Abstract
Background:
Assessing chemicals for their potential to cause male reproductive toxicity involves the evaluation of evidence obtained from experimental, epidemiological, and mechanistic studies. Although mechanistic evidence plays an important role in hazard identification and evidence integration, the process of identifying, screening and analyzing mechanistic studies and outcomes is a challenging exercise due to the diversity of research models and methods and the variety of known and proposed pathways for chemical-induced toxicity. Ten key characteristics of carcinogens provide a valuable tool for organizing and assessing chemical-specific data by potential mechanisms for cancer-causing agents. However, such an approach has not yet been developed for noncancer adverse outcomes.
Objectives:
The objective in this study was to identify a set of key characteristics that are frequently exhibited by exogenous agents that cause male reproductive toxicity and that could be applied for identifying, organizing, and summarizing mechanistic evidence related to this outcome.
Discussion:
The identification of eight key characteristics of male reproductive toxicants was based on a survey of known male reproductive toxicants and established mechanisms and pathways of toxicity. The eight key characteristics can provide a basis for the systematic, transparent, and objective organization of mechanistic evidence relevant to chemical-induced effects on the male reproductive system. https://doi.org/10.1289/EHP5045
Introduction
Exogenous Chemicals and Altered Male Reproductive Health
There has been a decline in sperm counts (Levine et al. 2017) and a simultaneous rise in the incidence of testicular cancer in most regions of the world (Skakkebaek et al. 2016). It has been proposed that environmental chemicals are contributors to a large proportion of these adverse effects on male reproduction and associated conditions such as testicular dysgenesis syndrome (Bergman et al. 2013; Skakkebaek et al. 2016). Further, several exogenous chemical exposures are associated with decreased human male fertility, including occupational exposures to lead, cadmium, welding fumes, certain pesticides (e.g., 1,2-dibromo-3-chloropropane), alcohol consumption, cigarette smoking, drug use (e.g., cocaine), and certain pharmaceuticals (Hotchkiss et al. 2008; du Plessis et al. 2015; Semet et al. 2017). The identification of chemicals that cause male reproductive effects, and the mechanisms underlying these effects, is critical to developing approaches to mitigate the risks of environmental, occupational, medical, and lifestyle exposures and to understand the etiology of population-level trends in dysfunction.
Apical End Points of Male Reproductive Toxicity
Proper development and structural organization of the male reproductive tract can be clinically assessed by examining testicular descent, anogenital distance, preputial separation, areola and nipple retention, external genitalia (e.g., hypospadias, cleft phallus) and reproductive organ size and morphology. Such evaluations can be performed visually and by ultrasound in humans and by necropsy in animals (e.g., small testes; presence/absence of vas deferens, seminal vesicles, epididymis, prostate) (Chapin and Creasy 2012; Foster and Gray 2013; Nikolaidis 2017). Analyses of gross pathology (e.g., size, organ weights, malformations) and histopathology can uncover developmental defects (e.g., abnormal/delayed sexual development, Sertoli cell–only tubules, spermatogenesis arrest) as well as insults at different life stages that interfere with male fertility (e.g., atrophy, edema, inflammation, seminiferous tubule vacuolation) (Creasy et al. 2012; Nikolaidis 2017). Detailed sperm evaluation at multiple levels is essential, including testicular spermatogenesis and spermiation, ejaculated semen analysis (count, concentration, motility, morphology), assessment of sperm functions (e.g., Catsper channels, capacitation, acrosome reaction, hyperactivation, DNA integrity), and fertilizing ability (e.g., sperm penetration assay) that individually contribute novel information and insights regarding the effects of potential reproductive toxicants (U.S. EPA 1996a; Creasy and Chapin 2018). Hormone measurements [e.g., luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone, estradiol, inhibin] can inform interference with the hypothalamic–pituitary–testicular axis, which systemically controls reproduction and aids in identifying primary versus secondary hypogonadism (Chapin and Creasy 2012). Finally, assessments of sexual behavior and performance (e.g., libido, erection, mounting behaviors, intromission, ejaculation) are key end points that affect fecundity. These activities may be adversely influenced by alterations in endocrine and neural systems and structural aberrations (Nikolaidis 2017).
Role of Mechanistic Evidence in Hazard Evaluation
Hazard identification as part of a human health risk assessment consists of an analysis of the available evidence on chemical-induced adverse health effects that are focused on cancer and noncancer outcomes including male reproductive toxicity. For example, the State of California maintains a list of carcinogens and reproductive toxicants and indicates those that are specific to male and/or female effects (OEHHA 2019). Evaluation of epidemiological and toxicological studies for direct evidence of effects after chemical exposures plays a critical role in the hazard identification process. Mechanistic evidence is also an important component. Analysis of events that are precursors to apical end points seen in animals and humans supports evidence of a hazard, identifies potential susceptible populations and life stages, and informs the human relevance of effects observed in animals (NRC 2007, 2017). Evaluation of in vivo and in vitro mechanistic evidence can also identify data gaps in the current understanding of how a chemical may cause adverse effects in experimental models and humans. Finally, there continues to be a shift away from whole animal testing for apical end points toward high-throughput in vitro testing and toxicogenomic profiling due to cost, timelines, ethical considerations, regulatory constraints on animal testing, and the large volume of chemicals needing evaluation (NRC 2007, 2017). Although some of these animal model alternatives may be useful for male reproductive toxicants, it is important to note that cell culture of germ cells through maturation in organoids and even tissue explants has had far less success than for some other tissues, which will limit their use for testicular analyses in the near future (Nakamura et al. 2019). Nonetheless, the authors concur that increasingly in the future, large, diverse, and complex mechanistic data sets will likely comprise much of the data available for evaluating the hazards of chemicals used in commerce and their breakdown products.
The identification of male reproductive toxicants that pose a hazard to human health typically requires the integration of evidence from epidemiological studies of different designs, populations, and exposures; animal evidence from studies of various apical end points and experimental designs; and consideration of the varied mechanistic data that support (or diminish support for) the chemical posing a reproductive hazard (Rooney et al. 2014; OHAT 2019). Various organizations provide evidence evaluation frameworks that can be used to categorize and analyze toxicological end points indicative of male reproductive toxicity (see Table S1), but currently there is no generally accepted approach for systematically and transparently identifying and organizing mechanistic data for male reproductive hazard identification. Consequently, there is a lack of uniformity in the mechanistic topics addressed across assessments and an absence of a standard procedure to efficiently identify, organize, and summarize the voluminous data from mechanistic studies. The proposed key characteristics of male reproductive toxicants were developed as a tool that can improve the systematic and transparent screening and evaluation of mechanistic data related to chemical-induced male reproductive toxicity.
Identifying the Key Characteristics of Male Reproductive Toxicants
Recently, the 10 key characteristics of human carcinogens were introduced to provide a uniform and objective approach for identifying and organizing mechanistic evidence to support cancer hazard identification (Smith et al. 2016; Guyton et al. 2018). The key characteristics were identified by an international working group of experts organized by the International Agency for Research on Cancer (IARC) (Smith et al. 2016). The 10 key characteristics of human carcinogens comprise the properties of known human carcinogens, including their ability, for example, to be genotoxic or immunosuppressive or to modulate receptor-mediated effects. Established human carcinogens commonly exhibit one or more of these characteristics, and therefore such data can provide independent evidence of carcinogenicity when human epidemiological evidence is lacking. Such data can also help in interpreting the relevance and importance of findings of cancer in animals and in humans. In its 2017 report “Using 21st Century Science to Improve Risk-Related Evaluations,” the National Academy of Sciences, Engineering and Medicine (NASEM) opined that the key characteristics approach “avoids a narrow focus on specific pathways and hypotheses and provides for a broad, holistic consideration of the mechanistic evidence” (NRC 2017). Indeed, the key characteristics of carcinogens have been successfully used by the IARC Monographs Programme to evaluate the mechanistic data compiled for the more than 30 agents evaluated in Meetings 112–121 of the IARC Monographs (Guyton et al. 2018). The 2017 NRC report also recommended that the key characteristics approach be expanded to other end points, including reproductive effects, endocrine disruption, and cardiovascular disease (NRC 2017).
Here, we have attempted to develop a set of key characteristics of male reproductive toxicants based on our current knowledge of the mechanisms by which chemicals cause reproductive toxicity. A working group consisting of regulatory experts, toxicologists, and epidemiologists with a background in reproductive biology and mechanisms associated with chemical-induced adverse health effects was convened at the University of California, Berkeley from 7 to 8 March 2018 to address this topic. The objective was to review the key characteristics approach and to determine whether this methodology can also be applied to the evaluation of male reproductive toxicants. The workgroup participants consulted comprehensive lists of chemicals known to target the male reproductive system (OEHHA 2019) and also addressed current male reproductive outcomes considered in evaluations of experimental and epidemiological evidence of toxicity induced by agents such as radiation and viral and bacterial pathogens. Peer-reviewed publications and U.S. government health assessments [e.g., U.S. EPA Integrated Risk Information System (https://cfpub.epa.gov/ncea/iris/search/index.cfm), U.S. Agency for Toxic Substances and Disease Registry (ATSDR)] provided reviews and examples of chemical-induced effects in the male reproductive system, including the “Toxicological Profile of Cadmium” (ATSDR 2012), the “Toxicological Profile of DDT” (ATSDR 2002), and the “Toxicological Review of Benzo[a]pyrene” (ATSDR 2002, 2012; U.S. EPA 2017). Based on the initial analysis, several key characteristics of male reproductive toxicants were identified (Figure 1 and Table 1), as well as examples of chemicals known to affect those specific characteristics (Table 2).
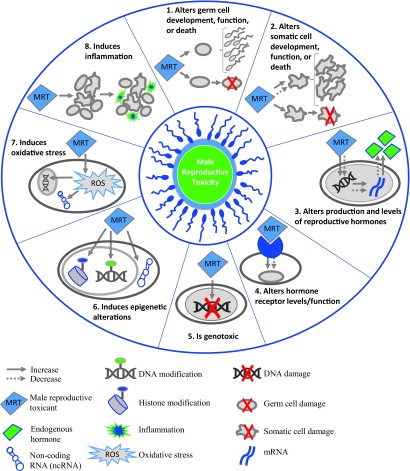
Figure 1.
Key characteristics of male reproductive toxicants. Exposure to male reproductive toxicants (MRT) resulting in (1) altered spermatogenesis, normal functions (e.g., acrosome reaction), or increased cell death; (2) disruptions in somatic cell development (e.g., increased or decreased proliferation), functions (e.g., alterations in blood–testis barrier), or death; (3) changes in hormone production/levels; (4) modifies hormone receptor functions/cellular levels; (5) increases DNA damage; (6) epigenetic alterations of cellular macromolecules (DNA, RNA, and/or proteins); (7) reactive oxygen species (ROS)-induced cellular damage; and (8) increases inflammation (e.g., elevated production/levels of pro-inflammatory cytokines and edema). In combination with the male-specific end points of reproductive toxicity described in Table S1, the key characteristics of male reproductive toxicants can be applied for the evaluation of toxicological and mechanistic evidence for hazard identification.
Table 1.
Key characteristics of male reproductive toxicants.
| Key characteristic | Examples of relevant evidence |
|---|---|
| 1. Alters germ cell development, function, or death | Increased germ cell apoptosis; alterations in sperm acrosome reaction and motility |
| 2. Alters somatic cell development, functions, or death | Increased Sertoli cell apoptosis; alterations in Sertoli cell functions, cytoskeleton, and interactions with germ cells; alterations in Leydig cell development |
| 3. Alters production and levels of reproductive hormones | Decreased Leydig cell steroidogenic functions; increased hepatic metabolism and excretion of sex hormones |
| 4. Alters hormone receptor levels/functions | Androgen receptor antagonism, estrogen receptor activation, decreased LH receptor expression |
| 5. Is genotoxic | DNA damage, chromosome fragmentation, altered sperm cell chromosome numbers |
| 6. Induces epigenetic alterations | Altered sperm ncRNAs, germ cell DNA methylation patterns, and histone retention sites |
| 7 Induces oxidative stress | Reduced tissue antioxidant levels |
| 8. Induces inflammation | Increased testicular expression of pro-inflammatory markers and prostaglandin levels |
Note: LH, luteinizing hormone; ncRNA, noncoding RNA.
Table 2.
Key characteristics of male reproductive toxicants and examples from chemicals known to affect the male reproductive system.
| Key characteristic | Example toxicants | Known mechanism/pathway associated with adverse male reproductive outcomes | References |
|---|---|---|---|
| 1. Alters germ cell development, function, or death | B[a]P | Increased spermatogenic cell apoptosis; altered sperm motility and acrosome reaction | Ramesh et al. 2017; U.S. EPA 2017 |
| Cadmium | Increased spermatogenic cell apoptosis, reduced sperm count, altered sperm motility | ATSDR 2012; Jenardhanan et al. 2016 | |
| Phthalates | Germ cell degeneration and reduced cell number | Howdeshell et al. 2008; Martino-Andrade and Chahoud 2010; Habert et al. 2014 | |
| 4-Methylbenzylidenecamphor | Altered sperm motility via disrupted channel function | Schiffer et al. 2014 | |
| Cocaine, sirolimus, sulfasalazine, cannabinoids, DES | Decreased sperm count and motility, altered sperm morphology | Li et al. 2003; Semet et al. 2017 | |
| 2. Alters somatic cell development, functions, or death | B[a]P | Increased Sertoli cell apoptosis | Ramesh et al. 2017 |
| Phthalates | Altered Sertoli-germ cell interactions; decreased testosterone production in Leydig cells | Boekelheide et al. 2005; Scott et al. 2009 | |
| Cadmium | Disruption of the blood–testis barrier via alterations in Sertoli cell actin filaments, and assembly of tight junctions | Siu et al. 2009; Gao et al. 2015; Li et al. 2016; de Angelis et al. 2017 | |
| PCBs | Decreased Sertoli cell metabolic functions and viability | Jenardhanan et al. 2016 | |
| Alcohol, phthalates | Increased Fas-mediated Sertoli and germ cell apoptosis | Lucas et al. 2009; Pourmasumi et al. 2017; Sansone et al. 2018 | |
| 3. Alters production and levels of reproductive hormones | DDT | Increased hepatic expression of CYP3A4 and metabolism of sex hormones | Laurenzana et al. 2002; Medina-Díaz et al. 2007 |
| Linuron | Decreased fetal androgen production/levels | Hotchkiss et al. 2008; Wilson et al. 2008; Scott et al. 2009; Dent et al. 2015 | |
| Phthalates, sirolimus | Decreased expression of steroidogenic enzymes and reduced androgen production | Hotchkiss et al. 2008; Bergman et al. 2013; Semet et al. 2017 | |
| Ketoconazole, prochloraz | Inhibition of the steroidogenic enzyme CYP17A1 activity | Scott et al. 2009; Dent et al. 2015 | |
| PCBs, B[a]P | Decreased serum levels of reproductive hormones; decreased androgen production in Leydig cells | Meeker and Hauser 2010; Jenardhanan et al. 2016; Ramesh et al. 2017; U.S. EPA 2017 | |
| Opiates | Reduced androgen levels and secretion of gonadotropin-releasing hormone; increased aromatase expression | Bawor et al. 2015; Drobnis and Nangia 2017; Semet et al. 2017 | |
| Cadmium | Alterations in LH associated with changes in prolactin secretion; decreased Leydig cell steroidogenic enzyme activity, cAMP levels, and expression of the LH receptor | Siu et al. 2009; Lafuente 2013 | |
| 4. Alters hormone receptor levels/functions | Prochloraz, linuron, procymidone, vinclozolin, flutamide, cyproterone acetate, DDT | AR antagonism | ATSDR 2002; Hotchkiss et al. 2008; Wilson et al. 2008; Scott et al. 2009; Dent et al. 2015; Semet et al. 2017 |
| DES | Activation of estrogen receptor | Henley and Korach 2006 | |
| B[a]P | Activation of AHR resulting in increased expression of xenobiotic metabolic enzymes and formation of reactive metabolites and ROS | Ramesh et al. 2017 | |
| Cadmium | Reduced levels of the LH Receptor | Gunnarsson et al. 2007; Wan et al. 2011 | |
| 5. Is genotoxic | Acrylamide | Increased germ cell formation of gylcidamide-DNA adducts | Estill and Krawetz 2016 |
| Cadmium, PCBs | Chromatin fragmentation, and ROS-dependent DNA damage in germ cells | Meeker and Hauser 2010; Tavares et al. 2016; de Angelis et al. 2017 | |
| B[a]P, cisplatin, carboplatin | Increased DNA adducts and DNA fragmentation in spermatozoa and testicular tissue | Vakalopoulos et al. 2015; Tavares et al. 2016; Ramesh et al. 2017; U.S. EPA 2017 | |
| Alcohol | Altered sperm chromosome number (aneuploidy), and increased DNA fragmentation | Kapp 2010; Pourmasumi et al. 2017 | |
| Chlorambucil, cyclophosphamide, procarbazine, melphalan | DNA alkylation, altered DNA structure and function | Vakalopoulos et al. 2015 | |
| Ethane-methane sulfonate | Increased/irreversible spermatogonia DNA damage resulting in necrosis | Woldemeskel 2017 | |
| 6. Induces epigenetic alterations | TCDD, methoxychlor, alcohol | Altered germ cell DNA methylation patterns | Anway et al. 2005; Paoloni-Giacobino 2014; Skinner 2016; Chastain and Sarkar 2017; Pilsner et al. 2017; Ding et al. 2018 |
| Vinclozolin | Altered sperm ncRNAs, DNA methylation, histone retention sites | Brieño-Enríquez et al. 2015, 2016; Ben Maamar et al. 2018 | |
| Diethylhexyl phthalate | Altered sperm ncRNAs associated with testicular dysgenesis syndrome in mice | Stenz et al. 2017 | |
| 7. Induces oxidative stress | Cadmium, B[a]P | Reduction in antioxidant enzyme activity, and antioxidant levels | Kapp 2010; Rezk and Sikka 2011; Lafuente 2013; de Angelis et al. 2017; Ramesh et al. 2017 |
| TCDD | Decreased tissue antioxidant levels | Lavranos et al. 2012 | |
| Lindane, methoxychlor | Reduction in antioxidant enzyme activity | Jenardhanan et al. 2016 | |
| 8. Induces inflammation | Cadmium, TCDD, silver nanoparticles | Increased testicular expression/levels of pro-inflammatory markers. Increased testicular edema | Siu et al. 2009; Sengupta 2013; de Angelis et al. 2017; Pilsner et al. 2017 |
| TCDD | Increased testicular prostaglandin levels | Bruner-Tran et al. 2014 |
Note: AHR, aryl hydrocarbon receptor; AR, androgen receptor; B[a]P, benzo[a]pyrene; , calcium ion; cAMP, cyclic adenosine monophosphate; CYP3A4, cytochrome P450 family 3 subfamily A member 4; CYP17A1, cytochrome P450 family 17 subfamily A member 1; DDT, dichlorodiphenyltrichloroethane; DES, diethylstilbestrol; LH, luteinizing hormone; ncRNA, noncoding RNA; PCBs, polychlorinated biphenyls; ROS, reactive oxygen species; TCDD, tetrachlorodibenzodioxin.
Each key characteristic is described in the context of mechanisms or pathways by which exposure to male reproductive toxicants (including environmental toxicants, pharmaceuticals, and drugs of abuse) can lead to adverse health effects. This is not intended to be an exhaustive discussion of all potentially available evidence from experimental studies. Instead, this narrative relies on previous literature reviews and mechanistic analyses of toxicant-induced adverse male reproductive health effects. Examples of chemicals known to affect the male reproductive system via mechanisms that fall under the key characteristics described herein are presented in Table 2.
The Eight Key Characteristics of Male Reproductive Toxicants
1. Alters Germ Cell Development, Function, or Death
The multistep process of sperm formation begins early in embryonic and fetal life. During the first trimester, migrating primordial germ cells embed in the developing gonadal primordium that contains somatic supportive cell precursors. At the time of colonization, the primordial germ cells and somatic supportive cell precursors are still bipotential. During the second and third trimesters, the somatic cell precursors are induced by the sex determining region Y (SRY) to differentiate into Sertoli cells in the gonadal anlage, whereas the pluripotent gonocyte lineage commits to differentiate into spermatogonia (Del Valle et al. 2017). During the first months after birth, a developmental stage known as minipuberty, characterized by an increased testosterone level, occurs due to a brief activation of the pituitary–gonadal axis (Grinspon and Rey 2010; Rey 2014). Throughout the duration of this developmental stage, some germ cells may still show fetal characteristics. However, germ cells are generally quiescent in childhood and classified as spermatogonia until meiosis starts at the beginning of puberty with the production of sperm (Müller and Skakkebæk 1983; Masliukaite et al. 2016). Spermarche (first production of sperm) occurs around 13 y of age (currently the data are limited to studies of Caucasian boys) (Nielsen et al. 1986; Del Valle et al. 2017; Creasy and Chapin 2018). Although the underlying mechanism behind the onset of puberty is not known, the initial maturation steps involve hypothalamic and pituitary centers leading to activation of the pituitary–gonadal axis, resulting in increased LH and testosterone levels, and the subsequent maturation of external genitalia (Sørensen et al. 2010).
Germ cells are vulnerable to external stressors during all developmental stages. Human genetic models (Lottrup et al. 2013) and experimental animal findings show that the differentiation process of pluripotent fetal germ cells into mature spermatogonia committed to spermatogenesis is particularly sensitive to toxicant-induced alterations in signaling from surrounding cells such as Sertoli and Leydig cells (Sharpe and Skakkebaek 2008). It is assumed that seminomas (germ cell tumors) and nonseminomas in young adults originate in fetal pluripotent germ cells that failed to differentiate correctly during the perinatal period. The fetal hypothesis also links germ cell cancer to other symptoms of the testicular dysgenesis syndrome, including cryptorchidism, hypospadias, and decreased spermatogenesis (Skakkebaek et al. 2016). Furthermore, animal studies consistently report alterations in germ cell development and germ cell loss after gestational exposure to phthalate esters (Martino-Andrade and Chahoud 2010; Albert and Jégou 2014).
The adult testis has been generally considered to be less sensitive than the immature gonad to toxicant-induced alterations. However, modern chemotherapy has—as proof of principle—shown that human spermatogenesis can be disrupted by exposures to chemicals (Brennemann et al. 1997). Further, recent studies have shown that endocrine-disrupting chemicals may mimic progesterone effects on CatSper, a calcium channel in the human sperm cell that regulates sperm motility and acrosomal exocytosis (Schiffer et al. 2014). Although the clinical significance of these findings remains to be established, they demand consideration in light of the high frequency of abnormal and immotile sperm among men in the western world (Levine et al. 2017). The workshop consensus is that there is a continued need for a comprehensive approach where combined male and female factors are considered together to elucidate a possible role of male reproductive toxicants during the fertilization process.
Environmental chemicals and other substances that directly or indirectly target proliferation, differentiation, or death processes in germ cells (at any stage of production) include heavy metals (e.g., cadmium), various phthalate esters, and drugs of abuse such as cocaine (Table 2). Exposure to these agents has been shown to lead to male reproductive toxicity.
2. Alters Somatic Cell Development, Functions, or Death
Exposures to toxic compounds has been shown to adversely affect the male reproductive system by targeting somatic cells in several organ systems that are essential for healthy reproduction outcomes. Under normal conditions, somatic cells provide structural support and nourishment and regulate endocrine functions that are necessary for normal spermatogenesis and fertility (Woldemeskel 2017; Creasy and Chapin 2018). Chemicals and substances that interfere with the development, integrity, or function of these somatic cells can have deleterious effects on male fertility (Boekelheide et al. 2005; Scott et al. 2009; Sansone et al. 2018). Within the testes is a network of seminiferous tubules where spermatogenesis occurs. Sertoli cells line the walls of these tubules and provide essential nourishment and support for developing gametes through paracrine factors and cell–cell communication. Peritubular myoid cells lie at the base of the tubules and provide contractile elements for sperm transport and contribute to the blood–testes barrier formed by Sertoli cell tight junctions. Between the tubules are the Leydig cells, which synthesize androgens and are essential for sperm production and growth of the male reproductive tract (van den Driesche et al. 2012). Chemicals such as hydrocarbons, cadmium, and some phthalate esters have been shown to induce Sertoli cell apoptosis, alterations in Sertoli cell cytoskeleton and Sertoli-germ cell interactions, and decreased steroidogenesis in Leydig cells (Table 2). In addition, resident macrophages that interact with Leydig cells as well as endothelial cells are present in the testicular interstitial space and can become targets for toxicant-induced damage (Hales 2002; Zheng et al. 2010; Harris et al. 2016). Accessory reproductive organs such as the epididymis and vas deferens are the excurrent ducts where sperm mature and are stored and properly transported during emission/ejaculation (Evans and Ganjam 2017). The prostate gland and seminal vesicles produce seminal plasma for sperm transport and capacitation. Normal accessory reproductive organ functions can be altered by exposure to reproductive toxicants such as vinclozolin and flutamide (Dent et al. 2015). The hypothalamus and pituitary play a critical role in male reproductive development and functions by regulating Sertoli and Leydig cell functions via LH and FSH secretion (Foster and Gray 2013), and exposure to environmental contaminants such as cadmium may impact normal gonadotropin secretion, resulting in altered testicular functions (Lafuente 2013). In addition, alterations in somatic cells of other organ systems can impact the male reproductive system. For example, dichlorodiphenyltrichloroethane (DDT) and nonylphenol may alter hepatic metabolism of endogenous hormones, which in turn alters available androgen and estrogen levels (Laurenzana et al. 2002; Kretschmer and Baldwin 2005; Medina-Díaz et al. 2007; Hotchkiss et al. 2008).
3. Alters Production and Levels of Reproductive Hormones
Gonadotropins, sex steroids, and thyroid hormones produced by the hypothalamic–pituitary–gonadal (HPG) axis, the hypothalamic–pituitary–adrenal axis, and the hypothalamic–pituitary–thyroid axis play an important role in the normal development and function of the male reproductive system (Dent et al. 2015). Chemical-induced alterations in reproductive hormones during developmental and sexually mature stages have been shown to result in adverse effects including malformations and infertility (Sharpe and Skakkebaek 2008; Scott et al. 2009; Mocarelli et al. 2011; Semet et al. 2017). Environmental chemicals such as DDT, linuron, polychlorinated biphenyls (PCBs), and phthalate esters have been shown to interfere with the normal development and function of the male reproductive system via alterations in hormone production, hormone levels, and the balance between male and female hormones (Table 2). Reproductive hormone production and levels have also been shown to be altered after exposure to pharmaceuticals (Semet et al. 2017) as well as drugs of abuse (Bawor et al. 2015; Drobnis and Nangia 2017; Semet et al. 2017). These compounds can alter reproductive steroid hormone levels by directly inhibiting steroidogenic enzyme activity, reducing their cellular expression, or accelerating their metabolism and excretion (Hotchkiss et al. 2008). For example, marijuana and its metabolites adversely affect the HPG axis—specifically, a reduction in LH levels—which in turn reduces testosterone levels and compromises spermatogenesis (du Plessis et al. 2015). Toxicant effects on steroid hormones are not restricted to reproductive organs; for example, hepatic expression and activity of CYP3A4, an enzyme known to metabolize androgens (Niwa et al. 2015), can be altered by DDT exposure (Medina-Díaz et al. 2007). Gonadotropic hormones (gonadotropic releasing hormone, FSH, LH, and inhibin), prolactin, and thyroid hormones also play an important role regulating development and normal functions of the male reproductive system (Wan et al. 2013; Reis et al. 2015), and levels of these hormones can be altered by several toxicants (Table 2).
4. Alters Hormone Receptor Levels/Functions
Chemicals that interact with steroid and protein hormone receptors may alter their normal functions. This can occur through various mechanisms, including binding to and activating target cellular receptors, occupying a receptor’s active site and blocking normal activation by endogenous hormones, modulating recruitment of co-activators (or co-repressors) to the transcriptional complex or interfering with normal crosstalk between membrane and nuclear hormone receptors (Wilson et al. 2008; Rezk and Sikka 2011; Yeung et al. 2011). Studies in androgen receptor knockout mice revealed ablation of the masculinization of reproductive organs and the emergence of female phenotypical appearance (Matsumoto et al. 2008). Similarly, chemicals that alter androgen receptor functions during critical periods of sex differentiation and maturation or in the adult can result in male reproductive toxicity by adversely affecting male sexual development, the male phenotype, and maintenance of reproductive functions. Some chemicals known to cause toxicity via this pathway include dicarboximide fungicides (e.g., vinclozolin and procymidone), linuron, and flutamide (Table 2). Estrogen receptors (, , G protein-coupled ER) have also been shown to play important roles in male reproductive function, and toxicants that agonize or antagonize these receptors, such as diethylstilbestrol (Gill et al. 1979), have been shown to have adverse effects on this system. As described above gonadotropic hormones regulate development and normal male reproductive system functions, and agents that interfere in their receptor signaling can lead to adverse effects (Wan et al. 2013). Several environmental chemicals (e.g., cadmium, lead) have been shown to interfere with expression of the LH receptor without affecting LH mRNA levels (Wan et al. 2013).
5. Is Genotoxic
Human evidence suggests that DNA damage in sperm is associated with lower fertility rates, poor embryo quality, and pregnancy loss (Zini and Sigman 2009; Rezk and Sikka 2011; Ioannou et al. 2016). Toxicant exposure can damage sperm DNA through direct mechanisms, for example, through DNA strand breaks or DNA binding, or indirectly, for example, through the induction of oxidative stress (Delbès et al. 2010). There are also a variety of intrinsic factors that contribute to sperm DNA damage, including protamine deficiency (which reduces sperm chromatin compaction) and high endogenous reactive oxygen species (ROS) levels (Zini and Sigman 2009; González-Marín et al. 2012). Mature sperm have no capacity for DNA repair, so epididymal or ejaculated sperm exposed to these stressors may become damaged with no possibility of repair prior to fertilization (González-Marín et al. 2012). Examples of chemicals known to promote DNA/chromosome damage in male germ cells include hydrocarbons {e.g., benzo[a]pyrene [B(a)P]}, acrylamide, chemotherapy drugs, alcohol, PCBs, and cadmium (Table 2).
Toxicant exposure can also cause aneuploidy in sperm, which is the gain or loss of whole chromosomes or segments of chromosomes as a result of nondisjunction that occurs when meiosis is disrupted during gametogenesis. Sperm aneuploidy is associated with infertility, pregnancy loss, and congenital abnormalities (Mandrioli et al. 2016; Ioannou et al. 2016). Sperm aneuploidy may result from exposure to chemicals classified as genotoxicants (Mandrioli et al. 2016) but might also result from indirect effects of endocrine-disrupting chemicals (Perry et al. 2016).
6. Induces Epigenetic Alterations
The term epigenetic alterations refers to stable changes in gene expression that are not caused by alterations in the DNA sequence and can be heritable across cell divisions (Tammen et al. 2013). Examples of epigenetic phenomena include changes in DNA methylation (Jirtle and Skinner 2007; Skinner 2016; Donkin and Barrès 2018), histone modification (Skinner 2016; Donkin and Barrès 2018), chromatin packaging, and noncoding RNA (ncRNA) (Donkin and Barrès 2018), all of which can affect the activity and availability of DNA for expression. Studies have shown that some endocrine-disrupting chemicals and other male reproductive toxicants alter DNA and histone methylation patterns, suggesting that epigenetic modifications are part of the mechanism of action for these chemicals (Crews and McLachlan 2006; Wu et al. 2015; Estill and Krawetz 2016). Epigenetic changes in germ cells may also be inherited by offspring with potential for transgenerational inheritance (Youngson and Whitelaw 2008). An example is the transgenerational inheritance of compromised male fertility following gestational exposure to the fungicide vinclozolin (Anway et al. 2005).
There is growing interest in the role played by the sperm epigenome in male fertility, embryonic development, and offspring health. During sperm DNA maturation, histones are largely replaced by specialized protamines, creating a tightly compacted packaging that protects sperm DNA from damage (Carrell et al. 2012). Nonetheless, the retained histones play fundamental roles in offspring development and transgenerational inheritance (Siklenka et al. 2015) and together with DNA methylation and ncRNA are sites for environmental reprograming (Siklenka et al. 2015; Belleau et al. 2018; Ben Maamar et al. 2018). The sperm epigenome is also involved in genomic imprinting, whereby methylation of certain genes in parental gametes determines the allelic expression of those genes in offspring Kitamura et al. (2015). Consequently, alterations in sperm DNA methylation patterns are suggested to have negative implications for male fertility (Aston et al. 2015; Urdinguio et al. 2015; Jenkins et al. 2016), embryonic development (Aston et al. 2015), and offspring disease susceptibility (Jenkins et al. 2014). Chemicals known to alter male germ cell DNA methylation patterns, ncRNAs, and histone methylation or retention include vinclozolin, alcohol, methoxychlor, and diethylhexyl phthalate (Table 2).
7. Induces Oxidative Stress
Oxidative stress is caused by an increased production of ROS that overwhelms cellular and tissue antioxidant capacity (Chen et al. 2013; Sabeti et al. 2016). Although ROS are necessary for normal sperm functions such as capacitation and oocyte fertilization, excessive production of oxygen radicals is associated with sperm abnormalities such as tail defects and acrosome abnormalities, increased DNA damage, and decreased sperm mobility and viability (Lavranos et al. 2012; Chen et al. 2013; Sabeti et al. 2016). Furthermore, male germ cells are susceptible to damage caused by oxygen radicals because sperm cells do not have DNA repair mechanisms (Agarwal et al. 2014; Sabeti et al. 2016), possess low levels of antioxidant enzymes (Sabeti et al. 2016), and have high levels of plasma membrane polyunsaturated fatty acids that are targets for lipid peroxidation (Chen et al. 2013; Agarwal et al. 2014). Oxygen radicals in the male reproductive tract are commonly produced by immune system cells (including leukocytes and macrophages) (Lavranos et al. 2012; Agarwal et al. 2014). ROS in sperm cells are produced in the mitochondria and the plasma membrane (Sabeti et al. 2016; Agarwal et al. 2014). Chemicals known to cause adverse effects in the male reproductive system (e.g., reduced sperm counts and motility, increased abnormal sperm morphology) via increased production of ROS and downstream cellular damage include pesticides, tetrachlorodibenzodioxin (TCDD), and heavy metals such as cadmium (Table 2).
8. Induces Inflammation
Acute or chronic inflammation of the male reproductive tract caused by infection, hormonal perturbations, or exposure to environmental contaminants are known causes of male factor infertility (Lavranos et al. 2012; Fijak et al. 2018). The defined clinical manifestations include urethritis, prostatitis, seminal vesiculitis, epididymitis, and orchitis. Although bacteria and other common uropathogens represent the most frequent cause of inflammatory conditions (Fijak et al. 2018), noninfectious causes of inflammation, including environmental contaminants such as cadmium, should also be considered (de Angelis et al. 2017). Inflammatory responses caused by pro-inflammatory agents are amplified by activated lymphocytes and macrophages through the release of a variety of cytokines, chemokines, and growth factors present in human semen. These factors include interleukins, tumor necrosis factor (TNF), TNF-related apoptosis-inducing ligand, soluble receptors and antagonists, granulocyte and macrophage colony-stimulating factors, interferons, chemokines, macrophage inflammatory proteins, transforming growth factor, monocyte chemotactic and activating factor, hepatocyte growth factor, and prostaglandins (Fraczek and Kurpisz 2007, 2015; Frungieri et al. 2015). Increased levels and/or production of these pro-inflammatory cytokines is associated with decreases in sperm number, motility, and concentration; increased ROS production; and reduced testosterone production by Leydig cells (Fraczek and Kurpisz 2007). Inflammation is also a source of oxidative stress (Bachir and Jarvi 2014; Ko et al. 2014) leading to an imbalance in the oxidant/antioxidant system, and it may cause scarring of the delicate ductal system leading to subsequent anatomic obstruction (Bachir and Jarvi 2014). Environmental contaminants such as cadmium and TCDD have been shown to induce inflammation in the male reproductive system (Table 2).
Discussion and Conclusions
The identification and analysis of mechanistic evidence for the evaluation of chemical-induced male reproductive toxicity is an increasingly essential aspect of hazard evaluation and human health risk assessment, especially when epidemiological and apical animal toxicity data are inadequate or sparse. Approaches that facilitate the systematic evaluation of toxicological and mechanistic evidence can improve the transparency and strength of evidence analyses performed as part of a risk assessment. Here, we have developed a set of eight key characteristics of male reproductive toxicants (Figure 1) that can provide a structure for systematically identifying and organizing the relevant literature on mechanistic information in support of an evaluation of a chemical for male reproductive toxicity.
The workshop participants concluded that chemicals that are male reproductive toxicants would be expected to have strong evidence for having one or more of the key characteristics, although it should be noted that simply the presence of one or even several of these characteristics does not conclusively identify a chemical as a male reproductive toxicant. Indeed, several key characteristics of male reproductive toxicants overlap with the key characteristics of carcinogens. The key characteristics of male reproductive toxicants, as well as the key characteristics of carcinogens, are not checklists but, instead, provide a starting point for identification, organization, and analysis of mechanistic data that may inform whether a chemical can cause male reproductive effects or cancer, respectively. The key characteristics of male reproductive toxicants are intended to be used as part of an evaluation of chemical-induced reproductive toxicity that includes data from studies of experimental animal and epidemiological outcomes, if available, and requires expert judgment. Evaluation of the available mechanistic evidence for male reproductive effects of a chemical should therefore be focused on organs and systems that can impact male reproductive functions.
The key characteristics can be leveraged at multiple steps in the chemical evaluation process, including literature identification and problem formulation efforts. For example, the key characteristics can be used to develop a targeted literature search strategy to identify mechanistic data using appropriate combinations of Medical Subject Headings (MeSH) search tools and keyword terms for end points that can ultimately inform mechanisms relevant to male reproductive toxicity (see Table S2). The resulting identified papers can then be further screened and evaluated in more detail, including considerations of study design, dose–response effects, life stage, and reporting features. Accordingly, a full evaluation of the mechanistic database available for each chemical can be achieved, and strength of the evidence descriptors may be assigned to each key characteristic, for example, whether they are strong, limited, or inadequate (IARC 2019).
The authors suggest another potential application of the key characteristics approach is the development of literature inventories that can facilitate the review of the available evidence informing each of the key characteristics and the proposed network by creating a summary-level sortable list of the available evidence. This becomes particularly relevant when working with a large database of studies using diverse experimental models and designs. The inventory should include study design features as well as a description of the findings that inform each of the key characteristics. Once completed, the assessor can use the inventory to navigate through the available evidence according to information categories captured in it (e.g., species, strain, dose, life stage, evidence for each of the key characteristics). An example of the types of information that can be captured in a literature inventory is presented in Excel Table S3. The studies captured in Excel Table S3 were identified from literature reviews by Siu et al. (2009), Lafuente (2013), and de Angelis et al. (2017).
The key characteristics of male reproductive toxicants may also be applied to guide prioritization of data-poor chemicals for further evaluation. In a hazard evaluation of a chemical for which animal and human data are sparse, the key characteristics could be used to organize and integrate relevant high-throughput, toxicogenomic and other mechanistic data and identify the potential for male reproductive toxicity. This would encompass inclusion and analyses of new high-throughput assays as well as new mapping of assays to the key characteristics. The analysis can carry less uncertainty when done in read across fashion wherein one or more structurally or mechanistically similar anchor chemicals have robust animal or human apical end point data sets showing adverse male reproductive outcomes (NRC 2017).
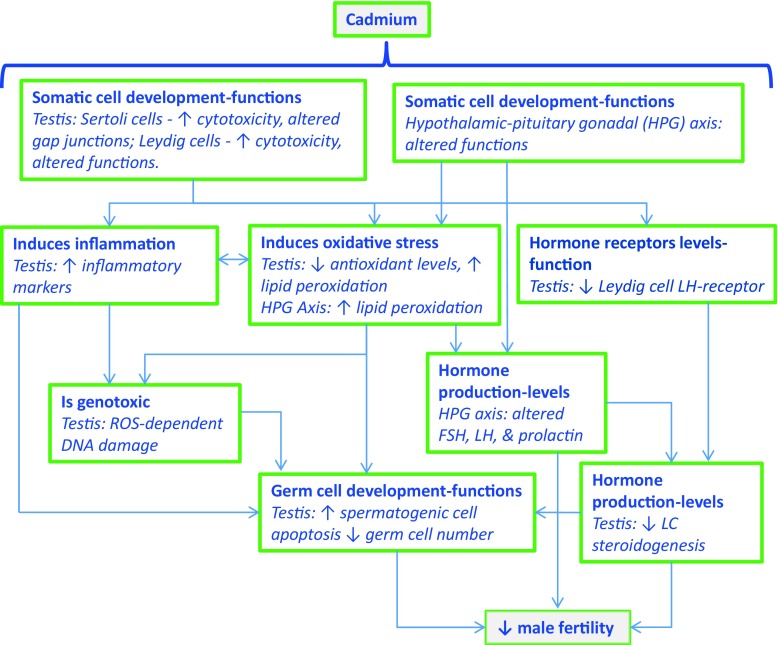
Information obtained from screened studies can be organized in a mechanistic network describing the potential pathways associated with chemical-induced male reproductive toxicity. An example using cadmium is presented in Figure 2 (Siu et al. 2009; Lafuente 2013; Wan et al. 2013; Gao et al. 2015; Jenardhanan et al. 2016; de Angelis et al. 2017; Flora and Agrawal 2017). Potential key characteristics involved in cadmium-induced male reproductive toxicity include a) alters germ cell development, functions, or death; b) alters somatic cell development, functions, or death; c) alters production and levels of reproductive hormones; d) alters hormone receptor levels/functions; e) is genotoxic; f) induces oxidative stress; and g) induces inflammation (Table 2).
Figure 2.
Application of the key characteristics to develop a network on cadmium-induced male reproductive effects.
Mode of action (MOA) analyses were developed in an attempt to link key events in a theoretical biological sequence so that a relatively simple hypothesis as to the mechanism involved in the toxic effect could be generated (U.S. EPA 1996b, 2005). Adverse outcome pathways (AOPs) are a more recent expansion of MOA concepts that include a molecular initiating event and an adverse outcome in an organism linked by all key events measured at various levels of organization (Carusi et al. 2018). Both MOAs and AOPs are linear, reductive models of complex physiology, but they may nonetheless be useful for understanding how chemicals exert their toxic effects (Escher et al. 2017). A challenge to the practical application of MOA and AOP approaches for chemical safety decision-making is limitations in the current understanding of disease processes that may be shown to be incorrect or incomplete (Guyton et al. 2009). This limitation was recognized by Sir Bradford Hill, who formalized the research of causality in humans while noting that, “what is biologically plausible depends upon the biological knowledge of the day” (Hill 1965). The authors believe the key characteristics are agnostic with respect to current or future knowledge of downstream adverse effects and of the precise mechanistic pathways leading to these outcomes. The value of this approach for reproductive toxicants is that, as for carcinogens, gaps in mechanistic data that delineate the complete pathway from exposure to adversity need not hamper the identification of reproductive toxicants. If strong evidence is present for one or more likely key characteristics, this can directly inform the hazard identification process. This emancipates the risk assessor from “connecting the dots” between a molecular initiating event and a specific adverse outcome that is the basis of the AOP approach to hazard identification. The key characteristics approach we describe here can be viewed as identifying molecular initiating events or early key events as described in the MOA and AOP frameworks based on our current knowledge of the molecular mechanisms of reproductive toxicant action and their role in health and disease. Using key characteristics to assemble mechanistic data about a putative reproductive toxicant does not require an exhaustive understanding of how the characteristics are causally linked to the adverse response or an a priori hypothesis about the MOA or AOP.
The proposed key characteristics of male reproductive toxicants thus form an objective approach for organizing and evaluating the complex and ever-accumulating mechanistic evidence on a given chemical or group of chemicals. This evaluation of the mechanistic evidence is inclusive of data from both human observational studies and in vivo and in vitro experimental systems, encompassing molecular epidemiological studies using biomarkers and high-throughput in vitro tests. Once the available evidence for a chemical (or group of chemicals) suspected of targeting the male reproductive system is extracted and sorted into the different key characteristics, biological networks can be developed if required to facilitate a qualitative and quantitative evaluation (Figure 2 presents an example using cadmium). Furthermore, mechanistic evidence analyses that rely on the key characteristics of male reproductive toxicants can also help identify significant data gaps and areas that could benefit from additional research and provide guidance on the development of high-throughput assays to systematically evaluate each of the proposed key characteristics. Finally, the key characteristics of male reproductive toxicants should be updated as new mechanisms and pathways for chemical-induced male reproductive toxicity are discovered and described in the peer-reviewed literature and as more chemical-specific case studies and health assessments using the key characteristics approach are conducted.
Supplementary Material
Acknowledgments
This project was supported by contract 17-E0023 from the California Environmental Protection Agency (CalEPA) Office of Environmental Health Hazard Assessment and by the Research Translation Core of the National Institute of Environmental Health Sciences (NIEHS) Superfund Research Center at Berkeley under National Institutes of Health (NIH) grant P42ES004705. The authors acknowledge funding from the NIH (grants R01ES009718, R01ES014370, R01ES022955, ES027027408 to R.H.; P30ES027792, R01ES02207, R01CA172220 to G.S.P.; and P42ES004705 to M.T.S.), the Office of Environmental Health Hazard Assessment (grant 17-0023 to M.T.S.). We thank all other members of the 2018 Working Group who attended the workshop in Berkeley, California, for important discussion, including the following: P. Browne, OECD; V. Cogliano, U.S. EPA; B. Eskenazi, UC Berkeley; K. Guyton, IARC, A. Kortenkamp, Brunel University; M. La Merrill, UC Davis; U. Luderer, UC Irvine; C. McHale, UC Berkeley; L. Rieswijk, UC Berkeley; M. Sandy, Office of Environmental Health Hazard Assessment (OEHHA); T. Schug, NIEHS; G. Solomon, UC San Francisco; H. Sone and O. Udagawa, NIES: National Institute for Environmental Studies, Japan; L. Vandenberg, University of Massachusetts; T. Woodruff, UCSF; T. Zoeller, University of Massachusetts; and L. Zhang, UC Berkeley. V. Wilson, U.S. EPA; J. Congleton, U.S. EPA; R. Subramaniam, U.S. EPA; K. Thayer, U.S. EPA; and C. Rider, NIEHS provided comments and suggestions during internal (U.S. EPA and NIEHS-National Toxicology Program) review of this manuscript.
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP5045).
The views expressed are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency (EPA), the California EPA, or the National Institute of Environmental Health Sciences.
Martyn T. Smith is retained as a consultant and potential expert witness in U.S. litigation involving chemical and pharmaceutical exposures and various disease outcomes, including cancer. The litigation does not involve male reproductive toxicity. All other authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Agarwal A, Virk G, Ong C, du Plessis SS. 2014. Effect of oxidative stress on male reproduction. World J Mens Health 32(1):1–17, PMID: 24872947, 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert O, Jégou B. 2014. A critical assessment of the endocrine susceptibility of the human testis to phthalates from fetal life to adulthood. Hum Reprod Update 20(2):231–249, PMID: 24077978, 10.1093/humupd/dmt050. [DOI] [PubMed] [Google Scholar]
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. 2005. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308(5727):1466–1469, PMID: 15933200, 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston KI, Uren PJ, Jenkins TG, Horsager A, Cairns BR, Smith AD, et al. . 2015. Aberrant sperm DNA methylation predicts male fertility status and embryo quality. Fertil Steril 104(6):1388–1397.e1–e5, PMID: 26361204, 10.1016/j.fertnstert.2015.08.019. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). 2002. Toxicological profile for DDT, DDE, and DDD. Atlanta, GA:ATSDR, U.S. Department of Health and Human Services, 93–99. https://www.atsdr.cdc.gov/ToxProfiles/tp35.pdf [accessed 4 June 2019]. [Google Scholar]
- ATSDR. 2012. Toxicological profile for cadmium. Atlanta, GA:ATSDR, U.S. Department of Health and Human Services, 170–174. https://www.atsdr.cdc.gov/toxprofiles/tp5.pdf [accessed 4 June 2019]. [Google Scholar]
- Bachir BG, Jarvi K. 2014. Infectious, inflammatory, and immunologic conditions resulting in male infertility. Urol Clin North Am 41(1):67–81, PMID: 24286768, 10.1016/j.ucl.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Bawor M, Bami H, Dennis BB, Plater C, Worster A, Varenbut M, et al. . 2015. Testosterone suppression in opioid users: a systematic review and meta-analysis. Drug Alcohol Depend 149:1–9, PMID: 25702934, 10.1016/j.drugalcdep.2015.01.038. [DOI] [PubMed] [Google Scholar]
- Belleau P, Deschênes A, Scott-Boyer MP, Lambrot R, Dalvai M, Kimmins S, et al. . 2018. Inferring and modeling inheritance of differentially methylated changes across multiple generations. Nucleic Acids Res 46(14):e85, PMID: 29750268, 10.1093/nar/gky362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Maamar M, Sadler-Riggleman I, Beck D, McBirney M, Nilsson E, Klukovich R, et al. . 2018. Alterations in sperm DNA methylation, non-coding RNA expression, and histone retention mediate vinclozolin-induced epigenetic transgenerational inheritance of disease. Environ Epigenet 4(2):dvy010, PMID: 29732173, 10.1093/eep/dvy010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman Å, Heindel JJ, Jobling S, Kidd KA, Zoeller RT, eds. 2013. State of the Science of Endocrine Disrupting Chemicals – 2012. Geneva, Switzerland:United Nations Environment Programme and the World Health Organization, 57–75. [Google Scholar]
- Boekelheide K, Johnson KJ, Richburg JH. 2005. Sertoli cell toxicants. In: Sertoli Cell Biology. Skinner MK, Griswold MD, eds. San Diego, CA:Elsevier Academic Press, 345–382. [Google Scholar]
- Brennemann W, Stoffel-Wagner B, Helmers A, Mezger J, Jäger N, Klingmüller D. 1997. Gonadal function of patients treated with cisplatin based chemotherapy for germ cell cancer. J Urol 158(3 Pt 1):844–850, PMID: 9258096, 10.1016/S0022-5347(01)64333-7. [DOI] [PubMed] [Google Scholar]
- Brieño-Enríquez MA, García-López J, Cárdenas DB, Guibert S, Cleroux E, Dĕd L, et al. . 2015. Exposure to endocrine disruptor induces transgenerational epigenetic deregulation of microRNAs in primordial germ cells. PloS One 10(4):e0124296, PMID: 25897752, 10.1371/journal.pone.0124296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieño-Enríquez MA, Larriba E, Del Mazo J. 2016. Endocrine disrupters, microRNAs, and primordial germ cells: a dangerous cocktail. Fertility and sterility 106(4):871–879, PMID: 27521771, 10.1016/j.fertnstert.2016.07.1100. [DOI] [PubMed] [Google Scholar]
- Bruner-Tran KL, Ding T, Yeoman KB, Archibong A, Arosh JA, Osteen KG. 2014. Developmental exposure of mice to dioxin promotes transgenerational testicular inflammation and an increased risk of preterm birth in unexposed mating partners. PloS One 9(8):e105084, PMID: 25127480, 10.1371/journal.pone.0105084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell EJ, Thorne CM, Tschumper GS. 2012. Basis set dependence of higher-order correlation effects in pi-type interactions. J Chem Phys 136(1):014103, PMID: 22239765, 10.1063/1.3671950. [DOI] [PubMed] [Google Scholar]
- Carusi A, Davies MR, De Grandis G, Escher BI, Hodges G, Leung KMY, et al. . 2018. Harvesting the promise of AOPs: an assessment and recommendations. Sci Total Environ 628–629:1542–1556, PMID: 30045572, 10.1016/j.scitotenv.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin RE, Creasy DM. 2012. Assessment of circulating hormones in regulatory toxicity studies II. Male reproductive hormones. Toxicol Pathol 40(7):1063–1078, PMID: 22552397, 10.1177/0192623312443321. [DOI] [PubMed] [Google Scholar]
- Chastain LG, Sarkar DK. 2017. Alcohol effects on the epigenome in the germline: role in the inheritance of alcohol-related pathology. Alcohol 60:53–66, PMID: 28431793, 10.1016/j.alcohol.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SJ, Allam JP, Duan YG, Haidl G. 2013. Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch Gynecol Obstet 288(1):191–199, PMID: 23543240, 10.1007/s00404-013-2801-4. [DOI] [PubMed] [Google Scholar]
- Creasy D, Bube A, de Rijk E, Kandori H, Kuwahara M, Masson R, et al. . 2012. Proliferative and nonproliferative lesions of the rat and mouse male reproductive system. Toxicol Pathol 40(6 Suppl):40S–121S, PMID: 22949412, 10.1177/0192623312454337. [DOI] [PubMed] [Google Scholar]
- Creasy DM, Chapin RE. 2018. Male reproductive system. In: Fundamentals of Toxicologic Pathology. Wallig MA, Haschek WM, Rousseaux CG, Bolon B, Mahler BW, eds., 3d ed London, UK:Academic Press, 459–516. [Google Scholar]
- Crews D, McLachlan JA. 2006. Epigenetics, evolution, endocrine disruption, health, and disease. Endocrinology 147(6 Suppl):S4–S10, PMID: 16690812, 10.1210/en.2005-1122. [DOI] [PubMed] [Google Scholar]
- de Angelis C, Galdiero M, Pivonello C, Salzano C, Gianfrilli D, Piscitelli P, et al. . 2017. The environment and male reproduction: the effect of cadmium exposure on reproductive function and its implication in fertility. Reprod Toxicol 73:105–127, PMID: 28774687, 10.1016/j.reprotox.2017.07.021. [DOI] [PubMed] [Google Scholar]
- Del Valle I, Buonocore F, Duncan AJ, Lin L, Barenco M, Parnaik R, et al. . 2017. A genomic atlas of human adrenal and gonad development. Wellcome Open Res 2:25, PMID: 28459107, 10.12688/wellcomeopenres.11253.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbès G, Hales BF, Robaire B. 2010. Toxicants and human sperm chromatin integrity. Mol Hum Reprod 16(1):14–22, PMID: 19812089, 10.1093/molehr/gap087. [DOI] [PubMed] [Google Scholar]
- Dent MP, Carmichael PL, Jones KC, Martin FL. 2015. Towards a non-animal risk assessment for anti-androgenic effects in humans. Environ Int 83:94–106, PMID: 26115536, 10.1016/j.envint.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Ding T, Mokshagundam S, Rinaudo PF, Osteen KG, Bruner-Tran KL. 2018. Paternal developmental toxicant exposure is associated with epigenetic modulation of sperm and placental Pgr and Igf2 in a mouse model. Biol Reprod 99(4):864–876, PMID: 29741588, 10.1093/biolre/ioy111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkin I, Barrès R. 2018. Sperm epigenetics and influence of environmental factors. Mol Metab 14:1–11, PMID: 29525406, 10.1016/j.molmet.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobnis EZ, Nangia AK. 2017. Pain medications and male reproduction. Adv Exp Med Biol 1034:39–57, PMID: 29256126, 10.1007/978-3-319-69535-8_6. [DOI] [PubMed] [Google Scholar]
- du Plessis SS, Agarwal A, Syriac A. 2015. Marijuana, phytocannabinoids, the endocannabinoid system, and male fertility. J Assist Reprod Genet 32(11):1575–1588, PMID: 26277482, 10.1007/s10815-015-0553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escher BI, Hackermüller J, Polte T, Scholz S, Aigner A, Altenburger R, et al. . 2017. From the exposome to mechanistic understanding of chemical-induced adverse effects. Environ Int 99:97–106, PMID: 27939949, 10.1016/j.envint.2016.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estill MS, Krawetz SA. 2016. The epigenetic consequences of paternal exposure to environmental contaminants and reproductive toxicants. Curr Environ Health Rep 3(3):202–213, PMID: 27357567, 10.1007/s40572-016-0101-4. [DOI] [PubMed] [Google Scholar]
- Evans TJ, Ganjam VK. 2017. Reproductive anatomy and physiology. In: Reproductive and Developmental Toxicology. Gupta RC, ed., 2nd ed San Diego CA:Elsevier, Academic Press, 7–37. [Google Scholar]
- Fijak M, Pilatz A, Hedger MP, Nicolas N, Bhushan S, Michel V, et al. . 2018. Infectious, inflammatory and ‘autoimmune’ male factor infertility: how do rodent models inform clinical practice? Hum Reprod Update 24(4):416–441, PMID: 29648649, 10.1093/humupd/dmy009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora JSW, Agrawal S. 2017. Arsenic, cadmium, and lead. In: Reproductive and Developmental Toxicology. Gupta RC, ed., 2nd ed San Diego CA:Elsevier, Academic Press, 537–566. [Google Scholar]
- Foster PM, Gray LE Jr. 2013. Toxic responses of the reproductive system. In: Casarett & Doull’s Toxicology: The Basic Science of Poisons. Klaassen CD, ed. 8th ed McGraw Hill, 861–906. [Google Scholar]
- Fraczek M, Kurpisz M. 2007. Inflammatory mediators exert toxic effects of oxidative stress on human spermatozoa. J Androl 28(2):325–333, PMID: 17079739, 10.2164/jandrol.106.001149. [DOI] [PubMed] [Google Scholar]
- Fraczek M, Kurpisz M. 2015. Cytokines in the male reproductive tract and their role in infertility disorders. J Reprod Immunol 108:98–104, PMID: 25796532, 10.1016/j.jri.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Frungieri MB, Calandra RS, Mayerhofer A, Matzkin ME. 2015. Cyclooxygenase and prostaglandins in somatic cell populations of the testis. Reproduction 149(4):R169–R180, PMID: 25504871, 10.1530/REP-14-0392. [DOI] [PubMed] [Google Scholar]
- Gao Y, Mruk DD, Cheng CY. 2015. Sertoli cells are the target of environmental toxicants in the testis – a mechanistic and therapeutic insight. Expert Opin Ther Targets 19(8):1073–1090, PMID: 25913180, 10.1517/14728222.2015.1039513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill WB, Schumacher GF, Bibbo M, Straus FH 2nd, Schoenberg HW. 1979. Association of diethylstilbestrol exposure in utero with cryptorchidism, testicular hypoplasia and semen abnormalities. J Urol 122(1):36–39, PMID: 37351. [DOI] [PubMed] [Google Scholar]
- González-Marín C, Gosálvez J, Roy R. 2012. Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int J Mol Sci 13(11):14026–14052, PMID: 23203048, 10.3390/ijms131114026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinspon RP, Rey RA. 2010. Anti-müllerian hormone and Sertoli cell function in paediatric male hypogonadism. Horm Res Paediatr 73(2):81–92, PMID: 20190544, 10.1159/000277140. [DOI] [PubMed] [Google Scholar]
- Gunnarsson D, Nordberg G, Selstam G. 2007. Differential effects of cadmium on the gene expression of seven-transmembrane-spanning receptors and GAPDH in the rat testis. Toxicol Lett 168(1):51–57, PMID: 17123754, 10.1016/j.toxlet.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Guyton KZ, Chiu WA, Bateson TF, Jinot J, Scott CS, Brown RC, et al. . 2009. A reexamination of the PPAR-α activation mode of action as a basis for assessing human cancer risks of environmental contaminants. Environ Health Perspect 117(11):1664–1672, PMID: 20049115, 10.1289/ehp.0900758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton KZ, Rusyn I, Chiu WA, Corpet DE, van den Berg M, Ross MK, et al. . 2018. Application of the key characteristics of carcinogens in cancer hazard identification. Carcinogenesis 39(4):614–622, PMID: 29562322, 10.1093/carcin/bgy031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habert R, Livera G, Rouiller-Fabre V. 2014. Man is not a big rat: concerns with traditional human risk assessment of phthalates based on their anti-androgenic effects observed in the rat foetus. Basic Clin Androl 24:14, PMID: 25780587, 10.1186/2051-4190-24-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales DB. 2002. Testicular macrophage modulation of Leydig cell steroidogenesis. J Reprod Immunol 57(1–2):3–18, PMID: 12385830, 10.1016/S0165-0378(02)00020-7. [DOI] [PubMed] [Google Scholar]
- Harris S, Shubin SP, Wegner S, Van Ness K, Green F, Hong SW, et al. . 2016. The presence of macrophages and inflammatory responses in an in vitro testicular co-culture model of male reproductive development enhance relevance to in vivo conditions. Toxicol In Vitro 36:210–215, PMID: 27511800, 10.1016/j.tiv.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley DV, Korach KS. 2006. Endocrine-disrupting chemicals use distinct mechanisms of action to modulate endocrine system function. Endocrinology 147(6 Suppl):S25–S32, PMID: 16690802, 10.1210/en.2005-1117. [DOI] [PubMed] [Google Scholar]
- Hill AB. 1965. The environment and disease: association or causation? Proc R Soc Med 58:295–300, PMID: 14283879. [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss AK, Rider CV, Blystone CR, Wilson VS, Hartig PC, Ankley GT, et al. . 2008. Fifteen years after “Wingspread”—environmental endocrine disrupters and human and wildlife health: where we are today and where we need to go. Toxicol Sci 105(2):235–259, PMID: 18281716, 10.1093/toxsci/kfn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL, Rider CV, Wilson VS, Gray LE Jr.. 2008. Mechanisms of action of phthalate esters, individually and in combination, to induce abnormal reproductive development in male laboratory rats. Environ Res 108(2):168–176, PMID: 18949836, 10.1016/j.envres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer). 2019. Mechanistic evidence. In: IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Preamble, 33–35. https://monographs.iarc.fr/wp-content/uploads/2019/01/Preamble-2019.pdf [accessed 4 June 2019].
- Ioannou D, Miller D, Griffin DK, Tempest HG. 2016. Impact of sperm DNA chromatin in the clinic. J Assist Reprod Genet 33(2):157–166, PMID: 26678492, 10.1007/s10815-015-0624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenardhanan P, Panneerselvam M, Mathur PP. 2016. Effect of environmental contaminants on spermatogenesis. Semin Cell Dev Biol 59:126–140, PMID: 27060550, 10.1016/j.semcdb.2016.03.024. [DOI] [PubMed] [Google Scholar]
- Jenkins TG, Aston KI, Meyer TD, Hotaling JM, Shamsi MB, Johnstone EB, et al. . 2016. Decreased fecundity and sperm DNA methylation patterns. Fertil Steril 105(1):51–57.e1–e3, PMID: 26453269, 10.1016/j.fertnstert.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins TG, Aston KI, Pflueger C, Cairns BR, Carrell DT. 2014. Age-associated sperm DNA methylation alterations: possible implications in offspring disease susceptibility. PLoS Genet 10(7):e1004458, PMID: 25010591, 10.1371/journal.pgen.1004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirtle RL, Skinner MK. 2007. Environmental epigenomics and disease susceptibility. Nat Rev Genet 8(4):253–262, PMID: 17363974, 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp RW., Jr. 2010. Male reproductive toxicology. In: Reproductive Toxicology, Kapp RW Jr, Tyl RW, 3rd ed New York, NY:Informa Healthcare, 249–269. [Google Scholar]
- Kitamura A, Miyauchi N, Hamada H, Hiura H, Chiba H, Okae H, et al. . 2015. Epigenetic alterations in sperm associated with male infertility. Congenit Anom (Kyoto) 55(3):133–144, PMID: 26212350, 10.1111/cga.12113. [DOI] [PubMed] [Google Scholar]
- Ko EY, Sabanegh ES Jr, Agarwal A. 2014. Male infertility testing: reactive oxygen species and antioxidant capacity. Fertil Steril 102(6):1518–1527, PMID: 25458618, 10.1016/j.fertnstert.2014.10.020. [DOI] [PubMed] [Google Scholar]
- Kretschmer XC, Baldwin WS. 2005. CAR and PXR: xenosensors of endocrine disrupters? Chem Biol Interact 155(3):111–128, PMID: 16054614, 10.1016/j.cbi.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Lafuente A. 2013. The hypothalamic–pituitary–gonadal axis is target of cadmium toxicity. An update of recent studies and potential therapeutic approaches. Food Chem Toxicol 59:395–404, PMID: 23811532, 10.1016/j.fct.2013.06.024. [DOI] [PubMed] [Google Scholar]
- Laurenzana EM, Weis CC, Bryant CW, Newbold R, Delclos KB. 2002. Effect of dietary administration of genistein, nonylphenol or ethinyl estradiol on hepatic testosterone metabolism, cytochrome P-450 enzymes, and estrogen receptor alpha expression. Food Chem Toxicol 40(1):53–63, PMID: 11731036, 10.1016/S0278-6915(01)00095-3. [DOI] [PubMed] [Google Scholar]
- Lavranos G, Balla M, Tzortzopoulou A, Syriou V, Angelopoulou R. 2012. Investigating ROS sources in male infertility: a common end for numerous pathways. Reprod Toxicol 34(3):298–307, PMID: 22749934, 10.1016/j.reprotox.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, et al. . 2017. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update 23(6):646–659, PMID: 28981654, 10.1093/humupd/dmx022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Xu L, Dunbar JC, Dhabuwala CB. 2003. Role of mitochondrial cytochrome c in cocaine-induced apoptosis in rat testes. Urology 61(3):646–650, PMID: 12639677, 10.1016/S0090-4295(02)02263-X. [DOI] [PubMed] [Google Scholar]
- Li N, Mruk DD, Lee WM, Wong CK, Cheng CY. 2016. Is toxicant-induced Sertoli cell injury in vitro a useful model to study molecular mechanisms in spermatogenesis? Semin Cell Dev Biol 59:141–156, PMID: 26779951, 10.1016/j.semcdb.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lottrup G, Jørgensen A, Nielsen JE, Jørgensen N, Duno M, Vinggaard AM, et al. . 2013. Identification of a novel androgen receptor mutation in a family with multiple components compatible with the testicular dysgenesis syndrome. J Clin Endocrinol Metab 98(6):2223–2229, PMID: 23589523, 10.1210/jc.2013-1278. [DOI] [PubMed] [Google Scholar]
- Lucas B, Fields C, Hofmann MC. 2009. Signaling pathways in spermatogonial stem cells and their disruption by toxicants. Birth Defects Res C Embryo Today 87(1):35–42, PMID: 19306349, 10.1002/bdrc.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrioli D, Belpoggi F, Silbergeld EK, Perry MJ. 2016. Aneuploidy: a common and early evidence-based biomarker for carcinogens and reproductive toxicants. Environ Health 15(1):97, PMID: 27729050, 10.1186/s12940-016-0180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino-Andrade AJ, Chahoud I. 2010. Reproductive toxicity of phthalate esters. Mol Nutr Food Res 54(1):148–157, PMID: 19760678, 10.1002/mnfr.200800312. [DOI] [PubMed] [Google Scholar]
- Masliukaite I, Hagen JM, Jahnukainen K, Stukenborg JB, Repping S, van der Veen F, et al. . 2016. Establishing reference values for age-related spermatogonial quantity in prepubertal human testes: a systematic review and meta-analysis. Fertil Steril 106(7):1652–1657.e2, PMID: 27717555, 10.1016/j.fertnstert.2016.09.002. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Shiina H, Kawano H, Sato T, Kato S. 2008. Androgen receptor functions in male and female physiology. J Steroid Biochem Mol Biol 109(3–5):236–241, PMID: 18434134, 10.1016/j.jsbmb.2008.03.023. [DOI] [PubMed] [Google Scholar]
- Medina-Díaz IM, Arteaga-Illán G, de León MB, Cisneros B, Sierra-Santoyo A, Vega L, et al. . 2007. Pregnane X receptor-dependent induction of the CYP3A4 gene by o,p′-1,1,1,-trichloro-2,2-bis (p-chlorophenyl)ethane. Drug Metab Dispos 35(1):95–102, PMID: 17035600, 10.1124/dmd.106.011759. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Hauser R. 2010. Exposure to polychlorinated biphenyls (PCBs) and male reproduction. Syst Biol Reprod Med 56(2):122–131, PMID: 20377311, 10.3109/19396360903443658. [DOI] [PubMed] [Google Scholar]
- Mocarelli P, Gerthoux PM, Needham LL, Patterson DG Jr, Limonta G, Falbo R, et al. . 2011. Perinatal exposure to low doses of dioxin can permanently impair human semen quality. Environ Health Perspect 119(5):713–718, PMID: 21262597, 10.1289/ehp.1002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J, Skakkebæk NE. 1983. Quantification of germ cells and seminiferous tubules by stereological examination of testicles from 50 boys who suffered from sudden death. Int J Androl 6(2):143–156, PMID: 6862671, 10.1111/j.1365-2605.1983.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Sloper DT, Del Valle PL. 2019. Evaluation of an in vitro mouse testis organ culture system for assessing male reproductive toxicity. Birth Defects Res 111(2):70–77, PMID: 30575315, 10.1002/bdr2.1431. [DOI] [PubMed] [Google Scholar]
- Nielsen CT, Skakkebæk NE, Richardson DW, Darling JAB, Hunter WM, Jørgensen M, et al. . 1986. Onset of the release of spermatozoa (spermarche) in boys in relation to age, testicular growth, pubic hair, and height. J Clin Endocrinol Metabol 62(3):532–535, PMID: 3944237, 10.1210/jcem-62-3-532. [DOI] [PubMed] [Google Scholar]
- Nikolaidis E. 2017. Relevance of animal testing and sensitivity of end points in reproductive and developmental toxicity. In: Reproductive and Developmental Toxicology. Gupta RC, ed., 2nd ed San Diego CA:Elsevier, Academic Press, 211–224. [Google Scholar]
- Niwa T, Murayama N, Imagawa Y, Yamazaki H. 2015. Regioselective hydroxylation of steroid hormones by human cytochromes P450. Drug Metab Rev 47(2):89–110, PMID: 25678418, 10.3109/03602532.2015.1011658. [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council). 2007. Toxicity Testing in the 21st Century: A Vision and a Strategy. Washington, DC:National Academies Press. [Google Scholar]
- NRC. 2017. Using 21st Century Science to Improve Risk-Related Evaluations. Washington, DC:National Academies Press. [PubMed] [Google Scholar]
- OEHHA (Office of Environmental Health Hazard Assessment). 2019. Chemicals Known to the State to Cause Cancer or Reproductive Toxicity (The Proposition 65 List). https://oehha.ca.gov/media/downloads/proposition-65//p65list030819.pdf [accessed 5 June 2019].
- OHAT (Office of Health Assessment and Translation). 2019. Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration, pp. 65–68. https://ntp.niehs.nih.gov/ntp/ohat/pubs/handbookmarch2019_508.pdf [accessed 5 June 2019].
- Paoloni-Giacobino A. 2014. Epigenetic effects of methoxychlor and vinclozolin on male gametes. Vitam Horm 94:211–227, PMID: 24388192, 10.1016/B978-0-12-800095-3.00008-0. [DOI] [PubMed] [Google Scholar]
- Perry MJ, Young HA, Grandjean P, Halling J, Petersen MS, Martenies SE, et al. . 2016. Sperm aneuploidy in Faroese Men with lifetime exposure to dichlorodiphenyldichloroethylene (p,p′-DDE) and polychlorinated biphenyl (PCB) pollutants. Environ Health Perspect 124(7):951–956, PMID: 26535963, 10.1289/ehp.1509779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilsner JR, Parker M, Sergeyev O, Suvorov A. 2017. Spermatogenesis disruption by dioxins: epigenetic reprograming and windows of susceptibility. Reprod Toxicol 69:221–229, PMID: 28286111, 10.1016/j.reprotox.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourmasumi S, Sabeti P, Rahiminia T, Mangoli E, Tabibnejad N, Talebi AR. 2017. The etiologies of DNA abnormalities in male infertility: an assessment and review. Int J Reprod Biomed (Yazd) 15(6):331–344, PMID: 29177237, 10.29252/ijrm.15.6.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh A, Harris KJ, Archibong AE. 2017. Reproductive toxicity of polycyclic aromatic hydrocarbons. In: Reproductive and Developmental Toxicology. Gupta RC, ed., 2nd ed San Diego CA:Elsevier, Academic Press, 745–763. [Google Scholar]
- Reis MM, Moreira AC, Sousa M, Mathur PP, Oliveira PF, Alves MG. 2015. Sertoli cell as a model in male reproductive toxicology: advantages and disadvantages. J Appl Toxicol 35(8):870–883, PMID: 25693974, 10.1002/jat.3122. [DOI] [PubMed] [Google Scholar]
- Rey RA. 2014. Mini-puberty and true puberty: differences in testicular function. Ann Endocrinol (Paris) 75(2):58–63, PMID: 24793991, 10.1016/j.ando.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Rezk BM, Sikka S. 2011. Developmental and reproductive disorders: role of endocrine disruptors in testicular toxicity. In: Reproductive and Developmental Toxicology. Gupta RC, ed. San Diego CA:Elsevier, Academic Press, 903–912. [Google Scholar]
- Rooney AA, Boyles AL, Wolfe MS, Bucher JR, Thayer KA. 2014. Systematic review and evidence integration for literature-based environmental health science assessments. Environ Health Perspect 122(7):711–718, PMID: 24755067, 10.1289/ehp.1307972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeti P, Pourmasumi S, Rahiminia T, Akyash F, Talebi AR. 2016. Etiologies of sperm oxidative stress. Int J Reprod Biomed (Yazd) 14(4):231–240, PMID: 27351024, 10.29252/ijrm.14.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone A, Di Dato C, de Angelis C, Menafra D, Pozza C, Pivonello R, et al. . 2018. Smoke, alcohol and drug addiction and male fertility. Reprod Biol Endocrinol 16(1):3, PMID: 29334961, 10.1186/s12958-018-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer C, Müller A, Egeberg DL, Alvarez L, Brenker C, Rehfeld A, et al. . 2014. Direct action of endocrine disrupting chemicals on human sperm. EMBO Rep 15(7):758–765, PMID: 24820036, 10.15252/embr.201438869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott HM, Mason JI, Sharpe RM. 2009. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev 30(7):883–925, PMID: 19887492, 10.1210/er.2009-0016. [DOI] [PubMed] [Google Scholar]
- Semet M, Paci M, Saïas-Magnan J, Metzler-Guillemain C, Boissier R, Lejeune H, et al. . 2017. The impact of drugs on male fertility: a review. Andrology 5(4):640–663, PMID: 28622464, 10.1111/andr.12366. [DOI] [PubMed] [Google Scholar]
- Sengupta P. 2013. Environmental and occupational exposure of metals and their role in male reproductive functions. Drug Chem Toxicol 36(3):353–368, PMID: 22947100, 10.3109/01480545.2012.710631. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Skakkebaek NE. 2008. Testicular dysgenesis syndrome: mechanistic insights and potential new downstream effects. Fertil Steril 89(2 Suppl):e33–38, PMID: 18308057, 10.1016/j.fertnstert.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Siklenka K, Erkek S, Godmann M, Lambrot R, McGraw S, Lafleur C, et al. . 2015. Disruption of histone methylation in developing sperm impairs offspring health transgenerationally. Science 350(6261):aab2006, PMID: 26449473, 10.1126/science.aab2006. [DOI] [PubMed] [Google Scholar]
- Siu ER, Mruk DD, Porto CS, Cheng CY. 2009. Cadmium-induced testicular injury. Toxicol Appl Pharmacol 238(3):240–249, PMID: 19236889, 10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Buck Louis GM, Toppari J, Andersson AM, Eisenberg ML, et al. . 2016. Male reproductive disorders and fertility trends: influences of environment and genetic susceptibility. Physiol Rev 96(1):55–97, PMID: 26582516, 10.1152/physrev.00017.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK. 2016. Endocrine disruptors in 2015: epigenetic transgenerational inheritance. Nat Rev Endocrinol 12(2):68–70, PMID: 26585656, 10.1038/nrendo.2015.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MT, Guyton KZ, Gibbons CF, Fritz JM, Portier CJ, Rusyn I, et al. . 2016. Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis. Environ Health Perspect 124(6):713–721, PMID: 26600562, 10.1289/ehp.1509912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen K, Aksglaede L, Petersen JH, Juul A. 2010. Recent changes in pubertal timing in healthy Danish boys: associations with body mass index. J Clin Endocrinol Metabol 95(1):263–270, PMID: 19926714, 10.1210/jc.2009-1478. [DOI] [PubMed] [Google Scholar]
- Stenz L, Escoffier J, Rahban R, Nef S, Paoloni-Giacobino A. 2017. Testicular dysgenesis syndrome and long-lasting epigenetic silencing of mouse sperm genes involved in the reproductive system after prenatal exposure to DEHP. PloS One 12(1):e0170441, PMID: 28085963, 10.1371/journal.pone.0170441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammen SA, Friso S, Choi SW. 2013. Epigenetics: the link between nature and nurture. Mol Aspects Med 34(4):753–764, PMID: 22906839, 10.1016/j.mam.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares RS, Escada-Rebelo S, Correia M, Mota PC, Ramalho-Santos J. 2016. The non-genomic effects of endocrine-disrupting chemicals on mammalian sperm. Reproduction 151(1):R1–R13, PMID: 26585413, 10.1530/REP-15-0355. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 1996a. Guidelines for Reproductive Toxicity Risk Assessment. EPA/630/R-96/009. Washington, DC:U.S. EPA, 25–34. [Google Scholar]
- U.S. EPA. 1996b. Proposed guidelines for carcinogen risk assessment. Docket No. FRL-5460-3. Fed Reg 61(79):17960–18011. [Google Scholar]
- U.S. EPA. 2005. Guidelines for Carcinogen Risk Assessment. EPA/630/P-03/001F. Washington, DC:U.S. EPA, Risk Assessment Forum, 1–10. [Google Scholar]
- U.S. EPA. 2017. Toxicological Review of Benzo[a]pyrene [CASRN 50-32-8]. EPA/635/R-17/0003Fa. Washington, DC:U.S. EPA, Office of Research and Development, 30–39. [Google Scholar]
- Urdinguio RG, Bayón GF, Dmitrijeva M, Toraño EG, Bravo C, Fraga MF, et al. . 2015. Aberrant DNA methylation patterns of spermatozoa in men with unexplained infertility. Hum Reprod 30(5):1014–1028, PMID: 25753583, 10.1093/humrep/dev053. [DOI] [PubMed] [Google Scholar]
- Vakalopoulos I, Dimou P, Anagnostou I, Zeginiadou T. 2015. Impact of cancer and cancer treatment on male fertility. Hormones (Athens) 14(4):579–589, PMID: 26732148, 10.14310/horm.2002.1620. [DOI] [PubMed] [Google Scholar]
- van den Driesche S, Kolovos P, Platts S, Drake AJ, Sharpe RM. 2012. Inter-relationship between testicular dysgenesis and Leydig cell function in the masculinization programming window in the rat. PloS One 7(1):e30111, PMID: 22253897, 10.1371/journal.pone.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan HT, Mruk DD, Wong CK, Cheng CY. 2013. Targeting testis-specific proteins to inhibit spermatogenesis: lesson from endocrine disrupting chemicals. Expert Opin Ther Targets 17(7):839–855, PMID: 23600530, 10.1517/14728222.2013.791679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan HT, Zhao YG, Wong MH, Lee KF, Yeung WS, Giesy JP, et al. . 2011. Testicular signaling is the potential target of perfluorooctanesulfonate-mediated subfertility in male mice. Biol Reprod 84(5):1016–1023, PMID: 21209418, 10.1095/biolreprod.110.089219. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Blystone CR, Hotchkiss AK, Rider CV, Gray LE Jr.. 2008. Diverse mechanisms of anti-androgen action: impact on male rat reproductive tract development. Int J Androl 31(2):178–187, PMID: 18315717, 10.1111/j.1365-2605.2007.00861.x. [DOI] [PubMed] [Google Scholar]
- Woldemeskel M. 2017. Toxicologic pathology of the reproductive system. In: Reproductive and Developmental Toxicology. Gupta RC, ed., 2nd ed San Diego CA:Elsevier, Academic Press, 1209–1241. [Google Scholar]
- Wu H, Hauser R, Krawetz SA, Pilsner JR. 2015. Environmental susceptibility of the sperm epigenome during windows of male germ cell development. Curr Environ Health Rep 2(4):356–366, PMID: 26362467, 10.1007/s40572-015-0067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung BH, Wan HT, Law AY, Wong CK. 2011. Endocrine disrupting chemicals: multiple effects on testicular signaling and spermatogenesis. Spermatogenesis 1(3):231–239, PMID: 22319671, 10.4161/spmg.1.3.18019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngson NA, Whitelaw E. 2008. Transgenerational epigenetic effects. Annu Rev Genomics Hum Genet 9:233–257, PMID: 18767965, 10.1146/annurev.genom.9.081307.164445. [DOI] [PubMed] [Google Scholar]
- Zheng SJ, Tian HJ, Cao J, Gao YQ. 2010. Exposure to di(n-butyl)phthalate and benzo(a)pyrene alters IL-1β secretion and subset expression of testicular macrophages, resulting in decreased testosterone production in rats. Toxicol Appl Pharmacol 248(1):28–37, PMID: 20655936, 10.1016/j.taap.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Zini A, Sigman M. 2009. Are tests of sperm DNA damage clinically useful? Pros and cons. J Androl 30(3):219–229, PMID: 19059901, 10.2164/jandrol.108.006908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.