Abstract
Pulmonary vascular disease and resultant pulmonary hypertension (PH) have been increasingly recognized in the preterm population, particularly among patients with bronchopulmonary dysplasia (BPD). Limited data exist on the impact of PH severity and right ventricular (RV) dysfunction at PH diagnosis on outcome. The purpose of this study was to evaluate if echocardiography measures of cardiac dysfunction and PH severity in BPD-PH were associated with mortality. The study is a retrospective analysis of the echocardiography at three months or less from time of PH diagnosis. Survival analysis using a univariate Cox proportional hazard model is presented and expressed using hazard ratios (HR). We included 52 patients with BPD and PH of which 16 (31%) died at follow-up. Average gestational age at birth was 26.3 ± 2.3 weeks. Echocardiography was performed at a median of 43.3 weeks (IQR: 39.0–54.7). The median time between PH diagnosis and death was 117 days (range: 49–262 days). Multiple measures of PH severity and RV performance were associated with mortality (sPAP/sBP: HR 1.02, eccentricity index: HR 2.02, tricuspid annular plane systolic excursion Z-score: HR 0.65, fractional area change: HR 0.88, peak longitudinal strain: HR 1.22). Hence, PH severity and underlying RV dysfunction at PH diagnosis were associated with mortality in BPD-PH patients. While absolute estimation of pulmonary pressures is not feasible in every screening echocardiography, thorough evaluation of RV function and other markers of PH may allow to discriminate the most at-risk population and should be considered as standard add-ons to the current screening at 36 weeks.
Keywords: pulmonary hypertension, prematurity, bronchopulmonary dysplasia, speckle-tracking echocardiography, strain
Introduction
Pulmonary hypertension (PH) has been increasingly recognized in the premature population with bronchopulmonary dysplasia (BPD),1–5 and we recently described the demographics, maternal, and perinatal risk factors, as well as the clinical evolution of a cohort of premature newborns with BPD and PH followed at a single institution.6 Recent guidelines have advocated for echocardiographic screening of PH in preterm infants with BPD at 36 weeks of post-menstrual age (PMA), but there is a lack of consensus on which echocardiographic parameters should be used for optimal detection of PH and assessment of its hemodynamic consequences in this population.3,7 Also, recommendations regarding the echocardiographic evaluation of patients with BPD during their screening and at follow-up have been proposed,7,8 but not formally evaluated in the context of a population of BPD-PH patients. There are currently limited data on the natural history of this disease and underlying cardiac mechanics. While right ventricular (RV) performance has been described as a key factor contributing to outcomes in primary PH for both adult9 and pediatric cohorts,10 this is not well described in BPD patients. A recent survey of neonatologists regarding the screening of PH in BPD patients highlighted the variability in practices and the need for better guidance in the standardization of assessments of this vulnerable population.11 Understanding the underlying hemodynamic status and contribution of RV dysfunction may allow for refinement of treatment paradigms.
Herein, we sought to better describe the cardiac function and the degree of PH using echocardiography in this premature population with BPD and PH. Our secondary objective was to evaluate if these echocardiography parameters were associated with mortality. We hypothesized that markers of RV dysfunction at PH detection were associated with mortality in BPD patients with PH.
Methods
Patient population
Clinical and echocardiography data of patients with prematurity and PH treated at a single institution were retrospectively collected between January 2000 and May 2017. All premature patients with PH diagnosed at ≥ 36 weeks PMA and born at ≤ 32 weeks of estimated gestational age (GA) were identified from clinical databases. Patients were excluded if they had major congenital anomalies, genetic syndromes or did not have an echocardiography performed at our institution within three months of the diagnosis of PH (when referred as outpatient). Study data were extracted from the electronic medical record and recorded in a secure electronic database hosted at the Stanford Center for Clinical Informatics.12 This study was approved by the Stanford University Institutional Review Board.
Clinical definitions
Percentile for birth weight was calculated according to GA and sex using the Fenton growth chart and small for gestational age (SGA) was defined as a birth weight ≤ 10th percentile.13 BPD was defined and stratified as per the National Institute of Child Health and Human development Workshop definition.14 Patients were considered to have had a necrotizing enterocolitis (NEC) if documented as grade 2 and above according to the Bell classification.15
Echocardiography data collection
The echocardiography available at our institution closest to the diagnosis of PH was analyzed for each patient. Images were acquired according to the American Society of Echocardiography guidelines16 using Philips iE33, Philips EPIQ 7 (Philips Medical Systems, Bothell, WA), Siemens SC2000 or Siemens Sequoia C512 (Siemens Medical Solutions USA, Mountain View, CA) systems. Individual echocardiography images were analyzed by a rater masked to clinical status of the patients, and each echocardiography parameter was remeasured. Offline measurements were done on Syngo Dynamics workstation (Siemens Medical Solutions USA).
Conventional measures of ventricular function
LV systolic function was measured using ejection fraction (LV-EF), and calculated according the 5/6 area × length method which provides an estimated end-diastolic volume (EDV) and end-systolic volume (ESV).17,18 LV-EF, LV-EDV, and LV-ESV were also calculated using the modified Simpson’s rule.16,19 RV systolic function was assessed by RV fractional area change (RV-FAC),20 and tricuspid annular plane systolic excursion (TAPSE). A threshold of less than 30% area change by FAC was established as being abnormal based on reported guidelines21 and literature on newborn RV function.22 TAPSE Z-score was derived according to previously published reference values23 and a threshold of < −2.0 was considered abnormal.
Ventricular output
The velocity time integral (VTI) of the pulsed wave Doppler in the RV outflow tract (RVOT), sampled at the level of the pulmonary valve (PV) attachments, was measured in the parasternal short axis view to calculate stroke distance. LV outflow tract (LVOT) VTI of the pulsed wave Doppler, sampled at the level of the aortic valve (AV) attachments, was measured in the apical three-chamber view. The resultant stroke distances relate to their respective ventricles’ cardiac output (CO).24,25 Diameter of the PV was measured in the parasternal short axis view and of the AV in the parasternal long axis view. Stroke volume was derived from the VTI multiplied by the corresponding outflow cross-sectional area: (PV or AV/2)2 × π. The CO was then estimated by multiplying the resultant stroke volume with the heart rate at time of VTI assessment, and divided by body weight in kilograms (kg).19,20
Deformation analysis of ventricular function
Stored digital imaging and communications in medicine images of the apical four-chamber view and of the parasternal short axis view at the level of the papillary muscle of the mitral valve were transferred to the velocity vector imaging (VVI) platform for strain analysis by speckle-tracking echocardiography (STE; VVI 3.01.45 by Siemens Medical Solutions USA, Inc.). VVI allows for vendor-independent deformation analysis26 and tracks endocardial border speckles through cardiac cycles.27 An endocardial tracing of the RV and LV was done manually and repeated to ensure appropriate tracking.28 The interventricular septum (IVS) was included in each ventricle. Two to three consecutive cardiac cycles were averaged for subsequent analysis. Peak RV and LV longitudinal systolic (pLS) strain and strain rate (pLSR), as well as circumferential strain and strain rate of the LV were calculated by the software. RV focused view in apical four chamber was used for assessment of RV deformation. Peak longitudinal early diastolic strain rate (LSRe) of the RV and LV were extracted from the average strain rate curves.27,29,30 When early and late strain rate curves were fused, peak diastolic strain rate was recorded as LSRe. VVI provides estimation of both LV-EF and RV-FAC by auto-tracking. A pLS of < −14% was considered normal as per current neonatal echocardiographic literature on RV function.31
Pulmonary artery pressure estimation
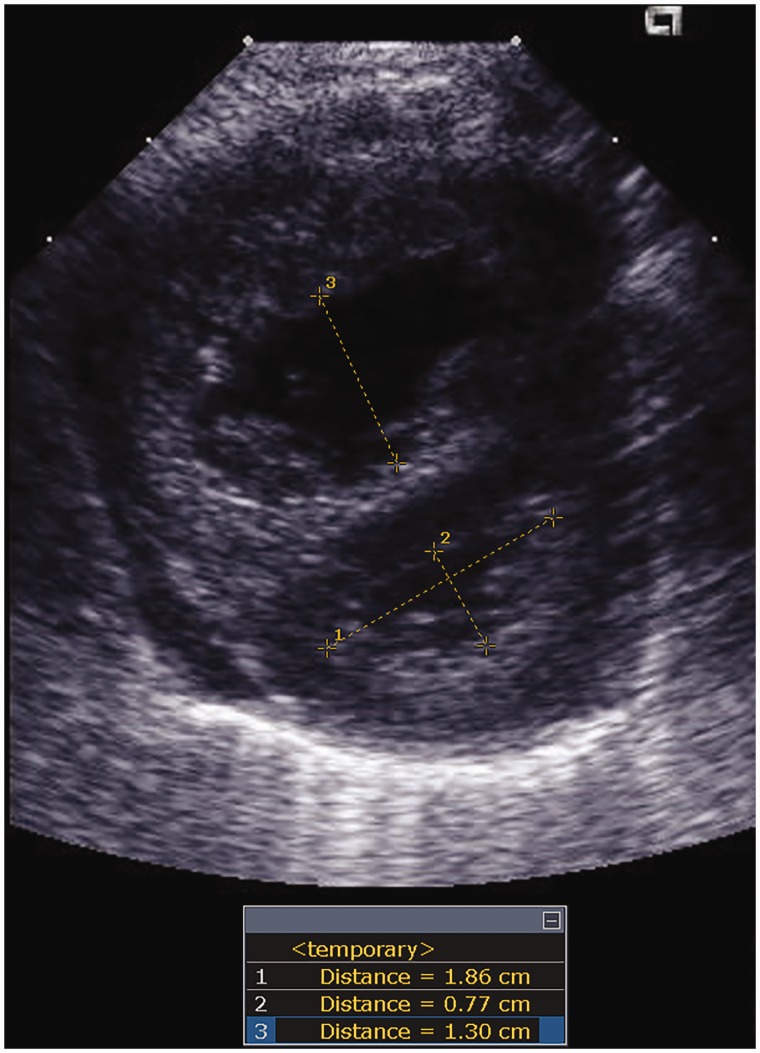
PH was diagnosed by echocardiography if any of the following were present: a mean pulmonary arterial pressure (mPAP) estimate ≥ 25 mmHg or an estimated systolic pulmonary arterial pressure (sPAP) ≥ 40 mmHg. If none of these quantifiable measures were available, flattening of the IVS at the end of systole was used as a surrogate of increased pulmonary pressure.32 mPAP was estimated by echocardiography using the full Doppler envelope of pulmonary insufficiency (PI), when available.20 sPAP was estimated by echocardiography using either: (a) tricuspid regurgitant jet (TRJ) ≥ 35 mmHg (plus expected right atrial pressure of 5 mmHg), when a full Doppler envelope was available, or by a (b) ventricular septal defect (VSD) or patent ductus arteriosus (PDA) velocity gradient.33 A ratio of the estimated sPAP to the systemic systolic blood pressure (sBP) at the time of the echocardiography was calculated. Main pulmonary artery (MPA) was measured as the largest diameter in the suprasternal notch view or parasternal short-axis view. Right atrial (RA) planimetry was measured in the apical four-chamber view. Z-scores for MPA and RA were derived according to previously published reference values.34 Pulmonary artery acceleration time to RV ejection time (PAAT/RVET) ratio was measured from the pulsed wave Doppler of the RVOT35 and a PAAT/RVET ratio less than 0.30 set as a marker of pulmonary vascular disease (PVD).33,35 Systolic to diastolic TRJ time ratio was measured from the continuous wave Doppler sampled at the tricuspid valve. LV eccentricity index (EI) at end of systole, a quantification of IVS distortion resulting from PH, was calculated (Fig. 1).33 EI is the ratio of the measure parallel to the septum to the measure perpendicular to the septum (with a perfect circle giving a ratio of 1.0). An abnormal LV EI was set as > 1.23.35 As a measure of RV dilation, the LV to RV ratio was calculated (ratio between the distance from the IVS to the LV free wall to the distance of IVS to the RV free wall at end of systole in parasternal short axis at papillary level).36 A ratio less than 1 has been associated with adverse outcomes in pediatric PH and correlates with invasive measurements of pulmonary pressures.37 Pulmonary vein stenosis (PVS) was defined as: (1) a monophasic Doppler flow profile by echocardiography with a mean gradient of more than 4 mmHg38 or (2) read on computed tomography with contrast or (3) as detected on cardiac catheterization via angiogram.
Fig. 1.
Eccentricity index and LV/RV ratio. Parasternal short-axis view at the papillary muscle level. LV eccentricity index is calculated as the ratio of the largest diameter of LV parallel to septum (distance 1) to the distance between septum and LV free wall (distance 2). LV/RV ratio is calculated as the ratio of the distance between septum and LV free wall (distance 2) and the distance between septum and RV free wall (distance 3).
Data analysis
Results are described as mean with standard deviation or median with interquartile range (IQR) for continuous variables and counts with proportions for categorical variables. The Fisher's exact and chi-square tests were used to compare categorical characteristics. Student t test and Wilcoxon–Mann–Whitney test were used to compare continuous variables for parametric and non-parametric variables, respectively. Patients were censored at last follow-up if alive and uncensored at time of death. Kaplan–Meier survival analysis was performed using log-rank test. Univariate associations between death and patient’s or echocardiography characteristics were analyzed using Cox proportional hazards regression, and expressed by hazard ratio (HR). Statistical analyses were done with Stata SE (Version 14.2, College Station, TX). The level of significance was set at 0.05 for all comparisons.
Results
Patient population
From 2000 through 2017, 86 premature patients with a diagnosis of PH were identified within our databases, of which 34 were excluded (n = 10 major congenital heart defect, n = 10 major congenital anomalies or syndrome, n = 14 referred with no available echocardiography within 3 months of diagnosis). Study cohort included 52 patients with BPD and PH, for which echocardiography images were available for analysis within three months of PH diagnosis. Sixteen patients (31%) died at follow-up. Information regarding the perinatal demographics are displayed in Table 1. Average GA at birth was 26.3 ± 2.3 weeks, birth weight of 728 ± 323 g and 38% were SGA. There was no statistically significant demographic difference between those who died and those who survived, except for males being overrepresented within the cohort who died.
Table 1.
Demography and clinical characteristics.
| All (n = 52) | Alive (n = 36) | Death (n = 16) | p value | |
|---|---|---|---|---|
| GA | 26.3 (2.3) | 26.1 (2.3) | 26.7 (2.2) | 0.32 |
| Inborn | 21 (40) | 16 (44) | 5 (31) | 0.37 |
| Admitted to our NICU | 42 (81) | 30 (83) | 12 (75) | 0.48 |
| Male | 28 (54) | 15 (42) | 13 (81) | 0.008 |
| Birth weight | 728 (323) | 738 (628) | 706 (328) | 0.48 |
| SGA | 20 (38) | 13 (36) | 7 (44) | 0.65 |
| Birth weight percentile | 34 (31) | 40 (4–63) | 13.5 (1.5–48) | 0.18 |
| Severe BPD | 44 (85) | 28 (78) | 16 (100) | 0.16 |
| NEC | 13 (25) | 9 (25) | 4 (25) | 1.00 |
| APGAR at 5 min | 7 (6–8) | 8 (6–9) | 7 (6–8) | 0.62 |
| PMA at diagnosis of PH | 41.4 (38.4–50.1) | 50.6 (27.2) | 42.4 (7.9) | 0.22 |
BPD: bronchopulmonary dysplasia; NEC: necrotizing enterocolitis; NICU: neonatal intensive care unit; PH: pulmonary hypertension; PMA: post-menstrual age; SGA: small for gestational age.
Expressed as mean (standard deviation), median (inter-quartile range) or count (percentage).
The initial echocardiography available for analysis at our center closest to the diagnosis of PH was at a median of one day of the diagnosis of PH (IQR: 0–19 days), as some patients were transferred from another institution with a diagnosis prior to arrival. Some patients (n = 12) were started on one or multiple pulmonary vasodilator medication(s) prior to transfer. Rate of treatment with pulmonary vasodilators was similar at echocardiography between those who died and those who survived. Analyzed echocardiography was at 43.3 weeks of PMA (IQR: 39.0–54.7 weeks). In patients who survived (n = 36), time between echocardiography and last follow-up was 988 days (IQR: 464–2001 days). In those who died, time from echocardiography to death was of 117 days (range: 49–262 days). Deaths were due to respiratory failure, pulmonary hypertensive crisis, cardiac arrest, and/or pneumonia. The vast majority of patients who died (94%) were treated with pulmonary vasodilator medications (iNO, milrinone, phosphodiesterase inhibitors, prostacyclin analogs or endothelin receptor antagonists) and most (11 out of 15 who were treated) were on multiple medications. Thirteen patients died during the initial hospitalization and required respiratory support until death. Three patients were discharged on home oxygen therapy and died later. Eleven patients (21%) were found to have signs of PVS at follow-up, of which eight had multiple veins involved, eight were on pulmonary vasodilator medications, and five died. None of these patients, described in a previous report,6 had signs of PVS at the diagnostic echocardiography analyzed for this study.
Conventional measures of ventricular function
The results of intergroup comparisons of conventional echocardiographic measures of function are summarized in Table 2. The LV-EF was within normal limits by three methods and similar between PH patients who died and those who survived, whereas RV systolic function was decreased in the group who died upon initial echocardiography by both TAPSE assessment (p = 0.0002) and FAC (by manual tracing: p = 0.00001, and auto-tracking: p = 0.001). RV function by FAC (HR = 0.88, 95% CI 0.83–0.94, p = 0.0001) and TAPSE Z-score (HR = 0.65, 95% CI 0.50–0.85, p = 0.002) were significantly associated with death at follow-up by Cox univariate analysis (Table 3). Ventricular outputs as assessed by LVOT VTI, RVOT VTI and calculated CO of both outflow tracts were not associated with mortality.
Table 2.
Echocardiography results.
| All (n = 52) | Alive (n = 36) | Death (n = 16) | p value | |
|---|---|---|---|---|
| PMA at echocardiography | 43.3 (39.0–54.7) | 52.8 (27.8) | 45.5 (9.1) | 0.38 |
| Days between PH Dx and echocardiography | 1 (0–19) | 1 (0–15) | 0 (0–37) | 0.97 |
| On PH-therapy at echocardiography (%) | 12 (23) | 6 (17) | 6 (38) | 0.10 |
| Weight at echocardiography in kg | 3.9 (2.8) | 4.2 (3.2) | 3.2 (1.4) | 0.27 |
| Systolic BP at echocardiography | 82 (13) | 84.8 (14.4) | 78.1 (12.0) | 0.13 |
| Diastolic BP at echocardiography | 48 (11) | 49.0 (10.5) | 45.2 (13.2) | 0.27 |
| Heart rate at echocardiography in bpm | 150 (22) | 150 (17) | 151 (30) | 0.24 |
| LV and RV function by echocardiography | ||||
| EF by Simpson's | 67.0 (6.4) | 66.2 (5.9) | 68.7 (7.4) | 0.21 |
| EF by 5/6 | 66.8 (6.8) | 65.7 (6.0) | 69.0 (8.1) | 0.12 |
| EF by VVI | 62.3 (5.7) | 61.4 (5.0) | 64.2 (6.7) | 0.14 |
| TAPSE | 9.2 (3.0) | 10.2 (2.9) | 7.0 (2.0) | 0.0002 |
| TAPSE Z-score | −1.08 (−2.66–0.45) | −0.08 (−1.4–0.84) | −2.69 (−3.27 to −1.59) | 0.0002 |
| FAC of RV | 31.3 (9.6) | 34.7 (8.6) | 23.5 (7.2) | 0.00001 |
| FAC by VVI | 31 (8) | 33.8 (7.4) | 25.6 (8.1) | 0.001 |
| Ventricular output | ||||
| VTI at PV | 0.13 (0.04) | 0.14 (0.04) | 0.13 (0.04) | 0.49 |
| VTI at AV | 0.13 (0.04) | 0.14 (0.04) | 0.13 (0.04) | 0.31 |
| RV CO | 476 (198) | 498 (223) | 433 (134) | 0.42 |
| LV CO | 260 (82) | 268 (79) | 243 (90) | 0.42 |
| Markers of pulmonary pressure | ||||
| TRJ systolic/diastolic time ratio | 2.46 (0.82) | 2.42 (0.84) | 2.56 (0.80) | 0.59 |
| sPAP | 74.63 (22.9) | 70.0 (22.9) | 86.5 (19.1) | 0.04 |
| sPAP/sBP | 88.9 (26.2) | 81.7 (23.5) | 107.0 (24.7) | 0.001 |
| Eccentricity index | 1.91 (0.76) | 1.74 (0.78) | 2.28 (0.58) | 0.02 |
| PAAT/RVET | 0.27 (0.08) | 0.27 (0.08) | 0.26 (0.07) | 0.53 |
| LV/RV ratio | 0.81 (0.36) | 0.89 (0.40) | 0.65 (0.19) | 0.04 |
| MPA Z-score | 4.33 (1.77) | 4.36 (1.69) | 4.28 (2.01) | 0.88 |
| RA planimetry Z-score | 2.7 (2.2) | 2.9 (2.2) | 2.3 (2.2) | 0.43 |
AV: aortic valve; Dx: diagnosis; CO: cardiac output; EDSR: early diastolic longitudinal strain rate; EF: ejection fraction; FAC: fractional area change; GA: gestational age; LV: left ventricle; MPA: main pulmonary artery; NEC: necrotizing enterocolitis; PAAT: pulmonary artery acceleration time; pLS: peak systolic longitudinal strain; pLSR: peak longitudinal systolic strain rate; PH: pulmonary hypertension; PMA: post-menstrual age; PV: pulmonary valve; RA: right atrium; RV: right ventricle; RVET: RV ejection time; VTI: velocity time integral; SGA: small for gestational age; sBP: systolic systemic blood pressure; sPAP: systolic pulmonary arterial pressure estimate; TAPSE: tricuspid annular plane systolic excursion; TRJ: tricuspid regurgitant jet.
Expressed as mean (standard deviation), median (inter-quartile range) or count (percentage).
Table 3.
Cox proportional hazards model – univariate analysis.
| HR | CI 5% | CI 95% | p value | |
|---|---|---|---|---|
| GA at birth | 0.96 | 0.9 | 1.02 | 0.16 |
| Male status | 0.24 | 0.07 | 0.85 | 0.03 |
| Birth weight | 1.00 | 0.998 | 1.001 | 0.76 |
| SGA status | 1.19 | 0.44 | 3.19 | 0.34 |
| NEC | 0.92 | 0.29 | 2.88 | 0.88 |
| PMA at PH diagnosis | 1.07 | 0.88 | 1.31 | 0.48 |
| sPAP | 1.03 | 1.002 | 1.05 | 0.04 |
| sPAP/sBP | 1.02 | 1.01 | 1.04 | 0.003 |
| Eccentricity index | 2.02 | 1.14 | 3.59 | 0.02 |
| LV/RV ratio | 0.16 | 0.03 | 0.9 | 0.04 |
| TAPSE Z-score | 0.65 | 0.50 | 0.85 | 0.002 |
| FAC of RV | 0.88 | 0.83 | 0.94 | 0.0001 |
| RV pLS | 1.22 | 1.06 | 1.41 | 0.007 |
| RV pLSR | 3.82 | 0.95 | 15.41 | 0.06 |
| RV LSRe | 0.29 | 0.10 | 0.83 | 0.02 |
LSRe: early diastolic longitudinal strain rate; FAC: fractional area change; GA: gestational age; HR: hazard ratio; LV: left ventricle; NEC: necrotizing enterocolitis; pLS: peak systolic longitudinal strain; pLSR: peak longitudinal systolic strain rate; PMA: post-menstrual age; RV: right ventricle; SGA: small for gestational age; sBP: systolic systemic blood pressure; sPAP: systolic pulmonary arterial pressure estimate; TAPSE: tricuspid annular plane systolic excursion.
Pulmonary artery pressure estimation
A PDA, a VSD, or a TRJ for assessment of sPAP was available in 75% (39/52) of patients. A full PI envelope was available in 13 patients (25%), of which 12 had a concomitant estimation of sPAP. Twelve were diagnosed with PH based on their septal configuration at the diagnostic echocardiography. At follow-up echocardiography, seven of these patients had an estimation of sPAP meeting criteria for PH and one had a confirmatory cardiac catheterization. The four remaining patients had progressive normalization of their IVS configuration.
The sPAP/sBP ratio (81.7 ± 23.5 vs. 107.0 ± 24.7, p = 0.001), the LV EI (1.74 ± 0.78 vs. 2.28 ± 0.58, p = 0.02), and the LV/RV ratio (0.89 ± 0.40 vs. 0.65 ± 0.19, p = 0.04) were significantly different and abnormal in the PH population who died, while PAAT/RVET ratio and systolic to diastolic time ratio of TRJ were not (Table 2). PAAT/RVET (0.27 ± 0.08), LV-EI (1.91 ± 0.76), and LV/RV ratio (0.81 ± 0.36) were meeting threshold described as abnormal in the overall BPD-PH population. The MPA was dilated in the overall cohort (Z-score 4.33 ± 1.77) and the RA was enlarged (Z-score 2.7 ± 2.2), but no differences were found between survivors and non-survivors. Association between absolute sPAP, sPAP/sBP, LV EI, and LV/RV ratio with death remained by univariate Cox analysis.
There was a significant association between PH severity assessment by sPAP/sBP ratio and RV function assessed by RV-FAC (R2 = 0.31, slope = −1.52, p < 0.001), as well as a weak association with TAPSE Z-score (R2 = 0.11, slope = −4.63, p = 0.047). Degree of PH by sPAP/sBP ratio was also significantly correlated to other markers of PH such as: LV EI (R2 = 0.34, slope = 21.02, p < 0.001), the systolic to diastolic TRJ time ratio (R2 = 0.20, slope = 14.9, p = 0.04) and the LV/RV ratio at end of systole (R2 = 0.35, slope = −39.0, p < 0.001).
Deformation analysis of ventricular function
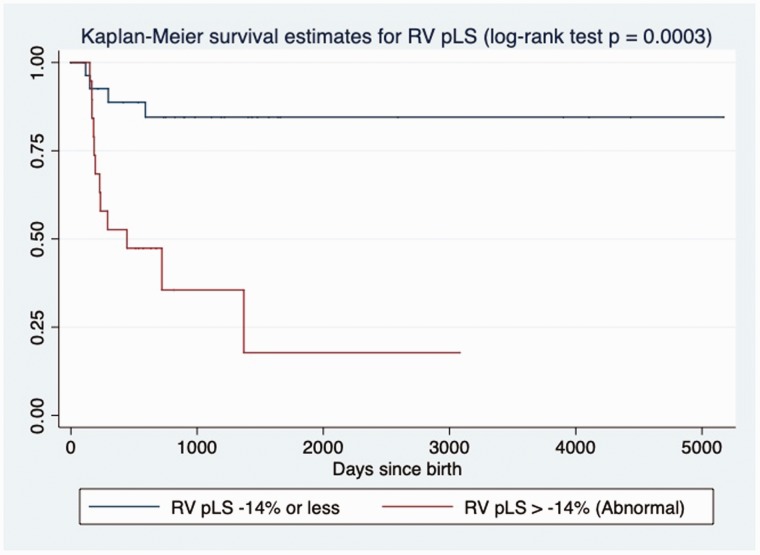
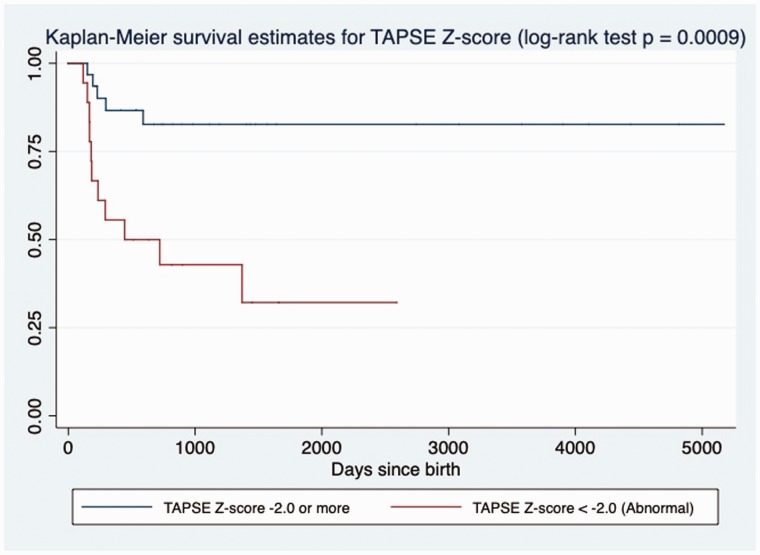
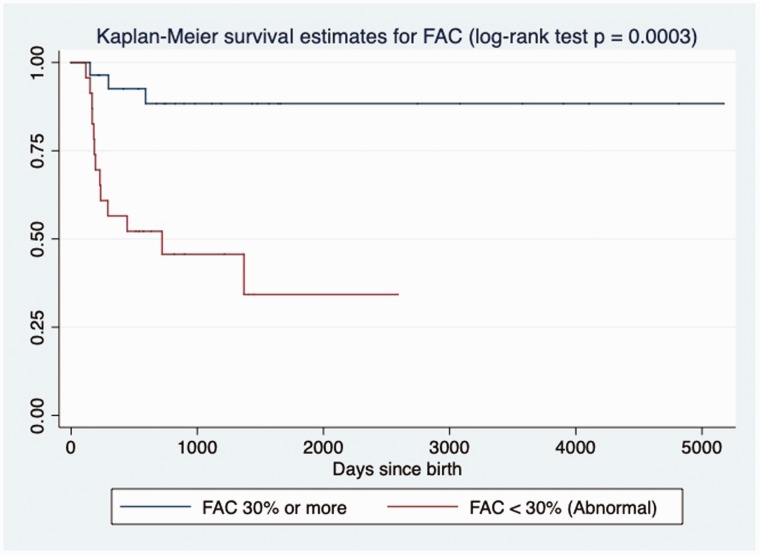
The results of intergroup comparisons of deformation measures are summarized in Table 4. The RV pLS, pLSR, and LSRe were decreased in non-survivors, while deformation parameters of the LV seemed to be preserved. RV pLS was also significantly correlated to the degree of PH by sPAP/sBP ratio (R2 = 0.27, slope = 3.45, p = 0.001) and to other conventional markers of RV function, such as: FAC (R2 = 0.66, slope = −1.69, p < 0.001) and TAPSE Z-score (R2 = 0.30, slope = −0.22, p < 0.001). Association between pLS and LSRe of RV with death remained by univariate Cox analysis (Table 3). Patients with RV pLS > −14% (log-rank test, p = 0.0003, Fig. 2), TAPSE Z-score < −2.0 (log-rank test, p = 0.0009, Fig. 3) or RV-FAC of < 30% (log-rank test, p = 0.0003, Fig. 4) had increased mortality at follow-up by Kaplan–Meier analysis.
Table 4.
Deformation analysis.
| All (n = 52) | Alive (n = 36) | Death (n = 16) | p value | |
|---|---|---|---|---|
| RV pLS | −15.6 (4.5) | −16.9 (4.1) | −13.1 (4.5) | 0.006 |
| RV pLSR | −1.37 (0.48) | −1.47 (0.50) | −1.18 (0.38) | 0.04 |
| RV LSRe | 1.73 (0.66) | 1.90 (0.64) | 1.39 (0.59) | 0.01 |
| LV pLS | −17.0 (4.4) | −17.6 (4.1) | −15.8 (4.8) | 0.21 |
| LV pLSR | −1.57 (0.64) | −1.57 (0.71) | −1.57 (0.51) | 0.51 |
| LV LSRe | 2.00 (0.64) | 2.00 (0.49) | 1.99 (0.90) | 0.40 |
| LV circumferential strain | −18.8 (6.0) | −18.9 (6.0) | −18.5 (6.1) | 0.83 |
| LV circumferential SR | −1.7 (−2.2 to −1.5) | −1.69 (1.17) | −1.71 (0.44) | 0.40 |
LSRe: early diastolic longitudinal strain rate; LV: left ventricle; pLS: peak systolic longitudinal strain; pLSR: peak longitudinal systolic strain rate; RV: right ventricle; SR: strain rate.
Fig. 2.
Kaplan–Meier assessment of RV peak longitudinal strain. RV peak LS > −14% at echocardiography closest to diagnosis of PH in BPD patients was significantly associated with death at follow-up in days (log-rank test; p = 0.0003).
Fig. 3.
Kaplan–Meier assessment of TAPSE Z-score. TAPSE Z-score < −2.0 at echocardiography closest to diagnosis of PH in BPD patients was significantly associated with death at follow-up in days (log-rank test; p = 0.0009).
Fig. 4.
Kaplan–Meier assessment of RV FAC. RV-FAC < 30.0% at echocardiography closest to diagnosis of PH in BPD patients was significantly associated with death at follow-up in days (log-rank test; p = 0.0003).
Discussion
In this cohort of patients with BPD and PH, echocardiographic indicators of PH and RV dysfunction at echocardiography closest to PH diagnosis were associated with mortality at a median of 117 days after the diagnostic echo. In addition, RV pLS correlated well with other indices of RV function (TAPSE and FAC), and the overall BPD-PH population had abnormal markers of PH (absolute sPAP estimates, LV-EI, PAAT/RVET, LV/RV ratio, as well as, MPA and RA measurements).
Echocardiography in BPD patients
Echocardiography allows for simultaneous assessment of cardiac function, cardiac structures, and pulmonary pressures33 and is the current modality advocated for screening in BPD patients.3 Echocardiography is, however, an imperfect tool, since it does not allow estimation of pulmonary pressures in every patients (nearly 1/4 of our cohort could not have their mPAP and/or sPAP estimated by echocardiography), is poor in the assessment of severity of PH39 and is not performed in the same hemodynamic conditions as during cardiac catheterization. Recently, inter-rater reliability of echocardiography readers evaluating PVD in the premature population at risk with BPD, revealed strong agreement (especially at 36 weeks of PMA).40 Despite the limitations of echocardiography, our data suggest that BPD-PH patients should be screened and followed using a comprehensive evaluation of the RV performance (by TAPSE, FAC, pLS) and of the pulmonary pressures (using direct estimation of PAP, as well as, indirect markers such as PAAT/RVET, LV/RV ratio and EI).
Correlates to the pediatric and adult population with PH
Limited data exist about the long-term impact of PVD in premature infants when reaching pediatric and adult age. Recently, a prospective longitudinal study looked at RV function, as assessed by FAC in preterm infants (23–28 weeks of GA at birth) without BPD. FAC at one month of age in that population was of 35 ± 5%.41 In our cohort, RV-FAC in those who survived (34.7 ± 8.6%) was similar to the FAC reported for those healthy preterm infants, while infants who died had a much lower FAC at diagnosis (23.5 ± 7.2%). Also, in a cohort of pediatric patients with BPD, RV pLS at one year of corrected age was found to be lower when compared to preterm infants without BPD.42 Similarly, another group evaluated children with prematurity and BPD at the age of three to five years and found that deformation analysis was abnormal compared to term controls, with a correlation between RV mechanics at pediatric age and the duration of invasive ventilation.43 Hence, respiratory phenotype of the preterm infant seems to reflect the abnormal cardiac performance even in the long term. In our study, a majority were in the severe BPD range. Thus, the association between degree of pulmonary disease and underlying differences in RV mechanics seems to be already present at term corrected age in the premature infants with PVD.
Markers of PH by echocardiography
The degree of PH was associated with increased mortality in our population. However, estimation of sPAP on a single echocardiography is not always feasible and warrants new objective measures to detect infants with PH or at risk for PH. We evaluated other markers: PAAT/RVET ratio, systolic to diastolic TRJ time ratio, LV/RV ratio, LV EI, and MPA size. All these markers were found to be abnormal in our cohort. The systolic to diastolic TRJ time ratio has been previously described as increased in the population of BPD with PH compared to BPD free of PH, and to controls.44 This measure is heart rate dependent and has been associated with cardiac performance in multiple pediatric populations.45,46 While a value above 1.1 has been described in BPD patients with PH,44 our cohort had much higher values (2.42 ± 0.82), but this time ratio was not associated with mortality (in the context of similar heart rates at echocardiography). The systolic to diastolic time ratio was significantly correlated to the degree of PH (sPAP/sBP) in our cohort, consistent with prior studies.44 LV/RV ratio has been used to quantify the degree of RV dilation by echocardiography due to the high subjectivity associated with qualitative assessment of RV dimensions.20 In a pediatric cohort with PH, a LV/RV ratio at the end of systole < 1.0 has been associated with adverse outcomes (initiation of intravenous prostacyclin treatment, atrial septostomy, transplant or mortality).20,37 Magnitude of LV/RV ratio was also correlated to pulmonary vascular resistance, mPAP, sPAP, and sPAP/sBP ratio by cardiac catheterization.37 Our cohort had an abnormal LV/RV ratio, and the magnitude of dilation was correlated to the degree of PH by sPAP/sBP by echocardiography. This ratio was significantly decreased in BPD patients who died, indicative of increased RV dilation secondary to RV failure. Similarly, LV EI is a measure of septal deformation and a ratio above 1.23 at end of systole has been associated with PH,35,47 and correlated with pulmonary vascular resistance, transpulmonary gradient as well as cardiac index by cardiac catheterization in adults.20,48 In this study, LV EI correlated with markers of RV dysfunction and the magnitude of increase in LV EI was associated with death. MPA size did not correlate with outcomes in our cohort, but was significantly dilated in the majority of patients (92% with a Z-score > 2.0). While the estimation of sPAP may only be present in 54% to 60% of pediatric patient undergoing screening for PH,39,44 the other described parameters indicative of PVD (PAAT/RVET, LV/RV ratio, LV EI, MPA size) can be measured in the absence of a full TRJ or PI envelope or a PDA/VSD gradient. Hence, in the absence of an estimation of pressure by echocardiography, these indirect measures may help identify at high-risk patients for PH and help tailoring management and follow-up.
RV function assessed by echocardiography
PH in BPD patients has been associated with long-term mortality,49,50 but the underlying RV dysfunction in our population also greatly impacted outcomes. RV performance is the key determinant of survival in adults and children with PH.9,10 Echocardiography allows for non-invasive assessment of RV and LV function. While the RV geometry makes the quantitative assessment of cardiac contractility challenging, novel markers such as deformation analysis by STE gives new insights on RV performance. Peak systolic longitudinal strain (pLS) of the RV has been described as significantly associated with survival in the adult population with PAH, where a pLS of ≥ −15.5% showed lower event-free survival.9 In our population, TAPSE (Z-score < 2.0), FAC (<30%), and RV pLS (>−14%) at echocardiography closest to diagnosis were discriminative markers. While strain analysis by STE might not be readily available in all centers, FAC and TAPSE are measures of RV systolic function that are rapid and easy to acquire. FAC in neonates has good inter- (93%) and intra-observer (94%) reliability and reference values in that population have been established.41 Z-scores for TAPSE in both preterm and term infants have also been derived from populational studies51,52 and this marker has been described as a reproducible index of RV function in infants with PH (96% intra-observer and 92% inter-observers agreement).53 With the increasing use of echocardiography for screening, degree of PH at diagnosis may be difficult to interpret when assessing individual patients. Our data suggest that a thorough assessment of RV performance may augment the detection of patients that are at the highest risk for poor outcomes.
Most patients in our study were on currently available vasodilator therapies, which were used in the hope to decrease pulmonary pressures and improve RV performance. While LV function seems to be preserved, some may develop post-capillary PH due to abnormal pulmonary venous vasculature. Indeed, 21% of our cohort did show signs of pulmonary venous stenosis at follow-up. Interestingly, patients with a diagnosis of PVS had all signs of abnormal Doppler flow at subsequent echocardiography, but none had anomalies at echocardiography closest to PH diagnosis. Recently, a group described that a median of five echocardiography (range 1–25) were necessary before the diagnosis of PVS in a retrospective multi-center study looking at premature infants.54 Hence, the later appearance warrants constant vigilance in this population which seems at high risk for development of acquired PVS. Indeed, a recent survey described that the vast majority of responding neonatologists had encountered a case of PVS in the context of prematurity.11 Assessing pulmonary veins by echocardiography with documentation of individual spectral Dopplers can be challenging. Delay in diagnosis may be related to technical difficulties but routine surveillance is indicated given the progressive nature of the condition.54 The impact of pulmonary vasodilation in the context of venous obstruction is unclear and warrants further studies. Finally, patients who died in our population had a severe pulmonary phenotype. Future research should evaluate if early screening and targeted management to decreased PH and augment RV performance can be translated in improved outcomes.
Limitations
This is a single center, retrospective study and its generalizability should be tested in a multi-institutional study with a larger sample size. Some patients were referred to our PH clinic from other hospitals and their echocardiography was not at the initial diagnosis. Hence, we had to exclude patients who presented later than three months after their PH diagnosis. There was no formalized screening protocol for PH at our institution, thus the population might be skewed towards the sickest patients who were identified due to clinical symptoms. Due to the absence of formal screening protocol, the analyzed echocardiography scans were not performed at a set age-point for the individual patients. Multiple echocardiographic platforms were used to image the patients, which could introduce variability in speckle presentations between vendors;55 however, the STE analysis was performed by a single multivendor software platform to decrease variability.56,57 STE analysis was done by a single reader and inter-reader reliability was not explored, since reports have described a high reproducibility of strain measurements by the same reader or a different reader using different strain vendors58 and using different echocardiography machines.56 Analyzed echocardiography images were originally acquired at a lower frame rate of 30–60 Hz, when 2D STE recommendations advocate for 80–100 Hz. However, a recent report observed no difference between strain measurements at lower (below 60 Hz) and higher (≥60 Hz) frame rates.57
In premature infants with BPD, degree of PH and underlying RV dysfunction were associated with mortality at follow-up. Specifically, RV FAC, TAPSE, and RV peak longitudinal strain were decreased in infants with poor outcomes. In addition to estimation of pulmonary pressures by echocardiography, other markers such as PAAT/RVET, LV/RV ratio, and LV EI may be of interest when assessing a premature patient with BPD for screening or follow-up of PH. A thorough evaluation of RV performance by echocardiography at time of PH screening should be done to identify the most at risk patients in this population. Future studies should investigate if screening and targeted management in those with signs of RV dysfunction can improve outcomes.
Supplemental Material
Supplemental material, PUL878598 Supplemental Material for Diminished right ventricular function at diagnosis of pulmonary hypertension is associated with mortality in bronchopulmonary dysplasia by Gabriel Altit, Shazia Bhombal, Jeffrey Feinstein, Rachel K. Hopper and Theresa A. Tacy in Pulmonary Circulation
Conflict of interest
The authors have no conflict of interest to declare.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Guarantor
Dr Gabriel Altit.
Contributorship
Dr Gabriel Altit collected the data and wrote the first draft of the manuscript. Drs Shazia Bhombal, Rachel Hopper, Jeffrey Feinstein, and Theresa Tacy contributed to design of the study (methods), data collection, data analysis, and manuscript revision.
Ethical approval
This study was approved by the institutional review board of Stanford University (protocol – IRB-39388).
Supplemental material
Supplemental material for this article is available online.
References
- 1.Subhedar N, Shaw N. Changes in pulmonary arterial pressure in preterm infants with chronic lung disease. Arch Dis Child Fetal Neonatal Ed 2000; 82: F243–F247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abman SH, Collaco JM, Shepherd EG, et al. Interdisciplinary care of children with severe bronchopulmonary dysplasia. J Pediatr 2017; 181: 12–28. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abman SH, Hansmann G, Archer SL, et al. Pediatric pulmonary hypertension: guidelines from the american heart association and american thoracic society. Cir 2015; 132: 2037–2099. [DOI] [PubMed]
- 4.Mourani PM, Sontag MK, Younoszai A, et al. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med 2015; 191: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhat R, Salas AA, Foster C, et al. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics 2012; 129: e682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altit G, Bhombal S, Hopper RK, et al. Death or resolution: the “natural history” of pulmonary hypertension in bronchopulmonary dysplasia. J Perinatol 2019; 39: 415–425. [DOI] [PubMed]
- 7.Krishnan U, Feinstein JA, Adatia I, et al. Evaluation and management of pulmonary hypertension in children with bronchopulmonary dysplasia. J Pediatr 2017; 188: 24–34.e21. [DOI] [PubMed] [Google Scholar]
- 8.Altit G, Dancea A, Renaud C, et al. Pathophysiology, screening and diagnosis of pulmonary hypertension in infants with bronchopulmonary dysplasia – a review of the literature. Paediatr Respir Rev 2017; 23: 16–26. [DOI] [PubMed] [Google Scholar]
- 9.Park J-H, Park MM, Farha S, et al. Impaired global right ventricular longitudinal strain predicts long-term adverse outcomes in patients with pulmonary arterial hypertension. J Cardiovasc Ultrasound 2015; 23: 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandoval J, Bauerle O, Gomez A, et al. Primary pulmonary hypertension in children: clinical characterization and survival. J Am Coll Cardiol 1995; 25: 466–474. [DOI] [PubMed] [Google Scholar]
- 11.Altit G, Lee HC, Hintz S, et al. Practices surrounding pulmonary hypertension and bronchopulmonary dysplasia amongst neonatologists caring for premature infants. J Perinatol 2018; 38: 361–367. [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 2013; 13: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001; 163: 1723–1729. [DOI] [PubMed] [Google Scholar]
- 15.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 1978; 187: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai WW, Geva T, Shirali GS, et al. Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr 2006; 19: 1413–1430. [DOI] [PubMed] [Google Scholar]
- 17.Lu JC, Ensing GJ, Yu S, et al. 5/6 Area length method for left-ventricular ejection-fraction measurement in adults with repaired tetralogy of Fallot: comparison with cardiovascular magnetic resonance. Pediatr Cardiol 2013; 34: 231–239. [DOI] [PubMed] [Google Scholar]
- 18.Wyatt H, Heng M, Meerbaum S, et al. Cross-sectional echocardiography. II. Analysis of mathematic models for quantifying volume of the formalin-fixed left ventricle. Circulation 1980; 61: 1119–1125. [DOI] [PubMed] [Google Scholar]
- 19.Mertens L, Seri I, Marek J, et al. Targeted neonatal echocardiography in the neonatal intensive care unit: practice guidelines and recommendations for training. Writing Group of the American Society of Echocardiography (ASE) in collaboration with the European Association of Echocardiography (EAE) and the Association for European Pediatric Cardiologists (AEPC). J Am Soc Echocardiogr 2011; 24: 1057–1078. [DOI] [PubMed] [Google Scholar]
- 20.Jone PN, Ivy DD. Echocardiography in pediatric pulmonary hypertension. Front Pediatr 2014; 2: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23: 685–713. [DOI] [PubMed] [Google Scholar]
- 22.Levy PT, Dioneda B, Holland MR, et al. Right ventricular function in preterm and term neonates: reference values for right ventricle areas and fractional area of change. J Am Soc Echocardiogr 2015; 28: 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koestenberger M, Ravekes W, Nagel B, et al. Reference values of the right ventricular outflow tract systolic excursion in 711 healthy children and calculation of z-score values. Eur Heart J Cardiovasc Imaging 2014; 15: 980–986. [DOI] [PubMed] [Google Scholar]
- 24.Ficial B, Finnemore AE, Cox DJ, et al. Validation study of the accuracy of echocardiographic measurements of systemic blood flow volume in newborn infants. J Am Soc Echocardiogr 2013; 26: 1365–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ristow B, Schiller NB. Obtaining accurate hemodynamics from echocardiography: achieving independence from right heart catheterization. Curr Opin Cardiol 2010; 25: 437–444. [DOI] [PubMed] [Google Scholar]
- 26.Arunamata A, Selamet Tierney ES, Tacy TA, et al. Echocardiographic measures associated with early postsurgical myocardial dysfunction in pediatric patients with mitral valve regurgitation. J Am Soc Echocardiogr 2015; 28: 284–293. [DOI] [PubMed] [Google Scholar]
- 27.Lorch SM, Ludomirsky A, Singh GK. Maturational and growth-related changes in left ventricular longitudinal strain and strain rate measured by two-dimensional speckle tracking echocardiography in healthy pediatric population. J Am Soc Echocardiogr 2008; 21: 1207–1215. [DOI] [PubMed] [Google Scholar]
- 28.Fine NM, Shah AA, Han IY, et al. Left and right ventricular strain and strain rate measurement in normal adults using velocity vector imaging: an assessment of reference values and intersystem agreement. Int J Cardiovasc Imaging 2013; 29: 571–580. [DOI] [PubMed] [Google Scholar]
- 29.Carasso S, Biaggi P, Rakowski H, et al. Velocity Vector Imaging: standard tissue-tracking results acquired in normals – the VVI-STRAIN study. J Am Soc Echocardiogr 2012; 25: 543–552. [DOI] [PubMed] [Google Scholar]
- 30.Punn R, Axelrod DM, Sherman-Levine S, et al. Predictors of mortality in pediatric patients on venoarterial extracorporeal membrane oxygenation. Pediatr Crit Care Med 2014; 15: 870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel N, Massolo AC, Paria A, et al. Early postnatal ventricular dysfunction is associated with disease severity in patients with congenital diaphragmatic hernia. J Pediatr 2018; 203: 400–407.e401. [DOI] [PubMed] [Google Scholar]
- 32.King M, Braun H, Goldblatt A, et al. Interventricular septal configuration as a predictor of right ventricular systolic hypertension in children: a cross-sectional echocardiographic study. Circulation 1983; 68: 68–75. [DOI] [PubMed] [Google Scholar]
- 33.Altit G, Dancea A, Renaud C, et al. Pathophysiology, screening and diagnosis of pulmonary hypertension in infants with bronchopulmonary dysplasia-a review of the literature. Paediatr Respir Rev 2017; 23: 16–26. [DOI] [PubMed]
- 34.Steven C. Boston Children's Hospital Heart Center – Z-score calculator. Boston Children's Hospital, http://zscore.chboston.org/ (2017, accessed April 2017).
- 35.Nagiub M, Lee S, Guglani LJE. Echocardiographic assessment of pulmonary hypertension in infants with bronchopulmonary dysplasia: systematic review of literature and a proposed algorithm for assessment. Echocardiography 2015; 32: 819–833. [DOI] [PubMed] [Google Scholar]
- 36.Koestenberger M, Friedberg MK, Nestaas E, et al. Transthoracic echocardiography in the evaluation of pediatric pulmonary hypertension and ventricular dysfunction. Pulm Circ 2016; 6: 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jone PN, Hinzman J, Wagner BD, et al. Right ventricular to left ventricular diameter ratio at end-systole in evaluating outcomes in children with pulmonary hypertension. J Am Soc Echocardiogr 2014; 27: 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalfa D, Belli E, Bacha E, et al. Primary pulmonary vein stenosis: outcomes, risk factors, and severity score in a multicentric study. Ann Thorac Surg 2017; 104: 182–189. [DOI] [PubMed] [Google Scholar]
- 39.Mourani PM, Sontag MK, Younoszai A, et al. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics 2008; 121: 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carlton EF, Sontag MK, Younoszai A, et al. Reliability of echocardiographic indicators of pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. J Pediatr 2017; 186: 29–33. [DOI] [PMC free article] [PubMed]
- 41.Levy PT, Dioneda B, Holland MR, et al. Right ventricular function in preterm and term neonates: reference values for right ventricle areas and fractional area of change. J Am Soc Echocardiogr 2015; 28: 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levy PT, Afif E-K, Patel MD, et al. Maturational patterns of systolic ventricular deformation mechanics by two-dimensional speckle-tracking echocardiography in preterm infants over the first year of age. J Am Soc Echocardiogr 2017; 30: 685–698. [DOI] [PMC free article] [PubMed]
- 43.Xie L, Chee YY, Wong KY, et al. Cardiac mechanics in children with bronchopulmonary dysplasia. Neonatology 2016; 109: 44–51. [DOI] [PubMed] [Google Scholar]
- 44.McCrary A, Malowitz J, Hornick C, et al. Differences in eccentricity index and systolic–diastolic ratio in extremely low-birth-weight infants with bronchopulmonary dysplasia at risk of pulmonary hypertension. Am J Perinatol 2016; 33: 057–062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friedberg MK, Silverman NH. The systolic to diastolic duration ratio in children with heart failure secondary to restrictive cardiomyopathy. J Am Soc Echocardiogr 2006; 19: 1326–1331. [DOI] [PubMed] [Google Scholar]
- 46.Friedberg MK, Silverman NH. The systolic to diastolic duration ratio in children with hypoplastic left heart syndrome: a novel Doppler index of right ventricular function. J Am Soc Echocardiogr 2007; 20: 749–755. [DOI] [PubMed] [Google Scholar]
- 47.Ryan T, Petrovic O, Dillon JC, et al. An echocardiographic index for separation of right ventricular volume and pressure overload. J Am Coll Cardiol 1985; 5: 918–924. [DOI] [PubMed] [Google Scholar]
- 48.López-Candales A, Rajagopalan N, Kochar M, et al. Systolic eccentricity index identifies right ventricular dysfunction in pulmonary hypertension. Int J Cardiol 2008; 129: 424–426. [DOI] [PubMed] [Google Scholar]
- 49.Khemani E, McElhinney DB, Rhein L, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics 2007; 120: 1260–1269. [DOI] [PubMed] [Google Scholar]
- 50.An HS, Bae EJ, Kim GB, et al. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circul J 2010; 40: 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koestenberger M, Nagel B, Ravekes W, et al. Systolic right ventricular function in preterm and term neonates: reference values of the tricuspid annular plane systolic excursion (TAPSE) in 258 patients and calculation of Z-score values. Neonatology 2011; 100: 85–92. [DOI] [PubMed] [Google Scholar]
- 52.Koestenberger M, Ravekes W, Everett AD, et al. Right ventricular function in infants, children and adolescents: reference values of the tricuspid annular plane systolic excursion (TAPSE) in 640 healthy patients and calculation of z score values. J Am Soc Echocardiogr 2009; 22: 715–719. [DOI] [PubMed] [Google Scholar]
- 53.Richardson C, Amirtharaj C, Gruber D, et al. Assessing myocardial function in infants with pulmonary hypertension: the role of tissue doppler imaging and tricuspid annular plane systolic excursion. Pediatr Cardiol 2017; 38: 558–565. [DOI] [PubMed] [Google Scholar]
- 54.Mahgoub L, Kaddoura T, Kameny AR, et al. Pulmonary vein stenosis of ex-premature infants with pulmonary hypertension and bronchopulmonary dysplasia, epidemiology, and survival from a multicenter cohort. Pediatr Pulmonol 2017; 52: 1063–1070. [DOI] [PubMed]
- 55.Brooks PA, Khoo NS, Hornberger LK. Systolic and diastolic function of the fetal single left ventricle. J Am Soc Echocardiogr 2014; 27: 972–977. [DOI] [PubMed] [Google Scholar]
- 56.Risum N, Ali S, Olsen NT, et al. Variability of global left ventricular deformation analysis using vendor dependent and independent two-dimensional speckle-tracking software in adults. J Am Soc Echocardiogr 2012; 25: 1195–1203. [DOI] [PubMed] [Google Scholar]
- 57.Liu MY, Tacy T, Chin C, et al. Assessment of speckle-tracking echocardiography-derived global deformation parameters during supine exercise in children. Pediatr Cardiol 2016; 37: 519–527. [DOI] [PubMed] [Google Scholar]
- 58.Costa SP, Beaver TA, Rollor JL, et al. Quantification of the variability associated with repeat measurements of left ventricular two-dimensional global longitudinal strain in a real-world setting. J Am Soc Echocardiogr 2014; 27: 50–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, PUL878598 Supplemental Material for Diminished right ventricular function at diagnosis of pulmonary hypertension is associated with mortality in bronchopulmonary dysplasia by Gabriel Altit, Shazia Bhombal, Jeffrey Feinstein, Rachel K. Hopper and Theresa A. Tacy in Pulmonary Circulation