Abstract
Background:
Although growing evidence links air pollution to stroke incidence, less is known about the effect of air pollution on atrial fibrillation (AF), an important risk factor for stroke.
Objectives:
We assessed the associations between air pollution and incidence of AF and stroke. We also sought to characterize the shape of pollutant–disease relationships.
Methods:
The population-based cohort comprised 5,071,956 Ontario residents, age 35–85 y and without the diagnoses of both outcomes on 1 April 2001 and was followed up until 31 March 2015. AF and stroke cases were ascertained using health administrative databases with validated algorithms. Based on annual residential postal codes, we assigned 5-y running average concentrations of fine particulate matter (), nitrogen dioxide (), and ozone () from satellite-derived data, a land-use regression model, and a fusion-based method, respectively, as well as redox-weighted averages of and () for each year. Using Cox proportional hazards models, we estimated the hazard ratios (HRs) and 95% confidence intervals (95% CIs) of AF and stroke with each of these pollutants, adjusting for individual- and neighborhood-level variables. We used newly developed nonlinear risk models to characterize the shape of pollutant–disease relationships.
Results:
Between 2001 and 2015, we identified 313,157 incident cases of AF and 122,545 cases of stroke. Interquartile range increments of , , , and were associated with increases in the incidence of AF [HRs (95% CIs): 1.03 (1.01, 1.04), 1.02 (1.01, 1.03), 1.01 (1.00, 1.02), and 1.01 (1.01, 1.02), respectively] and the incidence of stroke [HRs (95% CIs): 1.05 (1.03, 1.07), 1.04 (1.01, 1.06), 1.05 (1.03, 1.06), and 1.05 (1.04, 1.06), respectively]. Associations of similar magnitude were found in various sensitivity analyses. Furthermore, we found a near-linear association for stroke with , whereas , -, and relationships exhibited sublinear shapes.
Conclusions:
Air pollution was associated with stroke and AF onset, even at very low concentrations. https://doi.org/10.1289/EHP4883
Introduction
Atrial fibrillation (AF) is the leading sustained arrhythmia that frequently precipitates other severe cardiovascular outcomes (McManus et al. 2012). In particular, AF increases the risk of stroke 5-fold (Wolf et al. 1991). Complications from AF-related strokes place an enormous burden on health care systems, such as longer hospitalizations, greater disability and cognitive decline, and higher mortality (Lip 2013; Patel et al. 2014; Schnabel et al. 2015; Wang et al. 2015). In Canada, for example, AF patients who later developed a stroke incurred the highest long-term health care costs in comparison with other major cardiovascular comorbidities (Tawfik et al. 2016). Recent studies suggest that the economic burden of AF placed on health care systems is increasing, given the upward trajectory in terms of prevalence and incidence of AF worldwide (Chugh et al. 2014; Colilla et al. 2013; Lip et al. 2012). In the past two decades, the global incidence of AF has increased from 141.0 to 181.2 per 100,000 person-years among adult males and from 102.0 to 139.7 among females (Chugh et al. 2014). Thus, the prevention of AF, a potentially preventable stroke precursor, by identifying its modifiable risk factors is an important public health priority (The Lancet Neurology 2015).
Ambient air pollution has been increasingly recognized as an important risk factor for cardiovascular morbidity and mortality (Brook et al. 2010). Mechanistic studies have consistently linked air pollution exposure to adverse responses in the cardiovascular system, such as oxidative stress and systemic inflammation, endothelial and vascular dysfunction, and autonomic imbalance (Brook et al. 2010). Evidence from epidemiological (Pieters et al. 2012), animal (Chen and Hwang 2005; Corey et al. 2006), and panel studies (Pope et al. 2004; Schwartz et al. 2005) have also shown reduced heart rate variability and changes in sympathetic and parasympathetic tone from exposures to air pollution. However, epidemiological evidence supporting this hypothesis is limited for AF. To date, in only two studies were the associations between chronic exposure to air pollution and the incidence of AF investigated. A Danish study reported an association between the incident AF and nitrogen dioxide (), and a Swedish study did not report any association with particulate matter (PM) having aerodynamic diameters less than and ( and , respectively) (Monrad et al. 2016; Stockfelt et al. 2017). These findings raise questions about the potential impact of air pollution on AF onset.
In comparison with AF, more epidemiological studies on the association between air pollution and incident stroke have been conducted. A recent review of cohort studies of PM and incident stroke showed a positive association with long-term exposure to (Scheers et al. 2015). A positive association between and incident stroke has been reported in two studies (Andersen et al. 2012; Kim et al. 2017), but not in others (Atkinson et al. 2013; Oudin et al. 2009, 2011; Stafoggia et al. 2014). Furthermore, most of these previous epidemiological studies considered only a linear association between air pollution and stroke incidence, and thus the shape of the pollutant–disease relationship remains unclear. A more accurate characterization of the pollutant–disease relationship may have important implications for health impact assessment.
In the present study, we estimated the associations between 5-y past exposures to ambient , , , and the combined atmospheric oxidant capacity of and (referred to as ) and the incidence of AF and stroke in a large population-based cohort in Ontario, Canada, where air pollution levels are among the lowest in the world. We also characterized the pollutant–disease relationship for stroke and AF in association with these four pollutants.
Methods
Study Design and Participants
The present analysis used the population-based, retrospective Ontario Population Health and Environment Cohort (ONPHEC). We have previously described this cohort in detail (Chen et al. 2016). Briefly, ONPHEC comprises all long-term residents of Ontario (i.e., resided in Ontario for five or more years), age 35 y or older, and registered with the Ontario Health Insurance Plan as of 1 April 1996. This cohort was created by linking various health administrative databases at ICES using unique identifiers. Use of the data for this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a research ethics board.
In this study, we further restricted participants of ONPHEC to those subjects who were between 35 and 85 y of age and did not have any physician-diagnosed AF and history of hospitalization for stroke as of 1 April 2001. All individuals were followed up until the earliest diagnosis of AF or stroke (depending on the outcome under investigation), death, termination of Ontario health insurance (i.e., moving out of Ontario), or the end of the follow-up on 31 March 2015.
Exposure Assessment
We obtained estimates of surface concentrations of from satellite observations of aerosol optical depth based on the Moderate Resolution Imaging Spectroradiometer (MODIS) from the National Aeronautics and Space Administration (NASA) Terra satellite (van Donkelaar et al. 2015). Briefly, aerosol optical depth is a measure of the extinction of electromagnetic radiation by aerosols in an atmospheric column. These estimates of were calibrated using an optimal estimation algorithm in conjunction with a geographically weighted regression of urban land cover, elevation, and aerosol composition. This approach produced the annual average concentrations of surface below 70°N, which includes all of Ontario, at a resolution for the period 1998 through 2012 (van Donkelaar et al. 2014, 2015). These satellite-based estimates are closely aligned with ground measurements of at fixed-site monitors in North America ( for 2004–2008 5-y mean comparison) (van Donkelaar et al. 2015).
Similarly, residential exposure to was estimated from a national land-use regression (LUR) model developed using data from Environment Canada’s National Air Pollution Surveillance (NAPS) (Hystad et al. 2011). The LUR model was constructed by combining measurements of from fixed-site monitors with a range of predictors, including satellite estimates of for the years 2005–2011, road length, industrial land use, and summer rainfall. The resulting LUR model explained 73% of the variability in fixed-site monitor concentrations of in 2006 (Hystad et al. 2011). To incorporate fine-scale geographic variability of from vehicle emissions, a distance-decay gradient based on proximity to highways and major roads was added as a multiplier to the LUR model (Hystad et al. 2011).
To derive exposure to , we used an optimal interpolation technique by combining 8-h maximum concentrations from monitoring stations in the warm seasons (1 May to 31 October) with physically based air quality prediction models. This model, which accounts for meteorological and chemical patterns of , was developed by Environment and Climate Change Canada to produce an annual mean exposure surface of at resolution across Canada from 2002 to 2009 (Pudykiewicz et al. 1997; Robichaud and Ménard 2014). The cross-validation analysis for using 90% of the observations provided an absolute yearly averaged systematic error of by volume (ppbv) and random error of (Robichaud and Ménard 2014).
Because the exposure surface of was derived for 2006, we conducted yearly calibration of the exposure surface by scaling it by the ratio of the mean concentrations at fixed-site stations in Ontario for a given year and that in 2006 (Beelen et al. 2014; Chen et al. 2013b). Using this approach, we produced annual mean estimates of between 1996 and 2015. Additionally, we applied similar scaling approaches to derive exposures to and in all other years where the annual estimates were unavailable between 1996 and 2015 (Hystad et al. 2011; van Donkelaar et al. 2015), similar to previous studies (Beelen et al. 2014; Chen et al. 2017). Because annual estimates of were unavailable before 1998 and after 2012, we extrapolated the estimates in 1998 to years prior and the estimates in 2012 to years after by scaling the 1998 and 2012 surfaces with a ratio between the average concentrations of at all fixed-site monitors across Ontario in a given year in 1996–1998 and 2013–2015 with those in 1998 and 2012, respectively. Furthermore, we created annual exposure surfaces for using long-term average estimates for the years 2002–2009 and annual concentrations of at fixed-site monitors across Ontario between 1996 and 2015.
Both and have been used individually in many epidemiologic studies as the main oxidative atmospheric pollutants. has been shown to induce the release of reactive oxygen species from alveolar macrophage (Kienast et al. 1994), whereas is implicated in increased lipid peroxidation, generation of reactive oxygen species, and increased systemic oxidative stress (Bocci et al. 1998; Broeckaert et al. 2000; Kodavanti et al. 2000; Larini and Bocci 2005). These two gaseous pollutants, in conjunction with nitric oxide, further form a dynamic relationship through atmospheric chemistry and interchange over short time scales (Williams et al. 2014). As a result, there is mounting interest in the role of their combined oxidant capacity on health, especially exerting adverse effects via oxidative stress and other mechanisms such as protein nitration (Williams et al. 2014). Several epidemiological studies have examined the effects of on acute health events, such as daily mortality, respiratory inflammation, and emergency department visits for myocardial infarction (Weichenthal et al. 2016; Williams et al. 2014; Yang et al. 2016), but no studies to date have examined its impact on the incidence of major cardiovascular disease. To derive redox-weighted oxidant capacity of and , we mathematically used redox potentials as the weights such that can be obtained as follows: , as done in previous studies (Bratsch 1989; Weichenthal et al. 2016). This redox-weighted measure accounts for the fact that is a stronger oxidant than (Weichenthal et al. 2016).
For each of the four above metrics of air pollution, we assigned annual exposure estimates for the years 1996 to 2015 according to participants’ annual residential postal codes obtained from the Registered Persons Database, a registry of all Ontario residents with health insurance (Chen et al. 2013a). In urban areas, each postal code corresponds to a side of a street block or a large apartment building, and in rural areas these postal code areas can be much larger.
Case Ascertainment
Using population-based health administrative databases, we ascertained the cases of AF and stroke by applying algorithms, validated against medical records, throughout the study period. We obtained data on hospitalizations, emergency department visits, and physician billing claims through the Canadian Institute of Health Information Discharge Abstract Database (CIHI-DAD), the National Ambulatory Care Reporting System Database (NACRS), and Ontario Health Insurance Plan (OHIP) physician claims database, respectively. These population-based databases contain detailed diagnostic and procedural information for all these health care encounters in Ontario and have been previously validated (Hinds et al. 2016).
Incident AF, both paroxysmal and persistent, was defined as a hospitalization, an emergency department visit, or four physician billings in a 1-y period with a 30-d time interval between each physician billing code (Tu et al. 2016). Hospitalizations and emergency department visits were identified using International Classification of Disease, Ninth (ICD-9) and Tenth (ICD-10) codes 427.31 or 427.32 and I48, respectively. The OHIP diagnostic code for AF falls under 427. A recent validation study found the algorithm to have a sensitivity of 70.8% (95% CI: 64.4, 77.3), specificity of 99.2% (95% CI: 99.0, 99.4), positive predictive value of 70.8% (95% CI: 64.4, 77.3), and negative predictive value of 99.9% (95% CI: 99.0, 99.4) (Tu et al. 2016).
To identify the incidence of stroke, we used first-recorded hospitalization for stroke as the surrogate. Stroke-related hospitalizations have been used frequently in previous studies to determine the time trends of stroke incidence and their association with air pollution and were found to be highly predictive of overall stroke incidence (Kim et al. 2017; Koton et al. 2014; Rosengren et al. 2013; Wellenius et al. 2005). Similar to previous studies (Hall et al. 2016; Tu et al. 2013), we identified an incident diagnosis of stroke as a first-recorded hospitalization due to ischemic stroke (ICD-9 codes 434 and 436 or ICD-10 codes I63.x [excluding I63.6], I64, and H34.1) or hemorrhagic stroke (ICD-9 codes: 430 and 431; ICD-10 codes: I60 and I61). The algorithm used for ischemic stroke had a sensitivity of 79.6% (95% CI: 78.2, 81.0) and positive predictive value of 72.9% (95% CI: 71.5, 74.5). For hemorrhagic stroke, the algorithm had a sensitivity of 70.6% (95% CI: 67.1, 74.0) and positive predictive value of 42.5% (95% CI: 39.6, 45.4) (Hall et al. 2016). The lower sensitivity of incident strokes in comparison with that of other cardiovascular diseases, such as congestive heart failure, may be due to the characteristic symptoms of stroke being more episodic than chronic conditions that are likely to accrue administrative claims (Schultz et al. 2013). Cases of AF and stroke that occurred before baseline were considered prevalent cases and thus were excluded.
Statistical Analysis
We used single-pollutant Cox proportional hazards models to estimate the associations between exposures to , , , and and the incidence of AF and stroke, respectively. To capture longer-term exposure to these selected air pollutants, we assigned estimates of exposure using a 5-y moving average. For instance, an individual’s exposure in 2001 was estimated as the mean exposure over the years from 1996 to 2000. This approach accounted for the variability in exposures associated with residential mobility as well as secular trends of air pollution. In addition, to control for the potential differences in health status between individuals living in the Greater Toronto Area (GTA)—the most populous metropolitan area in Canada—and those elsewhere, we stratified our models by a regional variable indicating residence in the GTA at cohort inception.
We specified three incremental models that sequentially adjusted for known and suspected risk factors of stroke and AF. First, we adjusted for individual-level age at baseline and sex. We then added four time-varying neighborhood-level socioeconomic status variables obtained from the 1996, 2001, and 2006 Canadian Censuses at the dissemination area level: unemployment rate; proportions of residents age 15 y or older who had completed less than high school education; proportions of recent immigrants (i.e., an immigrant who first obtained their immigrant or permanent resident status within the five years before a given census); and community-specific income quintile, which is based on household income and accounts for household size (Statistics Canada 2011). The dissemination area is the smallest standard census geographical unit covering all of Canada, with a population between 400 and 700 persons. Next, we adjusted for two geographic indicator variables at baseline for potential regional patterns in the incidence of AF or stroke that may be caused by factors unrelated to air pollution: a regional indicator variable for southern/northern Ontario, based on the 14 Ontario Local Health Integration Networks; and rural/urban areas using the 2004 Rurality Index for Ontario (Kralj 2000). The last of the incremental models was considered the fully adjusted main model and was further used in sensitivity analyses.
To test the robustness of our analysis, we performed several sensitivity analyses. We additionally adjusted for each of the following covariates in the main model: a) comorbidities ascertained at baseline [including diabetes, hypertension, and congestive heart failure from validated databases in Ontario (Hux et al. 2002; Schultz et al. 2013; Tu et al. 2007), as well as coronary heart disease consisting of unstable angina, stable angina, and myocardial infarction] (Table S1); b) area-level material deprivation in year 2006 based on the Ontario Marginalization Index, which quantifies the degree of marginalization between areas and inequalities in health and social well-being in Ontario (Matheson et al. 2012); c) area-level access to physician care (i.e., the density of primary care and physician specialists at a census subdivision level) at baseline to account for its potential influence on disease diagnosis using the ICES Physician Database in Ontario, collectively measuring access to primary and related health care (Chan and Schultz 2005); d) area-level proportion of visible minorities (individuals belonging to nonwhite, non-Aboriginal ethnic groups) age 15 y or older at baseline; and e) time-varying calendar year as a linear term to adjust for time trends in air pollution and risk of outcomes. In addition, we evaluated the robustness of the standard Cox models by constructing random-effects Cox models with census divisions (equivalent to counties) at baseline as a random effect. Person-years with any missing data for covariates were excluded in the analyses. Last, we examined correlations among all the air pollutants and further conducted analyses using two- and three-pollutant models for both AF and stroke cohorts.
In addition, we applied an indirect adjustment method to assess the impact of unobserved individual-level behavioral factors, specifically smoking, obesity, physical activity, and alcohol consumption. Briefly, we used a method to mathematically adjust the hazard ratios (HRs) for these missing variables while simultaneously controlling for all measured variables (Shin et al. 2014). We estimated the association between the missing covariates and the exposure of interest (i.e., , , , and ) from the auxiliary dataset, which included data from three population-based health surveys, including the 1996 cycle of the National Population Health Survey and the 2000–2001 and 2003 cycles of the Canadian Community Health Survey (Statistics Canada 2007). We also obtained the estimates of association between the missing behavioral risk factors and AF or stroke from the literature, based on the strength of evidence from recent systematic reviews or large epidemiological studies on AF (Chamberlain et al. 2011; Kodama et al. 2011; Ofman et al. 2013; Wanahita et al. 2008) and stroke (Kyu et al. 2016; Larsson et al. 2016; O’Donnell et al. 2010; Strazzullo et al. 2010). Using this information, we mathematically adjusted the HRs for the missing covariates, while simultaneously controlling for the observed covariates (e.g., age and sex).
Moreover, to explore whether certain characteristics may modify the relationship between air pollution and stroke and AF, we conducted subgroup analyses and performed Cochran Q test to assess heterogeneity between subgroups. We examined selected individual-level variables, including age in 10-y intervals (35–44, 45–54, 55–64, 65–74, and 75–85), sex (men/women), comorbid hypertension and diabetes (yes/no), and stroke subtype (ischemic/hemorrhagic). We also conducted subgroup analyses by area-level household income (in quintiles).
Shape of the Concentration–Response Relationships
To characterize the shapes of the concentration–response associations among individual air pollutants with incidence of AF and stroke, we used a newly developed modeling framework, the Shape Constrained Health Impact Function (SCHIF). The detailed methodology is presented elsewhere (Nasari et al. 2016). Briefly, cohort studies have often used natural, restricted, or smoothing splines to assess the shapes of the association among air pollution, incidence of chronic disease, and mortality. in comparison with the spline-based approaches, the SCHIF approach can be used with any regression model and identifies the different shapes of the association between exposure and outcome in a monotonically nondecreasing manner, suitable for health-effect assessments. The SCHIF approach yields an estimate of the logarithm of HR for a unit change in the transformed concentration with a set of additional parameters that control the amount of curvature and shape. It defines transformations of concentration as the product of either a linear or log-linear function of concentration, multiplied by a logistic weighting function:
where was a parameterized, monotonic transformation of pollutant concentration z, and ; r the range in the pollutant concentration; the amount of curvature of the HR function where larger values represent less curvature; and or the two forms of concentration previously used in ambient air pollution cohort studies. The SCHIF permits capturing various nonlinear shapes, including supralinear, near-linear, and sublinear forms. This method has been employed in previous studies to examine the effects of air pollution on various health outcomes, including incidence of major chronic diseases and mortality, at diverse locations (Burnett et al. 2018; Lavigne et al. 2018; Pinault et al. 2017; Weichenthal et al. 2017).
Using the SCHIF, we examined the shape of the relationships for AF and stroke with each air pollutant, adjusting for all available individual and neighborhood-level risk factors used in our main analysis. In our analysis, we used , and () were unknown parameters estimated from the cohort survival data by method described elsewhere (Nasari et al. 2016). The estimation method was based on a routine that selects multiple values of (), and given these values, estimates of and its standard error were obtained using the Cox model. The combination of defined the transformation of the concentration. We restricted the search to values defined by the 0th, 25th, 50th, and 75th percentiles of the exposure distribution, and , resulting in eight model runs. We then identified the model form with the largest log-likelihood value and fixed f for the remainder of the search routing. We then varied up or down by five percentiles from identified in the initial search corresponding to the largest log-likelihood value, until log-likelihood was maximized. If in the initial search was the 0th percentile, we varied upwards only as we could not define percentiles of the exposure distribution below the minimum value.
Based on the ensemble transformations, where we determined the joint risk coefficient and uncertainty weighted by the likelihood values across all models examined, we characterized the shape of the association between each air pollutant, and AF and stroke, respectively (Nasari et al. 2016). Whenever nonlinear shapes for AF and stroke were depicted by SCHIF, we also derived and reported the ensemble HRs and the 95% confidence intervals (CI) within each quartile of air pollution concentrations (i.e., comparing the highest vs. the lowest concentration in each quartile) to describe the possible changes in air pollution risk by different levels of exposure.
We reported HRs and 95% CIs from linear associations and nonlinear associations from SCHIF. The linear associations were expressed per interquartile-range (IQR) increase of (), (), (), and () (referred to as ) to facilitate comparisons among the different air pollutants in this study population. We used the coxme library of R version statistical software (version 3.1.0; R Development Core Team) and SAS Enterprise Guide statistical software (version 6.1; SAS Institute Inc.).
Results
Descriptive Statistics
The cohort comprised 5,071,956 individuals, among whom a total of 313,157 first-recorded physician diagnoses of AF (total of 66,916,668 person-years of follow-up) and 122,545 hospitalizations for stroke (total of 67,869,896 person-years of follow-up) occurred during the follow-up. Of all stroke cases, ischemic stroke comprised 82%. At baseline, the mean age of the cohort was 53.2 y, and the mean age at diagnosis was ∼ 66 y for AF and for stroke (Table 1). In addition, individuals diagnosed with AF or stroke were more likely to be men and were twice as likely to have preexisting cardiovascular conditions as the overall population (e.g., of those diagnosed had hypertension vs. among the entire cohort).
Table 1.
Baseline characteristics of the cohort in Ontario, Canada, in 2001.
| Characteristic | Population | Stroke cases | AF cases |
|---|---|---|---|
| () | () | () | |
| Age [mean ± SD (years)] | 53.2 (12.9) | 65.6 (12.3) | 66.0 (11.7) |
| Age group [n (%)] | |||
| 35–44 | 1,608,823 (32) | 8,612 (7) | 17,824 (6) |
| 45–54 | 1,418,506 (28) | 17,057 (14) | 39,706 (13) |
| 55–64 | 941,155 (18) | 24,395 (20) | 66,544 (21) |
| 65–74 | 687,849 (14) | 37,722 (31) | 103,387 (33) |
| 75–85 | 415,623 (8) | 34,759 (28) | 85,696 (27) |
| Sex [n (%)] | |||
| Men | 2,432,072 (48) | 61,684 (50) | 163,732 (52) |
| Women | 2,639,884 (52) | 60,861 (50) | 149,425 (48) |
| Geographic indicators [n (%)] | |||
| Urban Areaa | 4,310,483 (85) | 103,424 (84) | 265,649 (85) |
| Rural Area | 761,473 (15) | 19,121 (16) | 47,508 (15) |
| Greater Toronto Area | 1,984,630 (39) | 44,184 (36) | 117,356 (37) |
| Non-Greater Toronto Area | 3,087,326 (61) | 78,361 (64) | 195,801 (63) |
| Northern Ontario | 410,828 (8) | 12,009 (10) | 27,558 (9) |
| Southern Ontario | 4,661,128 (91) | 110,536 (90) | 285,599 (91) |
| Comorbidity [n (%)] | |||
| Congestive heart failure | 88,790 (2) | 5,969 (5) | 21,099 (7) |
| Without congestive heart failure | 4,983,166 (98) | 116,576 (95) | 292,058 (93) |
| Hypertension | 1,326,723 (26) | 64,226 (52) | 166,467 (53) |
| Without hypertension | 3,745,233 (74) | 58,319 (48) | 146,690 (47) |
| Diabetes | 414,418 (8) | 23,915 (20) | 50,120 (16) |
| Without diabetes | 4,657,538 (92) | 98,630 (80) | 263,037 (84) |
| Coronary heart disease | 273,627 (5) | 17,370 (14) | 50,224 (16) |
| Without coronary heart disease | 4,798,329 (95) | 105,175 (86) | 262,933 (84) |
| Type of stroke [n (%)] | |||
| Ischemic | — | 100,964 (82) | — |
| Hemorrhagic | — | 21,581 (18) | — |
| Area-level risk factorsb (mean ± SD) | |||
| Proportion with c | 26.0 (10) | 27.6 (10) | 26.8 (10) |
| Unemployment ratec | 6.2 (3) | 6.5 (3) | 6.3 (3) |
| Proportion of recent immigrantsd | 3.8 (5) | 3.8 (6) | 3.8 (5) |
| Income quintile [n (%)] | |||
| Lowest | 869,887 (17) | 25,790 (21) | 58,484 (19) |
| Lower | 1,009,778 (20) | 27,185 (22) | 65,645 (21) |
| Middle | 1,043,174 (21) | 24,705 (20) | 63,458 (20) |
| Upper | 1,044,510 (21) | 22,508 (18) | 60,228 (19) |
| Uppermost | 1,104,607 (22) | 22,357 (18) | 65,342 (21) |
| Access to physician caree | 167 (573) | 142 (525) | 192 (615) |
| Material deprivation | (0.8) | (0.9) | (0.9) |
| Proportion of visible minorities | 0.1 (0.2) | 0.1 (0.2) | 0.2 (0.2) |
Urban areas are defined by Statistics Canada as continuously built-up areas with a population of and a population density of . All others were considered to be rural areas.
All area-level risk factors are derived at the census dissemination area levels, with the exception of access to physician care derived at the census subdivision (i.e., municipality) level. Less than 1% and 0.5% of all observations had missing information on material deprivation and proportion of visible minorities, respectively. All other variables had a complete set of information.
The area-level risk factors were derived from Canadian Census for percentage of population who are age 15 y or older.
Recent immigrants refer to individuals who obtained their immigrant or permanent resident status in Canada in the five years prior to the given census.
The density of primary care and physician specialists at a census subdivision level was derived as a proxy to access to physician care.
At cohort inception in 2001, the mean annual concentrations of , , , and were (), (), (), and (), respectively (Table S2).
Air Pollution and Associations with AF and Stroke
The associations between past exposures to air pollution and the incidence of AF and stroke over the 15-y period are presented in Tables 2 and 3, respectively. In the main model for AF, each IQR increase in exposure to was associated with a of 1.03 (95% CI: 1.01, 1.04), or HR per (i.e., ) of 1.07 (95% CI: 1.04, 1.10), after stratifying by GTA/non-GTA and adjusting for age, sex, area-level socioeconomic status, southern/northern Ontario, and rural/urban areas relative to our observed minimum concentration (Table 2). In addition, we observed positive associations between incident AF and exposures to , , and with of 1.02 (95% CI: 1.01, 1.03) or of 1.02 (95% CI: 1.00, 1.03), 1.01 (95% CI: 1.00, 1.02) or of 1.02 (95% CI: 1.00, 1.03), and 1.01 (95% CI: 1.01, 1.02) or of 1.04 (95% CI: 1.01, 1.06), respectively. Similarly, we observed positive associations between each of the four pollutants and stroke incidence, with varying between 1.04 and 1.05 (Table 3). In 10-unit increments, we found of 1.12 (95% CI: 1.09, 1.16) for , of 1.03 (95% CI: 1.02, 1.05) for , of 1.09 (95% CI: 1.06, 1.11) for , and of 1.13 (95% CI: 1.10, 1.16) for .
Table 2.
Association between 5-year average exposure to ambient , , and and incidence of atrial fibrillation in Ontario, Canada, per interquartile range increment, from 2001 to 2015.
| Hazard ratio (95% confidence interval) | ||||
|---|---|---|---|---|
| Incremental main analysis | ||||
| Age and sexa | 1.01 (1.00, 1.02) | 0.98 (0.97, 0.99) | 1.01 (1.00, 1.01) | 1.00 (0.99, 1.00) |
| b | 1.03 (1.02, 1.05) | 1.03 (1.01, 1.04) | 1.02 (1.01, 1.03) | 1.02 (1.01, 1.03) |
| c | 1.03 (1.01, 1.04) | 1.02 (1.01, 1.03) | 1.01 (1.00, 1.02) | 1.01 (1.01, 1.02) |
| Sensitivity Analysisd | ||||
| 1.03 (1.02, 1.04) | 1.03 (1.01, 1.04) | 1.01 (1.00, 1.02) | 1.02 (1.01, 1.02) | |
| 1.03 (1.01, 1.05) | 1.03 (1.01, 1.04) | 0.99 (0.98, 1.01) | 1.01 (1.00, 1.02) | |
| 1.03 (1.01, 1.04) | 1.02 (1.01, 1.03) | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.02) | |
| 1.03 (1.02, 1.04) | 1.03 (1.01, 1.04) | 1.01 (1.00, 1.01) | 1.01 (1.00, 1.02) | |
| 1.03 (1.01, 1.04) | 1.02 (1.00, 1.03) | 1.01 (1.00, 1.02) | 1.01 (1.01, 1.02) | |
| e | 1.02 (1.01, 1.04) | 1.02 (1.00, 1.03) | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.02) |
| 1-year moving averagef | 1.02 (1.01, 1.03) | 1.01 (0.99, 1.02) | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.01) |
| 3-year moving averageg | 1.02 (1.02, 1.03) | 1.01 (1.00, 1.03) | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.02) |
| Spatial random-effectsh | 1.02 (1.01, 1.03) | 1.01 (1.01, 1.02) | 1.00 (0.98, 1.03) | 1.01 (0.99, 1.03) |
Base model stratified by a dichotomous indicator for residing in the Greater Toronto Area (GTA) or outside the GTA.
Four area-level variables at dissemination area level were added to the base model: income quintile, proportion of individuals with less than high school education, unemployment rate, and proportion of recent immigrants who obtained landed immigrant or permanent residency status within five years of Canadian census.
Considered the main model, which included two geographic indicators (i.e., northern/southern Ontario and urban/rural areas) to the base model and all previous variables labeled with “b.”
Each sensitivity analysis variable was added to the main model “c.” Comorbidities included diabetes, hypertension, congestive heart failure, and coronary heart disease. Material deprivation and visible minorities were derived at the dissemination area level, access to physician care was derived at the census subdivision level, and the rest of covariates at individual level.
Indirectly adjusted for smoking, physical activity, obesity, and alcohol consumption.
Associations per interquartile range of (), (), (), and ().
Associations per interquartile range of (), (), (), and ().
One-level random-effects Cox models adjusted for covariates in the main model “c,” and random-effects represented by one level of spatial clusters defined by census divisions (equivalent to counties).
Table 3.
Association between 5-year average exposure to ambient , , , and and incidence of stroke in Ontario, Canada, per interquartile range increment from 2001 to 2015.
| Hazard ratio (95% confidence interval) | ||||
|---|---|---|---|---|
| Incremental Main Analysis | ||||
| Age, sexa | 1.01 (0.99, 1.04) | 0.98 (0.96, 1.01) | 1.01 (1.00, 1.02) | 1.00 (0.99, 1.01) |
| b | 1.05 (1.03, 1.07) | 1.05 (1.02, 1.08) | 1.02 (1.01, 1.03) | 1.03 (1.01, 1.04) |
| c | 1.05 (1.03, 1.07) | 1.04 (1.01, 1.06) | 1.05 (1.03, 1.06) | 1.05 (1.04, 1.06) |
| Sensitivity Analysisd | ||||
| 1.05 (1.04, 1.06) | 1.05 (1.03, 1.07) | 1.05 (1.04, 1.06) | 1.05 (1.04, 1.06) | |
| 1.08 (1.05, 1.10) | 1.05 (1.02, 1.08) | 1.08 (1.06, 1.11) | 1.09 (1.06, 1.12) | |
| 1.05 (1.03, 1.07) | 1.05 (1.02, 1.07) | 1.05 (1.02, 1.07) | 1.04 (1.03, 1.05) | |
| 1.05 (1.03, 1.07) | 1.04 (1.02, 1.07) | 1.04 (1.03, 1.06) | 1.04 (1.03, 1.06) | |
| 1.05 (1.03, 1.07) | 1.04 (1.01, 1.07) | 1.05 (1.03, 1.06) | 1.05 (1.04, 1.06) | |
| e | 1.05 (1.03, 1.06) | 1.04 (1.01, 1.06) | 1.05 (1.03, 1.06) | 1.04 (1.03, 1.05) |
| 1-year moving averagef | 1.03 (1.01, 1.04) | 1.02 (1.00, 1.04) | 1.06 (1.04, 1.07) | 1.04 (1.03, 1.05) |
| 3-year moving averageg | 1.04 (1.03, 1.05) | 1.04 (1.02, 1.07) | 1.05 (1.04, 1.06) | 1.05 (1.04, 1.06) |
| Spatial random effectsh | 1.05 (1.02, 1.07) | 1.04 (1.01, 1.07) | 1.04 (1.02, 1.07) | 1.05 (1.02, 1.07) |
Base model stratified by a dichotomous indicator for residing in the Greater Toronto Area (GTA) or outside the GTA.
Four area-level variables at dissemination area level were added to the model including base model: income quintile, proportion of individuals with less than high school education, unemployment rate, and proportion of recent immigrants who obtained landed immigrant or permanent residency status within five years of Canadian census.
Considered the main model, which included two geographic indicators (i.e., northern/southern Ontario and urban/rural areas) to the base model and all previous variables labeled with “b.”
Each sensitivity analysis variable was added to the main model “c.” Comorbidities included diabetes, hypertension, congestive heart failure, and coronary heart disease. Material deprivation and visible minorities were derived at the dissemination area level, access to physician care was derived at the census subdivision level, and the rest of covariates at individual level.
Indirectly adjusted for smoking, physical activity, obesity, and alcohol consumption.
Associations per interquartile range of (), (), (), and ().
Associations per interquartile range of (), (), (), and ().
One-level random-effects Cox models adjusted for covariates in the main model “c,” and random-effects represented by one level of spatial clusters defined by census divisions (equivalent to counties).
In the sensitivity analyses with AF, our indirect adjustment for smoking, alcohol consumption, obesity, and physical activity did not result in any appreciable change in association (Table 2). The estimated association was also insensitive to additional control for access to physician care, material deprivation, visible minorities, and adding random effects to account for potential spatial clustering. Similarly, for stroke, the associations with all four air pollutants remained robust to most of the sensitivity analyses (Table 3), with somewhat stronger associations observed after further adjusting for material deprivation with (HR 1.08; 95% CI, 1.05, 1.10) and (HR 1.09; 95% CI, 1.06, 1.12). Furthermore, in the analysis using multipollutant models, we found that for AF, associations with an IQR increase were stronger for than for the other three air pollutants. For stroke, on the other hand, we observed that associations with were stronger than associations with but weaker than associations with and in two-pollutant models, whereas in the three-pollutant model, the association with was weaker than the associations with both and (Table S3). Exposure to was moderately correlated with , whereas and had a weak inverse correlation, and all others were more strongly correlated (Table S4).
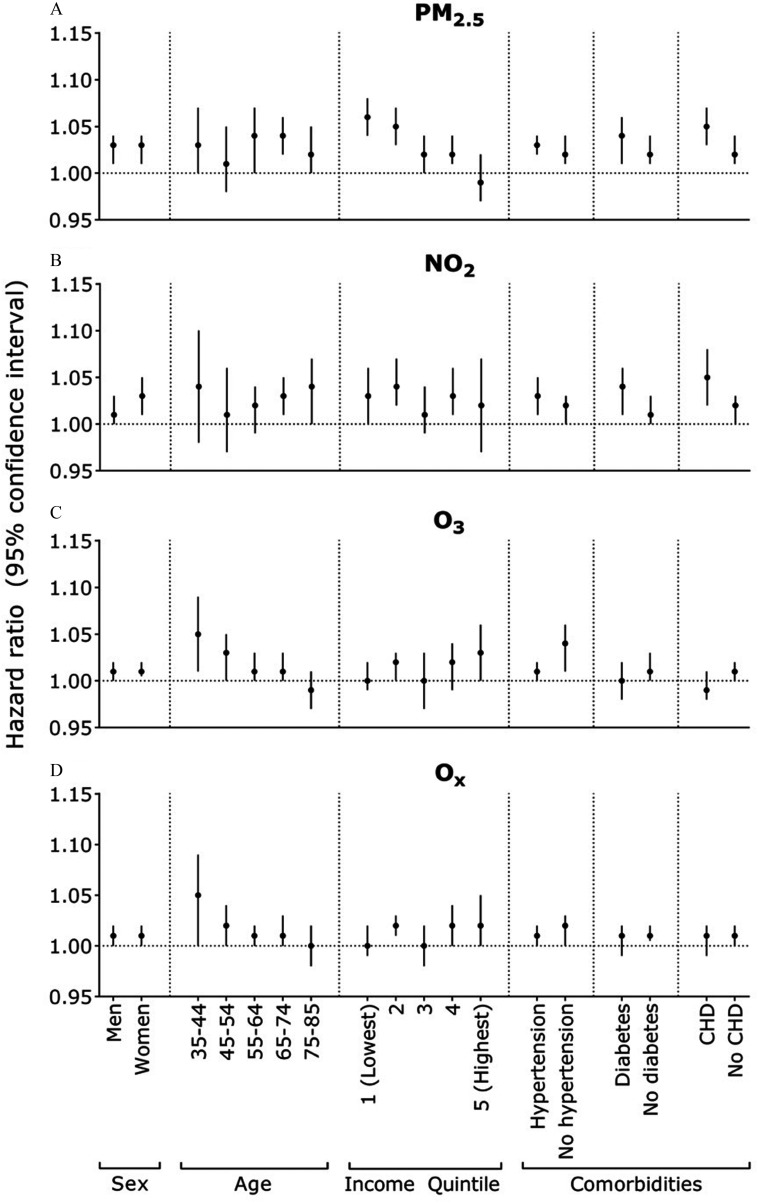
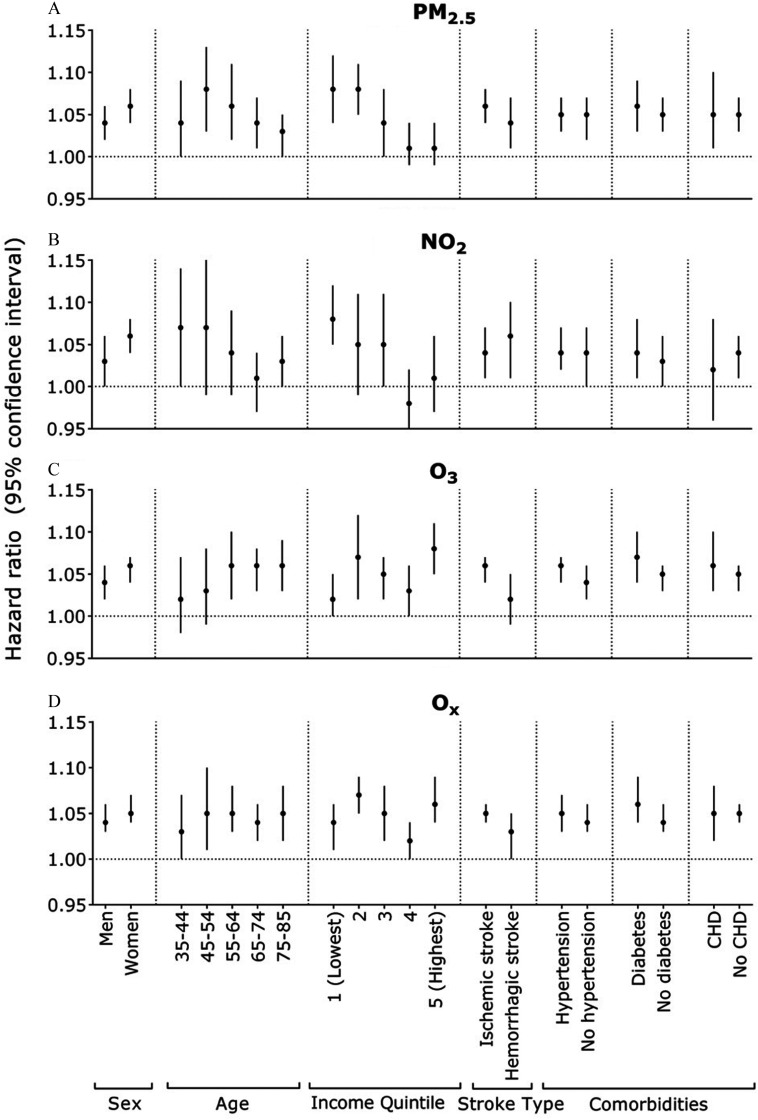
In our exploratory subgroup analyses, observed that individuals with lower incomes tended to exhibit a higher association for AF with ( 1.06; 95% CI: 1.04, 1.08 for the lowest income vs. 0.99; 95% CI: 0.97, 1.02 for the highest income) (Figure 1, Table S5). In addition, ischemic stroke was generally more strongly associated with most air pollutants than hemorrhagic stroke (e.g., 1.06; 95% CI: 1.04, 1.08 vs. 1.04; 95% CI: 1.01, 1.07 for ), with being the exception (Figure 2, Table S6). We observed a similar association for stroke and ( 1.08; 95% CI: 1.04, 1.12 for the lowest income vs. 1.01; 95% CI: 0.99, 1.04 for the highest income). Furthermore, for stroke, the younger age groups exhibited an elevated association with exposure to (Figure 2). A similar pattern was observed with across all age groups, with the exception of the youngest individuals. Conversely, stronger associations with and were found among older age groups.
Figure 1.
Hazard ratios for the associations between air pollution (for each interquartile range) and atrial fibrillation, stratified by certain characteristics. Interquartile range values for pollutants: (A) (), (B) (), (C) () and (D) (). Each subgroup analysis used the fully adjusted model, stratified by an indicator for living in the Greater Toronto Area or not, and adjusted for age, sex, area-level socioeconomic status (education, recent immigrants, unemployment rate, and income quintile), urban/rural area, and northern/southern Ontario, except for the subgroup variable of interest. CHD, coronary heart disease.
Figure 2.
Hazard ratios for the associations between air pollution (for each interquartile range) and stroke, stratified by certain characteristics. Interquartile range values for pollutants: (), (), () and (). Each subgroup analysis used the fully adjusted model, stratified by an indicator for living in the Greater Toronto Area or not, and adjusted for age, sex, area-level socioeconomic status (education, recent immigrants, unemployment rate, and income quintile), urban/rural area, and northern/southern Ontario, except for the subgroup variable of interest. CHD, coronary heart disease.
Concentration–Response Relationships
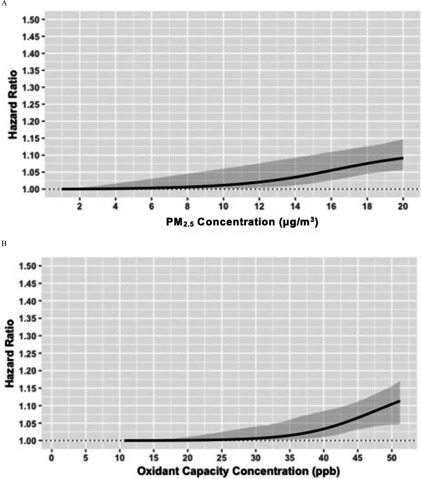
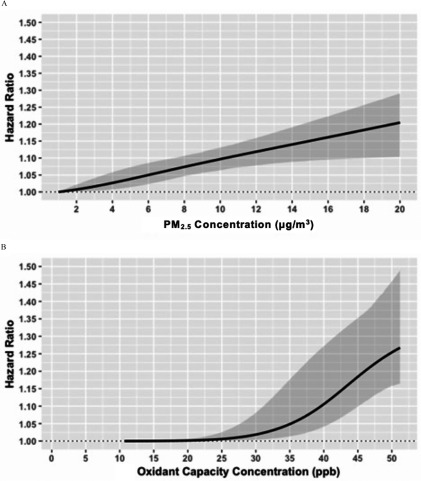
The shapes of the pollutant-disease relationships for AF and stroke with and are illustrated in Figures 3 and 4, respectively. For AF, we observed a tendency for sublinear relationships with both and (Figure 3), with some evidence for potential thresholds at relatively low levels of the two pollutants (at for and for ). For stroke, we also noted a sublinear association between and stroke, with no apparent association below . In contrast, we observed a near-linear association between stroke and , with a pronounced effect across the entire range of exposure. An important aspect that we observed was an increased association for incident stroke (HR 1.10; 95% CI: 1.06, 1.13) at for , which is the current Canadian Ambient Air Quality Standards and the World Health Organization (WHO) guideline (Environment and Climate Change Canada 2013; WHO), relative to the minimum concentration of . Furthermore, for both AF and stroke, we found supralinear and sublinear relationships with and , respectively (Figures S1 and S2).
Figure 3.
Shapes of the concentration–response relationship between atrial fibrillation, and . Gray area represents the 95% confidence interval (CI). Fully adjusted model, stratified by an indicator for living in the Greater Toronto Area or not, and adjusted for age, sex, area-level socioeconomic status (education, recent immigrants, unemployment rate, and income quintile), urban/rural area, and northern/southern Ontario.
Figure 4.
Shapes of the concentration–response relationships between stroke, and . Gray area represents the 95% confidence interval (CI). Fully adjusted model, stratified by an indicator for living in the Greater Toronto Area or not, and adjusted for age, sex, area-level socioeconomic status (education, recent immigrants, unemployment rate, and income quintile), urban/rural area, and northern/southern Ontario.
In view of some evidence of nonlinear associations between past exposures to air pollution and the incidence of AF and stroke in our cohorts, we provide an alternative description of the pollutant–disease relationships using the ensemble estimates of the HRs from all nonlinear models examined (Table S7). We compared the HRs of highest with the lowest concentrations in each quartile of air pollution concentrations. For example, for the -AF relationship, we compared the 25th with first percentiles (6.5 vs. ) in the lowest quartile, median with 25th percentile (8.7 vs. ) in the second quartile, 75th percentile with median (10.6 vs. ) in the third quartile, and 99th with 75th percentiles (14.6 vs. ) in the highest quartile. We found that the incidence of AF increased with a HR (95% CI) of 1.00 (0.98, 1.02) in the lowest quartile, 1.00 (0.97, 1.03) in the second, 1.01 (0.97, 1.05) in the third, and 1.03 (0.98, 1.08) in the highest quartile of . Similarly, we found an increase in association with HR (95% CI) of 1.011 (0.981, 1.042), 1.005 (0.964, 1.048), 1.006 (0.960, 1.054), and 1.019 (0.969, 1.07) in each quartile, respectively of . For stroke, we also observed that the incidence of stroke increased with with HR (95% CI) of 1.03 (1.00, 1.07) in the lowest quartile, 1.02 (0.98, 1.07) in the second, 1.02 (0.98, 1.07) in the third and 1.04 (0.98, 1.10) in the highest quartile. We found a similar pattern of association using quintiles of exposures (Table S8).
Discussion
In this population-based cohort study in Ontario, Canada, we observed modest associations between AF incidence and long-term exposure to , and to a lesser degree, with , , and . We also found that all four pollutants were consistently associated with higher incidence of hospitalizations for stroke. All pollutants with the exception of were more strongly associated with ischemic stroke than with hemorrhagic stroke. These results were robust to most sensitivity analyses, including further adjustments for comorbidities, proportion of visible minorities, and individual-level behavioral risk factors. Furthermore, we observed that individuals with lower income exhibited a heightened risk for developing AF and stroke as a result of exposure.
To date, epidemiologic evidence on the potential effect of ambient air pollution on AF is scarce. A handful of time–series studies examined the short-term impact of air pollution on episodes of AF, and most have found that exposure to air pollution, particularly and , is associated with an increased risk of AF (Bunch et al. 2011; Liao et al. 2011; Link et al. 2013; Milojevic et al. 2014; Rich et al. 2006). To our knowledge, only two cohort studies have examined the relationship with long-term exposure, and they reported inconsistent results (Monrad et al. 2016; Stockfelt et al. 2017). In a Swedish cohort study, Stockfelt et al. (2017) reported no associations for and with AF incidence. In contrast, we found positive associations between the incidence of AF and multiple air pollutants, especially . This finding was consistent with existing evidence on the proarrhythmic effects of , which may trigger changes in autonomic tone and reduce heart rate variability, as shown in previous animal studies (Chen and Hwang 2005; Corey et al. 2006) and panel studies (Pope et al. 2004; Schwartz et al. 2005). In addition, a Danish cohort study showed an 8% higher incidence of AF (95% CI: 1.01, 1.14) for every increase in and a 16% higher risk of AF (95% CI: 1.02, 1.32) between the highest and the lowest quintiles of exposure to nitrogen oxides () (Monrad et al. 2016). We also found that long-term exposures to and were positively (albeit marginally) associated with AF. Although some emerging evidence has linked and to short-term autonomic imbalance (Devlin et al. 2012; Gold et al. 2000; Srebot et al. 2009), more research is required on the possible proarrhythmic effects of air pollution.
In comparison with AF, there is converging epidemiological evidence on the effects of on stroke. A recent review of 10 epidemiological studies on air pollution and incident stroke reported the pooled HRs for each increment in as 1.06 (95% CIs: 1.02, 1.11) (Scheers et al. 2015). The positive association we found between and incident stroke ( 1.05; 95% CI: 1.03, 1.07) further strengthens this evidence. Furthermore, we found that the shape of the -stroke relationship was near linear without any discernible threshold. This result suggests that the adverse effect of on increasing stroke risk may exist even at very low concentrations. In contrast, less is known about the association between stroke incidence and gaseous pollutants (e.g., and ). In a population-based study conducted in South Korea, the risk of hospitalization for stroke was found to be positively associated with ( 2.65; 95% CI: 2.29, 3.06), but inversely associated with ( 0.60; 95% CI: 0.55, 0.65) (Kim et al. 2017). In another study conducted in Denmark, higher levels of were associated with ischemic stroke ( 1.05; 95% CI: 0.99, 1.11), but not with hemorrhagic stroke (Andersen et al. 2012). More recently, a cohort study from Stockholm County, Sweden, reported that traffic-related exposure was associated with a HR of 1.16 (95% CI: 0.83, 1.61) per increment for ischemic and hemorrhagic strokes combined (Korek et al. 2015). Furthermore, in the European Study of Cohorts for Air Pollution Effects (ESCAPE) study, the incidence of stroke was not linked to gaseous pollutants ( and ) (Stafoggia et al. 2014). In our study, we found that exposures to and were associated with a 4% to 5% increase in the risk of stroke over the IQR. The variability in results could be due to possible differences in outcome ascertainment; our study and some others (Andersen et al. 2012; Kim et al. 2017; Korek et al. 2015) used first-recorded hospitalizations, whereas others used interviews and medical records (Stafoggia et al. 2014).
For the first time, we investigated the possible combined oxidative effects of and () from long-term exposure. Ambient and are known to exert adverse health effects by generating reactive oxygen species, which then trigger oxidative stress in the cardiovascular system (Chen et al. 2007; Devlin et al. 2012; Ho et al. 2013; Srebot et al. 2009). Although individual and have been used extensively in epidemiological studies as the main oxidative pollutants, the impact of their combined oxidant capacity remains less clear. Though several studies have considered the effect of on acute health events, such as daily mortality, biomarkers of respiratory inflammation, and emergency department visits for myocardial infarction (Weichenthal et al. 2016; Williams et al. 2014; Yang et al. 2016), no studies to date have examined its long-term impact. In the present study, we used a redox-weighted approach, combining and to estimate . We found that incident AF had a modest positive association with ( 1.01; 95% CI: 1.01, 1.02), whereas incident stroke was associated with a 5% higher risk per IQR increase in (95% CI: 1.04, 1.06). Our findings of a detrimental effect on stroke and AF from corroborate the role of oxidative stress from air pollution on cardiovascular health.
Given that individual effects of and are difficult to separate by the complex chemical interrelationship, the weighted approach to measure oxidative capacity accounted for the chemical redox potentials and represented the total impact of these two gaseous pollutants on health through the oxidative stress mechanism. Thus, using as a single exposure metric may offer additional insights into the inextricable atmospheric chemistry in the combination and its true impact on human health (particularly, AF and stroke in this study) than either or alone. In addition, it has been shown that using may overcome the statistical limitations of collinearity, confounding, or differential measurement errors on the different exposures that may arise from assessing and in multipollutant models in traditional epidemiologic studies (Dominici et al. 2010; Vedal and Kaufman 2011). Given that few epidemiological studies have used as an exposure metric and only the short-term impact had been examined previously, more research is warranted to further consider to elucidate which metric(s) of common gaseous pollutants is most appropriate and relevant to health outcomes.
Contrary to the near-linear shape for the -stroke association, we found sublinear shapes for all other associations (, , and ), indicating population threshold concentrations below which an effect of air pollution on AF or stroke could not be detected. The steep curves beyond of for both stroke and AF suggest that continued efforts to reduce the annual average of below this level are warranted to reduce the detrimental cardiovascular effects of the combined oxidant capacity of and . Importantly, for stroke, we found a pronounced positive association of at concentrations below the current Canada-wide standards and WHO guidelines for annual average of (). These results collectively suggest that our efforts to maintain low concentrations of , even below the current WHO guidelines, could lead to tangible benefits in preventing AF and stroke, and the importance of considering the shapes of the pollutant–disease relationship when conducting health impact assessments of air pollution. Potential reasons for this finding of a population threshold may include biological damage to cardiovascular system at relatively high concentration levels to instigate a measurable oxidative stress response (Delfino et al. 2011), or increased exposure measurement errors at low levels of , given the relatively coarse resolution of the exposure surface.
Many experimental studies have examined the possible biological mechanisms for long-term exposure to pollution on cardiovascular health (Brook et al. 2010). Particularly, through inhalation and translocation to the blood may trigger adverse effects on the vascular system, such as endothelial dysfunction and atherosclerosis, which may reduce the blood supply to the atrial tissues and affect the contraction of the atria (Brook et al. 2010; Heeringa et al. 2007; Kido et al. 2011). In addition, exposure to and its deposition in the lung may lead to alterations in the autonomic nervous system, including induction of atrial electrophysiology changes (Liao et al. 2011; Perez et al. 2015). Several animal studies documented that exposure to concentrated ambient particles increased atherosclerotic plaque and temporary occlusion of coronary artery resulting in ischemia (Bartoli et al. 2009; Soares et al. 2009; Sun et al. 2008). Additionally, previous studies on air pollution and markers of cardiovascular health documented positive associations between and fibrinogen, a procoagulant in plasma that may induce alveolar inflammation, and increased serum levels of inflammatory interleukin-6 (IL-6) (Bind et al. 2013; Panasevich et al. 2009). Other studies have linked exposure to increased venous thrombosis, platelet aggregation, thrombin generation, and biomarkers of systemic inflammation, such as IL-6 (Dales et al. 2010; Rudež et al. 2009; Thompson et al. 2010). These effects, implicated in the pathogenic pathways of cardiovascular diseases, may in turn elevate the risk of AF and stroke.
AF has previously been shown to be a potent risk factor for stroke due to clinical complications, including cardiac thrombus formation and systemic embolism (Christiansen et al. 2016). Despite this pathway and accumulating evidence on the increased risk of stroke from exposure to air pollution, little is known about whether the burden of air pollution-related AF contributes to the increased risk of stroke associated with air pollution exposures. In our study, we found a positive association between ambient air pollution exposure and AF and stroke, separately. Given these results, further research, especially studies using formal causal mediation analysis, on the possibility of AF as a potential mediator in the association between ambient air pollution and incidence of stroke is warranted, which will further elucidate the pathological mechanism of stroke and its relationship with AF.
Our study has some limitations. Using health administrative databases, we were only able to identify physician-diagnosed cases of stroke and AF, and therefore may have missed undiagnosed cases of both conditions. Ischemic and hemorrhagic stroke cases were identified by a first hospitalization, and AF cases by a first hospitalization, emergency department visit, or a physician claim, reflecting only the severe cases that required immediate medical attention. We also used the date of physician diagnosis of AF, which may not represent the exact time of onset. Moreover, given the episodic nature of paroxysmal AF, which terminates spontaneously or with intervention in less than seven days, we may have underestimated the true incidence rate of paroxysmal AF in Ontario. This possible underestimation likely attenuated our associations towards the null, given that misclassification of AF diagnosis was expected to be nondifferential across Ontario. In addition, the exclusion of prevalent AF and stroke cases at cohort inception might have led to an underestimation of the associations. Furthermore, we lacked information on individual-level lifestyle and behavioral risk factors, which may have led to residual confounding. To assess the influence of potential residual confounding, we further adjusted for comorbidities expected to be related to lifestyle behaviors. In addition, we conducted an indirect adjustment for these variables. Although our results remained robust to these sensitivity analyses, we could not completely rule out the possibility of some residual confounding. We also lacked information on exposure to air pollution other than where people lived, including exposures from occupation, commuting, and pollution originating in indoor sources. Thus, we were unable to precisely characterize cumulative exposures to ambient and indoor air pollution. This lack of precision may have further attenuated our effect estimates due to nondifferential misclassification in our area-based exposure assessment. Last, the relatively coarse resolution of the exposure surface of reduced our ability to capture the finer variation in exposures or the photochemical reactions forms with other pollutants, which may result in larger uncertainties in characterizing the association for AF and stroke. Given that our area-based exposure assessment was likely subject to nondifferential misclassification, these factors may have attenuated our effect estimates.
The study has notable strengths. To our knowledge, this is the largest epidemiological study to date to examine the impact of air pollution on the incidence of AF, an important risk factor for stroke and other cardiovascular consequences. We also used a large population-based cohort of Ontario with virtually complete 14-y follow-up. Given Ontario’s universal access to physician care, potential for selection bias was minimized. In addition, using this large population-based cohort, we explored the shape of concentration–response relationships between common particulate and gaseous air pollutants and the incidence of AF and stroke. Furthermore, our study benefited from using as an exposure metric to examine the combined impact of exposures to and . We accounted for residential mobility by assigning exposures to the residential postal codes of all subjects for each year of follow-up, which reduced potential exposure misclassification. We also had access to a rich record of individual and area-level risk factors, such as individual health data and demographic characteristics, collected by health administrative databases, which allowed us to adjust for many important risk factors.
Conclusion
We found evidence of positive associations between long-term exposure to air pollution below the current standards, especially , and both stroke and AF. Our results highlight the importance of continuing to improve air quality, even in areas with relatively low concentrations such as Ontario, Canada.
Supplementary Material
Acknowledgments
This study was funded by Canadian Institutes of Health Research (MOP-133463) and Health Canada (MOU-HT421-17-2802). This study was also supported by ICES, which is funded by annual grants from the Ontario Ministry of Health and Long-Term Care (MOHLTC). This study also received support from Public Health Ontario (PHO). Parts of this material are based on data and information compiled and provided by Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions, and statements expressed herein are not necessarily those of CIHI, PHO, ICES, and MOHLTC. All copyrights and intellectual property conducted at Health Canada belong to the Crown, not to the authors. J.K. is supported by a Clinician Scientist Award from the Department of Family and Community Medicine, University of Toronto. K.T. is supported by a Research Scholar Award from the Department of Family and Community Medicine, University of Toronto.
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP4883).
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Andersen ZJ, Kristiansen LC, Andersen KK, Olsen TS, Hvidberg M, Jensen SS. 2012. Stroke and long-term exposure to outdoor air pollution from nitrogen dioxide: a cohort study. Stroke 43(2):320–325, PMID: 22052517, 10.1161/STROKEAHA.111.629246. [DOI] [PubMed] [Google Scholar]
- Atkinson RW, Carey IM, Kent AJ, Van Staa TP, Ross Anderson H, Cook DG. 2013. Long-term exposure to outdoor air pollution and incidence of cardiovascular diseases. Epidemiology 24(1):44–53, PMID: 23222514, 10.1097/EDE.0b013e318276ccb8. [DOI] [PubMed] [Google Scholar]
- Bartoli CR, Wellenius GA, Coull BA, Akiyama I, Diaz EA, Lawrence J, et al. 2009. Concentrated ambient particles alter myocardial blood flow during acute ischemia in conscious canines. Environ Health Perspect 117(3):333–337, PMID: 19337504, 10.1289/ehp.11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beelen R, Raaschou-Nielsen O, Stafoggia M, Andersen ZJ, Weinmayr G, Hoffmann B, et al. 2014. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet 383(9919):785–795, PMID: 24332274, 10.1016/S0140-6736(13)62158-3. [DOI] [PubMed] [Google Scholar]
- Bind M-A, Baccarelli A, Zanobetti A, Tarantini L, Suh H, Vokonas P, et al. 2013. Air pollution and markers of coagulation, inflammation, and endothelial function: associations and epigene-environment interactions in an elderly cohort. Epidemiology 23(2):332–340, PMID: 22237295, 10.1097/EDE.0b013e31824523f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocci V, Valacchi G, Corradeschi F, Aldinucci C, Silvestri S, Paccagnini E, et al. 1998. Studies on the biological effects of ozone: 7. Generation of reactive oxygen species (ROS) after exposure of human blood to ozone. J Biol Regul Homeost Agents 12(3):67–75, PMID: 9795834. [PubMed] [Google Scholar]
- Bratsch SG. 1989. Standard electrode potentials and temperature coefficients in water at 298.15 K. J Phys Chem Ref Data 18(1):1–21, 10.1063/1.555839. [DOI] [Google Scholar]
- Broeckaert F, Arsalane K, Hermans C, Bergamaschi E, Brustolin A, Mutti A, et al. 2000. Serum Clara cell protein: a sensitive biomarker of increased lung epithelium permeability caused by ambient ozone. Environ Health Perspect 108(6):533–537, PMID: 10856027, 10.1289/ehp.00108533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez-Roux AV, et al. 2010. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121(21):2331–2378, PMID: 20458016, 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Bunch TJ, Horne BD, Asirvatham SJ, Day JD, Crandall BG, Weiss JP, et al. 2011. Atrial fibrillation hospitalization is not increased with short-term elevations in exposure to fine particulate air pollution. Pacing Clin Electrophysiol 34(11):1475–1479, PMID: 21895725, 10.1111/j.1540-8159.2011.03200.x. [DOI] [PubMed] [Google Scholar]
- Burnett R, Chen H, Szyszkowicz M, Fann N, Hubbell B, Pope C, et al. 2018. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci USA 115(38):9592–9597, PMID: 30181279, 10.1073/pnas.1803222115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain AM, Agarwal SK, Folsom AR, Duval S, Soliman EZ, Ambrose M, et al. 2011. Smoking and incidence of atrial fibrillation: results from the Atherosclerosis Risk in Communities (ARIC) study. Hear Rhythm 8(8):1160–1166, PMID: 21419237, 10.1016/j.hrthm.2011.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan BT, Schultz SE. 2005. Supply and Utilization of General Practitioner and Family Physician Services in Ontario. ICES Investigative Report.
- Chen C, Arjomandi M, Balmes J, Tager I, Holland N. 2007. Effects of chronic and acute ozone exposure on lipid peroxidation and antioxidant capacity in healthy young adults. Environ Health Perspect 115(12):1732–1737, PMID: 18087591, 10.1289/ehp.10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Burnett RT, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, et al. 2013a. Risk of incident diabetes in relation to long-term exposure to fine particulate matter in Ontario. Environ Health Perspect 121(7):804–810, PMID: 23632126, 10.1289/ehp.1205958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Goldberg MS, Burnett RT, Jerrett M, Wheeler AJ, Villeneuve PJ. 2013b. Long-term exposure to traffic-related air pollution and cardiovascular mortality. Epidemiology 24(1):35–43, PMID: 23222554, 10.1097/EDE.0b013e318276c005. [DOI] [PubMed] [Google Scholar]
- Chen LC, Hwang JS. 2005. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice: IV. Characterization of acute and chronic effects of ambient air fine particulate matter exposures on heart-rate variability. Inhal Toxicol 17(4–5):209–216, PMID: 15804938, 10.1080/08958370590912789. [DOI] [PubMed] [Google Scholar]
- Chen H, Kwong JC, Copes R, Hystad P, van Donkelaar A, Tu K, et al. 2017. Exposure to ambient air pollution and the incidence of dementia: a population-based cohort study. Environ Int 108:271–277, PMID: 28917207, 10.1016/j.envint.2017.08.020. [DOI] [PubMed] [Google Scholar]
- Chen H, Kwong JC, Copes R, Villeneuve PJ, Goldberg MS, Weichenthal S, et al. 2016. Cohort profile: The ONtario Population Health and Environment Cohort (ONPHEC). Int J Epidemiol 1–11, PMID: 27097745, 10.1093/ije/dyw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen CB, Gerds TA, Olesen JB, Kristensen SL, Lamberts M, Lip GYH, et al. 2016. Atrial fibrillation and risk of stroke: a nationwide cohort study. Europace 18(11):1689–1697, PMID: 26838693, 10.1093/europace/euv401. [DOI] [PubMed] [Google Scholar]
- Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. 2014. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 129(8):837–847, PMID: 24345399, 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. 2013. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol 112(8):1142–1147, PMID: 23831166, 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- Corey LM, Baker C, Luchtel DL. 2006. Heart-rate variability in the apolipoprotein E knockout transgenic mouse following exposure to Seattle particulate matter. J Toxicol Environ Health. A 69(10):953–965, PMID: 16728373, 10.1080/15287390500362105. [DOI] [PubMed] [Google Scholar]
- Dales RE, Cakmak S, Vidal CB. 2010. Air pollution and hospitalization for venous thromboembolic disease in Chile. J Thromb Haemost 8(4):669–674, PMID: 20088925, 10.1111/j.1538-7836.2010.03760.x. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Vaziri ND.. 2011. Air pollution and circulating biomarkers of oxidative stress. Air Qual Atmos Heal 4(1):37–52, PMID: 23626660, 10.1007/s11869-010-0095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin RB, Duncan KE, Jardim M, Schmitt MT, Rappold AG, Diaz-Sanchez D. 2012. Controlled exposure of healthy young volunteers to ozone causes cardiovascular effects. Circulation 126(1):104–111, PMID: 22732313, 10.1161/CIRCULATIONAHA.112.094359. [DOI] [PubMed] [Google Scholar]
- Dominici F, Peng RD, Barr CD, Bell ML.. 2010. Protecting human health from air pollution: Shifting from a single-pollutant to a multipollutant approach. Epidemiol 21(2):187–194, PMID: 20160561, 10.1097/EDE.0b013e3181cc86e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environment and Climate Change Canada. 2013. Canadian Ambient Air Quality Standards. http://www.ec.gc.ca/default.asp?lang=En&n=56D4043B-1&news=A4B2C28A-2DFB-4BF4-8777-ADF29B4360BD [accessed 21 August 2018].
- Gold DR, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B, et al. 2000. Ambient pollution and heart rate variability. Circulation 101(11):1267–1273, PMID: 10725286, 10.1161/01.CIR.101.11.1267. [DOI] [PubMed] [Google Scholar]
- Hall R, Mondor L, Porter J, Fang J, Kapral MK. 2016. Accuracy of administrative data for the coding of acute stroke and TIAs. Can J Neurol Sci 43(6):765–773, PMID: 27426016, 10.1017/cjn.2016.278. [DOI] [PubMed] [Google Scholar]
- Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Rooij FJ, Lip GY, et al. 2007. Subclinical atherosclerosis and risk of atrial fibrillation: the Rotterdam study. Arch Intern Med 167(4):382–387, PMID: 17325300, 10.1001/archinte.167.4.382. [DOI] [PubMed] [Google Scholar]
- Hinds A, Lix LM, Smith M, Quan H, Sanmartin C. 2016. Quality of administrative health databases in Canada: a scoping review. Can J Public Health 107(1):e56–e61, PMID: 27348111, 10.17269/cjph.107.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho E, Karimi Galougahi K, Liu C-C, Bhindi R, Figtree GA. 2013. Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol 1:483–491, PMID: 24251116, 10.1016/j.redox.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hux JE, Ivis F, Flintoft V, Bica A. 2002. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 25(3):512–516, PMID: 11874939, 10.2337/diacare.25.3.512. [DOI] [PubMed] [Google Scholar]
- Hystad P, Setton E, Cervantes A, Poplawski K, Deschenes S, Brauer M, et al. 2011. Creating national air pollution models for population exposure assessment in Canada. Environ Health Perspect 119(8):1123–1129, PMID: 21454147, 10.1289/ehp.1002976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido T, Tamagawa E, Bai N, Suda K, Yang HHC, Li Y, et al. 2011. Particulate matter induces translocation of IL-6 from the lung to the systemic circulation. Am J Respir Cell Mol Biol 44(2):197–204, PMID: 20378751, 10.1165/rcmb.2009-0427OC. [DOI] [PubMed] [Google Scholar]
- Kienast K, Knorst M, Lubjuhn S, Müller-Quernheim J, Ferlinz R. 1994. Nitrogen dioxide-induced reactive oxygen intermediates production by human alveolar macrophages and peripheral blood mononuclear cells. Arch Environ Health 49(4):246–250, PMID: 8031179, 10.1080/00039896.1994.9937474. [DOI] [PubMed] [Google Scholar]
- Kim H, Kim J, Kim S, Kang S, Kim H, Kim H, et al. 2017. Cardiovascular effects of long‐term exposure to air pollution: a population‐based study with 900 845 person‐years of follow‐up. J Am Heart Assoc 6:e007170, PMID: 29118034, 10.1161/JAHA.117.007170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama S, Saito K, Tanaka S, Horikawa C, Saito A, Heianza Y, et al. 2011. Alcohol consumption and risk of atrial fibrillation: a meta-analysis. J Am Coll Cardiol 57(4):427–436, PMID: 21251583, 10.1016/j.jacc.2010.08.641. [DOI] [PubMed] [Google Scholar]
- Kodavanti UP, Schladweiler MC, Ledbetter AD, Watkinson WP, Campen MJ, Winsett DW, et al. 2000. The spontaneously hypertensive rat as a model of human cardiovascular disease: evidence of exacerbated cardiopulmonary injury and oxidative stress from inhaled emission particulate matter. Toxicol Appl Pharmacol 164(3):250–263, PMID: 10799335, 10.1006/taap.2000.8899. [DOI] [PubMed] [Google Scholar]
- Korek MJ, Bellander TD, Lind T, Bottai M, Eneroth KM, Caracciolo B, et al. 2015. Traffic-related air pollution exposure and incidence of stroke in four cohorts from Stockholm. J Expo Sci Environ Epidemiol 25(5):517–523, PMID: 25827311, 10.1038/jes.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koton S, Schneider ALC, Rosamond WD, Shahar E, Sang Y, Gottesman RF, et al. 2014. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA 312(3):259–268, PMID: 25027141, 10.1001/jama.2014.7692. [DOI] [PubMed] [Google Scholar]
- Kralj B. 2000. Measuring “rurality” for purposes of health-care planning: an empirical measure for Ontario. Ont Med Rev 33–52. [Google Scholar]
- Kyu H, Bachman V, Alexander L, Mumford J, Afshin A, Estep K, et al. 2016. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ 354:, PMID: 27510511, 10.1136/bmj.i3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larini A, Bocci V. 2005. Effects of ozone on isolated peripheral blood mononuclear cells. Toxicol Vitr 19(1):55–61, PMID: 15582356, 10.1016/j.tiv.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Larsson SC, Wallin A, Wolk A, Markus HS. 2016. Differing association of alcohol consumption with different stroke types: a systematic review and meta-analysis. BMC Med 14(1):178, PMID: 27881167, 10.1186/s12916-016-0721-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne É, Bélair M-A, Duque DR, Do MT, Stieb DM, Hystad P, et al. 2018. Effect modification of perinatal exposure to air pollution and childhood asthma incidence. Eur Respir J 51, PMID: 29419440, 10.1183/13993003.01884-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Shaffer ML, He F, Rodriguez-Colon S, Wu R, Whitsel EA, et al. 2011. Fine particulate air pollution is associated with higher vulnerability to atrial fibrillation-the APACR study. J Toxicol Environ Health 74(11):693–705, PMID: 21480044, 10.1080/15287394.2011.556056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link MS, Luttmann-Gibson H, Schwartz J, Mittleman MA, Wessler B, Gold DR, et al. 2013. Acute exposure to air pollution triggers atrial fibrillation. J Am Coll Cardiol 62(9):816–825, PMID: 23770178, 10.1016/j.jacc.2013.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lip GY. 2013. Stroke and bleeding risk assessment in atrial fibrillation: when, how, and why? Eur Heart J 34(14):1041–1049, PMID: 23257951, 10.1093/eurheartj/ehs435. [DOI] [PubMed] [Google Scholar]
- Lip GYH, Brechin C, Lane D, Al E. 2012. The global burden of atrial fibrillation and stroke: a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest 142(6):1489–1498, PMID: 22459778, 10.1378/chest.11-2888. [DOI] [PubMed] [Google Scholar]
- Matheson FI, Dunn JR, Smith KLW, Moineddin R, Glazier RH. 2012. Development of the Canadian Marginalization Index: a new tool for the study of inequality. Can J Public Health 103(8 suppl 2):S12–S16, PMID: 23618065, 10.17269/cjph.103.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus DD, Rienstra M, Benjamin EJ. 2012. An update on the prognosis of patients with atrial fibrillation. Circulation 126(10): e143–e146, PMID: 22949543, 10.1161/CIRCULATIONAHA.112.129759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milojevic A, Wilkinson P, Armstrong B, Bhaskaran K, Smeeth L, Hajat S. 2014. Short-term effects of air pollution on a range of cardiovascular events in England and Wales: case-crossover analysis of the MINAP database, hospital admissions and mortality. Heart 100(14):1093–1098, PMID: 24952943, 10.1136/heartjnl-2013-304963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monrad M, Sajadieh A, Christensen JS, Ketzel M, Raaschou-Nielsen O, Tjønneland A, et al. 2016. Long-term exposure to traffic-related air pollution and risk of incident atrial fibrillation: a cohort study. Environ Health Perspect 422–427, PMID: 27472911, 10.1289/EHP392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasari MM, Szyszkowicz M, Chen H, Crouse D, Turner MC, Jerrett M, et al. 2016. A class of non-linear exposure-response models suitable for health impact assessment applicable to large cohort studies of ambient air pollution. Air Qual Atmos Health 9(8):961–972, PMID: 27867428, 10.1007/s11869-016-0398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. 2010. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 376(9735):112–123, PMID: 20561675, 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- Ofman P, Khawaja O, Rahilly-Tierney CR, Peralta A, Hoffmeister P, Reynolds MR, et al. 2013. Regular physical activity and risk of atrial fibrillation: a systematic review and meta-analysis. Circ Arrhythmia Electrophysiol 6(2):252–256, PMID: 23515264, 10.1161/CIRCEP.113.000147. [DOI] [PubMed] [Google Scholar]
- Oudin A, Stroh E, Strömberg U, Jakobsson K, Björk J. 2009. Long-term exposure to air pollution and hospital admissions for ischemic stroke. A register-based case-control study using modelled NOx as exposure proxy. BMC Public Health 9:301, PMID: 19691845, 10.1186/1471-2458-9-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudin A, Strömberg U, Jakobsson K, Stroh E, Lindgren AG, Norrving B, et al. 2011. Hospital admissions for ischemic stroke: does long-term exposure to air pollution interact with major risk factors? Cerebrovasc Dis 31(3):284–293, PMID: 21196728, 10.1159/000322600. [DOI] [PubMed] [Google Scholar]
- Panasevich S, Leander K, Rosenlund M, Ljungman P, Bellander T, de Faire U, et al. 2009. Associations of long- and short-term air pollution exposure with markers of inflammation and coagulation in a population sample. Occup Environ Med 66(11):747–753, PMID: 19687019, 10.1136/oem.2008.043471. [DOI] [PubMed] [Google Scholar]
- Patel NJ, Deshmukh A, Pant S, Singh V, Patel N, Arora S, et al. 2014. Trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation 129(23):2371–2379, PMID: 24842943, 10.1161/CIRCULATIONAHA.114.008201. [DOI] [PubMed] [Google Scholar]
- Perez CM, Hazari MS, Farraj AK. 2015. Role of autonomic reflex arcs in cardiovascular responses to air pollution exposure. Cardiovasc Toxicol 15(1):69–78, PMID: 25123706, 10.1007/s12012-014-9272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters N, Plusquin M, Cox B, Kicinski M, Vangronsveld J, Nawrot TS. 2012. An epidemiological appraisal of the association between heart rate variability and particulate air pollution: a meta-analysis. Heart 98(15):1127, 10.1136/heartjnl-2011-301505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault LL, Weichenthal S, Crouse DL, Brauer M, Erickson A, Donkelaar A. V, et al. 2017. Associations between fine particulate matter and mortality in the 2001 Canadian Census Health and Environment Cohort. Environ Res 159:406–415, PMID: 28850858, 10.1016/j.envres.2017.08.037. [DOI] [PubMed] [Google Scholar]
- Pope CA, Hansen ML, Long RW, Nielsen KR, Eatough NL, Wilson WE, et al. 2004. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect, PMID: 14998750, 10.1289/ehp.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudykiewicz JA, Kallaur A, Smolarkiewicz PK. 1997. Semi-Lagrangian modelling of tropospheric ozone. Tellus B: Chem Phys Meteorol 49(3):231–248, 10.1034/j.1600-0889.49.issue3.1.x. [DOI] [Google Scholar]
- Rich DQ, Mittleman MA, Link MS, Schwartz J, Luttmann-Gibson H, Catalano PJ, et al. 2006. Increased risk of paroxysmal atrial fibrillation episodes associated with acute increases in ambient air pollution. Environ Health Perspect 114(1):120–123, PMID: 16393668, 10.1289/ehp.8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichaud A, Ménard R. 2014. Multi-year objective analyses of warm season ground-level ozone and PM2.5 over North America using real-time observations and Canadian operational air quality models. Atmos Chem Phys 14(4):1769–1800, 10.5194/acp-14-1769-2014. [DOI] [Google Scholar]
- Rosengren A, Giang KW, Lappas G, Jern C, Torén K, Björck L. 2013. Twenty-four-year trends in the incidence of ischemic stroke in Sweden from 1987 to 2010. Stroke 44(9):2388–2393, PMID: 23839506, 10.1161/STROKEAHA.113.001170. [DOI] [PubMed] [Google Scholar]
- Rudež G, Janssen NAH, Kilinc E, Leebeek FWG, Gerlofs-Nijland ME, Spronk HMH, et al. 2009. Effects of ambient air pollution on hemostasis and inflammation. Environ Health Perspect 117(6):995–1001, PMID: 19590696, 10.1289/ehp.0800437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheers H, Jacobs L, Casas L, Nemery B, Nawrot TS. 2015. Long-term exposure to particulate matter air pollution is a risk factor for stroke: meta-analytical evidence. Stroke 46(11):3058–3066, PMID: 26463695, 10.1161/STROKEAHA.115.009913. [DOI] [PubMed] [Google Scholar]
- Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, et al. 2015. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet 386(9989):154–162, PMID: 25960110, 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz SE, Rothwell DM, Chen Z, Tu K. 2013. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Canada 33:160–166, PMID: 23735455. [PubMed] [Google Scholar]
- Schwartz J, Litonjua A, Suh H, Verrier M, Zanobetti A, Syring M, et al. 2005. Traffic related pollution and heart rate variability in a panel of elderly subjects. Thorax 60(6):455–461, PMID: 15923244, 10.1136/thx.2004.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HH, Cakmak S, Brion O, Villeneuve P, Turner MC, Goldberg MS, et al. 2014. Indirect adjustment for multiple missing variables applicable to environmental epidemiology. Environ Res 134:482–487, PMID: 24972508, 10.1016/j.envres.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Soares SRC, Carvalho-Oliveira R, Ramos-Sanchez E, Catanozi S, da Silva LFF, Mauad T, et al. 2009. Air pollution and antibodies against modified lipoproteins are associated with atherosclerosis and vascular remodeling in hyperlipemic mice. Atherosclerosis 207(2):368–373, PMID: 19486979, 10.1016/j.atherosclerosis.2009.04.041. [DOI] [PubMed] [Google Scholar]
- Srebot V, Gianicolo EA, Rainaldi G, Trivella MG, Sicari R. 2009. Ozone and cardiovascular injury. Cardiovasc Ultrasound 7: , PMID: 19552797, 10.1186/1476-7120-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafoggia M, Cesaroni G, Peters A, Andersen ZJ, Badaloni C, Beelen R, et al. 2014. Long-term exposure to ambient air pollution and incidence of cerebrovascular events: Results from 11 European cohorts within the ESCAPE project. Environ Health Perspect 122(9):919–925, PMID: 24835336, 10.1289/ehp.1307301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics Canada. 2007. Canadian Community Health Survey – Annual component. http://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&Id=3359 [accessed 19 August 2018].
- Statistics Canada. 2011. User guide for the Survey of Household Spending, 2011. http://www.statcan.gc.ca/pub/62f0026m/2013001/part-partie1-eng.htm [accessed 19 August 2018].
- Stockfelt L, Andersson EM, Molnár P, Gidhagen L, Segersson D, Rosengren A, et al. 2017. Long-term effects of total and source-specific particulate air pollution on incident cardiovascular disease in Gothenburg, Sweden. Environ Res 158:61–71, PMID: 28600978, 10.1016/j.envres.2017.05.036. [DOI] [PubMed] [Google Scholar]
- Strazzullo P, D'Elia L, Cairella G, Garbagnati F, Cappuccio FP, Scalfi L. 2010. Excess body weight and incidence of stroke: meta-analysis of prospective studies with 2 million participants. Stroke 41(5):, PMID: 20299666, 10.1161/STROKEAHA.109.576967. [DOI] [PubMed] [Google Scholar]
- Sun Q, Yue P, Kirk RI, Wang A, Moatti D, Jin X, et al. 2008. Ambient air particulate matter exposure and tissue factor expression in atherosclerosis. Inhal Toxicol 20(2):127–137, PMID: 18236227, 10.1080/08958370701821482. [DOI] [PubMed] [Google Scholar]
- Tawfik A, Wodchis WP, Pechlivanoglou P, Hoch J, Husereau D, Krahn M. 2016. Using phase-based costing of real-world data to inform decision-analytic models for atrial fibrillation. Appl Health Econ Health Policy 14(3):313–322, PMID: 26924098, 10.1007/s40258-016-0229-2. [DOI] [PubMed] [Google Scholar]
- The Lancet Neurology. 2015. Time for action on atrial fibrillation. Lancet Neurol 14:867, PMID: 26293556, 10.1016/S1474-4422(15)00194-5. [DOI] [PubMed] [Google Scholar]
- Thompson AMS, Zanobetti A, Silverman F, Schwartz J, Coull B, Urch B, et al. 2010. Baseline repeated measures from controlled human exposure studies: associations between ambient air pollution exposure and the systemic inflammatory biomarkers IL-6 and fibrinogen. Environ Health Perspect 118(1):120–124, PMID: 20056584, 10.1289/ehp.0900550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu K, Campbell NR, Chen Z-L, Cauch-Dudek KJ, McAlister FA. 2007. Accuracy of administrative databases in identifying patients with hypertension. Open Med 1(1):e18–26, PMID: 20101286. [PMC free article] [PubMed] [Google Scholar]
- Tu K, Nieuwlaat R, Cheng SY, Wing L, Ivers N, Atzema CL, et al. 2016. Identifying patients with atrial fibrillation in administrative data. Can J Cardiol 32(12):1561–1565, PMID: 27742459, 10.1016/j.cjca.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Tu K, Wang M, Young J, Green D, Ivers NM, Butt D, et al. 2013. Validity of administrative data for identifying patients who have had a stroke or transient ischemic attack using EMRALD as a reference standard. Can J Cardiol 29(11):1388–1394, PMID: 24075778, 10.1016/j.cjca.2013.07.676. [DOI] [PubMed] [Google Scholar]
- van Donkelaar A, Martin RV, Brauer M, Boys BL. 2014. Use of satellite observations for long-term exposure assessment of global concentrations of fine particulate matter. Environ Health Perspect 110:135–143, PMID: 25343779, 10.1289/ehp.1408646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Donkelaar A, Martin RV, Spurr RJD, Burnett RT. 2015. High-resolution satellite-derived PM2.5 from optimal estimation and geographically weighted regression over North America. Environ Sci Technol 49(17):10482–10491, PMID: 26261937, 10.1021/acs.est.5b02076. [DOI] [PubMed] [Google Scholar]
- Vedal S, Kaufman JD. 2011. What does multi-pollutant air pollution research mean? Am J Respir Crit Care Med 183(1):4–6, PMID: 21193783, 10.1164/rccm.201009-1520ED. [DOI] [PubMed] [Google Scholar]
- Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. 2008. Atrial fibrillation and obesity-results of a meta-analysis. Am Heart J 155(2):310–315, PMID: 18215602, 10.1016/j.ahj.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Wang G, Joo H, Tong X, George MG. 2015. Hospital costs associated with atrial fibrillation for patients with ischemic stroke aged 18-64 years in the United States. Stroke 46(5):1314–1320, PMID: 25851767, 10.1161/STROKEAHA.114.008563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichenthal S, Lavigne E, Evans G, Pollitt K, Burnett RT. 2016. Ambient PM2.5 and risk of emergency room visits for myocardial infarction: impact of regional PM2.5 oxidative potential: a case-crossover study. Environ Health 15:46, PMID: 27012244, 10.1186/s12940-016-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichenthal S, Pinault LL, Burnett RT. 2017. Impact of oxidant gases on the relationship between outdoor fine particulate air pollution and nonaccidental, cardiovascular, and respiratory mortality. Sci Rep 7 (1):16401, PMID: 29180643, 10.1038/s41598-017-16770-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellenius GA, Schwartz J, Mittleman MA. 2005. Air pollution and hospital admissions for ischemic and hemorrhagic stroke among Medicare beneficiaries. Stroke 36(12):2549–2553, PMID: 16254223, 10.1161/01.STR.0000189687.78760.47. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization). Ambient (outdoor) air quality and health fact sheet. http://www.who.int/mediacentre/factsheets/fs313/en/ [accessed 15 June 2018].
- Williams ML, Atkinson RW, Anderson HR, Kelly FJ. 2014. Associations between daily mortality in London and combined oxidant capacity, ozone and nitrogen dioxide. Air Qual Atmos Health 7(4):407–414, PMID: 25431629, 10.1007/s11869-014-0249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf PA, Abbott RD, Kannel WB. 1991. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 22(8):983–988, PMID: 1866765, 10.1161/01.STR.22.8.983. [DOI] [PubMed] [Google Scholar]
- Yang C, Li H, Chen R, Xu W, Wang C, Tse LA, et al. 2016. Combined atmospheric oxidant capacity and increased levels of exhaled nitric oxide. Environ Res Lett 11(7):074014, 10.1088/1748-9326/11/7/074014. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.