Abstract
Background:
Race, psychiatric history, and adverse life events have all been independently associated with postpartum depression (PPD). However, the role these play together in Black and Latina women remains inadequately studied. Therefore, we performed a case-control study of PPD, including comprehensive assessments of symptoms and biomarkers, while examining the effects of genetic ancestry.
Methods:
We recruited our sample (549 cases, 968 controls) at six weeks postpartum from obstetrical clinics in North Carolina. PPD status was determined using the MINI-plus. Psychiatric history was extracted from medical records. Participants were administered self-report instruments to assess depression (Edinburgh Postnatal Depression Scale) and adverse life events. Levels of estradiol, progesterone, brain-derived neurotrophic factor (BDNF), oxytocin, and allopregnanalone were assayed. Principal components from genotype data were used to estimate genetic ancestry and logistic regression was used to identify predictors of PPD.
Results:
This population was racially diverse (68% Black, 13% Latina, 18% European). Genetic ancestry was not a predictor of PPD. Case status was predicted by a history of major depression (p = 4.01E-14), lifetime anxiety disorder diagnosis (p = 1.25E-34), and adverse life events (p = 6.06E-06). There were no significant differences between groups in any hormones or neurosteroids.
Conclusions:
Psychiatric history and multiple exposures to adverse life events were significant predictors of PPD in a population of minority and low-income women. Genetic ancestry and hormone levels were not predictive of case status. Increased genetic vulnerability in conjunction with risk factors may predict the onset of PPD, whereas genetic ancestry does not appear predictive.
BACKGROUND
Postpartum depression (PPD) is a subtype of major depressive disorder (MDD) that affects approximately 500,000 women annually in the U.S (Wisner et al., 2010, Marmorstein et al., 2004, Flynn et al., 2004, Hamilton et al., 2015). PPD is a common complication of the perinatal period and one the greatest causes of maternal mortality and morbidity (Gavin et al., 2005a, Gaynes et al., 2005). Moreover, PPD has been associated with increased risk for infanticide (Lindahl et al., 2005), poorer maternal-infant attachment, and impaired parenting behaviors (Flynn et al., 2004, Britton, 2007, Stein et al., 2014, Junge et al., 2017).
The major known risk factor for PPD is a past history of MDD, including PPD or MDD outside of the perinatal period (O’Hara and Swain, 1996, Gaynes et al., 2005, Howard et al., 2014, Suri et al., 2017). Perinatal anxiety, parity, marital conflict, perceived lack of partner support, stressful or adverse life events, unplanned pregnancy, and adverse pregnancy/birth outcomes have also been reported as risk factors (O’Hara and McCabe, 2013, Heron et al., 2004, Norhayati et al., 2015). In addition, there are complex biological changes that occur during the perinatal period that may contribute to vulnerability. These include significant fluctuations in estrogen and progesterone during the transition from pregnancy to the postpartum period. Estrogen and progesterone steadily increase to their highest physiological levels during pregnancy and drop precipitously with parturition. However, the levels of estrogen and progesterone do not predict the occurrence of PPD (Studd, 2011, Okun et al., 2011). Similarly, no consistent abnormality has been observed in PPD in levels of other hormones and biological markers including brain-derived neurotrophic factor (BDNF)(Christian et al., 2016, Gao et al., 2016), oxytocin (Cox et al., 2015, Stuebe et al., 2013), and allopregnanolone (Romeo et al., 1998, Strohle et al., 1999, Strous et al., 2006, Uzunova et al., 1998).
The role that race/ethnicity plays as an independent risk factor for PPD is largely unknown and has been inadequately studied (Liu and Tronick, 2014, Di Florio et al., 2016). While the prevalence of PPD is approximately 10–15% of women in the general population (largely based on studies in women of European ancestry)(Gavin et al., 2005b), the rate of PPD in Black women in the U.S. has been estimated to be up to two fold greater (Yonkers et al., 2001, Liu and Tronick, 2014). For Latinas living in the U.S., the prevalence is 30–43% for new mothers, around three times higher than that of the general U.S. population (Lucero et al., 2012, Kuo et al., 2004, Zayas et al., 2003). Among studies examining the role of race/ethnicity with PPD manifestation, there are conflicting conclusions (Liu and Tronick, 2014, Di Florio et al., 2016, Liu and Tronick, 2013). This may be due to using self-reported race, which refers to a person’s physical characteristics, and ethnicity, which refers to belonging to cultural, linguistic, or societal groups (Mersha and Abebe, 2015, Fujimura and Rajagopalan, 2011). Further, individuals identified with a given ethnicity (i.e. Hispanic) may have divergent racial backgrounds. Thus, the evaluation of race and ethnicity using self-report might be more influenced by societal or environmental factors rather than biological or genetic factors. In contrast, estimations of genetic ancestry group individuals on the basis of shared genetic variation, allowing an examination of how underlying genetic structure contributes to health disparities that may otherwise be attributed to race/ethnicity. The use of genetic ancestry is a more accurate indicator of the unique genetic composition of an individual, as it takes into account the complex and heterogeneous nature of an individual’s genome. It is important to note that genetic ancestry estimation is not the same as genome-wide association studies (Cross-Disorder Group of the Psychiatric Genomics et al., 2013), which identify specific risk loci associated with case status.
While the exact causes of PPD remain unknown, a genetic contribution is supported by the emerging literature demonstrating that the heritability of PPD is greater than MDD outside of the perinatal period (Treloar et al., 1999, Viktorin et al., 2016). The high prevalence of PPD among Black and Latina women in particular warrants further examination to identify the role of psychosocial contributions in addition to genetic risk. For example, other risk factors, such as daily stressors, adverse life events, and reproductive hormones have been shown to exert their effects via epigenetic changes (Guintivano et al., 2014, Kimmel et al., 2016). Despite the knowledge that Black and Latina women carry a disproportionately higher burden of exposure to the cumulative stress associated with adversity and trauma, these racial and ethnic groups are consistently underrepresented in studies that examine the predictors and associated contributors to PPD (Stockman et al., 2015). Therefore, we sought to address this critical gap in the literature by conducting a rigorous large case-control study of PPD in Black, Latina, and European women to examine the contributions of genetic ancestry, adverse life events, psychological and biological underpinnings to the development of PPD.
METHODS
Participant Recruitment and Screening
We followed the 2010 US Census terminology for describing the self-reported “race” and “ethnicity” (Hispanic or Non-Hispanic) of subjects. We refer to the participants as Latina (“of Latino, Hispanic, or Spanish origin”), Black (or African-American), and White (i.e., European ancestry, non-Hispanic). Women who reported being Black and Hispanic (n=15) were categorized as Black for analysis.
Recruitment of postpartum women aged 17–45 years occurred from 9/2012 to 6/2016 in four outpatient obstetrical clinics (University of North Carolina Women’s Hospital, Wake County Health Department, Alamance County Health Department, East Carolina University School of Medicine) during routine six-week postpartum visits (± 1–2 weeks). Detailed recruitment procedures can be found in Supplemental Methods. All women attending these clinics completed the Edinburgh Postnatal Depression Scale (EPDS). The 10-item EPDS is a commonly used PPD screening instrument with EPDS scores consistent with a PPD diagnosis by structured clinical interview (Cox et al., 1987, Gibson et al., 2009). Women with high EPDS scores (≥11) or low EPDS scores (≤7) were invited to participate. While the strict cut-off for PPD in the literature is >12 (Wisner et al., 2002), we included scores of 11 and 12 that may be considered minor depression, which is nonetheless associated with considerable morbidity and hence is clinically relevant. All cases were then compared with MINI diagnosis. For a full list of inclusion/exclusion criteria, see Supplemental Methods. Briefly, all participants had no indication of MDD during the first or second trimesters of pregnancy, singleton pregnancy, and live term birth (≥34 weeks gestation). This study was approved by the University of North Carolina Institutional Review Board Committee for the Protection of Human Subjects. All subjects provided written informed consent and signed the Health Insurance Portability and Accountability Act release.
Subject Assessments
All participants were administered the MINI International Neuropsychiatric Interview (MINI-Plus, version 6.0), a structured clinical interview for the assessment of psychiatric disorders (Otsubo et al., 2005, Sheehan et al., 1998). Experienced and certified (κ>0.8 versus criterion ratings) psychiatric research coordinators working in each clinic administered the MINI-Plus. Cases for this study were defined by having current MDD as assessed by the MINI-Plus. Controls did not have current MDD using the MINI-Plus. All study procedures could be performed in Spanish with a native speaker.
Subjects completed a battery of self-report instruments that are widely used and have proven validity (available in English or Spanish). These included the following: Abuse and Trauma Inventory (history of sexual or physical abuse)(Meltzer-Brody et al., 2007, Leserman et al., 1995, Leserman et al., 1996, Leserman, 2005), Everyday Stressors Index (ESI) (Hall et al., 1996), and Postpartum Bonding Questionnaire (PBQ)(Brockington et al., 2006). See Supplemental Methods for further details.
Biological Sampling
The Supplemental Methods section has full protocol details. Briefly, peripheral blood was sampled and immediately processed on-site at the time of subject assessment. All plasma and serum samples were then snap-frozen and kept at −80°C until analysis. Estradiol and progesterone were measured by radioimmunoassay from serum. Serum BDNF and allopregnanolone, and plasma oxytocin were each assayed using enzyme-linked immunosorbent assays (ELISA). Genomic DNA was extracted from aliquots of whole blood using Qiagen Autopure LS, which utilized Qiagen Puregene chemistry.
SNP Genotyping and Genetic Ancestry Determination
Genotypes were assessed using the Illumina Multi-Ethnic Genome Arrays (MEGA; Illumina, San Diego, CA, USA) through the Illumina Fast Track Genotyping service. GenomeStudio software version 2.0 (Illumina, San Diego, CA, USA) was used to call genotypes from raw Illumina data. We have described our quality control procedures for SNPs elsewhere (International Schizophrenia et al., 2009, Wang et al., 2010). Briefly, SNPs are removed for bad genome mapping, missingness (>0.01), and low MAF (<0.01). Any individual with high missingness was excluded. Genetic ancestry was determined using EigenSoft smartpca (Price et al., 2006) and fastStructure (Raj et al., 2014). Smartpca was run with HapMap(International HapMap, 2005) phase 3 reference populations. Subsequent principal components were standardized using z-score transformation. FastStructure was run with a range of K=1 through K=10. Optimum model complexity was chosen using chooseK within fastStructure. Percent ancestry for each K population subgroups was estimated for each participant.
Statistical Analyses
Analyses were conducted using SAS (v9.3, Cary, NC). Using a Shapiro-Wilk test, all distributions of data that rejected the null hypothesis of normality were subsequently evaluated with non-parametric tests. Descriptive statistics are reported using percentages for categorical variables and medians with interquartile ranges (IQR) for continuous variables. Bivariate analyses were conducted using χ2 statistics for categorical variables and analysis of variance for continuous variables. Univariate and multivariate logistic regression was performed to identify association with PPD and predictors of case status. A stringent significance threshold of p < 0.001 was used to account for multiple testing.
RESULTS
Genetic Ancestry and Self-Reported Race
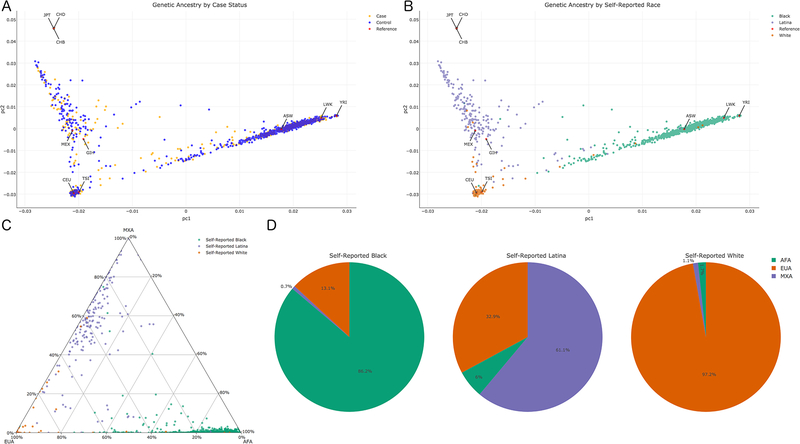
A total of 1517 women were included in these analyses (549 PPD cases, 968 controls). Participants self-identified with the following race/ethnicity: 67.4% Black, 14.4% Latina, and 18.2% White. To identify its role as a risk factor for PPD, genetic ancestry was estimated from genotype data in two ways: principal components analysis (PCA) and fastStructure. Principal components (PC) 1 and 2 are a standard metric to estimate genetic ancestry (Patterson et al., 2006, Price et al., 2006) and showed similar distribution of cases and controls (Figure 1a), indicating PC1 and PC2 were not segregating our samples by case status. Instead, PC1 and PC2 showed expected associations with Hapmap reference populations, providing validity of our genetic ancestry measures (Figure 1b). FastStructure determined three genetic groups within our cohort, which appear to represent three ancestry populations: African (AFA), European (EUA), and Mexican (MXA)(International HapMap, 2005, Joubert et al., 2010, Li et al., 2014). These estimated populations can also be observed with Hapmap references in Figure 1b. Ancestry proportions for each of these subgroups were estimated for each individual (Figure 1c) and for each self-reported racial group (Figure 1d). Self-report had varying degrees of association with genetic ancestry: self-reported Black women had mean proportions of 86.2% AFA, 13.1% EUA, and 0.7% MXA ancestry; self-reported Latinas had mean proportions of 61.1% MXA, 32.9% EUA, and 6% AFA ancestry; and self-reported White women had mean proportions of 97.2% EUA, 1.7% AFA, and 1.1% MXA ancestry (Figure 1d).
Figure 1.
Genetic Ancestry of Study Participants. A) Principal Component plot of cases (orange), controls (blue), and HapMap references (red). B) Principal Component plot of self-reported race: Black (green), Latina (purple), White (orange), and HapMap references (red). C) Ternary plot of fastStructure estimated percent ancestry for each participant. D) Average genetic ancestry composition for each self-reported racial group. Abbreviations: African ancestry in Southwest USA (ASW); Utah residents with Northern and Western European ancestry (CEU); Han Chinese in Beijing, China (CHB); Chinese in Metropolitan Denver, CO (CHD); Gujarati Indians in Houston, TX (GIH); Japanese in Tokyo, Japan (JPT); Luhya in Webuye, Kenya (LWK); Mexican ancestry in Los Angeles, CA (MEX); Toscani in Italia (TSI); Yoruba in Ibadan, Nigeria (YRI)
There was a significant difference between cases and controls in terms of self-reported race/ethnicity and genetic ancestry. With regards to self-reported race/ethnicity, cases comprised 58.8% Black, 14.7% Latina, and 26.6% White compared to controls with 72.2% Black, 14.3% Latina, and 13.5% White (; p = 8.17E-10). There was an association with PPD case status using genetic ancestry: PC1 (; p = 1.51E-02) and PC2 (; p = 2.93E-03), indicating differences in genetic ancestry between cases and controls. To identify the role genetic ancestry plays in association with various risk factors for PPD, we used PC1 and PC2 as covariates in multivariate logistical regression models for all subsequent analyses (denoted as “multivariate”) in addition to univariate logistic regression models.
Participant Demographics
Participants had a median age of 26.7 years and nearly half (46.5%) of participants reported being married. These women had a high school education (median = 12; IQR: 12 – 14), were overweight (median BMI = 30.4; IQR: 25.6 – 36.1), on government sponsored insurance (69.7%), and had given birth multiple times (only 3.6% primiparous). Demographic characteristics for cases and controls are shown in Table 1. Cases and controls did not differ in terms of demography with the exception of education (multivariate OR: 0.91; 95% CI: 0.86 – 0.96; p = 2.70E-04). The mean difference in education is less than half a year (Δ = −0.41) with cases having less. In addition, the multivariate model showed marital status was also significantly associated with case status (multivariate OR: 0.66; 95% CI: 0.52 – 0.85; p = 9.55E-04).
Table 1.
Demographic and Clinical Characteristics of Study Participants
| Univariate Model | Multivariate Model | ||||||
|---|---|---|---|---|---|---|---|
| Cases | Controls | OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| n | 549 | 968 | |||||
| Self-Reported Race/Ethnicity, % | 8.17E-10 | ||||||
| Black | 58.8 | 72.2 | |||||
| Latina | 14.7 | 14.3 | |||||
| White | 26.6 | 13.5 | |||||
| Age at enrollment, y | 1.01 (0.99 – 1.03) | 0.20 | 1.01 (0.99 – 1.03) | 0.26 | |||
| Median (IQR) | 27 (23 – 30) | 26 (22 – 30) | |||||
| Range | 17 – 45 | 17 – 43 | |||||
| Marital Status, % Married | 45.1 | 47.3 | 0.91 (0.74 – 1.13) | 0.40 | 0.66 (0.52 – 0.85) | 9.55E-04 | |
| Insurance Status, % Government | 70.6 | 69.2 | 1.07 (0.83 – 1.35) | 0.55 | 1.19 (0.92 – 1.55) | 0.18 | |
| Education, y | 12 (12 – 14) | 13 (12 – 14) | 0.92 (0.89 – 0.97) | 1.45E-03 | 0.91 (0.86 – 0.96) | 2.70E-04 | |
| BMI | 30.7 (25.8 – 36) | 30.3 (25.6 – 36.2) | 1.00 (0.99 – 1.00) | 0.47 | 1.00 (0.99 – 1.00) | 0.81 | |
| Num. prenatal visits | 11 (8 – 14) | 12 (9 – 14) | 0.98 (0.96 – 1.01) | 0.25 | 0.98 (0.95 – 1.01) | 0.14 | |
| Parity, % primiparous | 3.3 | 3.8 | 1.18 (0.66 – 2.18) | 0.59 | 1.00 (0.54 – 1.95) | 0.99 | |
| Total in household | 4 (3 – 5) | 4 (3 – 5) | 1.02 (0.95 – 1.10) | 0.62 | 1.01 (0.94 – 1.09) | 0.71 | |
| Total dependents | 2 (1 – 3) | 2 (1 – 2) | 1.09 (0.99 – 1.19) | 0.09 | 1.08 (0.98 – 1.20) | 0.11 | |
| Any breastfeeding, % | 44.1 | 47.8 | 0.86 (0.70 – 1.06) | 0.17 | 0.80 (0.64 – 1.00) | 0.05 | |
Psychiatric History
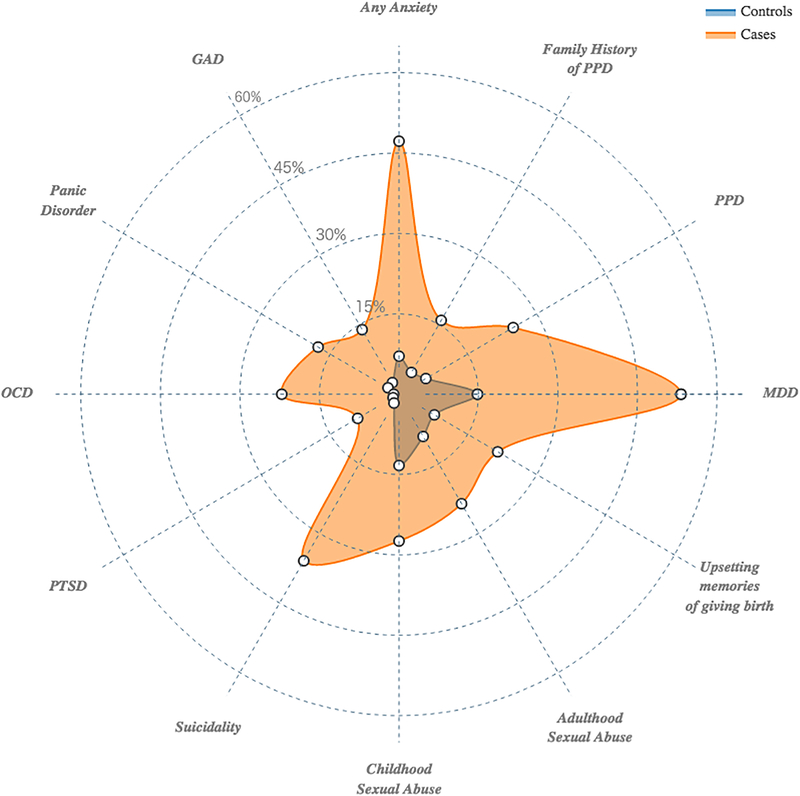
There were significantly higher rates of previous psychiatric diagnoses in cases, which includes adjustment for genetic ancestry (Figure 2 and Table 2). Cases had significantly higher EPDS total scores and higher rates of family history of PPD (15.9% v. 4.7%; multivariate OR: 3.74; 95% CI: 2.54 – 5.59; p = 1.56E-11), which is defined as mother, grandmother, sister, or aunt with lifetime PPD. Cases had significantly higher rates of previous diagnoses of MDD (53.2% v. 14.8%; multivariate OR: 6.28; 95% CI: 4.86 – 8.13; p = 3.82E-48) and PPD (24.9 v. 5.8%; multivariate OR: 5.39; 95% CI: 3.80 – 7.73; p = 7.67E-23). Additionally, 46.8% of cases were experiencing their first episode of MDD. Cases had significantly higher rates of suicidality in the month prior to assessment (35.9% v. 1.9%; multivariate OR: 26.15; 95% CI: 16.23 – 44.72; p = 2.78E-65).
Figure 2.
Radar plot representing the cumulative amount of risk factors (lifetime psychiatric disorders and adverse life events) experienced by cases compared to controls.
Table 2.
Psychiatric Characteristics and Trauma History
| Univariate Model | Multivariate Model | ||||||
|---|---|---|---|---|---|---|---|
| Cases | Controls | OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Major Depressive Disorder | |||||||
| EPDS score | 14 (12 – 17) | 2 (0 – 4) | |||||
| First depressive episode, % | 46.8 | 0.0 | |||||
| Previous MDD, % | 53.2 | 14.8 | 6.54 (5.13 – 8.37) | 7.94E-56 | 6.28 (4.86 – 8.13) | 3.82E-48 | |
| Previous PPD, % | 24.9 | 5.8 | 5.34 (3.84 – 7.49) | 2.03E-25 | 5.39 (3.80 – 7.73) | 7.67E-23 | |
| Family History PPD, % | 15.9 | 4.7 | 3.79 (2.61 – 5.58) | 1.08E-12 | 3.74 (2.54 – 5.59) | 1.56E-11 | |
| Anxiety Disorders, % | |||||||
| Lifetime anxiety, any | 47.2 | 7.1 | 11.64 (8.67 – 15.83) | 3.52E-71 | 11.13 (8.11 – 15.48) | 2.03E-59 | |
| GAD | 13.9 | 2.5 | 6.32 (4.00 – 10.33) | 4.16E-17 | 5.86 (3.59 – 9.95) | 9.20E-14 | |
| Panic Disorder | 17.6 | 2.4 | 8.61 (5.48 – 14.07) | 5.81E-25 | 8.13 (5.00 – 13.82) | 1.94E-20 | |
| OCD | 22.1 | 1.0 | 27.04 (14.78 – 55.52) | 1.09E-45 | 22.33 (12.12 – 46.08) | 5.32E-37 | |
| PTSD | 9.0 | 1.3 | 7.76 (4.23 – 15.42) | 6.26E-13 | 6.88 (3.62 – 14.19) | 2.50E-10 | |
| Suicidality, Past Month | 35.9 | 1.9 | 29.34 (18.33 – 49.92) | 7.29E-76 | 26.15 (16.23 – 44.72) | 2.78E-65 | |
| Abuse/Trauma Summary Score, median (IQR) | 2 (1 – 4) | 1 (0 – 2) | 1.37 (1.30 – 1.45) | 4.30E-31 | 1.36 (1.29 – 1.45) | 1.39E-27 | |
| Number of events, % | |||||||
| 0 | 20.5 | 40.4 | |||||
| 1 | 14.6 | 21.3 | 1.35 (0.95 – 1.93) | 0.09 | 1.32 (0.90 – 1.90) | 0.15 | |
| 2+ | 64.9 | 38.4 | 3.34 (2.55 – 4.39) | 9.30E-20 | 3.30 (2.49 – 4.39) | 9.90E-18 | |
| Child sexual abuse | 27.4 | 13.3 | 2.46 (1.86 – 3.25) | 2.51E-10 | 2.51 (1.87 – 3.37) | 9.18E-10 | |
| Adult sexual abuse | 23.6 | 9.1 | 3.07 (2.25 – 4.19) | 9.17E-13 | 2.87 (2.07 – 3.97) | 1.90E-10 | |
| Upsetting memories of giving birth | 21.5 | 7.7 | 3.49 (2.56 – 4.80) | 1.38E-15 | 3.70 (2.66 – 5.16) | 2.00E-15 | |
| Child having life-threatening illness or death | 9.2 | 7.7 | 1.21 (0.81 – 1.80) | 0.34 | 1.25 (0.82 – 1.91) | 0.30 | |
History of psychiatric disorders were prominent in PPD cases, and many followed the known comorbidities of MDD (Kessler et al., 2003, Kessler et al., 2005). Nearly half of all cases (47.2%) had a lifetime anxiety disorder diagnosis compared to 7.1% of controls (multivariate OR: 11.13; 95% CI: 8.11 – 15.48; p = 2.03E-59). This difference reflects multiple anxiety disorder subtypes: generalized anxiety disorder (13.9% vs. 2.5%; multivariate OR: 5.86; 95% CI: 3.59 – 9.95; p = 9.20E-14), panic disorder (17.6% vs. 2.4%; multivariate OR: 8.13; 95% CI: 5.00 – 13.82; p = 1.94E-20), obsessive-compulsive disorder (22.1% vs. 1.0%; multivariate OR: 22.33; 95% CI: 12.12 – 46.08; p = 5.32E-37), and post-traumatic stress disorder (9.0 vs. 1.3%; multivariate OR: 6.88; 95% CI: 3.62 – 14.19; p = 2.50E-10).
Abuse and Trauma History
Participants reported high rates of abuse and trauma with 66.6% reporting a history of at least one traumatic event. Figure 2 and Table 2 depict the rates of abuse and trauma in cases and controls. These events were prominent in cases. Cases had a greater proportion of multiple events (64.93% vs 38.35%). This is reflected in the summary score of the abuse and trauma inventory, which can be interpreted as the total number of traumatic events experienced. Cases had significantly higher summary scores (median of 2 vs. 1; multivariate OR: 1.36; 95% OR: 1.29 – 1.45 p = 1.39E-27). Those with PPD also had significantly higher scores for most items (9 out of 14) on the abuse and trauma inventory (Supplemental Table 1).
Women who experienced multiple adverse life events were three times more likely to have PPD (multivariate OR = 3.30; 95% CI: 2.49 – 4.39; p = 9.90E-18) compared to those who did not experience any. Multiple adverse life events also significantly increased the risk for previous MDD (multivariate OR = 4.06; 95% CI: 2.94 – 5.60; p = 1.40E-17), any lifetime anxiety disorder (multivariate OR = 3.68; 95% CI: 2.57 – 5.38; p = 8.60E-14), generalized anxiety disorder (multivariate OR = 2.78; 95% CI: 1.56 – 5.29; p = 3.32E-04), panic disorder (multivariate OR = 2.74; 95% CI: 1.61 – 4.90; p = 1.27E-04), obsessive-compulsive disorder (multivariate OR = 3.94; 95% CI: 2.30 – 7.23; p = 1.09E-07), and post-traumatic stress disorder (OR = 3.84; 95% CI: 1.81 – 9.51; p = 2.40E-04).
Everyday Stressors
Women with PPD had a median ESI total score more than three times higher than controls (19 vs. 6; multivariate OR: 1.15; 95% CI: 1.13 – 1.17 p = 6.83E-99). Table 3 depicts the total scores and five items from the ESI that show the largest case-control difference. The most significant stressors experienced by cases were “too many responsibilities,” “not enough time,” and “problems with marital status.” For the full list of items see Supplemental Table 2.
Table 3.
Everyday Stressors, Mother-Infant Attachment, and Levels of Steroid Hormones and Neurosteroids
| Univariate Model | Multivariate Model | ||||||
|---|---|---|---|---|---|---|---|
| Cases | Controls | OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Everyday Stressors Index | |||||||
| ESI Total Score | 19 (12 – 27) | 6 (2 – 11) | 1.14 (1.13 – 1.16) | 6.00E-103 | 1.15 (1.13 – 1.17) | 6.83E-99 | |
| Too many responsibilities | 2 (1 – 2) | 0 (0 – 1) | 3.54 (3.08 – 4.09) | 3.76E-909 | 3.62 (3.12 – 4.23) | 6.93E-83 | |
| Not enough time | 2 (1 – 3) | 1 (0 – 1) | 3.00 (2.64 – 3.42) | 8.00E-79 | 2.97 (2.60 – 2.40) | 4.29E-71 | |
| Problems with marital status | 1 (0 – 2) | 0 (0 – 1) | 2.42 (2.16 – 2.73) | 2.18E-58 | 2.49 (2.21 – 2.83) | 2.29E-55 | |
| Not enough money for necessities | 2 (1 – 3) | 0 (0 – 1) | 2.20 (1.98 – 2.44) | 6.88E-57 | 2.33 (2.09 – 2.61) | 8.36E-58 | |
| Problems getting along with family | 1 (0 – 2) | 0 (0 – 0) | 2.41 (2.11 – 2.76) | 8.96E-46 | 2.38 (2.08 – 2.74) | 1.46E-41 | |
| Postpartum Bonding Questionnaire | |||||||
| Total Score | |||||||
| Median (IQR) | 9.5 (5 – 16) | 3 (1 – 7) | 1.15 (1.13 – 1.18) | 2.88E-57 | 1.15 (1.13 – 1.18) | 3.41E-52 | |
| Pathological, % | 7.71 | 0.35 | 23.69(8.51 – 98.52) | 1.81E-14 | 21.58 (7.70 – 90.04) | 3.62E-13 | |
| Factor 1 | |||||||
| Median (IQR) | 6 (3 – 10) | 2 (0 – 5) | 1.21 (1.17 – 1.24) | 3.06E-47 | 1.23 (1.19 – 1.27) | 5.05E-46 | |
| Pathological, % | 16.33 | 4.43 | 4.21 (2.84 – 6.33) | 1.82E-13 | 4.42 (2.90 – 6.88) | 1.66E-12 | |
| Factor 2 | |||||||
| Median (IQR) | 1 (0 – 3) | 0 (0 – 0) | 1.48 (1.38 – 1.59) | 2.55E-44 | 1.47 (1.37 – 1.58) | 3.83E-39 | |
| Pathological, % | 2.64 | 0.11 | 25.45 (5.10 – 461.69) | 2.31E-06 | 24.17 (4.78 – 440.10) | 4.97E-06 | |
| Factor 3 | |||||||
| Median (IQR) | 2 (0 – 4) | 0 (0 – 2) | 1.40 (1.33 – 1.49) | 9.06E-39 | 1.38 (1.30 – 1.46) | 5.68E-33 | |
| Pathological, % | 2.42 | 0.42 | 5.84 (2.06 – 20.83) | 6.60E-04 | 4.93 (1.68 – 17.86) | 3.07E-03 | |
| Factor 4 | |||||||
| Median (IQR) | 0 (0 – 0) | 0 (0 – 0) | 1.74 (1.10 – 3.19) | 1.71EE-02 | 1.76 (1.08 – 3.47) | 2.07E-02 | |
| Pathological, % | 1.29 | 0.31 | 4.16 (1.15 – 19.37) | 2.93E-02 | 5.41 (1.28 – 36.75) | 2.06E-02 | |
| Hormones and Neurosteroids | |||||||
| Estradiol | 1.00 (1.00 – 1.00) | 0.88 | 1.00 (1.00 – 1.00) | 0.76 | |||
| n | 508 | 886 | |||||
| Concentration, pg/ml | 105.28 | 105.33 | |||||
| Progesterone | 0.99 (0.96 – 1.02) | 0.69 | 1.00 (0.97 – 1.03) | 0.99 | |||
| n | 462 | 784 | |||||
| Concentration, ng/ml | 1.46 | 1.57 | |||||
| Oxytocin | 1.02 (0.99 – 1.04) | 0.17 | 1.02 (0.99 – 1.04) | 0.16 | |||
| n | 492 | 827 | |||||
| Concentration, pg/ml | 13.30 | 11.85 | |||||
| BDNF | 1.00 (1.00 – 1.00) | 0.13 | 1.00 (1.00 – 1.00) | 0.36 | |||
| n | 509 | 891 | |||||
| Concentration, ng/ml | 44.07 | 45.17 | |||||
| Allopregnanalone | 0.99 (0.96 – 1.03) | 0.75 | 1.00 (0.96 – 1.03) | 0.84 | |||
| n | 255 | 259 | |||||
| Concentration, ng/ml | 4.5 (2.9 – 8.0) | 4.7 (3.0 – 7.4) | |||||
Mother-Infant Relationship
The PBQ assesses the degree of disordered mother-infant relationships (Table 3). Previously defined (Brockington et al., 2006) thresholds were used to determine disordered mother-infant relationships. Although infrequent, there were a significantly higher proportion of dysfunctional mother-infant relationships among cases (7.71% vs. 0.35%; multivariate OR: 21.58; 95% CI: 7.70 – 90.04; p = 3.97E-14). This pattern holds across all four factors with factor 1 having the largest proportion among cases, as well as the largest difference, compared to controls (16.33 vs. 4.43; multivariate OR: 4.42; 95% CI: 2.90 – 6.88; p = 5.11E-14). The complete list of PBQ items and responses can be viewed on Supplemental Table 3.
Reproductive Hormones and Neurosteroids
Five hormones/neurosteroids (estradiol, progesterone, oxytocin, BDNF, and ALLO) were assayed. As shown in Table 3, there were no significant differences between cases and controls for any of the five hormones. After adjustment for factors that may influence reproductive hormone or neurosteroid levels (maternal age, genetic ancestry, menstrual status, breastfeeding method, days since childbirth), logistic regression showed no association between case status and any of the hormones.
Predictors of Postpartum Depression Onset and Severity
Adverse life events, family history of PPD, marital status, and a previous history of an anxiety disorder or MDD were all significantly associated with case status in our previous logistic regression models (Tables 1 and 2). Multivariable logistic regression was performed using these independent factors along with PC1 and PC2 representing genetic ancestry. After an iterative process, the final model did not include PC1, PC2, family history of PPD, or marital status because they were not associated with case status (p > 0.001). Lifetime anxiety disorder (OR: 7.54; 95% CI: 5.39 – 10.64; p = 1.25E-34), previous MDD (OR: 3.23; 95% CI: 2.39 – 4.37; p = 4.01E-14), and adverse life events summary score (OR: 1.17; 95% CI: 1.09 – 1.25; p = 6.06E-06) were all significantly predictive of PPD status. With this model, we accounted for 23.68% of the variance associated with case status with a misclassification rate of 21.98%.
Using generalized linear modeling with EPDS total score as the outcome variable, lifetime anxiety diagnosis (; p = 2.90E-50), previous MDD (; p = 4.90E-17), and adverse life events summary score (; p = 1.72E-08), were also identified as predictors of EPDS score, a measure of PPD severity. This multivariate model is significant (; p = 3.00E-119) and provides the best fit for association with EPDS score (AIC = 8313.38) compared to any predictor alone or in other combination.
CONCLUSIONS
The purpose of this study was to examine the contribution of genetic ancestry, adverse life events, psychological and biological factors to PPD in a case-control sample of minority and low-income women, two groups that have been understudied to date (Liu and Tronick, 2014). We believe our findings represent the largest and most robustly phenotyped cohort of minority women with PPD. This study was also the first to examine genetic ancestry as it contributes to PPD risk. We found that genetic ancestry was not predictive of case status, despite the previously reported increased prevalence in Black and Latina women. Rather psychiatric history and exposure to adverse life events were predictors of PPD when examined in conjunction with genetic ancestry and other previously reported risk factors for PPD. Additionally, increased levels of perceived stress and altered mother-infant relationships were significantly associated with PPD case status.
We examined the role genetic ancestry played in association with PPD case status. While self-reported race/ethnicity may have been used, this approach does not account for individual variations in population stratification and may also be a reflection of environmental or cultural associations (Mersha and Abebe, 2015). Our study did however have high correlation between self-reported race/ethnicity and genetic ancestry (Figure 1c and 1d) for Black and White women, while Latinas were more ancestrally mixed. This discrepancy between self-report and genetic ancestry and the utility of PCA as a more accurate measure for population stratification (Price et al., 2006) was the impetus for using genetic ancestry as the main covariate in association with PPD case status. Correction for population stratification is important in any genetic epidemiological study, but even more important here due to differences in genetic ancestry (PC1 and PC2) between cases and controls. While we show that genetic ancestry does not appear to be predictive of case status, this does not rule out genetic contributors for PPD. Differences in genetic ancestry will affect the frequency of risk alleles present in certain genetic populations. Future studies will work to identify specific genetic variants that underlie PPD, just as they have for other psychiatric disorders (Sullivan, 2010).
Over half of all cases (53.2%) have a history of past MDD or PPD, which is significantly higher than previous depressive episodes observed in controls (14.8%). This finding is consistent with the current literature demonstrating that a prior history of either PPD or MDD outside of the perinatal period is associated with an increased risk for PPD onset (O’Hara and Swain, 1996, Gaynes et al., 2005). In addition, histories of any type of anxiety disorders are much more prevalent among cases. Our finding of high rates of lifetime psychiatric disorders is consistent with the literature (Postpartum Depression: Action Towards and Treatment, 2015) and may be partially explained by high genetic correlations among various psychiatric disorders (Bulik-Sullivan et al., 2015). Genetic vulnerability is believed to be a major contributor to the pathophysiology of PPD. The high heritability of PPD, along with its shared heritability with non-perinatal MDD, has only recently been examined (Viktorin et al., 2016, Treloar et al., 1999) but supports a genetic basis for PPD. This is further supported in our data by a nearly four-fold increased risk for PPD with a family history of PPD.
In this study, several types of adverse life events were assessed, with childhood (multivariate OR = 2.51) and adult sexual abuse (multivariate OR = 2.87) and life threatening attack (multivariate OR = 4.27) among the most predictive of case status (Supplemental Table 1). These findings support the existing literature that documents adverse life events increasing risk for PPD (O’Hara and McCabe, 2013, Gaillard et al., 2014, Sorbo et al., 2014, Qobadi et al., 2016). Adverse life events were prevalent among all study participants, though multiple exposures predicted PPD case status (multivariate OR = 3.30), previous MDD (multivariate OR = 4.06), any lifetime anxiety disorder (multivariate OR = 3.68), generalized anxiety disorder (multivariate OR = 2.78), panic disorder (multivariate OR = 2.74), obsessive-compulsive disorder (multivariate OR = 3.94), and post-traumatic stress disorder (multivariate OR = 3.85). The observed rates of adverse life events may be due in part to the overall lower socioeconomic status of the cohort (Brady and Matthews, 2002), which may be estimated from government sponsored insurance status and years of education (Shavers, 2007). Lower socioeconomic status may have had an effect on perceived stressors (Supplemental Table 2), which may have contributed to PPD severity and duration, but neither insurance nor education status distinguished cases from controls even when controlling for genetic ancestry. This may be the driver behind the observed increases in prevalence among Black and Latina women in the literature due to the concurrent increased prevalence of adverse life events among these populations (Roberts et al., 2011, Laskey et al., 2012, Assari and Lankarani, 2016), despite genetic ancestry not providing increased for PPD. One plausible mechanism linking adverse life events to PPD is via epigenetic modification. For example, adverse life events have been proposed to alter HPA-axis function via epigenetic changes (Kuhlman et al., 2015, Kimmel et al., 2016). Epigenetic changes may compound underlying genetic vulnerability to PPD. Additionally, these molecular marks may persist long after the adverse life events (Kimmel et al., 2016) and contribute to what is referred to as allostatic load, or cumulative somatic effects of lifetime stress (Geronimus et al., 2006, Myers et al., 2015).
Consistent with the current literature (Studd, 2011, Okun et al., 2011), hormone levels did not distinguish cases from controls. However, we did not assess changes in neurosteroid levels, which have been shown to differ between cases and controls in some previous reports (Christian et al., 2016, Gao et al., 2016, Cox et al., 2015, Stuebe et al., 2013, Romeo et al., 1998, Strohle et al., 1999). Further, women who developed PPD may have an altered response to the normal perinatal fluctuations in reproductive hormones. The transition from pregnancy to the postpartum period is characterized by a particularly large fluctuation in reproductive hormone levels during the reproductive life cycle (Schiller et al., 2016). In the current study, more than half of the cases experienced a recurrence of PPD. This group of women may be more genetically susceptible to the effects of reproductive hormones, resulting in PPD and perhaps other reproductive mood disorders (Guintivano et al., 2014).
This study has several strengths on which we capitalize in this analysis. Not only do these include the sample size and diversity of our cohort, but also, we collected data on a large number of both self-report and clinician-administered scales for each study participant, allowing for a comprehensive assessment of PPD in an understudied population. Prior studies that focused primarily on women of European ancestry may not be generalizable by failing to address differences that can arise with ancestry and culture (Di Florio et al., 2016). This study complements and extends the existing literature by providing a robust characterization of PPD in a population of women where such data was previously lacking. More importantly, this study shows that genetic ancestry does not appear to play as large a role in predicting PPD compared to exposures such as previous psychiatric episodes or abuse and trauma. Other previously identified risk factors, such as marital status or socioeconomic factors (insurance status, level of education) (O’Hara and McCabe, 2013), for PPD do not associate with case status when assessed in conjunction with these more significant predictors.
Our findings highlight the diversity of women who experience PPD and lead the way for genetic studies in the field. We have identified phenotypes that future studies should investigate to elucidate the genetic components of this disorder, just as they have for other psychiatric conditions (Cross-Disorder Group of the Psychiatric Genomics et al., 2013). Preliminary work regarding the role of epigenetic mechanisms in PPD onset has shown promise, but has been characterized by small sample sizes (Guintivano et al., 2014). Our data show that many environmental factors, such as cumulative adverse life events (which may alter epigenetic marks and increase allostatic load), contribute to PPD onset. The interaction of genetic risk with environmental (via epigenetic) changes can help explain the phenotypic heterogeneity of PPD identified in this study.
This study also has limitations that should be kept in mind when interpreting the results. First, our cohort is cross-sectional, examining PPD during the six-week postpartum visit. We are unable to speak to the duration of illness, as there was no prospective data collected, and we relied on patient report and review of obstetrical records to generate data. This approach also excluded some women during recruitment that may have exhibited PPD symptoms at an earlier time point (earlier than six weeks postpartum) and were euthymic at the six-week visit. Second, there are significantly different proportions of race and ethnicity between cases and controls. This difference was likely due to this cohort being a clinically ascertained sample with no strict matching rather than a strict epidemiological sample. We likely have ascertainment bias due to our inability to recruit matched controls for all cases. However, using logistic regression, we were able to show that this difference in proportions did not account for large variation (1.5%) in determining case status.
Clinical and Public Health Implications
This work contributes important information to our understanding of the complex factors associated with the development of PPD in a racially and ethnically diverse sample of women. Chiefly, we show that genetic ancestry was not predictive of case status despite reported rates of PPD differing among racial groups (Liu and Tronick, 2014, Yonkers et al., 2001, Zayas et al., 2002, Zayas et al., 2003, Lucero et al., 2012, Kuo et al., 2004). Rather, among all the phenotyping that was performed in this cohort, the best predictors for PPD are lifetime psychiatric disorders and history of abuse and trauma. These findings have clinical implications. The data provides a set of risk factors that together are significantly associated with case status and should be utilized in screening new mothers. These risk factors could be incorporated into the recent U.S. Preventive Services Task Force recommendations for increased depression screening during the perinatal period (Siu et al., 2016). A brief, single assessment for previous psychiatric and abuse/trauma history during the perinatal period, along with regular mood monitoring, may increase a clinicians’ ability to predict the onset of PPD.
While genetic ancestry does not play a role in our study in determination of case status, there are important ethnic and cultural differences that have been shown to have a meaningful role in the patient’s acceptance of different depression treatment options (Cooper et al., 2003). For example, Latinas and Black women may be less likely to find antidepressants an acceptable form of therapy (Cooper et al., 2003), which would mean also offering an array of psychotherapeutic treatment options. However, before any form of mental health treatment can be initiated, the patient must have appropriate access to care. There are currently significant barriers associated with access to mental health care in the U.S. that are highly influenced by race and socioeconomic status (Alegria et al., 2008) and will need to be addressed.
In sum, we believe that improving our understanding of the psychological, social, and biological predictors of PPD is vital to ultimately provide optimal care for all women that suffer with PPD. In particular, we must pay attention to the impact of cumulative adverse life events and past psychiatric history. Our findings demonstrate that the risk factors for PPD only account for 23.68% of the variation we observed in cases. Therefore, future work focused on underlying genetics and epigenetics may allow us to more fully understand the biologic triggers for postpartum depression.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Mental Health (D.R and S.M. grant 5R01MH095992–04)(J.G. grant 4T32MH093315-05)
Footnotes
Conflict of Interest: None
REFERENCES
- ALEGRIA M, CHATTERJI P, WELLS K, CAO Z, CHEN CN, TAKEUCHI D, JACKSON J & MENG XL 2008. Disparity in depression treatment among racial and ethnic minority populations in the United States. Psychiatric Services, 59, 1264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASSARI S & LANKARANI MM 2016. Stressful Life Events and Risk of Depression 25 Years Later: Race and Gender Differences. Frontiers in Public Health, 4, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADY SS & MATTHEWS KA 2002. The influence of socioeconomic status and ethnicity on adolescents’ exposure to stressful life events. Journal of Pediatric Psychology, 27, 575–83. [DOI] [PubMed] [Google Scholar]
- BRITTON JR 2007. Postpartum anxiety and breast feeding. Journal of Reproductive Medicine, 52, 689–95. [PubMed] [Google Scholar]
- BROCKINGTON IF, FRASER C & WILSON D 2006. The Postpartum Bonding Questionnaire: a validation. Archives of Women’s Mental Health, 9, 233–42. [DOI] [PubMed] [Google Scholar]
- BULIK-SULLIVAN B, FINUCANE HK, ANTTILA V, GUSEV A, DAY FR, LOH PR, REPROGEN C, PSYCHIATRIC GENOMICS C, GENETIC CONSORTIUM FOR ANOREXIA NERVOSA OF THE WELLCOME TRUST CASE CONTROL, C., DUNCAN L, PERRY JR, PATTERSON N, ROBINSON EB, DALY MJ, PRICE AL & NEALE BM 2015. An atlas of genetic correlations across human diseases and traits. Nature Genetics, 47, 1236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTIAN LM, MITCHELL AM, GILLESPIE SL & PALETTAS M 2016. Serum brain-derived neurotrophic factor (BDNF) across pregnancy and postpartum: Associations with race, depressive symptoms, and low birth weight. Psychoneuroendocrinology, 74, 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER LA, GONZALES JJ, GALLO JJ, ROST KM, MEREDITH LS, RUBENSTEIN LV, WANG NY & FORD DE 2003. The acceptability of treatment for depression among African-American, Hispanic, and white primary care patients. Medical Care, 41, 479–89. [DOI] [PubMed] [Google Scholar]
- COX EQ, STUEBE A, PEARSON B, GREWEN K, RUBINOW D & MELTZER-BRODY S 2015. Oxytocin and HPA stress axis reactivity in postpartum women. Psychoneuroendocrinology, 55, 164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COX JL, HOLDEN JM & SAGOVSKY R 1987. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry, 150, 782–6. [DOI] [PubMed] [Google Scholar]
- CROSS-DISORDER GROUP OF THE PSYCHIATRIC GENOMICS, C., LEE, RIPKE S, NEALE BM, FARAONE SV, PURCELL SM, PERLIS RH, MOWRY BJ, THAPAR A, GODDARD ME, WITTE JS, ABSHER D, AGARTZ I, AKIL H, AMIN F, ANDREASSEN OA, ANJORIN A, ANNEY R, ANTTILA V, ARKING DE, ASHERSON P, AZEVEDO MH, BACKLUND L, BADNER JA, BAILEY AJ, BANASCHEWSKI T, BARCHAS JD, BARNES MR, BARRETT TB, BASS N, BATTAGLIA A, BAUER M, BAYES M, BELLIVIER F, BERGEN SE, BERRETTINI W, BETANCUR C, BETTECKEN T, BIEDERMAN J, BINDER EB, BLACK DW, BLACKWOOD DH, BLOSS CS, BOEHNKE M, BOOMSMA DI, BREEN G, BREUER R, BRUGGEMAN R, CORMICAN P, BUCCOLA NG, BUITELAAR JK, BUNNEY WE, BUXBAUM JD, BYERLEY WF, BYRNE EM, CAESAR S, CAHN W, CANTOR RM, CASAS M, CHAKRAVARTI A, CHAMBERT K, CHOUDHURY K, CICHON S, CLONINGER CR, COLLIER DA, COOK EH, COON H, CORMAND B, CORVIN A, CORYELL WH, CRAIG DW, CRAIG IW, CROSBIE J, CUCCARO ML, CURTIS D, CZAMARA D, DATTA S, DAWSON G, DAY R, DE GEUS EJ, DEGENHARDT F, DJUROVIC S, DONOHOE GJ, DOYLE AE, DUAN J, DUDBRIDGE F, DUKETIS E, EBSTEIN RP, EDENBERG HJ, ELIA J, ENNIS S, ETAIN B, FANOUS A, FARMER AE, FERRIER IN, FLICKINGER M, FOMBONNE E, FOROUD T, FRANK J, FRANKE B, et al. 2013. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nature Genetics, 45, 984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DI FLORIO A, PUTNAM K, ALTEMUS M, APTER G, BERGINK V, BILSZTA J, BROCK R, BUIST A, DELIGIANNIDIS KM, DEVOUCHE E, EPPERSON CN, GUILLE C, KIM D, LICHTENSTEIN P, MAGNUSSON PK, MARTINEZ P, MUNK-OLSEN T, NEWPORT J, PAYNE J, PENNINX BW, O’HARA M, ROBERTSON-BLACKMORE E, ROZA SJ, SHARKEY KM, STUART S, TIEMEIER H, VIKTORIN A, SCHMIDT PJ, SULLIVAN PF, STOWE ZN, WISNER KL, JONES I, RUBINOW DR & MELTZER-BRODY S 2016. The impact of education, country, race and ethnicity on the self-report of postpartum depression using the Edinburgh Postnatal Depression Scale. Psychological Medicine, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLYNN HA, DAVIS M, MARCUS SM, CUNNINGHAM R & BLOW FC 2004. Rates of maternal depression in pediatric emergency department and relationship to child service utilization. General Hospital Psychiatry, 26, 316–22. [DOI] [PubMed] [Google Scholar]
- FUJIMURA JH & RAJAGOPALAN R 2011. Different differences: the use of ‘genetic ancestry’ versus race in biomedical human genetic research. Social Studies of Science, 41, 5–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAILLARD A, LE STRAT Y, MANDELBROT L, KEITA H & DUBERTRET C 2014. Predictors of postpartum depression: prospective study of 264 women followed during pregnancy and postpartum. Psychiatry Research, 215, 341–6. [DOI] [PubMed] [Google Scholar]
- GAO X, WANG J, YAO H, CAI Y & CHENG R 2016. Serum BDNF concentration after delivery is associated with development of postpartum depression: A 3-month follow up study. Journal of Affective Disorders, 200, 25–30. [DOI] [PubMed] [Google Scholar]
- GAVIN NI, GAYNES BN, LOHR KN, MELTZER-BRODY S, GARTLEHNER G & SWINSON T 2005a. Perinatal depression: a systematic review of prevalence and incidence. Obstetrics and Gynecology, 106, 1071–83. [DOI] [PubMed] [Google Scholar]
- GAVIN NI, GAYNES BN, LOHR KN, MELTZER-BRODY S, GARTLEHNER G & SWINSON T 2005b. Perinatal depression: a systematic review of prevalence and incidence. Obstetrics and gynecology, 106, 1071–1083. [DOI] [PubMed] [Google Scholar]
- GAYNES BN, GAVIN N, MELTZER-BRODY S, LOHR KN, SWINSON T, GARTLEHNER G, BRODY S & MILLER WC 2005. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evidence Reports/Technology Assessments, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERONIMUS AT, HICKEN M, KEENE D & BOUND J 2006. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. American Journal of Public Health, 96, 826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON J, MCKENZIE-MCHARG K, SHAKESPEARE J, PRICE J & GRAY R 2009. A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatrica Scandinavica, 119, 350–64. [DOI] [PubMed] [Google Scholar]
- GUINTIVANO J, ARAD M, GOULD TD, PAYNE JL & KAMINSKY ZA 2014. Antenatal prediction of postpartum depression with blood DNA methylation biomarkers. Molecular Psychiatry, 19, 560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL LA, KOTCH JB, BROWNE D & RAYENS MK 1996. Self-esteem as a mediator of the effects of stressors and social resources on depressive symptoms in postpartum mothers. Nursing Research, 45, 231–8. [DOI] [PubMed] [Google Scholar]
- HAMILTON BE, MARTIN JA, OSTERMAN MJ, CURTIN SC & MATTHEWS TJ 2015. Births: Final Data for 2014. National Vital Statistics Reports, 64, 1–64. [PubMed] [Google Scholar]
- HERON J, O’CONNOR TG, EVANS J, GOLDING J, GLOVER V & TEAM AS 2004. The course of anxiety and depression through pregnancy and the postpartum in a community sample. Journal of Affective Disorders, 80, 65–73. [DOI] [PubMed] [Google Scholar]
- HOWARD LM, MOLYNEAUX E, DENNIS CL, ROCHAT T, STEIN A & MILGROM J 2014. Non-psychotic mental disorders in the perinatal period. Lancet, 384, 1775–88. [DOI] [PubMed] [Google Scholar]
- INTERNATIONAL HAPMAP C 2005. A haplotype map of the human genome. Nature, 437, 1299–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INTERNATIONAL SCHIZOPHRENIA C, PURCELL SM, WRAY NR, STONE JL, VISSCHER PM, O’DONOVAN MC, SULLIVAN PF & SKLAR P 2009. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature, 460, 748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOUBERT BR, NORTH KE, WANG Y, MWAPASA V, FRANCESCHINI N, MESHNICK SR & LANGE EM 2010. Comparison of genome-wide variation between Malawians and African ancestry HapMap populations. Journal of Human Genetics, 55, 366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNGE C, GARTHUS-NIEGEL S, SLINNING K, POLTE C, SIMONSEN TB & EBERHARD-GRAN M 2017. The Impact of Perinatal Depression on Children’s Social-Emotional Development: A Longitudinal Study. Maternal and Child Health Journal, 21, 607–615. [DOI] [PubMed] [Google Scholar]
- KESSLER RC, BERGLUND P, DEMLER O, JIN R, KORETZ D, MERIKANGAS KR, RUSH AJ, WALTERS EE, WANG PS & NATIONAL COMORBIDITY SURVEY R 2003. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA, 289, 3095–105. [DOI] [PubMed] [Google Scholar]
- KESSLER RC, CHIU WT, DEMLER O, MERIKANGAS KR & WALTERS EE 2005. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62, 617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIMMEL M, CLIVE M, GISPEN F, GUINTIVANO J, BROWN T, COX O, BECKMANN MW, KORNHUBER J, FASCHING PA, OSBORNE LM, BINDER E, PAYNE JL & KAMINSKY Z 2016. Oxytocin receptor DNA methylation in postpartum depression. Psychoneuroendocrinology, 69, 150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUHLMAN KR, GEISS EG, VARGAS I & LOPEZ-DURAN NL 2015. Differential associations between childhood trauma subtypes and adolescent HPA-axis functioning. Psychoneuroendocrinology, 54, 103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUO WH, WILSON TE, HOLMAN S, FUENTES-AFFLICK E, O’SULLIVAN MJ & MINKOFF H 2004. Depressive symptoms in the immediate postpartum period among Hispanic women in three U.S. cities. Journal of Immigrant Health, 6, 145–53. [DOI] [PubMed] [Google Scholar]
- LASKEY AL, STUMP TE, PERKINS SM, ZIMET GD, SHERMAN SJ & DOWNS SM 2012. Influence of race and socioeconomic status on the diagnosis of child abuse: a randomized study. Journal of Pediatrics, 160, 1003–8 e1. [DOI] [PubMed] [Google Scholar]
- LESERMAN J 2005. Sexual abuse history: prevalence, health effects, mediators, and psychological treatment. Psychosomatic Medicine, 67, 906–15. [DOI] [PubMed] [Google Scholar]
- LESERMAN J, DROSSMAN DA & LI Z 1995. The reliability and validity of a sexual and physical abuse history questionnaire in female patients with gastrointestinal disorders. Behavioral Medicine, 21, 141–50. [DOI] [PubMed] [Google Scholar]
- LESERMAN J, DROSSMAN DA, LI Z, TOOMEY TC, NACHMAN G & GLOGAU L 1996. Sexual and physical abuse history in gastroenterology practice: how types of abuse impact health status. Psychosomatic Medicine, 58, 4–15. [DOI] [PubMed] [Google Scholar]
- LI H, GLUSMAN G, HU H, SHANKARACHARYA, CABALLERO J, HUBLEY R, WITHERSPOON D, GUTHERY SL, MAULDIN DE, JORDE LB, HOOD L, ROACH JC & HUFF CD 2014. Relationship estimation from whole-genome sequence data. PLoS Genetics, 10, e1004144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDAHL V, PEARSON JL & COLPE L 2005. Prevalence of suicidality during pregnancy and the postpartum. Archives of Women’s Mental Health, 8, 77–87. [DOI] [PubMed] [Google Scholar]
- LIU CH & TRONICK E 2013. Rates and predictors of postpartum depression by race and ethnicity: results from the 2004 to 2007 New York City PRAMS survey (Pregnancy Risk Assessment Monitoring System). Maternal and Child Health Journal, 17, 1599–610. [DOI] [PubMed] [Google Scholar]
- LIU CH & TRONICK E 2014. Prevalence and predictors of maternal postpartum depressed mood and anhedonia by race and ethnicity. Epidemiology and Psychiatric Sciences, 23, 201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCERO NB, BECKSTRAND RL, CALLISTER LC & SANCHEZ BIRKHEAD AC 2012. Prevalence of postpartum depression among Hispanic immigrant women. Journal of the American Academy of Nurse Practitioners, 24, 726–734. [DOI] [PubMed] [Google Scholar]
- MARMORSTEIN NR, MALONE SM & IACONO WG 2004. Psychiatric disorders among offspring of depressed mothers: associations with paternal psychopathology. American Journal of Psychiatry, 161, 1588–94. [DOI] [PubMed] [Google Scholar]
- MELTZER-BRODY S, LESERMAN J, ZOLNOUN D, STEEGE J, GREEN E & TEICH A 2007. Trauma and posttraumatic stress disorder in women with chronic pelvic pain. Obstetrics and Gynecology, 109, 902–8. [DOI] [PubMed] [Google Scholar]
- MERSHA TB & ABEBE T 2015. Self-reported race/ethnicity in the age of genomic research: its potential impact on understanding health disparities. Human Genomics, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYERS HF, WYATT GE, ULLMAN JB, LOEB TB, CHIN D, PRAUSE N, ZHANG M, WILLIAMS JK, SLAVICH GM & LIU H 2015. Cumulative burden of lifetime adversities: Trauma and mental health in low-SES African Americans and Latino/as. Psychological Trauma: Theory, Research, Practice and Policy, 7, 243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORHAYATI MN, HAZLINA NH, ASRENEE AR & EMILIN WM 2015. Magnitude and risk factors for postpartum symptoms: a literature review. Journal of Affective Disorders, 175, 34–52. [DOI] [PubMed] [Google Scholar]
- O’HARA MW & MCCABE JE 2013. Postpartum depression: current status and future directions. Annual review of clinical psychology, 9, 379–407. [DOI] [PubMed] [Google Scholar]
- O’HARA M & SWAIN A 1996. Rates and risk of postpartum depression—a meta -analysis. International Review of Psychiatry, 8, 37–54. [Google Scholar]
- OKUN ML, LUTHER J, PRATHER AA, PEREL JM, WISNIEWSKI S & WISNER KL 2011. Changes in sleep quality, but not hormones predict time to postpartum depression recurrence. Journal of Affective Disorders, 130, 378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTSUBO T, TANAKA K, KODA R, SHINODA J, SANO N, TANAKA S, AOYAMA H, MIMURA M & KAMIJIMA K 2005. Reliability and validity of Japanese version of the Mini-International Neuropsychiatric Interview. Psychiatry and Clinical Neurosciences, 59, 517–26. [DOI] [PubMed] [Google Scholar]
- PATTERSON N, PRICE AL & REICH D 2006. Population structure and eigenanalysis. PLoS Genetics, 2, e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTPARTUM DEPRESSION: ACTION TOWARDS, C. & TREATMENT C 2015. Heterogeneity of postpartum depression: a latent class analysis. Lancet Psychiatry, 2, 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRICE AL, PATTERSON NJ, PLENGE RM, WEINBLATT ME, SHADICK NA & REICH D 2006. Principal components analysis corrects for stratification in genome-wide association studies. Nature Genetics, 38, 904–9. [DOI] [PubMed] [Google Scholar]
- QOBADI M, COLLIER C & ZHANG L 2016. The Effect of Stressful Life Events on Postpartum Depression: Findings from the 2009–2011 Mississippi Pregnancy Risk Assessment Monitoring System. Maternal and Child Health Journal, 20, 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAJ A, STEPHENS M & PRITCHARD JK 2014. fastSTRUCTURE: variational inference of population structure in large SNP data sets. Genetics, 197, 573–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS AL, GILMAN SE, BRESLAU J, BRESLAU N & KOENEN KC 2011. Race/ethnic differences in exposure to traumatic events, development of post-traumatic stress disorder, and treatment-seeking for post-traumatic stress disorder in the United States. Psychological Medicine, 41, 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMEO E, STROHLE A, SPALLETTA G, DI MICHELE F, HERMANN B, HOLSBOER F, PASINI A & RUPPRECHT R 1998. Effects of antidepressant treatment on neuroactive steroids in major depression. American Journal of Psychiatry, 155, 910–3. [DOI] [PubMed] [Google Scholar]
- SCHILLER CE, JOHNSON SL, ABATE AC, RUBINOW DR & SCHMIDT PJ 2016. Reproductive Steroid Regulation of Mood and Behavior. Comprehensive Physiology, 6, 1135–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAVERS VL 2007. Measurement of socioeconomic status in health disparities research. Journal of the National Medical Association, 99, 1013–23. [PMC free article] [PubMed] [Google Scholar]
- SHEEHAN DV, LECRUBIER Y, SHEEHAN KH, AMORIM P, JANAVS J, WEILLER E, HERGUETA T, BAKER R & DUNBAR GC 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59 Suppl 20, 22–33;quiz 34–57. [PubMed] [Google Scholar]
- SIU AL, FORCE, U. S. P. S. T., BIBBINS-DOMINGO K, GROSSMAN DC, BAUMANN LC, DAVIDSON KW, EBELL M, GARCIA FA, GILLMAN M, HERZSTEIN J, KEMPER AR, KRIST AH, KURTH AE, OWENS DK, PHILLIPS WR, PHIPPS MG & PIGNONE MP 2016. Screening for Depression in Adults: US Preventive Services Task Force Recommendation Statement. JAMA, 315, 380–7. [DOI] [PubMed] [Google Scholar]
- SORBO MF, GRIMSTAD H, BJORNGAARD JH, LUKASSE M & SCHEI B 2014. Adult physical, sexual, and emotional abuse and postpartum depression, a population based, prospective study of 53,065 women in the Norwegian Mother and Child Cohort Study. BMC Pregnancy and Childbirth, 14, 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEIN A, PEARSON RM, GOODMAN SH, RAPA E, RAHMAN A, MCCALLUM M, HOWARD LM & PARIANTE CM 2014. Effects of perinatal mental disorders on the fetus and child. Lancet, 384, 1800–19. [DOI] [PubMed] [Google Scholar]
- STOCKMAN JK, HAYASHI H & CAMPBELL JC 2015. Intimate Partner Violence and its Health Impact on Ethnic Minority Women [corrected]. Journal of Women’s Health, 24, 62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STROHLE A, ROMEO E, HERMANN B, PASINI A, SPALLETTA G, DI MICHELE F, HOLSBOER F & RUPPRECHT R 1999. Concentrations of 3 alpha-reduced neuroactive steroids and their precursors in plasma of patients with major depression and after clinical recovery. Biological Psychiatry, 45, 274–7. [DOI] [PubMed] [Google Scholar]
- STROUS RD, MAAYAN R & WEIZMAN A 2006. The relevance of neurosteroids to clinical psychiatry: from the laboratory to the bedside. European Neuropsychopharmacology, 16, 155–69. [DOI] [PubMed] [Google Scholar]
- STUDD JW 2011. A guide to the treatment of depression in women by estrogens. Climacteric, 14, 637–42. [DOI] [PubMed] [Google Scholar]
- STUEBE AM, GREWEN K & MELTZER-BRODY S 2013. Association between maternal mood and oxytocin response to breastfeeding. Journal of Women’s Health, 22, 352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SULLIVAN PF 2010. The psychiatric GWAS consortium: big science comes to psychiatry. Neuron, 68, 182–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SURI R, STOWE ZN, COHEN LS, NEWPORT DJ, BURT VK, AQUINO-ELIAS AR, KNIGHT BT, MINTZ J & ALTSHULER LL 2017. Prospective Longitudinal Study of Predictors of Postpartum-Onset Depression in Women With a History of Major Depressive Disorder. Journal of Clinical Psychiatry. [DOI] [PubMed] [Google Scholar]
- TRELOAR SA, MARTIN NG, BUCHOLZ KK, MADDEN PA & HEATH AC 1999. Genetic influences on post-natal depressive symptoms: findings from an Australian twin sample. Psychological Medicine, 29, 645–54. [DOI] [PubMed] [Google Scholar]
- UZUNOVA V, SHELINE Y, DAVIS JM, RASMUSSON A, UZUNOV DP, COSTA E & GUIDOTTI A 1998. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proceedings of the National Academy of Sciences of the United States of America, 95, 3239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIKTORIN A, MELTZER-BRODY S, KUJA-HALKOLA R, SULLIVAN PF, LANDEN M, LICHTENSTEIN P & MAGNUSSON PK 2016. Heritability of Perinatal Depression and Genetic Overlap With Nonperinatal Depression. American Journal of Psychiatry, 173, 158–65. [DOI] [PubMed] [Google Scholar]
- WANG KS, LIU XF & ARAGAM N 2010. A genome-wide meta-analysis identifies novel loci associated with schizophrenia and bipolar disorder. Schizophrenia Research, 124, 192–9. [DOI] [PubMed] [Google Scholar]
- WISNER KL, MOSES-KOLKO EL & SIT DK 2010. Postpartum depression: a disorder in search of a definition. Archives of Women’s Mental Health, 13, 37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISNER KL, PARRY BL & PIONTEK CM 2002. Clinical practice. Postpartum depression. New England Journal of Medicine, 347, 194–9. [DOI] [PubMed] [Google Scholar]
- YONKERS KA, RAMIN SM, RUSH AJ, NAVARRETE CA, CARMODY T, MARCH D, HEARTWELL SF & LEVENO KJ 2001. Onset and persistence of postpartum depression in an inner-city maternal health clinic system. American Journal of Psychiatry, 158, 1856–63. [DOI] [PubMed] [Google Scholar]
- ZAYAS LH, CUNNINGHAM M, MCKEE MD & JANKOWSKI KR 2002. Depression and negative life events among pregnant African-American and Hispanic women. Womens Health Issues, 12, 16–22. [DOI] [PubMed] [Google Scholar]
- ZAYAS LH, JANKOWSKI KRB & MCKEE DM 2003. Prenatal and Postpartum Depression among Low-Income Dominican and Puerto Rican Women. Hispanic Journal of Behavioral Sciences, 25, 370–385. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.