Abstract
A new type of antibody–drug conjugate (ADC) has been prepared that contains a sulfur-bearing maytansinoid attached to an antibody via a highly stable tripeptide linker. Once internalized by cells, proteases in catabolic vesicles cleave the peptide of the ADC’s linker causing self-immolation that releases a thiol-bearing metabolite, which is then S-methylated. Conjugates were prepared with peptide linkers containing only alanyl residues, which were all l isomers or had a single d residue in one of the three positions. A d-alanyl residue in the linker did not significantly impair a conjugate’s cytotoxicity or bystander killing unless it was directly attached to the immolative moiety. Increasing the number of methylene units in the maytansinoid side chain of a conjugate did not typically affect an ADC’s cytotoxicity to targeted cells but did increase bystander killing activity. ADCs with the highest in vitro bystander killing were then evaluated in vivo in mice, where they displayed improved efficacy compared to previously described types of maytansinoid conjugates.
Keywords: Antibody−drug conjugates, maytansinoid, peptide
Antibody–drug conjugates (ADCs) are composed of an antibody attached to a cytotoxic payload via a linker.1,2 Most ADCs bind to target antigens expressed on the surface of cancer cells, then the conjugate internalizes into catabolic vesicles where the antibody, and possibly the linker, are degraded to release one or more cytotoxic metabolites. In some cases, these released metabolite(s) are membrane permeable, allowing them to diffuse into and kill neighboring cells in a tumor, an effect known as bystander killing.3 ADCs have been investigated for more than two decades; however, ado-trastuzumab emtansine (Kadcyla), for the treatment of metastatic breast cancer, is currently the only conjugate approved for a solid tumor indication.4 It is a maytansinoid bearing ADC that has a noncleavable linker and releases a charged metabolite that does not induce bystander killing.1 The high stability of noncleavably linked ADCs is expected to maximize delivery of cytotoxic molecules to tumors while reducing payload release in healthy tissues.2 However, the linker may not be ideal as many other conjugates using the same linker and payload as ado-trastuzumab emtansine were not successful in the clinic.5 Also, based on mouse xenograft studies, cleavably linked conjugates that induce bystander killing are typically more efficacious than noncleavably linked ADCs.6,7
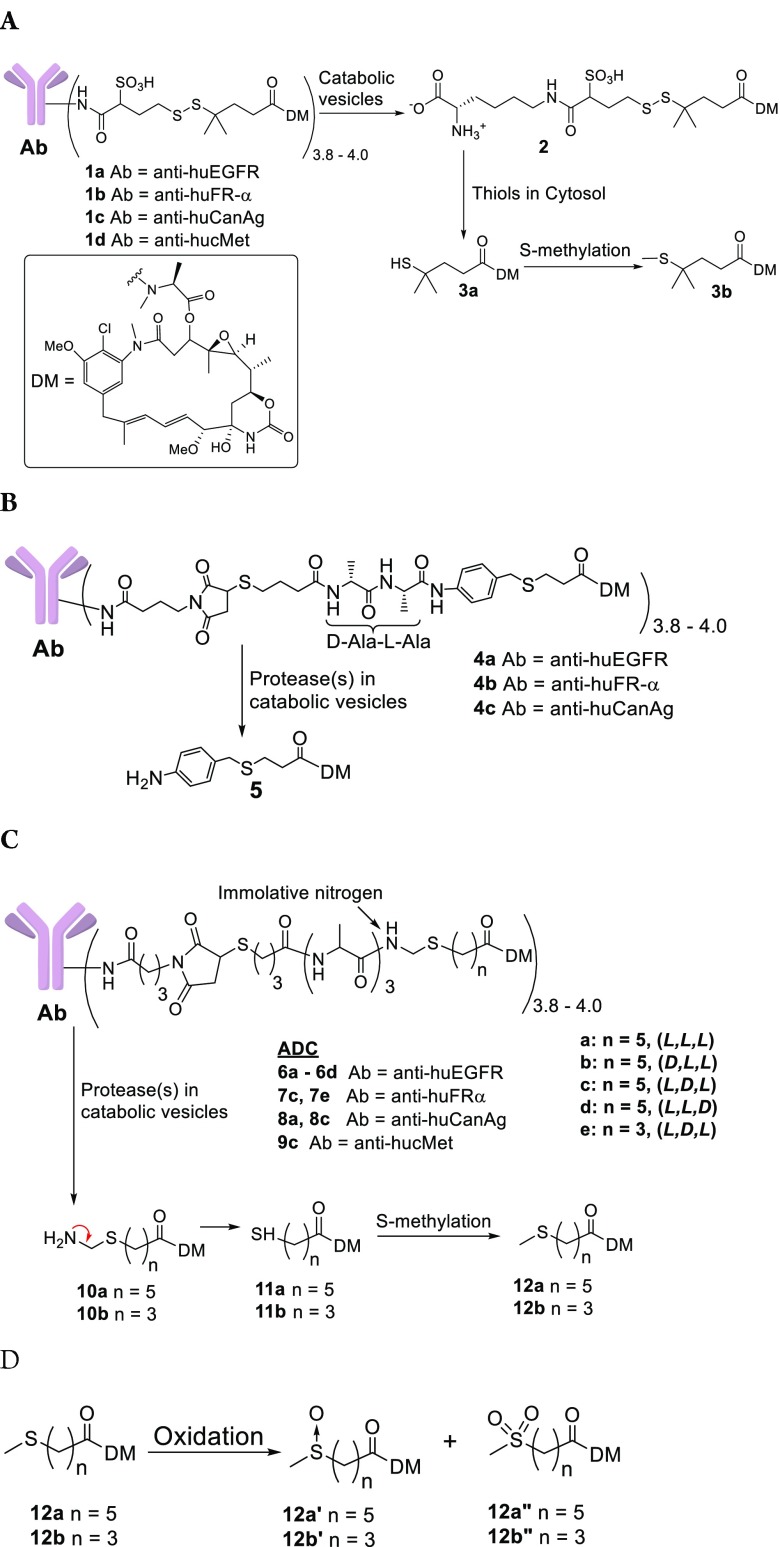
We have previously developed two types of maytansinoid ADCs that induce bystander killing, each have strengths but also potential for improvement. In the first type, the payload is linked to a lysine residue of an antibody via a disulfide bond, ADCs (1a–1d) are shown as examples, Figure 1A.8 After internalization by cells, the antibody of the ADC is catabolized to give 2 and most is reduced to give the thiol-containing maytansinoid (3a), some of which can then be S-methylated to 3b. Only 3a and 3b are hydrophobic, allowing them to diffuse into and kill bystander cells. Most of 3a that reaches the liver in vivo is S-methylated to 3b, which is efficiently oxidized to less potent compounds, potentially reducing systemic toxicity.9 We have previously shown that disulfide-linked ADCs typically show superior efficacy in mouse tumor xenograft models than conjugates bearing the same noncleavable linker used in ado-trastuzumab emtansine.6 The second type of maytansinoid conjugate that induces bystander killing has a peptide-anilino linkage, such as 4a–4c, Figure 1B.10 These ADCs are catabolized by cells to give the potent metabolite 5, which can induce bystander killing. However, linker half-lives of both disulfide-linked and anilino conjugates were only ∼5.5 days,10 which compares poorly to the stability of noncleavably linked conjugates (9.3 day).11
Figure 1.
(A) Structures of ADCs bearing disulfide linkers and the metabolites that are formed from their proteolysis in catabolic vesicles followed by disulfide cleavage and S-methylation. DM indicates the depicted maytansinoid moiety, derivatizable on the N-methyl alanyl amine. (B) Structures of ADCs with peptide-anilino linkers and the metabolite that is formed from proteolysis. (C) Structures of the new ADCs bearing peptide-immolative linkers and the metabolites that are expected to be formed in catabolic vesicles followed by S-methylation. Bracketed letters (l or d) indicate alanyl stereochemistry in the direction from antibody toward the maytansinoid. (D) Structures formed if the sulfur atoms of 12a–12c are oxidized.
ADCs can incorporate peptide linkers without anilino moieties; however, in order to maximize metabolite hydrophobicity, the conjugate should release noncharged compounds. Although their work was not on ADCs, Kingsbury and coauthors reported that a peptide could be enzymatically cleaved followed by immolation of the resulting hydrophilic amine to give a hydrophobic thiol-bearing compound.12 Based on these findings, the goal of this work was to design highly stable protease cleavable maytansinoid conjugates that release hydrophobic metabolites, capable of bystander killing, and determine if they are superior to disulfide-linked and anilino ADCs.
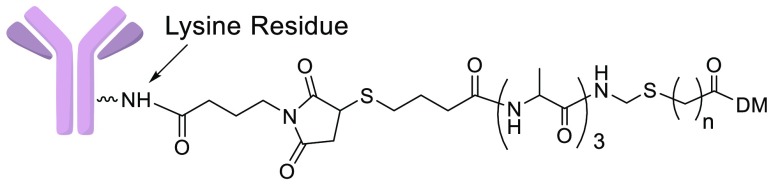
The humanized mAbs anti-huEGFR (targeting human epidermal growth factor 1),13 anti-huFRα (targeting human folate receptor-alpha),14 anti-huCanAg (targeting human CanAg antigen),15 and anti-hucMet (targeting human cMet antigen)16 were used in these studies. New immolative ADCs 6a–6d (Ab = anti-huEGFR), 7c, 7e (Ab = anti-huFRα), 8a, 8c (Ab = anti-huCanAg), and 9c (Ab = anti-hucMet) with tripeptide linkers were prepared, Figure 1C. After internalization into targeted Ag+ cells, the new ADCs were designed to be cleaved in lysosomes up to the immolative nitrogen atom, followed by release of the corresponding thiol-containing metabolite (11a or 11b), which in turn could potentially be S-methylated giving maytansinoids (12a or 12b). In order to simplify the interpretation of results, alanine was the only amino acid residue used in the linkers. Peptide linkers can often be stabilized by replacing an l-amino acid with its d-isomer.10,17 Thus, the effect of replacing one of the linker l-alanyl residues with its d isomer was investigated. Linker amino acids are listed in the direction from the antibody toward the maytansinoid, for example, (l, l, d) indicates that a d-alanyl residue is directly attached to the immolative nitrogen. Increasing a molecule’s hydrophobicity typically improves its diffusion into cells.18 Therefore, we wished to determine if changing the number of hydrophobic methylene units in the maytansinoid side chain, n, would affect ADC bystander killing.
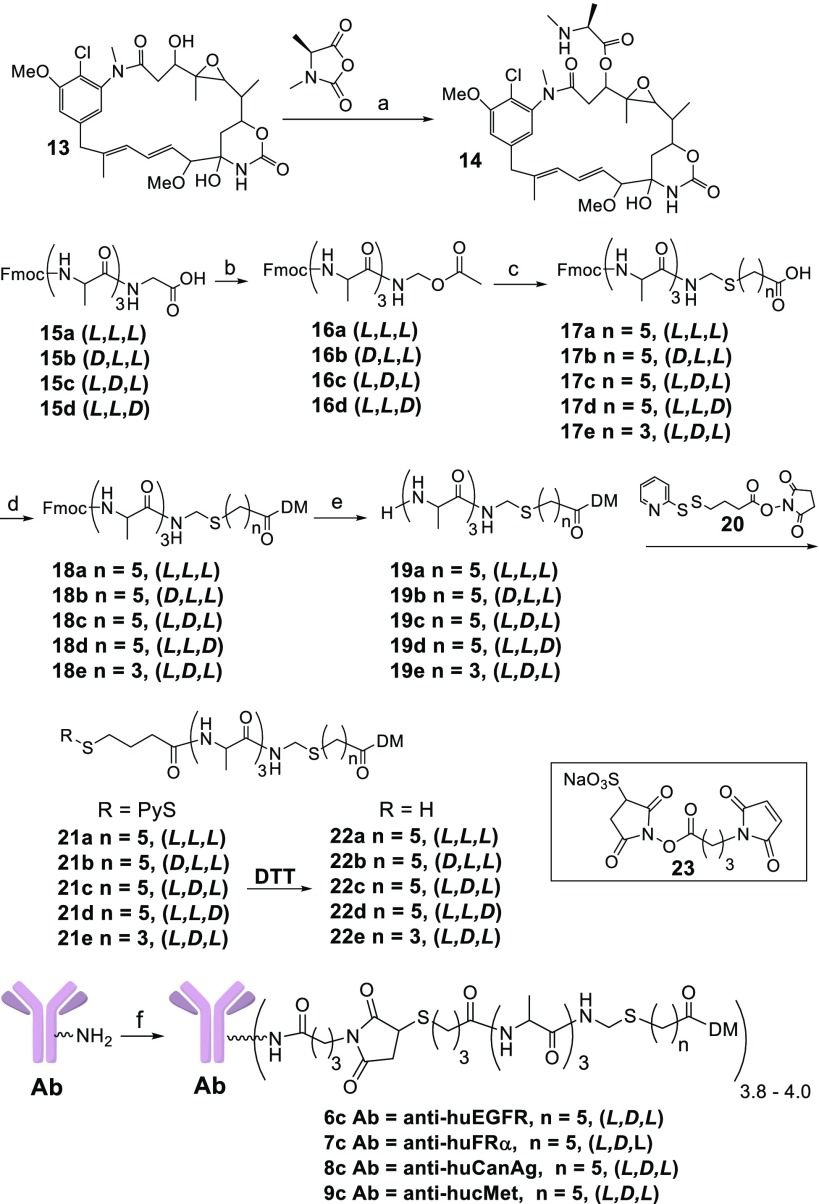
Immolative ADC payloads were prepared as depicted in Scheme 1. Maytansinol (13) was reacted with the carbonic anhydride of N-methyl alanine in the presence of zinc triflate and N,N-diisopropylethylamine (DIPEA) to give 14.19 Fmoc-protected alanyl peptides (15a–15d) were prepared by solid phase synthesis,20 then oxidatively decarboxylated with lead tetraacetate and acetic acid in dimethylformamide (DMF) to give 16a–16d respectively.21 Compounds 16a–16e were reacted with the appropriate thiol-bearing carboxylic acid in the presence of ∼20% trifluoroacetic acid (TFA) in dichloromethane to give 17a–17e. Compound 14 was coupled to 17a–17e using 1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide (EDC) to give 18a–18e, which were then deprotected with morpholine giving 19a–19e. The heterobifunctional linker (20) was coupled to 19a–19e to give 21a–21e, and disulfide reduction with 1,4-dithiothreitol (DTT) gave payloads (22a–22e). Immolative ADCs were prepared from one of these payloads and a mAb, as depicted for representative conjugations with 22c, Scheme 1. The heterobifunctional linker 23 was coupled to the thiol of 22c in a solution of buffer–organic solvent. The reaction solution, without purification, was reacted with a mAb, and the mixture was purified by size exclusion chromatography (SEC). Disulfide-linked and peptide anilino ADCs as well as their metabolites were prepared as previously described.10,22
Scheme 1. Synthesis of New Immolative Maytansinoid ADCs.
Reagents and conditions: (a) Zn(OTf)2, DIPEA; (b) Pb(OAc)4, AcOH; (c) HS-(CH2)n-COOH, TFA; (d) EDC, 14; (e) morpholine; (f) 22c, 23.
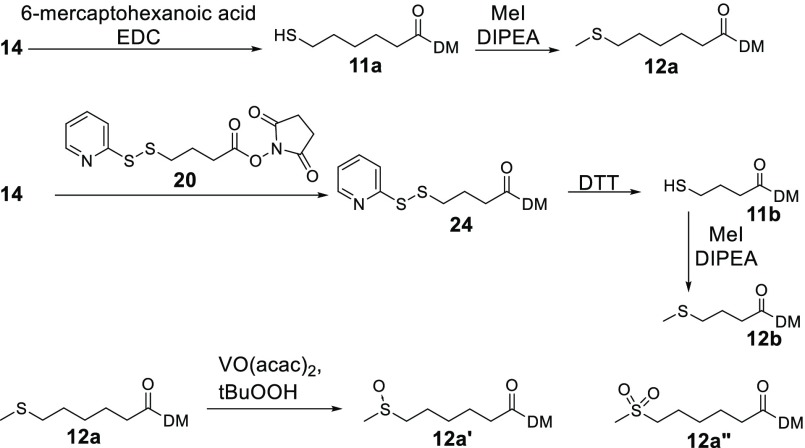
The compounds that were expected to be formed when cells metabolize immolative ADCs were prepared as depicted in Figure 2. Compound 14 was reacted with 6-mercaptohaxanoic acid to give 11a, which was then reacted with iodomethane to give 12a. Compound 14 was also coupled with 20 to give 2. The disulfide of 24 was then reduced with DTT to give 11b, which was reacted with iodomethane to give 12b. The S-methylated metabolites could potentially be oxidized on their sulfur atoms in liver. Representative compounds 12a′ and 12a″ were prepared by oxidizing 12a with tert-butyl hydroperoxide in the presence of vanadium acetylacetonate.
Figure 2.
Preparation of possible immolative ADC metabolites 12a, 12b, 12c, 12a′, and 12a″.
To determine if linker stereochemistry affected cytotoxicity, the anti-huEGFR immolative ADCs (6a–6d) were incubated with Ag+ CA922 cells, Figure 3A. Cytotoxicities for all ADCs were determined as previously described.23 Conjugate 6d (l, l, d) was the least potent, while the other conjugates exhibited similar high cytotoxicities. This indicated that ADC cytotoxicity was impaired when a d-alanine was directly attached to the immolative nitrogen, which will be designated as a d-linked conjugate. Conjugate 1a also had similar potency as 6a–6c against CA922 cells. In each case, the addition of a 1000-fold molar excess of the unconjugated anti-huEGFR antibody fully inhibited ADC potency, demonstrating that cytotoxicity was antigen-dependent.
Figure 3.
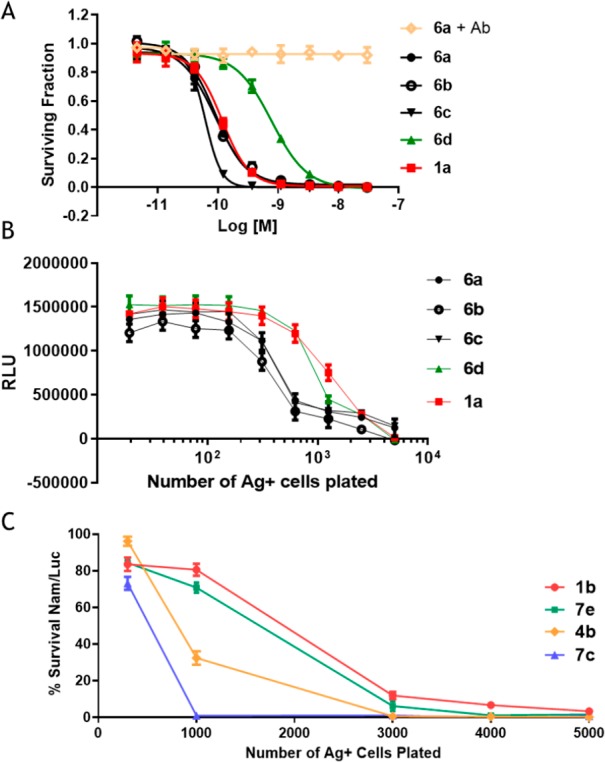
(A) In vitro cytotoxicities of immolative ADCs bearing peptides with different stereochemistries and of 1a against CA922 cells. (6a + Ab) indicates 6a plus 1000 equiv of anti-EGFR antibody. (B) Bystander killing (via assay 1) of immolative conjugates bearing peptides of different stereochemistries compared to 1a. RLU indicates relative luminescence units. (C) In vitro bystander killing activity (via assay 2) of immolative ADCs that have different n values and the disulfide-linked ADC 1b. The cell lines Jeg-3 cells (Ag+) and Nam\Luc (Ag−), which are Namalwa cells stably transfected with luciferase, were used.
Next, the effect of methylene side chain length (n) on ADC cytotoxicity to cells that homogeneously express target antigen was investigated. The cytotoxicities of 7e and 7c, having n values of 3 and 5, respectively, were most often similar to each other, and to 1b (Supplementary Table S1). ADCs 6a–6d were tested for their ability to induce bystander killing in vitro, Figure 3B. A fixed number of Ag– cells were incubated with ADC and a variable number of Ag+ cells.3 The Ag+ cells take up and degrade the ADC to release metabolite(s) that can kill these cells. Membrane permeable metabolite(s) may also diffuse into and kill a portion of the proximal Ag– cells (bystander cells). ADCs that induce the highest level of bystander killing require addition of the lowest number of added Ag+ cells to kill the Ag– cells in the mixed cell population. The relative number of HCC827 Ag+ cells needed to kill 50% of the MCF-7 Ag– cells were compared. Conjugates 6d and 1a required addition of ∼1250 Ag+ cells, while the other ADCs required only ∼400 Ag+ cells. Thus, 6a, 6b, and 6c induced ∼3-fold greater in vitro bystander killing than 6d and 1a.
As shown in Figure 3C, increasing the number of methylene units in the maytansinoid side chain increased the in vitro bystander killing of immolative ADCs. Conjugates 7e and 7c, having 3 and 5 methylene groups in the maytansinoid side chain, required approximately 1800 and 700 Jeg-3 Ag+ cells, respectively, to induce 50% Nam/Luc Ag– cell killing. Thus, 7c induced ∼2.5-fold more bystander killing than 7e. Conjugate 7c also induced more bystander killing than 4b and 1b, which required approximately 950 and 2000 Ag+ cells, respectively, to induce 50% cell killing. The similar cytotoxicities of 7c and 7e could be explained if their metabolites are produced with similar efficiencies and have similar potencies once inside cells. However, if the metabolites of 7c are more hydrophobic, as expected, then they could more easily diffuse into other cells to induce more bystander killing. Additional studies will be needed to determine if this is the case; however, because of their lower bystander killing, conjugates with n = 3 were not evaluated further.
The metabolites of 8c were identified by incubating the conjugate with the high processing cell line COLO-205 for 24 h followed by cell lysis with organic solvent, using a previously described method.8 As a control, COLO-205 cells were treated the same way but without prior exposure to 8c. Both samples were analyzed by UPLC/MS (Supplementary Figure S1). The 252 nm UPLC trace of cells treated with 8c showed two peaks that were not in the control, a major peak at 12.73 min and a minor peak at 7.72 min having about a fourth of the area of the major peak. The synthesized S-methylated standards 12a and its sulfoxide 12a′, shown in Figure 1D, were found to have the same retention times and mass spectra as the 12.73 and 7.72 min peaks, respectively. Sulfides are prone to air oxidation, and trace metals can act as catalysts.24 Indeed aqueous solutions of standard 12a were oxidized to 12a′ over time (data not shown). Indicating that 12a′ in cell lysates was likely an artifact due to oxidation of 12a by air.
The in vitro cytotoxicities of the synthesized S-methylated metabolites 12a–12b were evaluated on the H1703, H1975, and COLO-704 cell lines, Table 1. The potency of 12a (n = 5) was consistently ∼2.5 times that of 12b (n = 3), indicating that the cytotoxicity of free metabolites increased as hydrophobic methylene units were added. Also, 12a had a similar potency to the isomeric compound 3b. The synthesized sulfone 12a″ and the racemic sulfoxide 12a′ were at least 80-fold less cytotoxic than the precursor 12a. Indicating that if 12a is efficiently oxidized in liver it should be substantially inactivated.
Table 1. In Vitro Cytotoxicities of S-Methylated ADC Metabolites and the Oxidation Products of 12a (12a′ and 12a″).
| Cell
Line IC50 (M) |
|||
|---|---|---|---|
| metabolite | H1703 | H1975 | COLO-704 |
| 12a (n = 5) | 2.3 × 10–12 | 2.5 × 10–12 | 3.0 × 10–12 |
| 12b (n = 3) | 5.9 × 10–12 | 5.6 × 10–12 | 6.9 × 10–12 |
| 3b | 3.7 × 10–12 | 4.1 × 10–12 | 5.1 × 10–12 |
| 12a′ | 3.1 × 10–10 | 8.4 × 10–10 | 8.2 × 10–10 |
| 12a″ | 6.3 × 10–10 | 2.1 × 10–10 | 3.6 × 10–10 |
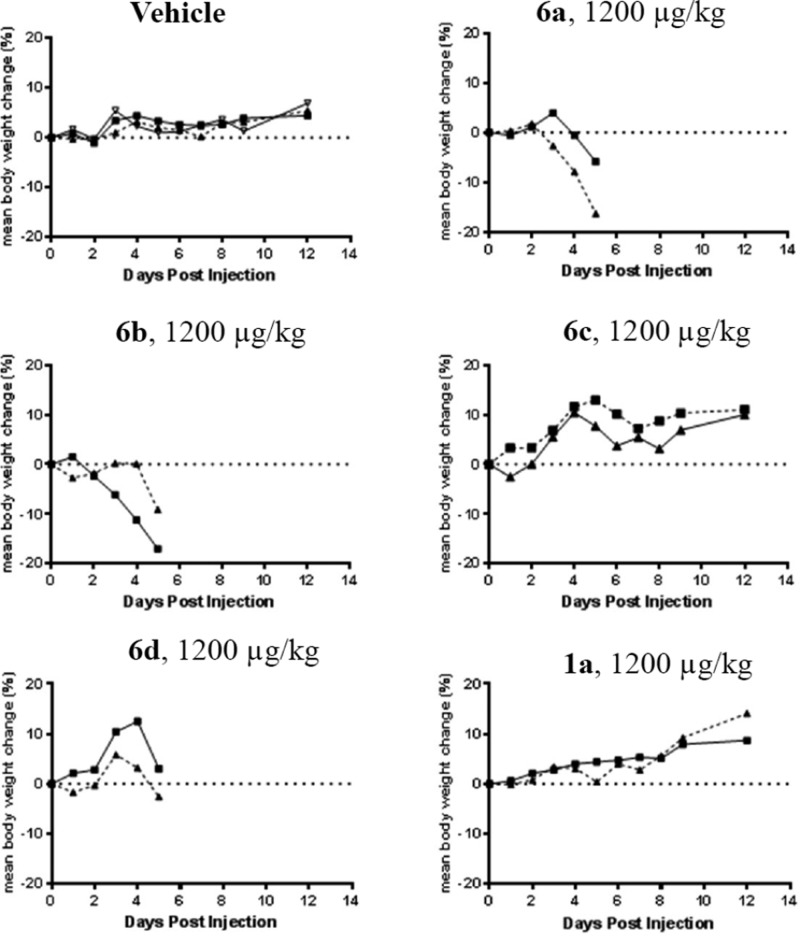
Earlier studies with maytansinoid ADCs indicated mouse tolerabilities between 1200–1500 μg/kg based on the mass of delivered payload.6,10 An initial study was conducted using anti-huEGFR ADCs (2 mice/group) at a dose of 1200 μg/kg based on the mass of delivered payload (47 mg/kg based on the mass of antibody) to determine if the tolerability of immolative conjugates was affected by having a single d-alanyl in the tripeptide linker and how these tolerabilities compared to a disulfide-linked ADC, Figure 4. The groups receiving vehicle, 1a, and 6c gained weight, although some skin reddening was noted in mice receiving 6c. In contrast, animals that had been treated with 6a and 6b lost weight and displayed pronounced skin reddening. The mice in the 6d group, d-linked conjugate, also displayed some skin toxicity, but weight loss was not as pronounced as the 6a and 6b groups. This study revealed that immolative ADCs bearing the l-Ala-d-Ala-l-Ala linker were better tolerated in mice than those bearing the other tripeptide linkers.
Figure 4.
Change in body weight of CD-1 mice treated with vehicle (3 mice/group), the immolative ADCs 6a–6d (2 mice/group), or with the disulfide-linked ADC 1a (2 mice/group).
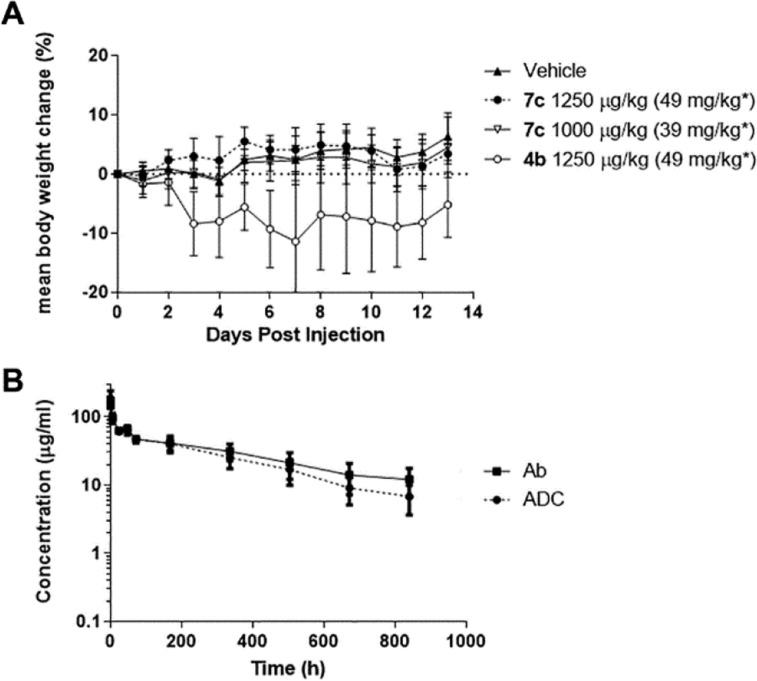
A second MTD study was undertaken, using ADCs bearing an anti-huFRα antibody, Figure 5A. The groups (8 mice/group) consisted of vehicle, 4b (1250 μg/kg), and the immolative ADC 7c (l, d, l) at 1000 and 1250 μg/kg. Mice in all groups gained weight, but the 7c (1250 μg/kg) group had some skin reddening. Because of their favorable tolerability, immolative ADCs bearing the l-Ala-d-Ala-l-Ala peptide were evaluated in further in vivo studies.
Figure 5.
(A) In vivo tolerability in mice (8/group) of the immolative ADC 7c vs the anilino ADC 4b and vehicle. * indicates the concentration of ADC based on the antibody component. (B) Pharmacokinetic study of 7c in mice where ■ is the concentration versus time plot of the antibody component and ● is the concentration versus time plot of the payload component.
The clearance of 7c at a dose of 10 mg/kg from circulation in nontumor bearing mice was determined by ELISA as previously described.25 The half-lives of the ADC’s antibody and drug components were ∼15.2 and ∼10.9 days, respectively, Figure 5B.
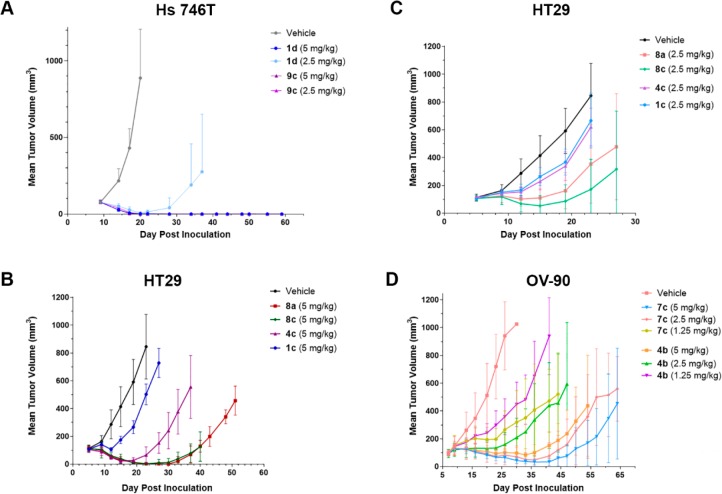
The in vivo efficacy of maytansinoid ADCs 9c and 1d was compared in mice (8/group) bearing Hs 746T tumor xenografts, where the target c-Met antigen is expressed homogeneously, Figure 6A. The H-Score for these cells,26 a measure of antigen expression, is 160. At the 2.5 mg/kg dose, complete regressions (CRs) were achieved for all mice in the 9c group, but only two CRs were seen in the 1d group, which latter regrew. Next anti-huCanAg ADCs (5 mg/kg) were compared in mice (6/group) bearing HT-29 xenografts, where CanAg is only expressed on 20–30% of cancer cells in the tumor, Figure 6B.27 The greatest antitumor activity was observed with the immolative ADCs 8a (l, l, l) and 8c (l, d, l), resulting in CRs in 6/6 mice, although tumors began to regrow at day 30. The anilino ADC 4c (2/6 CRs) and the disulfide-linked ADC 1c (0/6 CRs) were less efficacious. HT-29 xenograft bearing mice (6/group) were also treated at 2.5 mg/kg; group 8c (2 CRs) had a larger reduction in median tumor size than group 8a (1 CR), Figure 6C. The in vivo efficacy of anti-huFRα ADC was compared in mice bearing OV-90 xenografts, which express folate receptor heterogeneously (H-Score = 48), Figure 6D. The immolative ADC 7c was approximately twice as efficacious as the anilino ADC 4b, based on the similarity in the tumor volume plots for group 7c (1.25 mg/kg) and group 4b (2.5 mg/kg), and the similar or better response of group 7c (2.5 mg/kg) compared to group 4b (5 mg/kg).
Figure 6.
In vivo efficacy of maytansinoid ADCs against (A) homogeneously expressing mouse Hs746T xenografts (8 mice/group), (B) heterogeneously expressing HT-29 xenografts (6 mice/group, 5 mg/kg), (C) heterogeneously expressing HT-29 xenografts (6 mice/group, 2.5 mg/kg), and (D) heterogeneously expressing OV-90 xenografts (6 mice/group).
In conclusion, the in vitro bystander killing of immolative ADCs positively correlated with metabolite hydrophobicity. Conjugates with adjacent linker l-alanyl residues were not as well tolerated and were less efficacious than the (l-Ala-d-Ala-l-Ala) peptide-linked ADC. We postulate that ADCs with adjacent l-alanyl residues may be less stable in vivo, which would increase metabolite release in healthy tissues, and lower payload delivery to tumors. Finally, increasing both in vitro bystander killing, and linker stability resulted in ADCs having a better therapeutic window in mice than previously described maytansinoid conjugates.
Acknowledgments
We would like to thank Richard Bates for editing of the manuscript, Shan Jin for analysis of metabolites, Erica Hong for the preparation of some ADCs used in this work, and Richard Gregory, Joseph Kenny, and David Pleynet for helpful suggestions.
Glossary
ABBREVIATIONS
- ADC
antibody–drug conjugate
- CR
complete regression (tumor xenograft no longer detected)
- DIPEA
N,N-diisopropylethylamine
- DMF
dimethylformamide
- DTT
1,4-dithiothreitol
- EDC
1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide
- PR
partial regression (50% reduction of tumor xenograft size)
- Et3N
triethylamine
- SEC
size-exclusion chromatography
- TFA
trifluoroacetic acid
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.9b00310.
Detailed experimental procedures(PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This study was supported by ImmunoGen, Inc.
The authors declare no competing financial interest.
Supplementary Material
References
- Dan N.; Setua S.; Kashyap V. K.; Khan S.; Jaggi M.; Yallapu M. M.; Chauhan S. C. Antibody-drug conjugates for cancer therapy: chemistry to clinical implications. Pharmaceuticals (Basel) 2018, 11, E32. 10.3390/ph11020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargh J. D.; Isidro-Llobet A.; Parker J. S.; Spring D. R. Cleavable linkers in antibody–drug conjugates. Chem. Soc. Rev. 2019, 48, 4361. 10.1039/C8CS00676H. [DOI] [PubMed] [Google Scholar]
- Kovtun Y. V.; Audette C. A.; Ye Y.; Xie H.; Ruberti M. F.; Phinney S. J.; Leece B. A.; Chittenden T.; Blaettler W. A.; Goldmacher V. S. Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res. 2006, 66, 3214. 10.1158/0008-5472.CAN-05-3973. [DOI] [PubMed] [Google Scholar]
- Girish S.; Gupta M.; Wang B.; Lu D.; Krop I. E.; Vogel C. L.; Burris H. A. Iii; LoRusso P. M.; Yi J. H.; Saad O.; Tong B.; Chu Y. W.; Holden S.; Joshi A. Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody-drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother. Pharmacol 2012, 69, 1229. 10.1007/s00280-011-1817-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Goeij B. E.; Lambert J. M. New developments for antibody-drug conjugate-based therapeutic approaches. Curr. Opin. Immunol. 2016, 40, 14. 10.1016/j.coi.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Kellogg B. A.; Garrett L.; Kovtun Y.; Lai K. C.; Leece B.; Miller M.; Payne G.; Steeves R.; Whiteman K. R.; Widdison W.; Xie H.; Singh R.; Chari R. V.; Lambert J. M.; Lutz R. J. Disulfide-linked antibody-maytansinoid conjugates: optimization of in vivo activity by varying the steric hindrance at carbon atoms adjacent to the disulfide linkage. Bioconjugate Chem. 2011, 22, 717. 10.1021/bc100480a. [DOI] [PubMed] [Google Scholar]
- Drake P. M.; Rabuka D. An emerging playbook for antibody–drug conjugates: lessons from the laboratory and clinic suggest a strategy for improving efficacy and safety. Curr. Opin. Chem. Biol. 2015, 28, 174. 10.1016/j.cbpa.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Erickson H. K.; Park P. U.; Widdison W. C.; Kovtun Y. V.; Garrett L. M.; Hoffman K.; Lutz R. J.; Goldmacher V. S.; Blattler W. A. Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res. 2006, 66, 4426. 10.1158/0008-5472.CAN-05-4489. [DOI] [PubMed] [Google Scholar]
- Sun X.; Widdison W.; Mayo M.; Wilhelm S.; Leece B.; Chari R.; Singh R.; Erickson H. Design of antibody-maytansinoid conjugates allows for efficient detoxification via liver metabolism. Bioconjugate Chem. 2011, 22, 728. 10.1021/bc100498q. [DOI] [PubMed] [Google Scholar]
- Widdison W. C.; Ponte J. F.; Coccia J. A.; Lanieri L.; Setiady Y.; Dong L.; Skaletskaya A.; Hong E. E.; Wu R.; Qiu Q.; Singh R.; Salomon P.; Fishkin N.; Harris L.; Maloney E. K.; Kovtun Y.; Veale K.; Wilhelm S. D.; Audette C. A.; Costoplus J. A.; Chari R. V. Development of anilino-maytansinoid ADCs that efficiently release cytotoxic metabolites in cancer cells and induce high levels of bystander killing. Bioconjugate Chem. 2015, 26, 2261. 10.1021/acs.bioconjchem.5b00430. [DOI] [PubMed] [Google Scholar]
- Ponte J. F.; Sun X.; Yoder N. C.; Fishkin N.; Laleau R.; Coccia J.; Lanieri L.; Bogalhas M.; Wang L.; Wilhelm S.; Widdison W.; Pinkas J.; Keating T. A.; Chari R.; Erickson H. K.; Lambert J. M. Understanding how the stability of the thiol-maleimide linkage impacts the pharmacokinetics of lysine-linked antibody-maytansinoid conjugates. Bioconjugate Chem. 2016, 27, 1588. 10.1021/acs.bioconjchem.6b00117. [DOI] [PubMed] [Google Scholar]
- Kingsbury W. D.; Boehm J. C.; Perry D.; Gilvarg C. Portage of various compounds into bacteria by attachment to glycine residues in peptides. Proc. Natl. Acad. Sci. U S A 1984, 81, 4573. 10.1073/pnas.81.14.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K. C.; Deckert J.; Setiady Y. Y.; Shah P.; Wang L.; Chari R.; Lambert J. M. Evaluation of targets for maytansinoid ADC therapy using a novel radiochemical assay. Pharm. Res. 2015, 32, 3593. 10.1007/s11095-015-1633-2. [DOI] [PubMed] [Google Scholar]
- Ab O.; Whiteman K. R.; Bartle L. M.; Sun X.; Singh R.; Tavares D.; LaBelle A.; Payne G.; Lutz R. J.; Pinkas J.; Goldmacher V. S.; Chittenden T.; Lambert J. M. IMGN853, a Folate receptor-alpha (FRalpha)-targeting antibody-drug conjugate, exhibits potent targeted antitumor activity against FRalpha-expressing tumors. Mol. Cancer Ther. 2015, 14, 1605. 10.1158/1535-7163.MCT-14-1095. [DOI] [PubMed] [Google Scholar]
- Tolcher A. W.; Ochoa L.; Hammond L. A.; Patnaik A.; Edwards T.; Takimoto C.; Smith L.; de Bono J.; Schwartz G.; Mays T.; Jonak Z. L.; Johnson R.; DeWitte M.; Martino H.; Audette C.; Maes K.; Chari R. V.; Lambert J. M.; Rowinsky E. K. Cantuzumab mertansine, a maytansinoid immunoconjugate directed to the CanAg antigen: a phase I, pharmacokinetic, and biologic correlative study. J. Clin. Oncol. 2003, 21, 211. 10.1200/JCO.2003.05.137. [DOI] [PubMed] [Google Scholar]
- Lai K. C.; Li M.; Selvitelli K.; Sikka S.; Boulé S.; Gavrilescu L. C.; Hicks S. W.; Donahue K. Abstract 4817: Preclinical evaluation of a new, non-agonist ADC targeting MET amplified tumors with a peptide-linked maytansinoid. Cancer Res. 2019, 79, 1. 10.1200/JCO.2003.05.137. [DOI] [Google Scholar]
- Doronina S. O.; Bovee T. D.; Meyer D. W.; Miyamoto J. B.; Anderson M. E.; Morris-Tilden C. A.; Senter P. D. Novel peptide linkers for highly potent antibody-auristatin conjugate. Bioconjugate Chem. 2008, 19, 1960. 10.1021/bc800289a. [DOI] [PubMed] [Google Scholar]
- Bennion B. J.; Be N. A.; McNerney M. W.; Lao V.; Carlson E. M.; Valdez C. A.; Malfatti M. A.; Enright H. A.; Nguyen T. H.; Lightstone F. C.; Carpenter T. S. Predicting a drug’s membrane permeability: A computational model validated with in vitro permeability assay data. J. Phys. Chem. B 2017, 121, 5228. 10.1021/acs.jpcb.7b02914. [DOI] [PubMed] [Google Scholar]
- Akssira M.; Boumzebra M.; Kasmi H.; Dahdouh A. New Routes to 1,4- Benzodiazepin-2,5-diones. Tetrahedron 1994, 50, 9051. 10.1016/S0040-4020(01)85371-0. [DOI] [Google Scholar]
- Benoiton L.Chemistry of Peptide Synthesis; CRC Press, 2006. [Google Scholar]
- Kumar N. S.; Ratnayake R. M.; Widmalm G.; Jansson P. E. Selective cleavage of welan gum (S-130) by oxidative decarboxylation with lead tetraacetate. Carbohydr. Res. 1996, 291, 109. 10.1016/S0008-6215(96)00137-1. [DOI] [PubMed] [Google Scholar]
- Widdison W. C.; Wilhelm S. D.; Cavanagh E. E.; Whiteman K. R.; Leece B. A.; Kovtun Y.; Goldmacher V. S.; Xie H.; Steeves R. M.; Lutz R. J.; Zhao R.; Wang L.; Blattler W. A.; Chari R. V. Semisynthetic maytansine analogues for the targeted treatment of cancer. J. Med. Chem. 2006, 49, 4392. 10.1021/jm060319f. [DOI] [PubMed] [Google Scholar]
- Kovtun Y. V.; Audette C. A.; Mayo M. F.; Jones G. E.; Doherty H.; Maloney E. K.; Erickson H. K.; Sun X.; Wilhelm S.; Ab O.; Lai K. C.; Widdison W. C.; Kellogg B.; Johnson H.; Pinkas J.; Lutz R. J.; Singh R.; Goldmacher V. S.; Chari R. V. Antibody-maytansinoid conjugates designed to bypass multidrug resistance. Cancer Res. 2010, 70, 2528. 10.1158/0008-5472.CAN-09-3546. [DOI] [PubMed] [Google Scholar]
- Hoffmann M. R.; Lim B. C. Kinetics and mechanism of the oxidation of sulfide by oxygen: catalysis by homogeneous metal-phthalocyanine complexes. Environ. Sci. Technol. 1979, 13, 1406. 10.1021/es60159a014. [DOI] [Google Scholar]
- Xie H.; Audette C.; Hoffee M.; Lambert J. M.; Blattler W. A. Pharmacokinetics and biodistribution of the antitumor immunoconjugate, cantuzumab mertansine (huC242-DM1), and its two components in mice. J. Pharmacol. Exp. Ther. 2004, 308, 1073. 10.1124/jpet.103.060533. [DOI] [PubMed] [Google Scholar]
- Detre S.; Saclani Jotti G.; Dowsett M. A ″quickscore″ method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J. Clin. Pathol. 1995, 48, 876. 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.; Tadayoni B. M.; Bourret L. A.; Mattocks K. M.; Derr S. M.; Widdison W. C.; Kedersha N. L.; Ariniello P. D.; Goldmacher V. S.; Lambert J. M.; Blattler W. A.; Chari R. V. Eradication of large colon tumor xenografts by targeted delivery of maytansinoids. Proc. Natl. Acad. Sci. U. S. A. 1996, 93, 8618. 10.1073/pnas.93.16.8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.