Abstract
Background:
A few studies suggest that air pollution may decrease fertility, but prospective studies and examinations of windows of susceptibility remain unclear.
Objective:
We aimed to examine the association between time-varying exposure to nitrogen dioxide (), ozone (), fine particulate matter (), and black carbon (BC) on in vitro fertilization (IVF) outcomes.
Methods:
We included 345 women (522 IVF cycles) for the , , and analyses and 339 women (512 IVF cycles) for the BC analysis enrolled in a prospective cohort at a Boston fertility center (2004–2015). We used validated spatiotemporal models to estimate daily residential exposure to , , , and BC. Multivariable discrete time Cox proportional hazards models with four periods [ovarian stimulation (OS), oocyte retrieval to embryo transfer (ET), ET to implantation, implantation to live birth] estimated odds ratios (OR) and 95% confidence intervals (CI) of failing at IVF. Time-dependent interactions were used to identify vulnerable periods.
Results:
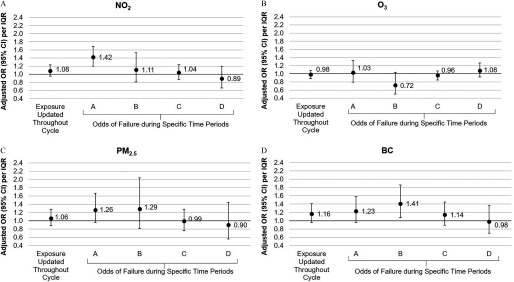
An interquartile range (IQR) increase in , , and BC throughout the IVF cycle was associated with an elevated odds of failing at IVF prior to live birth (, 95% CI: 0.95, 1.23 for ; , 95% CI: 0.88, 1.28 for ; and , 95% CI: 0.96, 1.41 for BC). This relationship significantly varied across the IVF cycle such that the association with higher exposure to air pollution during OS was strongest for early IVF failures. An IQR increase in , , and BC exposure during OS was associated with 1.42 (95% CI: 1.20, 1.69), 1.26 (95% CI: 0.96, 1.67), and 1.23 (95% CI: 0.96, 1.59) times the odds of failing prior to oocyte retrieval, and 1.32 (95% CI: 1.13, 1.54), 1.27 (95% CI: 0.98, 1.65), and 1.32 (95% CI: 1.10, 1.59) times the odds of failing prior to ET.
Conclusion:
Increased exposure to traffic-related pollutants was associated with higher odds of early IVF failure. https://doi.org/10.1289/EHP4601
Introduction
Chronic exposure to ambient air pollution is recognized as an important risk factor for adverse birth outcomes such as prematurity and low birth weight (Lamichhane et al. 2015; Shah and Balkhair 2011). Potential biological pathways mediating this link include oxidative stress (Møller et al. 2014), systemic inflammation (Panasevich et al. 2009), endothelial dysfunction (Wauters et al. 2013), and DNA damage (Risom et al. 2005). Although these pathways are all implicated in the etiology of earlier reproductive outcomes such as follicular and embryologic development (Germain et al. 2007; Gupta et al. 2007; Kwak-Kim et al. 2009; Sharma 2014; Yin et al. 2012), less research has focused on these end points. Furthermore, in the past decade, there has been an increasing number of reports linking air pollution to decreased fecundity and early pregnancy loss (Carré et al. 2017a; Checa Vizcaíno et al. 2016); however, the specific mechanisms underlying these associations and windows of susceptibility remain unclear. By studying a cohort of women undergoing in vitro fertilization (IVF), it is possible to directly observe many early reproductive outcomes that cannot be observed in couples attempting to conceive without medical assistance. Moreover, because key events in the IVF cycle are timed and triggered by a physician, exact periods of air pollution exposure can be determined, which is more challenging to determine in spontaneously conceived pregnancies.
Only a handful of studies to date have assessed the association between air pollution and IVF outcomes (Carré et al. 2017b; Choe et al. 2018; Legro et al. 2010; Perin et al. 2010a, 2010b). While all of these studies suggest an adverse effect of at least one specific air pollutant on IVF outcomes, all were retrospective and unable to account for many other lifestyle factors, namely, tobacco smoke and socioeconomic status (SES). All previous studies also relied upon nearest monitor or district/zip code–level air pollution exposures rather than using more precise prediction models based on the women’s residential address. The previous studies were also unable to assess more specific markers of traffic pollution, such as black carbon (BC), which might be particularly detrimental based on evidence from animal models (Januário et al. 2010; Veras et al. 2009). BC forms during combustion and is emitted when there is insufficient oxygen and heat available for the combustion process to burn the fuel completely (Janssen et al. 2012). Daily variations in BC in urban areas are most strongly associated with local traffic emissions, particularly from diesel vehicles, followed by minor contributions from biomass combustion (Janssen et al. 2012). We previously found a link between residential proximity to major roadways and lower probability of live birth following IVF (Gaskins et al. 2018); however, we were unable to determine whether this association was due to a specific air pollutant(s). To build on this previous literature, we leveraged existing validated spatiotemporal models of air pollution to evaluate the association between specific air pollutants and IVF outcomes in a prospective cohort of women undergoing IVF at a single fertility center.
Methods
Study Population
Participants were recruited into the Environment and Reproductive Health (EARTH) Study starting in November 2004 from patients presenting for infertility evaluation and treatment at the Massachusetts General Hospital (MGH) Fertility Center (Messerlian et al. 2018). All women 18–46 y at enrollment were eligible, and approximately 60% of eligible women contacted by the research staff participated in the study. Upon enrollment, all participants provided their residential address for reimbursement purposes. Women were then followed through their infertility treatment cycles until discontinuation or live birth.
Of the 696 IVF cycles contributed by EARTH participants to date, we excluded 30 donor oocyte recipient cycles and 91 cryopreservation thaw cycles, since our primary interest was in fresh, autologous IVF cycles. Fresh cycles were defined as those with an intended embryo transfer (ET) immediately following a cycle of ovarian stimulation (OS). From the remaining 575 IVF cycles, we further excluded 53 cycles that ended after December 2015 (due to the temporal constraints of some of the air pollution models). This resulted in 522 fresh, autologous IVF cycles contributed by 345 women for the analysis of nitrogen dioxide (), ozone (), and particulate matter in diameter (). For the analysis of BC, we further excluded six women (10 IVF cycles) who resided in Michigan, Maine, or northern New Hampshire (which were outside the range of this model). The EARTH Study was approved by the Human Studies Institutional Review Boards of the MGH and the Harvard T.H. Chan School of Public Health. All study participants signed an informed consent after the study procedures were explained by research study staff.
Air Pollution Exposures
The residential address of each woman was geocoded using ArcGIS version 10.6.1 (ESRI) and linked to the various spatiotemporal models of air pollution. Daily concentrations at the home address were estimated using a validated spatiotemporal model that uses satellite remote sensing data in combination with land use regression (Lee and Koutrakis 2014). Daily exposure at each participant’s residence was estimated using a validated spatiotemporal model based on satellite data, chemical transport models, vertical profiles, meteorological variables, land use terms, and other atmospheric compounds (Di et al. 2017). Daily residential exposures were estimated with a validated hybrid model of moderate-resolution imaging spectroradiometer satellite–derived aerosol optical depth measurements and land use terms (Kloog et al. 2014). We further evaluated residential exposure to BC because this measure of particles primarily emitted by traffic (specifically diesel vehicles) has been more strongly associated with health risks such as mortality, as compared with other traffic-related pollution indicators, such as PM (Janssen et al. 2011). Daily BC exposure at the home address was estimated by using a validated spatiotemporal model derived using ambient BC measurements from over 300 monitors in Massachusetts, Rhode Island, and southern New Hampshire over 12 y (Abu Awad et al. 2017).
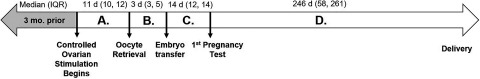
To evaluate possible critical time windows of exposure, five different exposures were calculated by averaging daily concentrations (Figure 1): a) 3 months prior to controlled OS, b) between initiation of controlled OS and oocyte retrieval (period A), c) between oocyte retrieval and ET (period B), d) between ET and first human chorionic gonadotropin (hCG) pregnancy test (i.e., implantation) (period C), and e) between a positive hCG test and live birth (period D). If an IVF cycle failed during the specified time window, air pollution concentrations were averaged through the day of failure.
Figure 1.
Timeline of exposure windows during a typical fresh in vitro fertilization cycle.
Outcome Assessment
All women are prospectively followed throughout their IVF cycle until their point of failure. Briefly, upon beginning IVF, women underwent a pretreatment cycle of oral contraceptives for 2–5 wk to suppress, unless contraindicated. Contraindications include women with a history of cancer or severe migraines and only account for of patients. On day 3 of induced menses, patients began controlled OS. Patients underwent one of three stimulation protocols as clinically indicated: luteal-phase gonadotropin-releasing hormone (GnRH) agonist protocol (generally used for normal/high responders), GnRH-antagonist protocol (generally used for normal/low responders), or a follicular-phase GnRH-agonist protocol (generally used for low responders). Patients were monitored during gonadotropin stimulation to ensure follicular development including serum estradiol, follicle size measurements and counts, and endometrial thickness. Once three or more lead follicles ( in diameter) were visualized and the estradiol level was , hCG was administered to induce oocyte maturation, and 35–37 h later, oocyte retrieval was performed using a transvaginal ultrasound–guided aspiration. On the day of hCG trigger, serum estradiol was measured with an automated electrochemiluminescence immunoassay at the MGH Core Laboratory (Mok-Lin et al. 2010), and endometrial thickness was measured via ultrasound. Women could fail during controlled OS due to a cycle cancellation or a conversion of the cycle to intrauterine insemination due to poor response.
Embryologists classified retrieved oocytes as germinal vesicle, metaphase I, metaphase II (MII), or degenerated. Total oocyte yield was defined as the sum of all oocytes retrieved regardless of type. Mature oocyte yield was the sum of all MII oocytes. For fertilization, couples underwent conventional insemination or intracytoplasmic sperm injection as clinically indicated. Successful fertilization was determined 17–20 h after insemination. The number of normally fertilized oocytes was defined as the number of oocytes with two pronuclei. On day 3 following oocyte retrieval, embryos were assessed for morphological quality and cleavage rate. Embryos were assigned a score between 1 (best) and 5 (worst), with grades 1 and 2 considered best quality and 3, 4, and 5 considered poor quality. Embryos that had reached six to eight cells were considered to be cleaving at a normal rate, embryos with less than or equal to five cells were considered to be slow cleaving, and embryos with greater than or equal to nine cells were considered to have accelerated cleavage. Incubators maintained at 5% oxygen, 6.6% carbon dioxide, and balanced nitrogen were used for all steps of IVF (days 0–6). Following egg retrieval, women could fail due to failed fertilization, arrested embryo development, or conversion to a freeze-all cycle. Following ET, implantation was defined as a serum level typically measured 17 d (range, 15–20 d) after oocyte retrieval and live birth as the delivery of a neonate on or after 24 wk gestation. Following ET, women could fail due to onset of menses or negative pregnancy test (both considered failures of implantation) or due to a biochemical or clinical pregnancy loss, as documented in the electronic medical record.
Covariate Assessment
At enrollment, height and weight were measured by trained research study staff to calculate body mass index (BMI) (), and data on demographics, medical history, and lifestyle characteristics were collected on a brief, study staff-administered questionnaire. Participants also completed a detailed take-home questionnaire with additional questions on lifestyle factors, reproductive health, and medical history. Self-reported education (reported as: did not graduate from high school, high school graduate, 1 or 2 y of college, 3 or 4 y of college, college graduate, or graduate degree) and census tract–level median family income in the past 12 months (in 2011 inflation-adjusted dollars) from the American Community Survey 2007–2010 were used as a proxies for SES (ACS 2007–2010). Clinical information, including infertility diagnosis and protocol type, was abstracted from electronic medical records. Average daily temperature values were derived from the Parameter-elevation Regressions on Independent Slopes Model (PRISM) and averaged for the same time periods as the pollutants (PRISM Climate Group 2019). All covariates had complete information, with the exception of BMI () and education (). In those rare instances, we assigned women the mean BMI in our population and the middle category of education (college graduate).
Statistical Analysis
Descriptive statistics were calculated based on a woman’s first in-study IVF cycle and compared across quartiles of air pollution exposure in the 3 months prior to starting IVF as well as across categories of IVF failure points. Kruskal-Wallis tests and chi-square tests were used to test for differences across categories for continuous and categorical variables, respectively. Spearman correlation coefficients () were used to measure the strength of association across pollutants as well as within pollutants over time.
Due to the high amount of right skew, square root data transformations were used for and concentrations. Each air pollutant was modeled continuously in interquartile range (IQR) increments (either on the original or transformed scale) so that the strength of the association for the different air pollution metrics could be compared. Multivariable Cox proportional hazards models for discrete survival time were used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) for the associations between the time-varying air pollutants and odds of failing IVF prior to live birth (Maity et al. 2014; Missmer et al. 2011). In all of these models, a robust sandwich covariance estimate was used to account for the multiple cycles per woman. The data was structured in long format so that the air pollution exposures could be updated for each corresponding time period. The ORs estimate the odds of failing an IVF cycle at any point, conditional on not failing at an earlier moment during the same cycle. Women were considered at risk of failing IVF for the duration of their initiated cycle until their specific point of failure. There were four periods during which women could fail a cycle: between a) initiation of controlled OS and oocyte retrieval, b) oocyte retrieval and ET, c) ET and implantation, and d) implantation and live birth. To evaluate whether there were specific windows of the IVF cycle that were more vulnerable to the effects of air pollution, we fit multivariate models with time-dependent interactions between the discrete time periods and air pollution exposures.
To assess whether any of the observed associations for the time-varying air pollution exposures were specific to the IVF time windows (as opposed to characteristics of long-term exposure), we compared the results from the time-varying exposure models to models using the average air pollution concentrations in the 3 months prior to starting the IVF cycle. We also ran the time-varying exposure models, further adjusting for the women’s 3-month average baseline concentrations prior to starting IVF to assess the independent effects of short-term variations in air pollutants (above and beyond a woman’s average exposure concentrations).
Multivariate generalized linear mixed models with random intercepts were used to evaluate the associations between average exposure to the air pollutants in the 3 months prior to IVF (for controlled OS outcomes only) and during controlled OS (for controlled OS outcomes and embryo quality outcomes) and intermediate IVF outcomes while accounting for within-person correlations in outcomes. A normal distribution and an identity link function were specified for peak estradiol and endometrial thickness (both normally distributed), a Poisson distribution and a log link function were specified for oocyte counts, and a binomial distribution and logit link function were specified for the embryo quality measures. For all controlled OS outcomes (e.g., estradiol levels at trigger, endometrial thickness, total oocyte yield, mature oocyte yield, and number of normally fertilized oocytes), we used inverse probability weights to control for potential selection bias introduced by restricting the analysis to cycles with a successful oocyte retrieval, as air pollution concentrations could influence the probability of succeeding until that point (Cole and Hernán 2008; Howe et al. 2016). Similar weights were constructed for the embryo quality outcomes (e.g., percentage poor-quality embryos, percentage high-quality embryos, percentage slow-cleavage embryos, and percentage accelerated embryo cleavage) to control for the bias induced by restricting the analysis to only cycles with a day 3 or 5 ET. Weights were comprised of factors associated with the probability of succeeding until that point in the cycle, specifically age, BMI, smoking status, infertility diagnosis, protocol, and and concentrations 3 months prior to IVF. These covariates were chosen, as they were all associated with the probability of succeeding with in the final multivariable model. The intuition behind these weights is that women who successfully made it to oocyte retrieval who had characteristics similar to the women missing due to attrition (e.g., failing prior to oocyte retrieval) were upweighted in the analyses of air pollution and outcomes of controlled OS, so as to represent their original contribution as well as the missing contributions.
Confounding was evaluated using prior knowledge and descriptive statistics from our cohort through the use of directed acyclic graphs (Weng et al. 2009) (Figure S1). Variables retained in the final multivariable models were maternal age (continuous), BMI (continuous), infertility diagnosis (categorical: female, male, unexplained), treatment protocol (categorical: luteal phase agonist, other), and mean temperature (continuous). Sensitivity analyses were also done, further adjusting for the other pollutants and including a random intercept for zip code (to account for potential spatial autocorrelation of model residuals). We considered two-sided significance levels as statistically significant.
Results
Women in our analysis () were, on average, 35.0 y of age and had a BMI of . The majority of women were white (86%), never smokers (72%), with a college education or higher (92%). Of the 522 initiated fresh cycles, 492 (94%) had oocyte retrieval, 467 (89%) had at least one embryo transferred, 279 (53%) had a positive test, and 199 (38%) resulted in a live birth (Figure S2). Women were followed for one (64%), two (24%), three (10%), or four to six (2%) fresh IVF cycles. Average air pollution exposure concentrations were generally low in our cohort ( for , for , for , and for BC in the 3 months prior to starting IVF) and were similar across the IVF time windows (Table S1). As expected, given similar sources, BC was moderately correlated with () and () (Table S2). had weak inverse correlations with () and BC (). Across the IVF cycle, specific air pollution concentrations were moderately to highly correlated, particularly for ( across all time points) (Table S3).
Women who had a live birth as a result of their first in study IVF cycle tended to be younger and leaner and less likely to have female factor as their primary infertility diagnosis at enrollment (Table 1). There were also significant differences in protocol type by timing of IVF failure with very early failures and live births more likely to have been treated with a luteal phase agonist protocol. Women with higher residential exposure to BC tended to live closer to major roadways (Table S4). Women with higher residential exposure to had slightly higher BMIs and tended to live in census tracts with higher median incomes, while women with higher exposure to tended to live closer to major roadways.
Table 1.
Baseline characteristics of 345 women in Environment and Reproductive Health (EARTH) Study (2004–2015) according to the outcome of their first fresh in vitro fertilization (IVF) cycle.
| Number of women | Entire cohort | Outcome of first fresh IVF cycle | p-Valuea | |||
|---|---|---|---|---|---|---|
| Failed prior to ET | Failed between ET and implantation | Failed between implantation and live birth | Live birth | |||
| Personal characteristicsb | ||||||
| Age, years | 0.001 | |||||
| BMI, | 0.09 | |||||
| Smoking status, (%) | — | — | — | — | — | 0.1 |
| Never smoker | 248 (71.9) | 22 (59.5) | 81 (68.6) | 43 (78.2) | 102 (75.6) | — |
| Ever smoker | 97 (28.1) | 15 (40.5) | 37 (31.4) | 12 (21.8) | 33 (24.4) | — |
| Race, (%) | — | — | — | — | — | 0.6 |
| White/Caucasian | 294 (85.2) | 34 (91.9) | 98 (83.1) | 46 (83.6) | 116 (85.9) | — |
| Other | 97 (28.1) | 3 (8.1) | 20 (17.0) | 9 (16.4) | 19 (14.1) | — |
| Education level, (%) | — | — | — | — | — | 0.6 |
| Less than college | 30 (8.7) | 3 (8.1) | 13 (11.0) | 7 (12.7) | 7 (5.2) | — |
| College degree | 112 (32.5) | 10 (27.0) | 37 (31.4) | 18 (32.7) | 47 (34.8) | — |
| Graduate degree | 203 (58.8) | 24 (65.9) | 68 (57.6) | 30 (54.6) | 81 (60.0) | — |
| Census tract median income, | 0.9 | |||||
| Employment status, (%) | — | — | — | — | — | 0.2 |
| Currently working | 331 (95.9) | 36 (97.3) | 114 (96.6) | 50 (90.9) | 131 (97.0) | — |
| Currently not working | 14 (4.1) | 1 (2.7) | 4 (3.4) | 5 (9.1) | 4 (3.0) | — |
| Distance to major roadway, m | 0.5 | |||||
| Pollutant concentrations 3 months prior to IVF | ||||||
| , ppb | 0.4 | |||||
| , ppb | 0.7 | |||||
| , | 0.3 | |||||
| BC, | 1.0 | |||||
| Mean temperature, °C | 0.9 | |||||
| Initial cycle characteristics | ||||||
| Gravidity, (%) | — | — | — | — | — | 0.3 |
| 0 | 211 (61.2) | 27 (73.0) | 67 (56.8) | 31 (56.4) | 86 (63.7) | — |
| 134 (38.8) | 10 (27.0) | 51 (43.2) | 24 (43.6) | 49 (36.3) | — | |
| Parity, (%) | — | — | — | — | — | 0.7 |
| 0 | 294 (85.2) | 33 (89.2) | 99 (83.9) | 45 (81.8) | 117 (86.7) | — |
| 51 (14.8) | 4 (10.8) | 19 (16.1) | 10 (18.2) | 18 (13.3) | — | |
| Infertility diagnosis, (%) | — | — | — | — | — | 0.02 |
| Male factor | 113 (32.8) | 6 (16.2) | 40 (33.9) | 15 (27.3) | 52 (38.5) | — |
| Female factor | 107 (31.0) | 19 (51.4) | 40 (33.9) | 14 (25.5) | 34 (25.2) | — |
| Unexplained | 125 (36.2) | 12 (32.4) | 38 (32.2) | 26 (47.3) | 49 (36.3) | — |
| Treatment protocol, (%) | — | — | — | — | — | |
| Luteal phase agonist | 262 (75.9) | 33 (89.2) | 74 (62.7) | 35 (63.6) | 120 (88.9) | — |
| Flare or antagonist | 83 (24.1) | 4 (10.8) | 44 (37.3) | 20 (36.4) | 15 (11.1) | — |
Note: All covariates had complete information with the exception of BMI () and education (). In those rare instances, we assigned women the mean BMI in our population and the middle category of education (college graduate). —, no data; BC, black carbon; BMI, body mass index; ET, embryo transfer; , nitrogen dioxide; , ozone; , particulate matter .
p-Values were calculated using Kruskal-Wallis tests for continuous variables and chi-square tests for categorical variables.
Data are presented as or (%).
An IQR increase in , , and BC throughout the IVF cycle was associated with an elevated odds of failing at IVF prior to live birth (, 95% CI: 0.95, 1.23 for ; , 95% CI: 0.88, 1.28 for ; , 95% CI: 0.96, 1.41 for BC) (Figure 2). The magnitude of the associations, however, were not constant across the four time windows of the IVF cycle. In general, associations between , , and BC and odds of failing at IVF were strongest prior to ET, and more specifically during controlled OS, and tended to decrease in magnitude as the cycle progressed. For instance, an IQR increase in exposure to , , and BC during controlled OS (period A) was associated with 1.42 (95% CI: 1.20, 1.69), 1.26 (95% CI: 0.96, 1.67), and 1.23 (95% CI: 0.96, 1.59) times the odds of failing prior to oocyte retrieval; however, no significant associations were observed between ET and implantation (period C) or implantation to live birth (period D). No significant associations were observed between concentrations and odds of IVF failure, although there was a slight protective association between higher concentrations between oocyte retrieval and ET and lower likelihood of failure (, 95% CI: 0.50, 1.04 per IQR increase). Results were similar after adjustment for other pollutants (Table S5) and after accounting for spatial autocorrelation of model residuals (Table S6).
Figure 2.
Association between time-varying nitrogen dioxide () (Panel A), ozone () (Panel B), particulate matter () (Panel C), and black carbon (Panel D) concentrations during the in vitro fertilization (IVF) cycle and odds of failing at IVF. Odds ratios (ORs) were estimated using a discrete time Cox proportional hazards model. A robust sandwich covariance estimate was used to account for the multiple cycles per woman. The ORs estimate the odds of failing the IVF cycle at any point, conditional on not failing at an earlier moment during the same cycle. Women were considered at risk of failing IVF for the duration of their initiated cycle until their specific point of failure. Data adjusted for age, body mass index, smoking status, infertility diagnosis, protocol, and mean temperature. An interquartile range (IQR) increase was 2 for , 1.3 for , for , and for BC. Period A, time between initiation of controlled ovarian stimulation and oocyte retrieval; period B, time between oocyte retrieval and embryo transfer; period C, time between embryo transfer and first human chorionic gonadotropin (hCG) pregnancy test (i.e., implantation); period D, time between a positive hCG test and live birth.
Associations for average , and concentrations in the 3 months prior to IVF and time-varying , and concentrations further adjusted for average exposure concentrations in the 3 months prior to IVF were similar to the time-varying effect estimates (Figure S3). This suggests that women with chronically higher exposure to and (rather than higher short-term exposure during controlled OS) may have higher odds of early IVF failure. However, given the high correlation within pollutants across time windows, particularly for , these results should be interpreted with caution. The effect estimates for BC exposure in the 3 months prior to IVF were slightly attenuated compared with the effect estimates for the time-varying exposure. Moreover, the effect estimates for time-varying BC were strengthened after further adjustment for average exposure in the 3 months prior to IVF, suggesting that increases in BC exposure during a woman’s IVF cycle (relative to a woman’s average exposure) may be more important than average concentrations of exposure.
Among women with successful oocyte retrieval, higher exposure to during controlled OS was associated with a slightly higher number of normally fertilized oocytes (Table 2). Women with higher exposure to BC in the 3 months prior to IVF and during controlled OS had significantly higher estradiol levels at hCG trigger and a higher number of mature and normally fertilized oocytes. The positive associations persisted (although effect estimates were attenuated) after excluding freeze-all cycles and those with peak estradiol levels , which are generally indicative of hyperresponse (Table S7). There were no associations between BC exposure during controlled OS and embryo quality parameters measured on day 3 (Table S8). The only significant associations were between higher exposure and lower percent accelerated cleavage embryos and higher and higher percent slow cleavage embryos on day 3. All associations with intermediate outcomes were small in magnitude.
Table 2.
Association between nitrogen dioxide (), ozone (), particulate matter (), and black carbon (BC) concentrations 3 months prior to in vitro fertilization (IVF) and during ovarian stimulation on controlled ovarian stimulation outcomes of IVF ( women, 492 IVF cycles with successful egg retrieval).
| Adjusted beta coefficients (95% CI)a per IQRb | Adjusted percent change (95% CI) per IQR | ||||
|---|---|---|---|---|---|
| Estradiol levels at hCG trigger, pmol/L | Endometrial thickness, mm | Total oocyte yield, | Mature oocyte yield, | Normally fertilized oocytes, | |
| 3 months prior to IVFc | |||||
| 25.5 (, 107.9) | (, 0.18) | 0.5 (, 4.6) | 0.1 (, 4.3) | 0.7 (, 5.6) | |
| (, 37.4) | (, 0.05) | (, 1.7) | (, 2.4) | 0.2 (, 3.2) | |
| 59.6 (, 193.3) | (, 0.24) | (, 3.5) | (, 5.3) | 0.4 (, 8.7) | |
| BC | 122.2 (24.8, 219.5) | 0.01 (, 0.28) | 2.7 (, 8.1) | 4.8 (, 10.5) | 7.0 (0.5, 13.8) |
| During ovarian stimulationd | |||||
| 31.9 (, 109.2) | (, 0.18) | 1.0 (, 4.9) | 0.7 (, 4.6) | 0.4 (, 5.0) | |
| (, 24.8) | (, 0.10) | (, 2.0) | 0.3 (, 2.5) | 0.7 (, 2.6) | |
| 39.3 (, 121.0) | 0.01 (, 0.20) | 1.2 (, 5.2) | 4.4 (0.2, 8.7) | 8.1 (3.1, 13.3) | |
| BC | 149.7 (63.7, 235.6) | 0.04 (, 0.27) | 6.8 (2.1, 11.8) | 9.0 (4.0, 14.2) | 8.8 (3.0, 14.9) |
Note: BC, black carbon; CI, confidence interval; ET, embryo transfer; hCG, human chorionic gonadotropin; IQR, interquartile range; , nitrogen dioxide; , ozone; , particulate matter .
Models were adjusted for age, body mass index (BMI), smoking status (ever, never), infertility diagnosis (female, male, unexplained), protocol (luteal, antagonist/flare), and mean temperature. All outcomes were run with inverse probability weights to control for potential selection bias introduced by restricting the analysis to women who had a successful oocyte retrieval ( cycles). Weights comprised factors associated with the probability of oocyte retrieval, including age, BMI, smoking status (ever, never), infertility diagnosis (female, male, unexplained), protocol (luteal, antagonist/flare), and and concentrations.
An IQR increase was 2 for , 1.3 for , for , and for BC.
Exposure of interest was the average air pollutant concentrations in the 3 months prior to starting IVF.
Exposure of interest was the average air pollutant concentrations between initiation of controlled ovarian stimulation and oocyte retrieval.
Discussion
In our prospective cohort study of women undergoing IVF at a Boston-based fertility center, we observed associations between higher time-varying , , and BC concentrations and reduced success of infertility treatment with IVF. The associations were particularly strong for early IVF failures, prior to ET, and tended to weaken in magnitude as the IVF cycle progressed. This may suggest specific adverse effects of these air pollutants on follicular and embryo development or that women particularly vulnerable to the effects of air pollution were more likely to fail at IVF before making it to ET. Given that the strongest results were for and BC followed by concentrations, our results suggest that traffic-related combustion products, particularly from diesel sources, may be the primary driver of these detrimental effects.
Our findings are in agreement with the results from five previous studies (spanning four continents) suggesting that higher exposure to traffic-related air pollutants (such as and PM) are associated with poorer IVF outcomes. Specifically, the first two studies ( and 531 women) from Brazil found an increased risk of pregnancy loss after both IVF and spontaneous conceptions in women exposed to the highest concentrations of PM () (defined using the citywide average concentration from 14 monitoring sites) during the follicular phase (Perin et al. 2010a, 2010b). A subsequent study from the United States ( women) found that increased concentrations at the centroid of a women’s residential zip code were related to lower chance of pregnancy and live birth during all phases of IVF but most significantly after ET (Legro et al. 2010). The other air pollutants, , , sulfur dioxide, and , did not have consistent associations with IVF outcomes (Legro et al. 2010). More recently, a cohort study from France ( women) found a negative effect of acute and exposure (defined based on daily averages from regional monitors) on ovarian response, number of top-quality embryos, and implantation rate (Carré et al. 2017b), while higher exposure to was associated with higher ovarian response and number of top-quality embryos. Finally, a large cohort study from Korea ( women) found that increased ambient concentrations of , , and carbon monoxide based on the woman’s residential district were associated with reduced probability of achieving intrauterine pregnancy following IVF (Choe et al. 2018). Our research further extends this to show that BC, a more specific marker of exposure to traffic-related pollution, may have stronger associations with IVF failure.
Although not directly comparable, the suggestion of a detrimental effect of traffic-related air pollution on fecundity is also supported by experimental studies in animals and observational studies in women attempting to conceive without medical assistance. For instance, mice exposed to nonfiltered air from a crossroads with high traffic density in Brazil had an extended estrus cycle, a decrease in the number of antral follicles, and an increase in mating time compared with mice exposed to filtered air (Veras et al. 2009). Among women attempting to conceive without medical assistance, those residing closer to major roadways were shown to have a higher risk of infertility (Mahalingaiah et al. 2016) and longer time to pregnancy (Mendola et al. 2017), and those with higher exposure to , , and had lower census tract–level fertility rates (Nieuwenhuijsen et al. 2014) and a lower likelihood of pregnancy during the first month of unprotected intercourse (Slama et al. 2013).
In our study, the association of traffic-related pollutants, in particular, BC, with higher odds of failure prior to ET, suggests that these compounds may have specific adverse effects on the ovary that disrupt its response to controlled OS resulting in cycle cancellations (due to poor response) and freeze-all cycles (due to hyperresponse). This proposed biological pathway is similar to what is observed for both passive and active exposure to cigarette smoke, where both estrogenic and antiestrogenic effects have been observed in experimental studies, depending on the stage and target of the steroidogenesis (Dechanet et al. 2011; Sadeu and Foster 2011). Similar to cigarette smoke, car exhaust has been shown to contain a myriad of substances with estrogenic, antiestrogenic, and antiandrogenic activities that could potentially affect gonadal steroidogenesis and gametogenesis (Oh et al. 2008; Takeda et al. 2004). Alternatively, it could also be that there are different subgroups of women that respond differently to BC exposure, where in some women, it reduces response to OS, and in others, it results in a greater response and a higher production of oocytes. Differing susceptibilities to air pollution have been noted in other disciplines and could be due to genetics (Yang et al. 2008), maternal comorbidities (Dubowsky et al. 2006), lifestyle factors (e.g., poor diet) (Kannan et al. 2006), or coexposure to other air pollutants, to name a few.
Given the conditional nature of IVF outcomes, where each subsequent outcome is only observable among women who succeeded in the previous step, alternative explanations for the divergent response to OS with higher BC exposure should also be considered. We were only able to assess the intermediate outcomes (e.g., estradiol levels and oocyte counts) among cycles with successful oocyte retrieval. During controlled OS, women are monitored as frequently as daily for evidence of increasing estradiol levels and sufficient development of the ovarian follicles. If this is not observed, it is concluded that the woman is not responding adequately to stimulation, and the cycle is canceled. Among women with higher exposure to BC, we observed higher cycle cancellation rates prior to oocyte retrieval, which made us suspect that these women had low estradiol levels and poor follicular development. Since these women did not ultimately undergo oocyte retrieval, they could not be included in the analysis of controlled OS outcomes. In theory, if these canceled cycles are excluded and there is no further effect of BC on later IVF outcomes, then the analysis should show that there is no association between BC and the controlled OS outcomes. However, if there is a depletion of susceptibles and the surviving women who had high exposure to BC actually have better success after oocyte retrieval compared with women with lower exposure, then the effect of BC on outcomes of controlled OS could potentially appear beneficial. While we tried to account for this type of selection bias using inverse probability weights, the effect estimates from these weighted models are only unbiased if the outcome in the women with oocyte retrieval truly represents the unobserved outcomes of the subjects who failed prior to retrieval (with the same values of predictors used in the weight models), which may be unlikely. Therefore, the positive associations we found between higher exposure to BC and estradiol concentrations and oocyte counts may be due to selection bias (or depletion of the susceptibles) rather than true biological effects.
Similarly, our effect estimates for the odds of failure following ET should be interpreted with caution because we saw the strongest effects of the air pollutants on early IVF failures. Therefore, it is likely that with time, the proportion of susceptible women progressively decreased among women with high exposure to air pollution. The bias induced by this differential selection of susceptible women over time would lead to built-in selection bias for later period-specific ORs (Hernan 2010). This may explain why the OR for a 1-IQR increase in , , and BC during period D is less than 1.0, even if these air pollutants truly have no protective effect in any woman at any time.
In all our analyses, we used ambient air pollution exposures as a proxy for personal exposures, potentially leading to exposure misclassification. For example, we did not collect information on the women’s work addresses or their time–activity patterns during the IVF cycle, which limited our ability to predict personal exposures, particularly for pollutants with important indoor sources (Ouidir et al. 2015; Schembari et al. 2013). We also did not update a woman’s address after enrollment into the EARTH Study, and therefore, any changes in address would have been missed. However, the spatiotemporal models we used have been validated and were specific to the woman’s home address, which is a substantial improvement from the five previous studies. The use of ambient exposures is also valuable because regulation typically focuses on these concentrations. We also lacked information on the air quality in the IVF laboratory, which may be the more relevant exposure in the days between egg retrieval and ET. As this was an observational study, residual or unmeasured confounding may still explain our associations despite our ability to control for many important confounders such as body weight, smoking status, and markers of SES. While most women in our study resided at the same address as their male partner, the strongest associations we observed for pollutants on IVF failures was prior to fertilization, when the male gametes were introduced. This supports that the associations we observed are female specific. We were, however, limited by the high correlation of pollutants over time within women, which made it hard to determine specific windows of susceptibility (above and beyond women’s average exposure). Finally, our analysis only included women undergoing IVF at a single academic medical center in Massachusetts. While this benefitted our analysis in terms of limiting confounding across regions and IVF centers, the air pollution concentrations were generally low, and thus, it is unclear how generalizable our results are to all women undergoing IVF across the United States, Europe, or other regions of the world where sources, patterns, and concentrations of air pollutants may be different. We also had very little variation in SES in our cohort and therefore were unable to address any potential interactions between SES, air pollution, and reproductive outcomes. This also potentially limits the generalizability of our findings given the strong differences often observed in exposure to air pollution across socioeconomic classes. Finally, as we observed detrimental associations between air pollutants and IVF end points in a region where pollution levels are generally low, future research in areas with higher exposure is warranted.
In conclusion, we found that women with higher exposure to traffic-related air pollutants (e.g., , BC, and ) had higher odds of IVF failure prior to ET. Moreover, short-term increases in BC exposure concentrations during the controlled OS window of a woman’s IVF cycle relative to her average exposure may confer an even higher risk of early failure. Whether this suggests specific adverse effects of traffic-related pollutants on follicular and embryo development or suggests that women particularly susceptible to the effects of air pollution tend to fail earlier in the IVF cycle remains to be determined. Future research comparing exposure windows from fresh and cryo-thaw cycles within a woman may be helpful in teasing this out further. Finally, from a methodological perspective, our results highlight the importance of analyzing IVF data within a survival framework and interpreting results within this context, particularly for exposures with possible adverse effects on early reproductive outcomes.
Supplementary Material
Acknowledgments
This work was supported by grants ES009718, ES022955, ES000002, and K99ES026648 from the National Institute of Environmental Health Sciences (NIEHS). This publication was made possible by the U.S. Environmental Protection Agency (U.S. EPA): RD-834798 and RD-83587201. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the U.S. EPA. Further, the U.S. EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP4601).
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Abu Awad Y, Koutrakis P, Coull BA, Schwartz J. 2017. A spatio-temporal prediction model based on support vector machine regression: Ambient black carbon in three New England states. Environ Res 159:427–434, PMID: 28858756, 10.1016/j.envres.2017.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Community Survey. 2007–2010. Census tract–level median family income in the past 12 months (in 2011 inflation-adjusted dollars). https://www.census.gov/acs/www/data/data-tables-and-tools/ [accessed 1 December 2017].
- Carré J, Gatimel N, Moreau J, Parinaud J, Léandri R. 2017a. Does air pollution play a role in infertility?: A systematic review. Environ Health 16(1):82, PMID: 28754128, 10.1186/s12940-017-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré J, Gatimel N, Moreau J, Parinaud J, Leandri R. 2017b. Influence of air quality on the results of in vitro fertilization attempts: A retrospective study. Eur J Obstet Gynecol Reprod Biol 210:116–122, PMID: 28012404, 10.1016/j.ejogrb.2016.12.012. [DOI] [PubMed] [Google Scholar]
- Checa Vizcaíno MA, Gonzalez-Comadran M, Jacquemin B. 2016. Outdoor air pollution and human infertility: A systematic review. Fertil Steril 106(4):897–904 e891, PMID: 27513553, 10.1016/j.fertnstert.2016.07.1110. [DOI] [PubMed] [Google Scholar]
- Choe SA, Jun YB, Lee WS, Yoon TK, Kim SY. 2018. Association between ambient air pollution and pregnancy rate in women who underwent IVF. Hum Reprod 33(6):1071–1078, PMID: 29659826, 10.1093/humrep/dey076. [DOI] [PubMed] [Google Scholar]
- Cole SR, Hernán MA. 2008. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 168(6):656–664, PMID: 18682488, 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechanet C, Anahory T, Mathieu Daude JC, Quantin X, Reyftmann L, Hamamah S, et al. 2011. Effects of cigarette smoking on reproduction. Hum Reprod Update 17(1):76–95, PMID: 20685716, 10.1093/humupd/dmq033. [DOI] [PubMed] [Google Scholar]
- Di Q, Rowland S, Koutrakis P, Schwartz J. 2017. A hybrid model for spatially and temporally resolved ozone exposures in the continental United States. J Air Waste Manag Assoc 67(1):39–52, PMID: 27332675, 10.1080/10962247.2016.1200159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowsky SD, Suh H, Schwartz J, Coull BA, Gold DR. 2006. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect 114(7):992–998, PMID: 16835049, 10.1289/ehp.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins AJ, Hart JE, Minguez-Alarcón L, Chavarro JE, Laden F, Coull BA, et al. 2018. Residential proximity to major roadways and traffic in relation to outcomes of in vitro fertilization. Environ Int 115:239–246, PMID: 29605676, 10.1016/j.envint.2018.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain AM, Romanik MC, Guerra I, Solari S, Reyes MS, Johnson RJ, et al. 2007. Endothelial dysfunction: a link among preeclampsia, recurrent pregnancy loss, and future cardiovascular events? Hypertension 49(1):90–95, PMID: 17116761, 10.1161/01.HYP.0000251522.18094.d4. [DOI] [PubMed] [Google Scholar]
- Gupta S, Agarwal A, Banerjee J, Alvarez JG. 2007. The role of oxidative stress in spontaneous abortion and recurrent pregnancy loss: A systematic review. Obstet Gynecol Surv 62(5):335–347, PMID: 17425812, 10.1097/01.ogx.0000261644.89300.df. [DOI] [PubMed] [Google Scholar]
- Hernan MA. 2010. The hazards of hazard ratios. Epidemiology 21:13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CJ, Cole SR, Lau B, Napravnik S, Eron JJ Jr.. 2016. Selection bias due to loss to follow up in cohort studies. Epidemiology 27(1):91–97, PMID: 26484424, 10.1097/EDE.0000000000000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen NA, Hoek G, Simic-Lawson M, Fischer P, van Bree L, ten Brink H, et al. 2011. Black carbon as an additional indicator of the adverse health effects of airborne particles compared with PM10 and PM2.5. Environ Health Perspect 119(12):1691–1699, PMID: 21810552, 10.1289/ehp.1003369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen NAH, Gerlofs-Nijland ME, Lanki T, Salonen RO, Cassee F, Hoek G, et al. 2012. Health Effects of Black Carbon. Copenhagen, Denmark: World Health Organization. [Google Scholar]
- Januário DA, Perin PM, Maluf M, Lichtenfels AJ, Nascimento Saldiva PH. 2010. Biological effects and dose-response assessment of diesel exhaust particles on in vitro early embryo development in mice. Toxicol Sci 117(1):200–208, PMID: 20525899, 10.1093/toxsci/kfq165. [DOI] [PubMed] [Google Scholar]
- Kannan S, Misra DP, Dvonch JT, Krishnakumar A. 2006. Exposures to airborne particulate matter and adverse perinatal outcomes: A biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ Health Perspect 114(11):1636–1642, PMID: 17107846, 10.1289/ehp.9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog I, Chudnovsky AA, Just AC, Nordio F, Koutrakis P, Coull BA, et al. 2014. A new hybrid spatio-temporal model for estimating daily multi-year PM2.5 concentrations across northeastern USA using high resolution aerosol optical depth data. Atmos Environ (1994) 95:581–590, PMID: 28966552, 10.1016/j.atmosenv.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak-Kim J, Yang KM, Gilman-Sachs A. 2009. Recurrent pregnancy loss: a disease of inflammation and coagulation. J Obstet Gynaecol Res 35(4):609–622, PMID: 19751318, 10.1111/j.1447-0756.2009.01079.x. [DOI] [PubMed] [Google Scholar]
- Lamichhane DK, Leem JH, Lee JY, Kim HC. 2015. A meta-analysis of exposure to particulate matter and adverse birth outcomes. Environ Health Toxicol 30:e2015011, PMID: 26796890, 10.5620/eht.e2015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Koutrakis P. 2014. Daily ambient NO2 concentration predictions using satellite ozone monitoring instrument NO2 data and land use regression. Environ Sci Technol 48(4):2305–2311, PMID: 24437539, 10.1021/es404845f. [DOI] [PubMed] [Google Scholar]
- Legro RS, Sauer MV, Mottla GL, Richter KS, Li X, Dodson WC, et al. 2010. Effect of air quality on assisted human reproduction. Hum Reprod 25(5):1317–1324, PMID: 20228391, 10.1093/humrep/deq021. [DOI] [PubMed] [Google Scholar]
- Mahalingaiah S, Hart JE, Laden F, Farland LV, Hewlett MM, Chavarro J, et al. 2016. Adult air pollution exposure and risk of infertility in the Nurses' Health Study II. Hum Reprod, PMID: 26724803, 10.1093/humrep/dev330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity A, Williams PL, Ryan L, Missmer SA, Coull BA, Hauser R. 2014. Analysis of in vitro fertilization data with multiple outcomes using discrete time-to-event analysis. Stat Med 33(10):1738–1749, PMID: 24317880, 10.1002/sim.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendola P, Sundaram R, Louis GMB, Sun L, Wallace ME, Smarr MM, et al. 2017. Proximity to major roadways and prospectively-measured time-to-pregnancy and infertility. Sci Total Environ 576:172–177, PMID: 27783935, 10.1016/j.scitotenv.2016.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C, Williams PL, Ford JB, Chavarro JE, Minguez-Alarcon L, Dadd R, et al. 2018. The Environment and Reproductive Health (EARTH) Study: A Prospective Preconception Cohort. Hum Reprod Open 2018, PMID: 29888739, 10.1093/hropen/hoy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missmer SA, Pearson KR, Ryan LM, Meeker JD, Cramer DW, Hauser R. 2011. Analysis of multiple-cycle data from couples undergoing in vitro fertilization: methodologic issues and statistical approaches. Epidemiology 22(4):497–504, PMID: 21558857, 10.1097/EDE.0b013e31821b5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok-Lin E, Ehrlich S, Williams PL, Petrozza J, Wright DL, Calafat AM, et al. 2010. Urinary bisphenol a concentrations and ovarian response among women undergoing IVF. Int J Androl 33(2):385–393, PMID: 20002217, 10.1111/j.1365-2605.2009.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller P, Danielsen PH, Karottki DG, Jantzen K, Roursgaard M, Klingberg H, et al. 2014. Oxidative stress and inflammation generated DNA damage by exposure to air pollution particles. Mutat Res Rev Mutat Res 762:133–166, PMID: 25475422, 10.1016/j.mrrev.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuijsen MJ, Basagaña X, Dadvand P, Martinez D, Cirach M, Beelen R, et al. 2014. Air pollution and human fertility rates. Environ Int 70:9–14, PMID: 24879367, 10.1016/j.envint.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Oh SM, Ryu BT, Chung KH. 2008. Identification of estrogenic and antiestrogenic activities of respirable diesel exhaust particles by bioassay-directed fractionation. Arch Pharm Res 31(1):75–82, PMID: 18277611, 10.1007/s12272-008-1123-8. [DOI] [PubMed] [Google Scholar]
- Ouidir M, Giorgis-Allemand L, Lyon-Caen S, Morelli X, Cracowski C, Pontet S, et al. 2015. Estimation of exposure to atmospheric pollutants during pregnancy integrating space-time activity and indoor air levels: Does it make a difference? Environ Int 84:161–173, PMID: 26300245, 10.1016/j.envint.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panasevich S, Leander K, Rosenlund M, Ljungman P, Bellander T, de Faire U, et al. 2009. Associations of long- and short-term air pollution exposure with markers of inflammation and coagulation in a population sample. Occup Environ Med 66(11):747–753, PMID: 19687019, 10.1136/oem.2008.043471. [DOI] [PubMed] [Google Scholar]
- Perin PM, Maluf M, Czeresnia CE, Januário DA, Saldiva PH. 2010a. Impact of short-term preconceptional exposure to particulate air pollution on treatment outcome in couples undergoing in vitro fertilization and embryo transfer (IVF/ET). J Assist Reprod Genet 27(7):371–382, PMID: 20405197, 10.1007/s10815-010-9419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin PM, Maluf M, Czeresnia CE, Nicolosi Foltran Januário DA, Nascimento Saldiva PH. 2010b. Effects of exposure to high levels of particulate air pollution during the follicular phase of the conception cycle on pregnancy outcome in couples undergoing in vitro fertilization and embryo transfer. Fertil Steril 93(1):301–303, PMID: 19631320, 10.1016/j.fertnstert.2009.06.031. [DOI] [PubMed] [Google Scholar]
- PRISM Climate Group. 2019. Prism data sets: recent years (Jan 1981 - Nov 2018). http://prism.oregonstate.edu/recent/ [accessed 13 March 2019].
- Risom L, Møller P, Loft S. 2005. Oxidative stress-induced DNA damage by particulate air pollution. Mutat Res 592(1–2):119–137, PMID: 16085126, 10.1016/j.mrfmmm.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Sadeu JC, Foster WG. 2011. Cigarette smoke condensate exposure delays follicular development and function in a stage-dependent manner. Fertil Steril 95(7):2410–2417, PMID: 21514584, 10.1016/j.fertnstert.2011.03.072. [DOI] [PubMed] [Google Scholar]
- Schembari A, Triguero-Mas M, de Nazelle A, Dadvand P, Vrijheid M, Cirach M, et al. 2013. Personal, indoor and outdoor air pollution levels among pregnant women. Atmos Environ 64:287–295, 10.1016/j.atmosenv.2012.09.053. [DOI] [Google Scholar]
- Shah PS, Balkhair T. 2011. Air pollution and birth outcomes: a systematic review. Environ Int 37(2):498–516, PMID: 21112090, 10.1016/j.envint.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Sharma S. 2014. Natural killer cells and regulatory T cells in early pregnancy loss. Int J Dev Biol 58(2-4):219–229, PMID: 25023688, 10.1387/ijdb.140109ss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slama R, Bottagisi S, Solansky I, Lepeule J, Giorgis-Allemand L, Sram R. 2013. Short-term impact of atmospheric pollution on fecundability. Epidemiology 24(6):871–879, PMID: 24051894, 10.1097/EDE.0b013e3182a702c5. [DOI] [PubMed] [Google Scholar]
- Takeda K, Tsukue N, Yoshida S. 2004. Endocrine-disrupting activity of chemicals in diesel exhaust and diesel exhaust particles. Environ Sci 11(1):33–45, PMID: 15746887. [PubMed] [Google Scholar]
- Veras MM, Damaceno-Rodrigues NR, Guimarães Silva RM, Scoriza JN, Saldiva PH, Caldini EG, et al. 2009. Chronic exposure to fine particulate matter emitted by traffic affects reproductive and fetal outcomes in mice. Environ Res 109(5):536–543, PMID: 19394924, 10.1016/j.envres.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Wauters A, Dreyfuss C, Pochet S, Hendrick P, Berkenboom G, van de Borne P, et al. 2013. Acute exposure to diesel exhaust impairs nitric oxide-mediated endothelial vasomotor function by increasing endothelial oxidative stress. Hypertension 62(2):352–358, PMID: 23798345, 10.1161/HYPERTENSIONAHA.111.00991. [DOI] [PubMed] [Google Scholar]
- Weng HY, Hsueh YH, Messam LL, Hertz-Picciotto I. 2009. Methods of covariate selection: Directed acyclic graphs and the change-in-estimate procedure. Am J Epidemiol 169(10):1182–1190, PMID: 19363102, 10.1093/aje/kwp035. [DOI] [PubMed] [Google Scholar]
- Yang IA, Fong KM, Zimmerman PV, Holgate ST, Holloway JW. 2008. Genetic susceptibility to the respiratory effects of air pollution. Thorax 63(6):555–563, PMID: 18511640, 10.1136/thx.2007.079426. [DOI] [PubMed] [Google Scholar]
- Yin LJ, Zhang Y, Lv PP, He WH, Wu YT, Liu AX, et al. 2012. Insufficient maintenance DNA methylation is associated with abnormal embryonic development. BMC Med 10:26, PMID: 22413869, 10.1186/1741-7015-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.