Abstract
Aromatase inhibitors (AIs) represent a treatment option for post-menopausal estrogen receptor-positive (ER+) breast cancer as monotherapy, or in combination with cyclin-dependent kinase 4/6 or mTOR inhibitors. Long-term treatment with these agents leads to dose-limiting toxicity and drug resistance. Natural substances provide testable alternatives to current therapy. Tabebuia avellanedae (TA) tree is indigenous to the Amazon rainforest. The inner bark of TA represents a medicinal dietary supplement known as Taheebo. Non-fractionated aqueous extract from TA is an effective growth inhibitor in the Luminal A and triple negative breast cancer models. The quinone derivative naphthofurandione (NFD) is a major bioactive agent in TA. The present study examined the efficacy of finely ground powder from the inner bark of TA, available under the name of Taheebo-NFD-Marugoto (TNM). The ER+ MCF-7 cells stably transfected with the aromatase gene MCF-7AROM represented a model for aromatase-expressing post-menopausal breast cancer. Anchorage-independent colony formation, cell cycle progression, pro-apoptotic caspase 3/7 activity, apoptosis-specific gene expression, aromatase activity and select estradiol (E2) target gene expression represented the mechanistic end points. Treatment of MCF-7AROM cells with TNM induced a dose-dependent reduction in E2-promoted anchorage-independent colony number. Mechanistic assays on TNM-treated MCF-7AROM cells demonstrated that TNM at a concentration of 10 µg (NFD content: 2 ng), induced S-phase arrest, increased pro-apoptotic caspase 3/7 activity, increased pro-apoptotic BAX and decreased anti-apoptotic BCL-2 gene expression, and inhibited aromatase activity. Additionally, TNM treatment downregulated ESR-1 (gene for ER-α), aromatase and progesterone gene expression and reduced mRNA levels of E2 target genes pS2, GRB2 and cyclin D1. Inhibition of aromatase activity, based on the NFD content of TNM was superior to the clinical AIs Letrozole and Exemestane. These data demonstrated the potential efficacy of TNM as a nutritional alternative for current therapy of aromatase positive, post-menopausal breast cancer.
Keywords: Tabebuia avelanedae, growth inhibition, aromatase inhibition, breast cancer cells
Introduction
Clinical treatment options for post-menopausal hormone receptor positive, human epidermal growth factor receptor-2 negative breast cancer include the use of selective estrogen receptor modulators and aromatase inhibitors (1,2). Aromatase inhibitor based monotherapy is frequently combined with selective small molecule inhibitors of CDK4/6 and mTOR pathways. Long-term treatment involving single agent therapy or multi-agent combination therapy is frequently associated with acquired drug resistance predominantly due to the emergence of cancer stem cells, thereby impacting therapeutic efficacy and promoting disease progression (3-5).
Aromatase CYP19 A1 functions as a critical enzyme for peripheral and intra-tumoral estrogen bio-synthesis via conversion of adrenal androstenedione to estrone (E1) and subsequently to estradiol (E2) thereby providing growth-promoting estrogens. Pharmacological agents Letrozole (LET) and Exemestane (EXM) are selective inhibitors of aromatase (1,2). These agents exhibit acquired tumor resistance in preclinical models for aromatase-expressing Luminal A breast cancer, as well as in estrogen receptor-positive clinical breast cancer (6-12).
Naturally occurring non-toxic substances including dietary supplements and natural botanicals are widely used in complementary and alternative medicine. Natural products exhibiting effective inhibition of aromatase activity may represent potential testable alternatives to the limitations of clinical aromatase inhibitors.
Tabebuia avellanedae (TA) is a tree native to the Amazon rainforest. Drinks from the bark of the TA tree have been traditionally used by the indigenous population to address a wide variety of health issues. A non-fractionated powder from the inner bark of TA, under the name Taheebo, is available from Taheebo Japan, Co., Ltd.. An aqueous extract of Taheebo has been documented to exhibit anti-cancer activity in animal models for organ site cancers (13), as well as growth inhibitory efficacy in human carcinoma-derived cell culture models for prostate and breast cancer via multiple mechanisms (14-16). Growth inhibitory efficacy of TA extract in a model for Luminal A breast cancer subtypes is associated with the differential expression of proliferation and apoptosis-specific genes (17). In a model for triple-negative breast cancer the inhibitory efficacy of TA is attributable to inhibition of G1 to S phase transition, induction of pro-apoptotic caspase 3/7 activity and modulation of the RB pathway (18).
Recently, Taheebo Japan invented a proprietary process capable of producing TA powder that is considerably more finely ground than the original TA powder. The new product marketed by Taheebo Japan under the name of Taheebo NFD Marugoto (TNM), is expected to provide superior results to their original Taheebo due to its reduced particle size and greater aqueous solubility.
In an effort to evaluate the growth inhibitory effects and anti-aromatase activity of TNM, the experiments in the present study were designed to i) examine the growth inhibitory efficacy of TNM in a model for aromatase-expressing post-menopausal breast cancer, ii) evaluate the effects of TNM on cellular aromatase activity, and iii) identify possible molecular mechanisms responsible for the efficacy of TNM.
Materials and methods
Experimental model
The MCF-7AROM cell line represented the experimental model for the present study. These ER+/PR+/HER-2- human mammary carcinoma-derived cells, stably transfected with the aromatase gene (6,10), possess the characteristics of aromatase-expressing, post-menopausal Luminal A molecular subtype of clinical breast cancer.
Test compounds
Taheebo NFD Marugoto (TNM)
This compound is comprised of finely ground powder from the inner bark of TA tree containing the bioactive agent Naphthofurandione (NFD) was provided by Taheebo Japan Co., Ltd.. The non-fractionated aqueous stock solution was prepared following the protocol provided by the supplier. This stock solution of TNM contains 200 ng of NFD (Personal Communication: Dr Fukuda, Taheebo Japan). The stock solution was serially diluted in the culture medium to obtain final concentrations of TNM for the dose response experiments, and to identify minimally effective, half maximal (IC50) and maximally cytostatic (IC90) concentrations that were used for the mechanistic assays.
Letrozole (LET)
Stock solution of LET (molecular mass: 285 kDa, Sigma-Aldrich; Merck KGaA) was prepared in DMSO and serially diluted in the culture medium to obtain the final concentration of 1 µM (285 ng).
Exemestane (EXM)
Stock solution of EXM (molecular mass: 296 kDa, Sigma-Aldrich; Merck KGaA) was prepared in DMSO and was serially diluted in the culture medium to obtain the final concentration of 10 µM (2,960 ng).
LET and EXM represent the prototypical aromatase inhibitors. The concentrations of 1 µM LET and 10 µM EXM are comparable to the effective concentrations traditionally used in the cell culture experiments, and represent clinically achievable effective concentrations. These compounds were used as positive controls for the present experiments.
Anchorage-independent growth
For this assay the stock solution of agar was prepared by mixing DNA grade agar (Sigma-Aldrich; Merck KGaA) with an appropriate volume of 2X RPMI-1640 medium. To prepare the basement layer, this stock solution was diluted to 0.6%, dispersed in a 6-well plate and allowed to solidify overnight at 37˚C. Suspension of MCF-7AROM cells, at a density of 5x105 per ml, was prepared in RPMI-1640 medium containing 0.33% agar, and this cell suspension was overlaid on the basement layer in the presence or absence of TNM. The cultures were incubated at 37˚C in a CO2 incubator for 21 days. The anchorage-independent (AI) colonies were stained with 0.005% crystal violet and colony counts were determined at x10 magnification. The data were expressed as number of AI colonies.
Cell cycle progression
For the cell cycle analysis, 5x105 cells were seeded in T-25 flasks and treated for 24 h. post-seeding with 1, 5 and 10 µg of TNM for 48 h. The cells were harvested by trypsinization, pelleted at 500 x g, and washed twice with cold PBS (Sigma-Aldrich; Merck KGaA). The cells were then fixed with cold 70% ethanol, washed with cold PBS, and stained with propidium iodide (PI, 50 µg/ml, Sigma-Aldrich; Merck KGaA, in PBS), followed by the addition of ribonuclease (10 µg/ml, Sigma-Aldrich; Merck KGaA) and incubation for 4 h in the dark. The cell cycle analysis was performed at the Core Facility of the University of Texas Health Sciences Center (San Antonio), using optimized protocols that included i) sorting of PI-stained cells, ii) use of a 488 nm excitation filter and a 520 nm band pass filter, and iii) gating of fluorescent events on forward versus side scatter. Cell cycle progression was monitored using a Becton Dickinson FACSCAN flow cytometer (BD Biosciences), and the data were analyzed using FACS Express software (De Novo Software). The data were expressed as a percentage of cells in G1, S and G2 phases of the cell cycle.
Caspase activity
Caspase 3/7 activity in the MCF-7AROM cells was measured using caspase-Glo assay kit (Promega). Briefly, the cells treated with TNM were homogenized by sonication in homogenization buffer (25 mmol/l HEPES, pH 7.5, 5 mmol/l MgCl2, and 1 mmol/l EGTA) and protease inhibitors (all from Sigma-Aldrich; Merck KGaA). The homogenate was centrifuged at 6,500 x g at 4˚C for 15 min, and the supernatant was collected. Subsequently, 10 µl of assay reagent was added to 10 µl of supernatant and the reaction mixture was incubated at room temperature for 2 h. Resulting luminescence was measured using a Fluoroskan Luminometer (Thermo Scientific Co.). The data were expressed as relative luminescent units (RLU).
Gene expression profiling
The effect of TNM on the expressions of apoptosis regulatory genes BAX and BCL-2, E2 regulatory target genes ESR-1, AROM and PR and E2 responsive target genes pS2, GRB-2 and cyclin D1 was examined using reverse transcription quantitative PCR (RT-qPCR) assay following published protocols (19). Briefly, RNA from TNM treated and untreated control cells was isolated using the RNeasy plus kit (Qiagen Inc.), with a genomic DNA removal step as per the manufacturer's protocol. Reverse transcription (RT) was carried out using the Applied Biosystems kit (Applied Biosystems). RT-qPCR and subsequent analyses were carried out using Smart Mix PCR beads (Cepheid) with 0.25X SYBR-Green in the Cepheid Smart Cycler to detect indicated E2 target genes and the housekeeping gene β-actin transcripts, representing normalization control. Melt curve analysis was performed after each RT-qPCR cycle to ascertain PCR product specificity. PCR reactions of the indicated primer sets for E2 target genes and for β-actin primer sets gave unique melt peaks, indicative of discrete amplification products. Reaction mix (25 µl) was prepared containing MgCl2 (2 mmol/l), 12.5 µl of 2X Taq PCR Master Mix (Qiagen, Inc.), 0.25X SYBR-Green (Fisher Scientific Co.) and gene-specific primer sets (0.3 µmol/l each, obtained from the Core Facility, University of Texas). The PCR reaction was set for 40 cycles, and the data were compared after normalization with β-actin RNA levels. RT-qPCR assays were performed in duplicate and repeated at least three times. These data were expressed as ΔΔCq values for quantification of relative gene expression (20). The primer sequences for the sense (S) and anti-sense (AS) strands for ESR-1 (gene for ER-α), AROM, PR, pS2, GRB2, cyclin D1, BCL-2, BAX and β-actin genes are represented from 5' to 3' (Table I).
Table I.
Primer sets used for reverse transcription-quantitative PCR analysis.
| Gene name | Primer sequence |
|---|---|
| ESR-1 | 5'-TGTGCAATGACTATGCTTCA-3' (S) |
| 5'-GCTCTTCCTCCTGTTTTTTA-3' (AS) | |
| AROM | 5'-AGCATGCTGTACCAGCCTGT-3' (S) |
| 5'-TCATCATCACCATGGCCATGT-3' (AS) | |
| PR | 5'-ACAGAATTCATGAGCCGGTCCGGG |
| TGCAAG-3' (S) | |
| 5'ACAAGATCTCCACCCAGAGCCCG | |
| AGGTTT-3' (AS) | |
| pS2 | 5'-CATCGACGTCCCTCCAGAAGAG-3' (S) |
| 5'-CTCTGGGACTAATCACCGTGCTG-3' (AS) | |
| GRB2 | 5'-AAATGCTCAGCAAACAGCGG-3' (S) |
| 5'-TGAAGTGCTGCACATCATTTCC-3' (AS) | |
| Cyclin D1 | 5'-ACGAAGGTCTGCGCGTGTT-3' (S) |
| 5'-CCGCTGGCCATGAACTACCT-3' (AS) | |
| BCL-2 | 5'-CCTGTGGATGACTGAGTACC-3' (S) |
| 5'-GAGACAGCCAGGAGAAATCA-3' (AS) | |
| BAX | 5'-GTTTCATCCAGGATCGAGCAG-3' (S) |
| 5'-CATCTTCTTCCAGATGGTGA-3' (AS) | |
| β-actin | 5'-GACCTCTATGCCAACACAGT-3' (S) |
| 5'-AGTACTTGCGCTCAGGAGGA-3' (AS) |
Aromatase activity
To measure aromatase enzymatic activity, the 3H2O release assay was used. [3H] androstenedione represented the substrate and its conversion to E1 represented a measure for aromatase activity. The MCF-7AROM cells grown in phenol-free RPMI-1640 medium supplemented with charcoal-stripped fetal bovine serum (Sigma-Aldrich; Merck KGaA) were suspended in an assay mixture containing 0.1% bovine serum albumin, 67 mmol/l KPO4 (pH 7.4), and 2.0 µmol/l progesterone. After sonication, 100 nmol/l of [3H] androstenedione (25.3 ci/mmol, NET-962; Perkin-Elmer, Inc.) was added and the mixture was incubated for 10 min at room temperature. NADPH was then added to a final concentration of 1.2 mmol/l, followed by 37˚C incubation and the addition of an equal volume of 5% trichloroacetic acid. The supernatant was collected and extracted with an equal volume of chloroform. Dextran-coated charcoal was added to the assay mixture, which was then vortexed and centrifuged at 6,500 x g at 40˚C for 15 min. The supernatant was then added to scintillation fluid and measured in a scintillation counter (Perkin-Elmer). The data were expressed as f mole E1 formed, per mg protein, per hour (21).
Statistical analysis
The experiments for dose response using the anchorage-independent growth assay were conducted in quadruplicate. Experiments for cell cycle progression, caspase 3/7 activity, aromatase activity and gene expression profiling were conducted in triplicate. The data were expressed as mean ± SD. Statistically significant differences between the control and treatment groups were assessed by the two-sample Student's t-test. P<0.05 was considered to indicate a statistically significant difference. Additionally, data from comparisons of multiple treatment groups were analyzed using analysis of variance (ANOVA) and Dunnett's test as a post-hoc test with a threshold of α=0.05 (Microsoft Excel 2013 XLSTAT-Base software).
Results
Effect of TNM on anchorage-independent growth
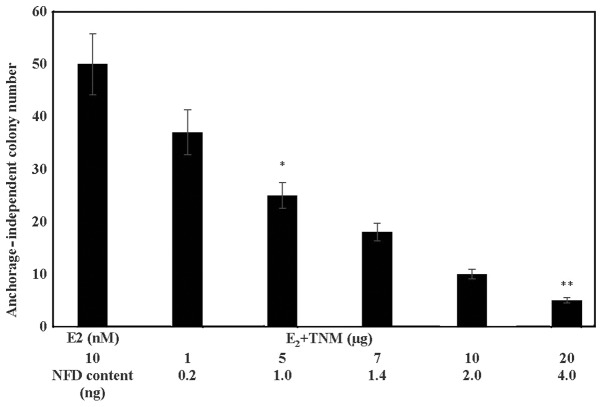
The data presented in Fig. 1 show a TNM dose-dependent reduction in the number of E2 promoted anchorage-independent colonies. These data identified the IC50 as 5 µg TNM (α=0.05), and IC90 as 20 µg TNM (α=0.05), relative to the E2-treated controls. The NFD content of TNM at these concentrations was estimated to be 1.0 and 4.0 ng, respectively.
Figure 1.
Effect of Taheebo NFD Marugoto (TNM) on anchorage-independent growth in MCF-7AROM cells. TNM treatment exhibited a dose-dependent decrease in the number of anchorage-independent colonies. TNM IC50: 5 µg* (α=0.05); IC90 20 µg** (α=0.05) vs. E2-treated control. Results were presented as mean anchorage-independent colony number ± SD, n=4 per treatment group. Data were analyzed by ANOVA and Dunnett's test. E2; 17β-estradiol, TNM, Taheebo NFD Marugoto; NFD; naphthofurandione, SD, standard deviation; ANOVA; analysis of variance.
Effect of TNM on cell cycle progression
The data presented in Table II examined the effect of TNM on the cell cycle progression of MCF-7AROM cells. TNM at the maximum cytostatic concentration of 10 µg resulted in 62.2% of cells arrested in the S phase of the cell cycle (P=0.04), relative to the untreated control. The inhibition in the G1 and G2 phases were modest and statistically non-significant.
Table II.
Inhibition of cell cycle progression by TNM in MCF-7AROM cells.
| Cell cycle phase | ||||
|---|---|---|---|---|
| Treatment | Concentration (µg) | % G1 | % S | % G2 |
| Control | - | 63.2±1.5 | 26.2±0.6a | 9.1±0.5 |
| TNM | 10 | 50.2±1.6 | 42.5±0.5b | 6.7±0.4 |
| Δ control | -20.6% | +63.2% | -26.4% | |
Results were expressed as mean ± SD, n=3 per treatment group.
a,bP=0.04. Data were analyzed by two-sample Student's t-test. TNM, Taheebo NFD Marugoto; SD, standard deviation.
Induction of pro-apoptotic activity by TNM
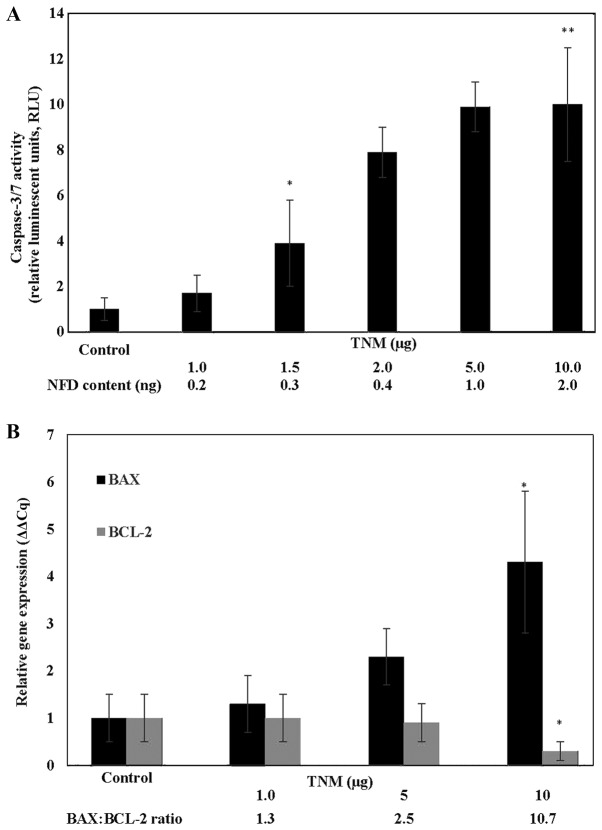
The induction of cellular apoptosis by treatment with TNM was examined by monitoring the status of caspase 3/7 activity. In response to treatment with TNM, caspase 3/7 activity exhibited a dose-dependent increase (Fig. 2A). Thus, relative to the untreated control, TNM at 1.5 µg, 5 µg and 10 µg exhibited a 2.9-fold (α=0.05), an 8.9-fold (α=0.05) and a 9-fold (α=0.05) increase in caspase 3/7 activity, respectively.
Figure 2.
(A) Induction of caspase activity by Taheebo NFD Marugoto (TNM) in MCF-7AROM cells. Treatment with TNM results in a dose-dependent increase in caspase 3/7 activity. Results were presented as RLU mean ± SD, n=3 per treatment group. Data were analyzed by ANOVA and Dunnett's test. Untreated control < 1.5 µg TNM*, untreated control <10 µg TNM** (α=0.05). TNM; Taheebo NFD Marugoto, NFD, naphthofurandione, RLU, relative luminescent units; SD, standard deviation; ANOVA, analysis of variance. (B) Modulated expression of apoptosis-specific genes in MCF-7AROM cells by Taheebo NFD Marugoto (TNM). Treatment with TNM results in upregulated expression of BAX and downregulated expression of BCL-2 genes. Results are presented as relative gene expression (ΔΔCq) mean ± SD, n=3 per treatment group. Data were analyzed by two-sample Student's t-test. BAX: *P=0.01 vs. untreated control. BCL-2: *P=0.02 vs. untreated control. TNM; Taheebo NFD Marugoto, BAX; BCL-2 associated X protein; BCL-2; B cell lymphoma-2; SD, standard deviation.
At the molecular level, TNM treatment resulted in a dose-dependent increase in pro-apoptotic BAX and a reciprocal decrease in anti-apoptotic BCL-2 gene expression. Thus, treatment with 10 µg TNM resulted in a 3.3-fold increase (P=0.01) in the pro-apoptotic BAX and a 70% decrease (P=0.02) in the anti-apoptotic BCL-2 expression, relative to the respective untreated controls. This reciprocal modulation in the expression of apoptosis-specific genes resulted in an increased BAX: BCL-2 ratio (Fig. 2B).
Inhibition of aromatase activity by TNM
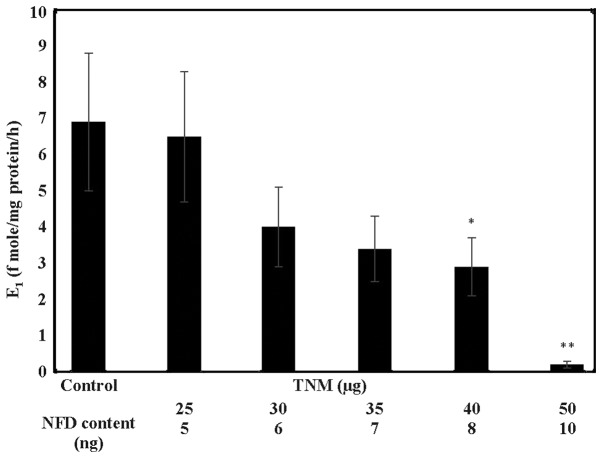
In response to treatment with TNM, MCF-7AROM cells exhibited dose-dependent inhibition in aromatase activity as measured by the extent of conversion of androstenedione to E1. Thus, TNM treatment at 30, 40 and 50 µg resulted in a 42% (α=0.05), a 57.9% (α=0.05) and a 97.1% (α=0.05) decrease in the aromatase activity, relative to the untreated control (Fig. 3).
Figure 3.
Inhibition of aromatase activity by Taheebo NFD Marugoto (TNM) in MCF-7AROM cells. Treatment with TNM results in a dose-dependent inhibition of aromatase activity. Results were presented as E1 formed (f mole/mg protein/h) mean ± SD, n=3 per treatment group. Untreated control >40 µg TNM* (α=0.05), untreated control >50 µg TNM** (α=0.05). Data were analyzed by ANOVA and Dunnett's test. TNM, Taheebo NFD Matugoto; NFD, naphthofurandione; E1; estrone, ANOVA, analysis of variance; SD, standard deviation.
Comparative inhibition of aromatase activity by TNM, LET and EXM
The comparative efficacy of TNM and LET for inhibition of aromatase activity revealed that the extent of inhibition of 63.8% (P=0.04) by 40 µg TNM (NFD content 8 ng) was essentially similar to 62.3% inhibition (P=0.04) induced by 285 ng (1 µM) of LET, relative to the untreated control (Table III).
Table III.
Comparative efficacy for aromatase inhibition by TNM and LET in MCF-7AROM cells.
| Treatment | Concentration | Aromatase activity (E1 fmole/mg protein/h) | Inhibition (% control) |
|---|---|---|---|
| Control | - | 6.9±0.4a | - |
| TNM (40 µg) | 8 ng NFD | 2.5±0.1b | 63.8 |
| LET (1 µM) | 285 ng | 2.6±0.1b | 62.3 |
Results were expressed as mean ± SD, n=3 per treatment group.
a,bP=0.04. Data were analyzed by the two-sample Student's t-test. E1, estrone; TNM, Taheebo NFD Marugoto; LET, Letrozole; NFD, naphthofurandione; SD, standard deviation.
The comparative efficacy of TNM and EXM for inhibition of aromatase activity is presented in Table IV. These data revealed that the extent of inhibition of 98.5% (P=0.01) by 100 µg TNM (NFD content 20 ng) was essentially similar to 97.1% inhibition (P=0.01) induced by 2,960 ng (10 µM) of EXM, relative to the untreated control.
Table IV.
Comparative efficacy for aromatase inhibition by TNM and EXM in MCF-7AROM cells.
| Treatment | Concentration | Aromatase Activity (E1 fmole/mg protein/h) | Inhibition (% control) |
|---|---|---|---|
| Control | - | 6.9±0.30a | - |
| TNM (100 µg) | 20 ng NFD | 0.1±0.08b | 98.5 |
| EXM (10 µM) | 2,960 ng | 0.2±0.10b | 97.1 |
Results were expressed as mean ±SD, n=3 per treatment group.
a,bP=0.01. Data were analyzed by two-sample Student's t-test. E1, estrone; TNM, Taheebo NFD Marugoto; EXM, exemestane; NFD, naphthafurandione; SD, standard deviation.
Inhibition of E2 regulated target gene expression by TNM
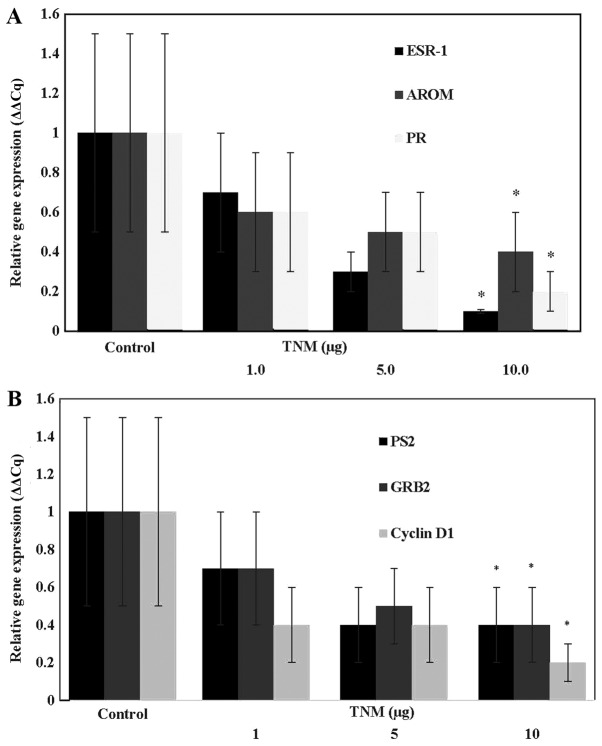
The data obtained from the effect of TNM on the expression of ESR-1 (gene for ER-α), AROM and PR genes are presented in Fig. 4A. The extent of inhibition at 10 µg of TNM for ESR-1 was 90% (P=0.01), for AROM it was 61% (p=0.04) and for PR it was 61% (P=0.04). Thus, treatment with TNM resulted in substantial downregulated expressions of select genes that are regulated by E2.
Figure 4.
(A) Inhibition of estrogen regulated gene expression by Taheebo NFD Marugoto (TNM) in MCF-7AROM cells. Treatment with TNM results in down-regulated expression of ESR-1, AROM and PR genes. Results were expressed as relative gene expression mean (ΔΔCq) ± SD, n=3 per treatment group. ESR-1: Untreated control >10 µg TNM* (α=0.05), AROM: untreated control >10 µg TNM* (α=0.05), PR: untreated control >10 µg TNM* (α=0.05). Data were analyzed by ANOVA and Dunnett's test. TNM, Taheebo NFD Marugoto; ESR-1, gene for ER-α. (B) Inhibition of estrogen responsive gene expression by Taheebo NFD Marugoto (TNM) in MCF-7AROM cells. Treatment with TNM results in downregulated expression of PS2, GRB2 and cyclin D1 gene. Results were presented as relative gene expression (ΔΔCq) mean ± SD, n=3 per treatment group. PS2: Untreated control >10 µg TNM* (α=0.05), GRB2: Untreated control >10 µg TNM*, cyclin D1: Untreated control > 10 µg TNM*. Data were analyzed by ANOVA and Dunnett's test. ESR-1: gene for estrogen receptor-α, AROM, aromatase; PR; progesterone, SD, standard deviation; TNM, Taheebo NFD Marugoto; PS2, estrogenresponsive gene; GRB2, growth factor receptor binding protein 2; ANOVA, analysis of variance; SD, standard deviation.
Inhibition of E2 responsive target gene expression by TNM
The data shown in Fig. 4B examined the effect of TNM on the expression of select E2 responsive genes. The extent of inhibition at 10 µg of TNM for pS2 was 62% (P=0.04), for GRB2 it was 61% (P=0.04), and for cyclin D1 it was 82% (P=0.01). Thus, TNM treatment resulted in a substantial downregulation of E2 responsive gene expressions.
Discussion
Metastatic breast cancer is a leading cause of cancer related mortality for women in the USA (22). The ER-α positive, aromatase-expressing Luminal A subtype of post-menopausal breast cancer responds to aromatase inhibitors (6,10). However, long-term therapy is frequently associated with acquired resistance that negatively impacts efficacy and facilitates disease progression.
In addition to the present MCF-7AROM model, other cellular models stably transfected with the aromatase gene have been developed from human mammary carcinoma derived MCF-7 and T47D cell lines. These models have been utilized to examine the effects of aromatase inhibitors and investigate the mechanisms responsible for resistance to AI-based endocrine therapy. For example, MCF-7AROM cells have exhibited resistance to Fluvestrant and cross resistance to Letrozole, Anastrazole, Exemestane (9) and T47DAROM cells have exhibited resistance to Letrozole and sensitivity to anti-progestin (23). Additionally, AI resistance has been documented to involve the upregulated expression of HER-2(10). Collectively, these three cellular models offer valuable experimental approaches to identify efficacious aromatase inhibitors and also to investigate molecular mechanisms responsible for acquired resistance to aromatase nihibitors.
Non-toxic natural nutritional products may represent testable alternatives for endocrine therapy-resistant post-menopausal breast cancer (17,18, 28-31), and thereby, may provide treatment options against the clinical limitations of current aromatase inhibitor-based therapy (2,3,6,9,10). Two species belonging to the Tabebuia genus have documented anti-cancer activity. The anti-cancer effects of T. avellanedae and T. chrisantha are documented in preclinical xeno-transplant models (13) and in mice carrying Ehrilch ascites tumor (24). The use of TA in traditional medicine is not well documented. However, the effect of an aqueous extract of TA has been examined on the status of quality of life in patients with multiple organ site cancers that are at advanced metastatic stages (25). Additionally, the effects of the TA quinone NFD have been documented on head and neck cancer and on a patient with lung metastasis (26).
Experiments in the present study were designed to examine the growth inhibitory efficacy of a non-fractionated aqueous extract from TNM on a cellular model for aromatase-expressing Luminal A subtype of post-menopausal breast cancer.
The ER-α-positive human mammary carcinoma-derived MCF-7 cell line is dependent on E2 for anchorage-independent growth in vitro and tumor development in vivo. These in vitro and in vivo end points exhibit a strong positive correlation (27). Thus, the in vitro endpoint that determines the number of AI colonies is a surrogate end point biomarker for cancer risk. E2 promoted anchorage-independent colony number was reduced in response to the treatment with TNM in MCF-7AROM cells. In this context, it is noteworthy that several mechanistically distinct nutritional herbs have demonstrated inhibitory effects on anchorage-independent colony formation in MCF-7 cells (28-31), suggesting their potential efficacy for the reduction of breast cancer risk.
At the mechanistic levels, the anti-proliferative effects of TNM were evidenced by induction of S-phase arrest and resultant inhibition of cell cycle progression. The pro-apoptotic effects of TNM were evidenced by the dose-dependent induction of caspase 3/7 activity. The pro-apoptotic BAX and anti-apoptotic BCL-2 genes are critical for the mitochondrial intrinsic apoptosis (32). TNM treatment resulted in a dose-dependent increase in the expression of pro-apoptotic BAX gene and decrease in the expression of anti-apoptotic BCL-2 gene. These data exhibiting reciprocal modulation of mRNA expression for these genes provide mechanistic leads that support the pro-apoptotic effect of TNM in the present MCF-7AROM model. In the E2-mediated signal transduction pathways pS2, GRB2 and cyclin D1 represent classical E2 responsive target genes (11,12). Thus, collectively, the inhibitory effects of TNM on E2 regulated ESR-1 (gene for ER-α), PR and AROM genes and on E2 responsive pS2, GRB2 and cyclin D1 genes provide mechanistic leads relevant to possible molecular targets for the efficacy of TNM via the ER signal transduction pathways.
Long-term treatment with the pharmacological inhibitors of aromatase is frequently associated with systemic toxicity and acquired drug resistance (2,3,6,10) leading to the emergence of therapy resistant stem cells. By contrast, naturally occurring TNM may exhibit lower systemic toxicity and may lack drug resistance that is induced by pharmacological inhibitors of aromatase activity. Inhibitory effect of TNM on aromatase activity is evidenced by its ability to reduce the conversion of androstenedione to E1 in MCF-7AROM cells in a dose-dependent manner. The specificity of this effect is indicated by the induction of aromatase inhibition by two selective pharmacological aromatase inhibitors LET and EXM. In this context, it is noteworthy that TNM at a concentration of 4 µg (NFD content: 8 ng) exhibits aromatase inhibition that is essentially comparable to 285 ng of LET. Therefore, based on the NFD content of TNM, it requires 35.6-fold higher concentration of LET which is indicative of a 35.6-fold greater potency of TNM. Additionally, TNM at a concentration of 100 µg (NFD content: 20 ng) exhibits aromatase inhibition, which is essentially comparable to 2,960 ng of EXM. Therefore, based on the NFD content of TNM, it requires 148-fold higher concentration of EXM which is indicative of a 148-fold greater potency of TNM. It is also noteworthy that extracts from natural products such as resveratrol and ellagitannin derivatives have documented anti-aromatase activity (33,34). In addition, natural products such as sulforaphane, benzyl iso-thiocyanate, a vitamin A derivative all-trans retinoic acid and a terpenoid carnosol have been documented to target cancer stem cells (35-37).
The present data identified several potential mechanistic leads responsible for TNM-mediated anti-aromatase activity. For example, the clinical aromatase inhibitor LET binds the active site of the aromatase enzyme, and EXM functions as a substrate analogue for the enzyme (1). TA downregulates ESR-1 and E2 metabolizing enzymes CYP1A1 and CYP1B1(17), and ER-α, and as its ligand E2 induces aromatase expression (38). Thus, TNM-mediated inhibition of the ER-α gene ESR1 and of AROM raise the possibility that TNM may be effective via one or more of the mechanisms discussed above.
At present, direct evidence to support efficacy of the active principle in TNM is equivocal. However, published evidence has demonstrated anti-cancer activity of NFD in animal models (13). In addition to NFD, another quinone β-lapachone (β-LAP) has been documented to be present in trace amounts in the non-fractionated aqueous extracts of TA and TNM. This minor constituent, at higher pharmacological concentrations, exhibits anti-cancer activity in preclinical xeno-transplant models of epithelial organ site cancers via distinct molecular mechanisms (15,16,39-41). However, it is important to recognize that at the low concentrations of TA or TNM used, the levels of β-LAP remain essentially undetectable (42).
The data from the present study have identified several potential mechanistic leads for the inhibitory efficacy of TNM in cellular models of human cancer. These leads include modulation of the RB signaling pathway, inhibition of cyclin-dependent kinase, inhibition of Cdc dual phosphatase, inhibition of cyclo-oxygenase-2, inhibition of telomerase and downregulated global expression of several genes that are involved in cell cycle progression, cellular apoptosis and hormone metabolism (14-18). Thus, collectively, these lines of evidence provide potential leads that the efficacy of TNM in the present model is likely due to its NFD content.
In conclusion, the data presented in this study, provide evidence for the growth inhibitory activity of TNM in a cellular model for aromatase-expressing post-menopausal breast cancer. More importantly, these data identify mechanistic leads as a proof of concept that TNM may be a superior naturally occurring substitute for clinical aromatase inhibitors. Overall, the present study validates an experimental approach for mechanistic evaluation of additional natural products that exhibit anti-aromatase activity. In this context, strong mechanistic leads for the efficacy of TNM in the present cell culture study identify future experimental approaches that are designed to provide clinically translatable therapeutic evidence for the use of TNM. These approaches may include experiments on the MCF-7AROM transplant model to examine the effects of TNM on tumor progression and on molecular characteristics of tumors relevant to altered E2-mediated signal transduction pathways, Additionally, for clinical translatability of the present preclinical study, experiments to obtain clinical data on absorption, distribution, metabolism and excretion (ADME) of TNM, and on data for human safety, tolerability and efficacy of TNM may provide valuable information..
Acknowledgements
Not applicable.
Funding
Major funding support for this research was provided by Taheebo Japan, Co., Ltd. (Osaka, Japan), and by the philanthropic contributions to the American Foundation for Chinese Medicine through Randall and Barbara Smith Foundation and the Sophie Stenbeck Family Foundation.
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
NT conceived the study design, formulated the experimental protocols, and prepared the manuscript. HBN conducted all the experiments, organized and analyzed the data and participated in the preparation of the manuscript. GYCW selected the test agent and contributed to data interpretation and preparation of the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Johnston SRD, Dowsett M. Aromatase inhibitors for breast cancer: Lessons from the laboratory. Nat Rev Cancer. 2003;3:821–831. doi: 10.1038/nrc1211. [DOI] [PubMed] [Google Scholar]
- 2.Ali S, Coombes RC. Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer. 2002;2:101–112. doi: 10.1038/nrc721. [DOI] [PubMed] [Google Scholar]
- 3.Ma CX, Reinert T, Chmielewska I, Ellis MJ. Mechanisms of aromatase inhibitor resistance. Nat Rev Cancer. 2015;15:261–275. doi: 10.1038/nrc3920. [DOI] [PubMed] [Google Scholar]
- 4.Ma CK, Gao F, Luo J, Northfeld DW, Goetz M, Forero A, Hoog J, Naughton M, Ademuyiwa F, Suresh R, et al. NeoPalAna: Neoadjuvant Palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and Anastrozole for clinical stage 2 or 3 estrogen receptor- positive breast cancer. Clin Cancer Res. 2017;23:4055–4065. doi: 10.1158/1078-0432.CCR-16-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taglieri L, De Iuliis F, Giuffrida A, Giantulli S, Silvestri I, Scarpa S. Resistance to the mTOR inhibitor everolimus is reversed by the downregulation of survivin in breast cancer cells. Oncol Lett. 2017;14:3832–3838. doi: 10.3892/ol.2017.6597. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Brodie A, Jelovac D, Long BJ. Predictions from a preclinical model: Studies of aromatase inhibitors and antiestrogens. Clin Cancer Res. 2003;9:455S–459S. [PubMed] [Google Scholar]
- 7.Boér K. Impact of palbociclib combinations on treatment of advanced estrogen receptor-positive/human epidermal growth factor 2-negative breast cancer. OncoTargets Ther. 2016;9:6119–6125. doi: 10.2147/OTT.S77033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alves CL, Elias D, Lyng M, Bak M, Kirkegaard T, Lykkesfeldt AE, Ditzel HJ. High CDK6 protects cells from Fulvestrant-mediated apoptosis and is predictor of resistance to Fulvestrant in estrogen receptor-positive metastatic breast cancer. Clin Cancer Res. 2016;22:5514–5526. doi: 10.1158/1078-0432.CCR-15-1984. [DOI] [PubMed] [Google Scholar]
- 9.Hole S, Pedersen AM, Hansen SK, Lundqvist J, Yde CW, Lykkesfeldt AE. New cell culture model for aromatase inhibitor-resistant breast cancer shows sensitivity to fulvestrant treatment and cross-resistance between letrozole and exemestane. Int J Oncol. 2015;46:1481–1490. doi: 10.3892/ijo.2015.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabnis G, Brodie A. Understanding resistance to endocrine agents: Molecular mechanisms and potential for intervention. Clin Breast Cancer. 2010;10:E6–E15. doi: 10.3816/CBC.2010.n.014. [DOI] [PubMed] [Google Scholar]
- 11.Moy B, Goss PE. Estrogen receptor pathway: Resistance to endocrine therapy and new therapeutic approaches. Clin Cancer Res. 2006;12:4790–4793. doi: 10.1158/1078-0432.CCR-06-1535. [DOI] [PubMed] [Google Scholar]
- 12.O'Hara J, Vareslija D, McBryan J, Bane F, Tibbitts P, Byrne C, Conroy RM, Hao Y, Gaora PO, Hill ADK, et al. AIB1:ERα transcriptional activity is selectively enhanced in aromatase inhibitor-resistant breast cancer cells. Clin Cancer Res. 2012;18:3305–3315. doi: 10.1158/1078-0432.CCR-11-3300. [DOI] [PubMed] [Google Scholar]
- 13.Ebina T. Anti-tumor effect of hot water extract of Taheebo tea: Comparison with other biological preparations. Biotherapy. 2002;16:321–327. [Google Scholar]
- 14.Brisson M, Nguyen T, Vogt A, Yalowich J, Giorgianni A, Tobi D, Bahar I, Stephenson CR, Wipf P, Lazo JS. Discovery and characterization of novel small molecule inhibitors of human Cdc25B dual specificity phosphatase. Mol Pharmacol. 2004;66:824–833. doi: 10.1124/mol.104.001784. [DOI] [PubMed] [Google Scholar]
- 15.Choi YH, Kang HS, Yoo MA. Suppression of human prostate cancer cell growth by β-lapachone via down-regulation of pRB phosphorylation and induction of Cdk inhibitor p21 (WAF1/CIP1) J Biochem Mol Biol. 2003;36:223–229. doi: 10.5483/bmbrep.2003.36.2.223. [DOI] [PubMed] [Google Scholar]
- 16.Lee JH, Cheong J, Park YM, Choi YH. Down-regulation of cyclooxygenase-2 and telomerase activity by β-lapachone in human prostate carcinoma cells. Pharmacol Res. 2005;51:553–560. doi: 10.1016/j.phrs.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee B, Telang N, Wong GYC. Growth inhibition of estrogen receptor positive human breast cancer cells by Taheebo from the inner bark of Tabebuia avellanedae tree. Int J Mol Med. 2009;24:253–260. doi: 10.3892/ijmm_00000228. [DOI] [PubMed] [Google Scholar]
- 18.Telang NT, Nair HB, Wong GYC. Efficacy of Tabebuia avellanedae extract on a cell culture model for triple negative breast cancer. Cancer Res. 2014;74 (Suppl) SABCS, P5-14-02. [Google Scholar]
- 19.Liu YG, Tekmal RR, Binkley PA, Nair HB, Schenken RS, Kirma NB. Induction of endometrial epithelial cell invasion and c-fms expression by transforming growth factor β. Mol Hum Reprod. 2009;15:665–673. doi: 10.1093/molehr/gap043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Nair HB, Luthra R, Kirma N, Liu YG, Flowers L, Evans D, Tekmal RR. Induction of aromatase expression in cervical carcinomas: Effects of endogenous estrogen on cervical cancer cell proliferation. Cancer Res. 2005;65:11164–11173. doi: 10.1158/0008-5472.CAN-05-1087. [DOI] [PubMed] [Google Scholar]
- 22. American Cancer Society: Cancer facts and figures. American Cancer Society, Atlanta, GA, 2018. [Google Scholar]
- 23.Gupta A, Mehta R, Alimirah F, Peng X, Murillo G, Wiehle R, Mehta RG. Efficacy and mechanism of action of Proellex, an antiprogestin in aromatase overexpressing and Letrozole resistant T47D breast cancer cells. J Steroid Biochem Mol Biol. 2013;133:30–42. doi: 10.1016/j.jsbmb.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Panda SP, Panigrahy UP, Panda S, Jena BR. Stem extract of Tabebuia chrysantha induces apoptosis by targeting sEGFR in Ehrlich Ascites Carcinoma. J Ethnopharmacol. 2019;235:219–226. doi: 10.1016/j.jep.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 25.Bacowsky H. Investigations on effects of Taheebo extract on various blood parameters and quality of life in 12 patients suffering from different form of cancer in different stages. J New Rem Clin. 2006;55:48–55. [Google Scholar]
- 26.Hirata S. An examination of supplement dose dependence and safety in integrative medicine for cancer: Based on the experience of Tabebuia avellanedae, a South American medicinal plant commonly known as Taheebo. Int J Integr Med. 2010;2:140–144. [Google Scholar]
- 27.Lippman ME, Osborne CK, Knazek R, Young N. In vitro model systems for the study of hormone-dependent human breast cancer. N Engl J Med. 1977;296:154–159. doi: 10.1056/NEJM197701202960307. [DOI] [PubMed] [Google Scholar]
- 28.Telang NT, Li G, Sepkovic DW, Bradlow HL, Wong GYC. Anti-proliferative effects of Chinese herb Cornus officinalis in a cell culture model for estrogen receptor-positive clinical breast cancer. Mol Med Rep. 2012;5:22–28. doi: 10.3892/mmr.2011.617. [DOI] [PubMed] [Google Scholar]
- 29.Telang N, Li G, Sepkovic D, Bradlow HL, Wong GYC. Comparative efficacy of extracts from Lycium barbarum bark and fruit on estrogen receptor positive human mammary carcinoma MCF-7 cells. Nutr Cancer. 2014;66:278–284. doi: 10.1080/01635581.2014.864776. [DOI] [PubMed] [Google Scholar]
- 30.Telang N, Li G, Katdare M, Sepkovic D, Bradlow L, Wong G. Inhibitory effects of Chinese nutritional herbs in isogenic breast carcinoma cells with modulated estrogen receptor function. Oncol Lett. 2016;12:3949–3957. doi: 10.3892/ol.2016.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Telang NT, Li G, Katdare M, Sepkovic DW, Bradlow HL, Wong GYC. The nutritional herb Epimedium grandiflorum inhibits the growth in a model for the Luminal A molecular subtype of breast cancer. Oncol Lett. 2017;13:2477–2482. doi: 10.3892/ol.2017.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tait SW, Green DR. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 33.Chottanapund S, Van Duursen MB, Navasumrit P, Hunsonti P, Timtavorn S, Ruchirawat M, Van den Berg M. Anti-aromatase effect of resveratrol and melatonin on hormonal positive breast cancer cells co-cultured with breast adipose fibroblasts. Toxicol In Vitro. 2014;28:1215–1221. doi: 10.1016/j.tiv.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Adams LS, Zhang Y, Seeram NP, Heber D, Chen S. Pomegranate ellagitannin-derived compounds exhibit antiproliferative and antiaromatase activity in breast cancer cells in vitro. Cancer Prev Res (Phila) 2010;3:108–113. doi: 10.1158/1940-6207.CAPR-08-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castro NP, Rangel MC, Merchant AS, MacKinnon G, Cuttitta F, Salomon DS, Kim YS. Sulforphane suppresses the growth of triple negative breast cancer stem-like cells in vitro and in vivo. Cancer Prev Res (Phila) 2019;12:147–158. doi: 10.1158/1940-6207.CAPR-18-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim SH, Singh SV. Role of Krüppel-like factor4-p21CIP1 axis in breast cancer stem-like cell inhibition by benzyl isothiocyanate. Cancer Prev Res (Phila) 2019;12:125–134. doi: 10.1158/1940-6207.CAPR-18-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Telang N. Targeting drug resistant stem cells in a human epidermal growth factor receptor-2-enriched breast cancer model. World Acad. Sci. J. 2019;1:86–91. doi: 10.3892/wasj.2019.9. [DOI] [Google Scholar]
- 38.Kinoshita Y, Chen S. Induction of aromatase (CYP19) expression in breast cancer cells through a nongenomic action of estrogen receptor α. Cancer Res. 2003;63:3546–3555. [PubMed] [Google Scholar]
- 39.Woo HJ, Choi YH. Growth inhibition of A549 human lung carcinoma cells by β-lapachone through induction of apoptosis and inhibition of telomerase activity. Int J Oncol. 2005;26:1017–1023. doi: 10.3892/ijo.26.4.1017. [DOI] [PubMed] [Google Scholar]
- 40.Jeon YJ, Bang W, Shin JC, Park SM, Cho JJ, Choi YH, Seo KS, Choi NJ, Shim JH, Chae JI. Downregulation of Sp1 is involved in β-lapachone-induced cell cycle arrest and apoptosis in oral squamous cell carcinoma. Int J Oncol. 2015;46:2606–2612. doi: 10.3892/ijo.2015.2972. [DOI] [PubMed] [Google Scholar]
- 41.Bang W, Jeon YJ, Cho JH, Lee RH, Park SM, Shin JC, Choi NJ, Choi YH, Cho JJ, Seo JM, et al. Shim JH ad Chae JI: β-lapachone suppresses the proliferation of human malignant melanoma by targeting specificity protein 1. Oncol Rep. 2016;35:1109–1116. doi: 10.3892/or.2015.4439. [DOI] [PubMed] [Google Scholar]
- 42.Queiroz ML, Valadares MC, Torello CO, Ramos AL, Oliveira AB, Rocha FD, Arruda VA, Accorci WR. Comparative studies of the effects of Tabebuia avellanedae barh extract and β-lapachone on hematopoietic response of tumor bearing mice. J Ethnopharmacol. 2008;117:228–235. doi: 10.1016/j.jep.2008.01.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.