Summary
Lecanosticta acicola causes brown spot needle blight (BSNB) of Pinus species. The pathogen occurs mostly in the Northern Hemisphere but has also been reported in Central America and Colombia. BSNB can lead to stunted growth and tree mortality, and has resulted in severe damage to pine plantations in the past. There have been increasingly frequent new reports of this pathogen in Europe and in North America during the course of the past 10 years. This is despite the fact that quarantine practices and eradication protocols are in place to prevent its spread.
Taxonomy
Kingdom Fungi; Phylum Ascomycota; Subphylum Pezizomycotina; Class Dothideomycetes; Subclass Dothideomycetidae; Order Capniodales; Family Mycosphaerellaceae; Genus Lecanosticta.
Host range and distribution
Lecanosticta spp. occur on various Pinus species and are found in North America, Central America, South America (Colombia), Europe as well as Asia.
Disease symptoms
Small yellow irregular spots appear on the infected pine needles that become brown over time. They can be surrounded by a yellow halo. These characteristic brown spots develop to form narrow brown bands that result in needle death from the tips down to the point of infection. Needles are prematurely shed, leaving bare branches with tufts of new needles at the branch tips. Infection is usually most severe in the lower parts of the trees and progresses upwards into the canopies.
Useful websites
The EPPO global database providing information on L. acicola (https://gd.eppo.int/taxon/SCIRAC)
Reference genome of L. acicola available on GenBank (https://www.ncbi.nlm.nih.gov/genome/?term=Lecanosticta+acicola)
JGI Gold Genome database information sheet of L. acicola sequenced genome (https://gold.jgi.doe.gov/organism?xml:id=Go0047147)
Keywords: brown spot needle blight, Lecanosticta acicola, Lecanosticta species, Mycosphaerella dearnessii, pine pathogen, Pinus spp
Introduction
Lecanosticta acicola is an ascomycete fungus that causes a disease of Pinus spp. known as brown spot needle blight (BSNB). The pathogen was first described by de Thümen (1878) and it owes its notoriety to a disease problem that arose in the southeastern USA on Pinus palustris, better known as long leaf pine in that area (Siggers, 1932). This tree species, which is highly susceptible to infection, is peculiar in having a so‐called ‘grass’ stage during the first five years of its growth. This mass of young needles provides a favourable environment for infection to occur.
The BSNB pathogen completes its life cycle (Fig. 1) on pine needles that are shed prematurely. This leads to reduced or stunted growth that can result in significant yield losses (Wakeley, 1970) or tree death. In some cases, pine plantations have been sufficiently damaged that they have needed to be cleared (Huang et al., 1995; Lévy, 1996; Markovskaja et al., 2011).
Figure 1.
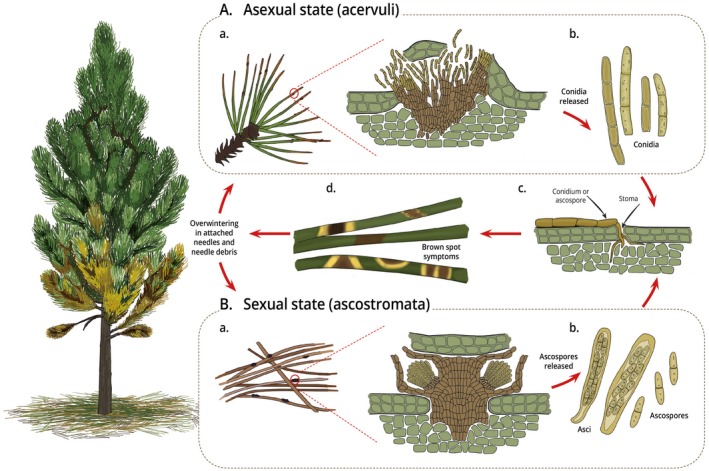
Life cycle of Lecanosticta acicola on Pinus spp. (A) Asexual state: acervuli (a) develop on attached needles and needle debris and release conidia (b). Infection occurs through the stomata of new season needles (c), resulting in brown spot symptoms (d). (B) Sexual state: ascostromata develop on dead needles associated with previous season infections (a) and release ascospores in spring (b). Infection occurs through the stomata of new season needles (c), resulting in brown spot symptoms (d).
Lecanosticta acicola has been recorded on 53 different Pinus species and hybrids in native and non‐native pine stands in the USA, Canada, several European countries and Asia as well as in Central America and Colombia (Table 1). Due to the severity of the disease, the pathogen has been afforded an A1 quarantine status in Africa, Argentina, Chile, Uruguay, Bahrain, Kazakhstan, Ukraine and Russia, and A2 quarantine status in Europe (https://gd.eppo.int/taxon/SCIRAC/categorization). However, reports of new outbreaks of the disease in various European countries have increased significantly since 2008 (Adamson et al., 2015, 2018; Anonymous, 2012; Cleary et al., 2019; Hintsteiner et al., 2012; Jankovský et al., 2009a; Markovskaja et al., 2011; Mullett et al., 2018; Ortíz de Urbina et al., 2017).
Table 1.
Host and geographical range of Lecanosticta species.
| Country, region, locality | Year collected | Host | Identification method and additional notes | Identification verified using molecular methods (*) | Reported severity of infections and applied eradication methods | Report references | |
|---|---|---|---|---|---|---|---|
| Lecanosticta acicola | |||||||
| Austria, Lower Austria, Valley of the river Ybbs | 1996–2000 | P. mugo, P. sylvestris | Morphological identifications of the pathogen were performed. | Infected trees were eradicated after which the disease was no longer detected (2001–2002). | Brandstetter and Cech (2003) | ||
| Austria, Lower Austria, Hollenstein/Ybbs | 2008–2009 | P. sylvestris | The pathogen was recognized during a forest survey. | Cech and Krehan (2008), Kessler (2009) | |||
| Austria, Lower Austria, Hollenstein/Ybbs | 2009–2010 | P. mugo subsp. mugo, P. mugo subsp. uncinata | Symptoms were observed in a survey. | Kessler and Krehan (2011) | |||
| Austria, Lower Austria | 1996 | P. mugo | Fruiting bodies were observed on pine needles. | Isolated occurrence in a garden. | Cech (1997) | ||
| Austria, Lower Austria, Hollenstein/Ybbs | 1998 | Pinus sp. | Symptoms were observed in the field. | Brandstetter and Cech (1999) | |||
| Austria, Lower Austria | 2004 | P. mugo, P. sylvestris | TEF 1 sequencing used for identification, both mating types were detected. | * | Janoušek et al. (2016) | ||
| Austria, Lower Austria | 2010 | P. mugo | TEF 1 sequencing used for identification, both mating types were detected. | * | Janoušek et al. (2016) | ||
| Austria, Upper Austria | 2010 | P. mugo | TEF 1 sequencing used for identification. Mating type 1 was detected. | * | Janoušek et al. (2016) | ||
| Austria, Upper Austria, Bregenz (Vorarlberg) | 2011 | P. mugo subsp. mugo | Symptoms were observed in a survey. | Kessler and Krehan (2011) | |||
| Austria, Upper Austria, Gmunden | 2011 | P. nigra var. nigra, P. mugo subsp. mugo | ITS sequencing used for identification. | * | Hintsteiner et al. (2012) | ||
| Austria, Upper Austria, Tyrol | 2011 | P. mugo subsp. uncinata | Symptoms were observed in a survey. | Kessler and Krehan (2011) | |||
| Austria, Upper Austria | 2012 | P. nigra | TEF 1 sequencing used for identification. Mating type 2 was detected. | * | Janoušek et al. (2016) | ||
| Austria, Upper Austria, Tyrol | 2015 | P. mugo subsp. mugo, P. mugo subsp. uncinata, P. sylvestris | The pathogen was detected during a forest survey and confirmed with laboratory tests (method not specified). | * | Detected in area covering more than 60 ha of forest. | EPPO (2015) | |
| Austria, Graz | 2016 | P. mugo | Infected needles were collected by I. Barnes. Isolations were made by I. Barnes and A. van der Nest, and identified by ITS sequencing. Mating type 2 was detected. | * | Trees heavily infected (see Fig. 2A,B). | I. Barnes, FABI, Pretoria, South Africa, personal communication | |
| Austria, Lower Austria | 2016 | P. mugo | Infected needles were collected by T. Cech. Isolations were made by I. Barnes and A. van der Nest, and identified by ITS sequencing. Mating type 2 was detected. | * | I. Barnes, FABI, Pretoria, South Africa, personal communication | ||
| Austria, Salzburg | 2016 | P. uncinata | Infected needles were collected by T. Cech. Isolations were made by I. Barnes and A. van der Nest, and identified by ITS sequencing. Mating type 2 was detected. | * | I. Barnes, FABI, Pretoria, South Africa, personal communication | ||
| Austria, Upper Austria | 2016 | P. mugo | Infected needles were collected by T. Cech. Isolations were made by I. Barnes and A. van der Nest, and identified by ITS sequencing. Mating type 1 was detected. | * | I. Barnes, FABI, Pretoria, South Africa, personal communication | ||
| Belize | 1981 | P. caribaea, P. oocarpa | Morphological identifications were made. Confirmation is needed as molecular identification did not reveal L. acicola in Central America (van der Nest et al., 2019). | Evans (1984) | |||
| Bulgaria, near Sofia | 1938 | P. nigra | The pathogen was identified based on morphological characteristics. However, the conidial descriptions are not typical of L. acicola and therefore this record is doubtful and should be verified. | Kovaćevski (1938) | |||
| Canada, Manitoba | 1965 | P. banksiana, P. contorta var. latifolia | Symptoms were observed in the field and the presence of the pathogen was confirmed with morphological identifications. | 50–90% of P. contorta var. latifolia was infected, 20% of P. banksiana was infected. | Laut et al. (1966) | ||
| Canada, New Brunswick, Quebec and Ontario | 2009 | P. strobus | L. acicola was reported to occur with Canavergella banfieldii on all trees sampled and confirmed based on morphological characteristics. | Laflamme et al. (2010) | |||
| Canada, Quebec | 2011 | P. strobus, P. mugo | TEF 1 sequencing used for identification, both mating types detected. | * | Janoušek et al. (2016) | ||
| China, Jiangsu | 1958 | P. thunbergii | Identification method not specified. | Insignificant damage was reported. | Ye and Wu (2011) | ||
| China, Fujian province | 1982–1985 | P. elliottii | Morphological identifications of the pathogen. | Li et al. (1987) | |||
| China, Anhui, Fujian, Guangdong, Guangxi, Jiangsu, Jiangxi and Zhejiang provinces | 1986 | P. caribaea, P. clausa, P. echinata, P. elliottii, P. palustris, P. taeda, P. thunbergii | Morphological characteristics were used to identify the pathogen. | P. elliottii, P. taeda and P. thunbergii were severely damaged. P. caribaea, P. clausa, P. echinata and P. palustris were reported as susceptible. | Li et al. (1986), Ye and Wu (2011) | ||
| China, Fujie | 1988 | P. elliottii | Morphological characteristics and RAPD analysis were used to identify the pathogen. TEF 1 sequencing was further used for identification and mating type 2 was detected by Janoušek et al. (2016). | * | Huang et al. (1995), Janoušek et al. (2016) | ||
| China, Zhejiang | 1991 | P. thunbergii | Morphological characteristics and RAPD analysis were used to identify the pathogen. | * | Huang et al. (1995) | ||
| China, Jiangxi | 1992 | P. elliottii, P. thunbergii | Morphological characteristics and RAPD analysis were used to identify the pathogen. | * | Huang et al. (1995) | ||
| China, Guanxi | 1992 | P. caribaea, P. elliottii | Morphological characteristics and RAPD analysis were used to identify the pathogen. | * | Huang et al. (1995) | ||
| Colombia, Piedras Blancas and Pereira | 1978 | P. radiata, P. elliottii, P. patula | Identification method not specified. | P. radiata severely defoliated but on P. elliottii and P. patula the pathogen was isolated from cast needles found underneath healthy trees. | Gibson (1980) | ||
| Colombia, Albán | 1981 | P. radiata | Morphological identification, sexual and asexual state were identified. | Plantations were severely defoliated. | Evans (1984) | ||
| Colombia, Refocosta | 2011 | P. caribaea | Infected needles were collected by C.A. Rodas. Isolations were made by I. Barnes. TEF 1 sequencing was used for identification and mating type 2 was detected by Janoušek et al. (2016). | * | Janoušek et al. (2016) | ||
| Costa Rica, Alajuela | 1980 | P. oocarpa | Morphological identification of pathogen. | Evans (1984) | |||
| Croatia, Dalmatia | 1975 | P. halepensis | Morphological identification of L. acicola. | The pathogen is not as aggressive as in the USA on this host and it seems to only be aggressive where dense canopies are present with high air humidity. Copper fungicides were applied. | Milatović (1976) | ||
| Croatia, Zadar | Not specified | P. halepensis | Forest surveys were conducted. It is not specified in the English abstract whether morphological identifications were performed. | 500 ha of P. halepensis was heavily infected with the pathogen. Highly infected trees and lower infected branches were cut down and it is reported that the trees recovered. | Glavaš and Margaletić (2001) | ||
| Croatia, Zadar | 2009 | P. halepensis | TEF 1 sequencing was used for identification. Mating type 2 was detected. | * | Janoušek et al. (2016) | ||
| Croatia, Kožino | 2015 | P. halepensis | TEF 1 sequencing was used for identification. Mating type 2 was detected. | * | Sadiković et al. (2019) | ||
| Cuba, Baracoa, Guantánamo, Plateau of Mayarí and Master Saw | 1980–1998 | P. caribaea, P. cubensis, P. maestrensis | Symptom identification and morphological confirmation of the fungus. | Mostly seedlings in nurseries were infected. | Lopéz Castilla et al. (2002) | ||
| Czech Republic, Southern Bohemia, Červené Blato Nature Reserve | 2007 | P. uncinata subsp. uliginosa | Morphological identifications were conducted as well as sequencing of the ITS region. The identity of the pathogen was again confirmed with TEF 1 sequencing by Janoušek et al. (2016). Both mating types were detected. | * | Heavy defoliation was reported in 2007. No control measures were taken as the incidence was reported in a natural nature reserve. | Jankovský et al. (2009b), Janoušek et al. (2016) | |
| Czech Republic, Southern Bohemia, Soběslav, Borkovická Blata National Nature Reserve | 2008 | P. uncinata subsp. uliginosa | Morphological identifications were conducted as well as sequencing of the ITS region. The identity of the pathogen was again confirmed with TEF 1 sequencing by Janoušek et al. (2016). Both mating types were detected. | * | No action was taken as the outbreak was in a natural reserve. | Jankovský et al. (2009a), Janoušek et al. (2016) | |
| Estonia, Hiiumaa Island and Käravere | 2014–2015 | P. mugo | Symptom identification was confirmed with conventional PCR directly from pine needles. Lecanosticta acicola was isolated from the needles and confirmed with ITS sequencing. Both mating types were detected. | * | Adamson et al. (2015) | ||
| Estonia, Tallinn Botanical Garden | 2006–2008 | P. ponderosa | Material of Dothistroma was collected and isolated but in culture it was determined to be L. acicola based on culture morphology. The TEF 1 sequences were later determined for representative isolates and mating type 2 was detected (Janoušek et al., 2016). | * | Drenkhan and Hanso (2009), Janoušek et al. (2016) | ||
| Estonia, Tallinn Botanical Garden | 2010–2013 | P. mugo, P. mugo var. pumilio, P. ponderosa, P. uncinata | Symptom identification was confirmed with conventional PCR directly from pine needles. Lecanosticta acicola was isolated from the needles and confirmed with ITS sequencing. Mating type 1 was detected. | * | Adamson et al. (2015) | ||
| Estonia, Tartu county | 2016 | P. sylvestris, P. mugo, Pinus × rhaetica | Visual symptom identification was confirmed with conventional PCR and selected isolates were identified using an ITS sequencing PCR. Both mating types were detected. | * | Adamson et al. (2018) | ||
| Estonia, Tori and Vasula | 2012, 2013 | P. mugo | Symptom identification was confirmed with conventional PCR directly from pine needles. Lecanosticta acicola was isolated from the needles and confirmed with ITS sequencing. | * | Adamson et al. (2015) | ||
| France, South‐West, Aquitaine and western Pyrénées | 1993 | P. attenuata × P. radiata | In field observations were made. | Severe tree mortality was observed. French authorities implemented eradication measures and destroyed 127 ha of trees. | Lévy (1996) | ||
| France, Gironde | 1995 | P. muricata | TEF 1 and BT 2 sequencing used for identification, mating type 1 detected. | * | Ioos et al. (2010), Janoušek et al. (2016) | ||
| France, Landes | 1995 | P. attenuata × P. radiata | TEF 1 and BT 2 sequencing used for identification, mating type 2 detected. | * | Ioos et al. (2010), Janoušek et al. (2016) | ||
| France, Pyrénées‐Atlantiques | 1995 | P. radiata | TEF 1 and BT 2 sequencing used for identification. | * | Ioos et al. (2010) | ||
| France, Ariège | 2009 | P. sylvestris | Forest surveys were conducted. | More than 50% of the trees were affected. | Alvère et al. (2010) | ||
| France, Tarn‐et‐Garonne | 2009 | P. nigra var. laricio | Forest surveys were conducted. | The trees were moderately affected. | Alvère et al. (2010) | ||
| France, Pyrénées‐Atlantiques | 2012 | P. radiata | TEF 1 sequencing used for identification, both mating types were detected. | * | Janoušek et al. (2016) | ||
| Germany, Bavaria | 1994 | P. mugo | The pathogen was identified based on morphological characteristics. | Pehl (1995) | |||
| Germany, Bavaria | 1994, 2000, 2010, 2011 | P. mugo | TEF 1 sequencing used for identification, both mating types were detected. | * | Janoušek et al. (2016) | ||
| Germany, Bavaria, Munich Botanical gardens | 2018 | P. mugo | Collected by I. Barnes. The identity was confirmed by ITS sequencing. Dothistroma septosporum was also present. | * | I. Barnes, FABI, Pretoria, South Africa, personal communication | ||
| Guatemala, El Progreso | 1983 | P. oocarpa | Morphological identification methods were used. As L. acicola was not identified in Central America using molecular identification techniques (van der Nest et al., 2019), this report will need to be verified. | Evans (1984) | |||
| Honduras | 1980–1983 | P. caribaea, P. maximinoi, P. oocarpa, P. tecunumanii, | Morphological identification methods were used. As L. acicola was not identified in Central America using molecular identification techniques (van der Nest et al., 2019), this report will need to be verified. | Evans (1984) | |||
| Ireland, Wexford county | 2016 | P. mugo, P. sylvestris | ITS sequencing was used for identification purposes. Mating type 1 was detected. | * | Mullett et al. (2018) | ||
| Italy, Brescia | 1997 | P. mugo | Symptoms were noted in the botanical garden and the presence of the pathogen was confirmed with morphological identifications. | Extensive necrosis and crown defoliation were observed in all 12 of the P. mugo trees present in the botanical garden. | La Porta and Capretti (2000) | ||
| Italy, Brescia | 2008 | P. mugo | TEF 1 sequencing used for identification and mating type 1 detected. | * | Janoušek et al. (2016) | ||
| Japan, Shimane Prefecture (Honshu) | 1996 | P. thunbergii, P. densiflora (tested in controlled environment) | The pathogen was morphologically identified. | P. thunbergii was severely infected. Inoculation trials on this host as well as P. densiflora also revealed that P. densiflora is susceptible although it was not reported in the host's natural environment. | Suto and Ougi (1998) | ||
| Japan, Shimane | 2010 | P. thunbergii | TEF 1 sequencing used for identification, mating type 2 was detected. | * | Janoušek et al. (2016) | ||
| Latvia, Salaspils | 2012 | P. pumila | Morphological identification. Later it was confirmed with PCR‐based methods. | * | Eradication measures were taken. | EPPO (2012a) | |
| Latvia, Salaspils | 2016 | P. mugo | Identification was done by ITS sequencing. Mating type 1 was detected. | * | Mullett et al. (2018) | ||
| Lithuania, Curonian Spit, Smiltynė Forest District | 2009 | P. mugo | Morphological characteristics as well as ITS sequencing and ITS‐RFLP was used to identify the pathogen. This material was again examined by Janoušek et al. (2016) and the identity confirmed with TEF 1. Mating type 1 was detected. | * | A monitoring programme was initiated and infected trees felled and burned. | Markovskaja et al. (2011), Janoušek et al. (2016) | |
| Lithuania, Curonian Spit, Smiltynė Forest District and Juodkrantė Forest District | 2010 | P. mugo | Morphological characteristics as well as ITS sequencing and ITS‐RFLP was used to identify the pathogen. | * | A monitoring programme was initiated and infected trees felled and burned. | Markovskaja et al. (2011) | |
| Lithuania, Curonian Spit, near Juodkrante | 2012 | P. mugo, P. sylvestris | Morphological identifications and PCR‐based methods. | * | Phytosanitary methods were implemented. | EPPO (2012b) | |
| Lithuania, Curonian Spit, Smiltyne Smiltynė Forest District and Juodkrantė Forest District | 2014 | P. mugo | Infected needles were collected by S. Markovskaja. Isolations were made by A. van der Nest. A multigene phylogenetic approach was used to determine the identity of the isolates. | * | van der Nest et al. (2019) | ||
| Mexico, Puebla | 1983 | P. patula | Morphological identification. | Evans (1984) | |||
| Mexico | 2000 | P. ayacahuite, P. cembroides, P. halepensis | Morphological characteristics were examined. | High disease severity was reported on P. halepensis. | Marmolejo (2000) | ||
| Mexico, Nuevo León | 2010, 2011 | P. halepensis | TEF 1 sequencing used for identification, both mating types detected. KJ938447–KJ938449 were later identified as L. variabilis (van der Nest et al., 2019) and the remaining isolates are part of L. acicola lineage 3. | * | Janoušek et al. (2016) | ||
| Nicaragua | 1981–1983 | P. caribaea, P. maximinoi, P. oocarpa, P. tecunumanii | Morphological identification methods were used. As L. acicola was not identified in Central America using molecular identification techniques (van der Nest et al., 2019), this report will need to be verified. Both the sexual and asexual state was observed. | Evans (1984) | |||
| Portugal, Minho | 2016 | P. radiata | Identification was done by ITS sequencing. Mating type 1 was detected. | * | Mullett et al. (2018) | ||
| Romania, Vrancea | 2017 | Pinus sp. | The pathogen was detected during a forest survey in a 30‐year‐old plantation. | Eradication reported to be under way in the 19‐hectare forest. | EPPO (2018) | ||
| Russia, Krasnodar region, Sochi | 2016 | P. mugo subsp. mugo, P. thunbergii | Identification was done by ITS sequencing. Mating type 2 was detected. | * | Mullett et al. (2018) | ||
| Slovenia, Bled | 2008–2009 | P. mugo, P. sylvestris | Morphological identifications. The identity of isolates on P. mugo were confirmed with TEF 1 sequencing and mating type 2 was detected (Janoušek et al., 2016; Sadiković et al., 2019). | * | All affected trees were eradicated. | Jurc and Jurc (2010), Janoušek et al. (2016), Sadiković et al. (2019) | |
| Slovenia, Čatež | 2015 | P. mugo | TEF 1 sequencing was used for identification. Mating type 1 was detected. | * | Sadiković et al. (2019) | ||
| Slovenia, Ljubljana | 2008–2009 | P. mugo, P. sylvestris | Morphological identifications. The identity of isolates from P. mugo were confirmed with TEF 1 sequencing by Sadiković et al. (2019). | * | All affected trees were eradicated. | Jurc and Jurc (2010), Sadiković et al. (2019) | |
| Slovenia, Ljubljana | 2013 | P. mugo | TEF 1 sequencing was used for identification. Mating type 1 was detected. | * | Sadiković et al. (2019) | ||
| Slovenia, Tolmin | 2016 | P. nigra | TEF 1 sequencing was used for identification. Mating type 1 was detected. | * | Sadiković et al. (2019) | ||
| Slovenia, Trenta | 2014–2015 | P. mugo | TEF 1 sequencing was used for identification. Mating type 2 was detected. | * | Sadiković et al. (2019) | ||
| South Korea, Naju | 2010–2011 | P. thunbergii | L. acicola symptoms were observed and confirmed with ITS sequencing. TEF 1 sequencing was used for identification by Janoušek et al. (2016) and mating type 2 was detected. | * | Low incidence, less than 1%. | Janoušek et al. (2016), Seo et al. (2012) | |
| Spain | 1942 | P. radiata | Probably oldest official report of L. acicola in Europe based on morphological identification. | Martínez (1942) | |||
| Spain, Cantabria | 2012 | P. radiata | TEF 1 sequencing used for identification, mating type 2 was detected. | * | Janoušek et al. (2016) | ||
| Spain, Spanish Atlantic climate region | 2015 | P. nigra, P. radiata | Sequenced directly from needles using conventional PCR (Ioos et al., 2010). Both mating types were detected. | * | Lecanosticta acicola was detected on 44.7% of trees that were surveyed. | Ortíz de Urbina et al. (2017) | |
| Sweden | 2017 | P. mugo ‘Hesse’ | Morphological identification and ITS sequencing. | * | Single tree in arboretum that was severely affected. | Cleary et al. (2019) | |
| Switzerland, Zollikon | 1995 | P. mugo, P. uncinata | Morphological identification of the pathogen. | Control measures were initiated in accordance with the phytosanitary policy of the EPPO. | Holdenrieder and Sieber (1995) | ||
| Switzerland, Canton St Gallen | 1999 | P. mugo | TEF 1 sequencing used for identification. | * | Janoušek et al. (2016) | ||
| Switzerland, Canton Zug | 2009 | P. mugo | Symptoms were observed in the field. Later, TEF 1 sequencing was used to confirm identification (Janoušek et al., 2016). Mating type 1 was detected. | * | Angst (2011), Janoušek et al. (2016) | ||
| Switzerland, Zürich | 2009 | P. mugo | Symptoms were observed in the field. Later, TEF 1 sequencing was used to confirm identification (Janoušek et al., 2016). Mating type 1 was detected. | * | Angst (2011), Janoušek et al. (2016) | ||
| Switzerland, Bern and Zürich | 2017 | P. mugo | Detection with qPCR and a conventional PCR directly from pine needles. | * | Schneider et al. (2019) | ||
| Switzerland, Schwyz | 2017 | P. sylvestris | Detection with qPCR and a conventional PCR directly from pine needles. | * | Schneider et al. (2019) | ||
| USA, Alabama | 1929 | P. palustris | Hedgcock reported on collections of the pathogen at the office of Forest Pathology at Washington, D.C. and the Mycological collections of the US Department of Agriculture. | Hedgcock (1929) | |||
| USA, Alabama | 1944 | P. echinata, P. palustris, P. taeda | Siggers reported Lecanosticta isolates that are in the collections in the Division of Forest Pathology in Louisiana and Maryland, USA. These reports should be verified. | Siggers (1944) | |||
| USA, Alabama | 1948–1967 | P. palustris | Symptoms were observed annually on seedlings and the proportion of seedlings affected were recorded. | In a 4‐year study, 78% or more seedlings were infected yearly with L. acicola. | Boyer (1972) | ||
| USA, Arkansas | 1929 | P. taeda | Hedgcock reported on collections of the pathogen at the office of Forest Pathology at Washington, D.C. and the Mycological collections of the US Department of Agriculture. | Hedgcock (1929) | |||
| USA, Arkansas | 1944 | P. taeda | Siggers reported Lecanosticta isolates that are in the collections in the Division of Forest Pathology in Louisiana and Maryland, USA. These reports should be verified. | Siggers (1944) | |||
| USA, Arkansas | 1967–1971 | P. sylvestris | Symptoms were observed in the field and the proportion of needles affected were noted. In some cases, microscopic examinations of conidia were used for identification. | Skilling and Nicholls (1974) | |||
| USA, Florida | 1929 | P. caribaea, P. glabra, P. palustris, P. taeda | Hedgcock reported on collections of the pathogen at the office of Forest Pathology at Washington, D.C. and the Mycological collections of the US Department of Agriculture. | Hedgcock (1929) | |||
| USA, Florida | 1944 | P. attenuata, P. caribaea, P. coulteri, P. jeffreyi, P. glabra, P. halepensis, P. latifolia, P. muricata, P. palustris, P. pinaster, P. pinea, P. ponderosa var. scopulorum, P. radiata, P. thunbergii | Siggers reported on Lecanosticta isolates that are in the collections in the Division of Forest Pathology in Louisiana and Maryland, USA. These reports should be verified. | Siggers (1944) | |||
| USA, Georgia | 1929 | P. palustris, P. taeda, P. virginiana | Hedgcock reported on collections of the pathogen at the office of Forest Pathology at Washington, D.C. and the Mycological collections of the US Department of Agriculture. | Hedgcock (1929) | |||
| USA, Georgia | 1944 | P. caribaea, P. palustris, P. taeda, P. virginiana | Siggers reported Lecanosticta isolates that are in the collections in the Division of Forest Pathology in Louisiana and Maryland, USA. These reports should be verified. | Siggers (1944) | |||
| USA, Idaho | 1929 | P. ponderosa | Hedgcock reported on collections of the pathogen at the office of Forest Pathology at Washington, D.C. and the Mycological collections of the US Department of Agriculture. According to Siggers (1944) the identification was based on characteristics that do not fit Lecanosticta and therefore this record should be verified. | Hedgcock (1929) | |||
| USA, Iowa | 1967–1971 | P. sylvestris | Symptoms were observed in the field and the proportion of needles affected were noted. In some cases, microscopic examinations of conidia were used for identification. | Skilling and Nicholls (1974) | |||
| USA, Kansas | 1929 | P. nigra var. austriaca | Hedgcock reported on collections of the pathogen at the office of Forest Pathology at Washington, D.C. and the Mycological collections of the US Department of Agriculture. According to Siggers (1944) the identification was based on characteristics that do not fit Lecanosticta and therefore this record should be verified. | Hedgcock (1929) | |||
| USA, Kansas | 1951 | P. nigra, P. ponderosa | Reports in the field and mycological identification. | Rogerson (1953) | |||
| USA, Kansas | 1967–1971 | P. sylvestris | Symptoms were observed in the field and the proportion of needles affected were noted. In some cases, microscopic examinations of conidia were used for identification. | Skilling and Nicholls (1974) | |||
| USA, Kentucky | 1929 | P. nigra var. austriaca | Hedgcock reported on collections of the pathogen at the office of Forest Pathology at Washington, D.C. and the Mycological collections of the US Department of Agriculture. According to Siggers (1944) the identification was based on characteristics that do not fit Lecanosticta and therefore this record should be verified. | Hedgcock (1929) | |||
| USA, Kentucky | 1967–1971 | P. sylvestris | Symptoms were observed in the field and the proportion of needles affected were noted. In some cases, microscopic examinations of conidia were used for identification. | Skilling and Nicholls (1974) | |||
| USA, Louisiana | 1929 |
P. palustris, Pinus
|
Hedgcock reported on collections of the pathogen at the office of Forest Pathology at Washington, D.C. and the Mycological collections of the US Department of Agriculture. | Hedgcock (1929) | |||
| USA, Louisiana | 1929–1930, 1960 | P. palustris | Symptoms were observed and the proportion of seedlings affected were recorded at 4–5 years of age and again at 30 years. | Most of the trees were affected. | Wakeley (1970) | ||
| USA, Louisiana | 1944 | P. attenuata, P. caribaea, P. contorta var. latifolia, P. echinata, P. nigra var. laricio, P. palustris, P. pinaster, P. ponderosa var. scopulorum, P. radiata, P. rigida, P. serotina, P. sabiniana, Pinus × sondereggeri, P. taeda | Siggers reported on Lecanosticta isolates that are in the collections in the Division of Forest Pathology in Louisiana and Maryland, USA. These reports should be verified. | Siggers (1944) | |||
| USA, Maine | 2011 | P. strobus | Isolates were collected and morphologically identified in a survey. These isolates were later identified with TEF 1 sequencing and both mating types were detected. | * | Munck et al. (2012), Janoušek et al. (2016) | ||
| USA, Maine | 2011–2012 | P. strobus | Lecanosticta acicola was identified as part of a complex of pathogens that cause white pine needle damage (WPND). Morphological identifications and selected ITS PCR sequencing was performed to confirm the presence of L. acicola. | * | It was observed that affected trees were defoliated annually. | Broders et al. (2015) | |
| USA, Michigan | 2016 | P. sylvestris | TEF 1 sequencing used for identification, mating type 2 was detected. | * | Janoušek et al. (2016) | ||
| USA, Minnesota | 1967–1971 | P. sylvestris | Symptoms were observed in the field and the proportion of needles affected were noted. In some cases, microscopic examinations of conidia were used for identification. | Skilling and Nicholls (1974) | |||
| USA, Minnesota and Wisconsin | 1970–1972 | P. banksiana, P. glauca, P. nigra, P. palustris, P. resinosa, P. strobus, P. sylvestris, Picea glauca | Symptoms were observed in the field and the proportion of needles affected were noted. | These species were tested for susceptibility in a field trial by planting the hosts underneath heavily infected P. sylvestris. Four varieties of P. sylvestris, as well as P. nigra and P. resinosa, were the most susceptible. P. strobus was moderately resistant. P. banksiana was the most resistant. Less than 1% of Picea glauca was infected. | Skilling and Nicholls (1974) | ||
| USA, Mississippi | 1929 | P. caribaea, P. palustris, P. taeda | Hedgcock reported on collections of the pathogen at the office of Forest Pathology at Washington, D.C. and the Mycological collections of the US Department of Agriculture. | Hedgcock (1929) | |||
| USA, Mississippi | 1944 | P. caribaea, P. palustris, P. pinaster, P. taeda, P. thunbergii | Siggers reported on Lecanosticta isolates that are in the collections in the Division of Forest Pathology in Louisiana and Maryland, USA. These reports should be verified. | Siggers (1944) | |||
| USA, Mississippi | 1952–1953 | P. palustris | Microscopic identification. Both the sexual and asexual states were observed. | Henry (1954) | |||
| USA, Mississippi | 1966–1967 | P. palustris | Morphological identifications. Both the sexual state and asexual state were observed throughout the year on infected P. palustris. | Kais (1971) | |||
| USA, Mississippi | 2012 | P. palustris, P. taeda | TEF 1 sequencing used for identification, both mating types detected. | * | Janoušek et al. (2016) | ||
| USA, Missouri | 1929 | P. nigra var. austriaca | Hedgcock reported on collections of the pathogen at the office of Forest Pathology at Washington, D.C. and the Mycological collections of the US Department of Agriculture. According to Siggers (1944) the identification was based on characteristics that do not fit Lecanosticta and therefore this record should be verified. | Hedgcock (1929) | |||
| USA, Missouri | 1947–1949 | P. ponderosa | Symptoms were observed in the field and morphological identifications were made. Both the sexual state and asexual state were observed. | All trees were affected. Excessive needle defoliation and in some cases tree mortality was observed. | Luttrell (1949) | ||
| USA, Missouri | 1967–1971 | P. sylvestris | Symptoms were observed in the field and the proportion of needles affected were noted. In some cases, microscopic examinations of conidia were used for identification. | Skilling and Nicholls (1974) | |||
| USA, New England | 2016 | P. strobus | Severe needle browning was observed and L. acicola was identified as part of a complex of species causing premature defoliation. This is possibly WPND although it was not defined as such. | Brazee (2016) | |||
| USA, New Hampshire | 2011 | P. strobus | Isolates were collected and morphologically identified in a survey. These isolates were later identified with TEF 1 sequencing and mating type 1 was detected. | * | Munck et al. (2012), Janoušek et al. (2016) | ||
| USA, New Hampshire | 2011–2012 | P. strobus | Lecanosticta acicola was identified as part of a complex of pathogens that cause WPND. Morphological identifications and selected ITS PCR sequencing confirmed the presence of L. acicola. | * | It was observed that affected trees were defoliated annually. | Broders et al. (2015) | |
| USA, New York | 1976 | P. mugo | Lecanosticta acicola was identified with morphological methods and brown spot needle blight symptoms confirmed on trees. Specimens are in the Cornell University Plant Pathology Herbarium. | Sinclair and Hudler (1980) | |||
| USA, North Carolina | 1929 | P. echinata, P. palustris, P. rigida, P. taeda, P. virginiana | Hedgcock reported on collections of the pathogen at the office of Forest Pathology at Washington, D.C. and the Mycological collections of the US Department of Agriculture. | Hedgcock (1929) | |||
| USA, North Carolina | 1944 | P. palustris, P. rigida, P. strobus, P. taeda, P. virginiana | Siggers reported on Lecanosticta isolates that are in the collections in the Division of Forest Pathology in Louisiana and Maryland, USA. These reports should be verified. | Siggers (1944) | |||
| USA, North Carolina | 1957, 1958 | P. strobus | Morphological identifications of L. acicola. | Boyce (1959) | |||
| USA, Ohio | 1944 | P. contorta var. latifolia, P. coulteri, P. jeffreyi | Siggers reported on Lecanosticta isolates that are in the collections in the Division of Forest Pathology in Louisiana and Maryland, USA. These reports should be verified. | Siggers (1944) | |||
| USA, Oregon | 1929 | P. attenuata | Hedgcock reported on collections of the pathogen at the office of Forest Pathology at Washington, D.C. and the Mycological collections of the US Department of Agriculture. | Hedgcock (1929) | |||
| USA, Oregon | 1944 | P. attenuata | Siggers reported on Lecanosticta isolates that are in the collections in the Division of Forest Pathology in Louisiana and Maryland, USA. These reports should be verified. | Siggers (1944) | |||
| USA, Pennsylvania | 1929 | P. rigida | Hedgcock reported on collections of the pathogen at the office of Forest Pathology at Washington, D.C. and the Mycological collections of the US Department of Agriculture. | Hedgcock (1929) | |||
| USA, Pennsylvania | 1987–1989 | P. strobus | Morphological identifications were done. | Stanosz (1990) | |||
| USA, South Carolina | 1876 | P. echinata (P. variabilis) | Morphological description of Cryptosporium acicolum. | de Thümen (1878) | |||
| USA, South Carolina | 1929 | P. caribaea, P. echinata, P. palustris, P. serotina, P. taeda | Hedgcock reported on collections of the pathogen at the office of Forest Pathology at Washington, D.C. and the Mycological collections of the US Department of Agriculture. | Hedgcock (1929) | |||
| USA, South Carolina | 1944 | P. caribaea, P. palustris, P. taeda | Siggers reported on Lecanosticta isolates that are in the collections in the Division of Forest Pathology in Louisiana and Maryland, USA. These reports should be verified. | Siggers (1944) | |||
| USA, Tennessee | 1929 | P. rigida, P. taeda | Hedgcock reported on collections of the pathogen at the office of Forest Pathology at Washington, D.C. and the Mycological collections of the US Department of Agriculture. | Hedgcock (1929) | |||
| USA, Tennessee | 1944 | P. palustris, P. ponderosa var. scopulorum, P. rigida, P. taeda | Siggers reported on Lecanosticta isolates that are in the collections in the Division of Forest Pathology in Louisiana and Maryland, USA. These reports should be verified. | Siggers (1944) | |||
| USA, Texas | 1929 | P. palustris, P. taeda | Hedgcock reported on collections of the pathogen at the office of Forest Pathology at Washington, D.C. and the Mycological collections of the US Department of Agriculture. | Hedgcock (1929) | |||
| USA, Texas | 1929 | P. palustris, P. taeda | Symptoms were observed on trees inside and surrounding the nurseries. | Low severity recorded. Nursery beds were sprayed with Bordeaux 4‐4‐50 with good results. | Webster (1930) | ||
| USA, Texas | 1944 | P. caribaea, P. palustris, P. pinaster, P. taeda | Siggers reported on Lecanosticta isolates that are in the collections in the Division of Forest Pathology in Louisiana and Maryland, USA. These reports should be verified. | Siggers (1944) | |||
| USA, Vermont | 2008 | P. mugo, P. resinosa, P. sylvestris, P. strobus | Forest surveys were conducted and the pathogen identified based on symptomology. | Gibbs and Sinclair (2008) | |||
| USA, Vermont | 2011 | P. strobus | Isolates were collected and morphologically identified in a survey. These isolates were later identified with TEF 1 sequencing and both mating types were detected. | * | Munck et al. (2012), Janoušek et al. (2016) | ||
| USA, Vermont | 2011–2012 | P. strobus | Lecanosticta acicola was identified as part of a complex of pathogens that cause WPND. Morphological identifications and selected ITS PCR sequencing confirmed the presence of L. acicola. | * | It was observed that affected trees were defoliated annually. | Broders et al. (2015) | |
| USA, Virginia | 1929 | P. rigida | Hedgcock reported on collections of the pathogen at the office of Forest Pathology at Washington, D.C. and the Mycological collections of the US Department of Agriculture. | Hedgcock (1929) | |||
| USA, Wisconsin | 1966–1970 | P. sylvestris | A forest survey was conducted and symptoms of L. acicola was observed. | Approximately 3000 acres in 55 plantations were severely infected. Short leaf French and Spanish P. sylvestris were severely affected. Long leaf P. sylvestris varieties were reported as resistant. | Prey and Morse (1971) | ||
| USA, Wisconsin | 1967–1971 | P. sylvestris | Symptoms were observed in the field and the proportion of needles affected were noted. In some cases, microscopic examinations of conidia were used for identification. | Skilling and Nicholls (1974) | |||
| USA, Wisconsin | 1970 | P. resinosa | Symptoms were observed in the field and morphological identifications were made. | After the pathogen was observed in pine stands, an inoculation trial revealed that P. resinosa is highly susceptible to L. acicola. | Nicholls and Hudler (1972) | ||
| USA, Wisconsin | 2010 | P. sylvestris | TEF 1 sequencing used for identification, mating type 2 was detected. | * | Janoušek et al. (2016) | ||
| Lecanosticta brevispora | |||||||
| Guatemala, Alta Verapaz, Santa Cruz Verapaz, near Tactíc | 2010 | P. oocarpa | Multigene phylogenetic analysis. | * | van der Nest et al. (2019) | ||
| Guatemala, Chimaltenango, Tecpán, Finca La Esperanza | 2010 | P. pseudostrobus | Multigene phylogenetic analysis. | * | van der Nest et al. (2019) | ||
| Guatemala, Lugar, La Soledad, Jalapa site II | 2012 | P. oocarpa | Multigene phylogenetic analysis. | * | van der Nest et al. (2019) | ||
| Honduras | 2010 | P. oocarpa | Multigene phylogenetic analysis. | * | van der Nest et al. (2019) | ||
| Mexico | 2000 | Pinus sp. | Multigene phylogenetic analysis. | * | Quaedvlieg et al. (2012) | ||
| Lecanosticta gloeospora | |||||||
| Mexico, Nuevo León, Iturbide‐Galeana | 1983 | P. pseudostrobus | Morphological identification. The type was later sequenced using multiple genes (van der Nest et al., 2019). | * | Evans (1984), Marmolejo (2000), van der Nest et al. (2019) | ||
| Lecanosticta guatemalensis | |||||||
| Guatemala, Baja Verapaz | 1983 | P. oocarpa | The type culture was previously identified as L. acicola based on morphological characteristics (Evans, 1984). Multigene phylogenetic analysis revealed it as a new species, L. guatemalensis. | * | Quaedvlieg et al. (2012), van der Nest et al. (2019) | ||
| Guatemala, Alta Verapaz, Santa Cruz Verapaz, near Tactíc | 2010 | P. oocarpa | Multigene phylogenetic analysis. | * | Very low. | van der Nest et al. (2019) | |
| Guatemala, Chiquimula | 2011 | P. oocarpa | Multigene phylogenetic analysis. | * | Very low. | van der Nest et al. (2019) | |
| Guatemala, Jalapa, Finca Forestal Soledad | 2012 | P. oocarpa | Multigene phylogenetic analysis. | * | Very low. | van der Nest et al. (2019) | |
| Guatemala, Coban, San Juan Chamelco | 2012 | P. oocarpa | Multigene phylogenetic analysis. | * | Very low. | van der Nest et al. (2019) | |
| Nicaragua | 1982 | P. tecunumanii | This isolate was previously identified as L. acicola based on morphological characteristics (Evans, 1984). Multigene phylogenetic analysis revealed it to be L. guatemalensis. | * | van der Nest et al. (2019) | ||
| Nicaragua, Matagalpa | 2010 | P. oocarpa | Multigene phylogenetic analysis. | * | Very low. | van der Nest et al. (2019) | |
| Honduras, Yoro | 1981 | P. caribaea, P. oocarpa | These isolates were previously identified as L. acicola based on morphological characteristics (Evans, 1984). Multigene phylogenetic analysis revealed it to be L. guatemalensis. | * | van der Nest et al. (2019) | ||
| Lecanosticta jani | |||||||
| Guatemala, Alta Verapaz, Santa Cruz Verapaz, near Tactíc | 2010 | P. oocarpa | Multigene phylogenetic analysis. | * | Very low. | van der Nest et al. (2019) | |
| Guatemala, Chiquimula | 2010 | P. oocarpa | Multigene phylogenetic analysis. | * | Very low. | van der Nest et al. (2019) | |
| Guatemala, Jalapa, Finca Forestal Soledad | 2012 | P. maximinoi | Multigene phylogenetic analysis. | * | Very low. | van der Nest et al. (2019) | |
| Guatemala, Jalapa, Finca La Soledad, Mataquescuintla | 2012 | P. tecunumanii | Multigene phylogenetic analysis. | * | Very low. | van der Nest et al. (2019) | |
| Nicaragua, Matagalpa | 2010 | P. oocarpa | Multigene phylogenetic analysis. | * | Very low. | van der Nest et al. (2019) | |
| Lecanosticta pharomachri | |||||||
| Guatemala, Baja Verapaz, San Jerónimo, Salamá | 2012 | P. tecunumanii | Multigene phylogenetic analysis. | * | Very low. | van der Nest et al. (2019) | |
| Guatemala, Jalapa, Finca La Soledad, Mataquescuintla | 2010–2012 | P. oocarpa | Multigene phylogenetic analysis. | * | Very low. | van der Nest et al. (2019) | |
| Honduras | 2010 | P. oocarpa | Multigene phylogenetic analysis. | * | Very low. | van der Nest et al. (2019) | |
| Lecanosticta tecunumanii | |||||||
| Guatemala, Baja Verapaz, San Jerónimo, Salamá | 2012 | P. tecunumanii | Multigene phylogenetic analysis. | * | Very low. | van der Nest et al. (2019) | |
| Lecanosticta variabilis | |||||||
| Guatemala, Alta Verapaz, Santa Cruz Verapaz, near Tactíc | 2010 | P. oocarpa | Multigene phylogenetic analysis. Both mating types were present (Janoušek et al., 2016). | * | Very low. | van der Nest et al. (2019) | |
| Guatemala, Jalapa, Finca Forestal Soledad | 2012 | P. maximinoi | Multigene phylogenetic analysis. | * | Very low. | van der Nest et al. (2019) | |
| Honduras, Santa Barbara, Lago de Yojoa | 1984 | P. caribaea | This isolate was previously identified as L. acicola in a morphological study by Evans (1984). A multigene phylogenetic analysis indicated that this is a new species, L. tecunumanii. | * | Very low. | Evans (1984), van der Nest et al. (2019) | |
| Mexico | 2000 | Pinus sp. | Multigene phylogenetic analysis. | * | van der Nest et al. (2019) | ||
| Mexico | 2010 | P. arizonica var. stormiae, P. halepensis | Multigene phylogenetic analysis. The isolates were previously identified as L. acicola (Janoušek et al., 2016) and both mating types were detected. | * | van der Nest et al. (2019) |
Quarantine measures rely on accurately identifying the presence of pathogens on symptomatic tissues. This is complicated in the case of L. acicola where the symptoms of BSNB closely resemble those of Dothistroma needle blight (DNB). DNB is caused by two species: Dothistroma septosporum and D. pini (Barnes et al., 2016). Due to their similar symptoms, field diagnoses of the causal agent based on symptoms and/or on morphology alone have commonly been incorrect (Shishkina and Tsanava, 1967; Siggers, 1944; Thyr and Shaw, 1964). Consequently, past reports of L. acicola based only on morphological descriptions and symptoms must be treated with caution and verified using molecular identification techniques (van der Nest et al., 2019).
Lecanosticta acicola has been well‐known in the southeastern USA since the early 1900s, but is rapidly spreading in northern parts of the USA, Canada and in some parts of Europe (Broders et al., 2015). Its complete host range is not known but appears to be expanding (Mullett et al., 2018). A recent taxonomic re‐evaluation of isolates previously identified as L. acicola, applying phylogenetic analyses based on DNA sequences, has led to various isolates being recognized as distinct species (Quaedvlieg et al., 2012; van der Nest et al., 2019). This and a number of recent publications (Adamson et al., 2018; Cleary et al., 2019; Mullett et al., 2018; Ondrušková et al., 2018; Ortíz de Urbina et al., 2017; Sadiković et al., 2019; Schneider et al., 2019; Wyka et al., 2017) justifies the need for a review of current knowledge regarding BSNB and the Lecanosticta species that cause this disease. This is the first review of the topic to be presented in 75 years subsequent to that of Siggers (1944).
Lecanosticta Species
The genus Lecanosticta, which includes nine species with the type species being L. acicola (previously known as Mycosphaerella dearnessii, Table 2), is characterized by stromata and septate, pigmented conidia. The genus was erected by Sydow and Petrak in 1922 (Sydow and Petrak, 1922). The taxonomic history and nomenclature of Lecanosticta acicola has been succinctly presented previously (Evans, 1984; Siggers, 1944) and is summarized and updated in Table 2.
Table 2.
A summarized history of the taxonomy and nomenclature of the genus Lecanosticta.
| Year | Species epithet | Reference | Sexual state reported | Country, location | Host | Description | Notes |
|---|---|---|---|---|---|---|---|
| Lecanosticta acicola | |||||||
| 1878 | Cryptosporium acicolum Thüm | de Thümen (1878) | Asexual | USA, South Carolina, Aiken | Pinus echinata (P. variabilis) | P. variabilis is a synonym of P. echinata. In Wolf and Barbour (1941), it was mentioned that it was in fact on P. caribaea and that the host was previously incorrectly identified. | |
| 1884 | Septoria acicola (Thüm) Sacc | Saccardo (1884) | Asexual | USA, Carolina, Aiken | P. variabilis | Saccardo moved C. acicolum to Septoria due to the characteristic septate conidia. | |
| 1922 | Lecanosticta pini | Sydow and Petrak (1922) | Asexual | USA, Arkansas and Oregon | P. taeda and P. palustris in Arkansas and P. attenuata in Oregon | The genus Lecanosticta was erected to accommodate L. pini, a fungus with erumpent stromata and pigmented conidia. | |
| 1924 | Lecanosticta acicola | Sydow and Petrak (1924) | Asexual | USA | – | The authors recognized that L. pini was C. acicolum. The genus was retained and the name L. acicola was proposed as the valid name. | |
| 1926 | Oligostroma acicola | Dearness (1926, 1928) | Sexual | USA, Florida, Silver Springs | P. palustris | The sexual state of L. acicola was isolated from old pine needles from which the asexual state was previously isolated. In 1928, it was proposed that the asexual state Septoria acicola fits better in the genus Cryptosporium and that Oligostroma acicola could be Cryptosporium acicolum’s sexual state (Dearness, 1928). | |
| 1939 | Schirria acicola | Siggers (1939) | Sexual | USA, Arkansas, Florida, Georgia, Louisiana, North Carolina, Texas | P. palustris, P. taeda, P. thunbergii | Ascospores as well as conidia were plated onto media and morphologically examined to come to the conclusion that the sexual and asexual state are connected. Oligostroma acicola was changed to Schirria acicola as erumpent acervuli were observed, characteristic of Schirria. | |
| 1941 | Systremma acicola | Wolf and Barbour (1941) | Sexual | USA | Pinus spp. | It was recognized that the pathogen was better suited in the Dothideaceae and therefore the fungus was moved to the genus Systremma and all the above names for the sexual state synonymized with S. acicola. | |
| 1967 | Dothistroma acicola | Shishkina and Tsanava (1967) | Asexual | – | – | The name D. acicola was incorrectly assigned to both D. pini and L. acicola. The name was not used in subsequent literature. | Due to similarities between symptoms caused by Dothistroma and Lecanosticta the asexual states of D. pini (presently D. septosporum) and L. acicola were synonymized and renamed as D. acicola and furthermore associated with the sexual state Systremma acicola. |
| 1972 | Mycosphaerella dearnessii | Barr (1972) | Sexual | USA | – | Systremma acicola was synonymized with Mycosphaerella dearnessii. Mycosphaerella dearnessii was assigned as the type for Mycosphaerella subgenus Mycosphaerella section Caterva. | MycoBank accession number: 318138. |
| 1996 | Eruptio acicola | Barr (1996) | Sexual | – | – | According to Barr (1996), Mycosphaerella dearnessii did not fit the description of Mycosphaerella and the new genus Eruptio M.E. Barr was erected to accommodate Eruptio acicola and Eruptio pini (sexual state of Dothistroma septosporum). | The validity of the genus Eruptio was questioned as Lecanosticta acicola phylogenetically clusters with other Mycosphaerella anamorphs (Crous et al. 2001). Eruptio is not widely used in literature. |
| 2012 | Lecanosticta acicola | Quaedvlieg et al. (2012) | Both | Europe and North America | Pinus spp. | As it was recognized that the genus Mycosphaerella should only be used with the anamorphic genus Ramularia (Crous et al., 2007; Crous, 2009), Lecanosticta acicola was selected as the valid name and type of the genus under the one fungus = one name rule. An epitype was designated for L. acicola in this study (Quaedvlieg et al., 2012). | MycoBank accession number: 255702 Epitype CBS H‐21113, Ex‐epitype CBS 133791. |
| Other Lecanosticta species | |||||||
| 1984 | Lecanosticta cinerea | Evans (1984); Marmolejo (2000) | Asexual | Honduras | Pinus sp. | The species name was proposed as the correct name for Gloeocoryneum cinereum. | This name is not validly published (Marmolejo, 2000) as no basionym was established. The new combination Leptomelanconium pinicola was proposed because it had previously been established that Gloeocoryneum cinereum is a synonym of Stilbospora pinicola and that the genera Gloeocoryneum and Leptomelanconium have the same characteristics (Marmolejo, 2000). |
| 1984 | Lecanosticta gloeospora | Evans (1984) | Asexual | Mexico, Iturbide‐Galeana, Nuevo León | P. pseudostrobus | The second valid species in the genus to be described based on morphological characteristics. | MycoBank accession number: 106975 Holotype IMI 283812 Ex‐type IMI 283812. |
| 2000 | Lecanosticta longispora | Marmolejo (2000) | Asexual | Mexico, Nuevo León | P. culminicola | The third species described in the genus based on morphological characteristics. | No type was assigned but Quaedvlieg et al. (2012) epitypified the species in 2012. |
| 2012 | Lecanosticta longispora | Quaedvlieg et al. (2012) | Asexual | Mexico, Nuevo León | P. culminicola | The first phylogenetic study to include this species. An epitype L. longispora was designated here. | MycoBank accession number: 466255 Epitype CBS H‐21111, Ex‐epitype CBS 133602. |
| 2012 | Lecanosticta brevispora | Quaedvlieg et al. (2012) | Asexual | Mexico | Pinus sp. | This isolate is the fourth species described in the genus based on phylogenetic inference and morphology. | MycoBank accession number: 801940 Holotype CBS H‐21110, Ex‐type CBS 133601. |
| 2012 | Lecanosticta guatemalensis | Quaedvlieg et al. (2012) | Asexual | Guatemala | P. oocarpa | The ex‐type of L. guatemalensis (IMI281598) was initially described as L. acicola based on morphological characteristics (Evans, 1984). The isolate was phylogenetically delineated as a new species in 2012 and subsequently described (Quaedvlieg et al., 2012). | MycoBank accession number: 801941 Holotype CBS H‐21108, Ex‐type IMI 281598. |
| 2018 | Lecanosticta jani | van der Nest et al. (2019) | Asexual | Guatemala, Nicaragua | P. maximinoi, P. oocarpa, P. tecunumanii | New species described based on phylogenetic and morphological data. | MycoBank accession number: 826875 Holotype PREM 62185, Ex‐type CBS 144456. |
| 2018 | Lecanosticta pharomachri | van der Nest et al. (2019) | Asexual | Guatemala, Honduras | P. oocarpa, P. tecunumanii | New species described based on phylogenetic and morphological data. | MycoBank accession number: 826876 Holotype PREM 62188, Ex‐type CBS 144448. |
| 2018 | Lecanosticta tecunumanii | van der Nest et al. (2019) | Asexual | Guatemala | P. tecunumanii | New species described based on phylogenetic and morphological data. | MycoBank accession number: 826877 Holotype PREM 62191, Ex‐type CBS 144450. |
| 2018 | Lecanosticta variabilis | van der Nest et al. (2019) | Asexual | Guatemala, Honduras | P. caribaea, P. maximinoi, P. oocarpa | New species described based on phylogenetic and morphological data. The ex‐type was initially described as L. acicola by Evans (1984) based on morphological observations. | MycoBank accession number: 826878 Holotype PREM 62195, Ex‐type CBS 144453 = IMI 281561. |
Lecanosticta acicola is the oldest known species in the genus and owes its notoriety to the disease of long leaf pine, which it was first associated with, in the southeastern USA (Chapman, 1926; Hedgcock, 1929). Although the pathogen was identified in Central America based on morphological characteristics (Evans, 1984), it is now recognized as a Northern Hemisphere pathogen for which phylogenetic analyses of the translation elongation factor 1‐α gene (TEF 1) sequences have revealed three distinct lineages (van der Nest et al., 2019). One of these lineages includes isolates from Canada, the northern parts of the USA (Maine, Michigan, New Hampshire, Vermont and Wisconsin) and Central and Northern Europe (Austria, Croatia, Czech Republic, Estonia, Germany, Italy, Lithuania, Slovenia, Switzerland) (van der Nest et al., 2019). A second lineage includes isolates from China, Colombia, France, Japan, Spain, South Korea and the southern part of the USA (Mississippi) (van der Nest et al., 2019). A third lineage includes isolates only from Mexico (van der Nest et al., 2019).
The eight other species described in Lecanosticta during the course of the past 35 years are present only in Mesoamerica (Tables 1 and 2) (Evans, 1984; Marmolejo, 2000; Quaedvlieg et al., 2012; van der Nest et al., 2019). Evans (1984) recognized considerable morphological variation amongst his collections of L. acicola. In that study, he described a second species, L. gloeospora from Pinus pseudostrobus in Mexico, and the fungus remains known only from Mexico on this host (Evans, 1984; Marmolejo, 2000). The novelty of this species was recently validated using DNA sequence data (van der Nest et al., 2019).
Lecanosticta longispora was first described based on morphological features from P. culminicola in Nuevo León, Mexico (Marmolejo, 2000). This species was characterized in a phylogenetic study by Quaedvlieg et al. (2012), and was distinguished from L. acicola based on differences in the TEF 1 and β‐tubulin 2 (BT 2) gene sequences. That study was the first to delineate species of Lecanosticta based on phylogenetic inference (Quaedvlieg et al., 2012). These authors included several samples from Central America that had previously been identified as L. acicola, as well as the collection used by Marmolejo (2000) to typify L. longispora. In their phylogenetic analyses (Quaedvlieg et al., 2012), L. acicola was not identified from Central America but two new species, L. brevispora and L. guatemalensis, were described (Tables 1 and 2).
Evans (1984) observed that ecotypes or morphotypes exist amongst isolates of L. acicola in Central America, depending on the altitude and hosts from which the isolations were made. He therefore hypothesized that Central America could be the centre of origin of Lecanosticta. This was later supported by analysis of TEF 1 sequence data that revealed high genetic diversity in this geographical region (Janoušek et al., 2016). An extensive collection of isolates from Central America was recently studied using a phylogenetic approach (van der Nest et al., 2019). Interestingly, L. acicola was not identified amongst isolates from Guatemala, Nicaragua or Honduras. Furthermore, the isolates considered to be L. acicola by Evans (1984) were sequenced and identified as L. guatemalensis and a new species, L. variabilis (van der Nest et al., 2019, Table 1). Lecanosticta brevispora was identified in Guatemala and Honduras on Pinus oocarpa and P. pseudostrobus (Table 1), expanding the host range and distribution for that species. Likewise, L. guatemalensis was also identified in Guatemala, Honduras and Nicaragua on P. caribaea, P. oocarpa and P. tecunumanii (Table 1). The study of van der Nest et al. (2019) introduced four new species, including Lecanosticta jani from Guatemala and Nicaragua, L. pharomachri from Guatemala and Honduras, L. tecunumanii from Guatemala and L. variabilis from Mexico, Guatemala and Honduras (van der Nest et al., 2019). Although Central America could not be confirmed as a centre of origin of L. acicola, the diversity of species recognized by van der Nest et al. (2019) suggests strongly that Mesoamerica is a centre of diversity for Lecanosticta.
With only one exception, which is probably a taxonomic incongruity, Lecanosticta species are all associated with Pinus species. Petrak (1954) described Phragmogloeum gaubae on Callistemon sieberi in Australia (Petrak, 1954). von Arx (1983) attempted to reduce various species with overlapping characteristics to fewer genera and found that Phragmogloeum had the same morphological characteristics as Lecanosticta. He proposed the new combination Lecanosticta gaubae. After the genus Eruptio was erected to accommodate Lecanosticta acicola and Dothistroma septosporum (Barr, 1996), Lecanosticta gaubae was transferred to that new genus (Crous, 1999). The genus Eruptio was further evaluated and it was found that L. acicola and D. septosporum were not congeneric (Crous, 2009). Consequently, Lecanosticta was selected as the correct name for Eruptio acicola following the one fungus one name convention (Crous et al., 2009; Hawksworth et al., 2011). Because Eruptio gaubae is morphologically similar to Lecanosticta, phylogenetic analyses are required to resolve this taxonomic confusion.
Lecanosticta acicola is the only species in the genus known to be a significant pathogen. This is particularly important because it is spreading rapidly in Europe and the northeastern parts of North America. Therefore, all data collected over time regarding Lecanosticta pertain to the organism that was assigned the name L. acicola, and the remainder of the review will focus on this species. However, it is relevant to recognize that other species of Lecanosticta cause symptoms similar to those of L. acicola and that they have the potential to emerge as pine pathogens if they were accidentally moved to new environments. They would then be recognized as members of a complex of BSNB pathogens.
Symptoms of Brown Spot Needle Blight
Symptoms of infection can vary depending on the host species affected. Typically, a small and yellow, sometimes light grey‐green or reddish brown, irregular circular spot, with defined margins, appears at the point of infection (Hedgcock, 1929) (Fig. 2C–E). These spots soon become brown as the infections mature and they are often surrounded by a yellow halo (Skilling and Nicholls, 1974). In severe cases, infections can occur on several parts of a needle, leading to more rapid necrosis (Fig. 2E). The characteristic brown spots are the first conspicuous symptoms on the pine needles and this has led to the common name ‘brown spot needle blight’ proposed by Siggers (1932). These brown spots can also appear resin‐soaked depending on the host species (Skilling and Nicholls, 1974). In some cases, as has been reported in P. strobus, symptoms may only be displayed as chlorosis of the needles without banding (Broders et al., 2015). Infected needles die from the apex to the base (Fig. 2B) and they are eventually shed from the trees (Hedgcock, 1929; Skilling and Nicholls, 1974). Usually only the second‐ and third‐year needles are affected, leaving healthy new growth at the tips of the branches. The new growth tips are then infected in the subsequent season by inoculum on older needles (Skilling and Nicholls, 1974). Generally, infection is more severe in the lower parts of the canopy and then progresses upwards in the trees (Sinclair and Lyon, 2005; Skilling and Nicholls, 1974).
Figure 2.

Symptoms of Lecanosticta acicola. (A) Pinus mugo in Austria displaying symptoms of both brown spot needle blight (BSNB) and Dothistroma needle blight (DNB) on the same branches. (B) Both the characteristic brown spots associated with BSNB (black arrow) and the red banding associated with DNB (white arrow) can be observed. (C)–(E) Symptoms of BSNB vary from only brown spots as observed on P. mugo (C) to distinct brown bands as observed on P. radiata (D) to irregular mosaic spots as observed on P. palustris (E). (F) Lecanosticta acicola conidiogenous cells giving rise to conidia on malt extract agar. (G) Lecanosticta acicola septate conidia with verruculose surfaces and truncate bases.
An asymptomatic phase in which L. acicola establishes within needles can last several days (Setliff and Patton, 1974) to 3 months (Skilling and Nicholls, 1974). This is dependent on the strain of the pathogen (Kais, 1972) and length of the wet season. This delay in symptom development could lead to the accidental movement of infected plants to new areas.
The symptoms of BSNB (Fig. 2) can easily be confused with those of DNB, which is caused by Dothistroma septosporum and D. pini (Barnes et al., 2004, 2016). On some host species, symptoms of DNB are similar to those of BSNB (Fig. 2B) but rather than the characteristic brown discoloration and spots, a distinct red band forms around the point of infection in the case of DNB (Pehl and Cech, 2008). However, in some cases the characteristic red banding pattern associated with DNB is not formed or alternatively the red bands are sufficiently dark to give a false impression of brown spots. This can easily lead to incorrect pathogen diagnoses (Barnes et al., 2016; Petrak, 1961).
Life Cycle
Lecanosticta acicola can occur in either its asexual or sexual state (Fig. 1) (Siggers, 1939). The pathogen overwinters in acervuli (asexual) (Fig. 1Aa) or ascostromata (sexual) (Fig. 1Ba) in the dead tissue of either dead or living pine needles. It can also overwinter as vegetative mycelium in the infected needles that remain attached to the host (Siggers, 1944). Conidia are released in gelatinous masses (Fig. 1Ab) or ascospores are released from asci in ascostromata (Fig. 1Bb) on the needles when the light, temperature and humidity are favourable (Kais, 1975; Tainter and Baker, 1996).
Conidia begin to germinate on the needle surfaces by developing one to four germ tubes, depending on the number of cells in the conidia (Setliff and Patton, 1974). It is uncertain whether the germ tubes are attracted to the stomata, or whether they grow randomly over the needle surface (Patton and Spear, 1978; Setliff and Patton, 1974). Light plays an indirect, but essential role in the infection process as it stimulates the opening of stomata, allowing the germ tube to penetrate the needle (Fig. 1c) (Kais, 1975). Infections can also occur through wounds (Kais, 1978). Once a germ tube enters the stomatal antechamber, it increases in diameter and becomes thick‐walled and melanized (Patton and Spear, 1978). Appresoria, such as those found in Dothistroma (Gadgil, 1967), have never been seen (Patton and Spear, 1978).
Once the mesophyll tissue has been invaded by L. acicola mycelium, conidiomata begin to form. These begin to integrate with the needle tissue and increase in size until they are visible to the naked eye (Wolf and Barbour, 1941). The conidiophores produce conidia towards the leaf exterior (Evans, 1984), which exerts pressure on the needle epidermis. This causes the epidermis to rupture, leaving a flap that partly covers the conidiomata (Wolf and Barbour, 1941). The conidia are released from the conidiomata during wet weather and the disease cycle is repeated.
In the case of the sexual state, asci are formed within the ascostromata on necrotic distal parts of living needles or on dead needles (Henry, 1954; Jewell, 1983). Ascospores are released from asci and dispersed through wind and rain. Asci and ascospores develop more rarely than conidia and have been reported only from Nicaragua, Honduras, Colombia and the southern parts of the USA (Table 1) (Evans, 1984; Henry, 1954; Kais, 1971; Luttrell, 1949; Siggers, 1944). The reports from Nicaragua and Honduras probably represent species other than L. acicola.
Toxin Production
Many plant pathogenic fungi have adapted to produce toxic secondary metabolites in their plant hosts and these could influence colonization and sporulation, as has been seen in D. septosporum (Kabir et al., 2015). Lecanosticta acicola is known to produce the toxic compounds LA‐I and LA‐II, which are heat‐resistant and non‐host specific phytotoxins (Yang et al., 2002, 2005). The two compounds interact with the host independently and do not promote or inhibit the interaction of one another (Yang et al., 2002). Different Pinus species have different reactions to LA‐I and LA‐II. When rooted cuttings of P. thunbergii were exposed to the toxin, they showed little sensitivity to it. In contrast, when P. elliottii and P. taeda, both highly susceptible to BSNB infection, were exposed to the toxin, the results showed high sensitivity to LA‐I (Ye and Qi, 1999). It seems likely that these toxins are involved in the destruction of mesophyll tissue of the pine needles at the point of infection (Jewell, 1983).
Biology and Dissemination
Conidia and ascospores are released throughout the year at temperatures ranging from –5.5 to 28 °C (Kais, 1971; Siggers, 1944; Wyka et al., 2018). However, warm and wet weather is particularly conducive for the development of BSNB, irrespective of whether infection takes place by sexual or asexual spores. The conidia do not germinate below 5 °C, although most survive this temperature and commence germination once the temperature increases (Siggers, 1944). At the other extreme, tolerance to high temperature was found to vary depending on the strain of Lecanosticta involved. It was shown that conidia of isolates from the northern parts of the USA could not germinate at 32 °C, whereas cultures isolated from the southern parts of the USA, as well as China, had a germination success of 80% at the same temperature (Huang et al., 1995). This physiological distinction is reflected in population genetic studies which define two lineages of the pathogen in the USA (Janoušek et al., 2016). The success of the pathogen may therefore be a result of isolates in each lineage adapting to local temperature conditions.
The maximum temperature for the germination of L. acicola conidia is 35 °C (Siggers, 1944). It was also found that high humidity pre‐ and post‐infection is required for high levels of infection (Kais, 1975). The optimal temperature for infection to occur is 30 °C during the day and 21 °C at night, and Kais (1975) showed that these temperatures gave positive results in inoculation trials.
Conidia are dispersed predominantly by rain splash to adjacent trees, and they contribute significantly to rapid disease build‐up in pine stands (Tainter and Baker, 1996). High levels of conidial dispersal were recorded during the rainy season in the USA, especially between late spring and summer, as well as when there were rain spells after a long period of dryness (Kais, 1971). In other reports, conidial production and dispersal were recorded throughout the year (Siggers, 1944). Dispersal was not influenced by the temperature range but conidial release was connected to rainfall patterns. In Wisconsin, two peaks of conidial release were recorded, with the first peak in early summer when young pine needles are present and the second in late summer (Skilling and Nicholls, 1974), which was similar to that found in the northeastern USA (Wyka et al. 2018). In Japan, it was found that conidia were produced by the pathogen from early spring to autumn with peak dispersal in mid‐summer. However, for a second year of infection, the dispersal was most abundant from late summer to mid‐autumn the following year (Suto, 2002). A study in Fujian province (China) showed that the greatest number of conidia were detected between early spring and mid‐summer and again in late summer to late autumn in Pinus elliottii plantations (Li et al., 1987). It consequently appears that conidial dispersal varies depending on the rainfall season in any particular geographical region.
Spore traps in several studies failed to capture ascospores (Kais, 1971; Siggers, 1939; Wyka et al., 2018). It was found, however, that conidia could be dispersed to a distance of up to 60 m (Wyka et al. 2018). A recent investigation of the dispersal of Dothistroma, where the mechanisms of conidial and ascospore dispersal are similar to those in L. acicola, showed that conidia could be naturally disseminated over more than 1 km (Mullett et al., 2016). The assumed distance of dispersal in L. acicola may, consequently, be similar.
The ascospores of L. acicola are forcibly expelled into the air (Wolf and Barbour, 1941) and dispersed by wind currents (Kais, 1971) or rain splash driven by wind (Siggers, 1939). Ascospores can also be released during periods of fog, rain and dew (Tainter and Baker, 1996). Ascospores were recorded in the USA mainly during periods when temperatures were above 15 °C and are found in late summer to autumn. Small numbers of ascospores were detected when temperatures were below 10 °C (Kais 1971).
The main component that facilitates spread of conidia and ascospores is moisture, but other factors may also aid in their dispersal. Insect dissemination was suggested as a mechanism of conidial spread when two Lepidopteran wing scales were found to have conidia attached to them (Skilling and Nicholls, 1974). Given the biology of L. acicola, it seems unlikely that insects are involved in its dissemination. It has also been suggested that animals grazing in forests might aid in dissemination of the conidia when spores stick to their coats or hooves (Skilling and Nicholls, 1974; Tainter and Baker, 1996). Again, this mode of dissemination seems unlikely to be particularly important.
Anthropogenic movement of infected plant material has contributed to the dissemination of many tree pathogens (Wingfield et al., 2015). This has been clearly demonstrated for Dothistroma septosporum (Barnes et al., 2014), which has a biology very similar to that of L. acicola. A study that used microsatellite markers has demonstrated that two separate lineages of L. acicola have most likely been introduced into Europe from North America (Janoušek et al., 2016). Long distance dispersal of L. acicola is, therefore, likely to be the result of anthropogenic movement of infected plant material. This would not include seed transmission as L. acicola conidia cannot survive on a pine seed's surface longer than 30 to 34 days and it is thus not considered seed‐borne (Jianren and Chuandao, 1988).
Disease Management
Several measures have been suggested to prevent BSNB during plantation establishment. The most effective is to plant disease‐free seedlings of superior quality (Cordell et al., 1990; Skilling and Nicholls, 1974). It is also advisable to avoid establishing new plantations alongside old, infected pines that could potentially serve as reservoirs of inoculum (Tainter and Baker, 1996). For natural pine stands, the application of thinning treatments was investigated as a silvicultural practice against pine needle diseases (McIntire et al., 2018). This practice, conducted on native stands of P. strobus in the USA, showed promise in reducing the fungal load of L. acicola, resulting in reduced severity of the disease over time in stands already infected with the pathogen (McIntire et al., 2018). This practice is recommended as a preventative measure in stands that are at risk of infection by L. acicola and other pine needle pathogens (McIntire et al., 2018).
Pruning of infected pines can contribute to the spread of BSNB if it is conducted during rainy or wet periods. This is because conidia are exuded during these conditions and can attach to the pruning shears, providing a means of spread from infected to healthy trees (Skilling and Nicholls, 1974). Cutting blades should be cleaned during pruning and clipped needles and shoots should be removed (Kais, 1978). In the case of infection on Pinus palustris, which begins growth as a grass stage, stimulation of growth during the first 3 years of growth reduces the levels of infection (Tainter and Baker, 1996). Because this treatment is economical, effective and environmentally safe, it is widely used in the southeastern USA (Cordell et al., 1990), where BSNB occurs on P. palustris.
Breeding for resistance to L. acicola has been successfully used to reduce the impact of the disease on P. palustris in Alabama. The source population of these trees found in southwestern Alabama was used in breeding programmes (Snyder and Derr, 1972) where seed was made available to the public (Phelps et al., 1978). Since 1982, resistant phenotypes of P. elliottii have also been selected for in plantations affected by BSNB in the Fujian province in China. Over time, and using artificial inoculations, resistant clones were selected and resistant seed orchards were established (Ye and Wu, 2011).
Fungicide treatment can protect pine seedlings from infection by L. acicola. For example, when P. palustris was sprayed with fungicide, the seedlings displayed increased diameter growth in a single growing season, compared to untreated plants (Siggers, 1932). Seedlings, seed orchard trees and Christmas tree plantations have been protected by Bordeaux mixture of copper sulphate and lime, which inhibits conidial germination, by a benomyl root treatment or by ferbam (Fermate®). Chlorothalonil, a broad‐spectrum organochlorine pesticide (products include Bravo®, Daconil® and Maneb®), has also been applied to provide efficient control against BSNB. Chlorothalonil is also very effective against Lophodermium needle cast, which could be advantageous when both pathogens are present (Cordell et al., 1990; Kais et al., 1986; Skilling and Nicholls, 1974). Practical details and recommendations concerning fungicide treatment can be found in Skilling and Nicholls (1974). However, the use of chemicals is not considered a desirable solution for disease control due to negative environmental factors and many of these treatments are no longer available.
Controlled burning in pine forests can eliminate competing vegetation and reduce the impact of needle pathogens, especially in P. palustris where a grass stage is relevant (Barnett, 1999; Chapman, 1932). This pine species is completely adapted to survive fires as it concentrates all its energy into root development during the first 5 years of growth (Chapman, 1932). Siggers (1934) showed that a single controlled fire can significantly decrease BSNB in P. palustris until the next season and that during the initial growth stage, before seedlings begin to increase in height, a winter burn every 3 years is the most beneficial for disease control. The efficacy of controlled burns differs depending on the Pinus spp. involved and on the ability to tolerate fire damage.
In countries and regions where L. acicola is a quarantine organism, it is suggested that complete eradication of diseased trees or pine stands should be performed once the disease is detected (Pehl and Cech, 2008). This is achieved by felling and burning of infected trees and litter found under infected trees (Sosnowski et al., 2009). In Lithuania, for instance, after positive identification of the pathogen in the Curonian Spit in 2009 and 2010, effective eradication measures were implemented (Markovskaja et al., 2011). Due to this rapid action, the disease has remained under control in that country and is under constant monitoring by the state plant service of the Ministry of Agriculture of Lithuania (https://gd.eppo.int/taxon/SCIRAC/distribution/LT). Eradication efforts are, however, not always effective, and the best preventative method is to limit the movement of plant material across borders and between regions. As new knowledge is emerging regarding different genetic entities of the pathogen, including strains of different mating types (Sadiković et al., 2019), the importance of avoiding new introductions is becoming increasingly obvious.
Host Range, Host Susceptibility and Geographic Distribution
In an effort to consolidate 140 years of literature with regards to the geographical distribution and host range of L. acicola, a detailed list of these data has been compiled (Table 1). This shows that the pathogen has been reported in 31 countries and on 53 pine species and pine hybrids. The majority of the host records on native and non‐native trees are from the Americas, followed by Europe. The pathogen has not been found in Africa, Australia or New Zealand and in South America it is known only in Colombia. Of the 69 reports of the pathogen (Table 1), 31 were made in the last decade (2009–2019). This suggests that incidences of the pathogen are most likely increasing.
In North America, the first report of L. acicola was in 1876 on native Pinus echinata as Cryptosporium aciculum (de Thümen, 1878). Since then, the pathogen has been reported in the USA on several susceptible species, including non‐native P. caribaea and P. pinea, and native P. elliottii, P. echinata, P. glabra, P. ponderosa, P. rigida, P. taeda and P. virginiana (Hedgcock, 1929; Siggers, 1944; Sinclair and Lyon, 2005; Webster, 1930) as well as on regionally planted exotic species such as P. attenuata, P. coulteri, P. muricata and P. sabiniana (Siggers, 1944). Pinus palustris seedlings are the most severely affected, largely due to the grass stage associated with early growth and where BSNB can cause complete defoliation (Siggers, 1934). Here it can result in mortality reaching 50% and higher in the southeastern USA (Cordell et al., 1990). New reports of L. acicola causing damage on P. strobus have emerged since 2005 in the northeastern USA and Canada and these have been attributed to changes in precipitation and climate in the regions (Broders et al., 2015; Wyka et al., 2017, 2018). Lecanosticta acicola is also recognized as a component of a complex of pathogens that cause white pine needle damage (WPND) in this region (Broders et al., 2015). Additionally, the pathogen has been reported on P. banksiana and P. contorta var. latifolia in Canada (Laut et al., 1966).
Lecanosticta acicola has been reported from 17 European countries (for a complete list of records see Table 1). The pathogen was first recorded in northern Spain in 1942 (Martínez, 1942), where it still occurs on P. radiata (Ortíz de Urbina et al., 2017). In southwest Europe, L. acicola has caused severe defoliation of P. radiata × P. attenuata, leading to the felling of 100 ha in the 1990s (Lévy, 1996). Lecanosticta acicola is spreading through the valleys in the Alps in Switzerland (Holdenrieder and Sieber, 1995), Austria (Cech, 1997; Hintsteiner et al., 2012), Italy (La Porta and Capretti, 2000) and Slovenia (Jurc and Jurc, 2010; Sadiković et al., 2019), which can be attributed to high humidity in deep valleys or the proximity of lakes. In Europe, L. acicola often infects P. mugo, a susceptible species on which it has recently caused severe outbreaks in Austria (https://gd.eppo.int/reporting/article-5139). It also infects other pine species such as P. sylvestris and P. nigra. The pathogen has been recorded in several peat bog sites in southern Bavaria (Germany) and southern Bohemia (Czech Republic). These locations are naturally humid throughout the year and the susceptible pine species P. mugo and/or P. uncinata subsp. uliginosa can be heavily infected, leading to considerable mortality. Similarly, L. acicola was recorded in the Baltic states (Drenkhan and Hanso, 2009) and, most recently, also in Sweden (Cleary et al., 2019). These records usually come from stands close to the sea or, very frequently, from botanical gardens or urban areas.
Other pine species such as Pinus × rhaetica and P. ponderosa have also been affected by L. acicola (Adamson et al., 2015, 2018). Lecanosticta acicola has been present in Croatia on P. halepensis for more than 40 years (Milatović, 1976; Sadiković et al., 2019). Interestingly, the pathogen was identified only at a single site in Ireland despite large‐scale screening throughout the British Isles (Mullett et al., 2018). From all these records, it is reasonable to conclude that L. acicola is spreading in Europe in native and non‐native pine species, in plantations and natural forests, and associated with different climatic conditions.
In Asia, BSNB has been reported in China in plantations of non‐native P. thunbergii, P. elliottii and P. taeda where the trees were severely damaged by the pathogen (Huang et al., 1995), and on P. caribaea, P. palustris, P. clausa and P. echinata that were reported to be susceptible to infection (Li et al., 1986). It was suggested that native pines such as P. taiwanensis, P. fenzeliana and P. massoniana were highly resistant to infection (Huang et al., 1995; Li et al., 1986). BSNB has been reported on native P. thunbergii in Japan (Suto and Ougi, 1998) as well as on native P. thunbergii in South Korea but the disease was not severe (Seo et al., 2012).
Although some species of Pinus seem to not be susceptible to infection by L. acicola, the pathogen has the potential to overcome host resistance in a favourable environment and expand its host range, as is suggested for D. septosporum and D. pini (Drenkhan et al., 2016). For example, L. acicola is rarely reported on native P. sylvestris in Europe. Considering the importance of P. sylvestris in Europe, it will be important to monitor the presence of the pathogen on this host. Only single incidences of L. acicola have been reported on P. sylvestris in Austria (Cech and Krehan, 2008), Slovenia (Jurc and Jurc, 2010) and most recently in Estonia (Adamson et al., 2018) and Ireland (Mullett et al., 2018). In contrast, L. acicola is an important pathogen of P. sylvestris grown as part of the Christmas tree industry since the 1960s in the USA (Skilling and Nicholls, 1974). This implies that under favourable conditions this host could be infected by the pathogen. Investigations on the impact of DNB on P. sylvestris revealed that there is high intraspecific variability of P. sylvestris in Europe and that susceptibility of the host to the pathogen varies between individuals (Perry et al., 2016a,b) and this could also influence the potential importance of L. acicola. Unusually high humidity associated with climate change could increase pathogen pressure on P. sylvestris (Perry et al., 2016a) and the single incidences in Europe should carefully be monitored. Caution must also be taken when planting susceptible exotic hosts alongside native forests, as this could influence the vulnerability of native forests (Piotrowska et al., 2018).
Of the 69 reports of L. acicola, only 22 used DNA sequence comparisons for species verification. This is of concern as there might be an over‐ or underestimation of hosts affected by BSNB globally. In Central America, for example, L. acicola was reported based on identifications using morphological characters. Because the pathogen has not yet been confirmed as occurring in this region using DNA sequences (Quaedvlieg et al., 2012; van der Nest et al., 2019), those reports could be erroneous and may represent different species which could possibly cause new outbreaks if not contained in their native environment.
Molecular Diagnostics and Future Prospects
Molecular markers used for species identification
Three molecular methods are currently being used to accurately identify L. acicola. These include sequencing of various gene regions, an ITS‐RFLP method and a conventional PCR that uses species‐specific primers. The most common of these approaches is comparison of DNA sequences for the ITS gene region (Adamson et al., 2015, 2018; Cleary et al., 2019; Markovskaja et al., 2011; Mullett et al., 2018). However, the TEF 1 (Fig. 3) and BT 2 gene regions have been recommended to distinguish between species of the Mycosphaerellaceae (Quaedvlieg et al., 2012). In order to accurately distinguish between different species of Lecanosticta, van der Nest et al. (2019) used a multi‐gene phylogenetic approach using sequences for the ITS, TEF 1, BT 1, MS204 and RPB 2 gene regions. The outcome was the discovery of four new species, with the ITS and TEF 1 proving to be the gene regions showing the best amplification success across all species. Pehl et al. (2004) developed an ITS‐RFLP method to distinguish between L. acicola, D. septosporum and ten other plant pathogens. However, whether this method remains valid after the recognition of various new species (van der Nest et al., 2019) will need to be established.
Figure 3.
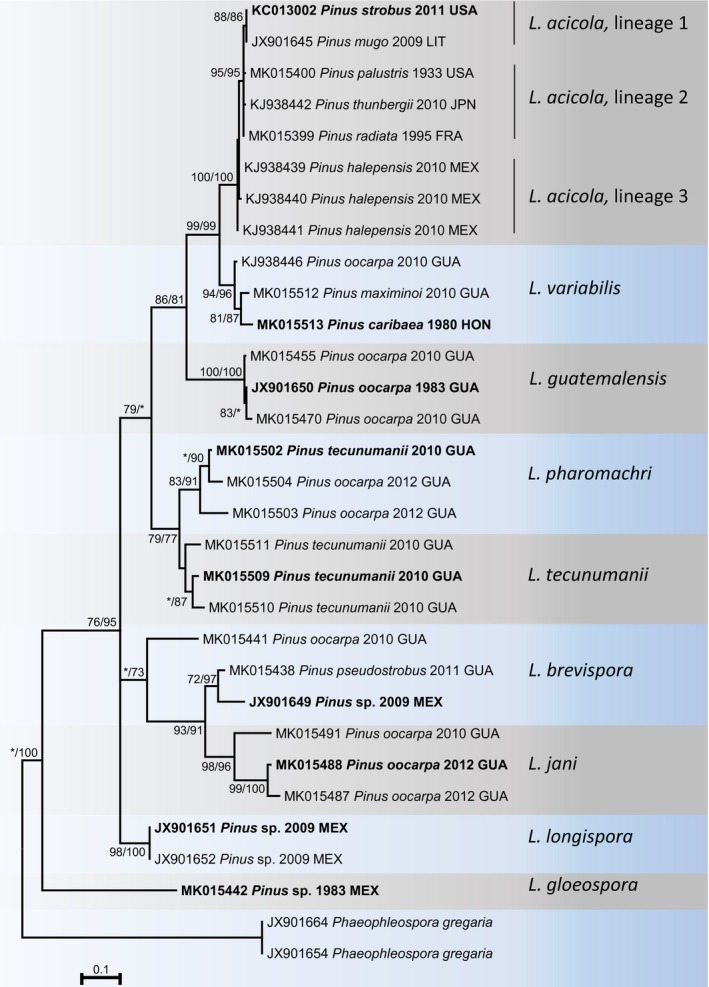
Maximum likelihood (ML) tree representing the nine known species of Lecanosticta as well as the three lineages of L. acicola generated from the translation elongation 1‐α region. ML bootstrap support (>70%) are indicated first, followed by maximum parsimony (MP) bootstrap support values (ML/MP, * = insignificant value). Phaeophleospora gregaria was used as the outgroup taxa. All represented type species are indicated in bold.
Another rapid method allowing for the identification of L. acicola, D. septosporum and D. pini is a conventional PCR that uses species‐specific primers (Ioos et al., 2010). These were developed to partially amplify the TEF 1 gene for L. acicola and D. pini, and partially amplify the BT 2 gene region in D. septosporum (Ioos et al., 2010). Importantly, this method can be used to identify the pathogens directly from infected needles (Adamson et al., 2015; Ortíz de Urbina et al., 2017; Schneider et al., 2019) and is now widely used for preliminary identification of L. acicola (Adamson et al., 2018; Sadiković et al., 2019). A multiplex qPCR was also recently developed to detect L. acicola as well as Dothistroma species from needles simultaneously using probe‐labelled primers developed by Ioos et al. (2010) and Schneider et al. (2019), which could become more widely used once that technology is more easily available.
Population genetic studies
Knowledge regarding the population structure and diversity of pathogens such as L. acicola allow for an understanding of migration patterns as well various aspects of their invasion biology. Eleven polymorphic microsatellite markers and mating type primers have been developed for this purpose (Janoušek et al., 2014). The first population genetic study using these markers revealed that two lineages of L. acicola were introduced into Europe, possibly on two separate occasions (Janoušek et al., 2016). These results are similar to an earlier study where RAPD analysis of L. acicola, collected in the northern and southern parts of the USA and China, showed that the Chinese population originated from the southern USA and that the collection from the northern USA was unique (Huang et al., 1995). A second population genetic study compared populations from Croatia and Slovenia and revealed four distinct populations with possible introductions from other sources within the two countries (Sadiković et al., 2019). Currently available knowledge suggests a Northern American centre of origin for this pathogen (Huang et al., 1995; Janoušek et al., 2016; van der Nest et al., 2019) but further sampling and analyses are required to support this hypothesis. In the population genetic study of Janoušek et al. (2016), the microsatellite markers amplified poorly for the L. acicola isolates from Mexico and Central America. A later study (van der Nest et al., 2019) showed that these isolates were L. variabilis, a new and recently described species.
The study by Janoušek et al. (2014) showed that L. acicola is heterothallic and that two individuals, one with a MAT1‐1‐1 idiomorph and the other with a MAT1‐2 idiomorph, are needed for sexual reproduction to occur. Consequently, to understand whether sexual recombination might occur in a region, it is important to have a knowledge of the mating type idiomorph distribution. Mating type primers that amplify the MAT1‐1‐1 and MAT1‐2 idiomorphs and that tested positive for Dothistroma species as well as L. acicola, L. guatemalensis and L. gloeospora have been developed (Janoušek et al., 2014). It is, however, not yet known whether these markers will amplify these gene regions for the other, newly described Lecanosticta species.
Janoušek et al. (2016) considered the global L. acicola population and showed that the ratio of mating type idiomorphs in Mississippi, Austria, France and Germany reflected sexual recombination in these regions/countries. In contrast, only asexual reproduction occurs in the Czech Republic and northern parts of America. Using the mating type markers of Janoušek et al. (2014), the distribution of MAT1 and MAT2 isolates was detected in studies with isolates from Croatia (Sadiković et al., 2019), Estonia (Adamson et al., 2015, 2018), Ireland, Portugal, Russia (Mullett et al., 2018) as well as Spain (Ortíz de Urbina et al., 2017). In Spain, both mating types were detected whereas only single mating types were detected in all other areas studied. However, in Estonia it was suggested that a second introduction of the pathogen occurred since only MAT1 was initially present but that later both mating types were detected in the same region (Adamson et al., 2015). In populations with equal ratios of mating types or with both mating types present, sexual reproduction could occur, possibly giving rise to more virulent strains. This emphasizes a need to exercise caution and thus to prevent introduction of new strains into regions where the pathogen is already present.
Future prospects in the age of genomics
Canada's Michael Smith Genome Sciences Centre has recently released a full genome for a L. acicola isolate from France (https://www.ncbi.nlm.nih.gov/assembly/GCA_000504345.2#/def). This genome has not yet been annotated but provides a valuable resource for future studies. Many other genomes of Dothidiomycetes, which have been sequenced and annotated, are available for comparative purposes (de Wit et al., 2012; Ohm et al., 2012). Annotation of putative genes of the L. acicola genome, utilizing knowledge of these other genomes, will provide insights into questions regarding many aspects of the biology of L. acicola. Opportunities also now arise to sequence the genomes of other Lecanosticta spp. and to compare these in order to better understand their relative importance. It will also be possible to follow the Dothistroma example where a transcriptomic study considered which genes are expressed during various stages in the infection of P. radiata (Bradshaw et al., 2016) and genome sequencing of global representatives of D. septosporum revealed that gene copy numbers could play a role in dothistromin production by the pathogen (Bradshaw et al., 2019).
Conclusions
Lecanosticta acicola has been known in the southern USA for many decades. Consequently, its life cycle, mode of infection, host susceptibility and strategies to prevent infection, particularly on P. palustris, have been extensively studied in that region. Yet there is evidence to show that the pathogen, which now has an extensive host range, is spreading rapidly northwards. The reasons for this host range and geographical expansion require further study. Contemporary knowledge has also shown that there have been two introductions of L. acicola into Europe. Consequently, BSNB is becoming a disease of great concern in Europe, where it is increasingly being discovered on both non‐native and native Pinus spp. There are many relevant hypotheses to explain the growing importance of BSNB and these include the effects of climate change, emergence of more aggressive strains of the pathogen and anthropogenic processes leading to new introductions. There is clearly a need for increased attention to and studies of L. acicola, particularly in Europe.
Recent studies have shown that there are eight species of Lecanosticta in addition to L. acicola. All of these other species appear to have a Mesoamerican origin. Much of the literature pertaining to L. acicola needs to be reconsidered given the fact that a single name has been widely used to refer to what we now know represents numerous cryptic species. Lecanosticta acicola identified based on DNA sequence comparisons has not been found in Central America, suggesting a North American centre of origin. Of the 69 reports of L. acicola, only 25 from 12 countries have been confirmed using DNA sequence‐based tools (Table 1). Many reports of the pathogen could thus be erroneous and there is an urgent need to resolve this important question.
All the available knowledge regarding BSNB relates to studies on L. acicola and these are predominantly from the USA. Nothing is known regarding the relative importance of the remaining eight species of Lecanostica. At least some of these are most likely also important pathogens and their relative threat to global forests and forestry needs to be assessed. A concerted effort must be made to prevent their accidental introduction into new regions of the world and as part of this process DNA sequence‐based techniques need to be routinely applied to allow for meaningful identification.
The development of new tools to study Lecanosticta spp. and BSNB provides many exciting opportunities to enhance our knowledge of this important group of pathogens. The population structure and diversity of L. acicola can now be easily studied in the USA as well as where new invasions occur in Europe, and at levels that were previously not possible. For example, application of the available microsatellite markers will enable a more comprehensive understanding of the pathogen as well as determination of its centre of origin.
Genome sequencing is rapidly becoming cheaper and more readily available, and an isolate of L. acicola is already available in the public domain for study. We envisage that all the species of Lecanosticta will be sequenced in the relatively near future and many isolates of some species will likely also be studied at this level. These studies, and others relating to the ‘omics’ level, will surely have a substantial impact on our understanding of a group of pathogens that is growing in importance and relevance. Overall, BSNB (including all species of Lecanosticta) has the potential to become a pine needle disease of global importance if proper preventative measures for the spread of the causal pathogens are not implemented.
Accession Numbers
The aligned dataset used to draw Fig. 3 is deposited in TreeBASE (No. S24301).
Acknowledgements
We thank Glenda Brits from the Department of Education Innovation for assistance in producing an illustration of the life cycle of L. acicola. We are also grateful to the National Research Foundation of South Africa (Thuthuka Grant no. 80670 and Grant no. 95875) as well as members of the Tree Protection Cooperative Program for financial support. Ariska van der Nest was supported by a Scarce Skills Doctoral Scholarship (no. 89086) provided by the National Research Foundation of South Africa. The authors have no conflicts of interest to declare.
References
- Adamson, K. , Drenkhan, R. and Hanso, M. (2015) Invasive brown spot needle blight caused by Lecanosticta acicola in Estonia. Scand. J. Forest Res. 30, 587–593. [Google Scholar]
- Adamson, K. , Laas, M. , Drenkhan, R. and Hanso, M. (2018) Quarantine pathogen Lecanosticta acicola, observed at its jump from an exotic host to the native Scots pine in Estonia. Balt. For. 24, 36–41. [Google Scholar]
- Alvère, M. , Aumonier, T. and Kersaudy, E. (2010) Bilan sylvosanitaire 2009 pour Midi‐Pyrénées In: Service régional de l’alimentation. (Département de la Santé des Forêts, ed), pp. 1–7. Direction régionale de l'alimentation, de l’agriculture et de la forêt d’Aquitaine, République Française. [Google Scholar]
- Angst, A. (2011) Braune Föhren in Gärten und Parks. Wald Holz, 92, 41–42. [Google Scholar]
- Anonymous (2012) First report of Mycosphaerella dearnessii in Latvia. Eur. Med. Plant Protec. Org. Bull., 8, 5–6. [Google Scholar]
- von Arx, J.A. (1983) Mycosphaerella and its anamorphs. P. K. Ned. Akad. C Biol. 86, 15–54. [Google Scholar]
- Barnes, I. , Crous, P.W. , Wingfield, B.D. and Wingfield, M.J. (2004) Multigene phylogenies reveal that red band needle blight of Pinus is caused by two distinct species of Dothistroma, D. septosporum and D. pini . Stud. Mycol. 50, 551–565. [Google Scholar]
- Barnes, I. , Wingfield, M.J. , Carbone, I. , Kirisits, T. and Wingfield, B.D. (2014) Population structure and diversity of an invasive pine needle pathogen reflects anthropogenic activity. Ecol. Evol. 4, 3642–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, I. , van der Nest, A. , Mullett, M.S. , Crous, P.W. , Drenkhan, R. , Musolin, D.L. and Wingfield, M.J. (2016) Neotypification of Dothistroma septosporum and epitypification of D. pini, causal agents of Dothistroma needle blight of pine. Forest Pathol. 46, 388–407. [Google Scholar]
- Barnett, J.P. (1999) Longleaf pine ecosystem restoration: the role of fire. J. Sustain. Forest. 9, 89–96. [Google Scholar]
- Barr, M.E. (1972) Preliminary studies on the Dothideales in temperate North America. Contributions from the University of Michigan Herbarium, 9, 523–638. [Google Scholar]
- Barr, M.E. (1996) Planistromellaeae, a new family in the Dothideales. Mycotaxon, 60, 433–442. [Google Scholar]
- Boyce, J.S. (1959) Brown spot needle blight on eastern white pine. Plant Dis. Rep. 43, 420. [Google Scholar]
- Boyer, W.D. (1972) Brown‐spot resistance in natural stands of longleaf pine seedlings. US Department of Agriculture Forest Service Research Paper, SO‐142, 1–4. [Google Scholar]
- Bradshaw, R.E. , Guo, Y. , Sim, A.D. , Kabir, M.S. , Chettri, P. , Ozturk, I.K. , Hunziker, L. , Ganley, R.J. and Cox, M.P. (2016) Genome‐wide gene expression dynamics of the fungal pathogen Dothistroma septosporum throughout its infection cycle of the gymnosperm host Pinus radiata . Mol. Plant Pathol. 17, 210–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw, R.E. , Sim, A.D. , Chettri, P. , Dupont, P.‐Y. , Guo, Y. , Hunziker, L. , McDougal, R.L. , van der Nest, A. , Fourie, A. , Wheeler, D. , Cox, M.P. and Barnes, I. (2019) Global population genomics of the forest pathogen Dothistroma septosporum suggest that chromosome duplications influence virulence factor levels. Mol. Plant Pathol. 20, 784–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstetter, M. and Cech, T.L. (1999) Neue Nadelkrankheiten an Kiefer. Osterreichishe Forstzeitung, 99, 35–36. [Google Scholar]
- Brandstetter, M. and Cech, T. (2003) Lecanosticta – Kiefernnadelbräune (Mycosphaerella dearnessii Barr) in Niederösterreich. Centralblatt für das gesamte forstwesen, 120, 163–176. [Google Scholar]
- Brazee, N.J. (2016) Dramatic needle browning and canopy dieback of eastern white pine (Pinus strobus) in southern New England (UMass Extension, Amherst, University of Massachusetts, ed), pp. 1–5. Massachusetts: United States Department of Agriculture. [Google Scholar]
- Broders, K. , Munck, I.A. , Wyka, S.A. , Iriarte, G. and Beaudoin, E. (2015) Characterization of fungal pathogens associated with white pine needle damage (WPND) in northeastern North America. Forests, 6, 4088–4104. [Google Scholar]
- Cech, T.L. (1997) Brown spot disease in Austria – the beginning of an epidemic. Forstschutz Aktuell, 19, 17. [Google Scholar]
- Cech, T.L. and Krehan, H. (2008) Lecanosticta‐Krankheit der Kiefer erstmals im Wald nachgewiesen. Forstschutz Aktuell, 45, 4–5. [Google Scholar]
- Chapman, H.H. (1926) Factors determining natural reproduction of longleaf pine on cut‐over lands in LaSalle Parish, Louisiana. Yale School of Forestry Bulletin, 16, 1–44. [Google Scholar]
- Chapman, H.H. (1932) Is the longleaf type a climax? Ecology, 13, 328–334. [Google Scholar]
- Cleary, M. , Laas, M. , Oskay, F. and Drenkhan, R. (2019) First report of Lecanosticta acicola on non‐native Pinus mugo in southern Sweden. Forest Pathol. 49, e12507. [Google Scholar]
- Cordell, C.E. , Anderson, R.L. and Kais, A.G. (1990) Brown‐Spot needle blight In: Southwide Forest Disease Workshop, (Boone A.J., Anderson R.L., Fenn P., Powers H.R. and Stambaugh W.J., eds.) pp. 18–19. Charleston, South Carolina: South Carolina Forestry Commission, Insect and Disease Section, Columbia, South Carolina. [Google Scholar]
- Crous, P.W. (1999) Species of Mycosphaerella and related anamorphs occurring on Myrtacea (excluding Eucalyptus). Mycol. Res. 103, 607–621. [Google Scholar]
- Crous, P.W. (2009) Taxonomy and phylogeny of the genus Mycosphaerella and its anamorphs. Fungal Divers. 38, 1–24. [Google Scholar]
- Crous, P.W. , Braun, U. and Groenewald, J.Z. (2007) Mycosphaerella is polyphyletic. Stud. Mycol., 58, 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous, P.W. , Kang, J. and Braun, U. (2001) A phylogenetic redefinition of anamorph genera in Mycosphaerella based on ITS rDNA sequence and morphology. Mycologia, 93, 1081–1101. [Google Scholar]
- Crous, P.W. , Summerell, B.A. , Carnegie, A.J. , Wingfield, M.J. , Hunter, G.C. , Burgess, T.I. , Andjic, V. , Barber, P.A. and Groenewald, J.Z. (2009) Unravelling Mycosphaerella: Do you believe in genera? Persoonia, 23, 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearness, J. (1926) New and noteworthy fungi: IV. Mycologia, 18, 236–255. [Google Scholar]
- Dearness, J. (1928) New and noteworthy fungi V. Mycologia, 20, 235–246. [Google Scholar]
- Drenkhan, R. and Hanso, M. (2009) Recent invasion of foliage fungi of pines (Pinus spp.) to the Northern Baltics. Forestry Studies, 51, 49–64. [Google Scholar]
- Drenkhan, R. , Tomešová‐Haataja, V. , Fraser, S. , Bradshaw, R.E. , Vahalík, P. , Mullett, M.S. , Martín‐García, J. , Bulman, L.S. , Wingfield, M.J. , Kirisits, T. , Cech, T.L. , Schmitz, S. , Baden, R. , Tubby, K. , Brown, A. , Georgieva, M. , Woods, A. , Ahumada, R. , Jankovský, L. , Thomsen, I.M. , Adamson, K. , Marçais, B. , Vuorinen, M. , Tsopelas, P. , Koltay, A. , Halasz, A. , La Porta, N. , Anselmi, N. , Kiesnere, R. , Markovskaja, S. , Kačergius, A. , Papazova‐Anakieva, I. , Risteski, M. , Sotirovski, K. , Lazarević, J. , Solheim, H. , Boroń, P. , Bragança, H. , Chira, D. , Musolin, D.L. , Selikhovkin, A.V. , Bulgakov, T.S. , Keča, N. , Karadžić, D. , Galovic, V. , Pap, P. , Markovic, M. , Poljakovic Pajnik, L. , Vasic, V. , Ondrušková, E. , Piškur, B. , Sadiković, D. , Diez, J.J. , Solla, A. , Millberg, H. , Stenlid, J. , Angst, A. , Queloz, V. , Lehtijärvi, A. , Doğmuş‐Lehtijärvi, H.T. , Oskay, F. , Davydenko, K. , Meshkova, V. , Craig, D. , Woodward, S. and Barnes, I. (2016) Global geographic distribution and host range of Dothistroma species: a comprehensive review. Forest Pathol. 46, 408–442. [Google Scholar]
- EPPO (2012a) First report of Mycosphaerella dearnessii in Latvia. EPPO Reporting Service no. 8. Num. article: 2012/168. Available at https://gd.eppo.int/reporting/article-2374 [accessed on April 17, 2019].
- EPPO (2012b) Mycosphaerella dearnessii detected again in Lithuania. EPPO Reporting Service no. 11. Num. article: 2012/240. Available at https://gd.eppo.int/reporting/article-2446 [accessed on April 17, 2019].
- EPPO (2015) Outbreak of Lecanosticta acicola in Tyrol, Austria. EPPO Reporting Service no. 10. Num. article: 2015/192. Available at https://gd.eppo.int/reporting/article-5139 [accessed on April 17, 2019].
- EPPO (2018) New data on quarantine pests and pests of the EPPO Alert List. EPPO Reporting Service no. 11. Num. article: 2018/212. Available at https://gd.eppo.int/reporting/article-6406 [accessed on April 17, 2019].
- Evans, H.C. (1984) The genus Mycosphaerella and its anamorphs Cercoseptoria, Dothistroma and Lecanosticta on pines. Myc. Papers, 153, 1–102. [Google Scholar]
- Gadgil, P.D. (1967) Infection of Pinus radiata needles by Dothistroma pini . New Zeal. J. Bot. 5, 498–503. [Google Scholar]
- Gibbs, J. and Sinclair, S. (2008) Forest insect and disease conditions in Vermont 2008: highlights and management recommendations In: Vermont Forest Insect and Disease Highlights . pp. 1–3. Waterbury, Vermont: Agency of Natural Resources, Department of Forests, Parks & Recreation. [Google Scholar]
- Gibson, I.A.S. (1980) Two pine needle fungi new to Colombia. Trop. Pest Manage. 26, 38–40. [Google Scholar]
- Glavaš, M. and Margaletić, J. (2001) Brown spot needle blight of aleppo pine and protection (English abstract) In: Znanost u potrajnom gospodarenju hrvatskim šumama: znanstvena knjiga, (Matić S., Krpanj A., Gračan J., eds), pp. 277–284. Jastrebarsko, Croatia/Zagreb, Croatia: šumarski fakultet. [Google Scholar]
- Hawksworth, D.L. , Crous, P.W. , Redhead, S.A. , Reynolds, D.R. , Samson, R.A. , Seifert, K.A. , Taylor, J.W. , Wingfield, M.J. , Abaci, Ö. , Aime, C. , Asan, A. , Bai, F.‐Y. , de Beer, Z.W. , Begerow, D. , Berikten, D. , Boekhout, T. , Buchanan, P.K. , Burgess, T. , Buzina, W. , Cai, L. , Cannon, P.F. , Crane, J.L. , Damm, U. , Daniel, H.‐M. , van Diepeningen, A.D. , Druzhinina, I. , Dyer, P.S. , Eberhardt, U. , Fell, J.W. , Frisvad, J.C. , Geiser, D.M. , Geml, J. , Glienke, C. , Gräfenhan, T. , Groenewald, J.Z. , Groenewald, M. , de Gruyter, J. , Guého‐Kellermann, E. , Guo, L.‐D. , Hibbett, D.S. , Hong, S.‐B. , de Hoog, G.S. , Houbraken, J. , Huhndorf, S.M. , Hyde, K.D. , Ismail, A. , Johnston, P.R. , Kadaifciler, D.G. , Kirk, P.M. , Kõljalg, U. , Kurtzman, C.P. , Lagneau, P.‐E. , Lévesque, C.A. , Liu, X. , Lombard, L. , Meyer, W. , Miller, A. , Minter, D.W. , Najafzadeh, M.J. , Norvell, L. , Ozerskaya, S.M. , Öziç, R. , Pennycook, S.R. , Peterson, S.W. , Pettersson, O.V. , Quaedvlieg, W. , Robert, V.A. , Ruibal, C. , Schnürer, J. , Schroers, H.‐J. , Shivas, R. , Slippers, B. , Spierenburg, H. , Takashima, M. , Taşkın, E. , Thines, M. , Thrane, U. , Uztan, A.H. , van Raak, M. , Varga, J. , Vasco, A. , Verkley, G. , Videira, S.I.R. , de Vries, R.P. , Weir, B.S. , Yilmaz, N. , Yurkov, A. and Zhang, N. (2011) The Amsterdam declaration on fungal nomenclature. IMA Fungus, 2, 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgcock, G.G. (1929) Septoria acicola and the brown‐spot disease of pine needles. Phytopathology, 19, 993–999. [Google Scholar]
- Henry, B.W. (1954) Sporulation by the brown spot fungus on longleaf pine needles. Phytopathology, 44, 385–386. [Google Scholar]
- Hintsteiner, M. , Cech, T.L. , Halmschlager, E. , Stauffer, C. and Kirisits, T. (2012) First report of Mycosphaerella dearnessii on Pinus nigra var. nigra in Austria. Forest Pathol. 42, 437–440. [Google Scholar]
- Holdenrieder, O. and Sieber, T.N. (1995) First report of Mycosphaerella dearnessii in Switzerland. Eur. J. For. Pathol. 25, 293–295. [Google Scholar]
- Huang, Z.‐Y. , Smalley, E.B. and Guries, R.P. (1995) Differentiation of Mycosphaerella dearnessii by cultural characters and RAPD analysis. Phytopathology, 85, 522–527. [Google Scholar]
- Ioos, R. , Fabre, B. , Saurat, C. , Fourrier, C. , Frey, P. and Marcais, B. (2010) Development, comparison, and validation of real‐time and conventional PCR tools for the detection of the fungal pathogens causing brown spot and red band needle blight of pine. Am. Phytopathol. Soc. 100, 105–114. [DOI] [PubMed] [Google Scholar]
- Jankovský, L. , Palovčíková, D. , Dvořák, M. and Tomšovský, M. (2009a) Records of brown spot needle blight related to Lecanosticta acicola in the Czech Republic. Plant Protect. Sci. 45, 16–18. [Google Scholar]
- Jankovský, L. , Palovčíková, D. and Tomšovský, M. (2009b) Brown spot needle blight associated with Mycosphaerella dearnessii occurs on Pinus rotundata in the Czech Republic. Plant Pathol. 58, 398. [Google Scholar]
- Janoušek, J. , Krumbock, S. , Kirisits, T. , Bradshaw, R.E. , Barnes, I. , Jankovský, L. and Stauffer, C. (2014) Development of microsatellite and mating type markers for the pine needle pathogen Lecanosticta acicola . Australas. Plant Path. 43, 161–165. [Google Scholar]
- Janoušek, J. , Wingfield, M.J. , Monsivais, J.G. , Jankovský, L. , Stauffer, C. , Konečný, A. and Barnes, I. (2016) Genetic analyses suggest separate introductions of the pine pathogen Lecanosticta acicola into Europe. Phytopathology, 106, 1413–1425. [DOI] [PubMed] [Google Scholar]
- Jewell, F.F. (1983) Histopathology of the brown spot fungus on longleaf pine needles. Phytopathology, 73, 854–858. [Google Scholar]
- Jianren, Y. and Chuandao, L. (1988) Study on seed transmission of brown spot fungus (Shcirrhia acicola). J. Nanjing Inst. For. 12, 21. [Google Scholar]
- Jurc, D. and Jurc, M. (2010) Mycosphaerella dearnessii occurs in Slovenia. Plant Pathol. 59, 808. [Google Scholar]
- Kabir, M.S. , Ganley, R.J. and Bradshaw, R.E. (2015) Dothistromin toxin is a virulence factor in dothistroma needle blight of pines. Plant Pathol. 64, 225–234. [Google Scholar]
- Kais, A.G. (1971) Dispersal of Schirria acicola spores in southern Mississippi. Plant Dis. Rep. 55, 309–311. [Google Scholar]
- Kais, A.G. (1972) Variation between southern and northern isolates of Schirrhia acicola In: Abstracts of papers for presentation at the sixty‐fourth annual meeting of the American Phytopathological Society, 6-10 August, pp. 768 Mexico City, Mexico. [Google Scholar]
- Kais, A.G. (1975) Environmental factors affecting brown‐spot infection on longleaf pine. Phytopathology, 65, 1389–1392. [Google Scholar]
- Kais, A.G. (1978) Pruning of longleaf pine seedlings in nurseries promotes brown‐spot needle blight. Tree Planter's Notes, 3–4. [Google Scholar]
- Kais, A.G. , Cordell, C.E. and Affeltranger, C.E. (1986) Benomyl root treatment controls brown‐spot disease on longleaf pine in the Southern United States. Forest Sci. 32, 506–511. [Google Scholar]
- Kessler, M. (2009) Aktuelle Verbreitung der Quarantänekrankheit Lecanosticta‐Nadelbräune der Kiefer (Mycosphaerella dearnessii M. E. Barr) in Hollenstein/Ybbs. Forstschutz Aktuell, 48, 29–30. [Google Scholar]
- Kessler, M. and Krehan, H. (2011) Neufunde von Quarantäneschadorganismen 2011 in Österreich. Forstschutz Aktuell, 53, 14–16. [Google Scholar]
- Kovaćevski, J.C. (1938) Parasitic fungi new for Bulgaria – Fifth contribution. Spisanie na Zemedelskite izpitatelni instituti v Bulgariya., 8, 3–13. [Google Scholar]
- Laflamme, G. , Côté, C. and Innes, L. (2010) White pine needle diseases in eastern Canada In: APS 2010 Northeastern Division Meeting Abstracts. Northampton, Massachusetts: Phytopathology, 101, S260. [Google Scholar]
- Laut, J.G. , Sutton, B. and Lawrence, J. (1966) Brown spot needle blight in Canada. Plant Dis. Rep. 50, 208. [Google Scholar]
- Lévy, A. (1996) Le point sur le champignon de quarantaine Scirrhia acicola dans le sud‐ouest de la France In: La Santé des Forêts, (Département de la Santé des Forêts, ed), pp. 28–30. France: Les Cahiers du DSF. [Google Scholar]
- Li, C. , Zku, X. , Han, Z. , Zhang, J. , Shen, G. , Zhang, Z. , Zheng, W. , Zou, K. and Shi, F. (1986) Investigation on Brown‐spot needle blight of pines in China. (English abstract). J. Nanjing Inst. For. 10, 11–18. [Google Scholar]
- Li, C. , Han, Z. , Jianren, Y. , Zheng, W. , Zhang, Z. , Zheng, R. and Zhang, H. (1987) Development of brown‐spot needle blight in slash pine plantations (English abstract). J. Nanjing Inst. For. 1, 1–7. [Google Scholar]
- Lopéz Castilla, R.A. , Duarte Casanova, A. , Guerra Rivero, C. , Cruz Escoto, H. and Triguero Issasi, N. (2002) Forest Nursery pest management in Cuba In: National Proceedings: Forest and Conservation Nursery Associations – 1999, 2000, 2001. (Dumroese R.K., Riley L.E., Landis T.D., technical coordinators, eds), pp. 213–218. Ogden, UT: USDA Forest Service, Rocky Mountain Research Station. [Google Scholar]
- Luttrell, E.S. (1949) Schirrhia acicola, Phaeocryptopus pinastri, and Lophodermium pinastri associated with the decline of ponderosa pine in Missouri. Plant Dis. Rep. 33, 397–401. [Google Scholar]
- Markovskaja, S. , Kacergius, A. and Treigiene, A. (2011) Occurrence of new alien pathogenic fungus Mycosphaerella dearnessii in Lithuania. Bot. Lith. 17, 29–37. [Google Scholar]
- Marmolejo, J.G. (2000) The genus Lecanosticta from Nuevo Leon, Mexico. Mycotaxon, 76, 393–397. [Google Scholar]
- Martínez, J.B. (1942) The mycoses of Pinus insignis in Guipúzcoa. Publ. Inst. for. Invest. Exp. 13, 1–72. [Google Scholar]
- McIntire, C.D. , Munck, I.A. , Ducey, M.J. and Asbjornsen, H. (2018) Thinning treatments reduce severity of foliar pathogens in eastern white pine. Forest Ecol. Manag. 423, 106–113. [Google Scholar]
- Milatović, I. (1976) Needle cast of pines caused by fungi Schirrhia pini Funk et Parker and Schirrhia acicola (Dearn.) Siggers in Yugoslavia. Poljoprivredna Znanstvena Smotra – Agriculturae Conspectus Scientificus, 39, 511–513. [Google Scholar]
- Mullett, M. , Tubby, K. , Webber, J. and Brown, A. (2016) A reconsideration of natural dispersal distances of the pine pathogen Dothistroma septosporum . Plant Pathol. 65, 1462–1472. [Google Scholar]
- Mullett, M. , Adamson, K. , Bragança, H. , Bulgakov, T. , Georgieva, M. , Henriques, J. , Jürisoo, L. , Laas, M. and Drenkhan, R. (2018) New country and regional records of the pine needle blight pathogens Lecanosticta acicola, Dothistroma septosporum and Dothistroma pini . Forest Pathol. 48, e12440. [Google Scholar]
- Munck, I.A. , Ostrofsky, W. and Burns, B. (2012) Pest Alert: Eastern white pine needle damage. NA‐PR‐01‐11 In: USDA Forest Service, Northeastern Area State and Private Forestry. Newtown, PA, USA. [Google Scholar]
- van der Nest, A. , Wingfield, M.J. , Ortiz, P.C. and Barnes, I. (2019) Biodiversity of Lecanosticta pine‐needle blight pathogens suggests a Mesoamerican Centre of origin. IMA Fungus. 1, 2 10.1186/s43008-019-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls, T.H. and Hudler, G.W. (1972) Red pine – a new host for brown spot (Schirrhia acicola). Plant Dis. Rep. 56, 712–213. [Google Scholar]
- Ohm, R.A. , Feau, N. , Henrissat, B. , Schoch, C.L. , Horwitz, B.A. , Barry, K.W. , Condon, B.J. , Copeland, A.C. , Dhillon, B. , Glaser, F. , Hesse, C.N. , Kosti, I. , LaButti, K. , Lindquist, E.A. , Lucas, S. , Salamov, A.A. , Bradshaw, R.E. , Ciuffetti, L. , Hamelin, R.C. , Kema, G.H.J. , Lawrence, C. , Scott, J.A. , Spatafora, J.W. , Turgeon, B.G. , de Wit, P.J.G.M. , Zhong, S. , Goodwin, S.B. and Grigoriev, I.V. (2012) Diverse lifestyles and strategies of plant pathogenesis encoded in the genomes of eighteen Dothideomycetes fungi. PLoS Pathog. 8, e1003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondrušková, E. , Hečková‐Jánošíková, Z. , Adamčík, S. , Kádasi Horáková, M. , Rakúsová‐Sládková, D. and Adamčíková, K. (2018) Needle blight caused by Dothistroma pini in Slovakia: distribution, host range and mating types. Scand. J. Forest Res. 1–7. [Google Scholar]
- Ortíz de Urbina, E. , Mesanza, N. , Aragonés, A. , Raposo, R. , Elvira‐Recuenco, M. , Boqué, R. , Patten, C. , Aitken, J. and Iturritxa, E. (2017) Emerging needle blight diseases in Atlantic Pinus ecosystems of Spain. Forests, 8, 1–18. [Google Scholar]
- Patton, R.F. and Spear, R.N. (1978) Scanning electron microscopy of infection of Scotch pine needles by Schirrhia acicola . Phytopathology, 68, 1700–1704. [Google Scholar]
- Pehl, V.L. (1995) Lecanosticta‐Nadelbräune. Eine neue Kiefernkrankheit in der Bundesrepublik Deutschland. Nachrichtenbl. Deut. Pflanzenscultzd, 47, 305–309. [Google Scholar]
- Pehl, L. and Cech, T. (2008) Mycosphaerella dearnessii and Mycosphaerella pini . Eur. Med. Plant Protec. Org. Bull., 38, 349–362. [Google Scholar]
- Pehl, L. , Burgermeister, W. and Wulf, A. (2004) Mycosphaerella‐Nadelpilze der Kiefer – Identifikation durch ITS‐RFLP‐Muster. Nachrichtenbl. Deut. Pflanzenscultzd, 56, 239–244. [Google Scholar]
- Perry, A. , Brown, A.V. , Cavers, S. , Cottrell, J.E. and Ennos, R.A. (2016a) Has Scots pine (Pinus sylvestris) coevolved with Dothistroma septosporum in Scotland? Evidence for spatial heterogeneity in the susceptibility of native provenances. Evol. Appl., 9, 982–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, A. , Wachowiak, W. , Brown, A.V. , Ennos, R.A. , Cottrell, J.E. and Cavers, S. (2016b) Substantial heritable variation for susceptibility to Dothistroma septosporum within populations of native British Scots pine (Pinus sylvestris). Plant Pathol., 65, 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrak, V.F. (1954) Phragmogloeum n. gen. eine neue Gattung der Sphaeropsideen. Sydowia, 8, 158–161. [Google Scholar]
- Petrak, F. (1961) Die Lecanosticta Krankheit der Fohren in Osterreich. Sydowia, 15, 252–256. [Google Scholar]
- Phelps, W.R. , Kais, A.G. and Nicholls, T.H. (1978) Brown‐spot needle blight of pines. US Department of Agriculture Forest Service, pp. 1–6. [Google Scholar]
- Piotrowska, M.J. , Riddell, C. , Hoebe, P.N. and Ennos, R.A. (2018) Planting exotic relatives has increased the threat posed by Dothistroma septosporum to the Caledonian pine populations of Scotland. Evol. Appl., 11, 350–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Porta, N. and Capretti, P. (2000) Mycosphaerella dearnessii, a needle‐cast pathogen on mountain pine (Pinus mugo) in Italy. Disease Notes, 84, 922. [DOI] [PubMed] [Google Scholar]
- Prey, A.J. and Morse, F.S. (1971) Brown spot needle blight of scotch pine christmas trees in Wisconsin. Plant Dis. Rep. 55, 648–649. [Google Scholar]
- Quaedvlieg, W. , Groenewald, J.Z. , Yáñez‐Morales, M.D.J. and Crous, P.W. (2012) DNA barcoding of Mycosphaerella species of quarantine importance to Europe. Persoonia, 29, 101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerson, C.T. (1953) Kansas mycological notes: 1951. Trans. Kans. Acad. Sci. 56, 53–57. [Google Scholar]
- Saccardo, P.A. (1884) Sylloge Fungorum. Patavii, Italy, 3, 507. [Google Scholar]
- Sadiković, D. , Piškur, B. , Barnes, I. , Hauptman, T. , Diminić, D. , Wingfield, M.J. and Jurc, D. (2019) Genetic diversity of the pine pathogen Lecanosticta acicola in Slovenia and Croatia. Plant Pathol. 68, 1120–1131. [Google Scholar]
- Schneider, S. , Jung, E. , Queloz, V. , Meyer, J.B. and Rigling, D. (2019) Detection of pine needle diseases caused by Dothistroma septosporum, Dothistroma pini and Lecanosticta acicola using different methodologies. Forest Pathol. 49, e12495. [Google Scholar]
- Seo, S.T. , Park, M.J. , Park, J.H. and Shin, H.D. (2012) First report of brown spot needle blight on Pinus thunbergii caused by Lecanosticta acicola in Korea. Disease Notes, 96, 914. [DOI] [PubMed] [Google Scholar]
- Setliff, E.C. and Patton, R.F. (1974) Germination behaviour of Scirrhia acicola conidia on pine needles. Phytopathology, 64, 1462–1464. [Google Scholar]
- Shishkina, A.K. and Tsanava, N.I. (1967) Systremma acicola (Dearn.) Wolf et Barbour: the perfect stage of Dothistroma acicola (Thüm.). A. Schischk. et N. Tzan. Novosti Sist. Nizsh. Rast. 276–277. [Google Scholar]
- Siggers, P.V. (1932) The brown‐spot needle blight of longleaf pine seedlings. J. Forest. 30, 579–593. [Google Scholar]
- Siggers, P.V. (1934) Observations on the influence of fire on the brown‐spot needle blight of longleaf pine seedlings. J. Forest. 32, 556–562. [Google Scholar]
- Siggers, P.V. (1939) Phytopathological note. Phytopathology, 29, 1076–1077. [Google Scholar]
- Siggers, P.V. (1944) The brown spot needle blight of pine seedlings. US Department of Agriculture, Washington, D.C. Technical Bulletin, 870, 1–36. [Google Scholar]
- Sinclair, W.A. and Hudler, G.W. (1980) Tree and shrub pathogens new or noteworthy in New York state. Plant Dis. 64, 590–592. [Google Scholar]
- Sinclair, W.A. and Lyon, H.H. (2005) Mycosphaerella diseases In: Diseases of Trees and Shrubs. Ithaca and London: Cornell University Press. [Google Scholar]
- Skilling, D.D. and Nicholls, T.H. (1974) Brown spot needle disease – biology and control in Scotch pine plantations. US Department of Agriculture. Forest Service Research Paper, NC‐109, 1–19. [Google Scholar]
- Snyder, E.B. and Derr, H.J. (1972) Breeding Longleaf pines for resistance to brown spot needle blight. Phytopathology, 62, 325–329. [Google Scholar]
- Sosnowski, M.R. , Fletcher, J.D. , Daly, A.M. , Rodoni, B.C. and Viljanen‐Rollinson, S.L.H. (2009) Techniques for the treatment, removal and disposal of host material during programmes for plant pathogen eradication. Plant Pathol. 58, 621–635. [Google Scholar]
- Stanosz, G. (1990) Premature needle drop and symptoms associated with brown spot needle blight on Pinus strobus in Northcentral Pennsylvania In: The American Phytophathological Society, Northeastern Division Annual Meeting, pp. 124 Phytopathology. [Google Scholar]
- Suto, Y. (2002) Seasonal development of symptoms and conidial production and dispersal of Lecanosticta acicola in Pinus thunbergii . Appl. For. Sci. 11, 17–22. [Google Scholar]
- Suto, Y. and Ougi, D. (1998) Lecanosticta acicola, causal fungus of brown spot needle blight in Pinus thunbergii, new to Japan. Mycoscience, 39, 319–325. [Google Scholar]
- Sydow, H. and Petrak, F. (1922) Ein beitrag zur kenntnis der Pilzflora Nordamerikas, insbesondere der nordwestlichen Staaten. Annales Mycologici, 20, 178–218. [Google Scholar]
- Sydow, H. and Petrak, F. (1924) Zweiter Beitrag zur Kenntnis der Pilzflora Nordamerikas, insbesondere der nordwestlichen Staaten. Annales Mycologici, 22, 387–409. [Google Scholar]
- Tainter, F.H. and Baker, F.A. (1996) Brown spot In: Principles of Forest Pathology, pp. 467–492. New York, USA: John Wiley. [Google Scholar]
- de Thümen, F. (1878) Fungorum americanorum triginta species novae. Flora, 61, 177–184. [Google Scholar]
- de Wit, P.J. , Van Der Burgt, A. , Ökmen, B. , Stergiopoulos, I. , Abd‐Elsalam, K.A. , Aerts, A.L. , Bahkali, A.H. , Beenen, H.G. , Chettri, P. and Cox, M.P. (2012) The genomes of the fungal plant pathogens Cladosporium fulvum and Dothistroma septosporum reveal adaptation to different hosts and lifestyles but also signatures of common ancestry. PLoS Genet. 8, e1003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyr, B.D. and Shaw, C.G. (1964) Identity of the fungus causing red band disease on pines. Mycologia, 56, 103–109. [Google Scholar]
- Wakeley, P.C. (1970) Thirty‐year effects of uncontrolled brown spot on planted longleaf pine. Forest Sci. 16, 197–202. [Google Scholar]
- Webster, C.B. (1930) Comments on "Brown‐Spot" disease of pine needles in Texas. J. Forest. 30, 763–778. [Google Scholar]
- Wingfield, M.J. , Brockerhoff, E.G. , Wingfield, B.D. and Slippers, B. (2015) Planted forest health: the need for a global strategy. Science, 349, 832–836. [DOI] [PubMed] [Google Scholar]
- Wolf, F.A. and Barbour, W.J. (1941) Brown‐spot needle disease of pines. Phytopathology, 31, 61–73. [Google Scholar]
- Wyka, S.A. , Smith, C. , Munck, I.A. , Rock, B.N. , Ziniti, B.L. and Broders, K. (2017) Emergence of white pine needle damage in the northeastern United States is associated with changes in pathogen pressure in response to climate change. Glob. Change Biol. 23, 394–405. [DOI] [PubMed] [Google Scholar]
- Wyka, S.A. , McIntire, C.D. , Smith, C. , Munck, I.A. , Rock, B.N. , Asbjornsen, H. and Broders, K.D. (2018) Effect of climatic variables on abundance and dispersal of Lecanosticta acicola spores and their impact on defoliation on eastern white pine. Phytopathology, 108, 374–383. [DOI] [PubMed] [Google Scholar]
- Yang, B. , Ye, J. , Bao, H. , Liu, J. and Dong, Z. (2002) Studies on the phytotoxic activities of LA‐I and LA‐II production by brown spot needlbe blight fungus (Lecanosticta acicola). Sci. Silvae Sin. 38, 84–88. [Google Scholar]
- Yang, B. , Ye, J. , Bao, H. , Lui, J. and Dong, Z. (2005) Separation and purification, structure of LA‐I toxin produced by brown spot needle blight fungus (Lecanosticta acicola). Sci. Silvae Sin. 41, 86–90. [Google Scholar]
- Ye, J. and Qi, G. (1999) Studies on the biological specialization of the toxin producing by brown spot needle blight fungus. J. Nanjing Inst. For. 23, 1–4. [Google Scholar]
- Ye, J. and Wu, X. (2011) Resistance in nature systems to brown‐spot needle blight of pine in China In: Fourth international workshop on the genetics of host–parasite interactions in forestry, pp. 107 Eugene, Oregon, USA: US Department of Agriculture. [Google Scholar]


