Abstract
Background:
Prenatal exposure to air pollution has been associated with childhood respiratory disease and other adverse outcomes. Epigenetics is a suggested link between exposures and health outcomes.
Objectives:
We aimed to investigate associations between prenatal exposure to particulate matter (PM) with diameter () or () and DNA methylation in newborns and children.
Methods:
We meta-analyzed associations between exposure to () and () at maternal home addresses during pregnancy and newborn DNA methylation assessed by Illumina Infinium HumanMethylation450K BeadChip in nine European and American studies, with replication in 688 independent newborns and look-up analyses in 2,118 older children. We used two approaches, one focusing on single cytosine-phosphate-guanine (CpG) sites and another on differentially methylated regions (DMRs). We also related PM exposures to blood mRNA expression.
Results:
Six CpGs were significantly associated [false discovery rate (FDR) ] with prenatal and 14 with exposure. Two of the CpGs mapped to FAM13A (cg00905156) and NOTCH4 (cg06849931) previously associated with lung function and asthma. Although these associations did not replicate in the smaller newborn sample, both CpGs were significant () in 7- to 9-y-olds. For cg06849931, however, the direction of the association was inconsistent. Concurrent exposure was associated with a significantly higher NOTCH4 expression at age 16 y. We also identified several DMRs associated with either prenatal and or exposure, of which two DMRs, including H19 and MARCH11, replicated in newborns.
Conclusions:
Several differentially methylated CpGs and DMRs associated with prenatal PM exposure were identified in newborns, with annotation to genes previously implicated in lung-related outcomes. https://doi.org/10.1289/EHP4522
Introduction
Many studies have reported adverse health effects of prenatal air pollution exposure in children, including adverse pregnancy outcomes, reduced lung growth, and increased risks of respiratory morbidity (Lamichhane et al. 2015; Korten et al. 2017; Horne et al. 2018). Findings from experimental models suggest that oxidative stress, inflammation, and mitochondrial dysfunction may contribute to health effects of particulate exposure, but our understanding of the involved mechanisms remains limited (Cassee et al. 2013; Niranjan and Thakur 2017). Recent studies demonstrate that environmental exposures may induce epigenetic modifications and that these changes can have long-lasting effects on gene expression and cell function (Desai et al. 2017; Gref et al. 2017). DNA methylation, the most studied epigenetic mechanism, entails cytosine modification with a methyl group at positions in DNA where a cytosine is located next to a guanine, a cytosine-phosphate-guanine (CpG) site. The crucial role of methylation in maintaining genomic stability and regulation of gene function makes it a potential mechanism by which environmental exposures contribute to the etiology of complex diseases.
Prenatal life is an important window of susceptibility to adverse effects of environmental hazards. In utero exposures may lead to epigenetic changes that influence fetal development and contribute to health outcomes throughout the life course (Barouki et al. 2018). Studies on prenatal exposures to cigarette smoke and traffic-related air pollution reported associations with modifications of the offspring epigenome (Joubert et al. 2016; Gruzieva et al. 2017). The majority of published studies investigated variability of DNA methylation in relation to air pollution either globally (i.e., overall methylation state of the genome) (Plusquin et al. 2017) or applying candidate-gene approaches (Somineni et al. 2016; Hew et al. 2015), but comprehensive evaluations of genome-wide DNA methylation patterns in children are limited (Breton et al. 2016; Gruzieva et al. 2017; Plusquin et al. 2018).
Epigenome-wide association studies (EWAS) of particulate air pollution exposure have so far been based almost exclusively on adult populations with inconclusive results. Epigenome-wide association studies of short-term exposure to particulate matter (PM) with an aerodynamic diameter of () reported associations with DNA methylation within genes involved in protein kinase and NFkB pathways (Jiang et al. 2014), as well as oxidative stress (Panni et al. 2016), although no robust associations could be demonstrated with long-term particulate exposure (Plusquin et al. 2017). We have previously found epigenome-wide cord blood DNA methylation differences in several mitochondria-related genes in relation to prenatal exposure to nitrogen dioxide, a marker of traffic-derived combustion pollutants (Gruzieva et al. 2017).
Earlier studies have focused on individual differentially methylated CpGs rather than differentially methylated regions (DMRs) (Breton et al. 2016; Gruzieva et al. 2017; Panni et al. 2016). DMR analysis is a statistically more powerful approach for detecting associations with exposures or health outcomes, as it uses the patterns of correlation between nearby CpGs to take advantage of the epigenomic structure (Pedersen et al. 2012; Peters et al. 2015). For the present study, we meta-analyzed genome-wide DNA methylation data in newborns in relation to maternal exposure to PM during pregnancy to identify both individual CpGs and regions of differential methylation. Furthermore, the associations found between maternal exposure to PM and cord blood DNA methylation were examined in independent data sets of newborn and older children. We also examined differences in peripheral blood gene expression for identified genes in relation to prenatal [in newborns from the Early Autism Risk Longitudinal Investigation (EARLI) cohort, ] and current air pollution exposure [in 16-y-olds from the Barn, Allergi, Miljö, Stockholm och Epidemiologi (BAMSE) cohort in Sweden (titled Children, Allergy, Milieu, Stockholm, Epidemiology in English), ].
Methods
Detailed information about each of the study cohorts in this analysis, including recruitment and eligibility; information about methods for measuring DMA methylation and gene expression, including quality control and normalization procedures; and detailed information about air pollution exposure estimation, are provided in Supplemental Material. Average concentrations of and throughout pregnancy were estimated at maternal home addresses through land-use regression (LUR) or equivalent models.
Discovery Study Population
A total of nine European and American studies participating in the Pregnancy and Childhood Epigenetics consortium (PACE) (Felix et al. 2017) were included in the discovery meta-analysis of particulate air pollution exposure during pregnancy and newborn DNA methylation (total ): INfancia y Medio Ambiente (INMA), Generation R, Southern California Children’s Health Study (CHS), Early Autism Risk Longitudinal Investigation (EARLI), the PRogramming of Intergenerational Stress Mechanisms (PRISM), Project Viva, Environmental Influences on Early Ageing (ENVIRONAGE), Rhea Mother and Child Cohort in Crete, Greece (Rhea), and Piccolipiù (Table 1).
Table 1.
Basic characteristics of cohorts included in the discovery EWAS meta-analysis.
| STUDY | Country | Enrollment period | Total N enrolled | Selection criteria for EWAS | Air pollution exposure assessment | DNA methylation measurement | Study reference (PMID) | Study website |
|---|---|---|---|---|---|---|---|---|
| INMA | Spain | 1997–2008 | 3768 | available DNA from one of the subcohorts (Sabadell) | LUR | Illumina | 21471022 | http://www.proyectoinma.org/ |
| Generation R | Netherlands | 2002–2006 | 9901 | European, complete follow-up | LUR | Illumina | 23086283, 25527369 | www.generationr.nl |
| CHS | USA | 1995–1997 | 5341 | non-Hispanic white/Hispanic white | Outdoor air pollution monitoring stations in each of the study communities | Illumina | 16675435, 22896588 | https://healthstudy.usc.edu/index.php |
| EARLI | USA | 2009–2012 | 232 | NA | used inverse distance-squared weighting | Illumina | 22958474 | http://www.earlistudy.org/ |
| PRISM | USA | 2012–2014 | 592 | Random sample | hybrid land use regression and satellite-based model | Illumina | 24476840, 25328835 | NA |
| Project Viva | USA | 1999–2003 | 2128 | Available cord blood or early/mid-childhood blood sample | hybrid land use regression and satellite-based model | Illumina | 24639442 | https://www.hms.harvard.edu/viva/index.html |
| ENVIRONAGE | Belgium | 2010–2016 | 1210 | Random sample | spatial-temporal interpolation method | Illumina | 23742113 | www.limburgsgeboortecohort.be |
| Piccolipiù | Italy | 2011–2015 | 3338 | Participants resident in the municipality of Turin with enough stored biological material and with 24-month follow-up data | LUR | Illumina | 24506846 | www.piccolipiu.it |
| Rhea | Greece | 2007–2008 | 1500 | Random sample | LUR | Illumina | 19713286 | www.rhea.gr |
Replication and Look-Up Study Populations
We performed a replication analysis of the FDR-significant findings in a separate sample of newborns () from the ALSPAC project (Relton et al. 2015). A look-up association analysis of the newborn findings at older ages was based on three independent samples of 7- to 9-y-olds: a) Mechanisms of the Development of ALLergy (MeDALL) comprising a pooled sample from two cohorts with uniform methylation measurements: BAMSE (Sweden) and Prevention and Incidence of Asthma and Mite Allergy (PIAMA; Netherlands), combined with an independent sample from the BAMSE cohort, BAMSE Epigene (total ) (Xu et al. 2018); b) Human Early Life Exposome (HELIX), a pooled sample from four cohorts (total ) (Vrijheid et al. 2014): Norwegian Mother and Child Cohort (MoBa), Etude de cohorte généraliste, menée en France sur les Déterminants pré et post natals précoces du développement psychomoteur et de la santé de l’Enfant (EDEN), Kaunas Cohort, Lithuania (KAUNAS), and Born in Bradford (BiB), Bradford, UK; c) Avon Longitudinal Study of Parents and Children (ALSPAC), UK (); as well as on two samples of 15- to 16-y-olds: BAMSE () and ALSPAC (). Consent for blood sampling was obtained from all parents. Ethical approval for each study was granted by local institutional review boards.
Statistical Analyses
Cohort-Specific Analyses.
For the cohort-specific analyses untransformed normalized methylation, beta values () were used. The value is a continuous variable ranging between 0 and 1, representing the ratio of the intensity of the methylated-probe signal to the total locus signal intensity. A of 0 corresponds to no methylation, and a value of 1 corresponds to 100% methylation at the specific CpG site measured. All included samples were analyzed on a cohort level, except the pooled HELIX study and the pooled MeDALL study with coordinated methylation measurements, as well as air pollution exposure assessment according to a harmonized protocol.
First, we examined the associations between exposure to PM and methylation levels across the genome in each cohort separately using multiple robust linear regression [rlm in the In functional analysis of expression data R package (version 3.3.2; R Core Team)] to account for potential outliers and heteroscedasticity in the data (Fox and Weisberg 2011). All analyses were adjusted for an a priori selected panel of covariates: child’s sex, maternal smoking ever during pregnancy (yes/no), cohort-specific batch indicator(s), and ancestry (in CHS). In addition, age at biosampling, municipality at birth (in BAMSE), and cohort indicator (in the pooled MeDALL and HELIX sample sets) were included in the analyses of the older children. To account for potential differences in DNA methylation that may arise from variability of cell composition in whole blood (Reinius et al. 2012), we estimated cell type composition in cord blood using a reference panel of cells isolated from cord blood (leukocytes and nucleated red blood cells) (Bakulski et al. 2016), and in the older children using an adult reference panel (Reinius et al. 2012), applying the estimateCellCounts function in the minfi Bioconductor package in R (Jaffe and Irizarry 2014). We adjusted for cell composition by including these estimated cell type fractions as covariates in the multivariable linear regression.
Air pollution concentrations were entered as continuous variables without transformation. The results are presented as difference in methylation per increase in average interquartile range (IQR) of and exposure levels across the cohorts corresponding to 5.6 and , respectively.
Meta-Analyses.
A total of 473,723 and 473,680 CpGs were included in the meta-analysis of and results, respectively, after quality control filtering, as well as exclusion of probes that mapped to the X () or Y () chromosomes. Cohort-specific results of the cord blood EWAS were subsequently meta-analyzed using fixed-effects inverse variance weighting in version 2011-03-25, METAL (http://www.sph.umich.edu/csg/abecasis/metal/) (Willer et al. 2010). We used the false discovery rate (FDR, for significance) procedure to account for multiple testing (Strimmer 2008). For replication and look-up analyses, a nominal was considered statistically significant. DNA methylation sites were annotated based on data provided by Illumina (Bibikova et al. 2011).
DMR Analyses.
Differentially methylated regions were identified using two methods available for use with meta-analysis results, comb-p (version 0.32), which identifies DMRs by regional clustering of low p-values from irregularly spaced p-values (Pedersen et al. 2012) and DMRcate (version 1.8.6; https://www.rdocumentation.org/packages/DMRcate), that identifies DMRs from tunable kernel smoothing process of association signals (Peters et al. 2015). Input files for both DMR analyses were our meta-analyzed single-CpG EWAS results on newborns: regression coefficients, standard deviations, uncorrected p-values for DMRcate and uncorrected p-values and chromosomal locations for comb-p. Significant DMRs were defined based on the following criteria: a) a DMR should contain more than one probe; b) regional information can be combined from probes within ; c) the region showed multiple-testing corrected in both methods (Sidak for comb-p and FDR for DMRcate). DMRs detected by both methods were considered significant in our analysis. Input parameters used for the DMR calling in both algorithms are provided in Table S1.
Functional Follow-Up
We investigated whether genes annotated to the significant CpGs were differentially expressed in cord blood in relation to air pollution exposure during pregnancy in the EARLI () or at the time of biosampling in the BAMSE cohort () by means of linear regression analysis. Furthermore, we analyzed the association of the FDR-significant CpG methylation with gene expression in cis ( window) in 3,075 adults in the Biobank-based Integrative Omics Studies (BIOS) consortium data set (Bonder et al. 2017), and used FDR correction as threshold.
To identify associations between methylation levels and the expression levels of nearby genes (cis-expression quantitative trait methylation, cis-eQTM), we regressed methylation M-value on gene expression, sex, sampling age, lymphocytes percentage, monocyte percentage, and RNA Flow Cell Number. The inflation of models is corrected by using “bacon” method (van Iterson et al. 2017). We mapped the eQTM in a window of around the identified 5,547 CpG sites. For this analysis, we used a total of 3,075 samples for which both methylation and gene expression data were available from four cohorts: Lifelines DEEP, Rotterdam, Leiden Longevity, and Netherlands Twin Register (NTR).
To identify plausible pathways associated with air pollution exposure, we performed the over-representation analysis based on CpGs significantly associated with prenatal PM exposure in the meta-analysis at an arbitrary cutoff of using ConsensusPathDB (Kamburov et al. 2013), as well as the R Bioconductor package missMethyl (version 1.10.0 gometh function), which performs one-sided hypergeometric tests taking into account and correcting for any bias derived from the use of differing numbers of probes per gene interrogated by the array (Phipson et al. 2016).
Finally, we investigated whether previously reported differentially methylated CpGs related to in utero tobacco smoke exposure [6,073 CpGs with FDR-significance (Joubert et al. 2016)] were differentially methylated in relation to prenatal PM exposure. We performed Fisher’s exact test for overrepresentation of smoking-related CpGs among nominally significant PM-related CpGs.
We additionally examined whether our FDR-significant CpGs overlapped with the list of potentially polymorphic and cross-reactive probes provided by Chen et al. (Chen et al. 2013), and applied the dip test (Hartigan and Hartigan 1985) for two overlapping CpGs to test for nonunimodal DNA methylation distribution using an independent publicly available data set of cord blood DNA methylation samples (Barrett et al. 2013; Rojas et al. 2015).
Results
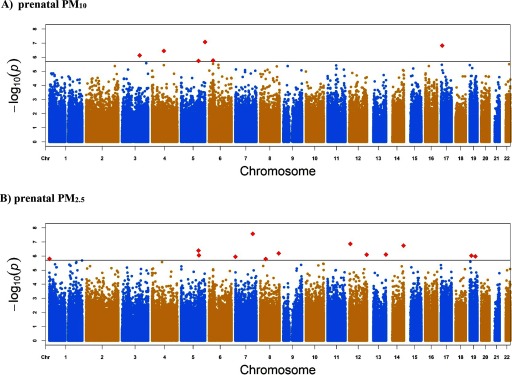
The baseline characteristics of the study populations are presented in Table 1 (and Table S2 in the online data supplement). Exposure contrasts were smallest for the PRISM ( IQR ) and RHEA ( IQR ) cohorts and were highest for the CHS ( and ). The discovery meta-analysis of cord blood methylation in relation to prenatal exposure included 1,949 newborns for and 1,551 for . The difference in sample sizes is due to missing prenatal data for Project Viva and PRISM cohorts, and missing prenatal data for the Generation R cohort. Minus log10(p-values) from the combined analysis of CpGs across the genome in cord blood samples are presented in Figure 1. The quantile–quantile plots did not reveal any noteworthy inflation in the distribution of observed p-values ( for exposure and 1.37 for ; Figure S1). Study-specific lambdas can be found in Table S3.
Figure 1.
Manhattan plot for epigenome-wide meta-analysis of the association between (A) prenatal () and (B) prenatal exposure () and cord blood DNA methylation. Note: The solid horizontal line corresponds to an FDR rate of 0.05. Manhattan plot for : Six CpGs were considered statistically significant using FDR correction (red squares): cg15082635 in GNB2L1;SNORD96A, cg20340716 in USP43, cg00905156 in FAM13A, cg24127244 in SRPRB, cg06849931 in NOTCH4, and cg18640183 in P4HA2. Manhattan plot for : Fourteen CpGs were considered statistically significant using FDR correction (red squares): cg16811875 in PLXNA4, cg12193649 in ZNF705A, cg11886880 upstream of C14orf2, cg16253537 in FNIP1, cg12044654 in COL22A1, cg19120073 in TMCO3, cg05479174 in SFRS8, cg06846669 downstream of NEUROG1, cg23270359 in MRI1, cg01011943 in PSG5, cg00348551 in C7orf50, cg24709511 downstream of MORN1, cg22038738 in PLAT, and cg02236896 in ZNF695.
Meta-Analyses Findings
We found epigenome-wide significant associations (FDR ) between exposure and DNA methylation for six CpGs, with higher exposure being associated with an increase in methylation for four CpGs mapping to GNB2L1; SNORD96A, FAM13A, SRPRB, and P4HA2, and a decrease for two CpGs within USP4, and NOTCH4 (Table 2). Effect sizes were generally small, i.e., 0.1% difference in methylation β-value per increase in prenatal exposure.
Table 2.
Statistically significant CpGs (FDR ) associated with IQR increases in prenatal () exposure and DNA methylation in newborns (discovery meta-analysis), and replication analyses in newborns, children (age 7–9 years) and adolescents (age 15–16 years).
| Chr | Positionb | CpG | Genec | Discovery: newbornsa | Replication: newborns | Replication: age 7–9 years | Replication: age 15–16 years | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| () | ALSPAC () | BAMSE () | HELIX () | ALSPAC () | BAMSE 16 years () | ALSPAC 15 years () | |||||
| (p-value) | Directiond | (p-value) | (p-value) | (p-value) | (p-value) | (p-value) | (P-value) | ||||
| 5 | 180670110 | cg15082635 | GNB2L1; SNORD96A | 0.001 (8.29E-08) | (0.17) | (1.00) | 0.0001 (0.75) | 0.0006 (0.02) | (0.63) | 0.00006 (0.05) | |
| 17 | 9559558 | cg20340716 | USP43 | (1.50E-07) | 0.0011 (0.50) | (0.73) | (0.39) | 0.0002 (0.89) | 0.0003 (0.19) | 0.0004 (0.15) | |
| 4 | 89744363 | cg00905156 | FAM13A | 0.001 (3.55E-07) | X | (0.33) | 0.0017 (0.03) | (0.84) | 0.0004 (0.15) | 0.0001 (0.72) | 0.00001 (0.90) |
| 3 | 133524572 | cg24127244 | SRPRB | 0.001 (7.33E-07) | (0.97) | (0.77) | 0.0002 (0.61) | (0.28) | (0.12) | 0.00004 (0.42) | |
| 6 | 32165893 | cg06849931 | NOTCH4 | (1.72E-06) | 0.0003 (0.81) | 0.0022 (0.03) | (0.002) | 0.0010 (0.33) | 0.00002 (0.95) | (0.30) | |
| 5 | 131563610 | cg18640183 | P4HA2 | 0.001 (1.80E-06) | 0.0003 (0.44) | 0.0006 (0.61) | 0.0009 (0.03) | (0.82) | 0.0001 (0.53) | 0.00001 (0.86) | |
Note: , coefficient for methylation with an IQR increase in prenatal exposure; CHR, chromosome.
Discovery meta-analysis does not include the PRISM or Project Viva cohorts due to missing prenatal data.
Chromosomal position based on NCBI human reference genome assembly Build 37.
UCSC annotated gene.
Direction of methylation for each cohort included in the analysis (INMA, Generation R, CHS, ENVIRONAGE, Rhea, Piccolipiù, EARLI): , , .
We found 14 CpGs significantly associated with prenatal using FDR correction, positioned in or near the following genes: PLXNA4, ZNF705A, downstream of C14orf2, FNIP1, COL22A1, TMCO3, SFRS8, upstream of NEUROG1, MRI1, PSG5, C7orf50, downstream of MORN1, PLAT, and ZNF695 (Table 3). The direction of the effect was negative for 11 of these CpGs, and positive for cg16253537 in FNIP1, cg01011943 in PSG5, and cg00348551 in C7orf50 in relation to higher exposure. The estimates ranged from to 0.3% difference in methylation level per IQR () increase in prenatal exposure.
Table 3.
Statistically significant CpGs (FDR ) associated with IQR increases in prenatal () exposure and DNA methylation in newborns (discovery meta-analysis), and replication in children (age 7–9 years) and adolescents (age 16 years).
| Discovery meta-analysis | Replication: age 7–9 years | Replication: age 16 years | ||||||
|---|---|---|---|---|---|---|---|---|
| Prenatal newborn methylation | BAMSE () | HELIX () | BAMSE () | |||||
| Chr | Positionb | CpG | Genec | (P-value) | Directiond | (P-value) | (P-value) | (P-value) |
| 7 | 132192823 | cg16811875 | PLXNA4 | (2.67E-08) | (0.41) | (0.19) | 0.0001 (0.86) | |
| 12 | 8324628 | cg12193649 | ZNF705A | (1.37E-07) | XXXX | X | (0.76) | X |
| 14 | 104376135 | cg11886880 | down C14orf2 | (1.81E-07) | X | (0.78) | (0.11) | 0.0002 (0.50) |
| 5 | 131132836 | cg16253537 | FNIP1 | 0.001 (4.10E-07) | (0.37) | 0.00008 (0.43) | 0.0001 (0.63) | |
| 8 | 139890342 | cg12044654 | COL22A1 | (6.42E-07) | 0.0004 (0.46) | (0.07) | 0.0001 (0.67) | |
| 13 | 114165365 | cg19120073 | TMCO3 | (7.77E-07) | (0.87) | (0.39) | (0.23) | |
| 12 | 132239000 | cg05479174 | SFRS8 | (7.99E-07) | 0.0002 (0.87) | (0.93) | 0.0005 (0.44) | |
| 5 | 134879739 | cg06846669 | up NEUROG1 | (8.92E-07) | (0.59) | 0.00007 (0.77) | (0.88) | |
| 19 | 13875381 | cg23270359 | MRI1 | (9.43E-07) | (0.84) | 0.00035 (0.01) | (0.71) | |
| 19 | 43690622 | cg01011943 | PSG5 | 0.003 (1.05E-06) | XXXX | X | (0.93) | X |
| 7 | 1177965 | cg00348551 | C7orf50 | 0.001 (1.13E-06) | XXXX | 0.0008 (0.13) | 0.00010 (0.51) | (0.32) |
| 1 | 2251570 | cg24709511 | down MORN1 | (1.57E-06) | (0.50) | (0.30) | 0.0004 (0.23) | |
| 8 | 42064673 | cg22038738 | PLAT | (1.61E-06) | 0.0008 (0.39) | (0.51) | 0.0006 (0.32) | |
| 1 | 247169036 | cg02236896 | ZNF695 | (2.05E-06) | X | (0.34) | (0.24) | (0.64) |
Note: , coefficient for methylation with an IQR increase in prenatal exposure; CHR, chromosome.
Discovery meta-analysis does not include the Generation R cohort due to missing prenatal data.
Chromosomal position based on NCBI human reference genome assembly Build 37.
UCSC annotated gene.
Direction of methylation for each cohort included in the analysis (INMA, Project Viva, CHS, PRISM, ENVIRONAGE, Rhea, Piccolipiù, EARLI): , , .
Two out of the 14 FDR-significant CpGs associated with prenatal , namely cg12193649 and cg01011943, overlapped with the list of potentially polymorphic and cross-reactive probes provided by Chen et al. (2013). However, results from the dip test applied to those two CpGs did not reveal statistically significant deviation from unimodality ( and , respectively).
Tests for heterogeneity did not display any major heterogeneity across studies: 8% and 9.9% of the examined - and CpGs, respectively, had heterogeneity , and median statistics for was 0% (ranging between 0–94%) and for (ranging between 0–88.7%). No significant heterogeneity was found for any of the identified FDR-significant CpGs (p-values for heterogeneity ranging within 0.08–0.81: see forest plots in Figure S2).
Analyses of Differentially Methylated Regions
By applying two different methods for DMR analysis of results, we identified 147 significant (FDR ) DMRs from DMRcate (Table S1) and 12 significant (Sidak ) DMRs from comb-p (Table S2), including 11 that were significant based on both approaches (Table 4). It is interesting to note that all genome-wide significant individual CpGs identified in the discovery meta-analysis were also found within the 147 DMRs found in DMRcate, with the exception of cg06849931 located in NOTCH4.
Table 4.
DMRs in Relation to prenatal exposure that overlap between DMRcate and comb-p methods.
| DMRcate | Comb-p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chr | Start | End | No. of probes | Max a | p-Valueb | Genec | Start | End | No. of probes | p-Valued |
| 7 | 27169674 | 27171528 | 25 | 1.58E-03 | 9.20E-10 | HOXA4 | 27169957 | 27171052 | 17 | 2.75E-12 |
| 11 | 2019730 | 2021243 | 29 | 1.45E-03 | 6.75E-06 | H19 | 2020101 | 2020418 | 10 | 4.30E-04 |
| 4 | 2366103 | 2367137 | 7 | 8.24E-04 | 6.05E-05 | ZFYVE28 | 2366555 | 2367138 | 5 | 1.68E-05 |
| 6 | 31963526 | 31964754 | 10 | 6.05E-05 | C4A | 31964193 | 31964392 | 5 | 2.42E-04 | |
| 1 | 75198211 | 75199117 | 11 | 4.93E-04 | 1.25E-04 | CRYZ; TYW3 | 75198403 | 75198842 | 6 | 7.01E-03 |
| 6 | 170596856 | 170598215 | 7 | 3.26E-04 | DLL1; FAM120B | 170597326 | 170597589 | 4 | 2.96E-03 | |
| 12 | 52400530 | 52401523 | 8 | 1.16E-04 | 5.36E-04 | GRASP | 52400530 | 52400908 | 5 | 3.02E-03 |
| 10 | 3823907 | 3825031 | 7 | 6.43E-04 | KLF6 | 3824387 | 3824688 | 4 | 7.18E-04 | |
| 1 | 1549799 | 1550886 | 12 | 8.50E-04 | MIB2 | 1550648 | 1550887 | 8 | 6.48E-03 | |
| 19 | 3970736 | 3971417 | 7 | 1.00E-03 | DAPK3 | 3971119 | 3971418 | 5 | 3.60E-04 | |
| 19 | 12876846 | 12877188 | 4 | 3.69E-03 | 2.65E-03 | HOOK2 | 12876846 | 12877189 | 4 | 7.51E-04 |
aFold change in DNA methylation .
Minimum FDR p-value for the region.
Annotated gene(s) in the region.
Sidak p-value.
We also found 272 significant (FDR ) DMRs from DMRcate (Table S3) and 33 significant (Sidak ) DMRs from comb-p (Table S4) in relation to prenatal exposure, of which 15 overlapped between the two methods (Table S4). Five out of 14 genome-wide significant individual CpGs identified in the discovery meta-analysis were also seen in the DMRs, namely related to genes C7orf50, ZNF705A, PLAT, PSG5, and MRI1.
Replication and Look-Up Analyses
None of the six FDR-significant CpGs identified as differentially methylated in relation to prenatal in our discovery meta-analysis sample of 1,949 newborns could be replicated in the 688 newborns of the ALSPAC study (Table 2). However, four out of these six CpGs showed significance later in childhood in associations with prenatal exposure; cg00905156 (FAM13A) and cg06849931 (NOTCH4) showed increased methylation in relation to exposure during pregnancy in the combined BAMSE Epigene and MeDALL samples () of 7- to 9-y-olds (), although the direction of association for cg06849931 was opposite to the one in the discovery analysis (Table 2). Furthermore, cg06849931 was also differentially methylated in the HELIX study (), along with cg18640183 (P4HA2) (), both demonstrating the same direction of association as those in the discovery meta-analysis. In addition, cg15082635 (GNB2L1; SNORD96A) was also nominally significant in 7-to 9-y-olds from the ALSPAC study with the same direction of association (). None of these six associations was present in adolescents from the BAMSE () and ALSPAC () studies (). Children’s concurrent exposure at the time of biosampling was not significantly associated with any of these six CpGs (; see Table S5).
Among the 14 epigenome-wide significant CpGs in newborns, none appeared to be statistically significant in children and adolescents, apart from cg23270359 (MRI1), which was significant in the HELIX sample (), although the direction of association was opposite to that in the discovery meta-analysis (Table 3).
Two significant gene regions from the discovery DMR analyses, including genes H19 and MARCH11, were also FDR-significant in analysis of the ALSPAC newborn sample using DMRcate (replication min FDR and , respectively).
Functional Follow-Up
The top three CpGs, including one within the FAM13A gene, as well as six out of 14 -associated CpGs, were significantly associated with gene expression in cis in BIOS (Table S6).
In functional analysis of expression data from the newborns in the EARLI cohort (), no significant association of in utero exposure with expression of genes annotated to the respective CpG was detected, whereas exposure was associated with expression of ZNF695 [, Log fold change per increase in exposure; Table 5]. In BAMSE (), current exposure at 16 y was associated with NOTCH4 (multiple transcripts, lowest , ) and USP43 expression levels in peripheral blood cells (, , per increase; Table 6). Among the associated genes, C7orf50 was significantly differentially expressed in relation to current exposure (, , per increase). Descriptive statistics of expression levels of genes associated with CpG methylation in response to maternal or exposure in the EARLI and BAMSE cohorts are provided in Table S7 and Table S8, respectively.
Table 5.
Associations between PM exposure and gene expression levels in newborn children of the EARLI cohort ().
| Chr | Gene | ProbeID | LogFC | p-Value |
|---|---|---|---|---|
| Prenatal | ||||
| 4 | FAM13A | 16977925 | 0.025 | 0.57 |
| 6 | NOTCH4 | 17017814 | 0.94 | |
| 6 | NOTCH4 | 17027038 | 0.90 | |
| 6 | NOTCH4 | 17029639 | 0.063 | 0.14 |
| 6 | NOTCH4 | 17034630 | 0.63 | |
| 6 | NOTCH4 | 17037128 | 0.018 | 0.51 |
| 6 | NOTCH4 | 17039839 | 0.19 | |
| 6 | NOTCH4 | 17042335 | 0.38 | |
| 5 | SNORD96A | 17119456 | 0.19 | |
| 5 | P4HA2 | 16999712 | 0.57 | |
| 17 | USP43 | 16831046 | 0.26 | |
| 3 | SRPRB | 16945907 | 0.46 | |
| Prenatal | ||||
| 7 | C7orf50 | 17054312 | 0.001 | 0.98 |
| 19 | ZNF606 | 16876074 | 0.21 | |
| 19 | PSG5 | 16872926 | 0.89 | |
| 1 | ZNF695 | 16701484 | 0.074 | 0.04 |
| 10 | MKX | 16712773 | 0.022 | 0.45 |
| 2 | CAPN10 | 16893222 | 0.15 | |
| 8 | COL22A1 | 17081580 | 0.008 | 0.63 |
| 12 | ZNF705A | 16747907 | 0.11 | |
| 5 | FNIP1 | 16999631 | 0.27 | |
| 7 | PLXNA4 | 17063005 | 0.17 | |
| 13 | TMCO3 | 16776883 | 0.32 | |
| 8 | PLAT | 17076726 | 0.26 | |
| 1 | VANGL2 | 16672635 | 0.21 | |
| 19 | MRI1 | 16858849 | 0.26 | |
Note: Results presented per increase in and increase in exposure for genes annotated to FDR significant CpGs in the discovery and EWAS. (one unit of the logFCs translates to a two-fold change in expression). Adjusted for sex, maternal smoking during pregnancy, and cell composition.
Table 6.
Associations between PM exposure and gene expression levels in 16-y-old children of the BAMSE cohort ().
| Chr | Gene | ProbeID | LogFC | p-Value |
|---|---|---|---|---|
| Concurrent | ||||
| 6 | NOTCH4 | TC6_mcf_hap5000165.hg.1 | 0.05 | 9.52E-05 |
| 6 | NOTCH4 | TC6_apd_hap1000098.hg.1 | 0.06 | 9.73E-05 |
| 6 | NOTCH4 | TC6_mann_hap4000155.hg.1 | 0.06 | 9.98E-05 |
| 6 | NOTCH4 | TC6_cox_hap2000190.hg.1 | 0.05 | 1.03E-04 |
| 6 | NOTCH4 | TC6_ssto_hap7000159.hg.1 | 0.05 | 1.10E-04 |
| 6 | NOTCH4 | TC06001564.hg.1 | 0.05 | 1.31E-04 |
| 6 | NOTCH4 | TC6_qbl_hap6000179.hg.1 | 0.05 | 1.42E-04 |
| 17 | USP43 | TC17000146.hg.1 | 0.05 | 4.98E-04 |
| 4 | FAM13A | TC04001380.hg.1 | 2.14E-01 | |
| 3 | SRPRB | TC03000725.hg.1 | 2.89E-01 | |
| Concurrent | ||||
| 7 | C7orf50 | TC07001077.hg.1 | 0.02 | 0.03 |
| 19 | PSG5 | TC19001582.hg.1 | 0.02 | 0.06 |
| 1 | VANGL2 | TC01001369.hg.1 | 0.02 | 0.08 |
| 1 | ZNF695 | TC01006392.hg.1 | 0.01 | 0.08 |
| 8 | COL22A1 | TC08001675.hg.1 | 0.02 | 0.10 |
| 8 | PLAT | TC08001175.hg.1 | 0.02 | 0.16 |
| 19 | MRI1 | TC19000239.hg.1 | 0.17 | |
| 2 | CAPN10 | TC02005015.hg.1 | 0.01 | 0.21 |
| 10 | MKX | TC10001133.hg.1 | 0.01 | 0.29 |
| 13 | TMCO3 | TC13000425.hg.1 | 0.30 | |
| 7 | PLXNA4 | TC07001877.hg.1 | 0.01 | 0.33 |
| 19 | ZNF606 | TC19001910.hg.1 | 0.96 | |
Note: Results presented per increase in and increase in exposure for genes annotated to FDR significant CpGs in the discovery and EWAS. (one unit of the logFCs translates to a two-fold change in expression). Adjusted for sex, maternal smoking during pregnancy, active smoking at the time of biosampling, age at biosampling, municipality at birth, doctor’s diagnosis of asthma, and cell composition.
Pathway Analysis
Twenty-eight of 31 unique gene identifiers extracted from the meta-analysis with exposure matched to ConsensusPathDB. Using FDR , six enriched pathways were identified including “Notch Signaling Pathway” (genes NOTCH4 and DVL2), “Rho GTPase cycle” (FAM13A; HMHA1; VAV2; and GMIP), “Neurotransmitter Release Cycle” (HSPA8; and RIMS1), and “GABA synthesis, release, reuptake and degradation” (HSPA8; and RIMS1). In the repeated pathway analysis using gometh function in missMethyl, no statistically significant pathways were found after correction for multiple testing; however, we observed the same top significant pathways as identified by ConsensusPathDB, i.e., related to regulation of GTPase activity (Table S9). No significantly enriched pathways were identified for .
Candidate-Gene Analysis of Smoking-Related CpGs
Out of 6,073 FDR-significant CpGs previously reported in relation to maternal smoking exposure (Joubert et al. 2016), 359 showed nominal significance () with prenatal and 390 with exposure, which is not more than expected by chance (Fisher’s exact test nonsignificant for overrepresentation of smoking-related CpGs among nominally significant PM-related CpGs). None of the genome-wide significant CpGs identified in our meta-analyses with and were among the 6,073 smoking-related sites.
Discussion
In this large-scale epigenome-wide meta-analysis evaluating the association between prenatal particulate air pollution exposure and DNA methylation in newborns, we found significant associations for and exposure during pregnancy with methylation differences in several genes of relevance for respiratory health, such as FAM13A and NOTCH4. Some of these associations were also seen in the older children. We also identified a number of unique DMRs associated with PM exposure by applying two independent methodologies. The observed differentially methylated genes in the newborn discovery data set represent novel associations in the context of air pollution exposure. One of the top significant hits, cg00905156, localizes in the gene FAM13A, which has been identified in multiple genome-wide association studies (GWAS) of pulmonary function and the related phenotype of COPD (Hobbs et al. 2017; Hancock et al. 2010). Research has shown that FAM13A interferes with the Wnt pathway, inducing degradation, which in turn may affect lung repair (Jiang et al. 2016). In vitro studies have also demonstrated differences in respiratory epithelial cell expression of FAM13A during differentiation into pulmonary type II cells (Wade et al. 2006).
Another significant CpG site, cg06849931, is located in the NOTCH4 gene, which has been identified in GWAS as a genetic marker of asthma-related traits (Li et al. 2013). Recently, an animal study proposed Notch4 as a susceptibility gene for ozone-induced lung injury (Verhein et al. 2015). Genome-wide transcriptomic analysis of lung tissue homogenates within the same study suggested that upregulation of NOTCH3 and NOTCH4 receptors may protect against inflammation. Our other observed differentially methylated CpGs reside in USP43, SRPRB, GNB2L1; SNORD96A, and a cytokine gene, P4HA2. GNB2L1 and P4HA2 have previously been suggested as candidate genes associated with the susceptibility and prognosis for lung cancer (Choi et al. 2015; Dong et al. 2012).
We were not able to replicate FDR-significant CpGs using a smaller independent methylation data set of newborns. However, in two out of three independent samples of school-age children, cg06849931 (NOTCH4) was found to be significantly differentially methylated in relation to prenatal . The direction of association in one of these two samples was opposite, however. Also, significant differential methylation was observed for CpGs in FAM13A, GNB2L1, and SNORD96A, as well as P4HA2 in one out of three independent samples of school-age children, with the same direction of association as those in the discovery EWAS. Furthermore, expression of the NOTCH4 gene in BAMSE participants at 16 years of age was increased in association with concurrent exposure to . Lack of replication in newborns may be attributed to generally weak effects of air pollution exposure that may be difficult to detect in a smaller sample. Furthermore, differences in exposure contrasts should be acknowledged, i.e., wide exposure range in the discovery analysis explained by inclusion of cohorts from areas with different exposure levels in comparison with the replication data set.
We found several significantly differentially methylated CpGs in relation to prenatal exposure, all of which were distinct from those related to prenatal . Unfortunately, no independent newborn data set with data was available for replication analysis. Look-up analysis in older children age 7–9 y suggested association of differential methylation of cg23270359 located in MRI1. Previous studies reported significant association between increased MRI1 methylation and severe asthma (Wysocki et al. 2015).
Earlier epigenome-wide association studies of long-term particulate exposure in adults failed to demonstrate robust associations (Plusquin et al. 2017; de FC Lichtenfels et al. 2018). This failure to demonstrate robust associations may be partly explained by limited statistical power. In addition to EWAS meta-analysis based on single probes, we also investigated regions of differential methylation. Several significant findings were discovered in relation to PM exposure, and the DMR results partly overlapped between the two methods applied, as well as with the genes identified in the single probe meta-analysis. Two of the DMRs comprising H19 and MARCH11 genes were also replicated in the independent newborn data set. The H19 gene is located in an imprinted region of chromosome 11 and is expressed only from the maternally inherited chromosome. Recent evidence suggests that H19 functions as an oncogene and inhibits the activity of tumor suppressor p53 but also plays an important role in embryonic development and growth control (Chen et al. 2018). DNA methylation levels at the H19 DMR have also been associated with being small for gestational age (Qian et al. 2016).
Our study is one of the first large-scale studies assessing the association of prenatal PM exposure on the neonatal blood methylome. Recently published EWAS meta-analysis of five cohorts () did not show any association of maternal exposure during pregnancy with DNA methylation in cord blood (Plusquin et al. 2018). However, pathway analysis of top hits revealed enriched pathways relating to the GABAergic synapse, as well as NOTCH signaling, which is in line with our results. Analysis based on the CHS demonstrated DNA methylation variability in newborn blood in relation to prenatal exposure to and , some of which were also associated with cardiorespiratory health outcomes later in childhood, including asthma and elevated blood pressure later in childhood (Breton et al. 2016). We have also recently reported associations of exposure during pregnancy with cord blood methylation differences in several genes involved in mitochondria function, and we noted that these associations with in utero exposure persisted into early childhood (Gruzieva et al. 2017). We did not, however, observe the same associations with PM exposure in the present study. It remains to be investigated whether those associations we observed with are pollutant-specific, or whether lack of overlap between and PM-related findings are attributed to difference in the sources of particulate pollution in different cities and locations (Eeftens et al. 2012). This difference in sources and chemical composition of PM may also be responsible for the lack of comparability between the present results with and exposures.
Some previous EWASs have identified and replicated extensive exposure-associated epigenetic alterations, for example in relation to exposure to maternal tobacco smoke (Joubert et al. 2016). Not only is particulate air pollution a different type of exposure, but also exposure levels are generally much lower than those related to tobacco smoking, which may explain differences in the magnitude of differential methylation patterns associated with exposure. Furthermore, measurement error in assignment of exposure to maternal smoking during pregnancy is likely much lower than for air pollution. Identifying robust signals at single CpG site level for complex exposures such as long-term air pollution may also require larger sample sizes than available in the present study. In addition, all the study populations were from countries with relatively low ambient levels of particulate air pollution. Inclusion of populations with higher exposures may help identify possible effects on DNA methylation.
This study has some weaknesses. We estimated individual concentrations only for outdoor air pollution at residential addresses, which are not equivalent to personal exposure. Also, due to lack of trimester-specific prenatal exposure data, we were not able to explore the importance of exposure time windows during pregnancy. Participants likely travel to several locations throughout the day and may spend more time at locations other than their residential addresses (e.g., workplaces), which may introduce some misclassification, although most likely nondifferential and thus would generally tend to attenuate the associations. However, ambient and levels have been consistently associated with negative health effects in multiple studies, including effects on fetal and neonatal outcomes (Lamichhane et al. 2015). Our analyses included studies based in western Europe and the United States, which have relatively lower air pollution concentrations in comparison with many other places. We should also acknowledge that the study included mainly white populations, and generalizability to other ethnic groups is uncertain.
Although we adjusted our analyses for predefined important covariates, residual confounding cannot be ruled out. Another possible limitation is that we used estimated cell counts in our analyses because measured cell types or single-cell methylation data were not available in all cohorts. However, such estimated cell type adjustment has been shown to be appropriate in epidemiological settings (Kaushal et al. 2017).
Methylation signatures are tissue and cell specific (Bakulski and Fallin 2014), and therefore, selection of relevant tissues and cells is of crucial importance for epigenetic analyses. The majority of previous studies have used peripheral blood cells to examine DNA methylation patterns associated with environmental exposures; however, air pollution exposure has also been associated with DNA methylation and expression changes in placenta (Cai et al. 2017; Saenen et al. 2017; Abraham et al. 2018), and lung epithelial cells (Clifford et al. 2017; Zhou et al. 2015). Clifford et al. reported differential methylation of CpG sites in HOXA4 in response to diesel exhaust following prior exposure to allergen (Clifford et al. 2017), which was also identified as DMR in our analysis with prenatal exposure. HOXA4 belongs to the family of Hox genes encoding homeodomain transcription factors that determine cell and tissue identities in the developing embryo and patterning of the developing mouse lung (Packer et al. 2000).
In conclusion, our epigenome-wide meta-analysis provides suggestive evidence of newborn methylation differences in several genes with relevance for airway disease, in relation to prenatal particulate air pollution exposure. Some of these associations were also observed later in childhood. Our results also point to the importance of considering the combined effect of nearby CpGs as DMRs when evaluating the impact of exposure on DNA methylation. Further studies are warranted to establish whether this epigenetic variability could potentially explain the influence of ambient air pollution on development of respiratory outcomes.
Supplementary Material
Acknowledgments
ALSPAC: The UK Medical Research Council and the Wellcome Trust (Grant ref. 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and P.Y. will serve as guarantors for the contents of this paper. A comprehensive list of grants funding is available on the ALSPAC website (http://www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf). This research was specifically funded by a joint grant from the UK Economic & Social and Biotechnology & Biological Sciences Research Councils (Grant ref. ES/N000498/1). ALSPAC was funded by the BBSRC (BBI025751/1 and BB/I025263/1). Air pollution exposure assessment was funded by Public Health England as part of the MRC-PHE Centre for Environment and Health, funded also by the UK Medical Research Council (Grant ref. MR/L01341X/1). This paper does not necessarily reflect the views of Public Health England or the Department of Health.
BAMSE was supported by The Swedish Research Council, The Swedish Heart-Lung Foundation, Freemason Child House Foundation in Stockholm, MeDALL (Mechanisms of the Development of ALLergy) a collaborative project conducted within the European Union (grant agreement No. 261357), Centre for Allergy Research, Stockholm County Council (ALF), Swedish Foundation for Strategic Research (SSF) (RBc08-0027), the Strategic Research Programme (SFO) in Epidemiology at Karolinska Institutet, The Swedish Research Council Formas, and the Swedish Environment Protection Agency. E.M. is supported by a grant from the European Research Council under the European Union (EU) Horizon 2020 (H2020) research and innovation programme (grant agreement number 757919, TRIBAL). O.G. is supported by Forte (Swedish Research Council for Health, Working Life and Welfare) and The Swedish Society for Medical Research.
CHS: This work was supported by NIEHS grants K01ES017801, R01ES022216, and P30ES007048.
EARLI: This work was supported by NIH grants R01ES016443, R01ES023780, and R01ES017646 as well as by Autism Speaks (AS 5938).
ENVIRONAGE: The ENVIRONAGE birth cohort is funded by the European Research Counsil (ERC-2012-StG.310898) and by funds of the Flemisch Scientific Research Council (FWO, N1516112/G.0.873.11N.10). The methylation assays were funded by the European Community's Seventh Framework Programme FP7/2007-2013 project EXPOsOMICS (grant no. 308610). Z.H. is supported by the Exposomics EC FP7 grant (Grant agreement no. 308610). ZH and A.G. and the Epigenetics Group at IARC are supported by grants from the Institut National du Cancer (INCa, Plan Cancer-EVA-Inserm, France) and Association pour la Recherche sur le Cancer (ARC, France).
Generation R Study: The general design of the Generation R Study is made possible by financial support from the Erasmus Medical Center (MC), Rotterdam, the Erasmus University Rotterdam, Netherlands Organization for Health Research and Development and the Ministry of Health, Welfare and Sport. The EWAS data was funded by a grant to VWJ from Netherlands Genomics Initiative (NGI)/Netherlands Organisation for Scientific Research (NWO) Netherlands Consortium for Healthy Aging (NCHA; project no. 050-060-810), by funds from the Genetic Laboratory of the Department of Internal Medicine, Erasmus MC. V.W.J. also received a grant from Netherlands Organization for Health Research and Development (VIDI 016.136.361) and a Consolidator Grant from the European Research Council (ERC-2014-CoG-648916). J.F.F. has received funding from the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement no. 633595 (DynaHEALTH). This project received funding from the European Union’s Horizon 2020 Research and Innovation Programme (733206, LIFECYCLE).
HELIX: The research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7/2007-206) under grant agreement no 308333 – the HELIX project. R.G. received the grant of the Lithuanian Agency for Science Innovation and Technology (No. 45 31V-66). The Norwegian Mother and Child Cohort Study (MoBa) is supported by the Ministry of Health and Care Services and the Ministry of Education and Research, NIH/NIEHS (contract no. N01-ES-75558), NIH/NINDS (grant no. 1 UO1 NS 047537-01 and grant no. 2 UO1 NS 047537-06A1).
INMA: This study was funded by grants from Instituto de Salud Carlos III (Red INMA G03/176), Generalitat de Catalunya-CIRIT 1999SGR 00241, and EU Commission (261357; 211250; 268479).
Piccolipiù: The study was approved and initially funded by the Italian National Centre for Disease Prevention and Control (CCM grant 2010) and by the Italian Ministry of Health (art 12 and 12bis Dl.gs.vo 502/92). The methylation assays were funded by the European Community's Seventh Framework Programme FP7/2007–2013 project EXPOsOMICS (grant no. 308610). Z.H. is supported by the Exposomics EC FP7 grant (Grant agreement no: 308610). Z.H. and A.G. and the Epigenetics Group at IARC are supported by grants from the Institut National du Cancer (INCa, Plan Cancer-EVA-INSERM, France) and Association pour la Recherche sur le Cancer (ARC, France).
Rhea: The methylation assays were funded by the European Community's Seventh Framework Programme FP7/2007-2013 project EXPOsOMICS (grant no. 308610). Z.H. is supported by the Exposomics EC FP7 grant (grant agreement no. 308610). ZH and A.G. and the Epigenetics Group at IARC are supported by grants from the Institut National du Cancer (INCa, Plan Cancer-EVA-INSERM, France) and Association pour la Recherche sur le Cancer (ARC, France).
PRISM: R.J.W. received funding for the PRISM cohort under R01 HL095606 and R01 HL1143396. A.C.J. is supported by R00 ES023450.
Project Viva: This Project Viva study was supported by grants from the NIH (NIH R01 HL 111108, R01 NR013945, R01 HD 034568, K24 HD069408, K23 ES022242, P01ES009825, R01AI102960, P30 ES000002) and the U.S. Environmental Protection Agency (EPA) (R832416, RD834798). This publication’s contents are solely the responsibility of the grantee and do not necessarily represent the official views of the U.S. Government, the U.S. Department of Health and Human Services or the NIH, or the EPA. Further, the EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
MeDALL: The methylation study of MeDALL cohorts was funded by MEDALL, a collaborative project supported by the European Union under the Health Cooperation Work Programme of the 7th Framework Programme (grant agreement no. 261357).
The Biobank-Based Integrative Omics Studies (BIOS) Consortium is funded by BBMRI-NL, a research infrastructure financed by the Dutch government (NWO 184.021.007).
Some of the results of the present study were previously reported in the form of a conference abstract (The 29th Annual Scientific Conference of the International Society of Environmental Epidemiology, ISEE, 2017).
ALSPAC: We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
BAMSE: We would like to thank all the families for their participation in the BAMSE study. In addition, we would like to thank E. Hallner, S. Nilsson, and A. Lauber at the BAMSE secretary for invaluable support, as well as Mutation Analysis Facility (MAF) at Karolinska Institutet for genome-wide methylation analysis, and I. Delin for excellent technical assistance. The computations were performed on resources provided by SNIC through Uppsala Multidisciplinary Center for Advanced Computational Science (UPPMAX) under Project b2014110.
CHS: We are indebted to the school principals, teachers, students, and parents in each of the study communities for their cooperation and especially to the members of the health testing field team for their efforts. We would like to express our sincere gratitude to S. Graham and R. Cooley at the California Biobank Program and Genetic Disease Screening Program within the California Department of Public Health for their assistance and advice regarding newborn bloodspots. The biospecimens and data used in this study were obtained from the California Biobank Program [SIS request number(s) 479] Section 6,555(b), 17 CCR. The California Department of Public Health is not responsible for the results or conclusions drawn by the authors of this publication.
EARLI: We thank collaborators for their help in collecting and generating the data from EARLI examined here, including C. Newschaffer, D. Fallin, I. Hertz-Picciotto, L. Croen, and F. Lurmann.
ENVIRonAGE: The authors acknowledge the participating mothers and neonates, as well as the staff of the maternity ward, midwives, and the staff of the clinical laboratory of East Limburg Hospital in Genk. We thank C. Cuenin for his help and dedication in the methylome experimental workflow and the Genetic Cancer Susceptibility Group at IARC for their assistance in these experiments.
Generation R Study: The Generation R Study is conducted by the Erasmus Medical Center (MC) in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam; the Municipal Health Service Rotterdam area, Rotterdam; the Rotterdam Homecare Foundation, Rotterdam; and the Stichting Trombosedienst & Artsenlaboratorium Rijnmond (STAR-MDC), Rotterdam. We gratefully acknowledge the contribution of children and parents, general practitioners, hospitals, midwives, and pharmacies in Rotterdam. The study protocol was approved by the Medical Ethical Committee of the Erasmus MC, Rotterdam. Written informed consent was obtained for all participants. The generation and management of the Illumina methylation array data (EWAS data) for the Generation R Study was executed by the Human Genotyping Facility of the Genetic Laboratory of the Department of Internal Medicine, Erasmus MC, Netherlands. We thank M. Verbiest, M. Jhamai, S. Higgins, M. Verkerk, and Dr. L. Stolk for their help in creating the EWAS database. We also thank Y. de Kluizenaar of the Department of Urban Environment and Safety, Netherlands Organization for Applied Scientific Research (TNO), Delft, Netherlands, for help in creating the air pollution data.
HELIX: The authors would particularly like to thank all the participants for their generous collaboration.
MoBa: We are grateful to all the participating families in Norway who take part in this ongoing cohort study.
EDEN: We acknowledge the participating families and midwife research assistants for data collection. The EDEN Mother–Child Cohort Study Group: I. Annesi-Maesano, J.Y. Bernard, J. Botton, M.A. Charles, P. Dargent-Molina, B. de Lauzon-Guillain, P. Ducimetière, M. de Agostini, B. Foliguet, A. Forhan, X. Fritel, A. Germa, V. Goua, R. Hankard, B. Heude, M. Kaminski, B. Larroque, N. Lelong, J. Lepeule, G. Magnin, L. Marchand, C. Nabet, F Pierre, R. Slama, M.J. Saurel-Cubizolles, M. Schweitzer, and O. Thiebaugeorges.
INMA: The authors would particularly like to thank all the participants for their generous collaboration. A full roster of the INMA Project Investigators can be found at http://www.proyectoinma.org/presentacion-inma/listado-investigadores/en_listado-investigadores.html.
Piccolipiù: We thank all the families who took part in the study, and the Piccolipiù team. We thank C. Cuenin for his help and dedication in the methylome experimental workflow and the Genetic Cancer Susceptibility Group at the International Agency for Research on Cancer (IARC) for their assistance in these experiments.
Rhea: We thank all of the participants of the Rhea study and the interviewers, statisticians, and hospital personnel for their cooperation and contribution to this study. We thank C. Cuenin for his help and dedication in the methylome experimental workflow and the Genetic Cancer Susceptibility Group at IARC for their assistance in these experiments.
Project Viva: The authors would like to thank Project Viva staff and participants. We thank collaborators for their help in directing the cohort study (E. Oken) and in collecting, generating, managing and interpreting the exposure and outcome data (J. Schwartz, B. Coull, M.-F. Hivert, S. Rifas-Shiman, and H. Gibson).
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP4522).
These authors contributed equally as senior authors.
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Abraham E, Rousseaux S, Agier L, Giorgis-Allemand L, Tost J, Galineau J, et al. 2018. Pregnancy exposure to atmospheric pollution and meteorological conditions and placental DNA methylation. Environ Int 118:334–347, PMID: 29935799, 10.1016/j.envint.2018.05.007. [DOI] [PubMed] [Google Scholar]
- Bakulski KM, Fallin MD. 2014. Epigenetic epidemiology: promises for public health research. Environ Mol Mutagen 55(3):171–183, PMID: 24449392, 10.1002/em.21850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakulski KM, Feinberg JI, Andrews SV, Yang J, Brown S, McKenney SL, et al. 2016. DNA methylation of cord blood cell types: applications for mixed cell birth studies. Epigenetics 11(5):354–362, PMID: 27019159, 10.1080/15592294.2016.1161875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouki R, Melén E, Herceg Z, Beckers J, Chen J, Karagas M, et al. 2018. Epigenetics as a mechanism linking developmental exposures to long-term toxicity. Environ Int 114:77–86, PMID: 29499450, 10.1016/j.envint.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, et al. 2013. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res 41(Database issue):D991–D995, PMID: 23193258, 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibikova M, Barnes B, Tsan C, Ho V, Klotzle B, Le JM, et al. 2011. High density DNA methylation array with single CpG site resolution. Genomics 98(4):288–295, PMID: 21839163, 10.1016/j.ygeno.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Bonder MJ, Luijk R, Zhernakova DV, Moed M, Deelen P, Vermaat M, et al. 2017. Disease variants alter transcription factor levels and methylation of their binding sites. Nat Genet 49(1):131–138, PMID: 27918535, 10.1038/ng.3721. [DOI] [PubMed] [Google Scholar]
- Breton CV, Gao L, Yao J, Siegmund KD, Lurmann F, Gilliland F. 2016. Particulate matter, the newborn methylome, and cardio-respiratory health outcomes in childhood. Environ Epigenet 2(2):dvw005, PMID: 29492287, 10.1093/eep/dvw005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Zhao Y, Liu P, Xia B, Zhu Q, Wang X, et al. 2017. Exposure to particulate air pollution during early pregnancy is associated with placental DNA methylation. Sci Total Environ 607–608:1103–1108, PMID: 28724248, 10.1016/j.scitotenv.2017.07.029. [DOI] [PubMed] [Google Scholar]
- Cassee FR, Héroux ME, Gerlofs-Nijland ME, Kelly FJ. 2013. Particulate matter beyond mass: recent health evidence on the role of fractions, chemical constituents and sources of emission. Inhal Toxicol 25(14):802–812, PMID: 24304307, 10.3109/08958378.2013.850127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YA, Lemire M, Choufani S, Butcher DT, Grafodatskaya D, Zanke BW, et al. 2013. Discovery of cross-reactive probes and polymorphic CpGs in the Illumina Infinium HumanMethylation450 microarray. Epigenetics 8(2):203–209, PMID: 23314698, 10.4161/epi.23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yang Q, Min D. 2018. Beyond brown adipogenesis the inheritance of imprinted H19. Non-coding RNA Investigation 2 http://ncri.amegroups.com/article/view/4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YY, Lee SY, Lee WK, Jeon HS, Lee EB, Lee HC, et al. 2015. RACK1 is a candidate gene associated with the prognosis of patients with early stage non-small cell lung cancer. Oncotarget 6(6):4451–4466, PMID: 25686824, 10.18632/oncotarget.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford RL, Jones MJ, MacIsaac JL, McEwen LM, Goodman SJ, Mostafavi S, et al. 2017. Inhalation of diesel exhaust and allergen alters human bronchial epithelium DNA methylation. J Allergy Clin Immunol 139(1):112–121, PMID: 27321436, 10.1016/j.jaci.2016.03.046. [DOI] [PubMed] [Google Scholar]
- de FC Lichtenfels AJ, van der Plaat DA, de Jong K, van Diemen CC, Postma DS, Nedeljkovic I, et al. 2018. Long-term air pollution exposure, genome-wide DNA methylation and lung function in the LifeLines Cohort Study. Environ Health Perspect 126(2):027004, PMID: 29410382, 10.1289/EHP2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai G, Chu L, Guo Y, Myneni AA, Mu L. 2017. Biomarkers used in studying air pollution exposure during pregnancy and perinatal outcomes: a review. Biomarkers 22(6):489–501, PMID: 28581828, 10.1080/1354750X.2017.1339294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Hu ZB, Wu C, Guo H, Zhou BS, Lv JC, et al. 2012. Association analyses identify multiple new lung cancer susceptibility loci and their interactions with smoking in the Chinese population. Nat Genet 44(8):895, PMID: 22797725, 10.1038/ng.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeftens M, Beelen R, de Hoogh K, Bellander T, Cesaroni G, Cirach M, et al. 2012. Development of Land Use Regression models for PM(2.5), PM(2.5) absorbance, PM(10) and PM(coarse) in 20 European study areas; results of the ESCAPE project. Environ Sci Technol 46(20):11195–11205, PMID: 22963366, 10.1021/es301948k. [DOI] [PubMed] [Google Scholar]
- Felix JF, Joubert BR, Baccarelli AA, Sharp GC, Almqvist C, Annesi-Maesano I, et al. 2017. Cohort profile: Pregnancy And Childhood Epigenetics (PACE) Consortium. Int J Epidemiol 47(1):22u–23u, PMID: 29025028, 10.1093/ije/dyx190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Weisberg S. 2011. Robust regression in R. In: An R Companion to Applied Regression. 2nd ed Thousand Oaks, CA:Sage. [Google Scholar]
- Gref A, Merid SK, Gruzieva O, Ballereau S, Becker A, Bellander T, et al. 2017. Genome-wide interaction analysis of air pollution exposure and childhood asthma with functional follow-up. Am J Respir Crit Care Med 195(10):1373–1383, PMID: 27901618, 10.1164/rccm.201605-1026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruzieva O, Xu CJ, Breton CV, Annesi-Maesano I, Antó JM, Auffray C, et al. 2017. Epigenome-wide meta-analysis of methylation in children related to prenatal NO2 air pollution exposure. Environ Health Perspect 125(1):104–110, PMID: 27448387, 10.1289/EHP36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock DB, Eijgelsheim M, Wilk JB, Gharib SA, Loehr LR, Marciante KD, et al. 2010. Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nat Genet 42(1):45–52, PMID: 20010835, 10.1038/ng.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartigan JA, Hartigan PM. 1985. The Dip Test of Unimodality. Ann Stat 13:70–84, 10.1214/aos/1176346577. [DOI] [Google Scholar]
- Hew KM, Walker AI, Kohli A, Garcia M, Syed A, McDonald-Hyman C, et al. 2015. Childhood exposure to ambient polycyclic aromatic hydrocarbons is linked to epigenetic modifications and impaired systemic immunity in T cells. Clin Exp Allergy 45(1):238–248, PMID: 25048800, 10.1111/cea.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs BD, de Jong K, Lamontagne M, Bossé Y, Shrine N, Artigas MS, et al. 2017. Genetic loci associated with chronic obstructive pulmonary disease overlap with loci for lung function and pulmonary fibrosis. Nat Genet 49(3):426–432, PMID: 28166215, 10.1038/ng.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne BD, Joy EA, Hofmann MG, Gesteland PH, Cannon JB, Lefler JS, et al. 2018. Short-term elevation of fine particulate matter air pollution and acute lower respiratory infection. Am J Respir Crit Care Med 198(6):759–766, PMID: 29652174, 10.1164/rccm.201709-1883OC. [DOI] [PubMed] [Google Scholar]
- Jaffe AE, Irizarry RA. 2014. Accounting for cellular heterogeneity is critical in epigenome-wide association studies. Genome Biol 15(2):R31, PMID: 24495553, 10.1186/gb-2014-15-2-r31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R, Jones MJ, Sava F, Kobor MS, Carlsten C. 2014. Short-term diesel exhaust inhalation in a controlled human crossover study is associated with changes in DNA methylation of circulating mononuclear cells in asthmatics. Part Fibre Toxicol 11:71, PMID: 25487561, 10.1186/s12989-014-0071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Lao T, Qiu W, Polverino F, Gupta K, Guo F, et al. 2016. A chronic obstructive pulmonary disease susceptibility gene, FAM13A, regulates protein stability of β-catenin. Am J Respir Crit Care Med 194(2):185–197, PMID: 26862784, 10.1164/rccm.201505-0999OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert BR, Felix JF, Yousefi P, Bakulski KM, Just AC, Breton C, et al. 2016. DNA methylation in newborns and maternal smoking in pregnancy: genome-wide consortium meta-analysis. Am J Hum Genet 98(4):680–696, PMID: 27040690, 10.1016/j.ajhg.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamburov A, Stelzl U, Lehrach H, Herwig R. 2013. The ConsensusPathDB interaction database: 2013 update. Nucleic Acids Res 41(Database issue):D793–800, PMID: 23143270, 10.1093/nar/gks1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal A, Zhang H, Karmaus WJJ, Ray M, Torres MA, Smith AK, et al. 2017. Comparison of different cell type correction methods for genome-scale epigenetics studies. BMC Bioinformatics 18(1):216, PMID: 28410574, 10.1186/s12859-017-1611-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korten I, Ramsey K, Latzin P. 2017. Air pollution during pregnancy and lung development in the child. Paediatr Respir Rev 21:38–46, PMID: 27665510, 10.1016/j.prrv.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Lamichhane DK, Leem JH, Lee JY, Kim HC. 2015. A meta-analysis of exposure to particulate matter and adverse birth outcomes. Environ Health Toxicol 30:e2015011, PMID: 26796890, 10.5620/eht.e2015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Hawkins GA, Ampleford EJ, Moore WC, Li H, Hastie AT, et al. 2013. Genome-wide association study identifies TH1 pathway genes associated with lung function in asthmatic patients. J Allergy Clin Immunol 132(2):313–320 e15, PMID: 23541324, 10.1016/j.jaci.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niranjan R, Thakur AK. 2017. The toxicological mechanisms of environmental soot (black carbon) and carbon black: focus on oxidative stress and inflammatory pathways. Front Immunol 8:763, PMID: 28713383, 10.3389/fimmu.2017.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer AI, Mailutha KG, Ambrozewicz LA, Wolgemuth DJ. 2000. Regulation of the Hoxa4 and Hoxa5 genes in the embryonic mouse lung by retinoic acid and TGFbeta1: implications for lung development and patterning. Dev Dyn 217(1):62–74, PMID: 10679930, . [DOI] [PubMed] [Google Scholar]
- Panni T, Mehta AJ, Schwartz JD, Baccarelli AA, Just AC, Wolf K, et al. 2016. Genome-wide analysis of DNA methylation and fine particulate matter air pollution in three study populations: KORA F3, KORA F4, and the Normative Aging Study. Environ Health Perspect 124(7):983–990, PMID: 26731791, 10.1289/ehp.1509966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BS, Schwartz DA, Yang IV, Kechris KJ. 2012. Comb-p: software for combining, analyzing, grouping and correcting spatially correlated P-values. Bioinformatics 28(22):2986–2988, PMID: 22954632, 10.1093/bioinformatics/bts545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters TJ, Buckley MJ, Statham AL, Pidsley R, Samaras K, V Lord R, et al. 2015. De novo identification of differentially methylated regions in the human genome. Epigenetics Chromatin 8:6, PMID: 25972926, 10.1186/1756-8935-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipson B, Maksimovic J, Oshlack A. 2016. missMethyl: an R package for analyzing data from Illumina's HumanMethylation450 platform. Bioinformatics 32(2):286–288, PMID: 26424855, 10.1093/bioinformatics/btv560. [DOI] [PubMed] [Google Scholar]
- Plusquin M, Chadeau-Hyam M, Ghantous A, Alfano R, Bustamante M, Chatzi L, et al. 2018. DNA methylome marks of exposure to particulate matter at three time points in early life. Environ Sci Technol 52(9):5427–5437, PMID: 29597345, 10.1021/acs.est.7b06447. [DOI] [PubMed] [Google Scholar]
- Plusquin M, Guida F, Polidoro S, Vermeulen R, Raaschou-Nielsen O, Campanella G, et al. 2017. DNA methylation and exposure to ambient air pollution in two prospective cohorts. Environ Int 108:127–136, PMID: 28843141, 10.1016/j.envint.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian YY, Huang XL, Liang H, Zhang ZF, Xu JH, Chen JP, et al. 2016. Effects of maternal folic acid supplementation on gene methylation and being small for gestational age. J Hum Nutr Diet 29(5):643–651, PMID: 27230729, 10.1111/jhn.12369. [DOI] [PubMed] [Google Scholar]
- Reinius LE, Acevedo N, Joerink M, Pershagen G, Dahlén SE, Greco D, et al. 2012. Differential DNA methylation in purified human blood cells: implications for cell lineage and studies on disease susceptibility. PLoS One 7(7):e41361, PMID: 22848472, 10.1371/journal.pone.0041361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relton CL, Gaunt T, McArdle W, Ho K, Duggirala A, Shihab H, et al. 2015. Data resource profile: Accessible Resource for Integrated Epigenomic Studies (ARIES). Int J Epidemiol 44(4):1181–1190, PMID: 25991711, 10.1093/ije/dyv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas D, Rager JE, Smeester L, Bailey KA, Drobná Z, Rubio-Andrade M, et al. 2015. Prenatal arsenic exposure and the epigenome: identifying sites of 5-methylcytosine alterations that predict functional changes in gene expression in newborn cord blood and subsequent birth outcomes. Toxicol Sci 143(1):97–106, PMID: 25304211, 10.1093/toxsci/kfu210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenen ND, Vrijens K, Janssen BG, Roels HA, Neven KY, Vanden Berghe W, et al. 2017. Lower placental leptin promoter methylation in association with fine particulate matter air pollution during pregnancy and placental nitrosative stress at birth in the ENVIRONAGE Cohort. Environ Health Perspect 125(2):262–268, PMID: 27623604, 10.1289/EHP38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somineni HK, Zhang X, Biagini Myers JM, Kovacic MB, Ulm A, Jurcak N, et al. 2016. Ten-eleven translocation 1 (TET1) methylation is associated with childhood asthma and traffic-related air pollution. J Allergy Clin Immunol 137(3):797–805 e5, PMID: 26684294, 10.1016/j.jaci.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strimmer K. 2008. fdrtool: a versatile R package for estimating local and tail area-based false discovery rates. Bioinformatics 24(12):1461–1462, PMID: 18441000, 10.1093/bioinformatics/btn209. [DOI] [PubMed] [Google Scholar]
- van Iterson M, van Zwet EW, Heijmans BT, BIOS Consortium. 2017. Controlling bias and inflation in epigenome- and transcriptome-wide association studies using the empirical null distribution. Genome Biology 18(1):19, PMID: 28129774, 10.1186/s13059-016-1131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhein KC, McCaw Z, Gladwell W, Trivedi S, Bushel PR, Kleeberger SR. 2015. Novel roles for Notch3 and Notch4 receptors in gene expression and susceptibility to ozone-induced lung inflammation in mice. Environ Health Perspect 123(8):799–805, PMID: 25658374, 10.1289/ehp.1408852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijheid M, Slama R, Robinson O, Chatzi L, Coen M, van den Hazel P, et al. 2014. The Human Early-Life Exposome (HELIX): project rationale and design. Environ Health Perspect 122(6):535–544, PMID: 24610234, 10.1289/ehp.1307204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade KC, Guttentag SH, Gonzales LW, Maschhoff KL, Gonzales J, Kolla V, et al. 2006. Gene induction during differentiation of human pulmonary type II cells in vitro. Am J Respir Cell Mol Biol 34(6):727–737, PMID: 16474099, 10.1165/rcmb.2004-0389OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer CJ, Li Y, Abecasis GR. 2010. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26(17):2190–2191, PMID: 20616382, 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki K, Conley Y, Wenzel S. 2015. Epigenome variation in severe asthma. Biol Res Nurs 17(3):263–269, PMID: 25288825, 10.1177/1099800414553463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CJ, Söderhäll C, Bustamante M, Baïz N, Gruzieva O, Gehring U, et al. 2018. DNA methylation in childhood asthma: an epigenome-wide meta-analysis. Lancet Respir Med 6(5):379–388, PMID: 29496485, 10.1016/S2213-2600(18)30052-3. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Liu Y, Duan F, Qin M, Wu F, Sheng W, et al. 2015. Transcriptomic analyses of the biological effects of airborne PM2.5 exposure on human bronchial epithelial cells. Plos One 10(9):e0138267, PMID: 26382838, 10.1371/journal.pone.0138267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.