Abstract
Background:
Given its hormonal activity, bisphenol S (BPS) as a substitute for bisphenol A (BPA) could actually increase the risk of endocrine disruption if its toxicokinetic (TK) properties, namely its oral availability and systemic persistency, were higher than those of BPA.
Objectives:
The TK behavior of BPA and BPS was investigated by administering the two compounds by intravenous and oral routes in piglet, a known valid model for investigating oral TK.
Methods:
Experiments were conducted in piglets to evaluate the kinetics of BPA, BPS, and their glucuronoconjugated metabolites in plasma and urine after intravenous administration of BPA, BPS, and BPS glucuronide (BPSG) and gavage administration of BPA and BPS. A population semiphysiologically based TK model describing the disposition of BPA and BPS and their glucuronides was built from these data to estimate the key TK parameters that drive the internal exposure to active compounds.
Results:
The data indicated that almost all the BPS oral dose was absorbed and transported into the liver where only 41% of BPS was glucuronidated, leading to a systemic bioavailability of 57.4%. In contrast, only 77% of the oral dose of BPA was absorbed and underwent an extensive first-pass glucuronidation either in the gut (44%) or in the liver (53%), thus accounting for the low systemic bioavailability of BPA (0.50%). Due to the higher systemic availability of BPS, in comparison with BPA, and its lower plasma clearance (3.5 times lower), the oral BPS systemic exposure was on average about 250 times higher than for BPA for an equal oral molar dose of the two compounds.
Conclusion:
Given the similar digestive tracts of pigs and humans, our results suggest that replacing BPA with BPS will likely lead to increased internal exposure to an endocrine-active compound that would be of concern for human health. https://doi.org/10.1289/EHP4599
Introduction
Due to concerns about its safety, the use of bisphenol A (BPA) has been restricted, and structural analogs, especially bisphenol S (BPS), have mainly replaced BPA in consumer products (ANSES 2013). As a result of its use to manufacture epoxy resins for inner coating of food cans and packaging containers, the dominant source of human exposure to BPS is considered to be contaminated food (Wu et al. 2018). The use of BPS as a color developer of thermal papers (Wu et al. 2018) may also contribute to human exposure through dermal and hand-to-mouth oral exposures. BPS has thus been detected in 89.4% of urine samples from a representative cohort of the U.S. population (, Lehmler et al. 2018), with median urinary BPS concentrations of . Similar median levels of BPS exposure were reported in a study conducted in pregnant women from Netherlands (; Philips et al. 2018). The increased frequency of BPS detection in urine samples collected between 2000 and 2014 () in U.S. adult volunteers reflects the reality of substituting BPA with BPS (Ye et al. 2015). The prevalence and level of human exposure may also be increased by potential accumulation of BPS in the environment resulting from its lower biodegradability, in comparison with BPA, in seawater (Danzl et al. 2009).
In vitro studies have demonstrated the endocrine activity of BPS, leading authors to raise the alert about its health hazard potential and the risk of such substitution (Rochester and Bolden 2015). BPS, like BPA, has been shown to display estrogenic activity via nuclear receptors, the potency of the effects depending on the in vitro assay systems used and their related endpoints. Hence, BPS showed less potent estrogen activities than BPA in a human ovarian adenocarcinoma cell line (Rosenmai et al. 2014) but was equipotent to BPA in human breast cancer MCF-7 cells (Kuruto-Niwa et al. 2005). The ability of BPS to act as an estrogen receptor (ER) agonist may result from its binding to ER and (Molina-Molina et al. 2013). The lower affinity of human for BPS than for BPA, reported by the former authors, is in agreement with its recently demonstrated lower potent estrogenic activity via human and in comparison with BPA (Kojima et al. 2019). Although these effects via nuclear receptors are observed at micromolar concentrations, some studies have demonstrated that at picomolar (pM) concentrations, BPS can activate nongenomic signaling pathways in pituitary cells, as does BPA (Viñas and Watson 2013). BPS has also been shown to decrease testosterone secretion ex vivo by mouse fetal testes at a lower concentration than BPA (Eladak et al. 2015), whereas, in vitro, the reduction of testosterone secretion by a human adrenocortical carcinoma cell line required higher BPS concentrations (Goldinger et al. 2015). Additionally, similar effects of BPA and BPS have been reported on lipid accumulation and the expression of adipogenic markers in human preadipocytes (Boucher et al. 2014, 2016), the potency of the adipogenic effects of BPS on a preadipocyte murine line being even greater than that of BPA (Ahmed and Atlas 2016). Although limited in number, in vivo studies have also evidenced similar effects of BPA and BPS treatments on mammary gland development that was accelerated in mice prenatally exposed to BPA or BPS (, Tucker et al. 2018), a higher incidence of mammary lesions being observed following prenatal exposure to BPS at . The decrease of protein contents and testosterone concentrations of the rat testes after subchronic oral treatments with of either BPA or BPS has been associated with a reduction in the height of the epithelial tissues of seminiferous tubules (Ullah et al. 2018). Adverse reproductive outcomes were also observed in female rats subcutaneously treated with either BPA or BPS (5 and ) during the neonatal period of life, including delayed onset of puberty, a disrupted pattern of estrous cyclicity, and detrimental effects on the ovarian development and function (Ahsan et al. 2018).
The harmful consequences of the chemical substitution of BPA with BPS for health may be further exacerbated if the toxicokinetic (TK) properties of BPS increase its bioavailability and enhance its persistence in the body, thereby resulting in higher plasma concentrations of BPS than of BPA for the same external exposure level. Indeed, the amounts of BPA that can reach the target tissues and exert effects are dependent on plasma concentrations, these latter being related to the dose by a key TK parameter, namely the blood (plasma) clearance in addition to the bioavailability. Bioavailability, which corresponds to the amount of substance reaching the systemic circulation unchanged, is determined by both the extent of gastrointestinal absorption and of gut and hepatic first-pass elimination when exposure occurs via the oral route. Due to the extensive first-pass glucuronidation of orally administered BPA (Völkel et al. 2002) and its high plasma clearance (Collet et al. 2015), the concentrations of unconjugated BPA in adult human plasma are predicted to be very low (in the pM range, Gauderat et al. 2017; Teeguarden et al. 2013), the predominant form of circulating BPA being BPA glucuronide (BPAG) (Völkel et al. 2002).
Due to their lack of estrogenicity (Matthews et al. 2001; Skledar et al. 2016), systemic exposure to bisphenol conjugated metabolites is not taken into account for risk assessment purposes. However, in vitro studies have suggested that BPAG may exert biological activities similar to (Boucher et al. 2015) or different from those of the parent compound (Viñas et al. 2013). Although it cannot be excluded that a possible back conversion of BPAG to its unconjugated form might account for its effect on adipocyte differentiation (Gayrard et al. 2015), the effect of BPAG on some estrogenic signaling pathways in estrogen-responsive prolactinoma cells, as opposed to that of BPA, suggests that BPAG on its own may have the ability to interfere with the actions of estrogens (Viñas et al. 2013). The fact that bisphenol glucuronides cannot be considered as totally inactive raised the need to evaluate systemic exposures to both the parent and its glucuronidated metabolites.
Currently, the limited TK and metabolic data of BPS suggest that although BPS, like BPA, is predominantly metabolized by conjugation reactions (Le Fol et al. 2015; Skledar et al. 2016; Zhou et al. 2014), the TK behavior of BPS may differ from that of BPA. Indeed, Oh et al. (2018) showed that after oral dosing in humans, the fraction of plasma BPS that was unconjugated (28%) was much higher than the corresponding value for BPA (0.5%; Thayer et al. 2015). Recently, these human biomonitoring data have been used to calibrate a physiologically based toxicokinetic model for BPS oral exposure (Karrer et al. 2018) and to predict human serum concentration profiles of unconjugated BPS. The modeled higher systemic exposure to BPS in comparison with BPA has highlighted the need for experimental data to further elucidate the TK behavior of BPS.
In that context, the objective of the present study was to develop a data-driven semiphysiologically based TK model from data obtained following intravenous administration of BPA, BPS, and BPS glucuronide (BPSG) and gavage administration of BPA and BPS in piglets, described as a relevant species for investigating oral TK in humans (Kararli 1995). This approach, by enabling a comparison of key TK parameters of BPA and BPS, namely plasma and renal clearances, oral bioavailability, and glucuronidation, should provide new insights for assessing the hazards of BPA substitution.
Methods
Animals
The animals used in this study were treated humanely and with regard for the alleviation of suffering. All animal procedures were carried out in accordance with accepted standards of humane animal care under agreement number 31-2011-142 from the French Ministry of Agriculture. The protocol was approved by the regional ethics committee (Midi-Pyrénées: protocol APAFiS #12,367). The experiments were carried out on 27 piglets of Large White breed (11 males and 16 females) originating from three farms in the south of France (GIE Villefranche Grand Sud-Villefranche de Rouergue, Marquié-Saint Ybars, and Calvignac-St. Vincent d’Autejac), age 28 to 42 d and with body weights (BW) between 8.75 and .
The piglets were housed in a force-ventilated room and had free access to drinking water. Piglets weighing less than were fed a postweaning food (PrimeFeed; Vilofoss), the piglets over were fed with a flour-based growth food (PS2; Solieval). The room was illuminated by artificial light on a 12-h light/dark cycle, and the temperature was maintained at ∼ 25°C.
Experimental Design and Dosing
Table 1 summarizes the protocol of the experiments and the plasma and urine datasets further considered for TK analyses. A first experiment (Exp. 1) was performed in 8 male piglets age 42 d ( BW) to investigate oral BPA TK. At a 7-d interval, piglets were administered BPA by intravenous route (IV) and by gavage at respective doses of (), and (), using a two-treatment, two-sequence, two-period crossover design. BPA data obtained after IV BPA administrations had already been used to evaluate human BPA clearance using an allometric approach (Collet et al. 2015).
Table 1.
Flowchart of the objectives, design and plasma and urine datasets in the experiments evaluating BPA and BPS toxicokinetics (TK).
| Experiment | Objectives | What was done | Data sets |
|---|---|---|---|
| 1 | Investigate BPA TK after IV and oral administrations | IV and oral BPA dosing in 8 piglets at a 7-d interval | - Serial jugular venous plasma samples obtained before and at 30 min and 1, 2, 3, 4, 6, 8, 12, 15, 18, and 24 h after BPA administration (and after 15 min for IV route) |
| - Total urine collected after each natural micturation until 24 h after BPA administration | |||
| 2 | Assess the elimination of BPA/BPAG in urine | IV administration of BPA at a dose of in 3 piglets | - Serial jugular venous plasma samples taken before and at 15min, 30 min, and 1, 2, 3, 4, 6, 8, 10, 12, and 24 h after BPA administration |
| - Total urine collected at 3, 6, 9, 12, 15, 18, 21, 24, 28, 31, 35, 37, 45, and 48.5 h after BPA administration | |||
| 3 | Investigate BPS and BPSG TK after IV BPS and BPSG administrations | IV administrations of BPS and BPSG at the respective doses of and in 6 piglets | - Serial jugular venous plasma samples taken before and at 15min, 30 min, and 1, 2, 3, 4, 6, 8, 10, 12, 24, 36, 48, and 72 h after IV BPS and BPSG administrations |
| - Total urine collected at 3, 6, 9, 12, 21, and 24 h after IVBPS and BPSG administrations | |||
| 4 | Evaluate the extent of BPS absorption by oral route and measure the oral bioavailability of BPS | IV BPS administration of BPS followed by BPS administration by orogastric gavage in 6 piglets | - Serial jugular venous plasma samples at 15 min, 30 min, and 1, 2, 3, 4, 6, 8, 10, 12, 24, 36, 48, and 72 h after BPS administration by IV route and orogastric gavage |
| - Total urine collected at 3, 6, 9, 12, 21, and 24 h after BPS administration by IV route and orogastric gavage (and after 1 h only for IV route) | |||
| 5 | Compare BPA and BPS disposition after IV and oral BPA administration using a cocktail approach | IV administrations of both BPA and BPS and administrations of both BPS and BPA by orogastric gavage in 4 piglets | - Serial jugular venous plasma samples taken before and at 2, 5, 15, 30 min, and 1, 2, 3, 4, 6, 8, 12, 24, 34, 48, and 72h after BPA and BPS administrations |
| - Total urine collected at 3, 6, 9, 12, and 24 h after BPA and BPS administrations |
Note: BPA, Bisphenol A; BPAG, Bisphenol A glucuronide; BPS, Bisphenol S; BPSG, Bisphenol S glucuronide; IV, Intravenous; TK, Toxicokinetic.
A second experiment (Exp. 2) designed to assess the elimination of BPA and BPAG in urine involved IV administration of BPA at a dose of in 3 male piglets age 38 d ( BW). A third experiment (Exp. 3) was performed in 6 female piglets age 28 d ( BW) to investigate both BPS and BPSG TK after IV administration. Each piglet received two successive IV administrations of BPS and BPSG at respective doses of () and (), 72 h apart.
A fourth experiment (Exp. 4) was performed in 6 female piglets age 28 d ( BW) to specifically evaluate the extent of BPS absorption by oral route and to measure the systemic bioavailability of BPS. The experiment was divided into two periods separated by 72 h, during which the piglets were administered BPS respectively at the dose of () by IV route and at a dose of () by orogastric gavage.
Finally, a fifth experiment (Exp. 5) was performed in 4 female piglets age 28 d ( BW) to directly compare BPA and BPS disposition after IV and oral administration using a cocktail approach. The experiment was divided into two periods separated by 4 d during which the piglets received administrations of both BPA and BPS by IV and oral routes at respective doses of ( for BPA and for BPS) and ( for BPA and for BPS), using a two-treatment, two-sequence, two-period crossover design. Selection of the IV and oral BPA and BPS doses was based on previous TK data to achieve plasma concentrations greater than the limit of quantification (LOQ, ) for about 8–10 h, i.e., sufficiently long to observe the terminal phase slope and allow calculation of TK parameters (Collet et al. 2015; Grandin et al. 2017).
Test Materials and Treatments
All materials for the preparation of solutions (including the materials used for sampling, processing, and analysis) were made of glass or BPA- and BPS-free plastic (polypropylene). BPA (purity 99%) and BPS (purity ) were purchased from Sigma-Aldrich. BPSG [(Bis(4-hydroxyphenyl) Sulfone Salt, purity 99.54%] was purchased from Toronto Research Chemicals. Carboxymethylcellulose sodium salt and sucrose were obtained from Sigma-Aldrich.
For the IV administrations, BPA powder was dissolved in ethanol–propylene glycol (1:49, vol:vol) at (Exp.1 and 2) and in ethanol–saline (1:2, vol:vol) at (Exp. 5). BPS powder was dissolved in ethanol–saline (1:2, vol:vol) at (Exp. 3 and 4) and (Exp. 5), and BPSG was dissolved in saline at .
For administrations by gavage, BPA powder was dissolved in ethanol–corn oil (1:9, vol:vol) at (Exp. 1). BPS powder was placed in a Luer-lock syringe, mixed with 2.5% (w/v) carboxymethylcellulose in phosphate buffer containing sucrose () using a syringe adaptor, to yield a carboxymethylcellulose gel containing of BPS (Exp. 4). For the cocktail approach, BPA and BPS were both dissolved in ethanol–corn oil (1:7, vol:vol) at (Exp. 5). The volume administered to animals was adjusted to the individual BW recorded during the 2 d preceding BPA, BPS and BPSG administrations. The concentrations of all dosing solutions were verified (Table S1) by Acquity ultra performance liquid chromatography coupled to a Xevo triple quadrupole mass spectrometer (UPLC-MS/MS, Waters Corporation; see the Sample Collection and Analysis section).
BPA and BPS solutions were administered intravenously via an indwelling catheter inserted into the auricular vein just before the administrations and orally by gastric intubation. The animals were fasted overnight prior to administration, had free access to drinking water, and were given a standard meal 3 h postdose. During the first 24 h of sampling, the piglets were housed individually in stainless steel cages.
Sample Collection and Analysis
Serial jugular venous blood samples ( for Exp. 3–5 and for Exp.1 and 2) were taken before and after BPA, BPS, and BPSG administrations. Total urine was collected before and after each micturition (Exp. 1) or at different times regularly spanned over 24 h after dosing (Exp. 2–5, Table 1). Blood samples were collected in heparinized polypropylene tubes, immediately chilled in ice and centrifuged for 10 min at at 4°C, and the supernatant plasma was stored in polypropylene tubes at until assay. Total urine was filtered through a nylon mesh () and collected in glass containers and chilled in ice. The volume of each urine sample and the sampling time were recorded. A urine sample was immediately chilled in ice and centrifuged for 10 min at at 4°C and stored at until assayed.
Analytes were quantified by Acquity UPLC-MS/MS (Waters Corporation). BPA, BPAG, BPS, and BPSG in plasma and urine samples were simultaneously quantified, without resorting to a hydrolysis step, according to the respective methods previously described for BPA and BPAG (Lacroix et al. 2011) and BPS and BPSG analyses (Grandin et al. 2017).
Briefly, samples () were purified by protein precipitation and quantified using , BPS-d8, BPA-G and BPS-G d8 (Toronto Research Chemicals) as internal standards. They were separated on a C18 column (with a water/acetonitrile gradient elution and detected in negative electrospray using the multiple reaction monitoring mode. Chromatographic data were monitored by Targetlynx® software (Waters Corporation). Blanks and quality-control samples were used to monitor potential contamination during analysis and the accuracy and precision of the method. The mean intra- and interday coefficients of variation for three concentration levels of BPA and BPAG and of BPS and BPSG were lower than 15%, respectively. The LOQ values for each molecule in plasma and urine are given for each experiment in the Table S2.
TK Analyses
All TK analyses were performed using the Phoenix® NLME (version 8.0; Pharsight Corporation). The plasma mass concentrations were converted into molar concentrations before analysis. The cumulated molar quantities of excreted BPA (BPAG, BPS, BPSG) in urine were calculated by multiplying the molar concentrations of BPA (BPAG, BPS, BPSG) by the volume of urine at each sampling time.
Noncompartmental Analysis
Plasma concentration–time profiles of BPA, BPAG, BPS, and BPSG were first analyzed according to a noncompartmental approach. The plasma analytes concentration–time data from individual piglets were used to derive the maximum concentration (Cmax), and the time to maximum concentration (Tmax). The area under the plasma concentration–time curves from dosing time to the time of the last measurable concentration () were calculated using the linear trapezoidal rule (Equation 1).
| (1) |
where is the concentration obtained at the time i ().
The area under the plasma concentration time curve from to infinity () was obtained using Equation 2.
| (2) |
where is the area extrapolated from the last observation to infinity, calculated according to Equation 3:
| (3) |
where is the last observed quantifiable plasma concentration, and is the slope of the terminal phase, as estimated by linear regression using the best fit option of Phoenix®. added less than 6% of .
Plasma clearance (Cl) of BPA, BPS and BPSG were obtained using Equation 4:
| (4) |
where Dose is the IV administered BPA (BPS, BPSG) dose and calculated as previously described.
Apparent clearance of BPAG and BPSG (Cl_F) was estimated after respective BPA and BPS IV administrations using the Equation 5:
| (5) |
where Dose is the IV administered BPA (BPS) dose and calculated for BPAG (BPSG) as previously described.
Apparent clearances of BPA and BPAG (BPS and BPSG) were also determined using Equation 5 after respective oral administration of BPA and BPS.
TK Modeling
A nonlinear mixed effect (NLME) modeling approach was then used to analyze data sets from the different experiments simultaneously and to provide robust estimates of the mean BPA and BPS TK parameters and of their between-subject variability. The NLME modeling approach is considered as a powerful tool for applying meta-analysis to diverse TK studies (Li et al. 2015). According to Food and Drug Administration guidance (FDA 1999), this TK approach can be used to analyze data from a variety of unbalanced designs, i.e., study designs in which not all the individuals supply the same amount of information. The NLME model was therefore developed by combining the plasma and urinary data sets from the five TK experiments carried out in piglets, except for the urinary BPA concentration obtained after oral administration of BPA. Indeed, after oral administration, the ratio of urinary BPAG/BPA concentrations can be very large () and even a minimal post-sampling hydrolysis of BPAG, e.g., ) could be sufficient to severely bias the observed urinary BPA concentrations. The urinary BPS concentrations obtained after oral administration of BPS were kept because fitting was systematically improved when considering these data. For plasma concentrations, only concentrations above the LOQ were considered; indeed as fewer than 10% of the plasma concentrations were below the LOQ, they could be ignored without the risk of bias (Byon et al. 2008). For urine the cumulative amount of BPA (BPS) and BPAG (BPSG) excreted at different sampling times was computed, and about 5 or 6 cumulated amounts, regularly spanned over the entire elimination period, were considered for data fitting.
Different compartmental models and submodels were tested and compared using the likelihood ratio test (LRT). The primary parameters of the model, namely the volumes of distribution (, , , ) and the first-order rate constants () and their associated precisions [standard error (SE)] were estimated by minimizing an objective function value (OFV) expressed as minus twice the log of the likelihood estimation ().
The between-subject variability (BSV) of the parameters was evaluated using the exponential model , where is the parameter estimated for the ith individual, is the typical population value of the parameter, and is the deviation associated with the ith individual from the corresponding value of the parameter at the population level. The distribution of was assumed to be normal with a mean of 0 and a variance . The BSV was reported as a coefficient of variation (CV) in the original scale . This random component was estimated for those parameters for which the shrinkage for calculated as was lower than 30%, SD (EBE) being the standard deviation of the individual values of the Empirical Bayesian Estimates (EBE) of .
The residual variance was modeled using a combined additive and proportional error model where and are respectively the jth observed and predicted concentrations for the ith individual and and the multiplicative and additive residual errors.
The bootstrap tool (20 replicates) was used to evaluate the mean, median, SE, and coefficient of variation of the parameter estimates. The predictive ability of the model was checked graphically by plotting Visual Predictive Check plots to compare the observed data with the selected quantiles (here 20th, 50th and 80th percentiles) of data simulated using the model and obtained from 200–1,000 replicates from each animal based on the structure of the original data (i.e., dosing, timing and number of samples).
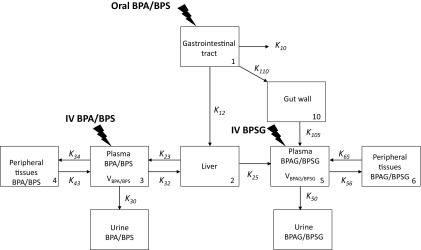
After exploration and comparison of different structural compartmental models, a 9-compartmental model based on the LRT, the Akaike Information Criterion (AIC), and inspection of different diagnostic plots (vide infra) was finally selected. For the LRT test, the critical value of the distribution considered for a given nominal risk and a given number of degrees of freedom was obtained using the Excel function CHISQ.INV.RT(). This model (Figure 1) was selected to better describe and understand the fate of BPA and BPS, i.e., to separately estimate absorption vs. bioavailability and to qualify the processes of the first-pass effect with a gut vs. a liver first-pass effect from the primary parameters of the compartmental model.
Figure 1.
Schematic representation of the compartmental model. Note: The model included both a central and a peripheral compartment, respectively CP3 and CP4 for BPA (BPS) and CP5 and CP6 for BPAG (BPSG). The volumes of the BPA (BPS) and BPAG (BPSG) central compartments are respectively and . The link between the two central compartments was modeled with a compartment (CP2) that should be viewed as the liver. For the oral route, BPA (BPS) is administered in the gastrointestinal tract represented by CP1. A fraction of BPA (BPS) is absorbed by the enterocytes by passive diffusion to pass directly into the portal blood via ; a second fraction () is absorbed by enterocytes and locally subjected to glucuronidation after which the formed BPAG (BPSG) is absorbed into the systemic circulation and reaches CP5 via . A third fraction of the oral BPA (BPS) dose is directly eliminated by the digestive tract via . The fraction of BPA passing into the portal blood via is subjected to a hepatic first-pass effect which transforms part of the BPA (BPS) reaching the liver via into BPAG (BPSG) that passes into CP5. The fraction of absorbed BPA that is not hepatically transformed reaches the CP3 via . The exchanges between CP2 and CP3 are bilateral, being the rate constant between CP3 and CP2 and the rate constant between CP2 and CP3. The urinary elimination of BPA (BPS) and BPAG (BPSG) was modeled with a first-order rate constant designated and for BPA (BPS) and BPAG (BPSG), respectively. It was assumed that no phase-two metabolite other than BPAG (BPSG) was formed. BPA, Bisphenol A; BPAG, Bisphenol A glucuronide; BPS, Bisphenol S; BPSG, Bisphenol S glucuronide; CP: Compartment; IV, Intravenous; , Rate constant corresponding to the unabsorbed BPA (BPS) from the gastrointestinal tract; , Rate constant of BPA (BPS) absorbed by the enterocytes passing directly into the portal blood; , , , and , Distribution rate constants between the plasma and the peripheral compartments for BPA (BPS); , , and Distribution rate constants between the plasma and the peripheral compartments for BPAG (BPSG); , BPA (BPS) elimination rate constant from plasma to urine; , BPAG (BPSG) elimination rate constant from plasma to urine; , Rate constant of BPA (BPS) absorbed by the enterocytes locally subjected to glucuronidation; , Rate constant of BPAG (BPSG) formed by the enterocytes absorbed into the systemic circulation; Volume of the BPA (BPS) central compartment; , Volume of the BPAG (BPSG) central compartment.
The urinary clearance values for bisphenols (BPs), BPA, and BPS, namely, , and their metabolites (BPs metabolite), BPAG and BPSG, namely , were estimated from their respective population primary parameters using Equation 6 and Equation 7.
| (6) |
where is the volume of the BPs central compartment, and is the BPs elimination rate constant from plasma to urine.
| (7) |
where is the volume of central compartment of the BPs metabolites, and is the elimination rate constant of the BPs metabolites from plasma to urine.
The fraction of BPA (BPS) that was absorbed by enterocytes (Fabs) was estimated using Equation 8:
| (8) |
where is the rate constant corresponding to the unabsorbed BPs from the gastrointestinal tract, is the rate constant of BPs absorbed by the enterocytes passing directly into the portal blood, and is the rate constant of BPs absorbed by the enterocytes locally subjected to glucuronidation.
The fraction of administered BPA (BPS) that was transformed into BPAG (BPSG) by the gut wall (Fgutwall) was estimated by Equation 9:
| (9) |
with , , and as defined above.
The fraction of administered BPA (BPS) that was absorbed as BPA (BPS) to reach the liver (Fliver) was estimated using Equation 10:
| (10) |
with , , and as defined above.
The fraction of the administered BPA (BPS) dose absorbed as BPA (BPS) and subsequently undergoing a hepatic first-pass effect (Hepatic_firstpass) was estimated with Equation 11:
| (11) |
where is the distribution rate constant between the liver and the plasma compartments for BPs, and is the distribution rate constant between the liver and the plasma compartments for BPs metabolites.
The overall bioavailability of BPA (BPS), i.e., the percentage of the BPA (BPS) dose that actually reached the systemic circulation was estimated by combining equations 10 and 11 as Equation 12.
| (12) |
with , , , ; as defined above.
Statistical Analyses
All results are presented as . ANOVA tests and SYSTAT 12 (version 12; Systat Software, Inc.) were used to analyze the differences between the mean log-transformed BPA and BPS TK parameter values, except those related to the highly dissimilar BPA and BPS plasma concentrations obtained after oral dosing (, Cmax/dose, Cl_F).
Results
BPA and BPS were not detected in any of the control samples obtained before the administrations, suggesting that little to no sample contamination had occurred during sample collection, processing, and assay.
Internal Exposure after IV and Oral Dosing
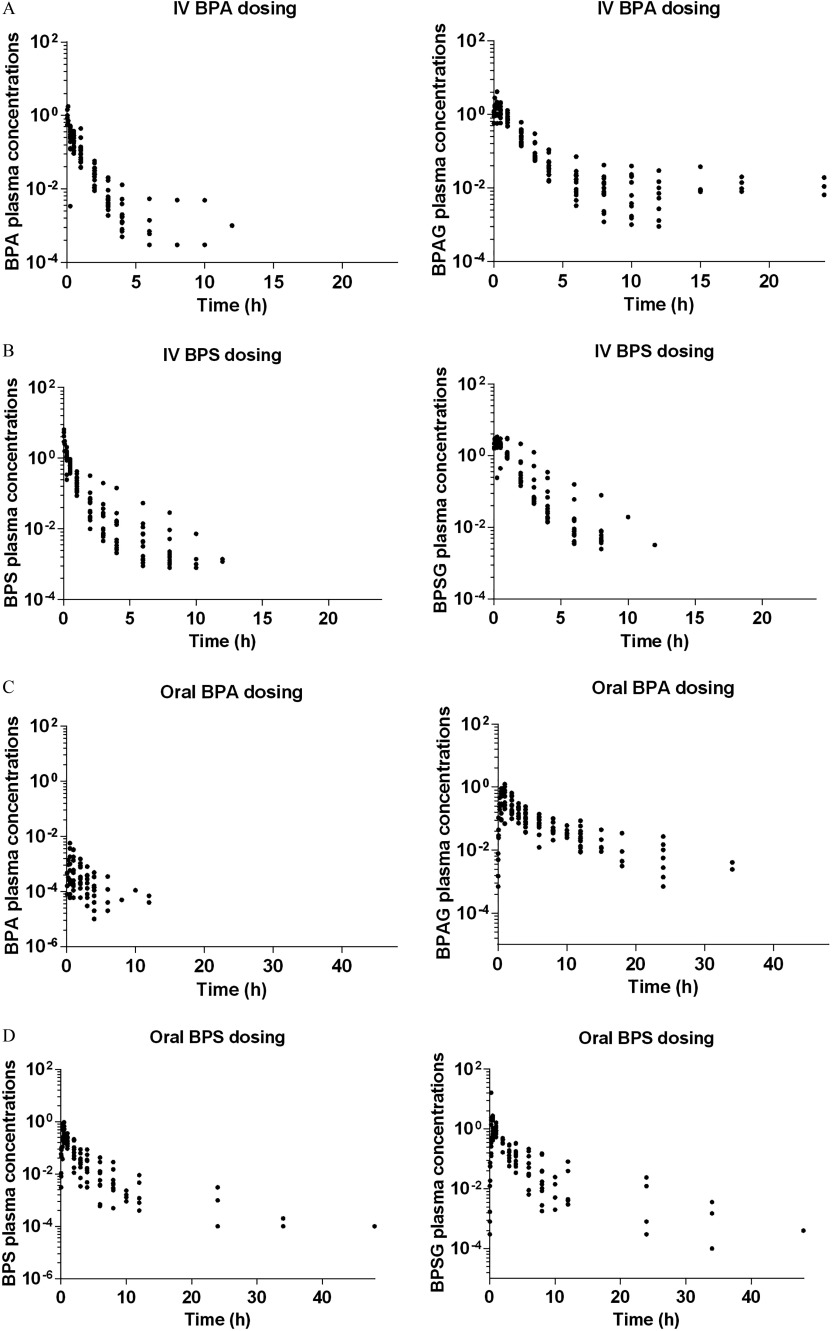
Figures 2A and 2B show the time course of individual plasma concentrations relative to the dose of BPA and BPAG (Figure 2A) and of BPS and BPSG (Figure 2B) after IV dosing. BPA, BPAG (Figure 2A), BPS, and BPSG plasma concentration–time plots (Figure 2B) were obtained after both a single IV administration of BPA at (Exp. 1 and 2, ) or BPS at (Exp. 3 and 4, ) and simultaneous IV administrations of BPA and BPS at (Exp. 5, ). Table 2 gives the values estimated by noncompartmental analysis for TK parameters of BPA and BPAG vs. BPS and BPSG after the corresponding IV BPA and BPS administrations. After IV administration to piglets, plasma BPA and BPS concentrations decreased rapidly to reach values below the LOQ, beyond 12 h after administration. Mean BPS plasma clearance was about 3.5 times lower than BPA clearance ( vs. , ). The time–concentration curves of BPAG and BPSG plasma concentrations observed after IV BPA and BPS dosing showed a comparable decay, with maximal plasma BPAG and BPSG concentrations being obtained about 20 min after administration and decreasing slowly to values below the LOQ of the assay, beyond about 12–24 h and 8–12 h after the IV administrations, respectively. The mean apparent clearance of BPSG was of the same order as that of BPAG ( vs. ). This value was close to the BPSG plasma clearance determined after direct IV BPSG dosing ().
Figure 2.
Semilogarithmic plots of individual plasma concentrations relative to dose ( BW) vs. time (h) of BPA and BPAG after IV (Figure 1A) and oral BPA dosing (Figure 1C) and of BPS and BPSG after IV (Figure 1B) and oral BPS dosing (Figure 1D) in piglets. BPA, Bisphenol A; BPAG, Bisphenol A glucuronide; BPS, Bisphenol S; BPSG, Bisphenol S glucuronide; BW, Body Weight; IV, Intravenous.
Table 2.
Toxicokinetic parameters of BPA and BPS () estimated by noncompartmental analysis after BPA and BPS IV dosing.
| Toxicokinetic parameter | BPA IV dosing () | BPS IV dosing () | ||
|---|---|---|---|---|
| BPA | BPAG | BPS | BPSG | |
| Cmax/dose ( BW) | * | |||
| Tmax (h) | NA | NA | ||
| () | * | |||
| Cl () | NA | * | NA | |
| Cl_F () | NA | NA | ||
Note: The toxicokinetic parameters after IV dosing were estimated from datasets obtained after a single IV administration of BPA at (Exp. 1–2, ), a single IV administration of BPS at (Exp. 3–4, ), and simultaneous IV administrations of BPA and BPS at the same dosage (Exp. 5, ). Dose scaled area under the plasma concentration–time curve from dosing time to the time of the last measurable plasma concentration;BPA, Bisphenol A; BPAG, Bisphenol A glucuronide; BPS, Bisphenol S; BPSG, Bisphenol S glucuronide.; BW, Body weight; Cl, Clearance; Cl_F, Apparent clearance; Cmax/dose, Dose scaled maximal plasma concentration; IV, Intravenous; NA, not applicable; Tmax, Time of Cmax.
Significantly different from mean values obtained with BPA (, ANOVA test).
Figures 2C and 2D show the time course of individual plasma concentrations relative to the dose of BPA and BPAG (Fig 2C) and of BPS and BPSG (Fig 2D) after oral dosing. BPA, BPAG (Figure 2C), BPS, and BPSG plasma concentration–time plots (Figure 2D) were obtained after both a single oral administration of BPA at (Exp. 1, ) or BPS at (Exp. 4, ) and simultaneous oral administrations of BPA and BPS at (Exp. 5, ). Table 3 gives the values estimated by noncompartmental analysis for TK parameters of BPA and BPAG vs. BPS and BPSG after respective oral BPA and BPS administrations.
Table 3.
Toxicokinetic parameters of BPA and BPS () estimated by non-compartmental analysis after BPA and BPS oral dosing.
| Toxicokinetic parameter | BPA oral dosing () | BPS oral dosing () | ||
|---|---|---|---|---|
| BPA | BPAG | BPS | BPSG | |
| Cmax/dose ( BW) | ||||
| Tmax (h) | ||||
| AUClast/dose ( BW) | ||||
| Cl_F () | ||||
Note: The toxicokinetic parameters after oral dosing were estimated from data sets obtained after a single oral administration of BPA at (Exp. 1, ), a single oral administration of BPS at (Exp. 3–4, ), and simultaneous oral administrations of BPA and BPS at (Exp. 5, ). AUClast/dose, Dose scaled area under the plasma concentration–time curve from dosing time to the time of the last measurable plasma concentration; BPA, Bisphenol A; BPAG, Bisphenol A glucuronide; BPS, Bisphenol S; BPSG, Bisphenol S glucuronide; BW, Body weight; Cl_F, Apparent clearance; Cmax/dose, Dose scaled maximal plasma concentration; IV, Intravenous; Tmax, Time of Cmax.
After oral administrations, the BPA and BPS plasma concentrations increased to maximal values about 1 h and 30 min after dosing, respectively. The average maximal BPS plasma concentrations were, in relation to the dose administered, more than 300 times higher than the maximal BPA values ranging from 173 to () scaled for a nominal dose of BW for BPS and from 0.059 to () scaled for a nominal dose of BW for BPA. The plasma concentrations of BPS remained above the LOQ of the assay for 3 times longer than those of BPA. The BPS systemic exposure after oral dosing was, on average, 262 times higher than that of BPA, the values relative to dose ranging from 323 to () scaled for a nominal dose of BW for BPS and from 0.23 to () scaled for a nominal dose of BW for BPA. The time course of BPAG and BPSG plasma concentrations observed after oral administrations of BPA and BPS, respectively, did not differ significantly.
Urinary Excretion of BPA and BPS
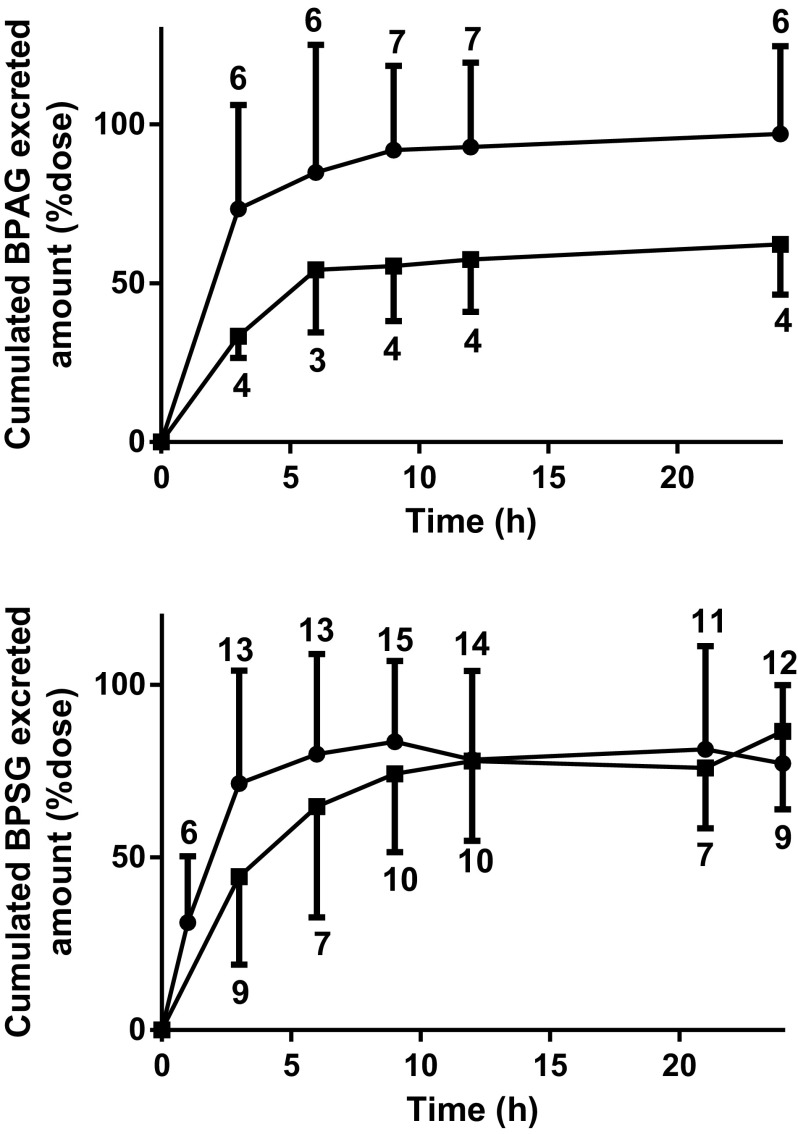
The cumulative urinary excretions of BPAG, BPS, and BPSG that were recovered at fixed intervals over 24 h (Exp. 2–5) are presented in Figure 3. By 24 h, the mean fractions () of the BPA dose recovered in urine as BPAG was approximately two times lower after oral BPA dosing (, range: 49.5–80.2%, ) than after IV BPA dosing (, 63.1–135.7%, , Figure 3). The mean fractional urinary excretion of BPS as BPSG was, respectively, (36.3–107.9%, ) and (65.5–126.8%, ) after IV and oral BPS dosing. About half of the BPA and BPS doses were eliminated 3 h after dosing. Two of the low BPSG recoveries () were attributed to loss of urine at one collection time corresponding to 6 h and 9 h post dosing.
Figure 3.
Cumulated urinary amounts of BPAG and BPSG in piglets after respective BPA and BPS IV (•, ) and oral (▪, mean−SD) dosing. BPAG urinary excretion was evaluated after both a single IV BPA administration at (Exp. 2, ) and simultaneous IV administrations of BPA and BPS at and after simultaneous oral administrations of BPA and BPS at (Exp. 5, ). BPSG urinary excretion was evaluated after both a single IV BPS administration at (Exp. 3–4, ) and simultaneous IV administrations of BPA and BPS at (Exp. 5, ) and after both a single oral BPS administration at (Exp. 4, ) and simultaneous oral administrations of BPA and BPS at (Exp. 5, ). Mean values were calculated at the time periods where at least four values were available. The numbers above or under the error bars indicate the number of animals for each time period. BPA, Bisphenol A; BPAG, Bisphenol A glucuronide; BPS, Bisphenol S; BPSG, Bisphenol S glucuronide; IV, Intravenous; Exp, Experiment; SD, Standard deviation.
Unconjugated BPA and BPS in urine represented, respectively, and of the IV BPA and BPS doses. The mean fraction of the IV BPSG dose recovered in urine was (40.1–69.8%), 24 h post dosing.
TK Modeling
For the present paper, the NLME modeling enabled several historical information data sets to be used to generate a single set of parameters (with their SE) for BPA and BPS. The estimated primary parameters (noted vector thetas), namely (), (), and the 13 of the model are reported with their SE and their coefficient of variation (Tables S3 and S4). The mean volume of the central compartment of BPA () was the highest in comparison with BPS (), BPSG (), and BPAG (). Tables S5 and S6 report the estimated variance matrix of () and BSV for the structural parameters of BPA and BPS TK model for which the shrinkage for was lower than 30% (, , , , , , , and ). The population predicted vs. observed plasma concentrations of BPA and BPAG and of BPS and BPSG (Figures S1 and S2) were evenly distributed around the line of identity of the diagnostic plots, suggesting that these data were appropriately described by the model. The goodness-of-fit plots for corresponding individual predicted values suggested the absence of any major bias in the random component of the model. The population predicted vs. observed quantities of BPA, BPAG, BPS, and BPSG excreted in urine (Figures S3A1–S5A1 and Figures S3B1–S5B1) were distributed vertically, suggesting that the model did not adequately predict these population values, especially the very low quantities of BPA and BPS excreted in urine (Figures S3A1–S5A1). Rather than discard these urinary values, we kept them not only to ensure the transparency of our results but also because the corresponding individual predicted values that were obtained by including a random component in the structural model were reasonably well predicted by the model (Figures S3–S5). Figures S6–S9 show the results of the Visual Predictive Check of the model for BPA and BPS TK, respectively. For each figure except those related to the very small quantities of BPA (Figure S7A) and BPS (Figure S9A and B) excreted in the urine after BPA and BPS IV or oral BPS dosing, the observed quantiles (20%, 50%, and 80%) are reasonably well overlaid by the corresponding predictive check quantiles.
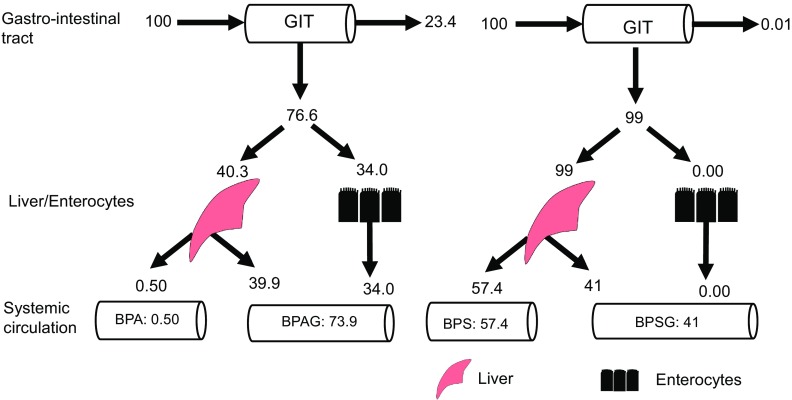
Tables 4 and 5 show the population secondary parameters for BPA/BPAG and BPS/BPSG, respectively, estimated from the primary parameters of the model. Figure 4 compares the fates of a same BPA and BPS dose given orally as predicted by the model. The estimated fraction of the oral BPA dose absorbed by the enterocytes was 76.6%, whereas BPS oral absorption was near total. According to the model, about 34% of the BPA dose (44% of the absorbed dose) was glucuronidated in the gut wall, the near totality (99%) of the remaining fraction (40.3%, i.e., 53% of the absorbed dose) being subjected to an hepatic first-pass effect (39.9%). In contrast, the model predicted that nearly 100% of the ingested BPS dose gained direct access to the liver, where the estimated hepatic first-pass effect was only 41%. The lack of a gut first-pass effect for BPS associated with a limited hepatic first-pass effect resulted in a much higher oral bioavailable fraction for BPS (57.4%) than for BPA (0.50%, Tables 4 and 5). The renal clearance values of BPA () and BPS () represented 0.06 and 0.037% of their respective plasma clearance values estimated by noncompartmental analysis, whereas the renal clearance values of BPAG () and BPSG () were close to their respective apparent clearance values (Tables 4 and 5).
Table 4.
Population secondary parameters of BPA/BPAG in pigs as obtained with a 9-compartment model.
| Parameters | Mean | Median | CV% |
|---|---|---|---|
| BPA fraction absorbed by the gastro-intestinal tract | 0.765 | 0.766 | 7.84 |
| Fraction of absorbed BPA undergoing a first-pass effect (gut and liver) | 0.988 | 0.989 | 0.61 |
| Fraction of administered BPA reaching the liver by the portal blood flow | 0.405 | 0.403 | 27.13 |
| Fraction of administered BPA glucuronidated by the gastro-intestinal tract | 0.360 | 0.340 | 24.39 |
| BPA renal clearance () | 0.0020 | 0.0020 | 23.35 |
| BPAG renal clearance () | 0.277 | 0.279 | 14.12 |
| BPA Bioavailability (%) | 0.005 | 0.005 | 31.73 |
Note: The bootstrap procedure was used to estimate the mean, median, and precision (coefficient of variation, CV%) of parameter estimates. BPA: Bisphenol A; BPAG, Bisphenol A glucuronide.
Table 5.
Population secondary parameters of BPS/BPSG in pigs as obtained with a 9-compartment model.
| Parameters | Mean | Median | CV% |
|---|---|---|---|
| BPS fraction absorbed by the gastro-intestinal tract | 0.986 | 0.989 | 0.956 |
| Fraction of absorbed BPS undergoing a first-pass effect (liver) | 0.435 | 0.408 | 17.412 |
| Fraction of administered BPS reaching the liver by the portal blood flow | 0.986 | 0.989 | 0.966 |
| Fraction of administered BPS glucuronidated by the gastro-intestinal tract | 0.00015 | 0.00014 | 66.823 |
| BPS renal clearance () | 0.00035 | 0.00035 | 16.325 |
| BPSG renal clearance () | 0.243 | 0.245 | 11.188 |
| BPS Bioavailability (%) | 0.557 | 0.574 | 12.935 |
Note: The bootstrap procedure was used to estimate the mean, median, and precision (coefficient of variation, CV%) of parameter estimates. BPS, Bisphenol S; BPSG, Bisphenol S glucuronide.
Figure 4.
Summary of the main results in terms of the fate of a dose of BPA and BPS administered by orogastric gavage. Note: The estimated absorption of BPA by the enterocytes was 76.6%, 23.4% of the BPA not being absorbed, whereas BPS absorption was near total (99%). Most of the BPA dose was subjected to intestinal (34%) and hepatic (40%) metabolism, resulting in a low oral BPA bioavailable dose (0.50%). The fraction of BPA undergoing an intestinal first-pass effect gained access to the blood and contributed to the systemic exposure to BPAG that accounted for 73.9% of the dose. By contrast, only 41% of the BPS oral dose reaching the portal blood was metabolized by the liver and contributed to the systemic exposure to BPSG (41%), with 57.4% of the BPS oral dose being bioavailable. The numbers represent the bootstrap estimates of the median, which explains why the sum is not 100. BPA, Bisphenol A; BPAG, Bisphenol A glucuronide; BPS, Bisphenol S; BPSG, Bisphenol S glucuronide; GIT, Gastrointestinal tract.
Discussion
The purpose of this study was to compare the oral TK of BPA and its major alternative, BPS, and to quantify the key TK mechanisms determining internal exposures. Our approach was based on compartmental TK modeling of BPA, BPS, BPSG, and BPAG in piglets, considered as a relevant species for translational research, because of their important anatomical and physiological similarities with humans, with regard to gastrointestinal function.
By developing the TK model that merged different data sets corresponding to the plasma and urinary concentrations of BPA, BPS, and their glucuronides after IV and oral administration, we were able to estimate, using a comprehensive NLME model, the relative contributions of the different pathways controlling BPA and BPS plasma concentrations following oral exposure. We estimated that the near totality (99%) of BPS was absorbed after gavage administration, whereas the absorption fraction represented only about 77% for BPA. According to the model, BPS metabolism did not occur in the enterocytes, whereas the gut was shown with the same model to contribute to the first-pass glucuronidation of 44% of the BPA absorbed fraction. Although only 41% of the BPS absorbed fraction was glucuronidated in the liver, the near totality (99%) of BPA unmetabolized in the enterocytes underwent an extensive hepatic first-pass glucuronidation. The considerable extent of BPS absorption associated with a lack of enterocyte first-pass metabolism and a moderate efficiency of hepatic first-pass BPS glucuronidation led to a systemic bioavailability of BPS that was about 100 times higher than that of BPA (57.4 vs. 0.50%).
The plasma clearance of BPS estimated by noncompartmental analysis was about 3.5-fold lower than that of BPA (0.95 vs. ), indicating that BPS is eliminated less efficiently than BPA, as previously shown in sheep (Grandin et al. 2017) and also explains its limited first-pass hepatic effect. Considering these clearance values, it was deduced that the renal clearance values of BPA (0.06%) and BPS (0.037%) estimated from the model are negligible in comparison with the metabolic hepatic clearance (higher than 99%). Assuming that the plasma (or serum) and blood concentrations of BPS are equal, the estimated hepatic extraction ratio of BPS, calculated from the ratio between the plasma clearance and the hepatic blood flow (estimated at in pigs from allometric scaling; Boxenbaum 1980), was 0.37. The agreement of this estimated value of the BPS fraction escaping presystemic metabolism (63%) with BPS oral bioavailability (57.4%) confirmed the predominant role of the liver for BPS clearance. The closeness of the estimated renal clearance values of BPAG () and BPSG () to the respective apparent clearance values obtained by noncompartmental analysis (0.51 vs. ) and by BPSG plasma clearance () is in agreement with the hypothesis that, as previously shown for BPA (Völkel et al. 2002), almost all the administered BPS is metabolized into BPSG, glucuronoconjugates being mainly excreted in the urine.
The great extent of BPA and BPS absorption may be explained by the relatively low molecular weights of BPA and BPS (228.29 and 250.27) respectively and their moderate water solubilities (LogP values of 3.3 and 1.65) respectively. The much higher oral bioavailability of BPS in comparison with that of BPA was rather unexpected due to the close chemical structure of the compounds. Using a database for 309 drugs in humans, Varma et al. (2010) showed that although lipophilicity favors absorption, the fractions of drugs escaping gut-wall and hepatic presystemic metabolism were decreased by lipophilicity, with compounds having logP values greater than 3 demonstrating the higher gut and hepatic extraction. This single physicochemical descriptor is however insufficient to explain the highly different extents of first-pass extraction of BPS in comparison with those of BPA. All of the five examined BPA analogs, except BPS, were predicted to be CYP2D6 substrates based on the in silico structure activity relationship (Rosenmai et al. 2014), which suggests that small changes in chemical structure could influence the binding sites to enzymes and metabolism.
The difference in the extent of BPS and BPA glucuronidation, both in the liver and the intestine as predicted by our model, is consistent with the relative intrinsic clearance values of BPS vs. BPA determined from the metabolic parameters (Vmax, Km) obtained for intestinal and hepatic glucuronidation in humans (Karrer et al. 2018). Hence, the liver and intestinal intrinsic clearances of BPS computed from these parameters (0.325 and ) are respectively 11 and 6 times lower than those of BPA (3.67 and ). The lack of enterocyte first-pass glucuronidation of BPS is also in agreement with the low glucuronidation rate ratio of BPS between human intestine and liver microsomes and the high activity of the hepatic recombinant human UDP-glucuronosyltransferase (UGT) 1A9 towards BPS in comparison with the intestinal UGT1A10 (Gramec Skledar et al. 2015).
In piglets, the -fold higher amount available as circulating BPS in comparison with BPA after oral dosing, combined with its 3.5 times lower plasma clearance, was responsible for the times higher systemic exposure to BPS resulting from oral dosing, as reflected by the AUC values, whereas the systemic exposures to BPAG and BPSG were very similar. The resulting mean fraction of the total BPS in plasma (BPS plus BPSG) that is BPS (23%) was much higher than the corresponding value for BPA (0.16%). The former fractions are in the same order of magnitude as those measured in humans after oral administration of BPA or BPS on a cookie, i.e., 28% for BPS (Oh et al. 2018) and 0.56% for BPA (Thayer et al. 2015) which underscores the relevance of our results for humans. A higher internal exposure to BPS in comparison with BPA for equal external exposures could explain the underestimation of BPS in vivo potency in the ovariectomized rat uterotrophic assay from in vitro predictions based on a bioassay of estrogenic activity (Conley et al. 2016). Although in vivo effects may involve more complex mechanisms than those targeted in vitro, the former study showed that BPS was a more potent oral estrogenic compound in vivo than BPA was, although it revealed a lower potential in vitro than BPA to affect estrogenic and androgenic endocrine activity.
Currently, the use of BPS is not regulated and no tolerable daily intake (TDI) has been defined for BPS despite its wide use as substitute for BPA. If, in the current state of knowledge, the estrogenic potency of BPS is considered to be similar to that of BPA, and a correction factor of 250 is applied to the TDI of BPA fixed temporarily at BW per day (EFSA CEF Panel 2015) to account for TK differences, the estimated safe level of BPS would be BW per day. This value is not very different from the estimated median daily dietary intake of BPS, of BW/d (Liao and Kannan 2014), which suggests that the margin of safety for BPS is very narrow.
Conclusion
Our study provides novel and critical information about BPS risk assessment. The finding that orally ingested BPS may be highly available as a circulating potentially endocrine-active compound provides a key insight for assessing substitution of BPA with BPS. Although the currently available toxicological data are considered insufficient for assessing the toxicity of BPA analogs, our results emphasize the need for TK studies to screen and discard those endocrine-active BPA substitutes to which human exposure is potentially high.
Supplementary Material
Acknowledgments
The authors thank N. Deschamps, C. Lacassagne, and J.P. Gau, for their assistance and involvement in animal care.
This work was supported by the French National Research Program for Environmental and Occupational Health of Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail (ANSES) (grant no. 2017/1/174).
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP4599).
The authors declare that they have no actual or potential competing financial interests
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Ahmed S, Atlas E. 2016. Bisphenol S- and bisphenol A-induced adipogenesis of murine preadipocytes occurs through direct peroxisome proliferator-activated receptor gamma activation. Int J Obes (Lond) 40(10):1566–1573, PMID: 27273607, 10.1038/ijo.2016.95. [DOI] [PubMed] [Google Scholar]
- Ahsan N, Ullah H, Ullah W, Jahan S. 2018. Comparative effects of Bisphenol S and Bisphenol A on the development of female reproductive system in rats; a neonatal exposure study. Chemosphere 197:336–343, PMID: 29407803, 10.1016/j.chemosphere.2017.12.118. [DOI] [PubMed] [Google Scholar]
- ANSES (Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail). 2013. Opinion on the assessment of the risks associated with bisphenol A for human health, and on toxicological data and data on the use of bisphenols S, F, M, B, AP, AF and BADGE www.anses.fr/en/content/opinion-assessment-risks-associated-bisphenol-human-health-and-toxicological-data-and-data [accessed 2 May 2018].
- Boucher JG, Ahmed S, Atlas E. 2016. Bisphenol S induces adipogenesis in primary human preadipocytes from female donors. Endocrinology 157(4):1397–1407, PMID: 27003841, 10.1210/en.2015-1872. [DOI] [PubMed] [Google Scholar]
- Boucher JG, Boudreau A, Ahmed S, Atlas E. 2015. In vitro effects of Bisphenol A β-D-Glucuronide (BPA-G) on adipogenesis in human and murine preadipocytes. Environ Health Perspect 123(12):1287–1293, PMID: 26018136, 10.1289/ehp.1409143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher JG, Boudreau A, Atlas E. 2014. Bisphenol A induces differentiation of human preadipocytes in the absence of glucocorticoid and is inhibited by an estrogen-receptor antagonist. Nutr Diabetes 4:e102, PMID: 24418828, 10.1038/nutd.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxenbaum H. 1980. Interspecies variation in liver weight, hepatic blood flow, and antipyrine intrinsic clearance: Extrapolation of data to benzodiazepines and phenytoin. J Pharmacokinet Biopharm 8(2):165–176, PMID: 6107379, 10.1007/BF01065191. [DOI] [PubMed] [Google Scholar]
- Byon W, Fletcher CV, Brundage RC. 2008. Impact of censoring data below an arbitrary quantification limit on structural model misspecification. J Pharmacokinet Pharmacodyn 35(1):101–116, PMID: 17963024, 10.1007/s10928-007-9078-9. [DOI] [PubMed] [Google Scholar]
- Collet SH, Picard-Hagen N, Lacroix MZ, Puel S, Viguié C, Bousquet-Melou A, et al. 2015. Allometric scaling for predicting human clearance of bisphenol A. Toxicol Appl Pharmacol 284(3):323–329, PMID: 25759244, 10.1016/j.taap.2015.02.024. [DOI] [PubMed] [Google Scholar]
- Conley JM, Hannas BR, Furr JR, Wilson VS, Gray LE. 2016. A demonstration of the uncertainty in predicting the estrogenic activity of individual chemicals and mixtures from an in vitro estrogen receptor transcriptional activation assay (T47D-KBluc) to the in vivo uterotrophic assay using oral exposure. Toxicol Sci 153(2):382–395, PMID: 27473340, 10.1093/toxsci/kfw134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzl E, Sei K, Soda S, Ike M, Fujita M. 2009. Biodegradation of bisphenol A, bisphenol F and bisphenol S in seawater. Int J Environ Res Public Health 6(4):1472–1484, PMID: 19440529, 10.3390/ijerph6041472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CEF Panel [EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF)]. 2015. Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA Journal 13(1):3978, 10.2903/j.efsa.2015.3978. [DOI] [Google Scholar]
- Eladak S, Grisin T, Moison D, Guerquin M-J, N'Tumba-Byn T, Pozzi-Gaudin S, et al. 2015. A new chapter in the bisphenol A story: Bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil Steril 103(1):11–21, PMID: 25475787, 10.1016/j.fertnstert.2014.11.005. [DOI] [PubMed] [Google Scholar]
- FDA (Food and Drug Administration). 1999. Guidance for industry. Population Pharmacokinetics. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/population-pharmacokinetics [accessed 30 April 2019].
- Gauderat G, Picard-Hagen N, Toutain P-L, Servien R, Viguié C, Puel S, et al. 2017. Prediction of human prenatal exposure to bisphenol A and bisphenol A glucuronide from an ovine semi-physiological toxicokinetic model. Sci Rep 7(1):15330, PMID: 29127374, 10.1038/s41598-017-15646-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayrard V, Gauderat G, Lacroix MZ, Viguié C, Bousquet-Melou A, Toutain P-L, et al. 2015. Comment on “In Vitro Effects of Bisphenol A β-D-Glucuronide (BPA-G) on Adipogenesis in Human and Murine Preadipocytes.” Environ Health Perspect 123(12):A289, PMID: 26623714, 10.1289/ehp.1510315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldinger DM, Demierre A-L, Zoller O, Rupp H, Reinhard H, Magnin R, et al. 2015. Endocrine activity of alternatives to BPA found in thermal paper in Switzerland. Regul Toxicol Pharmacol 71(3):453–462, PMID: 25579646, 10.1016/j.yrtph.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Gramec Skledar D, Troberg J, Lavdas J, Peterlin Mašič L, Finel M. 2015. Differences in the glucuronidation of bisphenols F and S between two homologous human UGT enzymes, 1A9 and 1A10. Xenobiotica 45(6):511–519, PMID: 25547628, 10.3109/00498254.2014.999140. [DOI] [PubMed] [Google Scholar]
- Grandin F, Picard-Hagen N, Gayrard V, Puel S, Viguié C, Toutain P-L, et al. 2017. Development of an on-line solid phase extraction ultra-high-performance liquid chromatography technique coupled to tandem mass spectrometry for quantification of bisphenol S and bisphenol S glucuronide: Applicability to toxicokinetic investigations. J Chromatogr A 1526:39–46, PMID: 29055528, 10.1016/j.chroma.2017.10.020. [DOI] [PubMed] [Google Scholar]
- Kararli TT. 1995. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos 16(5):351–380, PMID: 8527686, 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- Karrer C, Roiss T, von Goetz N, Gramec Skledar D, Peterlin Mašič L, Hungerbühler K. 2018. Physiologically based pharmacokinetic (PBPK) modeling of the bisphenols BPA, BPS, BPF, and BPAF with new experimental metabolic parameters: Comparing the pharmacokinetic behavior of BPA with its substitutes. Environ Health Perspect 126(7):077002, PMID: 29995627, 10.1289/EHP2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H, Takeuchi S, Sanoh S, Okuda K, Kitamura S, Uramaru N, et al. 2019. Profiling of bisphenol A and eight its analogues on transcriptional activity via human nuclear receptors. Toxicology 413:48–55, PMID: 30582956, 10.1016/j.tox.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Kuruto-Niwa R, Nozawa R, Miyakoshi T, Shiozawa T, Terao Y. 2005. Estrogenic activity of alkylphenols, bisphenol S, and their chlorinated derivatives using a GFP expression system. Environ Toxicol Pharmacol 19(1):121–130, PMID: 21783468, 10.1016/j.etap.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Lacroix MZ, Puel S, Collet SH, Corbel T, Picard-Hagen N, Toutain PL, et al. 2011. Simultaneous quantification of bisphenol A and its glucuronide metabolite (BPA-G) in plasma and urine: Applicability to toxicokinetic investigations. Talanta 85(4):2053–2059, PMID: 21872057, 10.1016/j.talanta.2011.07.040. [DOI] [PubMed] [Google Scholar]
- Le Fol V, Aït-Aïssa S, Cabaton N, Dolo L, Grimaldi M, Balaguer P, et al. 2015. Cell-specific biotransformation of benzophenone-2 and bisphenol-s in zebrafish and human in vitro models used for toxicity and estrogenicity screening. Environ Sci Technol 49(6):3860–3868, PMID: 25679259, 10.1021/es505302c. [DOI] [PubMed] [Google Scholar]
- Lehmler H-J, Liu B, Gadogbe M, Bao W. 2018. Exposure to bisphenol A, bisphenol F, and bisphenol S in U.S. adults and children: The National Health and Nutrition Examination Survey 2013-2014. ACS Omega 3(6):6523–6532, PMID: 29978145, 10.1021/acsomega.8b00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Gehring R, Lin Z, Riviere J. 2015. A framework for meta-analysis of veterinary drug pharmacokinetic data using mixed effect modeling. J Pharm Sci 104(4):1230–1239, PMID: 25641543, 10.1002/jps.24341. [DOI] [PubMed] [Google Scholar]
- Liao C, Kannan K. 2014. A survey of bisphenol A and other bisphenol analogues in foodstuffs from nine cities in China. Food Additives & Contaminants: Part A 31(2):319–329, PMID: 24262000, 10.1080/19440049.2013.868611. [DOI] [PubMed] [Google Scholar]
- Matthews JB, Twomey K, Zacharewski TR. 2001. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors alpha and beta. Chem Res Toxicol 14(2):149–157, PMID: 11258963, 10.1021/tx0001833. [DOI] [PubMed] [Google Scholar]
- Molina-Molina JM, Amaya E, Grimaldi M, Sáenz J-M, Real M, Fernández MF, et al. 2013. In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol Appl Pharmacol 272(1):127–136, PMID: 23714657, 10.1016/j.taap.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Oh J, Choi JW, Ahn Y-A, Kim S. 2018. Pharmacokinetics of bisphenol S in humans after single oral administration. Environ Int 112:127–133, PMID: 29272776, 10.1016/j.envint.2017.11.020. [DOI] [PubMed] [Google Scholar]
- Philips EM, Jaddoe VWV, Asimakopoulos AG, Kannan K, Steegers EAP, Santos S, et al. 2018. Bisphenol and phthalate concentrations and its determinants among pregnant women in a population-based cohort in the Netherlands, 2004-5. Environ Res 161:562–572, PMID: 29245124, 10.1016/j.envres.2017.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester JR, Bolden AL. 2015. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ Health Perspect 123(7):643–650, PMID: 25775505, 10.1289/ehp.1408989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmai AK, Dybdahl M, Pedersen M, Alice van Vugt-Lussenburg BM, Wedebye EB, Taxvig C, et al. 2014. Are structural analogues to bisphenol a safe alternatives? Toxicol Sci 139(1):35–47, PMID: 24563381, 10.1093/toxsci/kfu030. [DOI] [PubMed] [Google Scholar]
- Skledar DG, Schmidt J, Fic A, Klopčič I, Trontelj J, Dolenc MS, et al. 2016. Influence of metabolism on endocrine activities of bisphenol S. Chemosphere 157:152–159, PMID: 27213244, 10.1016/j.chemosphere.2016.05.027. [DOI] [PubMed] [Google Scholar]
- Teeguarden J, Hanson-Drury S, Fisher JW, Doerge DR. 2013. Are typical human serum BPA concentrations measurable and sufficient to be estrogenic in the general population? Food Chem Toxicol 62:949–963, PMID: 23959105, 10.1016/j.fct.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Thayer KA, Doerge DR, Hunt D, Schurman SH, Twaddle NC, Churchwell MI, et al. 2015. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ Int 83:107–115, PMID: 26115537, 10.1016/j.envint.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker DK, Hayes Bouknight S, Brar SS, Kissling GE, Fenton SE. 2018. Evaluation of prenatal exposure to bisphenol analogues on development and long-term health of the mammary gland in female mice. Environ Health Perspect 126(8):087003, PMID: 30102602, 10.1289/EHP3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah A, Pirzada M, Jahan S, Ullah H, Shaheen G, Rehman H, et al. 2018. Bisphenol A and its analogs bisphenol B, bisphenol F, and bisphenol S: Comparative in vitro and in vivo studies on the sperms and testicular tissues of rats. Chemosphere 209:508–516, PMID: 29940534, 10.1016/j.chemosphere.2018.06.089. [DOI] [PubMed] [Google Scholar]
- Varma MVS, Obach RS, Rotter C, Miller HR, Chang G, Steyn SJ, et al. 2010. Physicochemical space for optimum oral bioavailability: Contribution of human intestinal absorption and first-pass elimination. J Med Chem 53(3):1098–1108, PMID: 20070106, 10.1021/jm901371v. [DOI] [PubMed] [Google Scholar]
- Viñas R, Goldblum RM, Watson CS. 2013. Rapid estrogenic signaling activities of the modified (chlorinated, sulfonated, and glucuronidated) endocrine disruptor bisphenol A. Endocrine Disruptors 1(1):e25411, 10.4161/endo.25411. [DOI] [Google Scholar]
- Viñas R, Watson CS. 2013. Mixtures of xenoestrogens disrupt estradiol-induced non-genomic signaling and downstream functions in pituitary cells. Environ Health 12:26, PMID: 23530988, 10.1186/1476-069X-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völkel W, Colnot T, Csanády GA, Filser JG, Dekant W. 2002. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem Res Toxicol 15(10):1281–1287, PMID: 12387626, 10.1021/tx025548t. [DOI] [PubMed] [Google Scholar]
- Wu LH, Zhang XM, Wang F, Gao CJ, Chen D, Palumbo JR, et al. 2018. Occurrence of bisphenol S in the environment and implications for human exposure: A short review. Sci Total Environ 615:87–98, PMID: 28963899, 10.1016/j.scitotenv.2017.09.194. [DOI] [PubMed] [Google Scholar]
- Ye X, Wong L-Y, Kramer J, Zhou X, Jia T, Calafat AM. 2015. Urinary concentrations of bisphenol A and three other bisphenols in convenience samples of U.S. adults during 2000-2014. Environ Sci Technol 49(19):11834–11839, PMID: 26360019, 10.1021/acs.est.5b02135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Kramer JP, Calafat AM, Ye X. 2014. Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. J Chromatogr B Analyt Technol Biomed Life Sci 944:152–156, PMID: 24316527, 10.1016/j.jchromb.2013.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.