CLDN18.2 expression is highly prevalent in Japanese patients with gastric cancer, making it a targetable alteration, and supporting development of zolbetuximab as a therapeutic agent for this patient population.
Keywords: biomarkers, Claudin, gastric cancer, immunohistochemistry, prevalence
Abstract
Background
The monoclonal antibody zolbetuximab (formerly IMAB362), which is being developed as a potential treatment for gastric cancer (GC), targets Claudin 18.2 (CLDN18.2), a GC biomarker. This study aimed to determine the prevalence of CLDN18.2 in primary tumors and lymph node (LN) metastases of Japanese patients with GC.
Methods
CLDN18.2 expression was investigated in tissue samples from patients with gastric adenocarcinoma archived at Kurume University Medical Center, Japan, between 2000 and 2012. Expression of CLDN18.2 in tumor samples was evaluated by immunohistochemistry using the same detection antibody (43-14A) and assay used in the FAST clinical trial (NCT01630083), a phase 2 randomized trial that compared the safety and antitumor activity of the zolbetuximab-chemotherapy combination with chemotherapy alone. Samples showing any specific staining with ≥1+ intensity were defined as CLDN18.2-positive.
Results
Of 263 samples analyzed (134 primary gastric tumors and corresponding LN metastases; 128 primary tumors only; one LN metastases only), CLDN18.2 was detected in 87% (n = 228/262) of all primary tumors and 80% (n = 108/135) of LN metastases. Moderate-to-strong CLDN18.2 expression (≥2+ membrane staining intensity in ≥40% of tumor cells [FAST eligibility criterion]) was observed in 52% (n = 135/262) of primary tumors and 45% (n = 61/135) of (LN) metastases. CLDN18.2 expression was significantly higher in GCs of the diffuse histological subtype per Lauren classification and in high grade (G3) tumors.
Conclusions
The high prevalence of CLDN18.2 among Japanese patients with GC supports the therapeutic assessment of zolbetuximab in this population.
Introduction
Gastric cancer (GC) is among the malignancies with a high unmet medical need (1–3). It is one of the most commonly diagnosed cancers and among the leading causes of cancer-related deaths worldwide (3). Incidence rates are particularly high in East Asian countries (eg, Japan, Republic of Korea, China) (2) and incidences of proximal (cardia) anatomic and of diffuse histologic variants are rising (1,4,5). In general, GC has a poor prognosis with limited treatment options, and in countries where GC screening is not routinely performed, most cases are diagnosed at an advanced stage (6). Currently, trastuzumab and ramucirumab are the only targeted agents approved in a wide number of countries for GC, including in the United States, European Union, Japan, and the Republic of Korea (7–9); however, these treatment options have one or more shortcomings that restrict their utility, including modest survival benefits and the development of secondary resistance (10).
A limiting factor in developing highly effective targeted therapies for GC has been the scarcity of suitable biomarkers that can serve as targets (11–13). Claudin 18 splice variant 2 (CLDN18.2), a member of the Claudin family of tetraspanin proteins expressed at epithelial tight junctions, has been identified as an attractive biomarker for targeted therapy (14). Prior evidence demonstrates the tissue specificity of Claudin 18 isoforms and identifies CLDN18.2 as the dominant isoform of Claudin 18 expressed in normal gastric and gastric adenocarcinoma tissues (14,15). In normal tissue, CLDN18.2 expression is restricted to gastric mucosa cells (14,15); however, upon malignant transformation, perturbations in gastric mucosa cell polarity lead to the accessibility of CLDN18.2 to therapeutic antibodies (16). Furthermore, CLDN18.2 is aberrantly expressed in pancreatic, ovarian, biliary, and lung adenocarcinomas (14,17–23).
Zolbetuximab (formerly known as IMAB362) is a first-in-class monoclonal antibody specific to a CLDN18.2 epitope. Preclinical studies have demonstrated that zolbetuximab primarily binds to tumor cell surface CLDN18.2, but only to a limited extent to CLDN18.2 on normal gastric mucosa cells. Zolbetuximab mediates cell death through antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity (16,24). Early clinical studies (ClinicalTrials.gov Identifiers: NCT00909025, NCT01197885, NCT01671774) have found zolbetuximab to be well tolerated and showing evidence of antitumor activity (25,26). In a randomized phase 2 study (FAST; NCT01630083), the clinical effects of zolbetuximab in combination with EOX chemotherapy were evaluated in patients with histologically confirmed advanced gastric and gastro-esophageal junction cancers with moderate-to-strong CLDN18.2-expression (membrane staining intensity of ≥2+ by immunohistochemistry [IHC]) in ≥40% of cancer cells). In these patients, zolbetuximab, combined with chemotherapy, prolonged overall and progression-free survival over chemotherapy alone with acceptable safety and tolerability (25–28). Furthermore, in a subpopulation of patients with high CLDN18.2 expression (≥2+ staining intensity in ≥70% tumor cells), the observed antitumor activity was pronounced (29). In addition, health-related quality-of-life was maintained for a longer duration in patients who received EOX + zolbetuximab therapy compared with EOX alone (29).
Gastric cancer is a heterogeneous disease with significant variations in molecular characteristics observed across ethnic populations (30–32). Clinical trials of zolbetuximab to date have been conducted in Central and Eastern Europe (14); extension of the clinical program to Asia, and in particular to Japan, where GC is among the most frequently occurring neoplasms (33), can potentially have a great therapeutic impact. We therefore investigated CLDN18.2 expression in tissue samples from a cohort of Japanese patients using the same analytically validated CE-marked in vitro diagnostic IHC assay employing the same 43-14A detection antibody used in the FAST trial. The aims of this study were to establish the prevalence of CLDN18.2 expression in GC, and identify a patient population who may be eligible for future clinical assessment of zolbetuximab in the Japanese population.
Materials and methods
Tissue sample collection
Formalin-fixed, paraffin-embedded (FFPE) tissue blocks of gastric adenocarcinoma tissues including primary tumors, and in certain cases, lymph node (LN) metastases, were collected at Kurume University Medical Center in Japan between 2000 and 2012 from patients of Japanese ethnicity with approval by the local ethics committee. If primary tumor and LN metastasis were available from the same patient, these samples were referred to as ‘matched pairs.’ The histopathologic diagnosis, grading, and staging were performed according to the Japanese Classification of Gastric Carcinoma (34); diffuse and intestinal histopathologic subtypes were based on Lauren classification (35). Normal human stomach tissue, which is known to express CLDN18.2, was used as positive control tissue.
Immunohistochemistry and histologic assessment
All tissue samples were stained using the monoclonal mouse antibody clone 43-14A as part of the analytically validated histology kit. FFPE tissue sections (3 μm) from the primary tumor and LN metastatic tissue samples were mounted on Matsunami Platinum PRO Adhesive Microscope Slides (Matsunami Glass Ind., Ltd., Osaka, Japan) together with normal FFPE human stomach tissue sections as positive controls. Slides were incubated in a drying oven at 58−60°C for 1 hour, and subsequently deparaffinized, rehydrated, and washed in distilled water. Thereafter, slides were transferred into epitope retrieval solution (10 mM Tris, 1 mM EDTA, pH 9.0, preheated to 95−100°C) for 15 minutes at 95−99°C. Slides were cooled at room temperature for 10 minutes, washed, and then incubated for up to 20 minutes at room temperature. Endogenous peroxidases were blocked by incubation with 3% hydrogen peroxide for 10 minutes at room temperature. Slides were washed, blocked, and incubated with primary mouse 43-14A monoclonal antibody that was developed against a membrane epitope C-terminus of claudin 18 (Ganymed Pharmaceuticals AG, Mainz, DE), for 30 minutes at room temperature, followed by a 30-minute incubation with a ready-to-use visualization reagent containing a polymer reagent conjugated with horseradish peroxidase and goat anti-mouse Fab antibody fragments (Nichirei Biosciences, Inc., Tokyo, Japan). Antibody binding was visualized by incubation with the peroxidase substrate 3,3’-diaminobenzidine for 5 minutes. After counterstaining with Mayer’s hematoxylin, followed by dehydration, and mounting with X-TRA-Kitt (Medite, Burgdorf, Germany), tissue sections were analyzed using conventional light microscopy.
Evaluation of stained samples was performed by two independent scientists (RY, CR). To determine CLDN18.2 expression status, samples were analyzed according to a two-component scoring method: the intensity of staining (including complete basolateral as well as lateral membrane staining) and the percentage of tumor cells stained at the different staining intensities in relation to all tumor cells present in the respective tissue sample. The intensity of the staining was classified as 0 (no membrane or cytoplasmic reactivity), 1+ (weak membrane or cytoplasmic reactivity), 2+ (moderate membrane or cytoplasmic reactivity), and 3+ (strong membrane or cytoplasmic reactivity). Samples were defined as CLDN18.2-positive if they showed specific staining with at least 1+ intensity in any fraction of tumor cells (‘any positivity’). The staining of tumor samples was considered valid and included into the analysis only if the positive control (healthy stomach tissue) showed the expected staining pattern. Percentage of overall CLDN18.2 positive cells was determined by the estimated number of CLDN18.2+ cells divided by the estimated overall number of tumor cells in each sample including primary and corresponding LN met when available.
Statistical analysis
Statistical parameters were analyzed using GraphPad Prism 6.04 software (GraphPad Software, Inc., CA, US). Fisher’s exact tests were calculated using SAS Enterprise Guide version 6.1. Two data sets were compared with an unpaired nonparametric Mann-Whitney test (non-matched pairs), and a Wilcoxon matched-pairs signed rank test (for matched pairs of primary tumors and LN metastases). Three data sets were compared with a nonparametric Kruskal-Wallis test with Dunn’s multiple comparisons. The correlation between the percentage of CLDN18.2-positive tumor cells in primary tumors and corresponding LN metastases was determined using Spearman’s rank correlation. A two-tailed P value of < 0.05 was considered significant.
Results
CLDN18.2 expression in gastric tumors and lymph node metastases of Japanese patients
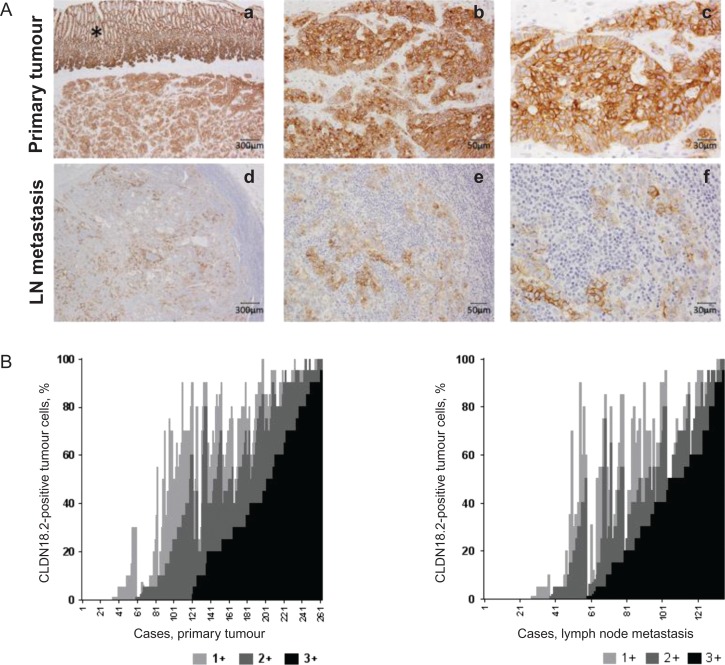
Tissue samples from 263 patients were collected; 134 primary gastric tumors/LN metastases matched pairs, 128 primary GC tumors only, and one LN metastasis only. These tissue samples were assessed and CLDN18.2 was detected in 87% (n = 228/262) of primary tumors and 80% (108/135) of LN metastases (Table 1). Micrographs of representative stained tissues are shown in Fig. 1A. Normal gastric epithelium, known to express CLDN18.2 (14), stained positive; individual tumor samples consisted of a mixture of tumor cells expressing CLDN18.2 at different staining intensities. As such, we correlated the fraction of tumor cells at each staining intensity (1+, 2+, 3+) with the fraction of all stained tumor cells for each individual sample. Primary tumor samples with a high fraction of CLDN18.2 reactivity had a large proportion of tumor cells stained with 3+ intensity (Spearman r = 0.8413; 95% CI 0.8006–0.8743; P < 0.0001, Fig. 1B, left panel). Similarly, a high fraction of tumor cells from LN metastasis showed the strongest correlation with 3+ staining intensity (Spearman r = 0.8297; 95% 0.7664–0.8770; P < 0.0001, Fig. 1B, right panel).
Table 1.
CLDN 18.2 expression in gastric tumor and LN metastases
| Pathological classification | Cases (N = 262) | CLDN18.2 expression | ||||
|---|---|---|---|---|---|---|
| N | % | Any positivity | 2-sided P-value, Fisher’s Exact |
Staining intensity ≥2+ in ≥40% of cells | 2-sided P-value, Fisher’s exact |
|
| n [%] | n [%] | |||||
| Primary stomach cancer | ||||||
| All samples | 262 | 100 | 228 [87.0] | -- | 135 [51.5] | -- |
| Histologic variant (Lauren classification) | ||||||
| Diffuse | 134 | 51.1 | 119 [88.8] | 77 [57.5] | ||
| Intestinal | 59 | 22.5 | 47 [79.7] | >0.05 | 23 [39.0] | 0.019 |
| Mucinous | 7 | 2.7 | 6 [85.7] | 1 [14.3] | ||
| Othera | 10 | 3.8 | 7 [70.0] | -- | 3 [30.0] | -- |
| Missingb | 52 | 19.9 | 49 [94.2] | -- | 31 [59.6] | -- |
| Grading | ||||||
| G1/2 | 69 | 26.3 | 54 [78.3] | 0.034 | 26 [37.7] | 0.005 |
| G3 | 144 | 55.0 | 129 [89.6] | 85 [59.0] | ||
| Missingb | 49 | 18.7 | 45 [91.8] | -- | 24 [49.0] | -- |
| N stage | ||||||
| pN0 | 128 | 48.9 | 117 [91.4] | 0.044 | 66 [51.6] | >0.05 |
| pN+ | 134 | 51.2 | 111 [82.8] | 69 [51.5] | ||
| T stage | ||||||
| pT1/2 | 70 | 26.7 | 65 [92.9] | >0.05 | 35 [50.0] | >0.05 |
| pT3/4 | 192 | 73.3 | 163 [84.9] | 100 [52.1] | ||
| LN metastases | ||||||
| All samples | 135 | 100 | 108 [80.0] | -- | 61 [45.2] | -- |
Abbreviations: %, percentage of N; [%], percentage of n; G, grade; LN, lymph node; N, total number of cases; n, number among N; n/a, not applicable.
aGroup ‘Other’ includes endocrine (n = 3), hepatoid (n = 4), and neuroendocrine (n = 3) tumors.
bGroup ‘Missing’ also includes cases where a classification was not applicable or not assessable.
Figure 1.
Expression of CLDN18.2 in primary gastric tumors and LN metastases.
- Intra-individually matched pairs of samples from a patient with strong CLDN18.2 expression in both the primary tumor (a-c) and corresponding lymph node metastasis (d-f) at three different magnifications.
- The graph depicts the distribution of CLDN18.2 staining intensities in tumor cells from patient samples of primary tumor and LN metastases. CLDN18.2 staining intensity in tumor cells from each tissue sample analyzed were classified as 1+, 2+, and 3+ CLDN18.2 reactivity and are shown.
CLDN18.2, claudin 18.2; LN, lymph node.
*Indicates normal stomach mucosal epithelium expressing CLDN18.2.
Among primary tumor samples, moderate-to-strong CLDN18.2 expression (≥2+ membrane staining intensity in ≥40% of tumor cells; eligibility criterion in FAST) was observed in 52% (n = 135/262) of primary tumors and 45% (n = 61/135) of LN metastases (Table 1). Furthermore, 24% (n = 64/262) of all specimens screened displayed 2+/3+ CLDN18.2 staining in at least 70% of the tumor cells.
As shown in Table 1, primary tumor samples with undifferentiated grade 3 tumors were more prevalent (55.0%, n = 144/262) than moderately differentiated or well-differentiated grade 1 and 2 tumors (26.3%, n = 69/262). The number of GC samples with pathologically positive LN metastases (pN+) was 51.2% (n = 134/262) and the number of those without LN metastases (pN0) was 48.9% (n = 128/262). More than half of the analyzed primary tumor samples were diffuse variants according to the Lauren classification (51.1%, n = 134/262), whereas the intestinal variant comprised the second largest group (22.5%, n = 59/262). The majority of samples were obtained from advanced tumors (pT3/4; 73.3%, n = 192/262). Examination of CLDN18.2 expression in tumors at different grades, stages, or histology revealed that loss of CLDN18.2 expression was significantly more likely in patients with differentiated lower-grade (G1/2) tumors (P = 0.034) and in nodal-positive disease (P = 0.044) than in undifferentiated and in nodal-negative tumors, respectively. Furthermore, these data suggest that moderate-to-strong and homogenous expression levels of CLDN18.2 (at least 40% of the tumor cells stained at 2+/3+ intensity) positively correlated with the diffuse histologic variant (57.5% vs 39.0%, for diffuse and intestinal variants, respectively; P = 0.019), as well as tumors with a higher grade of dedifferentiation (59.0% vs 37.7% for G3 and G1/2 tumors, respectively; P = 0.005). Taken together, these data suggest CLDN18.2 expression is well preserved in gastric adenocarcinoma from Japanese patients. Prevalence of CLDN18.2 expression in GC of Japanese patients is similar to those in the European population, and high expression of this biomarker correlates with unfavorable prognostic factors.
CLDN18.2 expression in matched pairs of primary tumors and lymph node metastases
The persistence of CLDN18.2 expression in metastases was assessed in 134 matched pairs of primary tumor and LN metastasis samples, as well as in the one LN metastasis-only sample (total 135). CLDN18.2 expression was detected in 80% (n = 108/135) of LN metastases; 45% (n = 61/135) of samples expressing CLDN18.2 exhibited 2+/3+ reactivity in at least 40% of tumor cells (Table 1).
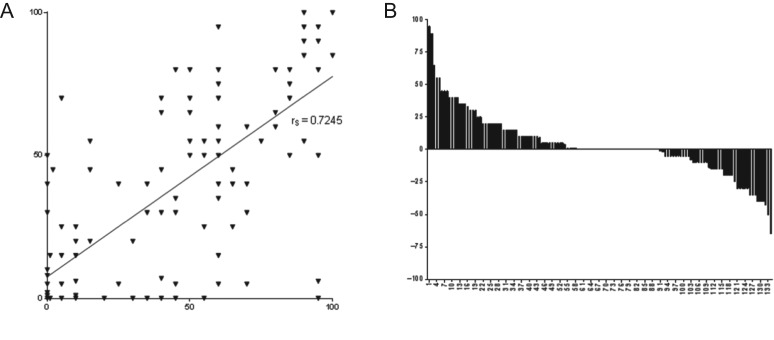
A comparison of metastatic lesions to the primary lesions from which they were derived showed 66% of the paired samples were both CLDN18.2-positive (n = 89/134) and 16% were both CLDN18.2-negative (n = 21/134). In a few cases, CLDN18.2 expression was observed either in the primary tumor (9%, n = 12/134) or in the LN metastasis (9%, n = 12/134). As detailed in Fig. 2A, the fraction of tumor cells expressing CLDN18.2 at any staining intensity strongly correlated between primary tumor and LN metastasis samples in matched pairs (Spearman r = 0.7245, P < 0.0001). In 43% (n = 58/134) of matched samples, the fractions of CLDN18.2-positive cells in matched primary tumors and LN metastases were comparable (within 5% of each other) (Fig. 2B). In 33% (n = 44/134) of samples, the percentage of stained cells was higher in the primary tumors than in LN metastases, and in 24% (n = 32/134) of samples, the percentage of stained cells was higher in LN metastases than in the primary tumors. Thus, CLDN18.2 expression was frequently maintained during the metastatic dissemination of GC within an individual patient.
Figure 2.
CLDN18.2 expression in matched pairs of primary tumors and corresponding LN metastases.
- The percentage of CLDN18.2-positive tumor cells in the primary tumor is indicated on the X axis, the percentage in the corresponding LN metastasis on the Y axis. Each ▾ represents one patient. Line shows Spearman’s correlation of stained tumor cells in primary tumors and LN.
- Difference in relative fraction of positive tumor cells for each case of intra-individually matched sample.
LN, lymph node.
Discussion
In GC, CLDN18.2 is targeted as a differentiation molecule that is highly specific for the gastric mucosa cell lineage, and largely maintained upon malignant transformation. The objective of this study was to assess the GC-associated expression of CLDN18.2 in a Japanese population with GC, with the assay recently used in the patient selection for FAST, a randomized phase 2 clinical trial investigating the anti-CLDN18.2 antibody zolbetuximab. Our key finding was that in this particular sample collection the prevalence of CLDN18.2 expression in Japanese patients with gastric adenocarcinoma (87%) was slightly higher than the European patient population (77%) (14). Previous reports described CLDN18 being expressed in 30–86% of Japanese patients with GC (15,36,37). As those IHC studies were performed with different detection monoclonal antibodies or immune sera using various scoring algorithms, the range of results was wide and it was not unequivocally clear how many Japanese patients would eventually be eligible for zolbetuximab treatment within a clinical trial. Our study, performed with the same validated assay as used in the FAST study, provides reliable information on the prevalence of CLDN18.2 expression in Japanese patients with GC and can be used as a guide to determine patient eligibility for further exploration of zolbetuximab therapy in the Japanese GC population. Furthermore, moderate-to-strong CLDN18.2 membrane staining in at least 40% of tumor cells was as frequent in Japanese samples as in samples from European GC populations who were treated with, and who experienced a survival benefit from, zolbetuximab in the FAST trial. The fraction of samples with any CLDN18.2 staining was also similar in the European and Japanese cohorts. We also found that high CLDN18.2 expression correlated with the diffuse histologic variant. Diffuse GCs generally also have less treatment options, since the trastuzumab target, HER2, is predominantly expressed in the intestinal variant (38–40).
Some reports suggest that CLDN18.2 expression decreases as the cancer progresses, contributing to the invasive potential of the tumor cells and formation of metastases (41,42). In our study, CLDN18.2 expression in the primary gastric tumors of Japanese patients with GC was maintained upon metastatic spread to the regional LNs. In 80% of samples with LN metastases, primary tumors displayed any CLDN18.2 positivity with about half the tumors showing strong expression. This is consistent with our findings from the FAST trial in Central and Eastern European patients with GC, but further studies are needed to clarify the role of CLDN18.2 in GC progression.
A limitation of this retrospective study is that all tumor samples came from one institution, which raises the possibility that these data do not accurately represent the overall Japanese patient population. However, the large cohort size, together with the long timespan over which these samples were collected, may reduce the risk of these factors having a substantial impact on the results. Moreover, our results show CLDN18.2 expression in Japanese patients with GC is very similar in levels and distribution pattern to that seen in the European population screened for the FAST trial. These data therefore provide a strong support for the feasibility of clinical testing of zolbetuximab in patients with GC in Japan.
Funding
This work was supported by a grant to Ganymed Pharmaceuticals GmbH (formerly Ganymed Pharmaceuticals AG) from the German Cutting Edge Cluster Initiative CI3 (BMBF). As of 2016, Ganymed Pharmaceuticals GmbH is a wholly owned subsidiary of Astellas Pharma, Inc. Financial support for this manuscript development, including medical writing and editorial assistance by Regina Switzer, PhD (SuccinctChoice Medical Communications, Chicago, IL), was provided by Astellas Pharma, Inc. (Northbrook, IL, USA).
Conflict of interest statement
For the work under consideration, RY and KI have nothing to disclose. SM and CR were employees at Ganymed Pharmaceuticals GmbH. ÖT and US (spouses) were co-founders of Ganymed Pharmaceuticals GmbH, both owned stock in Ganymed Pharmaceuticals GmbH, and held patents broadly related to the work. ÖT served as the CEO of Ganymed Pharmaceuticals GmbH until acquired by Astellas Pharma, Inc.
Access to study data
Access to anonymized individual participant level data will not be provided for this trial as it meets one or more of the exceptions described on www.clinicalstudydatarequest.com under ‘Sponsor Specific Details for Astellas.’
Author responsibilities
All authors confirm that they have not previously published or have not submitted the same manuscript elsewhere, all took a significant part in the work and approved the final version of the manuscript, have complied with ethical standards, agree to grant Oxford University Press a license to publish the accepted article when the manuscript is accepted, have obtained all necessary permissions to publish any figures or tables in the manuscript, and assure that the authors will pay for any necessary charges, and have informed all of the persons named in the acknowledgments of papers of their inclusion in this section.
Author contributions
US and ÖT were involved in the conception of the study. CR, RY, and KI contributed to study design and data acquisition. CR performed the statistical analysis. ÖT and SM were integral in the initial development of the manuscript. All authors were involved in data analysis and interpretation and were involved in manuscript review and editorial revisions. All authors are accountable for all aspects of the work, and have approved the manuscript for submission.
References
- 1. Ferro A, Peleteiro B, Malvezzi M, et al. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer 2014;50:1330–44. [DOI] [PubMed] [Google Scholar]
- 2. Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol 2014;20:4483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet 2016;388:2654–64. [DOI] [PubMed] [Google Scholar]
- 4. Abrams JA, Gonsalves L, Neugut AI. Diverging trends in the incidence of reflux-related and Helicobacter pylori-related gastric cardia cancer. J Clin Gastroenterol 2013;47:322–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006;12:354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maconi G, Manes G, Porro GB. Role of symptoms in diagnosis and outcome of gastric cancer. World J Gastroenterol 2008;14:1149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marrelli D, Polom K, de Manzoni G, Morgagni P, Baiocchi GL, Roviello F. Multimodal treatment of gastric cancer in the west: Where are we going? World J Gastroenterol 2015;21:7954–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mizrak Kaya D, Harada K, Shimodaira Y, Amlashi FG, Lin Q, Ajani JA. Advanced gastric adenocarcinoma: optimizing therapy options. Expert Rev Clin Pharmacol 2017;10:263–71. [DOI] [PubMed] [Google Scholar]
- 9. Shitara K, Ohtsu A. Advances in systemic therapy for metastatic or advanced gastric cancer. J Natl Compr Canc Netw 2016;14:1313–20. [DOI] [PubMed] [Google Scholar]
- 10. Jomrich G, Schoppmann SF. Targeted therapy in gastric cancer. Eur Surg 2016;48:278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jorgensen JT, Hersom M. HER2 as a prognostic marker in gastric cancer—a systematic analysis of data from the literature. J Cancer 2012;3:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Apicella M, Corso S, Giordano S. Targeted therapies for gastric cancer: failures and hopes from clinical trials. Oncotarget 2017;8:57654–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kulasingam V, Diamandis EP. Strategies for discovering novel cancer biomarkers through utilization of emerging technologies. Nat Clin Pract Oncol 2008;5:588–99. [DOI] [PubMed] [Google Scholar]
- 14. Sahin U, Koslowski M, Dhaene K, et al. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res 2008;14:7624–34. [DOI] [PubMed] [Google Scholar]
- 15. Sanada Y, Oue N, Mitani Y, Yoshida K, Nakayama H, Yasui W. Down-regulation of the claudin-18 gene, identified through serial analysis of gene expression data analysis, in gastric cancer with an intestinal phenotype. J Pathol 2006;208:633–42. [DOI] [PubMed] [Google Scholar]
- 16. Mitnacht-Kraus R, Kreuzberg M, Utsch M, et al. Preclinical characterization of IMAB362 for the treatment of gastric carcinoma. Poster presented at: European Society for Medical Oncology Annual Congress; September 8–12, 2017; Madrid, Spain.
- 17. Karanjawala ZE, Illei PB, Ashfaq R, et al. New markers of pancreatic cancer identified through differential gene expression analyses: claudin 18 and annexin A8. Am J Surg Pathol 2008;32:188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanaka M, Shibahara J, Fukushima N, et al. Claudin-18 is an early-stage marker of pancreatic carcinogenesis. J Histochem Cytochem 2011;59:942–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee JH, Kim KS, Kim TJ, et al. Immunohistochemical analysis of claudin expression in pancreatic cystic tumors. Oncol Rep 2011;25:971–78. [DOI] [PubMed] [Google Scholar]
- 20. Micke P, Mattsson JS, Edlund K, et al. Aberrantly activated claudin 6 and 18.2 as potential therapy targets in non-small-cell lung cancer. Int J Cancer 2014;135:2206–14. [DOI] [PubMed] [Google Scholar]
- 21. Woll S, Schlitter AM, Dhaene K, et al. Claudin 18.2 is a target for IMAB362 antibody in pancreatic neoplasms. Int J Cancer 2014;134:731–39. [DOI] [PubMed] [Google Scholar]
- 22. Keira Y, Takasawa A, Murata M, et al. An immunohistochemical marker panel including claudin-18, maspin, and p53 improves diagnostic accuracy of bile duct neoplasms in surgical and presurgical biopsy specimens. Virchows Arch 2015;466:265–77. [DOI] [PubMed] [Google Scholar]
- 23. Shinozaki A, Shibahara J, Noda N, et al. Claudin-18 in biliary neoplasms. Its significance in the classification of intrahepatic cholangiocarcinoma. Virchows Arch 2011;459:73–80. [DOI] [PubMed] [Google Scholar]
- 24. Heinz C, Mitnacht-Kraus R, Kreuzberg M, Wöll S, Sahin U, Türeci Ö Preclinical evaluation of the anti-CLDN18.2 antibody, IMAB362, in pancreatic carcinoma. Poster presented at: European Society for Medical Oncology Annual Congress; September 8–12, 2017; Madrid, Spain.
- 25. Schuler M, Al-Batran S, Zvirbule Z, et al. Final results of the FAST study, an international, multicenter, randomized, phase II trial of epirubicin, oxaliplatin, and capecitabine (EOX) with or without the anti-CLDN18.2 antibody IMAB362 as first-line therapy in patients with advanced CLDN18.2+ gastric and gastroesophageal junction (GEJ) adenocarcinoma. Ann Oncol 2016;27:vi218. [Google Scholar]
- 26. Trarbach T, Schuler M, Zvirbule Z, et al. Efficacy and safety of multiple doses of IMAB362 in patients with advanced gastro-esophageal cancer: results of a phase II study. Ann Oncol 2014;25:iv210–53. [Google Scholar]
- 27. Lordick F, Schuler M, Al-Batran S, et al. Claudin 18.2—a novel treatment target in the multicenter, randomized, phase II FAST study, a trial of epirubicin, oxaliplatin, and capecitabine (EOX) with or without the anti-CLDN18.2 antibody IMAB362 as 1st line therapy in advanced gastric and gastroesophageal junction (GEJ) cancer. Ann Oncol 2016;27:ix68–85. [Google Scholar]
- 28. Al-Batran SE, Schuler MH, Zvirble Z, et al. FAST: an international, multicenter, randomized, phase II trial of epirubicin, oxaliplatin, and capecitabine (EOX) with or without IMAB362, a first-in-class anti-CLDN18.2 antibody, as first-line therapy in patients with advanced CLDN18.2+ gastric and gastroesophageal junction (GEJ) adenocarcinoma [abstract LBA4001]. J Clin Oncol 2016;34:LBA4001. [Google Scholar]
- 29. Morlock R, Turnbull J, Blahut S, Krukas-Hampell M, Hawryluk E, Türeci Ö. Health-related quality-of-life results from the FAST study, a phase II trial of epirubicin, oxaliplatin, and capecitabine with or without IMAB362 in patients with advanced CLDN18.2+ gastric and gastroesophageal junction adenocarcinoma. J Clin Oncol 2018;36:54. [Google Scholar]
- 30. Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 2015;21:449–56. [DOI] [PubMed] [Google Scholar]
- 31. Lin SJ, Gagnon-Bartsch JA, Tan IB, et al. Signatures of tumour immunity distinguish Asian and non-Asian gastric adenocarcinomas. Gut 2015;64:1721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steinberg ML, Hwang BJ, Tang L, Shah MA. E-cadherin gene alterations in gastric cancers in different ethnic populations. Ethn Dis 2008;18:S2–70–4. [PubMed] [Google Scholar]
- 33. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 34. Japanese Gastric Cancer Association Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101–12. [DOI] [PubMed] [Google Scholar]
- 35. Fenoglio-Preiser C, Muñoz N, Carneiro F, et al. Gastric carcinoma In: Hamilton S, Aaltonen L, editors. Pathology and Genetics of Tumours of the Digestive System. Lyon, France: IARC Press, 2000;37–50. [Google Scholar]
- 36. Matsuda Y, Semba S, Ueda J, et al. Gastric and intestinal claudin expression at the invasive front of gastric carcinoma. Cancer Sci 2007;98:1014–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sentani K, Oue N, Tashiro T, et al. Immunohistochemical staining of Reg IV and claudin-18 is useful in the diagnosis of gastrointestinal signet ring cell carcinoma. Am J Surg Pathol 2008;32:1182–89. [DOI] [PubMed] [Google Scholar]
- 38. Cappellesso R, Fassan M, Hanspeter E, et al. HER2 status in gastroesophageal cancer: a tissue microarray study of 1040 cases. Hum Pathol 2015;46:665–72. [DOI] [PubMed] [Google Scholar]
- 39. Tanner M, Hollmen M, Junttila TT, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase II alpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol 2005;16:273–78. [DOI] [PubMed] [Google Scholar]
- 40. Stahl P, Seeschaaf C, Lebok P, et al. Heterogeneity of amplification of HER2, EGFR, CCND1 and MYC in gastric cancer. BMC Gastroenterol 2015;15:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oshima T, Shan J, Okugawa T, et al. Down-regulation of claudin-18 is associated with the proliferative and invasive potential of gastric cancer at the invasive front. PLoS One 2013;8:e74757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jun KH, Kim JH, Jung JH, Choi HJ, Chin HM. Expression of claudin-7 and loss of claudin-18 correlate with poor prognosis in gastric cancer. Int J Surg 2014;12:156–62. [DOI] [PubMed] [Google Scholar]