Abstract
Background
Cardiovascular disease (CVD) is the leading cause of death worldwide. Some studies have suggested anassociation between serum uric acid levels and cardiovascular mortality; however, the results have not been summarized in a meta-analysis.
Methods
A comprehensive search of all related studies until April 2018was performed in MEDLINE/PubMed and Scopus databases DerSimonianand Laird random-effects models were used to combine hazard ratios (HRs) with 95% confidence intervals (CIs). Dose-response analysis was also carried out.
Results
Thirty-two studies containing forty-four arms with 1,134,073 participants reported association between uric acid and risk of CVD mortality were included in our analysis. Pooled results showed a significant positive association between uric acid levels and risk of CVD mortality (HR 1.45, 95% CI 1.33–1.58, I2 = 79%). Sub-group analysis showed this relationshipwasstronger in women compared to men. Moreover, there was a significant non-linear association between uric acid levels and the risk of CVD mortality (r = 0.0709, p = 0.001).
Conclusion
Our analysis indicates a positive dose-response association between SUA and CVD mortality risk.
Keywords: Uric acid, Serum uric acid, Cardiovascular diseases mortality, CVD mortality, Longitudinal, Cohort
Background
Cardiovascular diseases (CVD) are the first leading cause of death worldwide [1]. This might be due to an increased incidence of chronic diseases such as obesity, diabetes, hypertension, dyslipidemia, and hyperuricemia [2]. Uric acid (UA) is considered the ultimate product of purine metabolism in humans. Evidence suggests that increased levels of serum uric acid (SUA) are associated with the development of hypertension, coronary heart disease (CHD), cardiovascular stroke, cerebrovascular accidents (CVA), and cardiovascular disease [3].SUA concentrations can reflect the amount of purine intake from the diet, inborn purine metabolism, changes in UA secretion (reduced glomerular filtration and tubular secretion, or increased tubular reabsorption), and intestinal degeneration [4]. The relationship between SUA and CVD was first reported more than 50 years ago, and several epidemiological studies were conducted to assess the association between hyperuricemia (HU) and CVD [5]. Although many studies have been conducted assessing the relationship between UA and CVD, there is disagreement about this relationship [6]. These controversies are due to the dual effect of UA in the body [7]. The atherogenic effects of UA include induction of oxidative stress in cells, which reduces the bioavailability of nitric oxide - associated with the activity of platelets and endothelial cells and the differentiation of smooth muscle cells in the vascular system. On the other hand, UA can also have antioxidant properties that can prevent atherosclerosis and improve endothelial function [8].
To gain a greater understanding of the prognostic value of SUA for future clinical decision making, we conducted a meta-analysis of prospective cohort studies with dose– response analysis to determine the relationship between SUA and CVD mortality.
Methods
This meta-analysis conducted by following the Meta-analysis of Observational Studies in Epidemiology study guidelines (MOOSE) [9]. A comprehensive literature search was carried out by two reviewers (MM) and (FRS) independently on PubMed/MEDLINE (https://www.ncbi.nlm.nih.gov/pubmed/) and Scopus (https://www.scopus.com/search/) databases up to April 2019 without any time restriction. Following keyword was followed for systematic search: in PubMed/MEDLINE: (((“Uric Acid”[Mesh] OR uric acid [Title/Abstract]) OR serum uric acid [Title/Abstract]) AND ((((“cardiovascular disease mortality”[Title/Abstract] OR “cardio vascular mortality”[Title/Abstract]) OR “cardiovascular mortality”[Title/Abstract]) OR “CVD mortality”[Title/Abstract]) OR CVD-mortality [Title/Abstract])) AND (((prospective [Title/Abstract] OR longitudinal [Title/Abstract]) OR follow-up [Title/Abstract]) OR cohort [Title/Abstract]), in Scopus: ((TITLE-ABS-KEY (prospective) OR TITLE-ABS-KEY (longitudinal) OR TITLE-ABS-KEY (follow-up) OR TITLE-ABS-KEY (cohort))) AND ((TITLE-ABS-KEY (uric AND acid) OR TITLE-ABS-KEY (serum AND uric AND acid))) AND ((TITLE-ABS-KEY (cardiovascular AND disease AND mortality) OR TITLE-ABS-KEY (cardiovascular AND mortality) OR TITLE-ABS-KEY (cardio AND vascular AND mortality) OR TITLE-ABS-KEY (cvd AND mortality) OR TITLE-ABS-KEY (cvd-mortality))).
Exclusion and inclusion criteria and data extraction
Non-English articles, reviews papers, editorials, non CVD mortality, non-human studies, in vitro research, case reports, and letters without sufficient data were excluded. Studies that met the following inclusion criteria were included in this meta-analysis:
1) Cohort study design with CVD mortality outcome.
2) Hazard ratio (HR) and the corresponding 95% confidence interval (CI) of CVD mortality were reported based on uric acid levels.
The first author, publication year, country, study design, number of participants, mean age, gender, years of follow-up, hazard ratios and 95% CIs of CVD mortality information extracted from included studies.
Statistical analysis
The STATA 14.0 statistical software (Stata Corporation, College Station, Texas, USA) was used for statistical analyses. Combined results of hazard ratio of CVD mortality conducted by Random-effects model [10]. P < 0.10 and I2 < %50 were considered as heterogeneity detection among included studies. In order to find source of heterogeneity, subgroup analysis was conducted based on gender, whereas meta-regression analysis was conducted based on follow-up years, age of participants and HR of CVD mortality. Non-linear association was examined by modeling concentration level using restricted cubic splines [10]. The publication bias among included studies evaluated by Funnel plot, Begg’s test, and Egger’s regression test.
Results
Literature search
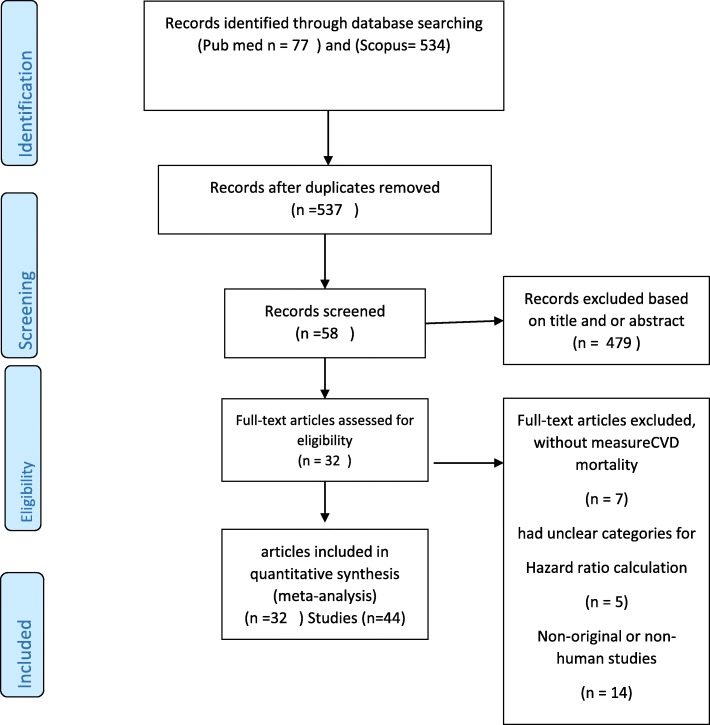
A flow chart of included studies is shown in Fig. 1. In the primary search 611 records were identified, after excluding 74 duplicates studies and 479 irrelevant studies from title and abstract screening, 58 studies remained for full text screening. After reviewing the full text, 26 studies were excluded because they did not meet the inclusion criteria. Finally, 32 studies, containing 44 arms and 1,134,073 participants, were included in the meta analysis.
Fig. 1.
Flow chart of studies reviewed
Study characteristics and quality assessment
Table 1 shows characteristics of included studies. Studies were published between 2000 and 2018. The mean age of participants was 55.9 years and the mean duration of follow-up was 9 years from 1 to 18 years. Seventeen studies were performed in Asia and Australia [11–27], and fifteen in Europe and America [28–42]. Twenty-two arms were conducted in both genders, eight in women, and fourteen in men.
Table 1.
Baseline Characteristics of Included Studies in the Meta-analysis
| Studies | Author | Year | Country | Follow up (year) | Sex (1-women, 2-men, 3-both) | Patients, n |
|---|---|---|---|---|---|---|
| 1 | Silbernagel, G. | 2013 | Germany | 7.3 | 3 | 3245 |
| 2 | Lin, G. M. | 2013 | Taiwan | 3.2 | 3 | 1054 |
| 3 | Hu, S. L. | 2012 | Taiwan | 10 | 3 | 1093 |
| 4 | Wen, C. P. | 2010 | Taiwan | 8.5 | 3 | 230,508 |
| 5 | Spoon, D. B. | 2010 | USA | 2 | 3 | 1916 |
| 6 | Strasak, A. M. | 2008 | Austria | 21 | 1 | 28,613 |
| 7 | Meisinger, C. | 2008 | Germany | 11.7 | 2 | 3604 |
| 8 | Niskanen, L. K. | 2004 | Finland | 11.9 | 2 | 1423 |
| 9 | Dawson, J. | 2013 | Scotland | 10 | 1 and 2 | 6984 |
| 10 | Ford, E. S. | 2011 | USA | 14 | 3 | 13,802 |
| 11 | Chen, J. H. | 2009 | Taiwan | 8.2 | 1 and 2 | 90,393 |
| 12 | Strasak, A. | 2008 | Austria | 13.6 | 2 | 83,638 |
| 13 | Tomita, M. | 2000 | Japan | 5.4 | 2 | 49,413 |
| 14 | Fang, J. | 2000 | USA | 16.4 | 1 and 2 | 5926 |
| 15 | Tseng,W | 2018 | Taiwan | 5.8 | 3 | 127,771 |
| 16 | Lopez-Pineda, A. | 2018 | Spain | 3 | 3 | 1119 |
| 17 | Tscharre, M. | 2018 | Austria | 5.5 | 3 | 1215 |
| 18 | Hu, W. S. | 2017 | china | 8.8 | 3 | 381,963 |
| 19 | Zhang, W. | 2016 | Japan | 23 | 1 and 2 | 36,313 |
| 20 | Nossent, J. | 2016 | Australia | 15 | 3 | 3475 |
| 21 | Li, Q. | 2016 | china | 3.9 | 3 | 1799 |
| 22 | Kamei, K. | 2016 | Japan | 8 | 1 and 2 | 3487 |
| 23 | Zhu, L. | 2015 | china | 12 | 3 | 588 |
| 24 | Zalawadiya, S. K. | 2015 | USA | 14.5 | 3 | 11,009 |
| 25 | Wu, C. Y. | 2015 | Taiwan | 5 | 1 and 2 | 77,541 |
| 26 | Wang, J. | 2015 | china | 6 | 3 | 2585 |
| 27 | Von Lueder, T. G. | 2015 | Norway | 2.7 | 3 | 12,677 |
| 28 | Shimizu, T. | 2015 | Japan | 1.67 | 3 | 424 |
| 29 | Mayer, F. J | 2015 | Austria | 6.3 | 3 | 959 |
| 30 | Kleber, M. E. | 2015 | Germany | 10 | 3 | 3315 |
| 31 | Beberashvili, I. | 2015 | Israel | 2 | 3 | 261 |
| 32 | Xia, X. | 2014 | China | 2.1 | 3 | 985 |
Main results of the meta-analysis
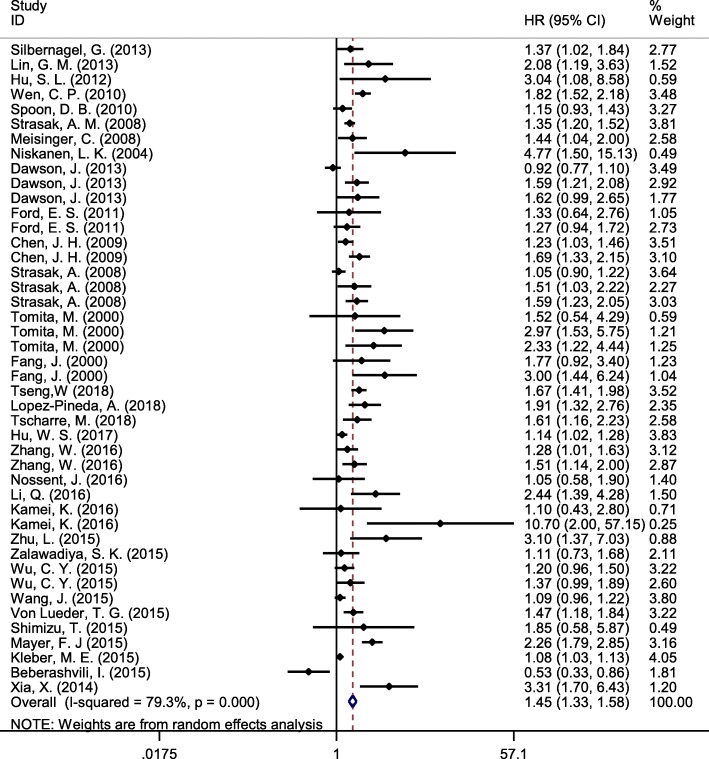
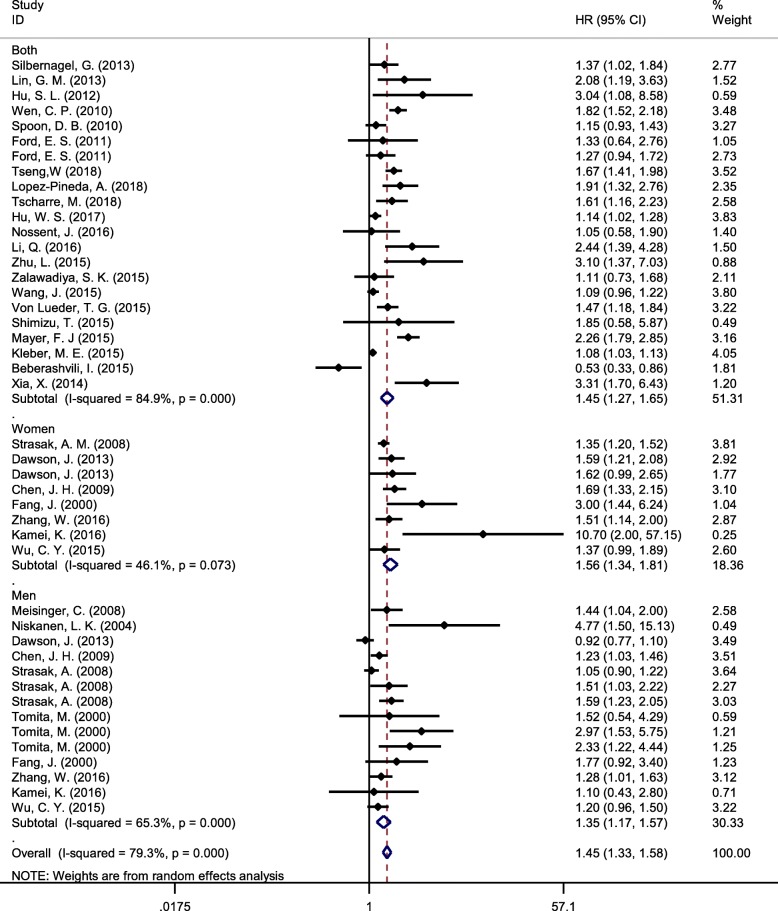
In 44 arms, pooled results from the random effects model showed a positive association between SUA and risk of CVD mortality in highest versus lowest category of SUA (HR 1.45, 95% CI 1.33–1.58, I2 = 79%) (Fig. 2). Subgroup analysis based on a gender showed a stronger relationship in women compared with men (Fig. 3). Meta-regression analysis did not show any significant relationship between SUA and risk of CVD mortality based on participant age (p = 0.86) or duration of follow-up(p = 0.44).
Fig. 2.
The forest plot between highest versus lowest categories of serum uric acid and cardiovascular disease mortality
Fig. 3.
The forest plot between highest versus lowest categories of serum uric acid and cardiovascular disease mortality based on gender
Dose-response analysis
The pooled HR from the random-effects dose-response model of included studies showed a significant positive association between SUA and CVD mortality (r = 0.0709, p = 0.001) (Fig. 4).
Fig. 4.
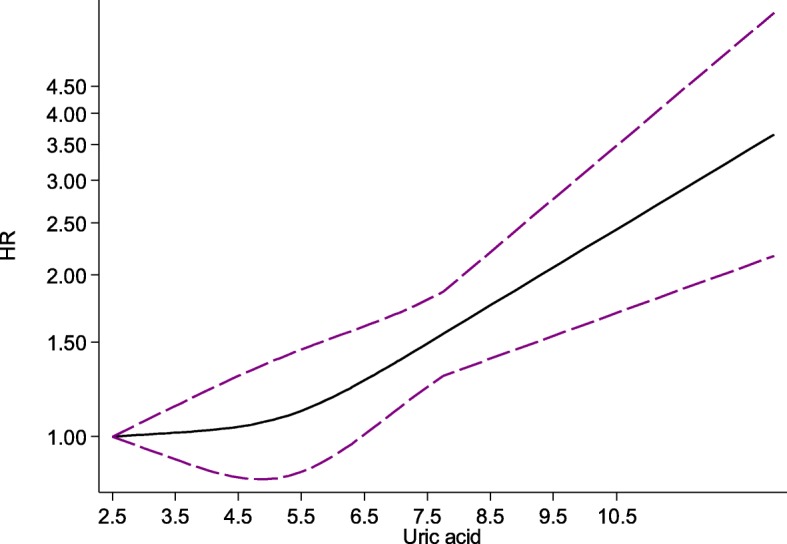
Dose-response association between serum uric acid (mg/dL) and cardiovascular disease mortality (HR)
Publication bias
Figure 5 shows funnel plots of CVD mortality. There was publication bias among the studies (the Begg’s p = 0.04 and Egger’s test). Using the ‘Trim and fill’ method to adjust for publication bias and random effects model showed 62 arms with pooled results HR = 1.19 (CI:1.09–1.30).
Fig. 5.
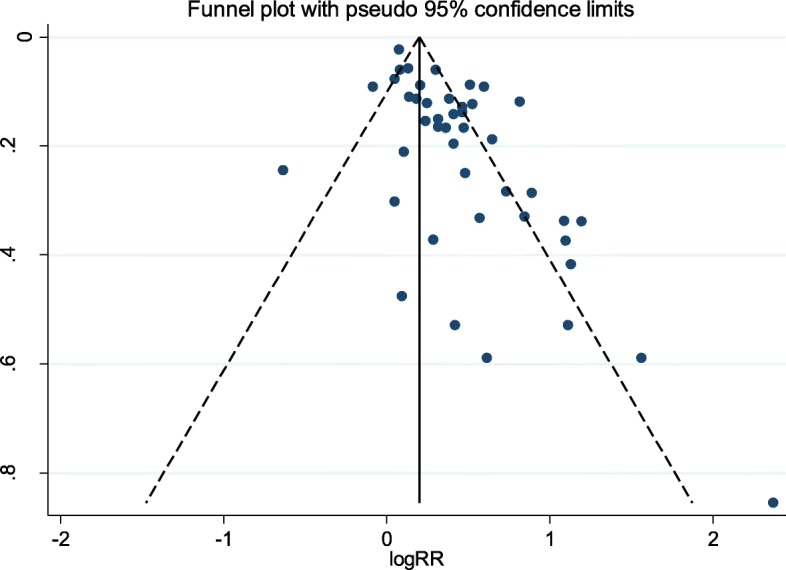
Funnel plots to assessment publication bias
Discussion
The present meta-analysis of cohort studies revealed that there is a strong relationship between SUA levels and risk of CVD mortality. Moreover, the pooled HR from the random-effects dose-response model indicated that this positive association is stable when SUA is greater than 6 mg/dL. Although the exact mechanism of the relationship between SUA and CVD mortality risk has not yet been elucidated, previous studies have suggested some possible explanations..
An experimental study has reported that using an inhibitor of uricase can elevate blood pressure in rats by activating the renin-angiotensin system and inhibition of nitric oxide synthase [43]. Moreover, it has been shown that patients with hypertension and hyperuricemia had higher carotid intima media thickness in comparison to patients without hyperuricemia [44]. Furthermore, it has been reported that blood atherosclerotic platelets consist of a great amount of UA and higher levels of SUA can simply boost thrombus development which may lead to slow coronary flow (SCF) [45]. In contrast, UA may have some anti-proliferative influence on the endothelium or can damage the process of nitric oxide production [16].
It has been suggested that UA can significantly slow down coronary flow by promoting the calcification of coronary arteries [45, 46]. In addition, higher levels of SUA may induce oxidative stress by oxidation of low-density lipoprotein cholesterol which may lead to SCF [45]. Another possible mechanism might be explained by the effects of hyperuricemia in induction of crystal shaping on vascular walls that impair endothelial and smooth muscle function leading to atherosclerosis by renin-angiotensin system activation [43, 47]. Even, crystals of urate have several noxious effects that activate neutrophilsand macrophage cells to set proteases free and stimulate the coagulation cascade [48].
The main strength of this meta-analysis was the application of cohort studies containing over one million participants, and subsequent dose-response analysis. This study has some possible limitation. The heterogeneity in the study populations is the main limitation of this study. Moreover, the duration of follow-up in our included studies differed. Further, there is an insufficient number of randomized clinical trials to confirm the effects of decreasing SUA levels on CVD deaths; however, our results suggest higher levels of SUA is an independent risk factor associated with CVD mortality. Furthermore, although we searched the literature extensively, we did not explore grey or unpublished literature to limit the possibility of publication bias.
Conclusion
In conclusion, our analysis indicates a positive dose-response association between SUA and CVD mortality risk; however, further clinical trials are needed to confirm these findings and to determine the possible cause-effect relationship.
Acknowledgements
This study is related to the project NO.1397/69621 from Student Research Committee, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
We also appreciate the “Student Research Committee” and “Research & Technology Chancellor” at Shahid Beheshti University of Medical Sciences for their financial support of this study.
Abbreviations
- CI
Confidence interval
- CVD
Cardiovascular diseases
- HR
Hazard ratio
- SCF
Slow coronary flow
- SUA
Serum uric acid
- UA
Uric acid
Authors’ contributions
FR designed the study. FR, MM, JR, NB and AH were involved in the selection of publications and data collection for the meta-analysis. AH reviewed the selected studies. FR, JR, and MM participated in data analysis. FR and AH wrote the core manuscript, and all authors reviewed and approved of the final manuscript.
Funding
The study was supported financially by the “Student Research Committee” and “Research & Technology Chancellor” at Shahid Beheshti University of Medical Sciences. The funding body did not play any role in design of the study, data analysis, and interpretation, and manuscript writing.
Availability of data and materials
All data are presented within the manuscript. Raw data can be available by corresponding author per request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dhindsa DS, Khambhati J, Sandesara PB, Eapen DJ, Quyyumi AA. Biomarkers to predict cardiovascular death. Card Electrophysiol Clin. 2017;9(4):651–664. doi: 10.1016/j.ccep.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Mora-Ramirez M, Estevez-Garcia IO, Irigoyen-Camacho ME, Bojalil R, Gonzalez-Pacheco H, Amezcua-Guerra LM. Hyperuricemia on admission predicts short-term mortality due to myocardial infarction in a population with high prevalence of cardiovascular risk factors. Rev Investig Clin. 2017;69(5):247–253. doi: 10.24875/ric.17002167. [DOI] [PubMed] [Google Scholar]
- 3.Zhao G, Huang L, Song M, Song Y. Baseline serum uric acid level as a predictor of cardiovascular disease related mortality and all-cause mortality: a meta-analysis of prospective studies. Atherosclerosis. 2013;231(1):61–68. doi: 10.1016/j.atherosclerosis.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 4.Vassalle Cristina, Mazzone Annamaria, Sabatino Laura, Carpeggiani Clara. Uric Acid for Cardiovascular Risk: Dr. Jekyll or Mr. Hide? Diseases. 2016;4(1):12. doi: 10.3390/diseases4010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braga F, Pasqualetti S, Ferraro S, Panteghini M. Hyperuricemia as risk factor for coronary heart disease incidence and mortality in the general population: a systematic review and meta-analysis. Clin Chem Lab Med. 2016;54(1):7–15. doi: 10.1515/cclm-2015-0523. [DOI] [PubMed] [Google Scholar]
- 6.Xu Q, Zhang M, Abeysekera IR, Wang X. High serum uric acid levels may increase mortality and major adverse cardiovascular events in patients with acute myocardial infarction. Saudi Med J. 2017;38(6):577–585. doi: 10.15537/smj.2017.6.17190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanbay M, Segal M, Afsar B, Kang DH, Rodriguez-Iturbe B, Johnson RJ. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart. 2013;99(11):759–766. doi: 10.1136/heartjnl-2012-302535. [DOI] [PubMed] [Google Scholar]
- 8.Kei Anastazia, Koutsouka Freideriki, Makri Andromachi, Elisaf Moses. Uric acid and cardiovascular risk: What genes can say. International Journal of Clinical Practice. 2017;72(1):e13048. doi: 10.1111/ijcp.13048. [DOI] [PubMed] [Google Scholar]
- 9.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 10.Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat Med. 2010;29(12):1282–1297. doi: 10.1002/sim.3602. [DOI] [PubMed] [Google Scholar]
- 11.Beberashvili I, Sinuani I, Azar A, Shapiro G, Feldman L, Stav K, et al. Serum uric acid as a clinically useful nutritional marker and predictor of outcome in maintenance hemodialysis patients. Nutrition. 2015;31(1):138–147. doi: 10.1016/j.nut.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Chen JH, Chuang SY, Chen HJ, Yeh WT, Pan WH. Serum uric acid level as an independent risk factor for all-cause, cardiovascular, and ischemic stroke mortality: a Chinese cohort study. Arthritis Rheum. 2009;61(2):225–232. doi: 10.1002/art.24164. [DOI] [PubMed] [Google Scholar]
- 13.Hsu Wei-Liang, Li Szu-Yuan, Liu Jia-Sin, Huang Po-Hsun, Lin Shing-Jong, Hsu Chih-Cheng, Lin Yao-Ping, Tarng Der-Cherng. High Uric Acid Ameliorates Indoxyl Sulfate-Induced Endothelial Dysfunction and Is Associated with Lower Mortality among Hemodialysis Patients. Toxins. 2017;9(1):20. doi: 10.3390/toxins9010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu SL, Liu CS, Lin CH, Davidson LE, Li CI, Lin CC, et al. Uric acid and mortality in elderly chinese: a 10-year population-based cohort study. J Am Geriatr Soc. 2012;60(9):1783–1785. doi: 10.1111/j.1532-5415.2012.04138.x. [DOI] [PubMed] [Google Scholar]
- 15.Kamei K, Konta T, Ichikawa K, Sato H, Suzuki N, Kabasawa A, et al. Serum uric acid levels and mortality in the Japanese population: the Yamagata (Takahata) study. Clin Exp Nephrol. 2016;20(6):904–909. doi: 10.1007/s10157-016-1228-1. [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Zhang Y, Ding D, Yang Y, Chen Q, Liu C, et al. Association between serum uric acid and mortality among Chinese patients with coronary artery disease. Cardiology. 2016;134(3):347–356. doi: 10.1159/000443518. [DOI] [PubMed] [Google Scholar]
- 17.Lin GM, Li YH, Zheng NC, Lai CP, Lin CL, Wang JH, et al. Serum uric acid as an independent predictor of mortality in high-risk patients with obstructive coronary artery disease. A prospective observational cohort study from the ET-CHD registry, 1997-2003. J Cardiol. 2013;61(2):122–127. doi: 10.1016/j.jjcc.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Nossent J, Raymond W, Divitini M, Knuiman M. Asymptomatic hyperuricemia is not an independent risk factor for cardiovascular events or overall mortality in the general population of the Busselton health study. BMC Cardiovasc Disord. 2016;16(1):256. doi: 10.1186/s12872-016-0421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimizu T, Yoshihisa A, Kanno Y, Takiguchi M, Sato A, Miura S, et al. Relationship of hyperuricemia with mortality in heart failure patients with preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2015;309(7):H1123–H1129. doi: 10.1152/ajpheart.00533.2015. [DOI] [PubMed] [Google Scholar]
- 20.Tomita M, Mizuno S, Yamanaka H, Hosoda Y, Sakuma K, Matuoka Y, et al. Does hyperuricemia aeffect mortality? A prospective cohort study of japanese male workers. J Epidemiol. 2000;10(6):403–409. doi: 10.2188/jea.10.403. [DOI] [PubMed] [Google Scholar]
- 21.Tseng WC, Chen YT, Ou SM, Shih CJ, Tarng DC, Yang CY, et al. U-shaped association between serum uric acid levels with cardiovascular and all-cause mortality in the elderly: The role of malnourishment. J Am Heart Assoc. 2018;7(4) English. [DOI] [PMC free article] [PubMed]
- 22.Wang J, Wang Y, Zhao D, Guo X, Zhong JQ. Association between serum uric acid and mortality in a Chinese population of hypertensive patients. Ren Fail. 2015;37(1):73–76. doi: 10.3109/0886022X.2014.964148. [DOI] [PubMed] [Google Scholar]
- 23.Wen CP, David Cheng TY, Chan HT, Tsai MK, Chung WS, Tsai SP, et al. Is high serum uric acid a risk marker or a target for treatment? Examination of its independent effect in a large cohort with low cardiovascular risk. Am J kidney Dis. 2010;56(2):273–288. doi: 10.1053/j.ajkd.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 24.Wu CY, Hu HY, Chou YJ, Huang N, Chou YC, Lee MS, et al. High serum uric acid levels are associated with all-cause and cardiovascular, but not Cancer, mortality in elderly adults. J Am Geriatr Soc. 2015;63(9):1829–1836. doi: 10.1111/jgs.13607. [DOI] [PubMed] [Google Scholar]
- 25.Xia X, He F, Wu X, Peng F, Huang F, Yu X. Relationship between serum uric acid and all-cause and cardiovascular mortality in patients treated with peritoneal dialysis. Am J Kidney Dis. 2014;64(2):257–264. doi: 10.1053/j.ajkd.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Iso H, Murakami Y, Miura K, Nagai M, Sugiyama D, et al. Serum uric acid and mortality form cardiovascular disease: EPOCH-JAPAN study. J Atheroscler Thromb. 2016;23(6):692–703. doi: 10.5551/jat.31591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu L, Wang J, Wang Y, Jia L, Sun K, Wang H, et al. Plasma uric acid as a prognostic marker in patients with hypertrophic cardiomyopathy. Can J Cardiol. 2015;31(10):1252–1258. doi: 10.1016/j.cjca.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Dawson J, Jeemon P, Hetherington L, Judd C, Hastie C, Schulz C, et al. Serum uric acid level, longitudinal blood pressure, renal function, and long-term mortality in treated hypertensive patients. Hypertension. 2013;62(1):105–111. doi: 10.1161/HYPERTENSIONAHA.113.00859. [DOI] [PubMed] [Google Scholar]
- 29.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971-1992. National Health and nutrition examination survey. JAMA. 2000;283(18):2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 30.Ford ES. Uric acid and mortality from all-causes and cardiovascular disease among adults with and without diagnosed diabetes: findings from the National Health and nutrition examination survey III linked mortality study. Diabetes Res Clin Pract. 2011;93(2):e84–ee6. doi: 10.1016/j.diabres.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Kleber ME, Delgado G, Grammer TB, Silbernagel G, Huang J, Krämer BK, et al. Uric acid and cardiovascular events: a Mendelian randomization study. J Am Soc Nephrol. 2015;26(11):2831–2838. doi: 10.1681/ASN.2014070660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez-Pineda A, Cordero A, Carratala-Munuera C, Orozco-Beltran D, Quesada JA, Bertomeu-Gonzalez V, et al. Hyperuricemia as a prognostic factor after acute coronary syndrome. Atherosclerosis. 2018;269:229–235. doi: 10.1016/j.atherosclerosis.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 33.Mayer FJ, Mannhalter C, Minar E, Schillinger M, Chavakis T, Siegert G, et al. The impact of uric acid on long-term mortality in patients with asymptomatic carotid atherosclerotic disease. J Stroke Cerebrovasc Dis. 2015;24(2):354–361. doi: 10.1016/j.jstrokecerebrovasdis.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 34.Meisinger C, Koenig W, Baumert J, Döring A. Uric acid levels are associated with all-cause and cardiovascular disease mortality independent of systemic inflammation in men from the general population the MONICA ORA cohort study. Arterioscler Thromb Vasc Biol. 2008;28(6):1186–1192. doi: 10.1161/ATVBAHA.107.160184. [DOI] [PubMed] [Google Scholar]
- 35.Niskanen LK, Laaksonen DE, Nyyssonen K, Alfthan G, Lakka HM, Lakka TA, et al. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: a prospective cohort study. Arch Intern Med. 2004;164(14):1546–1551. doi: 10.1001/archinte.164.14.1546. [DOI] [PubMed] [Google Scholar]
- 36.Silbernagel G, Hoffmann MM, Grammer TB, Boehm BO, Marz W. Uric acid is predictive of cardiovascular mortality and sudden cardiac death in subjects referred for coronary angiography. Nutr Metab Cardiovasc Dis. 2013;23(1):46–52. doi: 10.1016/j.numecd.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Spoon DB, Lerman A, Rule AD, Prasad A, Lennon RJ, Holmes DR, et al. The association of serum uric acid levels with outcomes following percutaneous coronary intervention. J Interv Cardiol. 2010;23(3):277–283. doi: 10.1111/j.1540-8183.2010.00555.x. [DOI] [PubMed] [Google Scholar]
- 38.Strasak A, Ruttmann E, Brant L, Kelleher C, Klenk J, Concin H, et al. Serum uric acid and risk of cardiovascular mortality: a prospective long-term study of 83,683 Austrian men. Clin Chem. 2008;54(2):273–284. doi: 10.1373/clinchem.2007.094425. [DOI] [PubMed] [Google Scholar]
- 39.Strasak AM, Kelleher CC, Brant LJ, Rapp K, Ruttmann E, Concin H, et al. Serum uric acid is an independent predictor for all major forms of cardiovascular death in 28,613 elderly women: a prospective 21-year follow-up study. Int J Cardiol. 2008;125(2):232–239. doi: 10.1016/j.ijcard.2007.11.094. [DOI] [PubMed] [Google Scholar]
- 40.Tscharre M, Herman R, Rohla M, Hauser C, Farhan S, Freynhofer MK, et al. Uric acid is associated with long-term adverse cardiovascular outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Atherosclerosis. 2018;270:173–179. doi: 10.1016/j.atherosclerosis.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Von Lueder TG, Girerd N, Atar D, Agewall S, Lamiral Z, Kanbay M, et al. Serum uric acid is associated with mortality and heart failure hospitalizations in patients with complicated myocardial infarction: findings from the high-risk myocardial infarction database initiative. Eur J Heart Fail. 2015;17(11):1144–1151. doi: 10.1002/ejhf.419. [DOI] [PubMed] [Google Scholar]
- 42.Zalawadiya SK, Veeranna V, Mallikethi-Reddy S, Bavishi C, Lunagaria A, Kottam A, et al. Uric acid and cardiovascular disease risk reclassification: findings from NHANES III. Eur J Prev Cardiol. 2015;22(4):513–518. doi: 10.1177/2047487313519346. [DOI] [PubMed] [Google Scholar]
- 43.Mazzali M, Kanellis J, Han L, Feng L, Xia Y-Y, Chen Q, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiology Renal Physiol. 2002;282(6):F991–F9F7. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 44.Tavil Y, Kaya MG, Oktar SÖ, Sen N, Okyay K, Yazıcı HU, et al. Uric acid level and its association with carotid intima–media thickness in patients with hypertension. Atherosclerosis. 2008;197(1):159–163. doi: 10.1016/j.atherosclerosis.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Xia S, Deng SB, Wang Y, Xiao J, Du JL, Zhang Y, et al. Clinical analysis of the risk factors of slow coronary flow. Heart Vessel. 2011;26(5):480–486. doi: 10.1007/s00380-010-0081-5. [DOI] [PubMed] [Google Scholar]
- 46.Kaya EB, Yorgun H, Canpolat U, Hazirolan T, Sunman H, Ulgen A, et al. Serum uric acid levels predict the severity and morphology of coronary atherosclerosis detected by multidetector computed tomography. Atherosclerosis. 2010;213(1):178–183. doi: 10.1016/j.atherosclerosis.2010.08.077. [DOI] [PubMed] [Google Scholar]
- 47.Patetsios P, Song M, Shutze WP, Pappas C, Rodino W, Ramirez JA, et al. Identification of uric acid and xanthine oxidase in atherosclerotic plaque. Am J Cardiol 2001;88(2):188–191, a6. PubMed PMID: 11448423. Epub 2001/07/13. eng. [DOI] [PubMed]
- 48.Gordon T, Terkeltaub R, Ginsberg M. Gout: crystal-induced inflammation. Inflammation: Basic Principles and Clinical Correlates New York, NY, Raven 1988:775–83.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are presented within the manuscript. Raw data can be available by corresponding author per request.