Abstract
Background:
Between 2013 and 2015, concentrations of poly- and perfluoroalkyl substances (PFAS) in public drinking water supplies serving at least six million individuals exceeded the level set forth in the health advisory established by the U.S. Environmental Protection Agency. Other than data reported for contaminated sites, no systematic or prospective data exist on the relative source contribution (RSC) of drinking water to human PFAS exposures.
Objectives:
This study estimates the RSC of tap water to overall PFAS exposure among members of the general U.S. population.
Methods:
We measured concentrations of 15 PFAS in home tap water samples collected in 1989–1990 from 225 participants in a nationwide prospective cohort of U.S. women: the Nurses’ Health Study (NHS). We used a one-compartment toxicokinetic model to estimate plasma concentrations corresponding to tap water intake of PFAS. We compared modeled results with measured plasma PFAS concentrations among a subset of 110 NHS participants.
Results:
Tap water perfluorooctanoic acid (PFOA) and perfluorononanoic acid (PFNA) were statistically significant predictors of plasma concentrations among individuals who consumed cups of tap water per day. Modeled median contributions of tap water to measured plasma concentrations were: PFOA 12% (95% probability interval 11%–14%), PFNA 13% (8.7%–21%), linear perfluorooctanesulfonic acid (nPFOS) 2.2% (2.0%–2.5%), branched perfluorooctanesulfonic acid (brPFOS) 3.0% (2.5%–3.2%), and perfluorohexanesulfonic acid (PFHxS) 34% (29%–39%). In five locations, comparisons of PFASs in community tap water collected in the period 2013–2016 with samples from 1989–1990 indicated increases in quantifiable PFAS and extractable organic fluorine (a proxy for unquantified PFAS).
Conclusions:
Our results for 1989–1990 compare well with the default RSC of 20% used in risk assessments for legacy PFAS by many agencies. Future evaluation of drinking water exposures should incorporate emerging PFAS. https://doi.org/10.1289/EHP4093
Introduction
Poly- and perfluoroalkyl substances (PFAS) are a broad class of fluorinated aliphatic compounds that are widely used by industry and for commercial applications and have been detected in the serum of 98% of U.S. individuals (Khalil et al. 2016). Exposure to some PFAS has been associated with developmental, metabolic, and immune disorders in humans (Grandjean and Budtz-Jørgensen 2013; Liu et al. 2018; Vaughn et al. 2013). Exposure sources for PFAS are diverse and include consumer products, food, indoor dust, and drinking water (Domingo and Nadal 2017; Haug et al. 2011; Miralles-Marco and Harrad 2015). Between 2013 and 2015, public drinking water supplies serving at least six million people exceeded the lifetime health advisory level for PFAS established by the U.S. Environmental Protection Agency (U.S. EPA) (Hu et al. 2016). However, little information is available on the relative contribution of drinking water to observed levels in human plasma among the general population and how such exposures have changed over time.
Near contaminated sites, drinking water PFAS concentrations more than two orders of magnitude higher than the U.S. EPA health advisory level guideline ( range) have been reported (Emmett et al. 2006; Gyllenhammar et al. 2015; Landsteiner et al. 2014; Worley et al. 2017). At such locations, drinking water can account for up to 75% of total PFAS exposure (Emmett et al. 2006; Hoffman et al. 2010; Seals et al. 2011; Vestergren and Cousins 2009). Away from point sources, drinking water PFAS concentrations approximately 1,000-fold lower (low range) have been more commonly measured (Ericson et al. 2008; Quiñones and Snyder 2009). Even these lower concentrations have been associated with elevated serum concentrations in U.S. women (Hurley et al. 2016). Many other sources of PFAS (consumer products, dust, food) are known to be important for overall exposure of different human populations (Sunderland et al. 2018; Tokranov et al. 2018). Additional data are thus needed to better understand the contribution of drinking water to total PFAS exposures among the general U.S. population.
Interindividual variability in consumption rates and toxicokinetics can affect the contribution of drinking water to serum PFAS concentrations. Uptake and elimination of PFAS varies depending on age, race, menstrual status, childbirth, and breastfeeding (Gribble et al. 2015; Wong et al. 2014; Zhang et al. 2013). Regulatory agencies often use a term known as the relative source contribution (RSC) during risk assessments to represent the fraction of total PFAS exposure allocated to drinking water (Table S1). Most agencies, including the U.S. EPA, have adopted a default RSC value for drinking water of 20% for all PFAS (DeWitt 2015; Minnesota Department of Health 2017).
Here we estimate the RSC of drinking water collected in 1989–1990 to measured concentrations of PFAS in plasma from women in a nationwide prospective cohort [the Nurses’ Health Study (NHS)]. We focus on plasma samples because plasma is the available matrix in the NHS. We also examine temporal changes in PFAS concentrations in drinking water by comparing selected measurements from 1989–1990 to matched sampling locations between 2013 and 2016. We use these data to better understand the changing significance of drinking water as an exposure pathway for PFAS in the U.S. general population.
Methods
Study Population
In 1976, 121,700 female registered nurses between 30 and 55 years(y) of age were enrolled in the NHS (Belanger et al. 1978). Study participants have responded to mailed questionnaires on their medical, lifestyle, and health-related history every two years since that time. As part of the 1988 questionnaire cycle, study participants provided information on their tap water consumption. Their county of residence was obtained and updated from the mailing address provided during each questionnaire cycle.
Blood and Tap Water Sample Collection
Between May 1989 and September 1990, 32,826 NHS participants between the ages of 43 and 69 y provided matched blood and tap water samples. Blood samples were collected by the participants themselves using a blood collection kit containing three Sodium Heparin Collection tubes. Tap water samples were collected concurrently with blood samples from participants’ home kitchens using high-density polyethylene (HDPE) bottles with screw caps. Blood samples and frozen water samples were couriered overnight to the Brigham and Women’s Hospital in Boston, Massachusetts. Collection instructions specified that blood should be drawn between Monday and Thursday to ensure that the kit reached the lab on a weekday for immediate processing. Across all blood samples, 97% arrived within 26 h of being drawn.
Upon return to the lab, whole blood samples were immediately separated into plasma, white blood cells, and red blood cells, and stored in the vapor phase of liquid nitrogen freezers at . Tap water samples were thawed, acidified with nitric acid to , and stored at room temperature in a warehouse in Malden, Massachusetts. Storage at room temperature is acceptable for this study because PFAS are extremely stable in solution and do not fully degrade in water under these conditions (Wang et al. 2015).
We tested for potential absorption of PFAS to sampling bottles following previous methods established for soil extractions (Guelfo and Higgins 2013; Houtz et al. 2013). Briefly, we added ammonium hydroxide with methanol to an evacuated bottle that was then vortexed and sonicated for 0.5–1 h and placed on a shaker table for 2 h. We concentrated the aqueous sample with nitrogen prior to analysis for PFAS by high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS). Results confirmed the sample bottles did not contain a fluoropolymer lining (Figure S1).
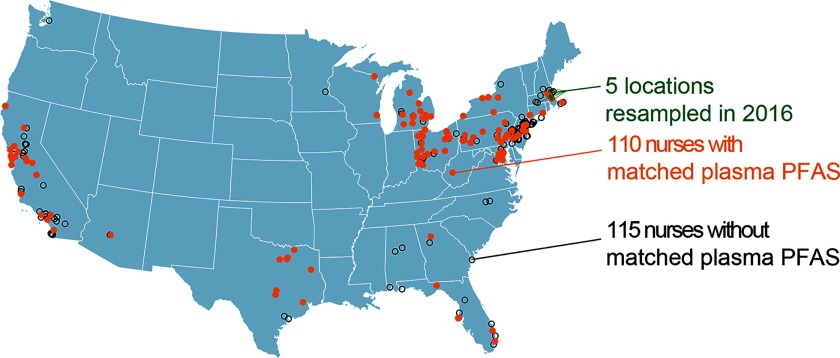
We selected 225 study participants living in 22 states for home tap water PFAS measurements (Figure 1, Table 1). Sample selection was intended to cover geographically diverse areas and to overlap with plasma samples from 110 individuals analyzed for PFAS as part of a separate epidemiological investigation. Individuals included in this study had demographic, biometric, and lifestyle factors that were similar to the rest of cohort (Table S2). The study protocol was approved by the institutional review boards of Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health. Return of self-administered questionnaires was considered informed consent. We also obtained informed consent for collecting blood and water samples.
Figure 1.
Locations of 225 home tap water samples obtained in 1989–1990.
Table 1.
Overview of Nurses’ Health Study (NHS) participants ( women, 1989–1990 data) included in this study.
| Tap water intakea | ||||
|---|---|---|---|---|
| 0–2 cups/day | 3–5 cups/day | 6–9 cups/day | 10 or more | |
| 5 | 26 | 135 | 59 | |
| Age, y | ||||
| White | 5 (100%) | 25 (96%) | 129 (96%) | 58 (98%) |
| BMI, | ||||
| Weight, lb | ||||
| Parity | ||||
| No birth | 1 (20%) | 4 (15%) | 2 (1%) | 3 (5%) |
| 1–3 birth | 4 (80%) | 13 (50%) | 86 (64%) | 43 (73%) |
| births | 0 (0%) | 9 (35%) | 47 (35%) | 13 (22%) |
| Breastfeeding durationc | ||||
| Never | 2 (40%) | 12 (46%) | 56 (41%) | 23 (39%) |
| 1 (20%) | 7 (27%) | 50 (37%) | 18 (31%) | |
| 2 (40%) | 7 (27%) | 29 (21%) | 18 (31%) | |
| Menstruation status | ||||
| Premenopause | 1 (20%) | 4 (15%) | 21 (16%) | 18 (31%) |
| Postmenopause | 4 (80%) | 22 (85%) | 114 (84%) | 41 (69%) |
| Seafood, servings/day | ||||
| Popcorn, servings/day | ||||
| Years residing at current location | ||||
| 0 (0%) | 3 (12%) | 6 (4%) | 5 (8%) | |
| 2–4 | 1 (20%) | 2 (8%) | 22 (16%) | 9 (15%) |
| 4–14 | 2 (40%) | 11 (42%) | 39 (29%) | 22 (37%) |
| 2 (40%) | 10 (38%) | 68 (50%) | 23 (39%) | |
Daily tap water consumption calculated as the sum of tap water consumed at all locations.
Racial category is dichotomized to white and nonwhite due to the small number of women belonging to other racial categories. White is defined without regard to Hispanic ethnicity.
Breastfeeding duration based on total months spent nursing all children reported in 1986 NHS questionnaire data.
Analysis of PFAS in Tap Water
Each archived tap water sample was homogenized by shaking vigorously before subsampling for analysis of 15 PFASs by LC-MS/MS at Harvard University. The perfluorosulfonic acids (PFSAs) measured included: perfluorobutane sulfonic acid (PFBS, four carbon chain length: C-4), linear and branched perfluorohexane sulfonic acid (nPFHxS, brPFHxS: C-6), linear and branched perfluorooctanesulfonic acid (nPFOS, brPFOS: C-8), and perfluorodecanesulfonic acid (PFDS: C-10). The perfluorocarboxylic acids (PFCAs) measured included: perfluoropentanoic acid (PFPeA: C-5), perfluoroheptanoic acid (PFHpA: C-7), linear and branched PFOA (nPFOA, brPFOA: C-8), linear and branched perfluorononanoic acid (nPFNA, brPFNA: C-9), perfluorodecanoic acid (PFDA: C-10), perfluoroundecanoic acid (PFUnDA: C-11), and perfluorododecanoic acid (PFDoA: C-12). The limit of detection (LOD) was calculated as the blank plus the average concentration at which the sample signal-to-noise ratio was three (Table S3). Perfluorohexanoic acid (PFHxA), N-methyl perfluorooctanesulfonamidoacetic acid (N-MeFOSAA) and N-ethyl perfluorooctanesulfonamidoacetic acid (N-EtFOSAA) were not detected in any samples and, therefore, are not included as analytes.
Each sample was adjusted to a pH between 5 and 7 so that all target analytes were in deprotonated state by adding ammonium hydroxide solution, and spiked with of a mass-labeled PFAS mixture (Wellington Laboratories) as internal standards for quantification. PFASs were extracted using an Oasis Wax solid phase extraction (SPE) cartridge (, sorbent, particle size) following methods established in prior work (Taniyasu et al. 2005; Zhang et al. 2016). Sample detection for native PFAS was performed using an Agilent 6460 triple quadrupole LC-MS/MS instrument (Agilent Technologies) equipped with an online SPE system described elsewhere (Zhang et al. 2016). One positive control (deionized water spiked with native PFAS) and one negative control (field blank or procedural blank) were included in every batch of 10 samples. The whole method recovery was between 80% and 98% for all analytes (Table S4). We excluded PFDS from our subsequent data analysis due to a low recovery rate of 44%. Variability between duplicates was less than 20% for all samples. Our method performance was comparable to a recently published ultrasensitive method for analyzing PFAS in small sample volumes (Dasu et al. 2017).
We quantified branched isomers for PFOS, PFOA, PFNA, and PFHxS using calibration standards for the linear isomers, assuming the same instrumental response factor, following previous work (Pellizzaro et al. 2018; Ullah et al. 2011; Zhang et al. 2016). PFDA and PFBS were detected in less than 20% of the 225 archived tap water samples and were excluded from subsequent analyses (Table 2).
Table 2.
PFAS Concentrations in archived tap water samples collected from the Nurses’ Health Study (NHS) participants’ residences in 1989–1990 ().
| (%) | Median (IQR)a [ng/L] | Max [ng/L] | |
|---|---|---|---|
| PFCAs | |||
| PFPeA | 84 (37) | 0.61 (0.13, 1.72) | 62.6 |
| PFHpA | 69 (31) | 0.71 (0.35, 1.15) | 9.65 |
| nPFOA | 134 (60) | 0.57 (0.33, 1.36) | 92.8 |
| brPFOA | 64 (28) | 0.39 (0.17, 0.51) | 12.0 |
| nPFNA | 62 (28) | 0.23 (0.13, 0.29) | 17.9 |
| brPFNA | 22 (10) | 0.19 (0.10, 0.34) | 16.1 |
| PFDAb | 43 (19) | 0.22 (0.19, 0.94) | 27.6 |
| PFUnDA | 109 (48) | 1.15 (0.32, 2.42) | 48.4 |
| PFDoDA | 76 (34) | 2.31 (0.47, 6.40) | 51.0 |
| PFSAs | |||
| PFBSb | 12 (5) | 0.20 (0.19, 0.25) | 2.97 |
| nPFHxS | 134 (60) | 0.51 (0.23, 1.43) | 28.0 |
| brPFHxS | 76 (34) | 0.44 (0.18, 0.99) | 4.93 |
| nPFOS | 107 (48) | 0.69 (0.29, 1.80) | 66.5 |
| brPFOS | 77 (34) | 0.94 (0.51, 2.04) | 18.7 |
| PFDSc | 27 (12) | 0.11 (0.08, 0.52) | 35.8 |
Median (IQR) were calculated for samples only.
PFDA and PFBS were excluded from subsequent analyses due to the proportion of nondetects.
PFDS was excluded from further analyses due to low recovery rate, even though its detection frequency is low as well.
For the remaining PFAS, we used the Robust Regression on Order Statistics (ROS) for data containing multiple detection limits to impute values for nondetects following established methods (Lee and Helsel 2005). First, a linear model was used to regress uncensored observed concentrations against their normal quantiles (order statistics). Censored concentrations were modeled using the parameters of the linear regression and normal quantiles of the censored observations, estimated by the exceedance probability of each censoring limit and the observation's rank. ROS assumes data are left-censored, and the relationship between concentrations and normal quantiles satisfies the assumptions of a linear regression. Water PFAS concentrations were log-transformed before ROS to correct for heteroskedasticity.
Analysis of PFAS in Plasma
Plasma samples for 110 individuals collected at the same time as tap water samples (1989–1990) were analyzed for PFAS by LC-MS/MS in Denmark as part of a separate epidemiological study. For this study, plasma concentrations of 12 PFAS were determined using online SPE followed by high-pressure LC-MS/MS at the University of Southern Denmark, following the methods reported elsewhere (Haug et al. 2009). The analytical system consisted of a Thermo Scientific EQuan MAX system connected to a TSQ Quantum Ultra Triple Stage Quadrupole mass spectrometer (Thermo Fisher Scientific). The online SPE was performed on a Betasil C8 () column, and the separation was performed on a Betasil C8 () column (Thermo Fisher Scientific). The LOD for all plasma PFAS was . Splits of blinded quality control samples were inserted in the different batches and used to calculate a between-batch coefficient of variation (CV) (Table S5). Measured PFAS concentrations were adjusted for batch effects following established methods (Rosner et al. 2008). A linear model was first fit to regress PFAS concentrations on batch indicator dummy variables. PFAS concentrations were then recalibrated by subtracting the difference between the coefficient of each individual batch and the average of the coefficients of all batches. All analyses reported here were based on the recalibrated values of plasma PFAS.
Comparability of Cross-Lab Water and Plasma Measurements
Two labs were used to conduct PFAS analysis in this study (Harvard University for water samples and the University of Southern Denmark for plasma samples) using parallel analytical methods. Each lab specializes in the respective matrix analyzed to ensure the highest-quality data were produced for this study. Rigorous QA protocols were followed for both water and plasma measurements reported here, as summarized in Table S4.
Statistical Methods
For the five major PFAS (PFOA, PFNA, nPFOS, brPFOS, PFHxS), we used a generalized additive model (GAM) to regress log-transformed plasma PFAS against log-transformed tap water PFAS with a cubic spline smoothing function (Hastie 2016). The relative importance of tap water to PFAS exposure is expected to vary according to tap water intake. We therefore stratified the GAM analysis by tap water consumption rates of greater or less than 8 cups per day. We calculated Cook’s distance statistic to identify data points with high leverage. The adjusted GAM included age, race/ethnicity, body weight, menstruation status, parity, breastfeeding history, years residing at current address, seafood consumption, and popcorn consumption. Our analysis was based on responses to the 1990 questionnaire, which were modeled as continuous variables or categorized as shown in Table 1. We calculated the duration of residence (years) at the tap water sample location based on residential addresses in 1976, 1986, 1988, and 1990. Statistical analyses were performed using R (version 3.3.2; R Development Core Team), and statistical significance was defined as . Concentrations for tap water and plasma PFAS were log-transformed (ln) before fitting a general additive model.
To illustrate the implications of high method reporting limits (MRL) in data from the U.S. EPA’s third Unregulated Contaminants Monitoring Rule (UCMR3) for human exposure, we reported effect sizes based on a hypothetical increase in tap water PFAS concentrations from the median of 1989–1990 samples to the MRL in U.S. EPA UCMR3. PFOS isomers are not reported separately in UCMR3, and here we assume 70% of PFOS is linear and 30% of PFOS is branched, based on literature values (Yu et al. 2015).
Toxicokinetic (TK) Model Simulations of Plasma PFAS Concentrations
One-compartment toxicokinetic (TK) models have been successfully used to relate external PFAS exposures from sources such as drinking water to serum PFAS concentrations (Gomis et al. 2017; Lorber and Egeghy 2011; Thompson et al. 2010). TK models can be used to assess the relative importance of different exposure sources (Lorber and Egeghy 2011; Trudel et al. 2008; Vestergren and Cousins 2009) and to establish benchmark doses for PFAS from toxicological and epidemiological studies (Goeden et al. 2019). For example, Grandjean and Budtz-Jørgensen (2013) used such modeling to suggest a health advisory level for perfluorooctanoic acid (PFOA) in drinking water of based on vaccine antibody responses in children and an uncertainty factor of 10 (Grandjean and Budtz-Jørgensen 2013).
We used the difference between measured plasma PFAS concentrations for 110 individuals and modeled contributions to plasma based on measured levels in tap water in 1989–1990 to estimate the RSC of drinking water to overall PFAS exposure. For individuals whose tap water PFAS concentrations were below detection, we assumed tap water was a negligible fraction of their overall exposure and excluded them from TK modeling. For example, consumption of of water per day for a woman at our analytical detection limit for PFOA (average ) corresponds to a plasma PFOA value of , which is 1.7% of the measured mean in the NHS plasma samples. TK modeling was conducted only for plasma PFAS with a , namely PFOA, PFNA, nPFOS, brPFOS, and PFHxS (Table S5).
For TK modeling, we assumed steady state from chronic drinking water exposure and simulated the resulting plasma concentrations as follows:
where (L/day) is the tap water consumption rate reported in NHS questionnaire data; (day) is the half-life in the human body for a given PFAS; (kg) is individual body weight reported in the NHS survey data; (mL/kg) is the volume of distribution, which is the theoretical volume that reflects how PFAS distribute throughout the body; and (ng/L) is the measured drinking water PFAS concentration in this study.
We used Monte Carlo (MC) simulations to investigate how variability in input parameters, such as self-reported water consumption rates, chemical half-lives, and the volume of distribution, affected the modeled relative source contribution of tap water. We ran 300 iterations of the TK model using input parameters that were randomly sampled from specified distributions (Table S6). Means and variances for the elimination half-life in the human body and volumes of distribution were based on a literature review (search terms are provided in Supplemental Information Section S1) and modeled using a truncated () lognormal distribution (Table S7). No information is available on the volume of distribution of PFAS in humans (Thompson et al. 2010); therefore, our parameterization was based on two animal studies (Ohmori et al. 2003; Sundström et al. 2012). A precedent for the use of animal data when human TK parameters are not available has been established in prior work (Gomis et al. 2016; Zhang et al. 2013). Data on half-lives of PFAS in human plasma are available from multiple studies, and we thus used a weighted average based on the inverse of the variance reported in each study in our TK model.
NHS participants reported tap water consumption inside and outside the home in the following categories as part of the 1988 questionnaire: none (zero), 1 to 2, 3 to 5, 6 to 9, and 10 or more cups per day. We used a uniform distribution with the lower and upper limit of each category, except for the “none” (assigned zero) and “10 or more” (assigned 11) categories. We calculated the mean and 95% probability interval among the 300 iterations for the median, mean, 25th and 75th percentile of the RSC of drinking water. We quantified the contribution of different input parameters to the total variability in the RSC of drinking water as the square of the correlation coefficient normalized to the sum of the squared correlation coefficients (Wang et al. 2016).
Temporal Changes in Tap Water Exposures to PFAS
To better understand temporal shifts in drinking water exposures to PFAS, we matched the locations of each of the 225 NHS participant’s homes to the U.S. EPA’s UCMR3 database by county. For the 2013–2015 data at each location, we calculated both the maximum PFAS concentration and detection frequency. This calculation was necessary because the UCMR3 method reporting limits (range ) were much higher than the detection limits in this study (Table S3). We calculated the Spearman’s rank order correlation coefficient between tap water PFAS concentrations in 1989–1990 and those in the period 2013–2015. In our TK modeling, we replaced drinking water PFAS concentrations measured in 1989–1990 with those from the UCMR database (2013–2016) to simulate potential changes in plasma concentrations. We used two approaches to account for samples below detection in the UCMR database. First, we replaced nondetects with the MRL divided by square root of 2. Second, we replaced nondetects with a concentration of zero. We then compared the sensitivity of modeled results to the treatment of nondetects. We did not use regression-based imputation methods such as ROS because the detection frequency in UCMR database was only 4%.
Recent work indicates many precursors to PFAS are present in environmental samples that are not detectable by targeted analyses (Barzen-Hanson et al. 2017; Wang et al. 2017). We thus conducted a pilot investigation that measured total extractable organofluorine (EOF) as a proxy for the total burden of fluorinated compounds in tap water from five cities where we had participant samples. In 2016, we collected samples from the same municipal water supplies as the original participants’ homes (Table S8). At each location, the tap water was flushed for 2 to 3 min before duplicate samples were collected in prerinsed HDPE bottles. One field blank was prepared at each sampling location using HPLC-grade water. Two duplicates were taken at each of the five sampling locations. For targeted PFAS in the 2016 water samples, we followed the same analytical procedures as reported above for the 1989–1990 tap water samples, except that no pH adjustment was needed and was used in the analysis. Field blanks for the five locations sampled in the summer of 2016 were all below the LOD for the targeted 15 PFAS. The relative percentage difference identified in duplicate samples for 15 PFAS was . Average concentrations measured at each location are reported here.
EOF was measured on paired 1989–1990 and 2016 tap water samples at Örebro University in Örebro, Sweden, using extraction methods that separate inorganic and organic fluorine and combustion ion chromatography, following previously established methods (Miyake et al. 2007). Briefly, SPE and dilution procedures were optimized to adequately separate inorganic fluorine (such as fluoride used for water fluoridation) from the organic fraction. Then, all the organic fluorine was converted into hydrogen fluoride (HF) during combustion and absorbed into a hydrogen peroxide solution. The concentration of was quantified by ion chromatography. The method detection limit (MDL) for EOF () was calculated from the average concentration of three procedure blanks plus three times the SD. Samples reported for EOF have been blank corrected and additional QA/QC information is provided in Table S4. Organofluorine concentrations contributed by quantifiable PFAS are calculated as:
where is the number of fluorine atoms in a single PFAS, is the molecular weight of fluorine (g/mol), is the molecular weight of the corresponding PFAS (g/mol), is the concentration of PFAS (ng/L).
To help interpret drivers of temporal differences in tap water PFAS between 1989–1990 and 2013–2016, we obtained data on the number of industries known to release PFAS between 1987 and 2015 (U.S. EPA 2018). No information on the magnitudes of PFAS releases is available from the EPA Toxic Release Inventory (TRI) database. Relevant industrial sources were thus identified following the methods outlined in previous work using the North American Industrial Classification System (NAICS) code (Table S9) (Zhang et al. 2016).
Results
Concentrations of PFAS in Matched Tap Water and Plasma Samples
Concentrations of the 15 PFAS measured in 1989–1990 tap water samples from the NHS study are shown in Table 2. The most frequently detected PFAS were nPFOA (60%), nPFHxS (60%), nPFOS (48%), PFUnDA (48%), and PFPeA (37%). We detected 11 out of the 15 PFAS targeted for analysis in 28% of the samples. Median concentrations of PFAS were in the low ng/L range. Highest concentrations measured in tap water collected in 1989–1990 were for nPFOA, for nPFOS, and for nPFHxS.
All five PFAS included in toxicokinetic modeling (PFOA, PFNA, nPFOS, brPFOS, and PFHxS) were detected in 100% of plasma samples from NHS participants (Table 3). Median concentrations measured in NHS participants were: PFOA (), PFOS (sum of linear and branched isomers, ), PFHxS (), and PFNA (). Median concentrations of PFAS in plasma for NHS participants reported here were similar to those for adult women who participated in the 1999–2000 NHANES survey (PFOA: , PFOS: , PFHxS: , PFNA: ) (Calafat et al. 2007).
Table 3.
PFAS Concentrations in tap water and plasma collected in 1989–1990 from Nurses’ Health Study (NHS) participants ().
| Plasma PFAS | Tap water PFAS | |||||
|---|---|---|---|---|---|---|
| (%) | Median (IQR) [ng/mL] | Max [ng/mL] | (%) | Median (IQR)a [ng/L] | Max [ng/L] | |
| PFOA | 110 (100) | 4.78 (3.56, 6.47) | 77.72 | 50 (45) | 0.57 (0.46, 1.65) | 104.74 |
| PFNA | 110 (100) | 0.61 (0.41, 0.89) | 11.53 | 31 (28) | 0.13 (0.12, 0.35) | 1.98 |
| nPFOS | 110 (100) | 15.86 (12.27, 22.00) | 79.83 | 58 (53) | 0.43 (0.25, 1.43) | 16.56 |
| brPFOS | 110 (100) | 11.99 (9.10, 16.13) | 47.45 | 50 (45) | 1.12 (0.44, 1.91) | 10.38 |
| PFHxS | 110 (100) | 1.89 (1.04, 2.85) | 52.66 | 67 (61) | 0.57 (0.10, 1.86) | 12.19 |
Medians (IQR) were only calculated for samples greater than the LOD (limit of detection).
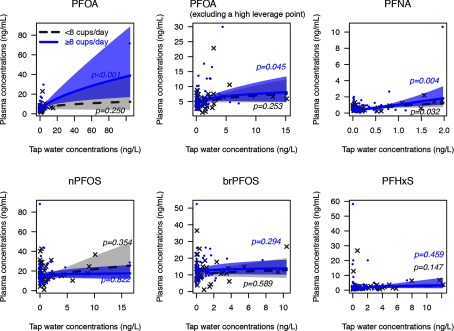
Measured tap water PFOA and PFNA concentrations were statistically significant predictors of plasma concentrations among the 66 NHS participants who consumed cups of tap water per day (Figure 2). We estimated the expected increases in plasma PFOA and PFNA associated with an increase in tap water concentrations from the 1989–1990 median to the U.S. EPA UCMR3 method reporting limit (Table 4). These associations remained statistically significant after adjustment for age, race/ethnicity, body weight, menstruation status, parity, breastfeeding history, years residing in current address, seafood consumption, and popcorn consumption. After adjustments for covariates, point estimates changed by for PFOA and for PFNA. We included and excluded one individual in the PFOA model because Cook’s distance statistic suggested this was a high leverage point. Excluding this observation did not change the statistical significance of the unadjusted association. The effect size was reduced from 250% (95% confidence interval (CI): 130%, 450%) to 80% (95% CI: 0.20%, 220%).
Figure 2.
Associations between tap water and plasma PFAS concentrations among NHS participants in 1989–1990, estimated with a Generalized Additive Model (GAM) with a cubic spline smoothing function. Model estimates were not adjusted for covariates. Shaded area showed 95% confidence interval (CI). Participants who consume cups of tap water per day () are shown in light gray and participants who consume cups of tap water per day () are shown in blue. The PFOA model was run twice after removing one individual with high leverage. For a description of the models, see Table 4.
Table 4.
Modeled difference in plasma PFAS concentrations (%) with a hypothetical increasea in tap water PFAS concentrations.
| MRL | Exposure changeb | Unadjusted | Adjustede | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ng/L | ng/L | % Change (95% CI) | p-value | % Change (95% CI) | p-value | % Change (95% CI) | p-value | % Change (95% CI) | p-value | |
| PFOA | 20.0 | 19.4 | 253 (127, 448) | 73.9 (, 215) | 0.3 | 227 (23.3, 766) | 23.5 (, 220) | 0.4 | ||
| PFOAc | 20.0 | 19.4 | 79.8 (0.2, 223) | 0.04 | 70.2 (, 202) | 0.3 | 55.2 (, 346) | 0.2 | 24.4 (, 219) | 0.3 |
| PFNA | 20.0 | 19.9 | 2720 (444, 14522) | 618 (91.4, 2592) | 0.03 | 1540 (98.1, 13483) | 0.06 | 275.2 (, 1917) | 0.2 | |
| nPFOSd | 28.0 | 27.6 | 13.3 (, 81.6) | 0.8 | 105 (, 375) | 0.4 | (, 165) | 1.0 | 75 (, 587) | 0.4 |
| brPFOS | 12.0 | 10.9 | 21.1 (, 70.6) | 0.3 | 8.7 (, 79.2) | 0.6 | 12.8 (, 194) | 0.6 | (, 104) | 0.3 |
| PFHxS | 30.0 | 29.4 | 157 (, 1025) | 0.5 | 54.6 (, 190) | 0.1 | 134 (, 1660) | 0.5 | 28 (, 470) | 0.4 |
Exposure change estimated based on a hypothetical increase in tap water PFAS concentrations from the median of 1989–1990 samples to the method reporting limits (MRL) in U.S. EPA UCMR3. Concentrations for tap water and plasma PFAS were log-transformed before fitting a general additive model.
The difference between the MRL and median tap water PFAS concentrations in 1989–1990.
PFOA model with one high leverage data point removed. This individual had a tap water PFOA concentration of and a plasma PFOA concentration of . She maintained the same residential location between 1986 and 1990 but moved at least once in the preceding decade (1976 to 1986).
PFOS isomers are not reported separately in UCMR3. Here we assume based on literature values that 70% of PFOS is linear and 30% of PFOS is branched (Yu et al, 2015).
Adjusted for age, race/ethnicity, body weight, menstruation status, parity, breastfeeding history, years residing at current address, seafood consumption, and popcorn consumption.
TK Modeling Results
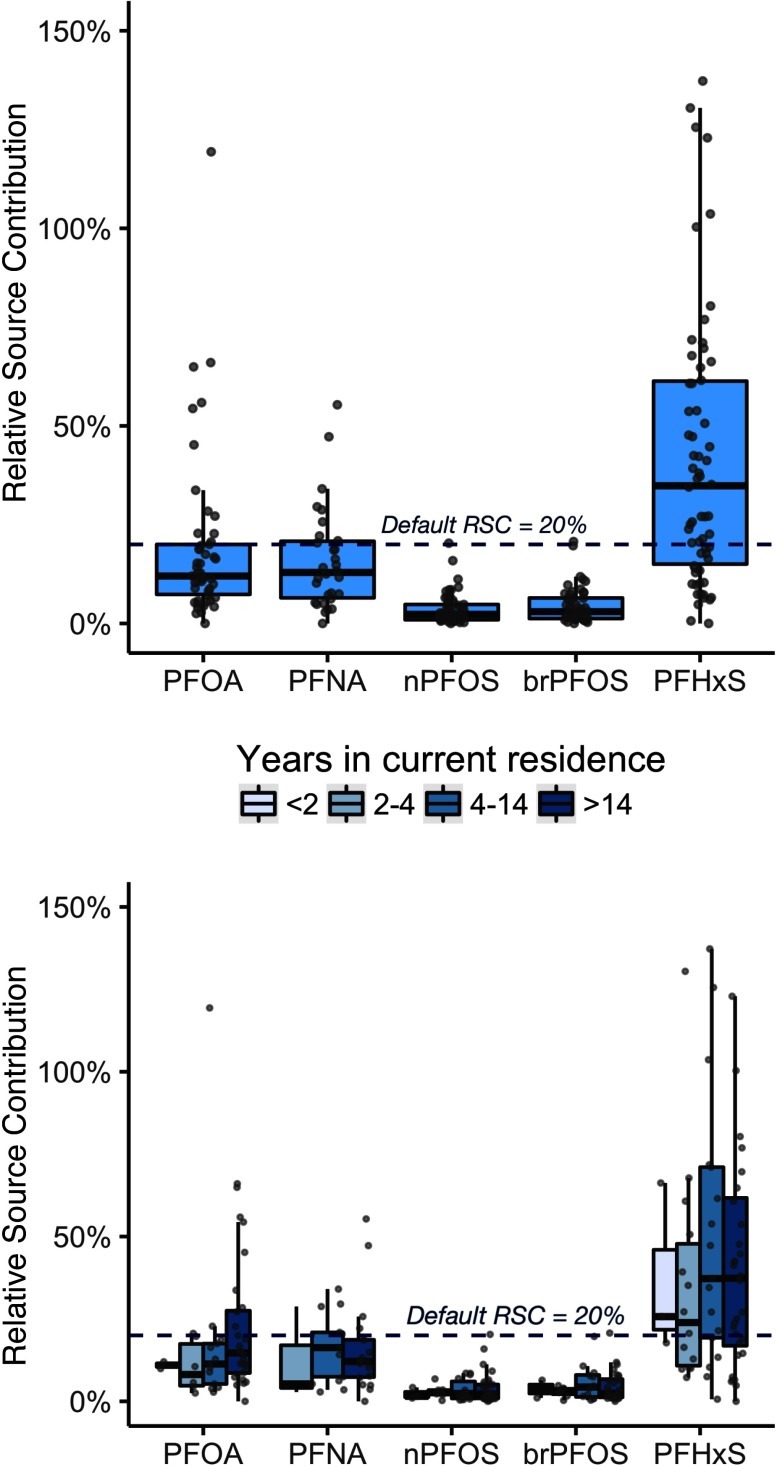
One-compartment TK modeling suggested that, in 1989–1990, the median relative contribution of tap water consumption to plasma concentrations was 12% [interquartile range (IQR): 7.7%– 20%] for PFOA, 13% (IQR: 6.4%–21%) for PFNA, 2.2% (IQR: 0.9%–.8%) for nPFOS, 3.0% (IQR: 1.2%–6.5%) for brPFOS, and 34% (IQR: 15%–61%) for PFHxS (Figure 3, Table S10). There was no statistically significant difference between the RSC of tap water to plasma PFAS concentrations across different groups of residential years at the same address (Table S11).
Figure 3.
Estimated relative source contribution (RSC) of tap water to overall PFAS exposure using a one-compartment toxicokinetic model. The dashed line represents the default RSC (20%) used in risk assessment to derive drinking water advisory levels. The upper panel shows the RSC among 110 Nurses’ Health Study (NHS) participants in 1989–1990. The lower-panel shows the RSC stratified by number of years at the same residential location. Box and whisker plots show the fifth, 25th, 50th, 75th and 95th percentiles among each group. Individual estimates are denoted by gray dots with small random variation added to their horizontal position for better separation. All data used to generate this figure are provided in Tables S8 and S9.
Median RSCs based on MC simulations were similar () to deterministic (single central point) estimates. However, the probabilistically estimated mean RSCs for PFNA and PFHxS increased by more than 5% (Table S12). The modeled RSCs for PFOA and PFOS showed less variability than did PFNA and PFHxS (Figure S2). For PFNA, uncertainty in the chemical half-life accounted for 60% of the variability in the modeled RSC. For PFHxS, the volume of distribution and drinking water intake were the two most important determinants of overall variability, contributing to 47% and 43%, respectively (Figure S3).
Temporal Changes in PFAS Concentrations in Tap Water
The U.S. EPA’s UCMR3 database includes data on six legacy PFAS measured between 2013 and 2015 in U.S. drinking water supplies (Hu et al. 2016). Four of the UCMR3 compounds (PFOA, PFNA, PFOS, and PFHxS) overlap with PFAS detected in this work. Comparing the 1989–1990 NHS tap water sampling locations to UCMR3 data revealed that 46% (representing 53 out of 144 counties) overlapped with sampling locations that had detectable concentrations of at least one PFAS in the UCMR3 database (U.S. EPA 2015). PFOS concentrations measured in 1989–1990 NHS samples were significantly and positively correlated with both the maximum concentration (Spearman correlation , ), and the detection frequency (, ) in the 2013–2015 UCMR3 samples. PFNA concentrations measured in 1989–1990 NHS samples were significantly and positively correlated with the maximum concentration in the 2013–2015 UCMR3 samples (, ). We did not find significant correlations for the maximum concentration for PFOA (, ), and PFHxS (, ), or the detection frequency for PFOA (, ), PFNA (, ), and PFHxS (, ).
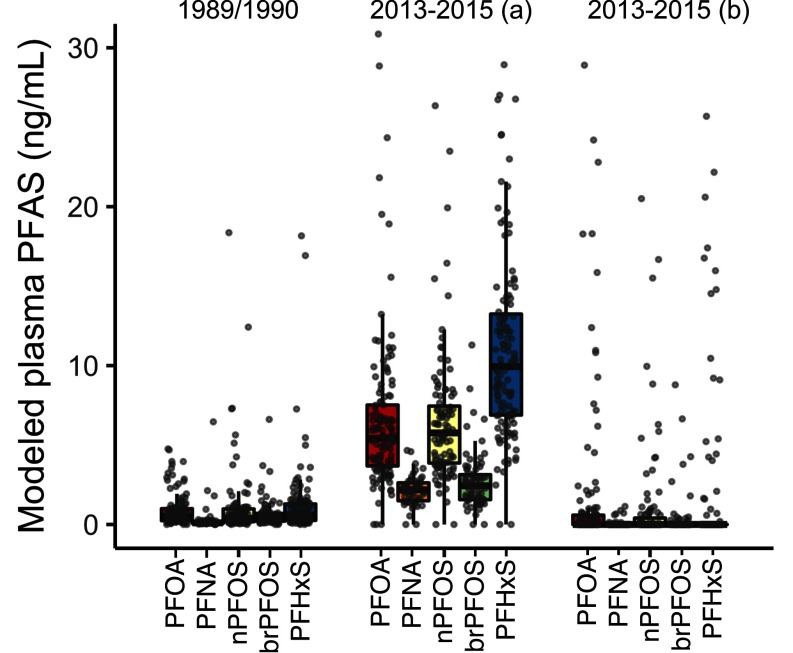
Figure 4 shows modeled median plasma concentrations are sensitive to the treatment of nondetects in the UCMR3 database due to its relatively high MRL of in comparison with our work. We replaced nondetects for PFOA in the UCMR3 database by the MRL divided by the square root of 2. This replacement results in a median modeled plasma concentration in 2013–2015 that is more than 10-fold higher () than modeled contributions of drinking water in 1989–1990 (). Alternately, replacing nondetects by zero results in median modeled plasma concentrations in 2013–2015 that are also zero.
Figure 4.
Estimated plasma PFAS concentrations for 225 individuals using a one-compartment toxicokinetic model and paired recent (2013–2015) measurements of PFASs in tap water with those from 1989–1990. Data under “2013–2015(a)” show nondetects replaced by the method ; Data under “2013–2015(b)” show nondetects replaced by zero. Box and whisker plots indicate fifth, 25th, 50th, 75th and 95th percentiles among each group. Individual estimates are shown as gray dots with small random variation added to their horizontal position for better separation.
We measured a 5- to 320-fold increase in total EOF among the tap water samples collected from the same city in 1989–1990 and 2016 (Table 5). The fraction of unquantified organic fluorine (unknown PFAS) was above 60% (range: 60%–94%) in all the tap water samples collected in 2016, in comparison with 8%– 89% in 1989–1990 samples. It is possible that some degradation of polyfluoroalkyl precursors to PFAS occurred over time in the archived tap water samples from 1989–1990, but this degradation would not affect the total EOF values reported.
Table 5.
Extractable organic fluorine levels from tap water samples matched by city at five locations in Massachusetts (MA1 – MA5) in 1989–1990a and 2016b
| Extractable organic fluorine (ng/L) | MA1 | MA2 | MA3 | MA4 | MA5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1989–1990 | 2016 | 1989–1990 | 2016 | 1989–1990 | 2016 | 1989–1990 | 2016 | 1989–1990 | 2016 | |
| PFOA | 0.2 | 6.2 | 0.5 | 1.7 | 0.9 | 4.8 | 0.6 | 0.9 | 1.3 | 0.9 |
| PFOS | 0.4 | 1.6 | 0.4 | 0.8 | 1.2 | 4.2 | 0.5 | 0.3 | 0.6 | 0.3 |
| Other PFCAs | 0.1 | 7.4 | 0.8 | 4.2 | 1.3 | 9.6 | 0.6 | 1.7 | 0.0 | 5.1 |
| Other PFSAs | 0.3 | 4.3 | 0.3 | 1.7 | 1.5 | 5.6 | 0.2 | 0.7 | 0.4 | 0.1 |
| PFOS precursors | 0.0 | 0.0 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Unknown | 6.7 | 135.6 | 19.8 | 105.2 | 2.9 | 39.4 | 0.2 | 58.5 | 5.4 | 9.6 |
1989–1990 tap water samples were collected from five participants’ home addresses, one sample at each location.
2016 tap water samples were collected in the cities from the same municipal water supplies as original participant’s homes. Two samples were collected at each location.
Discussion
In U.S. women without known occupational exposure to PFAS, we found significant positive associations between tap water and plasma PFOA and PFNA concentrations in samples collected in 1989–1990. These relationships were strongest among individuals who reported consuming cups of tap water per day. A default RSC of 20% is used by U.S. federal and state agencies in risk assessments for deriving drinking water advisory levels for legacy PFAS (Post et al. 2017; U.S. EPA 2016). Our results suggest that this RSC was reasonable for legacy PFAS in 1989–1990. However, recent increases in legacy PFAS concentrations have been reported in many of the same municipal water supplies in the U.S. EPA UCMR database. Our analysis shows large increases in unknown EOF (PFAS not quantified in the targeted analysis) in five paired tap water samples from 1989–1990 and 2016.
Tap water PFAS concentrations measured in 1989–1990 in this study were mostly in the low ng/L range and below current drinking water guidelines. Only 14 out of 225 samples exceeded the state of Michigan’s guideline for PFOS () and six samples exceeded the state of New Jersey’s advisory level for PFOA () and PFNA (). Concentrations reported in this study for 1989–1990 are much lower than those more recently measured near contaminated sites, and thus these archived samples provide unique insight into past drinking water exposures among the U.S. general population (Emmett et al. 2006; Hoffman et al. 2010; Hölzer et al. 2008).
Concentrations and detection of PFOS in drinking water samples collected in 2013–2015 as part of the U.S. EPA UCMR3 database were positively correlated with tap water concentrations measured in 1989–1990. No statistically significant temporal correlations were observed for the other three PFAS examined (PFHxS, PFNA, PFOA). This observation may reflect the large quantities of PFOS released to the environment between 1951 and 2002 in comparison with the other compounds. For example, global production of perfluorooctanesulfonyl fluoride (POSF), the parent chemical to PFOS, was 5 to 37 times greater than that of PFCAs over that time period (Earnshaw et al. 2014; Paul et al. 2009; Wang et al. 2014).
The number of potential PFAS point sources across the United States has fluctuated between 1990 and 2015 (Figure S4). Source abundance peaked in both 1990 and the early 2000s but declined in the later parts of both decades. Over the same time period, there has been a phaseout in production and use of legacy PFAS such as PFOS and PFOA in North America and Europe and rapid growth in the number and diversity of replacement compounds (Stockholm Convention 2009; U.S. EPA 2006; Wang et al. 2014; Wang et al. 2017). It is currently unclear how these changes have affected exposures to PFAS from drinking water.
Data collected in this work provide suggestive evidence of an increase in the estimated plasma PFAS concentrations of legacy PFAS (PFOS, PFOA, PFNA, and PFHxS) through tap water consumption between 1989–1990 and 2013–2015 (Figure 4). This finding is sensitive to the treatment of nondetects in the UCMR3 database. We also report an increase in the burden of unquantified organofluorine compounds based on pilot data of five pairs of tap water samples (Table 5). These pilot data provide preliminary evidence of a potential increase in accumulation of unknown organofluorine compounds in drinking water that are not included in routine environmental monitoring and targeted analysis. Such patterns are consistent with increasing numbers of PFAS produced and released to the environment in recent years (Ritscher et al. 2018; Strynar et al. 2015; Wang et al. 2013). This increase contrasts with serum trends in PFOS and PFOA in many human populations in the United States and Europe that have been widely observed to decline since the early 2000s, reflecting the success of phaseouts in chemical production and stewardship programs (Dassuncao et al. 2018).
Tap water concentrations were statistically significant predictors of plasma PFOA and PFNA concentrations for NHS participants, but not of PFOS and PFHxS. The high median RSC of tap water for PFHxS (34.1%) among individuals with detectable tap water concentrations likely reflects its long half-life (7.3 y) in human plasma (Olsen et al. 2007).
TK modeling suggests median concentrations of the five major PFAS measured in the NHS drinking water samples from 1989–1990 would result in exposures equivalent to between 0.01 and in plasma, assuming consumption of of water per day for a woman. This finding is relevant for the U.S. general population because plasma PFAS concentrations among NHS participants in 1989–1990 and adult women in the 1999–2000 NHANES survey are almost identical (Calafat et al. 2007). The median RSC of drinking water for PFOA exposures in 1989–1990 was 12%, with an IQR between 7.7% to 20%. This finding is similar to values reported in prior work (Post et al. 2012; Post et al. 2017), although to the best of our knowledge our study represents the earliest time period for general population exposures.
Our results are consistent with existing studies on the relative importance of different exposure pathways (diet, drinking water, dust, consumer product, inhalation). Dietary ingestion was thought to be the major exposure pathway for PFOS among the general adult populations in North America and Europe between 2011 and 2016. Estimated dietary PFOS exposures ranged from 66% to 99%, whereas drinking water contributed 0.10% to 22% of total exposure (Egeghy and Lorber 2011; Gebbink et al. 2015; Shan et al. 2016). Drinking water has been estimated to account for between 0.70% and 37% of overall exposure to PFOA (Tian et al. 2016; Vestergren and Cousins 2009). Our study is the first to estimate the relative importance of drinking water for PFHxS and PFNA.
The RSC of drinking water to total PFAS exposure is important for designing guidelines that are health protective. If the RSC of drinking water is overestimated, total exposures of the general population may exceed the reference dose, even when drinking water PFAS concentrations fall below the guideline. For PFAS other than PFOS and PFOA, the RSCs of tap water have not been resolved. Our analyses suggest tap water can be an equally important contributor for PFNA (median RSC is 13%), and an even more important contributor for PFHxS (median RSC is 34%). Pilot data on EOF suggest that unknown PFAS in drinking water may have increased and that static RSC values may be problematic.
This study has several strengths and limitations. It is nested in a well-established cohort study that includes comprehensive information on diet, lifestyle, and medical history collected using validated questionnaires. A detailed residential history is available for participants and the cohort employs stringent laboratory quality control procedures, allowing the development of a novel combination of both multivariate statistical and toxicokinetic models. Data presented in this study are limited in terms of sample size, which restricts their statistical power to inform the potential temporal changes of PFAS in tap water over the past 27 years. We measured PFAS in matched tap water and plasma samples at a single point in time. However, PFAS have long half-lives in human body, and plasma PFAS concentrations are not prone to short-term, within-person variability. Finally, information needed to quantify the magnitudes of nonwater sources of PFAS exposure, such as consumer product use, dietary consumption preferences for PFAS-containing items, and indoor exposure, is not available in the NHS. Such data are needed to evaluate the relative importance of other exposure pathways for PFAS.
Conclusions
The default RSC value (20%) for tap water currently used in PFAS risk assessments of drinking water are consistent with findings from this study, based on archived tap water and plasma samples from 1989–1990. We estimated that tap water contributed between 2.2% and 34% of plasma concentrations for the five PFAS examined in 1989–1990 among a subsample of participants in the NHS. Temporal and geographic shifts in PFAS production and use may have altered the RSC from drinking water since that time.
The ongoing presence of PFOS in drinking water samples collected between 2013–2016 reinforces the concern that accumulation of legacy PFAS in the environment can elevate human exposure long after the cessation of global production. The rapid decline in human exposure to legacy PFAS since 2000 has been predominantly driven by the phaseout of PFOS and its precursors (Dassuncao et al. 2018). If exposures from consumer product use decrease due to regulatory actions and voluntary phaseouts, environmental sources may drive future exposures (Gomis et al. 2017; Vestergren and Cousins 2009). We conclude that a more holistic approach that targets the entire class of fluorinated compounds would enable more health-protective drinking water guidelines (Blum et al. 2015). Our pilot data showing temporal changes in EOF suggest that consideration of drinking water exposures to emerging PFAS is warranted for the general U.S. population (Sunderland et al. 2018).
Supplementary Material
Acknowledgments
The authors thank the participants and staff of the NHS. This study was supported by the Harvard National Institute of Environmental Health and Sciences (NIEHS) Center Grant (P30 ES000002) and NIH grants, CA186107, CA49449, and ES021372. X.C.H., P.G., and E.M.S. were partially supported by the NIH Superfund Research Program P42ES027706. The authors thank C. Wagner, R. Stern, and G. Zong (Harvard) for assistance with water sample collection and statistical analyses.
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP4093).
P.G. served as a health expert for the state of Minnesota in a lawsuit against a PFAS-producing company. All other authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Barzen-Hanson KA, Roberts SC, Choyke S, Oetjen K, McAlees A, Riddell N, et al. 2017. Discovery of 40 classes of per- and polyfluoroalkyl substances in historical aqueous film-forming foams (AFFFs) and AFFF-impacted groundwater. Environ Sci Technol 51(4):2047–2057, PMID: 28098989, 10.1021/acs.est.6b05843. [DOI] [PubMed] [Google Scholar]
- Belanger CF, Hennekens CH, Rosner B, Speizer FE. 1978. The Nurses' Health Study. Am J Nurs 78(6):1039–1040, PMID: 248266. [PubMed] [Google Scholar]
- Blum A, Balan SA, Scheringer M, Trier X, Goldenman G, Cousins IT, et al. 2015. The Madrid statement on poly-and perfluoroalkyl substances (PFASs). Environ Health Perspect 123(5):A107, PMID: 25932614, 10.1289/ehp.1509934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS, Needham LL. 2007. Serum concentrations of 11 polyfluoroalkyl compounds in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES). Environ Sci Technol 41(7):2237–2242, PMID: 17438769. [DOI] [PubMed] [Google Scholar]
- Dassuncao C, Hu XC, Nielsen F, Weihe P, Grandjean P, Sunderland EM. 2018. Shifting global exposures to poly- and perfluoroalkyl substances (PFASs) evident in longitudinal birth cohorts from a seafood-consuming population. Environ Sci Technol 52(6):3738–3747, PMID: 29516726, 10.1021/acs.est.7b06044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasu K, Nakayama SF, Yoshikane M, Mills MA, Wright JM, Ehrlich S. 2017. An ultra-sensitive method for the analysis of perfluorinated alkyl acids in drinking water using a column switching high-performance liquid chromatography tandem mass spectrometry. J Chromatogr A 1494:46–54, PMID: 28336137, 10.1016/j.chroma.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt JC. 2015. Toxicological effects of perfluoroalkyl and polyfluoroalkyl substances. New York: Humana Press. [Google Scholar]
- Domingo JL, Nadal M. 2017. Per- and polyfluoroalkyl substances (PFASs) in food and human dietary intake: a review of the recent scientific literature. J Agric Food Chem, PMID: 28052194, 10.1021/acs.jafc.6b04683. [DOI] [PubMed] [Google Scholar]
- Earnshaw MR, Paul AG, Loos R, Tavazzi S, Paracchini B, Scheringer M, et al. 2014. Comparing measured and modelled PFOS concentrations in a UK freshwater catchment and estimating emission rates. Environ Int 70:25–31, PMID: 24879369, 10.1016/j.envint.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Egeghy PP, Lorber M. 2011. An assessment of the exposure of Americans to perfluorooctane sulfonate: a comparison of estimated intake with values inferred from NHANES data. J Expo Sci Environ Epidemiol 21(2):150–168, PMID: 20145679, 10.1038/jes.2009.73. [DOI] [PubMed] [Google Scholar]
- Emmett EA, Shofer FS, Zhang H, Freeman D, Desai C, Shaw LM. 2006. Community exposure to perfluorooctanoate: relationships between serum concentrations and exposure sources. J Occup Environ Med 48(8):759–770, PMID: 16902368, 10.1097/01.jom.0000232486.07658.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson I, Nadal M, van Bavel B, Lindström G, Domingo JL. 2008. Levels of perfluorochemicals in water samples from Catalonia, Spain: is drinking water a significant contribution to human exposure? Environ Sci Pollut Res Int 15(7):614–619, PMID: 18763004, 10.1007/s11356-008-0040-1. [DOI] [PubMed] [Google Scholar]
- Gebbink WA, Berger U, Cousins IT. 2015. Estimating human exposure to PFOS isomers and PFCA homologues: the relative importance of direct and indirect (precursor) exposure. Environ Int 74:160–169, PMID: 25454233, 10.1016/j.envint.2014.10.013. [DOI] [PubMed] [Google Scholar]
- Goeden HM, Greene CW, Jacobus JA. 2019. A transgenerational toxicokinetic model and its use in derivation of minnesota pfoa water guidance. J Expo Sci Environ Epidemiol 29:183–195, PMID: 30631142, 10.1038/s41370-018-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis MI, Vestergren R, MacLeod M, Mueller JF, Cousins IT. 2017. Historical human exposure to perfluoroalkyl acids in the United States and Australia reconstructed from biomonitoring data using population-based pharmacokinetic modelling. Environ Int 108:92–102, PMID: 28818713, 10.1016/j.envint.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Gomis MI, Vestergren R, Nilsson H, Cousins IT. 2016. Contribution of direct and indirect exposure to human serum concentrations of perfluorooctanoic acid in an occupationally exposed group of ski waxers. Environ Sci Technol 50(13):7037–7046, PMID: 27304840, 10.1021/acs.est.6b01477. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Budtz-Jørgensen E. 2013. Immunotoxicity of perfluorinated alkylates: calculation of benchmark doses based on serum concentrations in children. Environ Health 12(1):35, PMID: 23597293, 10.1186/1476-069X-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble MO, Bartell SM, Kannan K, Wu Q, Fair PA, Kamen DL. 2015. Longitudinal measures of perfluoroalkyl substances (PFAS) in serum of Gullah African Americans in South Carolina: 2003–2013. Environ Res 143(Pt. B):82–88, PMID: 25819541, 10.1016/j.envres.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelfo JL, Higgins CP. 2013. Subsurface transport potential of perfluoroalkyl acids at aqueous film-forming foam (AFFF)-impacted sites. Environ Sci Technol 47(9):4164–4171, PMID: 23566120, 10.1021/es3048043. [DOI] [PubMed] [Google Scholar]
- Gyllenhammar I, Berger U, Sundström M, McCleaf P, Eurén K, Eriksson S, et al. 2015. Influence of contaminated drinking water on perfluoroalkyl acid levels in human serum – a case study from Uppsala, Sweden. Environ Res 140:673–683, PMID: 26079316, 10.1016/j.envres.2015.05.019. [DOI] [PubMed] [Google Scholar]
- Hastie T. 2016. GAM: Generalized Additive Models. R Package version 1.14. https://cran.r-project.org/src/contrib/Archive/gam/ [accessed 15 October 2017]. [Google Scholar]
- Haug LS, Huber S, Becher G, Thomsen C. 2011. Characterisation of human exposure pathways to perfluorinated compounds—comparing exposure estimates with biomarkers of exposure. Environ Int 37(4):687–693, PMID: 21334069, 10.1016/j.envint.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Haug LS, Thomsen C, Becher G. 2009. A sensitive method for determination of a broad range of perfluorinated compounds in serum suitable for large-scale human biomonitoring. J Chromatogr A 1216(3):385–393, PMID: 19026423, 10.1016/j.chroma.2008.10.113. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Webster TF, Bartell SM, Weisskopf MG, Fletcher T, Vieira VM. 2010. Private drinking water wells as a source of exposure to perfluorooctanoic acid (PFOA) in communities surrounding a fluoropolymer production facility. Environ Health Perspect 119(1):92–97, PMID: 20920951, 10.1289/ehp.1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzer J, Midasch O, Rauchfuss K, Kraft M, Reupert R, Angerer J, et al. 2008. Biomonitoring of perfluorinated compounds in children and adults exposed to perfluorooctanoate-contaminated drinking water. Environ Health Perspect 116(5):651–657, PMID: 18470314, 10.1289/ehp.11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtz EF, Higgins CP, Field JA, Sedlak DL. 2013. Persistence of perfluoroalkyl acid precursors in afff-impacted groundwater and soil. Environ Sci Technol 47(15):8187–8195, PMID: 23886337, 10.1021/es4018877. [DOI] [PubMed] [Google Scholar]
- Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, et al. 2016. Detection of poly- and perfluoroalkyl substances (PFASs) in U.S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ Sci Technol Lett 3(10):344–350, PMID: 27752509, 10.1021/acs.estlett.6b00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley S, Houtz E, Goldberg D, Wang M, Park J-S, Nelson DO, et al. 2016. Preliminary associations between the detection of perfluoroalkyl acids (PFAAs) in drinking water and serum concentrations in a sample of California women. Environ Sci Technol Lett 3:264–269, 10.1021/acs.estlett.6b00154. [DOI] [Google Scholar]
- Khalil N, Chen A, Lee M, Czerwinski SA, Ebert JR, DeWitt JC, et al. 2016. Association of perfluoroalkyl substances, bone mineral density, and osteoporosis in the U.S. population in NHANES 2009-2010. Environ Health Perspect 124(1):81–87, PMID: 26058082, 10.1289/ehp.1307909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsteiner A, Huset C, Williams A, Johnson J. 2014. Biomonitoring for perfluorochemicals in a Minnesota community with known drinking water contamination. J Environ Health 77(5):14–19, PMID: 25619022. [PubMed] [Google Scholar]
- Lee L, Helsel D. 2005. Statistical analysis of water-quality data containing multiple detection limits: S-language software for regression on order statistics. Computers & Geosciences 31:1241–1248, 10.1016/j.cageo.2005.03.012. [DOI] [Google Scholar]
- Liu G, Dhana K, Furtado JD, Rood J, Zong G, Liang L, et al. 2018. Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: a prospective study. PLOS Med 15(2):e1002502, PMID: 29438414, 10.1371/journal.pmed.1002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorber M, Egeghy PP. 2011. Simple intake and pharmacokinetic modeling to characterize exposure of Americans to perfluoroctanoic acid, PFOA. Environ Sci Technol 45(19):8006–8014, PMID: 21517063, 10.1021/es103718h. [DOI] [PubMed] [Google Scholar]
- Minnesota Department of Health. 2017. Toxicological Summary for: Perfluorooctanoic acid. http://www.health.state.mn.us/divs/eh/risk/guidance/gw/pfoa.pdf [accessed 16 January 2018].
- Miralles-Marco A, Harrad S. 2015. Perfluorooctane sulfonate: a review of human exposure, biomonitoring and the environmental forensics utility of its chirality and isomer distribution. Environ Int 77:148–159, PMID: 25728452, 10.1016/j.envint.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Yamashita N, Rostkowski P, So MK, Taniyasu S, Lam PK, et al. 2007. Determination of trace levels of total fluorine in water using combustion ion chromatography for fluorine: a mass balance approach to determine individual perfluorinated chemicals in water. J Chromatogr A 1143(1–2):98–104, PMID: 17229428, 10.1016/j.chroma.2006.12.071. [DOI] [PubMed] [Google Scholar]
- Ohmori K, Kudo N, Katayama K, Kawashima Y. 2003. Comparison of the toxicokinetics between perfluorocarboxylic acids with different carbon chain length. Toxicology 184(2–3):135–140, PMID: 12499116, 10.1016/S0300-483X(02)00573-5. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115(9):1298–1305, PMID: 17805419, 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul AG, Jones KC, Sweetman AJ. 2009. A first global production, emission, and environmental inventory for perfluorooctane sulfonate. Environ Science Technol 43(2):386–392, PMID: 19238969, 10.1021/es802216n. [DOI] [PubMed] [Google Scholar]
- Pellizzaro A, Zaggia A, Fant M, Conte L, Falletti L. 2018. Identification and quantification of linear and branched isomers of perfluorooctanoic and perfluorooctane sulfonic acids in contaminated groundwater in the Veneto region. J Chromatogr A 1533:143–154, PMID: 29269145, 10.1016/j.chroma.2017.12.036. [DOI] [PubMed] [Google Scholar]
- Post GB, Cohn PD, Cooper KR. 2012. Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: a critical review of recent literature. Environ Res 116:93–117, PMID: 22560884, 10.1016/j.envres.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Post GB, Gleason JA, Cooper KR. 2017. Key scientific issues in developing drinking water guidelines for perfluoroalkyl acids: contaminants of emerging concern. PLoS Biol 15(12):e2002855, PMID: 29261653, 10.1371/journal.pbio.2002855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones O, Snyder SA. 2009. Occurrence of perfluoroalkyl carboxylates and sulfonates in drinking water utilities and related waters from the United States. Environ Sci Technol 43(24):9089–9095, PMID: 20000497, 10.1021/es9024707. [DOI] [PubMed] [Google Scholar]
- Ritscher A, Wang Z, Scheringer M, Boucher JM, Ahrens L, Berger U, et al. 2018. Zurich statement on future actions on per- and polyfluoroalkyl substances (PFASs). Environ Health Perspect 126(8):084502, 10.1289/EHP4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner B, Cook N, Portman R, Daniels S, Falkner B. 2008. Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol 167(6):653–666, PMID: 18230679, 10.1093/aje/kwm348. [DOI] [PubMed] [Google Scholar]
- Seals R, Bartell SM, Steenland K. 2011. Accumulation and clearance of perfluorooctanoic acid (PFOA) in current and former residents of an exposed community. Environ Health Perspect 119(1):119–124, PMID: 20870569, 10.1289/ehp.1002346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan G, Wang Z, Zhou L, Du P, Luo X, Wu Q, et al. 2016. Impacts of daily intakes on the isomeric profiles of perfluoroalkyl substances (PFASs) in human serum. Environment Int 89–90:62–70, PMID: 26826363, 10.1016/j.envint.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Stockholm Convention. 2009. The new POPs under the Stockholm Convention. http://chm.pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511/Default.aspx [accessed 15 January 2018].
- Strynar M, Dagnino S, McMahen R, Liang S, Lindstrom A, Andersen E, et al. 2015. Identification of novel perfluoroalkyl ether carboxylic acids (PFECAs) and sulfonic acids (PFESAs) in natural waters using accurate mass time-of-flight mass spectrometry (TOFMS). Environ Sci Technol 49(19):11622–11630, PMID: 26392038, 10.1021/acs.est.5b01215. [DOI] [PubMed] [Google Scholar]
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. 2018. A review of the pathways of human exposure to poly-and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 29(2):131.– , PMID: 30470793, 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström M, Chang S-C, Noker PE, Gorman GS, Hart JA, Ehresman DJ, et al. 2012. Comparative pharmacokinetics of perfluorohexanesulfonate (PFHxS) in rats, mice, and monkeys. Reprod Toxicol 33(4):441–451, PMID: 21856411, 10.1016/j.reprotox.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Taniyasu S, Kannan K, So MK, Gulkowska A, Sinclair E, Okazawa T, et al. 2005. Analysis of fluorotelomer alcohols, fluorotelomer acids, and short- and long-chain perfluorinated acids in water and biota. J Chromatogr A 1093(1–2):89–97, PMID: 16233874, 10.1016/j.chroma.2005.07.053. [DOI] [PubMed] [Google Scholar]
- Thompson J, Lorber M, Toms L-ML, Kato K, Calafat AM, Mueller JF. 2010. Use of simple pharmacokinetic modeling to characterize exposure of Australians to perfluorooctanoic acid and perfluorooctane sulfonic acid. Environ Int 36(4):390–397, PMID: 20236705, 10.1016/j.envint.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Tian Z, Kim S-K, Shoeib M, Oh J-E, Park J-E. 2016. Human exposure to per- and polyfluoroalkyl substances (PFASs) via house dust in Korea: implication to exposure pathway. Sci Total Environ 553:266–275, PMID: 26933964, 10.1016/j.scitotenv.2016.02.087. [DOI] [PubMed] [Google Scholar]
- Tokranov AK, Nishizawa N, Amadei CA, Zenobio JE, Pickard HM, Allen JG, et al. 2018. How do we measure poly-and perfluoroalkyl substances (PFASs) at the surface of consumer products? Environ Sci Technol Lett 6:38–43, 10.1021/acs.estlett.8b00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel D, Horowitz L, Wormuth M, Scheringer M, Cousins IT, Hungerbühler K. 2008. Estimating consumer exposure to PFOS and PFOA. Risk Anal 28(2):251–269, PMID: 18419647, 10.1111/j.1539-6924.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2006. Risk management for per- and polyfluoroalkyl substances (PFASs) under TSCA. PFOA Stewardship Program. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/risk-management-and-polyfluoroalkyl-substances-pfass-tab-3 [accessed 15 January 2018].
- U.S. EPA. 2015. Third unregulated contaminant monitoring rule. https://www.epa.gov/dwucmr/occurrence-data-unregulated-contaminant-monitoring-rule-3 [accessed 23 May 2016].
- U.S. EPA. 2016. Drinking water health advisory for perfluorooctanoic acid (PFOA). EPA Document 822‐R‐16‐005 https://www.epa.gov/sites/production/files/2016-05/documents/pfoa_health_advisory_final-plain.pdf [accessed 3 January 2018].
- U.S. EPA. 2018. Toxic release inventory (tri) program: Tri basic data files: Calendar years 1987–2016. https://www.epa.gov/toxics-release-inventory-tri-program/tri-basic-data-files-calendar-years-1987-2017 [accessed 1 March 2018].
- Ullah S, Alsberg T, Berger U. 2011. Simultaneous determination of perfluoroalkyl phosphonates, carboxylates, and sulfonates in drinking water. J Chromatogr A 1218(37):6388–6395, PMID: 21791340, 10.1016/j.chroma.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Vaughn B, Winquist A, Steenland K. 2013. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect 121(11–12):1313–1318, PMID: 24007715, 10.1289/ehp.1306615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergren R, Cousins IT. 2009. Tracking the pathways of human exposure to perfluorocarboxylates. Environ Sci Technol 43(15):5565–5575, PMID: 19731646, 10.1021/es900228k. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cousins IT, Scheringer M. 2015. Comment on “The environmental photolysis of perfluorooctanesulfonate, perfluorooctanoate, and related fluorochemicals.” Chemosphere 122:301–303, PMID: 24746526, 10.1016/j.chemosphere.2014.03.066. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Buck RC, Hungerbühler K. 2014. Global emission inventories for C4–C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, Part I: Production and emissions from quantifiable sources. Environ Int 70:62–75, PMID: 24932785, 10.1016/j.envint.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Hungerbühler K. 2013. Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFASs) and their potential precursors. Environ Int 60:242–248, PMID: 24660230. [DOI] [PubMed] [Google Scholar]
- Wang Z, DeWitt JC, Higgins CP, Cousins IT. 2017. A never-ending story of per- and polyfluoroalkyl substances (PFASs)? Environ Sci Technol 51(5):2508–2518, PMID: 28224793, 10.1021/acs.est.6b04806. [DOI] [PubMed] [Google Scholar]
- Wang D-G, Dong Q-Q, Du J, Yang S, Zhang Y-J, Na G-S, et al. 2016. Using Monte Carlo simulation to assess variability and uncertainty of tobacco consumption in a city by sewage epidemiology. BMJ Open 6(2):e010583, PMID: 26888732, 10.1136/bmjopen-2015-010583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F, MacLeod M, Mueller JF, Cousins IT. 2014. Enhanced elimination of perfluorooctane sulfonic acid by menstruating women: evidence from population-based pharmacokinetic modeling. Environ Sci Technol 48(15):8807–8814, PMID: 24943117, 10.1021/es500796y. [DOI] [PubMed] [Google Scholar]
- Worley RR, Moore SM, Tierney BC, Ye X, Calafat AM, Campbell S, et al. 2017. Per-and polyfluoroalkyl substances in human serum and urine samples from a residentially exposed community. Environ Int 106:135–143, PMID: 28645013, 10.1016/j.envint.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu N, Wang X, Zhang B, Yang J, Li M, Li J, et al. 2015. Distribution of perfluorooctane sulfonate isomers and predicted risk of thyroid hormonal perturbation in drinking water. Water Res 76:171–180, PMID: 25813491, 10.1016/j.watres.2015.02.047. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Beesoon S, Zhu L, Martin JW. 2013. Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life. Environ Sci Technol 47(18):10619–10627, PMID: 23980546, 10.1021/es401905e. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lohmann R, Dassuncao C, Hu XC, Weber AK, Vecitis CD, et al. 2016. Source attribution of poly- and perfluoroalkyl substances (PFASs) in surface waters from Rhode Island and the New York metropolitan area. Environ Sci Technol Lett 3(9):316–321, PMID: 28217711, 10.1021/acs.estlett.6b00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.