Abstract
Background:
We aimed to evaluate the prognostic role of programmed-death receptor ligand (PD-L1) in a multinational cohort of patients with localized renal cell carcinoma (RCC).
Methods:
Formalin-fixed paraffin-embedded blocks of 1017 patients from the Latin American Renal Cancer Group were analyzed. Tissue microarrays were immunostained for PD-L1 using a commercially available monoclonal antibody. Expression of PD-L1 in ⩾5% tumor cells was considered positive. PD-1 expression in immune cells was also assessed. All cases were reviewed twice based on antibody expression and compared with a positive control. Cox proportional hazard regression models were used to identify predictors of recurrence-free survival (RFS) and overall survival (OS).
Results:
A total of 738 cases with complete follow up met criteria. Median age was 57 [interquartile range (IQR): 49–64] years, and median follow up was 34 (IQR: 15–62.9) months. Median tumor size was 5 cm (IQR: 3.0–7.5 cm). Approximately 8.2% and 7.6% of tumors were PD-L1 and programmed cell-death 1 (PD-1) positive, respectively. PD-L1 and PD-1 positivity were significantly associated with higher tumor stage (both p < 0.001), and presence of tumor necrosis and lymphovascular multivariable analyses; PD-L1 positivity was found as a predictor of worse RFS [hazard ratio (HR) = 2.08, p = 0.05] and OS (HR = 2.61, p = 0.02).
Conclusions:
PD-L1 positivity was significantly associated with worse outcomes for patients with localized RCC at intermediate follow up. This marker may help stratify patients for stricter surveillance after surgical treatment and provide a basis for checkpoint-inhibitor therapy in the adjuvant setting.
Keywords: programmed-death ligand, renal cell carcinoma, stratification
Introduction
Renal cell carcinoma (RCC) is a lethal urologic malignancy corresponding to 62,700 new cases and 14,240 deaths per year in the Unites States.1 For years, agents targeting the vascular epithelial growth factor (VEGF) and the mammalian target of rapamycin have been the standard of care for patients with metastatic disease and clear-cell component.2,3 Despite the improvement from the pretargeted-therapy era, prognosis continues to be poor, with a 2-year overall survival (OS) of approximately 47% after treatment with tyrosine kinase inhibitors (TKIs).4 Current new combinations with checkpoint inhibitors are being tested and approved due to improved survival, thus highlighting the need for continued understanding of newer therapeutic agents with novel mechanisms of action.
Increased understanding of tumor cell signaling and interactions with immune-cell receptors have resulted in an increased understanding of immune-checkpoint inhibition.5 The blockade of cytotoxic T-lymphocyte-associated antigen-4 and programmed cell-death-1 (PD-1) have shown promising results in several malignancies, including advanced RCC.6–8 These emergent immunomodulatory approaches have been recently incorporated in the treatment armamentarium of RCC and have changed treatment paradigms. Despite these breakthroughs, the role of immune-checkpoint inhibition in the earlier stages of the disease has not yet been elucidated.
Current trials are evaluating the role of checkpoint inhibition as adjuvant therapy for high-risk RCC after nephrectomy, and are expected to alter treatment strategies for localized RCC. Herein, we aimed to assess the prognostic value of PD-1 and ligand (PD-L1) expression in a large, multicenter cohort of patients with localized RCC. We compared clinicopathologic characteristics and survival outcomes based on immunohistochemical (IHC) expression status for patients who underwent extirpative surgery for nonmetastatic clear-cell RCC (ccRCC).
Material and methods
Patient selection and sample collection
The cohort was derived from an international collaborative study group of large academic referral centers in Latin America and the United States.9 The Institutional Review Boards approved the study, and all patients signed an informed consent form to participate. Based on tissue availability, tissue microarrays (TMAs) were prepared from archival formalin-fixed paraffin-embedded specimens from 1017 cases of nonmetastatic, surgically treated ccRCC.
TMA construction and immunohistochemical analysis
Hematoxylin and eosin slides of each archival specimen were evaluated, and a block representing the overall tumor was chosen for TMA preparation. Three cores of 1 mm diameter per case were selected. Sections were cut 4 µm thick and placed on positively charged slides for immunostaining. Staining was performed using the Ventana Discovery XT automated system (Ventana Medical Systems, Tucson, AZ, USA) using commercially available antibodies against PD-1 on tissue-infiltrating lymphocytes (clone NAT105, diluted 1:100; Abcam, Cambridge, UK) and PD-L1 on tumor cell membranes (clone E1L3N, diluted 1:200; Cell Signaling Technology, Beverly, MA, USA). These assays had been previously validated using Formalin-Fixed Paraffin-Embedded cell-line controls.10
All TMAs were reviewed twice, based on antibody expression and compared with a positive control. Scoring was performed by an experienced uropathologist who was blinded to clinical outcomes. PD-L1 expression was evaluated based on the intensity and proportion of tumoral cells showing membranous or cytoplasmic staining, and was scored as follows: 0, negative (no immunoreactivity); 1, weak (5% to less than 25% of cells); 2, moderate (between 25 and 60% of cells); and 3, strong (more than 60% of cells). The numbers of PD-1 cytoplasmic-positive lymphocytes were assessed semiquantitatively.11
Statistical analysis
Pathologic stage was assigned according to the 2010 7th American Joint Committee on Cancer (AJCC) staging manual.12 Associations were assessed with χ2 tests or Fisher’s exact test to compare histopathologic features between clone-positive and -negative tumors. The Kaplan–Meier method was used to evaluate recurrence-free survival (RFS) and OS, with the log-rank test used for comparison. Based on sensitivity analyses, univariable Cox’s proportional hazards regression was used to assess pertinent clinicopathologic variables. A backward-stepwise selection procedure was used for the multivariable model. The hazard proportionality was verified by computing the log minus log against time. Statistical analysis was performed using the SPSS 25.0 software package (SPSS, Chicago, IL, USA).
Results
Patient characteristics
The final study cohort consisted of 738 patients after exclusion criteria were applied (Supplementary Figure 1). Median age for the cohort was 57 [interquartile range (IQR): 49–64] years, and the median follow up was 34 (IQR: 15.0–62.9) months. The median tumor size was 5 cm (3.0–7.5 cm). The majority of tumors (68%) were of AJCC pT1 type. Approximately 52% of patients were treated with radical nephrectomy and the rest treated with partial nephrectomy. Patient characteristics are summarized in Supplementary Table 1. There were a total of 119 recurrences (16.2%). Approximately 83% of patients were alive at the time of analysis.
Expression profile
On IHC staining, 8.3% and 7.6% of tumors were found to be PD-L1 and PD-1 positive, respectively (Figure 1). Approximately 4.9%, 2.0%, and 1.4% tumors received a PD-L1 score of 1, 2, and 3, respectively; whereas 4.5% and 3.1% received a PD-1 score of 1 and 2, respectively. On univariable analysis, PD-L1-positive tumors had a higher AJCC pT stage (p < 0.001), and were associated with tumor necrosis (p = 0.012) and lymphovascular invasion (p = 0.037). PD-1-positive tumors were also higher stage (p < 0.001), and associated with tumor necrosis (p = 0.019), lymphovascular invasion (p < 0.001), and higher Fuhrman grade (p < 0.001; Table 1).
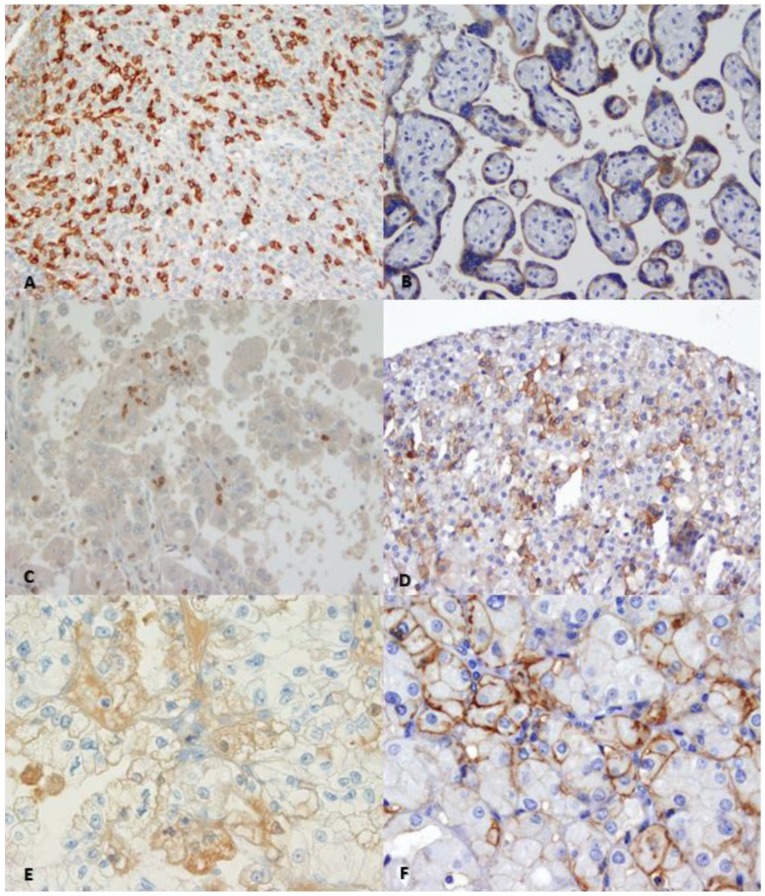
Figure 1.
Immunohistochemical staining showing PD-1 (a) and PD-L1 (b) positive controls. PD-1 positive immune cells in 20X (c) and 40X (e) magnification. PD-L1 positive tumor cells in 20X (d) and 40X magnification (f).
Table 1.
Association of PD-L1 and PD-1 expression with histopathological parameters.
| PD-L1− | PD-L1+ | p value | PD-1− | PD-1+ | p value | |
|---|---|---|---|---|---|---|
| Patients | 677 | 61 | 682 | 56 | ||
| AJCC tumor stage | <0.001 | <0.001 | ||||
| pT1 | 475 (70.2) | 29 (47.5) | 471 (69.1) | 23 (41.1) | ||
| pT2 | 152 (22.5) | 11 (18) | 159 (23.3) | 11 (19.6) | ||
| ⩾pT3 | 50 (7.3) | 21 (34.4) | 52 (7.6) | 22 (39.3) | ||
| Tumor necrosis | 0.012 | 0.0185 | ||||
| No | 519 (76.7) | 38 (62.3) | 512 (75) | 34 (60.7) | ||
| Yes | 158 (23.3) | 23 (37.7) | 170 (25) | 22 (39.3) | ||
| Lymphovascular invasion | 0.037 | <0.001 | ||||
| No | 604 (89.2) | 49 (80.4) | 610 (89.4) | 41 (73.2) | ||
| Yes | 73 (10.8) | 12 (19.6) | 72 (10.6) | 15 (26.8) | ||
| Fuhrman grade | 0.237 | <0.001 | ||||
| ⩽2 | 386 (57) | 30 (49.2) | 392 (57.5) | 19 (33.9) | ||
| ⩾3 | 291 (43) | 31 (50.8) | 290 (42.5) | 37 (66.1) |
Bold values indicate statistical significance.
AJCC, American Joint Committee on Cancer; PD-1, programmed cell-death 1; PD-L1, programmed cell-death ligand 1.
Survival analysis
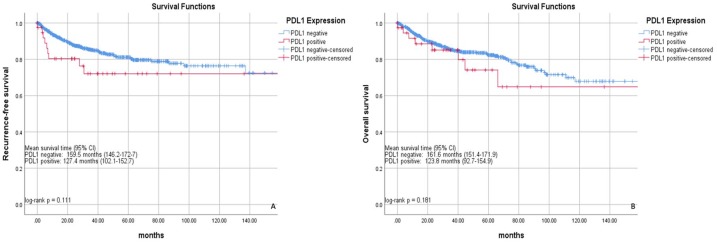
Kaplan–Meier analyses demonstrated nonsignificant, worse RFS and OS for PD-L1-positive cases (log rank p = 0.111 and 0.181, respectively; Figure 2). After adjusting for age, Eastern Cooperative Oncology Group performance status, tumor stage, grade, and presence of lymphovascular invasion or tumor necrosis, PD-L1 positivity was a significant predictor for worse RFS [hazard ratio (HR) = 2.08, 95% confidence interval (CI): 1.01–4.34] and OS (HR = 2.61, 95% CI: 1.15–5.96; Table 2).
Figure 2.
Recurrence-free (a) and overall survival (b) stratified by PD-L1 expression.
CI, confidence interval; PD-L1, programmed cell-death ligand 1.
Table 2.
Cox proportional hazard regression analysis for recurrence-free and overall survival.
| Survival | Univariable |
Multivariablea |
||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value* | |
| Recurrence-free survival | ||||
| PD-L1 positivity | 1.73 (0.87–3.42) | 0.116 | 2.08 (1.01–4.34) | 0.050 |
| PD-1 positivity | 2.35 (1.09–5.05) | 0.029 | – | |
| Overall survival | ||||
| PD-L1 positivity | 1.59 (0.80–3.31) | 0.185 | 2.61 (1.15–5.96) | 0.022 |
| PD-1 positivity | 0.84 (0.27–2.65) | 0.769 | – | |
Results of backward selection multivariable analysis.
–Dropped from statistical model.
Covariables included age, performance status, tumor stage, grade, and presence of lymphovascular invasion or tumor necrosis.
CI, confidence interval; HR, hazard ratio; PD-1, programmed cell-death 1; PD-L1, programmed cell-death ligand 1.
Discussion
In this large multi-institutional study, we report the prognostic value of PD-L1 expression for patients with clinically localized ccRCC. We found expression to be associated with high-risk histopathologic features on univariable analyses. After adjustment for pertinent covariates, we found PD-L1 positivity to be a significant predictor of worse RFS and OS. Our findings highlight PD-L1 positivity as a driver of inferior outcomes notwithstanding other patient and tumor characteristics. These patients merit strict follow-up surveillance and consideration for clinical trial enrollment.
Immunomodulatory agents targeting PD-1/PD-L1 have revolutionized treatment of several malignancies. PD-1 is a member of the B7-CD28 family and serves as a cell-surface inhibitory receptor on T cells.13,14 Expression of PD-1 and PD-L1 has been associated with poor outcomes in several tumor types.15–19 Thompson and colleagues were one of the first groups to demonstrate PD-L1 expression as a predictor of cancer progression and mortality in a subset of 268 patients with localized RCC.20 Abbas and colleagues demonstrated PD-L1 positivity associated with lymph node and distant metastasis, higher AJCC stage, and advanced disease.21 This same group also performed analyses on nonclear-cell histologies, although expression did not significantly impact tumor aggressiveness or clinical outcome.22 Studies of metastatic RCC have shown an increased risk of death for those with PD-L1 positivity on IHC staining.23–25 Our findings corroborate those studies, as PD-L1 positivity was associated with poor histopathologic features and worse outcomes in a multi-institutional cohort of patients of lower-stage ccRCC.
Recent clinical trials have focused on angiogenesis inhibitors such as TKIs and other molecular-targeted agents. So far, randomized phase III trials of adjuvant therapy have led to conflicting results. Sunitinib has shown improved disease-free survival (DFS) by a median of 1.2 years when compared with placebo, while two other studies involving sunitinib, sorafenib, or pazopanib had no impact on DFS while suffering from a high discontinuation rate due to treatment toxicity.26–28 On the other hand, immunotherapy trials involving nivolumab, or combination of nivolumab and ipilimumab, have shown a survival benefit over VEGF-targeted therapy for patients with previously treated or untreated advanced RCC, respectively.7,8 This leads us to believe that immunotherapy could have a beneficial role when given at an earlier stage for patients with high-risk RCC after nephrectomy.
Immunotherapeutic agents are now being investigated in the adjuvant and perioperative setting. Ongoing randomized phase III trials with pembrolizumab, atezolizumab, and nivolumab [ClinicalTrials.gov identifiers: NCT03142334, NCT03024996, NCT03055013, respectively] are currently underway and will help elucidate the role of checkpoint inhibition for localized RCC. Our results bear clinical importance, given the potential role for checkpoint inhibition for those with ccRCC PD-L1-positive tumors.
We acknowledge the limitations to this study that cannot be overcome. All cases included only retrospectively collected tissue samples. However, our sample is relatively large and interpreted by an experienced uropathologist blinded to clinical outcomes. Heterogeneity of RCC is well recognized and may not be representative of PD-L1 positivity for all tumor samples. Our study was performed on TMAs, which may not be representative of whole-tissue specimens and could lead to discordance in the expression of PD-L1 between tumor samples and primary tumors. However, we used three cores per patient to more adequately represent the expression of each antigen.29 Our short follow up and low number of events limit our conclusions. We limited our study to clear cell and no other histological subtypes. Although we correlate PD-L1 expression with cancer outcomes, causality cannot be established. Prospective studies are needed to further characterize the pathophysiological role of PD-L1 and its prognostic and therapeutic implications.
Conclusion
Tumor PD-L1 positivity was a significant predictor of worse outcomes for patients with clinically localized RCC at short follow up. This immunohistochemical marker may help identify patients that merit strict surveillance after nephrectomy and could potentially benefit from checkpoint-inhibitor therapy in the adjuvant setting.
Supplemental Material
Supplemental material, Supplementary_table_1 for Prognostic value of PD-L1 expression for surgically treated localized renal cell carcinoma: implications for risk stratification and adjuvant therapies by Juan Chipollini, Walter Henriques da Costa, Isabela Werneck da Cunha, Felipe de Almeida e Paula, Paulo Guilherme O. Salles, Mounsif Azizi, Philippe E. Spiess, Diego Abreu and Stênio de Cássio Zequi in Therapeutic Advances in Urology
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the National Institute of Science and Technology in Oncogenomics and Therapeutic Innovation.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iDs: Juan Chipollini  https://orcid.org/0000-0003-2603-0382
https://orcid.org/0000-0003-2603-0382
Walter Henriques da Costa  https://orcid.org/0000-0002-2940-4995
https://orcid.org/0000-0002-2940-4995
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Juan Chipollini, Department of Surgery, The University of Arizona College of Medicine, 1501 North Campbell Avenue, PO Box 245077, Tucson AZ 85724-5077, USA.
Walter Henriques da Costa, Division of Urology, AC Camargo Cancer Center, São Paulo, Brazil.
Isabela Werneck da Cunha, Division of Urology, AC Camargo Cancer Center, São Paulo, Brazil; National Institute for Science and Technology in Oncogenomics and Therapeutic Innovation, AC Camargo Cancer Center, São Paulo, São Paulo, Brazil.
Felipe de Almeida e Paula, Serviço de Urologistas, Hospital do Câncer de Presidente Prudente, São Paulo, Brazil.
Paulo Guilherme O. Salles, Division of Pathology, Instituto Mario Penna and Biocor Instituto, Belo Horizonte, Brazil.
Mounsif Azizi, Department of Genitourinary Oncology, Moffitt Cancer Center, Tampa, FL, USA.
Philippe E. Spiess, Department of Genitourinary Oncology, Moffitt Cancer Center, Tampa, FL, USA
Diego Abreu, Servicio de Urología, Hospital Pasteur, Montevideo, Uruguay.
Stênio de Cássio Zequi, Division of Urology, AC Camargo Cancer Center, São Paulo, Brazil; National Institute for Science and Technology in Oncogenomics and Therapeutic Innovation, AC Camargo Cancer Center, São Paulo, São Paulo, Brazil.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol 2004; 22: 454–463. [DOI] [PubMed] [Google Scholar]
- 3. Coppin C, Kollmannsberger C, Le L, et al. Targeted therapy for advanced renal cell cancer (RCC): a Cochrane systematic review of published randomised trials. BJU Int 2011; 108: 1556–1563. [DOI] [PubMed] [Google Scholar]
- 4. Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009; 27: 5794–5799. [DOI] [PubMed] [Google Scholar]
- 5. Tsai HF, Hsu PN. Cancer immunotherapy by targeting immune checkpoints: mechanism of T cell dysfunction in cancer immunity and new therapeutic targets. J Biomed Sci 2017; 24: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McDermott DF, Drake CG, Sznol M, et al. Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol 2015; 33: 2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015; 373: 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018; 378: 1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zequi SC, Clavijo DA. The creation, development and diffusion of the LARCG Latin American renal cancer group. Int Braz J Urol 2017; 43: 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maruse Y, Kawano S, Jinno T, et al. Significant association of increased PD-L1 and PD-1 expression with nodal metastasis and a poor prognosis in oral squamous cell carcinoma. Int J Oral Maxillofac Surg 2018; 47: 836–845. [DOI] [PubMed] [Google Scholar]
- 11. Kwon D, Kim S, Kim PJ, et al. Clinicopathological analysis of programmed cell death 1 and programmed cell death ligand 1 expression in the tumour microenvironments of diffuse large B cell lymphomas. Histopathology 2016; 68: 1079–1089. [DOI] [PubMed] [Google Scholar]
- 12. Felsenstein KM, Theodorescu D. Precision medicine for urothelial bladder cancer: update on tumour genomics and immunotherapy. Nat Rev Urol. Epub ahead of 14 November 2017. DOI: 10.1038/nrurol.2017.179. [DOI] [PubMed] [Google Scholar]
- 13. Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992; 11: 3887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26: 677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thompson RH, Dong H, Lohse CM, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res 2007; 13: 1757–1761. [DOI] [PubMed] [Google Scholar]
- 16. Mu CY, Huang JA, Chen Y, et al. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol 2011; 28: 682–688. [DOI] [PubMed] [Google Scholar]
- 17. Kammerer-Jacquet SF, Crouzet L, Brunot A, et al. Independent association of PD-L1 expression with noninactivated VHL clear cell renal cell carcinoma-a finding with therapeutic potential. Int J Cancer 2017; 140: 142–148. [DOI] [PubMed] [Google Scholar]
- 18. McDermott DF, Atkins MB. PD-1 as a potential target in cancer therapy. Cancer Med 2013; 2: 662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hino R, Kabashima K, Kato Y, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 2010; 116: 1757–1766. [DOI] [PubMed] [Google Scholar]
- 20. Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res 2006; 66: 3381–3385. [DOI] [PubMed] [Google Scholar]
- 21. Abbas M, Steffens S, Bellut M, et al. Intratumoral expression of programmed death ligand 1 (PD-L1) in patients with clear cell renal cell carcinoma (ccRCC). Med Oncol 2016; 33: 80. [DOI] [PubMed] [Google Scholar]
- 22. Abbas M, Steffens S, Bellut M, et al. Do programmed death 1 (PD-1) and its ligand (PD-L1) play a role in patients with non-clear cell renal cell carcinoma? Med Oncol 2016; 33: 59. [DOI] [PubMed] [Google Scholar]
- 23. Choueiri TK, Figueroa DJ, Fay AP, et al. Correlation of PD-L1 tumor expression and treatment outcomes in patients with renal cell carcinoma receiving sunitinib or pazopanib: results from COMPARZ, a randomized controlled trial. Clin Cancer Res 2015; 21: 1071–1077. [DOI] [PubMed] [Google Scholar]
- 24. Crispin H, Agarwal AM, Salama ME, et al. Correlation of tumor programmed death ligand-1 (PD-L1) expression and response to treatment with high-dose interleukin-2 (HD IL-2) in clear cell metastatic renal cell carcinoma (ccmRCC). J Clin Oncol 2014; 32: e15584. [Google Scholar]
- 25. Iacovelli R, Nolè F, Verri E, et al. Prognostic role of PD-L1 expression in renal cell carcinoma. A systematic review and meta-analysis. Target Oncol 2016; 11: 143–148. [DOI] [PubMed] [Google Scholar]
- 26. Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 2016; 387: 2008–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Motzer RJ, Haas NB, Donskov F, et al. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma. J Clin Oncol 2017; 35: 3916–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med 2016; 375: 2246–2254. [DOI] [PubMed] [Google Scholar]
- 29. Khouja MH, Baekelandt M, Sarab A, et al. Limitations of tissue microarrays compared with whole tissue sections in survival analysis. Oncol Lett 2010; 1: 827–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_table_1 for Prognostic value of PD-L1 expression for surgically treated localized renal cell carcinoma: implications for risk stratification and adjuvant therapies by Juan Chipollini, Walter Henriques da Costa, Isabela Werneck da Cunha, Felipe de Almeida e Paula, Paulo Guilherme O. Salles, Mounsif Azizi, Philippe E. Spiess, Diego Abreu and Stênio de Cássio Zequi in Therapeutic Advances in Urology