unprotected dug well, surface water
In IDP camps in Sudan it is common for households to rely on delivery of water in recycled oil barrels. Households obtaining water from vendors were present in 6 of the 36 camps surveyed, with 96% (173/181) of households surveyed in these 6 camps identifying such water vendors as their water source. In these 6 camps the proportion of children aged 1–9 y with TF was 21.1% (57/270) compared with 5.2% (81/1472) in the other 30 camps, the difference being highly statistically significant (p<0.0001, χ2 test).
Factors associated with TT in those aged ≥15 y
Univariable associations with TT are presented in Table 5. In the final multivariable model, TT was strongly independently associated with being aged ≥40 y (OR 22.0, 95% CI 2.8 to 171.6) and living alone (OR 6.9, 95% CI 2.1 to 22.7, Table 6).
Table 5.
Univariable associations of trachomatous trichiasis (TT) in those aged ≥15 y, internally displaced persons camps, Darfur States, Sudan, 2014–2015
| Individual | TT (%) | N (%) | OR (95% CI)a | p-valueb |
|---|---|---|---|---|
| Age | ||||
| ≥40 y | 15 (2.6) | 575 (34.0) | 31.9 (4.2–244.3) | 0.0009 |
| 15–39 y | 1 (0.1) | 1117 (66.0) | 1 | |
| Gender | ||||
| Female | 13 (1.1) | 1183 (69.9) | 1.9 (0.5–6.6) | 0.3284 |
| Male | 3 (0.6) | 509 (30.1) | 1 | |
| Household | ||||
| Household members | ||||
| At least one other person | 10 (0.6) | 1609 (95.0) | 1 | |
| Lives alone | 6 (7.2) | 83 (4.9) | 14.6 (4.8–44.5) | <0.0001 |
| Number of children aged 1–9 y in the household | ||||
| ≥1 | 5 (0.4) | 1236 (73.0) | 1 | 0.0007 |
| 0 | 11 (2.4) | 456 (27.0) | 6.5 (2.2–19.2) | |
| Water access c | ||||
| Main source of water for drinking | ||||
| Improved sourced | 13 (1.1) | 1131 (66.8) | 1 | 0.4893 |
| Unimproved sourcee | 1 (0.3) | 313 (18.5) | 0.3 (0.0–2.4) | |
| Water vendor | 2 (0.8) | 248 (14.7) | 0.7 (0.1–3.7) | |
| Time to main source of drinking water | ||||
| Up to 30 min round-trip | 8 (0.7) | 1241 (73.4) | 1 | |
| ≥30 min | 8 (1.8) | 451 (26.7) | 2.7 (0.9–7.8) | 0.0683 |
| Main source of water for washing | ||||
| Improved sourced | 13 (1.1) | 1131 (66.8) | 1 | 0.4894 |
| Unimproved sourcee | 1 (0.3) | 313 (18.5) | 0.3 (0.0–2.4) | |
| Water vendor | 2 (0.8) | 248 (14.7) | 0.7 (0.1–3.7) | |
| Time to main source of washing water | ||||
| Less than 30 min round-trip | 8 (0.6) | 1247 (73.7) | 1 | 0.062 |
| ≥30 min | 8 (1.8) | 445 (26.3) | 2.8 (0.9–8.0) | |
| Latrine access c | ||||
| Private latrine | 6 (0.8) | 723 (42.7) | 1 | 0.6169 |
| Shared latrine access | 5 (1.3) | 388 (22.9) | 1.7 (0.5–6.5) | |
| Latrine facilities absent (use of open defecation) | 5 (0.9) | 581 (34.3) | 0.8 (0.2–3.4) | |
| Latrine typef | ||||
| Pit latrine with slab (improved pit latrine) | 3 (0.7) | 417 (24.7) | 1 | 0.737 |
| Pit latrine without slab (unimproved pit latrine) | 8 (1.1) | 706 (41.7) | 1.5 (0.4–6.4) | |
| No facilities, bush or field | 5 (0.9) | 569 (33.6) | 0.9 (0.2–5.0) | |
| Otherg | 0 (0.0) | 9 (0.5) | - | |
| Camp | ||||
| Climate/environment k | ||||
| Rainfalll | ||||
| Desert (annual rainfall <500 mm) | 6 (0.6) | 1095 (64.7) | 0.3 (0.1–0.9) | |
| ≥500 mm | 10 (1.7) | 597 (35.3) | 1 | 0.0296 |
| Mean temperature annuallyl | ||||
| ≥25°C | 11 (0.9) | 1168 (69.0) | 0.9 | |
| <25°C | 5 (0.9) | 524 (31.0) | 1 | 0.9443 |
| Maximum temperature of hottest monthl | ||||
| ≥38°C | 11 (1.0) | 574 (33.9) | 1.1 (0.3–3.8) | 0.8746 |
| <38°C | 5 (0.9) | 1118 (66.1) | 1 | |
| Major land cover type | ||||
| Grasslands | 12 (0.9) | 1311 (77.5) | 1 | 0.7379 |
| Otherg | 4 (1.0) | 381 (22.5) | 1.3 (0.3–4.8) |
aUnadjusted OR and 95% CI from logistic regression
bp-value from Wald’s χ2, statistically significant associations highlighted in bold (p≤0.05)
cFocused interview with head of household
dImproved water source—piped water into dwelling, piped water to yard/plot, public tap or standpipe, Tubewell or borehole, protected dug well, protected spring, rainwater; 15 households had different washing/drinking water sources
eUnprotected spring, unprotected dug well, surface water
fDirect observation by data recorders
gNot specified; no TT outcome in group, so excluded from univariable analysis.
kExtracted from 2.5 arc-min (~5 km at equator) raster data from worldclim.org
iNo association in linear model, categorical data presented here
gOpen shrublands, cropland/natural vegetation mosaic, barren or sparsely vegetated
Table 6.
Multivariable logistic regression model of trachomatous trichiasis (TT) in those aged ≥15 y, internally displaced persons camps, Darfur States, Sudan, 2014–2015
| Variable | OR (95% CI)a | p-valueb |
|---|---|---|
| Age ≥40 y | 25.4 (3.2–200.0) | 0.002 |
| Living alone | 6.9 (2.1–22.7) | 0.001 |
aAdjusted OR and 95% CI from two-level mixed effects logistic regression; sex included a priori
bp-value from likelihood ratio test for variable nested in full model
Discussion
We found clustering of TF at state, IDP camp and household levels. The strongest effect of clustering was found at household level, consistent with the evidence presented by other studies,38–42 and a two-level hierarchical model was used to account for this. At an individual level, in IDP camps in Sudan, younger age was strongly associated with TF, another common finding in the trachoma literature from other contexts.38,39,42,43 We found no association between TF and gender, in keeping with trachoma’s close-contact mode of transmission, in which exposure would be expected to be similar in children of both genders;44–46 however, we included gender in all models a priori.
Household access to a private latrine of any kind was associated with decreased odds of TF. This could be considered a small validation of the WHO/UNICEF Joint Monitoring Programme for Water Supply, Sanitation and Hygiene’s definition of an improved latrine as one used by a single household (among several other criteria). Private latrine ownership is believed to confer a greater incentive to keep the latrine clean and well maintained, which in turn encourages more consistent use. However, the literature to support this having a protective association against trachoma is lacking: a number of studies suggest that the use of any latrine is protective, without identifying any additional benefit conferred by the latrine being private.47–49 In addition, several studies have suggested that community WASH coverage thresholds, rather than household-level WASH outcomes, are a better indicator of the protective association of access to sanitation, probably because of the decreased availability of feces in open areas to facilitate the spread of Musca spp. flies.50,51 In our data, similar odds of TF were observed between residents of households that practiced open defecation and residents of households using shared latrines. It is of course possible that the protective association of private latrine access here is mediated through other (unmeasured) factors, such as the health or educational advantages enjoyed by those with sufficient resources to enable maintenance of a private latrine.
At household level, sourcing drinking water primarily from a water vendor was strongly independently associated with TF in children. This relationship was also seen using the main source of washing water as the explanatory variable, although both source of drinking water and source of washing water were not included in the full model due to collinearity between the two variables (as most households’ sources of drinking and washing water were the same). A higher number of people living in the household was not associated with TF. Anecdotally, oil barrels for holding water are stored in the home and water from them is used for all drinking, cooking and cleaning by the household; in this context it would be understandable if use of the water for personal hygiene purposes was not prioritized because of the associated cost. However, the relationship between water cost or distance-to-source and use is known to be complex, and so we are wary of overinterpreting these data.52,53
IDP camps present a difficulty for trachoma elimination ambitions. There is relatively little experience in the implementation of the SAFE strategy in conflict-related settings such as these, and the question of whether decisions regarding interventions in these camps should be considered under the same guidance as non-IDP populations arises. Despite the IDP camps being seen as relative hotspots for trachoma, existing WHO guidelines make no recommendations as to how healthcare providers should respond. Understanding how best to undertake trachoma elimination interventions in these areas has to be a priority on disease control (as well as humanitarian) grounds.
Studies on the efficacy of health outcomes of hygiene and sanitation interventions in humanitarian crises are lacking, with published studies usually presenting data on the incidence of diarrheal illness. Few studies look at specific WASH interventions, including the fidelity of implementation and levels of utilization, so that to date, evidence of the impact of such interventions in this setting is scarce.54–61
In the case of trachoma, the association between disease and limited water access has been described before,45,62,63 but with notable exceptions where such a relationship was not evident;64–67 the disparity probably relates to the fact that ready access to water does not necessarily mean frequent use of water for personal hygiene.68 Beyond associated data, studies that demonstrate a significant impact of water or sanitation interventions on trachoma prevalence are still needed. Implementing sanitation interventions, like implementing the other components of the SAFE strategy, is likely to present specific challenges in the IDP camp setting, although this should not discourage us from working towards achievement of the Sustainable Development Goals for equitable and sustainable access to water and sanitation.
Authors’ contributions: CKM, KHM, BEE, BC and AWS contributed to study design; KHM, BEE, HES, NC, RW, BC and AWS were responsible for study implementation; CKM, KHM, BEE, AH, NC, RW and AWS carried out analysis and interpretation of data; CKM, KHM, BEE, AH, BC and AWS made major contributions to writing the manuscript. All authors read and approved the final draft.
Acknowledgements: We are grateful to Yael Velleman (WaterAid UK, London) and Sophie Boisson (World Health Organization, Geneva) for their guidance and review of the draft manuscript.
Funding: This study was principally funded by the Global Trachoma Mapping Project (GTMP) grant from the United Kingdom’s Department for International Development (DFID; ARIES: 203145) to Sightsavers, which led a consortium of non-governmental organizations and academic institutions to support health ministries to complete baseline trachoma mapping worldwide. The GTMP was also funded by the United States Agency for International Development (USAID), through the ENVISION project implemented by RTI International under cooperative agreement number AID-OAA-A-11-00048, and the END in Asia project implemented by FHI360 under cooperative agreement number OAA-A-10-00051. A committee established in March 2012 to examine issues surrounding completion of global trachoma mapping was initially funded by a grant from Pfizer to the International Trachoma Initiative. AWS was a Wellcome Trust Intermediate Clinical Fellow (098521) at the London School of Hygiene & Tropical Medicine, and is now a staff member of the World Health Organization. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated. None of the funders had any role in project design, in project implementation or analysis or interpretation of data, in the decisions on where, how or when to publish in the peer-reviewed press, or in preparation of the manuscript.
Competing interests: The authors report no conflicts of interest. The authors alone are responsible for the writing and content of this article.
Ethical approval: The study was conducted in accordance with the declaration of Helsinki, with the protocol for primary data collection approved by the Sudanese Federal Ministry of Health Ethics committee, and the ethics committee of the London School of Hygiene & Tropical Medicine (Refs: 6319 and 8355).
Verbal informed consent was obtained from community leaders and all study participants. Parents or guardians provided consent if participants were aged <18 y. The use of a smartphone application to record consent was considered acceptable in this setting by each ethics committee. These secondary analyses of anonymized data were considered by the ethics committee of the World Health Organization (0002988) to be exempt from full review.
Appendix
The Global Trachoma Mapping Project Investigators are: Agatha Aboe (1,11), Liknaw Adamu (4), Wondu Alemayehu (4,5), Menbere Alemu (4), Neal D. E. Alexander (9), Ana Bakhtiari (2,9), Berhanu Bero (4), Sarah Bovill (8), Simon J. Brooker (1,6), Simon Bush (7,8), Brian K. Chu (2,9), Paul Courtright (1,3,4,7,11), Michael Dejene (3), Paul M. Emerson (1,6,7), Rebecca M. Flueckiger (2), Allen Foster (1,7), Solomon Gadisa (4), Katherine Gass (6,9), Teshome Gebre (4), Zelalem Habtamu (4), Danny Haddad (1,6,7,8), Erik Harvey (1,6,10), Dominic Haslam (8), Khumbo Kalua (5), Amir B. Kello (4,5), Jonathan D. King (6,10,11), Richard Le Mesurier (4,7), Susan Lewallen (4,11), Thomas M. Lietman (10), Chad MacArthur (6,11), Colin Macleod (3,9), Silvio P. Mariotti (7,11), Anna Massey (8), Els Mathieu (6,11), Siobhain McCullagh (8), Addis Mekasha (4), Tom Millar (4,8), Caleb Mpyet (3,5), Beatriz Muñoz (6,9), Jeremiah Ngondi (1,3,6,11), Stephanie Ogden (6), Alex Pavluck (2,4,10), Joseph Pearce (10), Serge Resnikoff (1), Virginia Sarah (4), Boubacar Sarr (5), Alemayehu Sisay (4), Jennifer L. Smith (11), Anthony W. Solomon (1,2,3,4,5,6,7,8,9,10,11), Jo Thomson (4); Sheila K. West (1,10,11), Rebecca Willis (2,9).
1. Advisory Committee; 2. Information Technology, Geographical Information Systems and Data Processing; 3. Epidemiological Support; 4. Ethiopia Pilot Team; 5. Master Grader Trainers; 6. Methodologies Working Group; 7. Prioritisation Working Group; 8. Proposal Development, Finances and Logistics; 9. Statistics and Data Analysis; 10. Tools Working Group; 11. Training Working Group.
References
- 1. United Nations Refugee Agency UNHCR Statistical Yearbook 2014. Geneva: UN High Commissioner for Refugees (UNHCR); 2015. [Google Scholar]
- 2. Internal Displacement Monitoring Centre Internal displacement: global overview of trends and developments. Geneva: Norwegian Refugee Council/Internal Displacement Monitoring Centre (NRC/IDMC); 2015. [Google Scholar]
- 3. Feikin DR, Adazu K, Obor D, et al. Mortality and health among internally displaced persons in western Kenya following post-election violence, 2008: novel use of demographic surveillance. Bull World Health Organ. 2010;88(8):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petersen E, Baekeland S, Memish ZA, et al. Infectious disease risk from the Syrian Conflict. Int J Infect Dis. 2013;17:e666–e667. [Google Scholar]
- 5. Sharara SL, Kanj SS. War and infectious diseases: challenges of the Syrian civil war. PLoS Pathog. 2014;10(10):e1004438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guerrier G, Zounoun M, Delarosa O, et al. Malnutrition and mortality patterns among internally displaced and non-displaced population living in a camp, a village or a town in Eastern Chad. PLoS One. 2009;4(11):e8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olwedo MA, Mworozi E, Bachou H, et al. Factors associated with malnutrition among children in internally displaced person’s camps, northern Uganda. Afr Health Sci. 2008;8(4):244–52. [PMC free article] [PubMed] [Google Scholar]
- 8. Singh KP, Bhoopathy SV, Worth H, et al. Nutrition among men and household food security in an internally displaced persons camp in Kenya. Public Health Nutr. 2016;19(4):723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sheikh TL, Abdulaziz M, Agunbiade S, et al. Correlates of depression among internally displaced persons after post-election violence in Kaduna, North Western Nigeria. J Affect Disord. 2015;170:46–51. [DOI] [PubMed] [Google Scholar]
- 10. Sheikh TL, Mohammed A, Agunbiade S, et al. Psycho-trauma, psychosocial adjustment, and symptomatic post-traumatic stress disorder among internally displaced persons in Kaduna, Northwestern Nigeria. Front Psychiatry. 2014;5:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Siriwardhana C, Stewart R. Forced migration and mental health: prolonged internal displacement, return migration and resilience. Int Health. 2013;5(1):19–23. [DOI] [PubMed] [Google Scholar]
- 12. Haggaz AA, Radi EA, Adam I. High maternal mortality in Darfur, Sudan. Int J Gynaecol Obstet. 2007;98(3):252–3. [DOI] [PubMed] [Google Scholar]
- 13. Hynes M, Sakani O, Spiegel P, et al. A study of refugee maternal mortality in 10 countries, 2008–2010. Int Perspect Sex Reprod Health. 2012;38(4):205–13. [DOI] [PubMed] [Google Scholar]
- 14. Wirtz AL, Pham K, Glass N, et al. Gender-based violence in conflict and displacement: qualitative findings from displaced women in Colombia. Confl Health. 2014;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amowitz LL, Reis C, Lyons KH, et al. Prevalence of war-related sexual violence and other human rights abuses among internally displaced persons in Sierra Leone. JAMA. 2002;287(4):513–21. [DOI] [PubMed] [Google Scholar]
- 16. Grayston JT, Wang SP, Yeh LJ, et al. Importance of reinfection in the pathogenesis of trachoma. Rev Infect Dis. 1985;7(6):717–25. [DOI] [PubMed] [Google Scholar]
- 17. Gambhir M, Basáñez M-G, Burton MJ, et al. The development of an age-structured model for trachoma transmission dynamics, pathogenesis and control. PLoS Negl Trop Dis. 2009;3(6):e462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bourne RRA, Stevens GA, White RA, et al. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health. 2013;1(6):e339–49. [DOI] [PubMed] [Google Scholar]
- 19. Habtamu E, Wondie T, Aweke S, et al. Trachoma and relative poverty: a case-control study. PLoS Negl Trop Dis. 2015;9(11):e0004228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Emerson PM, Bailey RL, Walraven GE, et al. Human and other faeces as breeding media of the trachoma vector Musca sorbens. Med Vet Entomol. 2001;15:314–20. [DOI] [PubMed] [Google Scholar]
- 21. Mabey DCW, Solomon AW, Foster A. Trachoma. Lancet. 2003;362(9379):223–9. [DOI] [PubMed] [Google Scholar]
- 22. World Health Organization WHO Strategic and Technical Advisory Group for Neglected Tropical Diseases. Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases. Geneva: World Health Organization; 2012. [Google Scholar]
- 23. Francis V, Turner V. Achieving Community Support for Trachoma Control (WHO/PBL/93.36). Geneva: World Health Organization; 1993. [Google Scholar]
- 24. Solomon A, Mabey D. Trachoma. BMJ Clin Evid. 2007;2007:0706. [PMC free article] [PubMed] [Google Scholar]
- 25. West SK, Congdon N, Katala S, et al. Facial cleanliness and risk of trachoma in families. Arch Ophthalmol. 1991;109(6):855–7. [DOI] [PubMed] [Google Scholar]
- 26. Emerson PM, Lindsay SW, Alexander N, et al. Role of flies and provision of latrines in trachoma control: cluster-randomised controlled trial. Lancet. 2004;363(9415):1093–8. [DOI] [PubMed] [Google Scholar]
- 27. Evans JR, Solomon AW. Antibiotics for trachoma. Cochrane Database Syst Rev 2011;3:CD001860. [DOI] [PubMed] [Google Scholar]
- 28. Rabiu M, Alhassan MB, Ejere HOD, et al. Environmental sanitary interventions for preventing active trachoma. Cochrane Database Syst Rev 2012;2:CD004003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ejere H, Alhassan MB, Rabiu M. Face washing promotion for preventing active trachoma. Cochrane Database Syst Rev 2004;3:CD003659. [DOI] [PubMed] [Google Scholar]
- 30. United Nations Office for the Coordination of Humanitarian Affairs (UNOCHA) Humanitarian Bulletin Sudan. March 2016, Issue 13; 2016. [Google Scholar]
- 31.Central Bureau of Statistics. Sudan Census Report 2008—Total Population Expected to States for the Period 2009–2018. Republic of Sudan: Central Bureau of Statistics (Sudan); 2013. [Google Scholar]
- 32. Elshafie BE, Osman KH, Macleod C, et al. The epidemiology of trachoma in Darfur States and Khartoum State, Sudan: results of 32 population-based prevalence surveys. Ophthalmic Epidemiol. 2016;23(6):381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Solomon AW, Pavluck A, Courtright P, et al. The Global Trachoma Mapping Project: methodology of a 34-country population-based study. Ophthalmic Epidemiol. 2015;22(3):214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Solomon AW, Willis R, Pavluck AL, et al. Quality assurance and quality control in the Global Trachoma Mapping Project. Am J Trop Med Hyg. 2018;99(4):858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. World Health Organisation(WHO)/UNICEF Improved and unimproved water sources and sanitation facilities. WHO/UNICEF Joint Monitoring Programme(JMP) for Water Supply and Sanitation. http://www.wssinfo.org/definitions-methods/watsan-categories/ (accessed 26 October 2015).
- 36. Exley JLR, Liseka B, Cumming O, et al. The sanitation ladder, what constitutes an improved form of sanitation? Environ Sci Technol. 2015;49(2):1086–94. [DOI] [PubMed] [Google Scholar]
- 37. Leyland A, Goldstein H. Multilevel Modelling of Health Statistics. Chichester, UK: Wiley; 2001. [Google Scholar]
- 38. Last AR, Burr SE, Weiss HA, et al. Risk factors for active trachoma and ocular chlamydia trachomatis infection in treatment-naïve trachoma-hyperendemic communities of the Bijagós Archipelago, Guinea Bissau. PLoS Negl Trop Dis. 2014;8(6):e2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bailey R, Osmond C, Mabey DC, et al. Analysis of the household distribution of trachoma in a Gambian village using a Monte Carlo simulation procedure. Int J Epidemiol. 1989;18(4):944–51. [DOI] [PubMed] [Google Scholar]
- 40. Blake IM, Burton MJ, Bailey RL, et al. Estimating household and community transmission of ocular Chlamydia trachomatis. PLoS Negl Trop Dis. 2009;3(3):e401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Katz J, Zeger SL, Tielsch JM. Village and household clustering of xerophthalmia and trachoma. Int J Epidemiol. 1988;17(4):865–9. [DOI] [PubMed] [Google Scholar]
- 42. Hägi M, Schémann J-F, Mauny F, et al. Active trachoma among children in Mali: clustering and environmental risk factors. PLoS Negl Trop Dis. 2010;4(1):e583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Abdou A, Nassirou B, Kadri B, et al. Prevalence and risk factors for trachoma and ocular Chlamydia trachomatis infection in Niger. Br J Ophthalmol. 2007;91(1):13–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Harding-Esch EM, Edwards T, Sillah A, et al. Risk factors for active trachoma in The Gambia. Trans R Soc Trop Med Hyg. 2008;102(12):1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Golovaty I, Jones L, Gelaye B, et al. Access to water source, latrine facilities and other risk factors of active trachoma in Ankober, Ethiopia. PLoS One. 2009;4(8):e6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yalew KN, Mekonnen MG, Jemaneh AA. Trachoma and its determinants in Mojo and Lume districts of Ethiopia. Pan Afr Med J. 2012;13(Suppl 1):8. [PMC free article] [PubMed] [Google Scholar]
- 47. Heijnen M, Cumming O, Peletz R, et al. Shared sanitation versus individual household latrines: a systematic review of health outcomes. PLoS One. 2014;9(4):e93300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Montgomery MA, Desai MM, Elimelech M. Assessment of latrine use and quality and association with risk of trachoma in rural Tanzania. Trans R Soc Trop Med Hyg. 2010;104(4):283–9. [DOI] [PubMed] [Google Scholar]
- 49. Montgomery MA, Desai MM, Elimelech M. Comparing the effectiveness of shared versus private latrines in preventing trachoma in rural Tanzania. Am J Trop Med Hyg. 2010;82(4):693–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oswald WE, Stewart AE, Kramer MR, et al. Active trachoma and community use of sanitation, Ethiopia. Bull World Health Organ. 2017;95(4):250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Garn JV, Boisson S, Willis R, et al. Sanitation and water supply coverage thresholds associated with active trachoma: modeling cross-sectional data from 13 countries. PLoS Negl Trop Dis. 2018;12(1):e0006110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. White G, Bradley D, White A. Drawers of Water: Domestic Water Use in East Africa. Chicago: University of Chicago Press; 1972. [Google Scholar]
- 53. Feachem R, Burns E, Cairncross S, et al. Water, Health and Development: An Interdisciplinary Evaluation. London: Tri-Med Books; 1978. [Google Scholar]
- 54. Doocy S, Burnham G. Point-of-use water treatment and diarrhoea reduction in the emergency context: an effectiveness trial in Liberia. Trop Med Int Health. 2006;11(10):1542–52. [DOI] [PubMed] [Google Scholar]
- 55. Elsanousi S, Abdelrahman S, Elshiekh I, et al. A study of the use and impacts of LifeStraw in a settlement camp in southern Gezira, Sudan. J Water Health. 2009;7(3):478–83. [DOI] [PubMed] [Google Scholar]
- 56. Moll DM, McElroy RH, Sabogal R, et al. Health impact of water and sanitation infrastructure reconstruction programmes in eight Central American communities affected by Hurricane Mitch. J Water Health. 2007;5(1):51–65. [DOI] [PubMed] [Google Scholar]
- 57. Peterson EA, Roberts L, Toole MJ, et al. The effect of soap distribution on diarrhoea: Nyamithuthu Refugee Camp. Int J Epidemiol. 1998;27(3):520–4. [DOI] [PubMed] [Google Scholar]
- 58. Roberts L, Chartier Y, Chartier O, et al. Keeping clean water clean in a Malawi refugee camp: a randomized intervention trial. Bull World Health Organ. 2001;79(4):280–7. [PMC free article] [PubMed] [Google Scholar]
- 59. Walden VM, Lamond E-A, Field SA. Container contamination as a possible source of a diarrhoea outbreak in Abou Shouk camp, Darfur province, Sudan. Disasters. 2005;29(3):213–21. [DOI] [PubMed] [Google Scholar]
- 60. Ramesh A, Blanchet K, Ensink JHJ, et al. Evidence on the effectiveness of WAter, Sanitation, and Hygiene (WASH) interventions on health outcomes in humanitarian crises: a systematic review. PLoS One. 2015;10(9):e0124688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Garn JV, Sclar GD, Freeman MC, et al. The impact of sanitation interventions on latrine coverage and latrine use: a systematic review and meta-analysis. Int J Hyg Environ Health. 2017;220(2):329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Burton MJ, Hu VH, Massae P, et al. What is causing active trachoma? The role of nonchlamydial bacterial pathogens in a low prevalence setting. Invest Ophthalmol Vis Sci. 2011;52(8):6012–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Baggaley RF, Solomon AW, Kuper H, et al. Distance to water source and altitude in relation to active trachoma in Rombo district, Tanzania. Trop Med Int Health. 2006;11(2):220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. de Abreu Freitas HS, Medina NH, de Fátima Costa Lopes M, et al. Trachoma in indigenous settlements in Brazil, 2000–2008. Ophthalmic Epidemiol. 2016:23:354–9. [DOI] [PubMed] [Google Scholar]
- 65. Sokana O, Macleod C, Jack K, et al. Mapping trachoma in the Solomon Islands: results of three baseline population-based prevalence surveys conducted with the Global Trachoma Mapping Project. Ophthalmic Epidemiol. 2016;23(supp 1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ward B. The prevalence of active trachoma in Fiji. Am J Ophthalmol. 1965;59:458–63. [DOI] [PubMed] [Google Scholar]
- 67. Mathew AA, Keeffe JE, Le Mesurier RT, et al. Trachoma in the Pacific Islands: evidence from Trachoma Rapid Assessment. Br J Ophthalmol. 2009;93(7):866–70. [DOI] [PubMed] [Google Scholar]
- 68. Bailey R, Downes B, Downes R, et al. Trachoma and water use; a case control study in a Gambian village. Trans R Soc Trop Med Hyg. 1991;85(6):824–8. [DOI] [PubMed] [Google Scholar]