Abstract
Background
Household contacts (HHCs) of TB patients are at high risk of developing evidence of latent TB infection (LTBI) and active disease from the index patient. We estimated the age-specific prevalence of LTBI and the force of infection (FI), as a measure of recent transmission, among HHCs of active TB patients.
Methods
A cross-sectional analysis of HHCs of pulmonary TB patients enrolled in a prospective study, ‘CTRIUMPh’, was conducted at two sites in India. LTBI was defined as either a positive tuberculin skin test (induration ≥5 mm) or QuantiFERON–Gold in tube test (value ≥0.35 IU/ml) and was stratified by age. FI, which is a measure of recent transmission of infection and calculated using changes in age-specific prevalence rates at specific ages, was calculated. Factors associated with LTBI were determined by logistic regression models.
Results
Of 1020 HHCs of 441 adult pulmonary TB cases, there were 566 (55%) females and 289 (28%) children aged ≤15 y. While screening for the study 3% of HHC were diagnosed with active TB. LTBI prevalence among HHCs of pulmonary TB was 47% at <6 y, 53% between 6–14 y and 78% between 15–45 y. FI increased significantly with age, from 0.4 to 1.15 in the HHCs cohort (p=0.05).
Conclusion
This study observed an increased prevalence of LTBI and FI among older children and young adults recently exposed to infectious TB in the household. In addition to awareness of coughing etiquette and general hygiene, expanding access to TB preventive therapy to all HHCs, including older children, may be beneficial to achieve TB elimination by 2035.
Keywords: close contacts, force of infection, latent TB, transmission
Introduction
WHO estimated that globally in 2017 there were 10.0 million (9.0–11.1 million) incident cases of TB and 1.3 million (1.2–1.4 million) deaths from TB among HIV-negative individuals.1 The first step in the development of active TB is contact with an infectious TB patient, followed by the acquisition of infection with Mycobacterium tuberculosis (M.tb). Contacts exposed to patients with TB are at substantial risk of developing latent TB infection (LTBI) as well as progressing to active TB disease.2
One quarter of the global population, a reservoir of approximately 1.7–2.3 billion individuals, are reported to be latently infected with M.tb.3,4 However, many countries with a high burden of TB disease do not have nationwide prevalence data on LTBI. Also, mass screening and treatment for LTBI have not shown any significant effect in TB control in a high burden setting.5 Targeted screening and intervention for LTBI should be considered. While a few studies have assessed LTBI in young children6 not much work has been done in higher age groups.7 High risk of TB has been shown in pediatric contacts and countries recommend TB preventive therapy for children aged <6 y.8 Very little support is offered to the household contacts (HHCs), although the exposure of all HHCs to an infectious TB index case likely occurred months before that index case was diagnosed.
A better understanding of the burden of TB infection among HHCs and the relative contribution of all household members to the burden of TB infection, irrespective of age, will help guide TB preventive therapy for close contacts in the family. A systematic review in low- and middle-income settings has shown that LTBI prevalence among all contacts is 51.5% (95% CI: 47.1 to 55.8%).2
Tuberculin skin test (TST) and interferon-gamma release assays (IGRA) are the two commonly available tools for identification of individuals with TB infection in order to calculate the burden of LTBI.9,10 However, these tests have their limitations—TST may be affected by the BCG vaccine or environmental nontuberculous mycobacteria, while an individual’s immune status can modify IGRA. Alternatively, a new mathematical technique can be used to estimate the burden of TB infection that is called force of infection (FI). This concept is based on the principle that prevalence is a function of incidence and duration of illness or infectivity and is utilised to estimate the incidence of disease where true incidence is difficult to measure like HIV.11 FI is a measure of recent transmission of infection and is calculated using changes in age-specific prevalence rates and measures the proportion of susceptible individuals who have become infected with M.tb over a specified period.12–14 It is basically a product of TB disease prevalence and mixing pattern. This knowledge about recent transmission will also support implementation guidelines for preventive therapy for close contacts with household exposure in areas of the world with high TB burden. In the present analysis, we show the age-specific prevalence of LTBI by the available test along with FI in HHCs of active pulmonary TB patients in two cities of India, the country with the largest burden of TB in the world.
Materials and Methods
This study was part of an ongoing prospective study, ‘CTRIUMPh’, the details of which are described elsewhere.15 In brief, CTRIUMPh is a prospective cohort study of adult pulmonary TB patients and their HHCs to evaluate the response to anti-TB treatment as the active TB cohort and to evaluate M.tb infection and progression to TB disease among the HHCs cohort. The study is ongoing at the National Institute for Research in Tuberculosis, Chennai, and Byramjee Jeejeebhoy Government Medical College, Pune, India, through academic and operational partnerships with the Johns Hopkins University (JHU), USA, since August 2014.
We defined HHCs as adults and children living in the same household as the index case during the 3 mo before diagnosis of the index TB case. Active TB was ruled out in the HHCs before study enrolment. All HHCs underwent a symptom screen, physical examination, sputum testing for AFB by smear and culture, a blood sample for IGRA by Quantiferon-Gold In tube test (QFT-GIT), TST and chest x-ray upon study entry. We collected blood for QFT-GIT assay on the same day as TST was administered but before placement of TST. Venous blood samples were collected and processed according to the manufacturer’s instructions (QIAGEN, Germany), IFN-ɣ levels (IU/ml) were estimated using an ELISA reader (ELx808, BioTek, USA) and the results were reported as positive or negative QFT-GIT analysis software version 2.62 (Cellestis, Carnegie, Australia). The TST was performed by trained staff according to the Mantoux technique.16 On enrolment to the study, 0.1 ml of 2TU PPD (RT23; Span Diagnostics, India) was injected intradermally on the volar aspect of the forearm. Two trained staff read the transverse diameter of the TST induration 48–72 h after the injection.16 A positive QFT-GIT result was the value of the TB antigen minus nil control of ≥0.35 IU/ml and >25% of nil value. A positive TST was an induration diameter of ≥5mm.17 HHCs with a positive symptom screen with sputum smear or culture positive for AFB or chest x-ray suggestive of TB were considered to have active TB disease.
LTBI was defined as the presence of a positive TST or QFT-GIT test result at baseline or study entry without evidence of clinical, bacteriological or radiological evidence of TB disease. Prevalence of LTBI was calculated as the number of positives by either TST or QFT-GIT tests divided by the total number of HHCs tested. FI was calculated for specific ages for the pool of individuals who remained uninfected (absolute difference in prevalence between the baseline and month 4/[1-prevalence at baseline]). This shows the change in the prevalence of LTBI between age groups.12
Univariate and multivariate logistic regression models were used to measure the association between various factors and LTBI positivity. The multiple logistic models included potential confounding factors as well as factors found to be significant from the backward elimination method. ORs and 95% CIs were reported for each factor in the univariate and for the age groups alone in the final multivariate models by tests with combinations. Cox Stuart test was used to evaluate the trend in prevalence and FI. Statistical significance was determined at p<0.05. Analyses were conducted in Stata version 15.0 (StataCorp LP, College Station, TX, USA).
The Institutional Ethics Committee of the National Institute for Research in Tuberculosis, Chennai, and Byramjee Jeejeebhoy Government Medical College, Pune, India, approved the study, and all adult participants provided informed signed consent before study enrolment. For child participants, parental written consent and written assent from the child (wherever applicable) were obtained. The ethics committee of JHU, Baltimore, also approved the study protocol.
Results
Patient characteristics and TST diameters
Of 1050 HHCs of 441 adult pulmonary TB cases screened for the study, 30 HHCs (2.9%) were diagnosed with active TB disease and were excluded from further analysis. Of the 1020 who enrolled, 566 (55%) were females, 289 (28%) were children aged ≤15 y, 1% were HIV-infected and 10% of the adults were known diabetics. All HHCs received TST and the induration sizes ranged from 0 to 28 mm (median =5 mm; IQR: 3–10). Seventy-four HHCs (8%) had a TST induration of 0 mm, 459 (47%) had an induration of 1–5 mm and 189 (19%) had an induration of 6–9 mm. When assessing a higher TST cut-off, 254 (26%) had an induration of >10 mm. There was a significant difference in the median size of TST induration among HHCs with BCG scar compared with those without (4.5 vs 6 mm, p=0.001) and between malnourished (body mass index [BMI] <16 kg/m2) and well-nourished HHCs (4 vs 5 mm, p<0.001). However, QFT-GIT did not show any difference in results between those with or without BCG scars or between malnourished or well-nourished individuals.
Prevalence of LTBI and age stratification
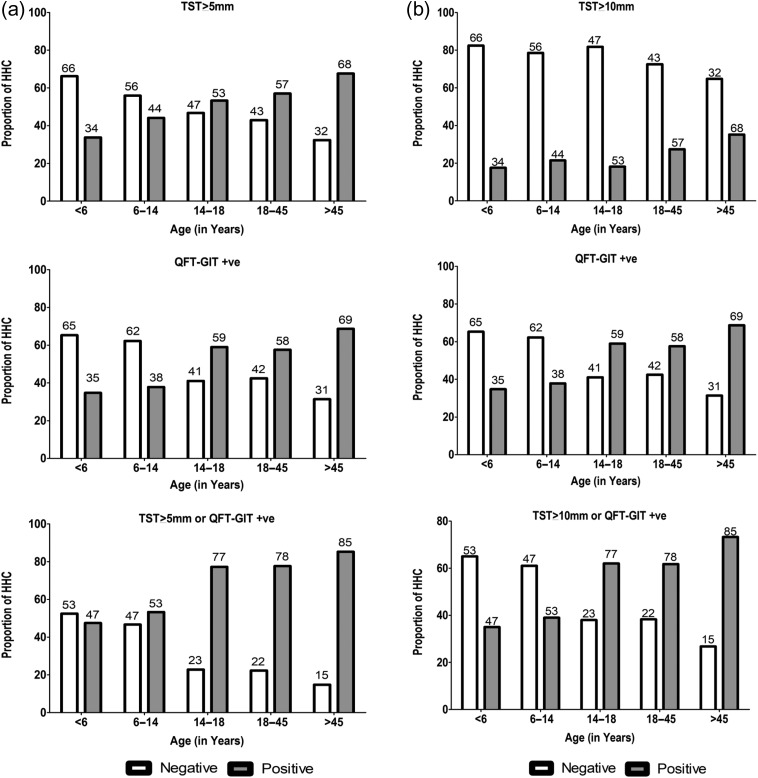
Defining LTBI as either TST induration of >5 mm or QFT-GIT ≥0.35 IU/L, the overall prevalence of LTBI in this cohort was 72% (95% CI: 69 to 75%). Absence of BCG scar (OR: 1.50, 95% CI: 1.13 to 2.00, p=0.006), BMI >18.5 kg/m2 (OR: 2.35, 95% CI: 1.77 to 3.12, p<0.001), time spent with the index patient (OR: 2.15, 95% CI: 1.17 to 3.96, p=0.014) along with the disease severity of the index patient in terms of sputum AFB smear grading (OR: 1.68, 95% CI: 1.18 to 2.38, p=0.004) were significantly different between the TB-infected HHCs (LTBI-positive) and the non-infected HHCs (LTBI-negative) (Table 1). Figure 1A shows the age-stratified prevalence of LTBI for the HHCs, with TST induration of ≥5 mm or QFT-GIT positivity. As a few countries follow TST induration of >10 mm as a positive cut-off, Figure 1B depicts the LTBI prevalence with a higher TST cut-off, which is significantly different when compared with a TST cut-off of 5 mm (p<0.001). The logistic regression model shows that the odds of being LTBI-positive were three times higher among HHCs aged >15 y compared with those aged <15 y (Table 1). Similarly, HHCs who spent more time with the index case had twice the risk of being latently infected. Also, HHCs of patients with a higher load of M.tb bacilli in their sputum were at a higher risk of latent infection. Multivariate logistic regression model showed that the age groups 15–18 y (adjusted OR [aOR]: 3.4, 95% CI: 1.7 to 7.1, p=0.001), 18–45 y (aOR: 3.2, 95% CI: 1.8 to 5.8, p<0.001) and >45 y (aOR: 7.1, 95% CI: 3.3 to 15.3, p<0.001), time spent with the index case (aOR 2.0, 95% CI: 1.1 to 3.7, p=0.04) and higher bacillary load in the sputum of index patient (aOR 1.08, 95% CI 1.2 to 2.6, p=0.004) were associated with the presence of LTBI, after adjusting for gender, BMI, BCG status, diabetes, smoking status, alcohol use and area of residence. This was similar irrespective of the TST positive induration of either 5 or 10 mm (Table 2). There is an increase in the odds of positivity as age increases (Table 3).
Table 1.
Characteristics of household contacts with latent TB infection
| LTBI negative (n=283) | LTBI positive (n=725) | OR (95% CI) | p-value | |
|---|---|---|---|---|
| Age (y) | ||||
| <6 | 42 (53%) | 38 (48%) | 1.00 | |
| 6–14 | 85 (47%) | 97 (53%) | 1.26 (0.74 to 2.14) | 0.388 |
| 15–18 | 18 (23%) | 61 (77%) | 3.75 (1.89 to 7.43) | <0.001 |
| 19–45 | 117 (22%) | 408 (78%) | 3.85 (2.37 to 6.26) | <0.001 |
| >45 | 21 (15%) | 121 (85%) | 6.37 (3.36 to 12.06) | <0.001 |
| Gender | ||||
| Male | 128 (29%) | 320 (71%) | 1.00 | 0.754 |
| Female | 155 (28%) | 405 (72%) | 1.05 (0.79 to 1.38) | |
| BMI (kg/m2) | ||||
| <18.5 | 142 (40%) | 217 (60%) | 1.00 | <0.001 |
| ≥18.5 | 141 (22%) | 507 (78%) | 2.35 (1.77 to 3.12) | |
| BCG scar | ||||
| No | 94 (23%) | 310 (77%) | 1.50 (1.13 to 2.00) | 0.006 |
| Yes | 189 (31%) | 415 (69%) | 1.00 | |
| Symptomatic | ||||
| No | 230 (28%) | 605 (72%) | 1.00 | 0.416 |
| Yes | 53 (31%) | 120 (69%) | 0.86 (0.60 to 1.23) | |
| HIV-infected | ||||
| No | 279 (28%) | 713 (72%) | 1.00 | 0.783 |
| Yes | 4 (25%) | 12 (75%) | 1.17 (0.38 to 3.67) | |
| Residential area | ||||
| Rural | 81 (25%) | 238 (75%) | 1.00 | 0.197 |
| Urban | 202 (29%) | 487 (71%) | 0.82 (0.61 to 1.11) | |
| Work involves outstation | ||||
| No | 274 (28%) | 694 (72%) | 1.00 | 0.425 |
| Yes | 9 (23%) | 31 (78%) | 1.36 (0.64 to 2.89) | |
| Time spent with index case | ||||
| No | 270 (29%) | 657 (71%) | 1.00 | 0.014 |
| Yes | 13 (16%) | 68 (84%) | 2.15 (1.17 to 3.96) | |
| Average number of meals shared with index case | ||||
| 0 | 16 (31%) | 35 (69%) | 1.00 | |
| 1 | 77 (28%) | 196 (72%) | 1.20 (0.63 to 2.29) | 0.586 |
| ≥2 | 190 (28%) | 494 (72%) | 1.22 (0.66 to 2.27) | 0.522 |
| Sleeping with index case | ||||
| Same room/bed | 74 (25%) | 220 (75%) | 1.42 (0.64 to 3.14) | 0.393 |
| Same room, different bed | 106 (26%) | 298 (74%) | 1.34 (0.61 to 2.93) | 0.466 |
| Same house, different room | 93 (33%) | 186 (67%) | 0.95 (0.43 to 2.11) | 0.904 |
| Different building | 10 (32%) | 21 (68%) | 1.00 | |
| Diabetesa | ||||
| No | 124 (20%) | 494 (80%) | 1.00 | 0.309 |
| Yes | 17 (25%) | 50 (75%) | 0.74 (0.41 to 1.32) | |
| Educationa | ||||
| Literate | 122 (21%) | 455 (79%) | 1.00 | 0.403 |
| Illiterate | 19 (18%) | 89 (82%) | 1.26 (0.74 to 2.14) | |
| Employmenta | ||||
| Employed | 119 (21%) | 453 (79%) | 1.00 | 0.748 |
| Unemployed | 22 (19%) | 91 (81%) | 1.09 (0.65 to 1.81) | |
| Smokera | ||||
| No | 127 (21%) | 477 (79%) | 1.00 | 0.435 |
| Yes | 14 (17%) | 67 (83%) | 1.27 (0.69 to 2.34) | |
| Alcohol usea | ||||
| No | 114 (21%) | 439 (79%) | 1.00 | >0.950 |
| Yes | 27 (20%) | 105 (80%) | 1.01 (0.63 to 1.62) | |
| Index case with coughb | ||||
| No | 5 (22%) | 18 (78%) | 1.00 | 0.496 |
| Yes | 278 (28%) | 707 (72%) | 0.71 (0.26 to 1.92) | |
| Cavity status of indexb | ||||
| No | 169 (29%) | 414 (71%) | 1.00 | 0.45 |
| Yes | 114 (27%) | 311 (73%) | 1.11 (0.84 to 1.47) | |
| AFB smear of index caseb | ||||
| Negative | 111 (34%) | 217 (66%) | 1.00 | |
| <2+ | 100 (27%) | 272 (73%) | 1.39 (1.01 to 1.92) | 0.046 |
| ≥2+ | 72 (23%) | 236 (77%) | 1.68 (1.18 to 2.38) | 0.004 |
BMI, body mass index; diabetes: known diabetes or HbA1c≥6.5 or glucose ≥200; LTBI, latent TB infection: tuberculin skin test (TST)≥5 mm or Quantiferon-Gold In tube test (QFT-GIT) positive; smoker: defined as those who had smoked 100 cigarettes in their life times.
aonly those who were aged ≥18 y were considered.
bOR was calculated after adjusting for cluster effects.
Figure 1.
(A) Age-stratified prevalence of latent TB infection (TST≥5 mm or QFT-GIT positive). (B) Age-stratified prevalence of latent TB infection (TST≥10 mm or QFT-GIT positive). QFT-GIT, Quantiferon-Gold In tube test; TST, tuberculin skin test.
Table 2.
Age-specific risk of latent TB infection among household contacts
| Age, y | OR (95% CI) | Significance | Adjusted OR (95% CI) | Significance |
|---|---|---|---|---|
| TST≥5 mm | ||||
| <6 | 1.00 | 1.00 | ||
| 6–15 | 1.54 (0.88 to 2.72) | 0.132 | 1.53 (0.86 to 2.71) | 0.149 |
| 15–18 | 2.23 (1.16 to 4.31) | 0.017 | 1.99 (0.99 to 3.97) | 0.052 |
| 18–45 | 2.60 (1.56 to 4.35) | <0.001 | 2.21 (1.20 to 4.05) | 0.011 |
| >45 | 4.09 (2.25 to 7.45) | <0.001 | 3.96 (1.96 to 8.00) | <0.001 |
| TST≥10 mm | ||||
| <6 | 1.00 | 1.00 | ||
| 6–15 | 1.28 (0.64 to 2.58) | 0.484 | 1.40 (0.69 to 2.84) | 0.358 |
| 15–18 | 1.04 (0.45 to 2.40) | 0.922 | 1.22 (0.51 to 2.91) | 0.660 |
| 18–45 | 1.78 (0.95 to 3.33) | 0.074 | 2.47 (1.18 to 5.14) | 0.016 |
| >45 | 2.55 (1.28 to 5.11) | 0.008 | 4.14 (1.83 to 9.34) | 0.001 |
| QFT-GIT positive | ||||
| <6 | 1.00 | 1.00 | ||
| 6–15 | 1.14 (0.64 to 2.04) | 0.651 | 1.20 (0.66 to 2.17) | 0.544 |
| 15–18 | 2.70 (1.39 to 5.24) | 0.003 | 3.08 (1.51 to 6.25) | 0.002 |
| 18–45 | 2.55 (1.52 to 4.27) | <0.001 | 2.87 (1.54 to 5.34) | 0.001 |
| >45 | 4.12 (2.24 to 7.56) | <0.001 | 5.97 (2.88 to 12.35) | <0.001 |
| QFT-GIT positive and TST≥5 mm | ||||
| <6 | 1.00 | 1.00 | ||
| 6–15 | 1.67 (0.81 to 3.42) | 0.162 | 1.78 (0.86 to 3.69) | 0.123 |
| 15–18 | 2.34 (1.07 to 5.13) | 0.034 | 2.62 (1.15 to 6.00) | 0.023 |
| 18–45 | 2.43 (1.26 to 4.67) | 0.008 | 2.83 (1.33 to 6.00) | 0.007 |
| >45 | 4.43 (2.17 to 9.04) | <0.001 | 6.33 (2.76 to 14.52) | <0.001 |
| QFT-GIT positive or TST≥5 mm | ||||
| <6 | 1.00 | 1.00 | ||
| 6–15 | 1.26 (0.74 to 2.14) | 0.388 | 1.25 (0.73 to 2.15) | 0.414 |
| 15–18 | 3.75 (1.89 to 7.43) | <0.001 | 3.42 (1.65 to 7.07) | 0.001 |
| 18–45 | 3.85 (2.37 to 6.26) | <0.001 | 3.19 (1.75 to 5.80) | <0.001 |
| >45 | 6.37 (3.36 to 12.06) | <0.001 | 7.08 (3.28 to 15.3) | <0.001 |
| QFT-GIT positive or TST≥10 mm | ||||
| <6 | 1.00 | 1.00 | ||
| 6–15 | 1.19 (0.69 to 2.05) | 0.538 | 1.24 (0.71 to 2.18) | 0.448 |
| 15–18 | 3.03 (1.59 to 5.79) | 0.001 | 3.22 (1.62 to 6.42) | 0.001 |
| 18–45 | 2.99 (1.83 to 4.90) | <0.001 | 3.21 (1.77 to 5.81) | <0.001 |
| >45 | 5.08 (2.82 to 9.18) | <0.001 | 7.49 (3.68 to 15.26) | <0.001 |
The model was adjusted for multiple confounders such as gender, body mass index, BCG, diabetes, smoking, alcohol use, area of residence, time spent with the index case, sleeping with the index case, presence of cough and smear grading of the index case; QFT-GIT, Quantiferon-Gold In tube test; TST, tuberculin skin test.
Table 3.
Force of infection across different age groups along with latent TB infection
| Test | Age, y | N | Mean (μ) | Number of positive (%) | OR (95% CI) | Force of infection |
|---|---|---|---|---|---|---|
| TST≥5 mm | <6 | 74 | 3.2 | 25 (34) | 0.51 (0.32 to 0.83) | 0.049 |
| 6–14 | 177 | 10.0 | 78 (44) | 0.79 (0.59 to 1.06) | 0.192 | |
| 15–18 | 77 | 16.4 | 41 (53) | 1.14 (0.73 to 1.78) | 0.328 | |
| 19–45 | 510 | 31.9 | 291 (57) | 1.33 (1.12 to 1.58) | 0.216 | |
| >45 | 139 | 54.9 | 94 (68) | 2.09 (1.46 to 2.98) | 0.138 | |
| Total | 977 | 27.8 | 529 (54) | 0.158 | ||
| p-value | 0.050 | |||||
| QFT-GIT | <6 | 72 | 3.2 | 25 (35) | 0.53 (0.33 to 0.86) | 0.442 |
| 6–14 | 164 | 10.0 | 62 (38) | 0.61 (0.44 to 0.83) | 0.360 | |
| 15–18 | 78 | 16.4 | 46 (59) | 1.44 (0.92 to 2.26) | 0.806 | |
| 19–45 | 497 | 31.9 | 286 (58) | 1.36 (1.13 to 1.62) | 0.483 | |
| >45 | 134 | 54.9 | 92 (69) | 2.19 (1.52 to 3.16) | 1.094 | |
| Total | 945 | 27.8 | 511 (54) | 0.571 | ||
| p-value | 0.059 | |||||
| LTBI | <6 | 80 | 3.2 | 38 (48) | 0.90 (0.58 to 1.40) | 0.438 |
| 6–14 | 182 | 10.0 | 97 (53) | 1.14 (0.85 to 1.53) | 0.363 | |
| 15–18 | 79 | 16.4 | 61 (77) | 3.39 (2.00 to 5.73) | 0.696 | |
| 19–45 | 525 | 31.9 | 408 (78) | 3.49 (2.84 to 4.28) | 1.153 | |
| >45 | 142 | 54.9 | 121 (85) | 5.76 (3.63 to 9.16) | 1.254 | |
| Total | 1008 | 27.8 | 725 (72) | 0.820 | ||
| p-value | 0.052 | |||||
Force of infection: calculated at specific ages for the pool of individuals who remained uninfected (absolute change in prevalence/[1-baseline prevalence]); LTBI, latent TB infection; N, total participants; OR was calculated by considering all other subgroups as reference; QFT-GIT, Quantiferon-Gold In tube test; TST, tuberculin skin test.
FI
As shown in Table 3, the FI was high for children aged <6 y (FI=0.438), indicating recent transmission within the household as they are less likely to have been previously infected or exposed to the community. In older children, adolescents and young adults, a significant excess FI was observed (15–18 y: FI=0.696 and 19–45 y: FI=1.15), indicating increasing rates of recent transmission (p=0.05) (Table 3).
Further follow-up
These HHCs, both LTBI-positive and –negative, are part of the follow-up to study the progression of latent infection to active TB disease over the next 24 mo and identify the risk factors/predictors for progression of infection to disease.
Discussion
This is one of the few studies to report the age-specific prevalence of LTBI and FI among HHCs, including adolescents and young adults exposed to an active index case in a TB endemic setting. In this study, we have shown an increased prevalence of LTBI from 47% at <6 y to 53% between 6–14 y, 78% between 15–45 y and so on. A prevalence survey among a population of 35 000, conducted by our institute in the same area in 1963, found the prevalence of LTBI among those aged 10–19 y to be around 33%.18 South African investigators also found an increasing prevalence of LTBI among a younger age group.19–21 A HHCs study from Peru demonstrated that the age-specific risk of infection from contact with a culture-positive index case increases up to approximately 30 y of age.22
However, the standard practice for TB prevention in many TB high burden and resource-constrained countries include administrating a single BCG vaccine at birth and administering daily isoniazid tablets or syrup for the pediatric contacts (<6 y of age) of pulmonary TB patients. Still, we found that the LTBI prevalence of 53% among the HHCs aged 6–14 y was much higher than the Kenyan survey for LTBI, which found a prevalence rate of 10.2% among children aged 6–14 y.23 It has been suggested that this low rate of TB transmission in Kenya over the last decade could be the result of activities undertaken by the TB control programme, which has been sufficient to keep TB transmission steady. Also, the settings where the studies were conducted in India, South Africa (rural) and Kenya can influence LTBI prevalence rates. Also, it is very clear from our cohort that as age advances, the burden of LTBI among HHCs also increases.
The high-risk environments of schools and workplaces can explain the higher rate of LTBI prevalence among young adults aged 15–18 y, with their greater frequency of social contacts, contact time and use of public transits, in addition to generally mixing with the index patient at home and being exposed to higher proportions of rebreathed air.24–26 Also, poor ventilation and overcrowding in many of these places contributes significantly to the transmission of TB spread by droplet nuclei.25 Given the above background and the increasing prevalence of LTBI with increasing age, one may deduce that a substantial proportion of TB transmission also occurs outside of the household. Molecular epidemiology from South Africa has shown that only 19% of TB cases arise from transmission within one’s own family.27 This is also supported by mathematical modelling that estimated that only 16% of TB transmission occurs within households.21
Similar to the South African group, we also found that the FI increased with advancing age.12 Although the FI is a measure of recent transmission, the higher rates noticed with advancing age can also be explained by the increased social mixing patterns associated with advancing age.24 The FI in younger children is explained primarily by household exposure to an active index TB patient, while that in adolescents and young adults can be attributed to both household and community exposure.22 FI in this older age group would result in a significant proportion of primary and secondary TB infection against the background of a high prevalence of smoking, indoor air pollution, malnutrition and diabetes in this community. All of these factors are associated with an increased risk of progression from TB infection to active disease.28
To summarise, LTBI is of relevance to both the TB control programme and public health programmes of a country, as control of TB epidemics requires the involvement of an increasing proportion of non-infected individuals in the population. Awareness of coughing etiquette, personal hygiene and protective masks should be emphasised both among the community and HHCs. Because of a high prevalence of LTBI in HHCs, taken in conjunction with the FI, especially among older children who may have been infected very recently, and considering the fact that treatment of LTBI may prevent progression to active disease,29 we suggest expanding the use of preventive therapy to HHCs aged >6 y. Zellner et al. also found an excess infection risk among children and young adult HHCs secondary to household exposure and suggested a beneficial effect of expanded access to preventive therapy for older children and young adults.22 Hence, prevention and treatment of LTBI, either in the form of a vaccine or chemoprophylaxis, should be recommended for HHCs, especially older children and young adults, to prevent the progression of LTBI to active TB disease.
Conclusions
This study observed an increased prevalence of LTBI and FI among older children and young adults recently exposed to infectious TB in the household. In addition to awareness of coughing etiquette and general hygiene, expanding access to TB preventive therapy to all HHCs, including older children, may be beneficial to achieve TB elimination by 2035.
Authors’ contributions: AG conceived the study; AG, CP, VM, NG and AkG designed the study protocol; CKD, CP, RL, AKi, MP, SBM, LM, SG, YP, SS, SP, NP, VK, SVBYS, MP, AKa, BTK, NS and VM carried out the study implementation; NG, KT, AkG, CKD, VM, CP, SBM and AG carried out the analysis and interpretation of the study data; CP, CKD, KT, NG, VM and AG drafted the manuscript with major contributions; RL, AKi, MP, SVB, SP, NP, AkG and PK critically revised the manuscript for intellectual content. All authors read and approved the final manuscript.
Acknowledgements: We acknowledge support from Persistent Systems for IT support in kind, HP for computer donations, and the Ujala Foundation, Wyncote Foundation and Gilead Foundation.
Funding: Data in this manuscript were collected as part of the Regional Prospective Observational Research for Tuberculosis (RePORT) India Consortium. This project has been funded in whole or in part with Federal funds from the Government of India’s (GOI) Department of Biotechnology (DBT), the Indian Council of Medical Research (ICMR), the United States National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID), Office of AIDS Research (OAR), and distributed in part by Civilian Research Development Foundation (CRDF) Global. The research reported in this publication (analysis and manuscript preparation) was also supported by the NIH study, Impact of Diabetes on TB Treatment Outcomes (R01AI097494), the NIH Byramjee Jeejeebhoy Government Medical College HIV Clinical Trials Unit (UM1AI069497), and the Fogarty International Center, National Institutes of Health D43TW009574.
The contents of this publication are solely the responsibility of the authors and do not represent the official views of the DBT, the ICMR, the NIH, JHU or CRDF Global. Any mention of trade names, commercial projects or organisations does not imply endorsement by any of the sponsoring organisations. The sponsors had no role in the study design and writing of this report.
Competing interests: None declared.
Ethical approval: The study was approved by the institutional ethics committees of the participating institutes.
Collaborators: The CTRIUMPH team, listed in alphabetical order: Aarti Kinikar, Akshay Gupte, Amita Gupta, Amita Nagraj, Anand Kumar, Andrea DeLuca, Anita More, Anju Kagal, Archana Gaikwad, Ashwini Nangude, Balaji S, Beena Thomas, Bency Joseph, Bharath TK, Brindha B, Chandrasekaran Padmapriyadarsini, David Dowdy, Deepak Pole, Devanathan A, Devi Sangamithrai, Dileep Kadam, Divyashri Jain, Dolla Chandrakumar, Gabriela Smit, Gangadarsharma R, Geetha Ramachandran, Hanumant Chaugule, Hari Koli, Hemanth kumar, Jeeva J, Jessica Elf, Jonathan Golub, Jyoti Chandane, Kanade Savita, Kannan M, Kannan Thiruvengadam, Karthikesh M, Karunakaran S, Kelly Dooley, Lakshmi Murali, Lavanya M, Luke E. Hannah, Madasamy S, Madeshwaran A, Mageshkumar M, Mangaiyarkarasi S, Mahesh Gujare, Manoharan S, Michel Premkumar M, Munivardhan P, Murugesan S, Gomathy NS, Nagaraj, Neeta Pradhan, Nikhil Gupte, Nishi Suryavanshi, Chandrasekaran Padmapriyadarsini, Ponnuraja C, Premkumar N, Rahul Lokhande, Rajkumar S, Ranganathan K, Rani S, Rani V, Renu Bharadwaj, Renu Madewar, Rengaraj R, Rewa Kohli, Robert Bollinger, Rosemarie Warlick, Rupak Shivakoti, Sahadev Javanjal, Sameer Joshi, Sandhya Khadse, Sathyamurthi P, Shalini Pawar, Shashank Hande, Shital Muley, Shital Sali, Shri Vijay Bala Yogendra Shivakumar, Subapriya K, Shyam Biswal, Silambu Chelvi K, Smita Nimkar, Soumya Swaminathan, Sriram Selvaraj, Sundeep Salvi, Sushant Meshram, Surendhar S, Swapnil Raskar, Uma Devi, Vandana Kulkarni, Vidula Hulyalkar, Vidya Mave, Vinod Tayawade, Vrinda Bansode and Yogesh Daware.
References
- 1.Global TB Report 2018. World Health Organisation. Available from: http://apps.who.int/iris/bitstream/handle/10665/274453/9789241565646-eng.pdf?ua=1 (accessed 28 September 2018).
- 2. Fox GJ, Barry SE, Britton WJ, et al. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2013;41:140–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Getahun H, Matteelli A, Chaisson RE, et al. Latent mycobacterium tuberculosis infection. N Engl J Med. 2015;372(22):2127–35. [DOI] [PubMed] [Google Scholar]
- 4. Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Churchyard GJ, Felding KL, Lewis JJ, et al. A trial of mass isoniazid preventive therapy for tuberculosis control. N Engl J Med. 2014;370:301–10. [DOI] [PubMed] [Google Scholar]
- 6. Middelkoop K, Bekker LG, Myer L, et al. Rates of tuberculosis transmission to children and adolescents in a community with a high prevalence of HIV infection among adults. Clin Infect Dis. 2008;47:349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shanaube K, Sismanidis C, Ayles H, et al. Annual risk of tuberculous infection using different methods in communities with a high prevalence of TB and HIV in Zambia and South Africa. PLoS One. 2009;4(11):e7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8(6):359–68 [DOI] [PubMed] [Google Scholar]
- 9. American Thoracic Society (ATS), Centers for Disease Control and Prevention (CDC), Infectious Diseases Society of America (IDSA) Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000;161:S221–47. [DOI] [PubMed] [Google Scholar]
- 10. Pai M, Denkinger CM, Kik SV, et al. Gamma interferon release assays for detection of mycobacterium tuberculosis infection. Clin Microbiol Rev. 2014;27(1):3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hallett TB, Zaba B, Todd J, et al. Estimating incidence from prevalence in generalized HIV epidemics: methods and validation. PLoS Med 2008;5:e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Middelkoop K, Bekker LG, Liang H, et al. Force of tuberculosis infection among adolescents in a high HIV and TB prevalence community: a cross-sectional observational study. BMC Infect Dis. 2011;11:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu P, Lau EHY, Cowling BJ, et al. The transmission dynamics of tuberculosis in a recently developed Chinese city. PLoS One 2010;5(5):e10468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mathema B, Andrews JR, Cohen T, et al. Drivers of tuberculosis transmission. J Infect Dis. 2017;216 (6) :S644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gupte A, Padmapriyadarsini C, Mave V, et al. Cohort for Tuberculosis Research by the Indo-US Medical Partnership (CTRIUMPH): protocol for a multicentric prospective observational study. BMJ Open. 2016;6(2):e010542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nayak S, Acharjya B. Mantoux test and its interpretation. Indian Dermatol Online J. 2012;3(1):2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chandrasekaran P, Mave V, Thiruvengadam K, et al. Tuberculin skin test and QuantiFERON-Gold In-Tube assay for diagnosis of latent TB infection among household contacts of pulmonary TB patients in high TB burden setting. PLoS One. 2018;13(8):e0199360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Narain R, Geser A, Jambunathan MV, et al. Some aspects of a tuberculosis prevalence survey in a South Indian district. Bull World Health Organ. 1963;29:641–64. [PMC free article] [PubMed] [Google Scholar]
- 19. Mahomed H, Ehrlich R, Hawkridge T, et al. TB incidence in an adolescent cohort in South Africa. PLoS One. 2013;8(3):e59652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mahomed H, Ehrlich R, Hawkridge T, et al. Screening for TB in high school adolescents in a high burden setting in South Africa. Tuberculosis (Edinb). 2013;93(3):357–62. [DOI] [PubMed] [Google Scholar]
- 21. Andrews JR, Morrow C, Walensky RP, et al. Integrating social contact and environmental data in evaluating tuberculosis transmission in a South African township. J Infect Dis. 2014;210(4):597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zellner JL, Murray MB, Becerra MC, et al. Age-specific risks of tuberculosis infection from household and community exposures and opportunities for interventions in a high-burden setting. Am J Epidemiol. 2014;180(8):853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kwamanga D, Chakaya J, Sitienei J, et al. Tuberculosis transmission in Kenya: results of the third National Tuberculin Survey. Int J Tuberc Lung Dis. 2010;14(6):695–700. [PubMed] [Google Scholar]
- 24. Middelkoop K, Bekker LG, Morrow C, et al. Childhood tuberculosis infection and disease: a spatial and temporal transmission analysis in a South African township. S Afr Med J. 2009;99:738–43. [PMC free article] [PubMed] [Google Scholar]
- 25. Wang PD, Lin RS. Tuberculosis transmission in the family. J Infect. 2000;41(3):249–51. [DOI] [PubMed] [Google Scholar]
- 26. Nardell EA. Transmission and institutional infection control of tuberculosis. Cold Spring Harb Perspect Med. 2016;6(2):a018192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Verver S, Warren RM, Munch Z, et al. Proportion of tuberculosis transmission that takes place in households in an high-incidence area. Lancet. 2004;363:212–4. [DOI] [PubMed] [Google Scholar]
- 28. Narasimhan P, Wood J, MacIntyre CR, et al. Risk factors for tuberculosis. Pulm Med. 2013;2013:82893923476764 [Google Scholar]
- 29. Zellner JL, Murray MB, Becerra MC, et al. Bacillus Calmette-Guerin and isoniazid preventive therapy protect contacts of patients with tuberculosis. Am J Respir Crit Care Med. 2014;189(7):853–9. [DOI] [PMC free article] [PubMed] [Google Scholar]